Abstract
Purpose
Phosphodiesterase-5 (PDE5) inhibitors were shown to exert powerful protection in various animal models of cardiomyopathy. Tadalafil is a long-acting and highly specific PDE5 inhibitor, which makes it the most attractive in its class for long-term management of patients with heart failure. We studied the effects of tadalafil in attenuating ischemic cardiomyopathy in mice.
Methods and Results
Adult male mice underwent myocardial infarction (MI) by permanent left coronary artery ligation and were treated daily with tadalafil (1 mg/kg; ip) or volume-matched 10 % DMSO for 4 weeks. Twenty four hours after coronary ligation, infarct size, measured by TTC staining, was reduced from 70.1±3.1 % in DMSO-treated group to 49.3± 2.6 % with tadalafil (P<0.05). Similarly, tadalafil treatment yielded a smaller fibrotic area (8.8±2.8 % of LV), assessed by Masson’s trichrome staining, as compared to DMSO group (21.9±3.9 %, P<0.05). Apoptosis, measured by TUNEL assay, also declined with tadalafil (2.1±0.2 %) as compared to DMSO (6.7±0.4 %, P<0.05) at 28 days post MI. Tadalafil also attenuated the increase in cardiac hypertrophy and pulmonary edema following infarction. These parameters reflect diminished left ventricular (LV) adverse remodeling and preserved fractional shortening with tadalafil at 7 and 28 days post infarction.
Conclusions
Tadalafil attenuates ischemic cardiomyopathy in mice and preserves LV function.
Keywords: Infarct size, Apoptosis, LV function, Cardiomyopathy, Phosphodiesterase-5 inhibitors
Introduction
Heart failure (HF) secondary to acute myocardial infarction (AMI) continues to be a major complication in patients. Cell death from both apoptosis and necrosis contributes to acute and chronic alterations in the heart, collectively known as ventricular remodeling [1]. As a result, there is a progressive reduction in left ventricular (LV) systolic function, signifying a poor prognosis for patients with AMI.
Augmentation of the nitric oxide-cyclic GMP-protein kinase G (NO-cGMP-PKG) axis has been shown to exert anti-ischemic protective effects in the heart [2]. In particular, phosphodiesterase-5 (PDE5) inhibitors prevent the degradation of cGMP. Our laboratory has reported that the PDE5 inhibitor sildenafil reduces infarct size, apoptosis, fibrosis, and systolic dysfunction when given before ischemia, at reperfusion, and chronically in permanent coronary ligation [3–6]. These cardioprotective effects were mediated by activation of PKG, enhanced expression of endothelial and inducible nitric oxide synthases (eNOS & iNOS), and increased Bcl-2/Bax ratio [5, 7]. The cardioprotective effects of sildenafil were not only restricted to adult animal models as we also demonstrated that sildenafil promotes myocardial protection in infant rabbits through post-ischemic preservation and elevation in LV cardiac output and aortic velocity time integral while reducing infarct size [8].
Recently, our laboratory reported that the long-acting PDE5 inhibitor, Tadalafil, attenuated ischemia/reperfusion (I/R) injury via PKG-dependent generation of hydrogen sulfide [9]. In these studies, a single dose treatment of Tadalafil 60 min prior to ischemia caused a 67 % reduction in infarct size. Moreover, we showed that Tadalafil treatment mitigated I/R injury in the type 2 diabetic heart in db/db mice by attenuating inflammation and oxidative stress [10, 11]. Tadalafil is particularly attractive because its selectivity for PDE5 is 10,000-fold over PDE1 to PDE4 and 700-fold over PDE6 [12]. In addition, the absorption of tadalafil is relatively unaffected by food while it reaches maximal plasma concentration in 2 h. Its half-life is 17.6 h, making its effect last up to 36 h [12]. Moreover, tadalafil along with sildenafil, vardenafil, and avanafil are Food and Drug Administration (FDA)-approved for erectile dysfunction while tadalafil and sildenafil are also FDA-approved for treatment of pulmonary arterial hypertension [13].
The safety and pharmacokinetic profile of tadalafil make it an attractive long-term therapy. However, the long-term effect of tadalafil in ischemic cardiomyopathy is unknown. We sought to determine if tadalafil treatment immediately following permanent ligation of the left coronary artery in a mouse model could limit the progression of HF by reducing infarct size, apoptosis, and preserving cardiac function.
Materials and Methods
Animals
Adult male ICR mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). The mean body weight was 33.6± 0.3 g. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). The animal protocol was approved by the Institutional Animal Care & Use Committee (IACUC) at Virginia Commonwealth University.
Drugs and Chemicals
Triphenyltetrazolium chloride (TTC), dimethyl sulfoxide (DMSO) and 10 % formalin were purchased from Sigma-Aldrich (St. Louis, MO). Tadalafil powder was kindly provided by Lily ICOS (Indianapolis, IN).
Myocardial Infarction Protocol
Adult ICR mice underwent permanent occlusion of the LAD coronary artery as previously described [5]. In brief, the animals were anesthetized (pentobarbital, 70 mg/kg, ip), intubated orotracheally and ventilated on a positive-pressure ventilator. The tidal volume was set at 0.2 ml, and the respiratory rate was adjusted to 133 cycles/min. All surgical procedures were carried out under sterile conditions. A left thoracotomy was performed at the fourth intercostal space, and the heart was exposed by stripping the pericardium. The left descending coronary artery was then identified and permanently occluded by a 7.0 silk ligature that was placed around it. After coronary artery occlusion, the air was expelled from the chest. The animals were extubated and then received intramuscular doses of analgesia (buprenex; 0.02 mg/kg) and antibiotic (Gentamicin; 0.7 mg/kg; for 3 days).
Experimental Groups
Following MI protocol, mice were randomly assigned to 1 of 3 groups: 1- DMSO (vehicle, volume-matched, ip, once daily), 2-Tadalafil (1 mg/kg, ip, once daily), 3- sham: left thoracotomy (as control for the surgical procedure), but no MI or treatment until heart collection. The treatments began immediately after coronary artery ligation until sacrifice. In both groups, LV function (n=5–9/group) was assessed via echocardiography at 7, and 28 days post-MI. Infarct size was measured in both groups at 24 h post-MI. At 28 days post-MI, hearts (n=4–6/group) were sampled for histological examinations.
Survival
Survival rate was determined based on the animals that survived the experimental protocol starting at recovery following surgery until 28 days after infarction. Animals that died during surgical recovery were excluded.
Infarct Size Assessment
After 24 h of reperfusion, the heart was quickly removed and mounted on a Langendorff apparatus as described previously [5]. The areas of infarcted tissue, risk zone, and whole left ventricle were determined using TTC staining by computer morphometry using a Bioquant imaging software.
Echocardiography
M-mode echocardiography was performed using the Vevo 770™ imaging system (VisualSonics, Inc.; Toronto, Canada) before surgery (baseline) and on days 7, and 28 post-MI before the animals were sacrificed as detailed previously [5]. Heart rate (HR), LV end-diastolic diameter (LVEDD), end-systolic diameter (LVESD), anterior wall diastolic thickness (AWDT), and anterior wall systolic thickness (AWST) were measured. LVFS was calculated as (LVEDD–LVESD)/LVEDD* 100.
Histology
Tranverse sections of the median third of the LV (n=4–6/ group) were fixed in 10 % formalin for at least 48 h, embedded in parrafin, and sectioned (5 µm). Apoptosis was examined using the terminal deoxynucleotidyl transferase–mediated (dUTP) nick-end labeling (TUNEL) assay (ApopTag; Millipore, Billerica, MA) according to manufacturer’s instructions. Apoptotic rate within in the peri-infarct regions was calculated at 40× magnification under light microscopy.
Myocardial fibrosis was examined to address prevalence of scar formation within the LV. Heart sections (5 µm) were stained with Masson’s trichrome (Sigma-Aldrich; St Louis, MO). Fibrotic area was computed using computer morphometry (Bioquant 98) and expressed as a percentage of left ventricle.
Evaluation of Cardiac Hypertrophy and Pulmonary Edema
On day 28 post-MI, the mice were weighed, and tibia length was obtained before sacrifice. The hearts and lungs were weighed immediately after collection. Cardiac hypertrophy was calculated as the ratio of heart weight to tibia length, and pulmonary edema was calculated as the ratio of lung weight to tibia length.
Statistics
All measurements of infarct size and risk areas are expressed as group means±SEM. Changes in echocardiography parameters and infarct size were analyzed using the random effects ANOVA for repeated-measures to determine the main effect of time, group, time-by-group interaction, and the post-hoc 2-sided Dunnett test to compare 2 groups at a time. Statistical differences were considered significant if the probability value was <0.05. Discrete variables were presented as absolute and percent value. The Chi-square test (or the Fisher exact test when appropriate) was used to compare discrete variable in different groups. The Bonferroni correction for posthoc analysis was used when comparing 2 groups from 3 or more groups. Kaplan Meyer analysis was used to test for differences in survival.
Results
Survival at 28 days
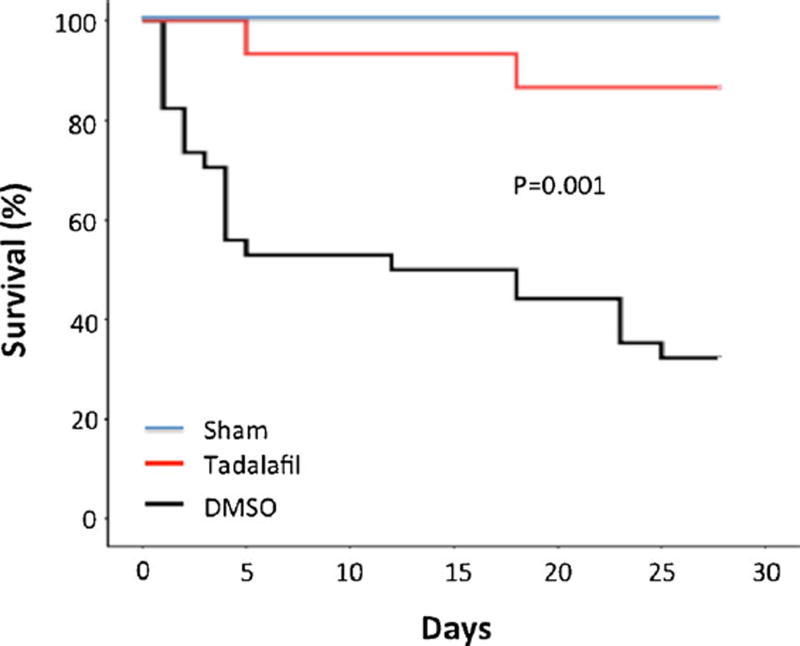
A total of 89 mice were used in this study, 48 of which were allocated for 28-day survival. 12 out of 14 mice survived with tadalafil (86 %) as compared to 11 out of 34 with DMSO (32 %, P<0.05, Fig. 1). The survival rate was 100 % in sham-operated mice.
Fig. 1.
Survival curve. Kaplan-Meier survival curve in DMSO-treated myocardial infarcted mice (n=34) and infarcted mice (n=14) treated with tadalafil (1 mg/kg/day) for 28 days. Note that tadalafil-treated mice exhibited a significant increase in survival as compared with the DMSO-treated animals (P=0.001)
Infarct Size
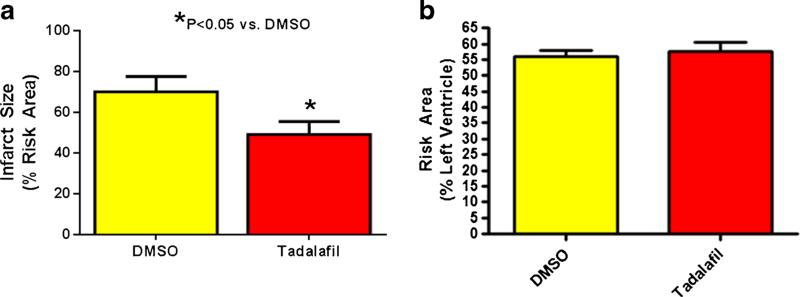
The infarct size (% of risk area) was reduced from 70.1±3.1 in the DMSO-treated group to 49.3±2.6 in the tadalafil-treated mice 24 h after infarction (P<0.05, Fig. 2a). The risk areas (% LV) were not statistically different between the groups (Fig. 2b).
Fig. 2.
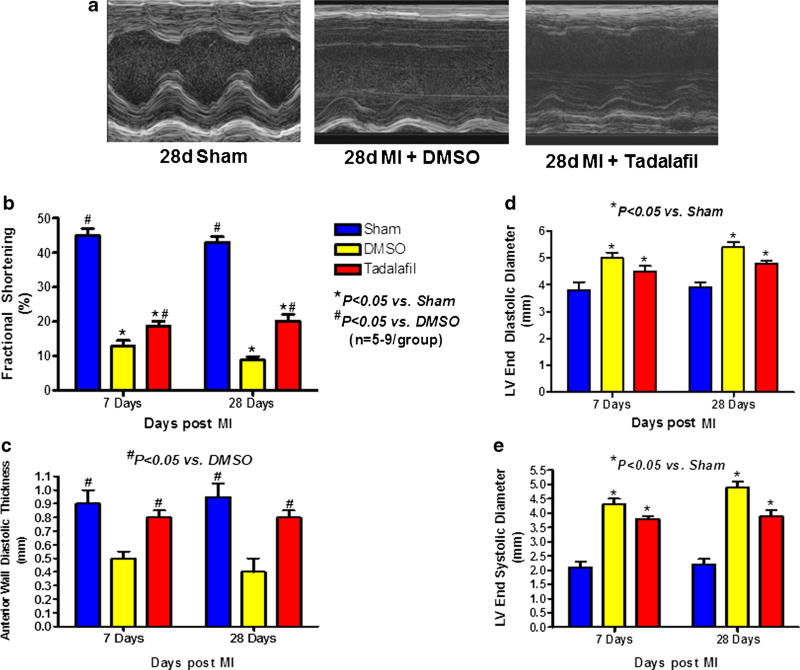
M-mode echocardiography. a Representative echocardiography images of sham-operated, DMSO-treated, and tadalafil-treated mice at 28d post-MI. b Fractional shortening. c Anterior Wall Diastolic Thickness. d Left ventricular end diastolic diameter. e Left ventricular end systolic diameter. Tadalafil treatment ameliorated left ventricular remodeling following permanent coronary occlusion at 7 and 28 days by reducing dilatation, preserving fractional shortening and anterior wall thickness, P<0.05 vs. saline at 7 and 28 days post-infarction; n=5–9/ group
Left Ventricular Remodeling and Function
Representative M-mode images of sham operated, DMSO-treated and tadalafil-treated mice at 28 days post MI are shown in Fig. 3a. Tadalafil treatment mitigated LV dilatation (smaller LVEDD) and preserved LV contractility (smaller LVESD and greater FS) on days 7 and 28, respectively, as compared to DMSO-treated group (P<0.05, Fig. 3b, d, and e). AWDT was also preserved in tadalafil-treated animals (vs. DMSO-treated animals, P<0.05, Fig. 3c) on days 7 and 28 post MI.
Fig. 3.
Infarct size and Risk Area. a Infarct size. Tadalafil reduced infarct size (% of Risk Area) at 24 h following infarction when compared to DMSO treatment, P<0.05; n=6/group. b Area at risk was similar in both groups
Fractional shortening of sham-operated mice was 43± 1.0 % at 28 days post left thoracotomy. Baseline LVEDD was 3.5±0.1 mm. An increase in LVEDD and a decrease in FS were seen in DMSO- and tadalafil-treated mice as compared to baseline and sham-operated mice (P<0.05) on days 7 and 28.
Apoptosis
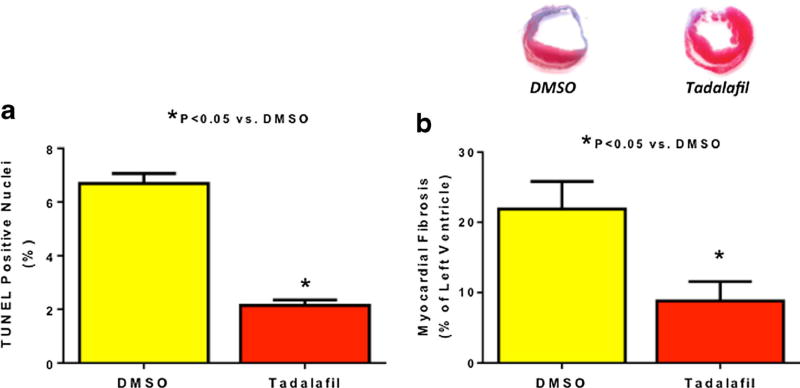
The TUNEL positive apoptotic cells in DMSO-treated hearts were 6.7±0.4 % cells as compared to 2.1±0.4 % in the tadalafil group on day 28 post MI in the peri-infarct area (P<0.05, N=4). (Fig. 4a).
Fig. 4.
Myocardial Fibrosis and Apoptosis. a Apoptosis. Apoptosis (% of TUNEL positive nuclei) were less in the tadalafil group at 28 days following infarction, P<0.05; n=4–6/group. b Representative heart sections with Masson’s trichrome staining illustrating myocardial fibrosis. Tadalafil-treated mice had less fibrosis (% of left ventricle) than DMSO-treated mice at 28 days post infarction, signifying reduced LV scar formation, P<0.05; n=4–6/group. Representative heart sections stained with Masson’s trichrome from each group
Cardiac Fibrosis, Hypertrophy, and Pulmonary Edema
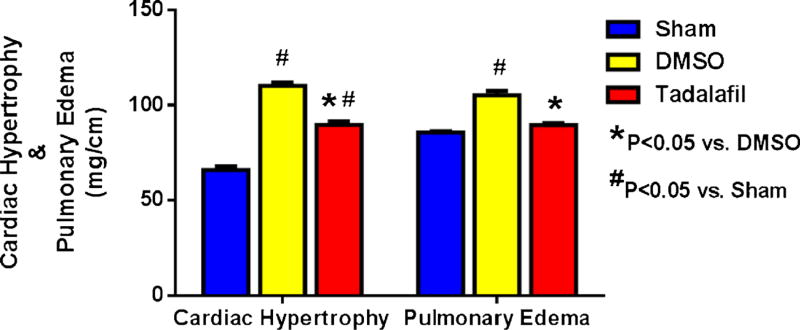
Myocardial fibrosis (% of left ventricle) was higher in DMSO treatment than tadalafil treatment at day 28 days post-MI (21.9±3.9 % vs. 8.8±2.8 % respectively, P<0.05, Fig. 4b). The heart-to-tibia length ratio (an index of cardiac hypertrophy) was increased in both DMSO (110.2±4.1 mg/cm) and tadalafil groups (89.7±5.5 mg/cm), as compared to the sham group (66±2.9 mg/cm, P<0.05, Fig. 4) at 28 days post-MI. However, cardiac hypertrophy in the tadalafil-treated group was significantly lower than in DMSO-treated mice (P<0.05). In addition, the ratio of lung weight to tibia length was 85.6± 1.3 mg/cm in the sham-operated animals, which increased to 105.3±4.9 mg/cm in the DMSO-treated group indicative of pulmonary edema (P<0.05, Fig. 5) and was reduced by tadalafil to 89.7±3.3 mg/cm (P<0.05 vs. DMSO, P>0.05 vs. sham).
Fig. 5.
Cardiac Hypertrophy and Pulmonary Edema. Both heart weight-to-tibia length (an index for cardiac hypertrophy) and lung weight-to-tibia length (an index for pulmonary edema) were attenuated with tadalafil treatment as compared to saline, P<0.05; n=5–9/group
Discussion
We have previously shown that chronic treatment with sildenafil in a severe ischemia model by way of permanent coronary artery ligation attenuates ischemic cardiomyopathy in mice up to 28 days [5]. The treatment regimen involved a clinically relevant dose of sildenafil (0.7 mg/kg) twice daily. Since tadalafil is a longer acting PDE5 inhibitor than sildenafil with a half-life of 17.5 h, we contemplated that a single daily dose of tadalafil would be sufficient to mitigate ischemic cardiomyopathy following severe ischemia in mice. Indeed, our results indicate that chronic treatment with tadalafil (1 mg/kg/day) starting immediately after permanent LAD ligation attenuates the progression of ischemic cardiomyopathy in mice. Tadalafil effectively preserved FS, reduced LV dilatation, and conserved AWDT as compared to DMSO treatment at 28 days post MI. While the chronic anti-remodeling effect is likely attributable, at least in part, to the acute infarct-sparing effect observed at 24 h, cardiomyocyte apoptosis and myocardial fibrosis were also attenuated at 28 days post-MI in the tadalafil-treated mice as compared to the DMSO group. Both cardiac hypertrophy and pulmonary edema, hallmarks of heart failure, were also mitigated with tadalafil treatment. Taken together, our results demonstrate that tadalafil exerts protective effects within the infarct and peri-infarct regions post MI. Not surprisingly, we observed a significantly higher rate of survival in the tadalafil-treated as compared to DMSO-treated mice (Fig. 1). Although this might be explained by the preservation of LV function in this group, it may also be influenced by a reduction in ventricular arrhythmias. Previous studies by Nagy et al. [14] showed that PDE5 inhibition with sildenafil decreased the incidence and severity of ventricular arrhythmias during coronary artery occlusion.
Tadalafil primarily prevents the degradation of NO-driven cGMP by targeting PDE5 and thus increases myocardial PKG activity as we demonstrated previously [9]. The enzyme PDE5 is expressed in pulmonary artery smooth muscle as well as adult murine [15] and canine [16] hearts. In the heart, PDE5 inhibition was cardioprotective when administered as a preconditioning mimetic or at reperfusion [3, 4, 17]. We also established that chronic treatment with sildenafil protects against permanent myocardial ischemia when given chronically following MI by increasing eNOS and iNOS expression, thus leading to infarct-sparing effects, which were abolished by the non-selective NOS inhibitor NG-nitro-L-arginine-methyl-ester (L-NAME) [5]. Moreover, chronic sildenafil treatment caused an increase in Bcl-2/Bax ratio, which resulted in a decreased apoptotic rate [5]. The salutary effects of sildenafil in this model were also observed when the treatment regimen was initiated well beyond infarct development. In fact, we demonstrated that chronic treatment with sildenafil (starting at 3 days post-MI) attenuated the progression of ischemic cardiomyopathy through PKG-dependent inhibition of RhoA/Rho-kinase [6]. In a less severe model of ischemia/ reperfusion (I/R) injury, tadalafil was shown to protect against necrosis and preserve LV function through PKG-dependent generation of hydrogen sulfide (H2S) in adult male mice [9]. This cardioprotective effect was absent in mice with genetic deletion of cystathionine-γ-lyase, the enzyme responsible for H2S production in the cardiovascular system. More recently, we also indicated that tadalafil exerts protection against I/R injury in the type 2 diabetic heart by reducing infarct size, apoptosis, inflammation and oxidative stress [10, 11].
To explain how sildenafil was able to induce protection against necrosis or apoptosis in the non-perfused ischemic zone in our previous studies, we speculated that the upregulation of eNOS and iNOS after sildenafil treatment [5] would induce NO generation in the adjacent non-ischemic tissue, which would diffuse into the ischemic region and preserve cardiomyocytes possibly through opening of mitoKATP channels and preventing Ca2+ overload. This was corroborated by the finding that L-NAME completely blocked the infarct-sparing effect of sildenafil. A similar conclusion was drawn by Li et al. [18] where iNOS gene transfer reduced infarct size by an average of 67 % despite the fact that only 18 % of the risk region exhibited transgene expression. Since in our more recent study we demonstrated that tadalafil can also increase myocardial H2S generation, similar to our previous explanation for NO, it is plausible that tadalafil-induced H2S in the non-risk area may also diffuse into the non-reperfused risk zone and lessen myocardial injury. In fact, we recently showed that exogenous H2S treatment by way of donor (Na2S) attenuated ischemic and inflammatory injury in mice [19]. In this study, we demonstrated for the first time that H2S inhibits inflammasome formation and activation in the heart and cardiomyocytes following ischemia. In this respect, it is possible that tadalafil also exerts anti-inflammasome effects via H2S generation therefore attenuating adverse cardiac remodeling post MI. Future studies are warranted to validate this hypothesis.
Both myocardial fibrosis and apoptosis in the peri-infarct region contribute to HF by exacerbating systolic dysfunction, hypertrophy, and chronic adverse remodeling [1, 20]. The present study demonstrated that tadalafil treatment limited the extent of LV scar formation and apoptosis at 28 days post-MI. The lower cardiac hypertrophy index in tadalafil-treated mice may well be due to the protective effect of tadalafil against myocardial fibrosis. Fibrosis is a hallmark in the development of HF due to several causes, including non-ischemic cardiac hypertrophy secondary to hypertension. In fact, fibrosis plays a key role in angiotensin II-induced HF in animal models as reflected by an increase in interstitial collagen I and collagen III in the heart [21]. Interestingly, a recent study demonstrated that PDE5 inhibition with sildenafil prevented the increase in collagen deposition and attenuated TGF-β signaling following chronic angiotensin II administration. TGF-β has been shown to mediate cardiac fibrosis in several models of heart disease, including ischemic and aortic valve disease [22]. Since sildenafil has been shown to attenuate fibrosis via inhibition of TGF-β, it is plausible that the long-term anti-fibrotic benefits observed with tadalafil treatment post MI in the present study are mediated through a similar mechanism involving attenuation of TGF-β. Further studies are needed to explore this idea. In addition to the inhibitory effects of tadalafil on cardiac fibrosis, the absence of pulmonary edema in the tadalafil-treated group corroborates a better LV systolic function.
Numerous clinical studies have examined PDE5 inhibition with sildenafil as a potential therapy for cardiovascular diseases. In patients with HF, PDE5 was upregulated, while overexpression of PDE5 in transgenic mice showed increased LV remodeling in MI-induced heart failure [23]. The chronic use of sildenafil improved LV function and clinical status in patients with New York Heart Association Class II-III systolic HF [24]. Moreover, sildenafil increased oxygen capacity and improved exercise performance in patients with HF [25, 26]. Recently, Giannetta et al. reported that PDE5 inhibition was associated with better LV dynamics and cardiac geometry in patients with diabetic cardiomyopathy [27]. Additionally, the RELAX trial which examined chronic sildenafil treatment in patients with HF with preserved ejection fraction (HFpEF) was recently concluded [28, 29]. Although the outcomes of the trial did not show any significant improvement in exercise capacity or clinical status over placebo in these patients, our present study was designed to test the effects of tadalafil in a more severe ischemic murine model with reduced EF. Collectively, our results support the clinical potential of PDE5 inhibition in ischemic heart failure with reduced ejection fraction (HFrEF).
The development of tadalafil offers an attractive alternative to sildenafil for long-term therapy. The specificity of tadalafil for PDE5 over sildenafil would provide a more targeted inhibition of PDE5 with less cross-reactivity with other PDE isoforms. Although chronic treatment with sildenafil up to 240 mg/day has been documented to be safe in clinical trials [24, 28, 30], sildenafil at high dosages can be associated with tachyarrhythmias in patients [31]. In support of this notion, sildenafil at 100 mg can decrease ventricular fibrillation threshold in swine [32]. Although the exact mechanism remains unknown, it has been postulated that in patients with poor drug clearance (HF, chronic kidney disease, or hepatic dysfunction), sildenafil plasma concentration can become supra-therapeutic. The result may lead to alterations in cardiac electrophysiology, thus making the myocardium vulnerable to arrhythmias [31]. In addition, sildenafil may act as PDE3 inhibitor at high dosages, leading to an arrhythmogenic state [33]. Further studies are needed to examine the safety of chronic management with high-dose sildenafil. Given its pharmacokinetic profile, tadalafil may bypass this phenomenon at a much lower dose and once daily regimen. In addition to lower treatment frequency, the use of tadalafil may also increase medication compliance by reducing ‘polypharmacy’ in patients with HF. In the trials Pulmonary Arterial Hypertension and Response to Tadalafil 1 and 2 (PHIRST-1 and 2), tadalafil up to 40 mg once daily was effective and safe at 52 weeks for treatment of pulmonary hypertension [34]. Moreover, tadalafil (20 mg once daily) was shown to improve LV relaxation, which suggests a potential benefit of its use as an adjunct to treat symptomatic subjects with LV hypertrophy and diastolic dysfunction such as patients with resistant hypertension [35].
Study Limitation
The dose of tadalafil (1 mg/kg) in our present study translates to 70 mg once daily for a male weighing 70 kg. Such a high dose of tadalafil must be further studied for its efficacy and safety profile in clinical trials of patients with HF.
Conclusion
We have demonstrated for the first time the effectiveness for chronic use of tadalafil immediately following MI in attenuating ischemic heart failure. The cardioprotective effects of tadalafil were primarily reflected by a decrease in necrosis, apoptosis, and myocardial fibrosis, thereby blunting adverse LV remodeling. We propose that tadalafil may be an attractive therapy for patients with HFrEF.
Acknowledgments
This work was supported by grants from the American Heart Association (10SDG3770011 and 14GRNT20010003) and the Virginia Commonwealth University Presidential Research Quest Fund to Fadi N. Salloum and grants from the National Institutes of Health (HL-51045, HL-79424, and HL-93685) to Rakesh C. Kukreja.
Tadalafil was kindly provided by Lily ICOS.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 2.Kukreja RC, Salloum FN, Das A. Cyclic guanosine monophosphate signaling and phosphodiesterase-5 inhibitors in cardioprotection. J Am Coll Cardiol. 2012;59:1921–7. doi: 10.1016/j.jacc.2011.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283:H1263–9. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- 4.Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K, et al. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol. 2007;42:453–8. doi: 10.1016/j.yjmcc.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 6.Chau VQ, Salloum FN, Hoke NN, Abbate A, Kukreja RC. Mitigation of the progression of the progression of heart failure with sildenafil involves the inhibition of RhoA/Rho-Kinase pathway. Am J Physiol Heart Circ Physiol. 2011;300:H2272–9. doi: 10.1152/ajpheart.00654.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem. 2006;281:38644–52. doi: 10.1074/jbc.M606142200. [DOI] [PubMed] [Google Scholar]
- 8.Bremer YA, Salloum F, Ockaili R, Chou E, Moskowitz WB, Kukreja RC. Sildenafil citrate (viagra) induces cardioprotective effects after ischemia/reperfusion injury in infant rabbits. Pediatr Res. 2005;57:22–7. doi: 10.1203/01.PDR.0000147736.27672.15. [DOI] [PubMed] [Google Scholar]
- 9.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, et al. Phosphodiesterase-5 Inhibitor, Tadalafil, Protects against Myocardial Ischemia/Reperfusion through Protein-Kinase G Dependent Generation of Hydrogen Sulfide. Circulation. 2009;120:S31–6. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varma A, Das A, Hoke NN, Durrant DE, Salloum FN, Kukreja RC. Anti-Inflammatory and Cardioprotective Effects of Tadalafil in Diabetic Mice. PLoS ONE. 2012;7:e45243. doi: 10.1371/journal.pone.0045243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koka S, Das A, Salloum FN, Kukreja RC. Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic Biol Med. 2013;60:80–8. doi: 10.1016/j.freeradbiomed.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Daugan A, Grondin P, Ruault C, de AC LMG, Coste H, Kirilovsky J, et al. The discovery of tadalafil: a novel and highly selective PDE5 inhibitor: 1:5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione analogues. J Med Chem. 2003;46:4525–32. doi: 10.1021/jm030056e. [DOI] [PubMed] [Google Scholar]
- 13.Frey MK, Lang I. Tadalafil for the treatment of pulmonary hypertension. Expert Opin Pharmacother. 2012;13:747–55. doi: 10.1517/14656566.2012.662220. [DOI] [PubMed] [Google Scholar]
- 14.Nagy O, Hajnal A, Parratt JR, Vegh A. Sildenafil (Viagra) reduces arrhythmia severity during ischaemia 24 h after oral administration in dogs. Br J Pharmacol. 2004;141:549–51. doi: 10.1038/sj.bjp.0705658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor, sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis: essential role of NO signaling. J Biol Chem. 2005;280:12944–55. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 16.Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–26. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 17.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–7. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Guo Y, Xuan YT, Lowenstein CJ, Stevenson SC, Prabhu SD, et al. Gene therapy with inducible nitric oxide synthase protects against myocardial infarction via a cyclooxygenase-2-dependent mechanism. Circ Res. 2003;92:741–8. doi: 10.1161/01.RES.0000065441.72685.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toldo S, Das A, Mezzaroma E, Chau VQ, Marchetti C, Durrant D, et al. Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ Cardiovasc Genet. 2014;7:311–20. doi: 10.1161/CIRCGENETICS.113.000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLellan WR, Schneider MD. Death by design. Programmed cell death in cardiovascular biology and disease. Circ Res. 1997;81:137–44. doi: 10.1161/01.res.81.2.137. [DOI] [PubMed] [Google Scholar]
- 21.Westermann D, Becher PM, Lindner D, Savvatis K, Xia Y, Fröhlich M, et al. Selective PDE5A inhibition with sildenafil rescues left ventricular dysfunction, inflammatory immune response and cardiac remodeling in angiotensin II-induced heart failure in vivo. Basic Res Cardiol. 2012;107:308. doi: 10.1007/s00395-012-0308-y. [DOI] [PubMed] [Google Scholar]
- 22.Munjal C, Opoka AM, Osinska H, James JF, Bressan GM, Hinton RB. TGF-β mediates early angiogenesis and latent fibrosis in an Emilin1-deficient mouse model of aortic valve disease. Dis Model Mech. 2014;7:987–96. doi: 10.1242/dmm.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, et al. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408–16. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 25.Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol. 2004;44:2339–48. doi: 10.1016/j.jacc.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 27.Giannetta E, Isidori AM, Galea N, Carbone I, Mandosi E, Vizza CD, et al. Chronic Inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopathy: a randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation. 2012;125:2323–33. doi: 10.1161/CIRCULATIONAHA.111.063412. [DOI] [PubMed] [Google Scholar]
- 28.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, et al. PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale design. Circ. Heart Fail. 2012;5:653–9. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirostko BM, Tressler C, Hwang LJ, Burgess G, Laties AM. Ocular safety of sildenafil citrate when administered chronically for pulmonary arterial hypertension: results from phase III, randomised, double masked, placebo controlled trial and open label extension. BMJ. 2012;344:e554. doi: 10.1136/bmj.e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varma A, Shah KB, Hess ML. Phosphodiesterase inhibitors, congestive heart failure, and sudden death: time for Re-evaluation. Congest Heart Fail. 2012;18:229–33. doi: 10.1111/j.1751-7133.2012.00293.x. [DOI] [PubMed] [Google Scholar]
- 32.Kanlop N, Shinlapawittayatorn K, Sungnoon R, Chattipakorn S, Lailerd N, Chattipakorn N. Sildenafil citrate on the inducibility of ventricular fibrillation and upper limit of vulnerability in swine. Med Sci Monit. 2008;14:BR205–9. [PubMed] [Google Scholar]
- 33.Amsallem E, Kasparian C, Haddour G, Boissel JP, Nony P. Phosphodiesterase III inhibitors for heart failure. Cochrane Database Syst Rev. 2005;1 doi: 10.1002/14651858.CD002230.pub2. CD002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oudiz RJ, Brundage BH, Galiè N, Ghofrani HA, Simonneau G, Botros FT, et al. Tadalafil for the treatment of pulmonary arterial hypertension: a double-blind 52-week uncontrolled extension study. J Am Coll Cardiol. 2012;60:768–74. doi: 10.1016/j.jacc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Santos RC, de Faria AP, Barbaro NR, Modolo R, Ferreira-Melo SE, Matos-Souza JR, et al. Eur J Clin Pharmacol. 2013;70:147–54. doi: 10.1007/s00228-013-1611-8. [DOI] [PubMed] [Google Scholar]