Abstract
Concomitant prevention of SARS-CoV-2 and extensively drug-resistant bacteria transmission is a difficult challenge in intensive care units dedicated to COVID-19 patients. We report a nosocomial cluster of four patients carrying NDM-1 plasmid-encoded carbapenemase-producing Enterobacter cloacae. Two main factors may have contributed to cross-transmission: misuse of gloves and absence of change of personal protective equipment, in the context of COVID-19-associated shortage. This work highlights the importance of maintaining infection control measures to prevent CPE cross-transmission despite the difficult context and that this type of outbreak can potentially involve several species of Enterobacterales.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-021-01022-6.
Keywords: Enterobacter cloacae, Carbapenemase, Cross transmission, Personal protective equipment, Intensive care unit, COVID-19
Introduction
The SARS-CoV-2 pandemic and antimicrobial resistance are two major public health problems that have so far rarely been intertwined [1, 2]. Unfortunately, in regions with high prevalence of carbapenemase-producing Enterobacterales (CPE) such as the Southwest Indian Ocean area (SIOA), some tertiary hospitals may be simultaneously confronted with both phenomena [3]. We describe here a nosocomial cluster of NDM-1 carbapenemase-producing Enterobacter cloacae (NDM-1 Ec) in an intensive care unit (ICU) of the University Hospital of Reunion Island (UHRI).
Reunion Island is a French overseas territory located close to Madagascar with similar healthcare standards to those found in mainland France. Patients with COVID-19 hypoxemic pneumonia from the SIOA requiring ICU are evacuated to the UHRI, considered as the reference hospital in the region. During the SARS-CoV-2 epidemic, one of the three subunits of the ICU (subunit C) was dedicated to suspected or confirmed COVID-19 patients.
Methods
Infection control policies and CPE screening
The ICU of the UHRI consists of three subunits (A, B and C), each containing 10 single rooms. UHRI's policies consist in accordance to the French guidelines of isolation precautions and screening of all admitted patients coming from a foreign country (including Mayotte Island) or recently hospitalized in another healthcare facility. In addition, patients hospitalized in the ICU were systematically screened for carriage of multidrug-resistant microorganisms, upon admission and weekly thereafter. Patients were kept on contact precautions until the first negative result was obtained. Furthermore, excreta management was optimally handled with bedpans equipped with gel bags and additionally with protective bags in case of CPE colonization (“Care Bag” type); and washed between each use in a bedpan washer. Regular meetings and audits were organized between the intensive care and infection control teams to evaluate these infection prevention practices. CPE screening was performed on rectal swabs by culture on selective chromogenic agar (ChromID CARBA Smart, BioMérieux, Marcy l’Etoile, France) according to the manufacturer’s recommendations.
CPE identification and antimicrobial susceptibility testing
Bacterial species was identified using MALDI-TOF mass spectrometry (Microflex, Bruker daltonics, Bremen, Germany). Carbapenemase production was confirmed using immuno-chromatographic assay (NG Rapid Test CARBA-5, NG Biotech, Guipry, France) and multiplex PCR (Xpert Carba, Cepheid, Sunnywale, USA). Antibiotic susceptibility testing was performed by agar diffusion according to the 2020 EUCAST recommendations (www.eucast.org).
Molecular genotyping and bioinformatics analysis
Clonality between isolates was evaluated by pulsed-field gel electrophoresis and multi-locus sequence typing as previously described [3]. Conjugation experiments were performed using the azide-resistant laboratory strain Escherichia coli J53 as previously described [4]. Plasmid DNA of the transconjugant was extracted using the QIAGEN Maxiprep kit according to the manufacturer’s recommendations. Sequencing of the extracted plasmid and of the genome of E. cloacae donor strain was performed on the MinION using an R9 (FLO-MIN106) flowcell. Antibiotic resistance genes were detected by querying the ResFinder database v. 4.0 [5]. The plasmid sequence generated is available on the Genbank database under the accession number MW464182. The detailed bioinformatics protocol is supplied in the supplementary material (Additional file 1).
Results
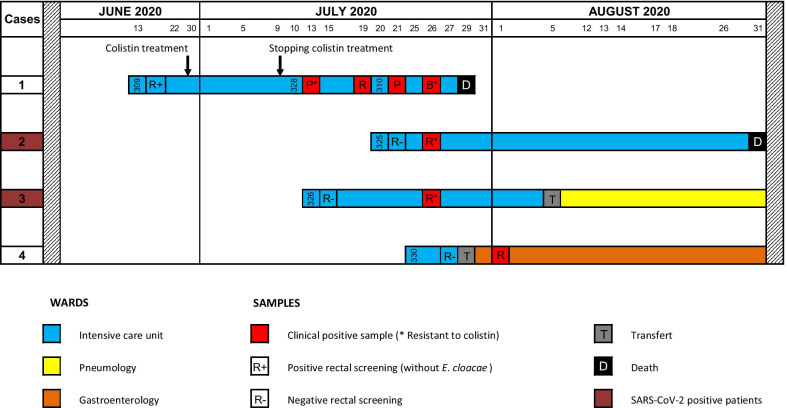
Case 1, considered as the index case, was hospitalized in ICU on 2020 June 13 (COVID-19 suspected pneumonia). He was positive for Escherichia coli and Klebsiella pneumoniae both producing NDM-1 on admission and for NDM-1 Ec for the first time on July 13, one month after its admission (Fig. 1). During July and August 2020, three other patients hospitalized in adjacent rooms tested positive for NDM-1 Ec. Case 1, originating from Reunion Island, died from bacteremia with NDM-1 Ec; the other three cases were colonized. Cases 2 and 3 had been transferred from Madagascar and were hospitalized for acute respiratory distress due to COVID-19. Case 4 came from Reunion Island and was hospitalized for 3 days in subunit C for a digestive haemorrhage (due to saturation of the 2 other subunits). The systematic screening on admission was negative for Cases 2, 3 and 4. During the period of the outbreak, 201 patients were hospitalized in the ICU, of which 94 were hospitalized in subunit C; all (exept the four cases) were tested negative for NDM-1 Ec. A total of seven isolates were collected (Fig. 1). Three isolates were not susceptible to all β-lactams (including cefiderocol), fluoroquinolones, sulfamethoxazole-trimethoprim, amikacin, and four were additionally resistant to colistin (Minimal Inhibitory Concentration—MIC, 16 mg/L) and to tigecycline (MIC, 2 mg/L). They remained susceptible only to gentamicin (MIC, 1 mg/L), fosfomycin (MIC, 16 mg/L) and the combination of aztreonam and avibactam (cumulated MIC, 0.5 mg/L).
Fig. 1.
Timeline of patients infected/colonized with NDM-1 producing Enterobacter cloacae (NDM-1 Ec) hospitalized in intensive care unit of the University Hospital of Reunion Island, Saint-Denis, France, June–August 2020 (n = 4). Each NDM-1 Ec positive patient is represented by a line and each positive sample is symbolized by a red box. For the positive clinical samples, letters in the red boxes indicate the specimen source: B for blood origin, P for pulmonary origin and R for rectal origin. Numbers in the boxes are those of the room in ICU. The death of Case 2 was considered not attributable to NDM-1 Ec.
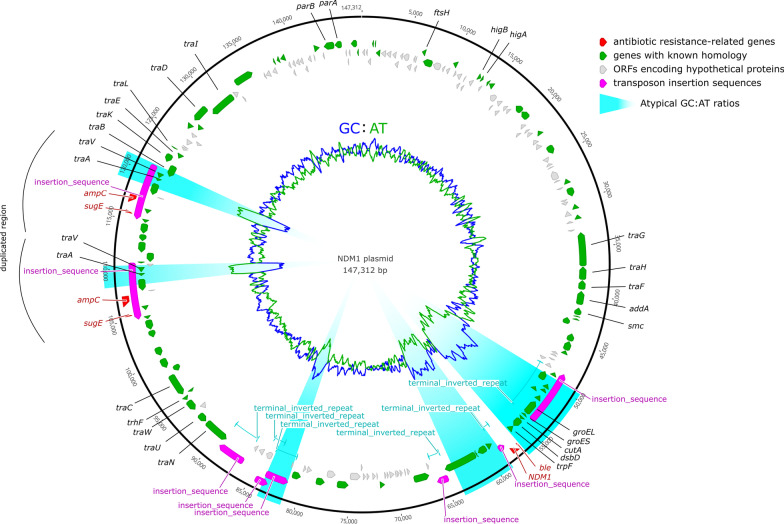
The analysis of five isolates of NDM-1 Ec (two isolates—one susceptible and one resistant to colistin—were analyzed from Case 1) shared the same pulsotype and belonged to Sequence Type 90. Resistance to carbapenem could be transferred by conjugation to the E. coli J53. The resistance gene blaNDM-1 was carried by an IncC plasmid of 147,312 bp and associated with the bleomycin resistance gene ble in a truncated ISAba125 insertion sequence (Fig. 2). This plasmid also harbored a duplicated region with ampC and sugE genes (3rd cepholosporin and quaternary ammonium resistances, respectively), interspersed between components of the tra operon (Fig. 2). The resistance mechanism to colistin, especially mutations in the PmrA/B and PhoP/Q two-component systems, is being explored.
Fig. 2.
Schematic map of the putative NDM-1 containing plasmid identified by conjugative transfer and MinION long-read sequencing. Genes associated with plasmid replication and maintenance as well as mobile elements related to the acquisition of antibiotic resistance are labelled. Other gene labels are left off for clarity. The plasmid carried 2 insertion sequences included resistances gene: (1) a truncated ISAba125 with blaNDM-1 and ble genes and (2) a duplicated region with ampC and sugE genes, interspersed between components of the tra operon
Discussion
A cluster of CPEs shared by four patients is unusual in this ward, where paramedical staff are well trained to preventive measures of cross-transmission and where enhanced contact precautions often need to be applied for medical evacuation [6]. Adherence of the ICU’s health care workers to these preventive practices had been regularly and favourably evaluated by the infection control team. In our opinion, two main factors could have favoured the transmission of NDM-1 Ec—most likely imported into the ICU by the Case 1—to the other three patients: (1) the misuse of gloves in nursing acts, and (2) the absence of change of over-gowns between patient rooms, considering that the whole subunit was as a COVID-positive sector. This way of using personal protective equipment (PPE) was implemented to limit the risk of contamination of nursing staff during undressing and to reduce demand in a context of a PPE shortage. Concomitant positive screening of Cases 2, 3, and 4 within a short time interval of seven days corroborates the probable health-care workers carrying transmission (Fig. 1). As the ratio of healthcare workers to patients remained constant during this period, the overload of the teams probably did not favor this cross-transmission. Moreover, these three patients had a negative rectal screening at their admission into the ICU, confirming the nosocomial transmission.
We immediately implemented infection control measures including (1) simultaneous screening of all patients in the subunit and (2) strict application of enhanced contact precautions with glove and over-gown change between each room. In addition, a hygienist nurse was assigned to the ICU to advise and retrain the nursing staff in the preventive measures of cross-transmission. Since, no new cases of infection or colonization with this NDM-1 Ec clone have occurred to date, three months after the episode.
NDM carbapenemase-producing E. cloacae ST90 clone has been very rarely described in the literature. Hence, only one study refers to an NDM-1 carbapenemase-producing E. cloacae ST90 from Henan Province in China [7]. This ST has also been described as potential vector of multidrug resistance by being associated with the blaVIM-2 and blaVIM-4 carbepenemase-encoding genes in Argentina and Poland, respectively [8, 9]. Although the carbapenemase-encoding gene may be carried by the same species of Enterobacterales (as described here), one should be aware that carbapenemase-resistance determinants are highly transferable via mobile genetic elements and can easily spread to other co-occurring species of the gut flora, like K. pneumoniae or E. coli, as in Case 1, and described elsewhere [10]. IncC plasmids (as well as IncFII and IncX3) are well known to be the genetic carrier and a vector for transmission of blaNDM genes between Enterobacterales [11–13]. As recently stated in an update of the French national guidelines, it is therefore important to consider the type of enzyme produced by the enterobacteria (such as OXA-48-like or NDM) independently of the bacterial species to define whether patients are part of the same outbreak [6]. Lastly, the adaptive resistance to colistin of the NDM-1 Ec isolate found in Case 1 was probably induced, as this patient had been treated with colistin (Fig. 1).
Conclusions
Here, we point out a new situation where the admission of COVID-19 patients can influence the long-established infection control behaviours. Such “collateral damage” has recently been described by authors who investigated the first nosocomial clusters in COVID-19 units [14–16]. Our observation highlights the importance of maintaining measures to control CPE despite the additional pressure put on ICUs by the large number of incoming COVID-19 cases. Finally, clinicians and hygienists should be aware that an CPE’s outbreak can affect several species of Enterobacterales due to the high transferability of the carbapenemase-encoding resistance genes.
Supplementary Information
Additional file 1. Detailed bioinformatics analyses.
Acknowledgements
We thank Julien Houivet and Karine Gambarotto for their help in the investigation and control of the outbreak.
Authors' contributions
GM designed the study and wrote the manuscript. TG, MD, NA, JA contributed to acquisition, analysis and interpretation of data. PC and DW made molecular genotyping and bioinformatics analysis. NL, OB, XB, DH and PM critically revised the manuscript. GM and PM coordinated the study. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the French Society of Infectious Diseases. The need for informed consent was waived, as the study was non-interventional and followed our usual protocol. However, all patients or their legally authorised representatives were verbally informed about the process of data collection, and could refuse to participate in the study.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miltgen G, Cholley P, Martak D, Thouverez M, Seraphin P, Leclaire A, et al. Carbapenemase-producing Enterobacteriaceae circulating in the Reunion Island, a French territory in the Southwest Indian Ocean. Antimicrob Resist Infect Control. 2020;9(1):36. doi: 10.1186/s13756-020-0703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lartigue MF, Poirel L, Aubert D, Nordmann P. In vitro analysis of ISEcp1B-mediated mobilization of naturally occurring b-lactamase gene blaCTX-M of Kluyvera ascorbata. Antimicrob Agents Chemother. 2006;50:1282–1286. doi: 10.1128/AAC.50.4.1282-1286.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75(12):3491–3500. doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haut Conseil de Santé Publique. Actualisation des recommandations relatives à la maîtrise de la diffusion des bactéries hautement résistantes aux antibiotiques émergentes (BHRe). Décembre 2019. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=758.
- 7.Liu C, Qin S, Xu H, Xu L, Zhao D, Liu X, et al. New Delhi Metallo-β-Lactamase 1(NDM-1), the dominant carbapenemase detected in carbapenem-resistant Enterobacter cloacae from Henan Province, China. PLoS ONE. 2015;10(8):e0135044. doi: 10.1371/journal.pone.0135044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Belder D, Faccone D, Tijet N, Melano RG, Rapoport M, Petroni A, et al. Novel class 1 integrons and sequence types in VIM-2 and VIM-11-producing clinical strains of Enterobacter cloacae. Infect Genet. 2017;54:374–378. doi: 10.1016/j.meegid.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Izdebski R, Baraniak A, Zabicka D, Sekowska A, Gospodarek-Komkowska E, Hryniewicz W, et al. VIM/IMP carbapenemase-producing Enterobacteriaceae in Poland: epidemic Enterobacter hormaechei and Klebsiella oxytoca lineages. J Antimicrob Chemother. 2018;73(10):2675–2681. doi: 10.1093/jac/dky257. [DOI] [PubMed] [Google Scholar]
- 10.Lo S, Goldstein V, Petitjean M, Rondinaud E, Ruppe E, Lolom I, et al. Analyse génomique d’une épidémie hospitalière d’entérobactéries productrices de NDM. CO-067. Congrès RICAI 2019. https://interactive-programme.europa-organisation.com/slides/programme_ricai-2019/CO-067.pdf.
- 11.Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. NDM metallo-β-Lactamases and their bacterial producers in health care settings. Clin Microbiol Rev. 2019;32(2):e00115. doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai Y, Lee S, Teng L, Ma Z, Hilliard NB, May RJ, et al. Dissemination mechanisms of NDM genes in hospitalized patients. JAC-Antimicrob Resist. 2021;3(1):dlab032. doi: 10.1093/jacamr/dlab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Touati A, Manseur L, Mehidi I, Mairi A. Epidemiological and genetic features of plasmids carrying blaNDM genes: an in silico analysis with emphasis on replicon types, and resistome. Microb Drug Resist. 2021. [DOI] [PubMed]
- 14.Stevens MP, Doll M, Pryor R, Godbout E, Cooper K, Bearman G. Impact of COVID-19 on traditional healthcare-associated infection prevention efforts. Infect Control Hosp Epidemiol. 2020;1–2. [DOI] [PMC free article] [PubMed]
- 15.Farfour E, Lecuru M, Dortet L, Guen ML, Cerf C, Karnycheff F, et al. Carbapenemase-producing Enterobacterales outbreak: another dark side of COVID-19. Am J Infect Control. 2020;S0196–6553(20):30895–30896. doi: 10.1016/j.ajic.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kampmeier S, Tönnies H, Correa-Martinez CL, Mellmann A, Schwierzeck V. A nosocomial cluster of vancomycin resistant enterococci among COVID-19 patients in an intensive care unit. Antimicrob Resist Infect Control. 2020;9(1):154. doi: 10.1186/s13756-020-00820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Detailed bioinformatics analyses.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.