Abstract

We have studied for the first time the role of hydrophobicity of the mesoporous silicate SBA-15 on the activity and the service life of a catalyst in the peroxide oxidation of sulfur-containing compounds. Immobilization of the molybdate anion on the SBA-15 support via ionic bonding with triethylammonium groups allows us not only to decrease the reaction temperature to a relatively low value of 60 °C without a drop in the dibenzothiophene conversion degree but also to increase the service life of the catalyst to many times that of the known analogs. The support and catalyst structures were investigated by low-temperature nitrogen adsorption/desorption, Fourier-transform infrared spectroscopy, X-ray fluorescence analysis, and transmission electron microscopy. Immobilization of the molybdate anion on the SBA-15 support, modified with ammonium species, prevents the leaching of active sites. However, only alkyl-substituted ammonium species minimize DBT sulfone adsorption, which significantly increases the catalyst’s service life. The synthesized catalyst Mo/Et3N-SBA-15 with hydrophobic properties is not sensitive to the initial sulfur content and hydrogen peroxide amount and retains its activity for at least six cycles of oxidation without regeneration. These catalysts can be efficiently used for clean fuel production.
1. Introduction
Currently, more than 95% of vehicles worldwide use internal combustion engines.1 However, the amount of light oil reserves decreases worldwide, and deposits containing heavy high-sulfur oil are being developed.2 An increase in the sulfur content in oil leads to an increase in sulfur compounds in the oil fractions obtained from it. This impairs the quality of the refined products and affects the refining processes themselves since sulfur compounds are corrosive and poison the expensive refining catalysts. This trend calls for improving the existing methods for cleaning raw materials from sulfur and developing new ones. Among the alternative methods of desulfurization that do not involve hydrogen (adsorption, extraction, biodesulfurization, and oxidative desulfurization), the latter is the most widespread technique.3 This method consists of two main stages: the oxidation of sulfur compounds and their subsequent extraction from the hydrocarbon medium.4,5 Hydrogen peroxide is the most widely used one among the oxidizing agents due to its environmental friendliness (the side product of the oxidation reaction is just water) and relative cheapness. However, non-catalytic oxidation with hydrogen peroxide under mild conditions (temperature up to 80 °C, atmospheric pressure) can provide no more than 20% conversion of sulfur compounds. To increase the oxidation conversion, it is necessary to use a catalyst, a key element of the oxidative desulfurization process.
The literature describes liquid-phase catalysts based on acids and salts of molybdenum and tungsten, reducing the sulfur content to values below EURO-5.1 The main drawback of such catalytic systems, which limits their wide application in the industry, is the complexity and, in most cases, the impossibility of their regeneration. Therefore, the interest of researchers is currently focused on heterogeneous systems. They are regenerable and can be easily separated from the reaction mixture at the end of the reaction. Zeolites and mesoporous carriers are most often used as the catalyst support due to their high mechanical strength, resistance to oxidants, and relative ease of modification.6−8 Zeolite catalysts are highly active in the oxidation of sulfides and unbranched benzo- and dibenzothiophenes. However, when the latter has alkyl tails, the catalysts’ efficiency drops sharply due to steric difficulties since the oxidation reaction takes place inside the catalyst’s pores. In this regard, mesoporous materials are the most optimal materials used as carriers for oxidative desulfurization catalysts. Among them, mesoporous materials such as MCM-41 and SBA-15 are the most widespread ones.9,10
The active phase of heterogeneous mesoporous catalysts most often consists of salts and oxides of molybdenum, which can form active peroxocomplexes that act as sources of active oxygen.11 Despite the high activity of mesoporous catalysts, oxidation results in rapid pore clogging by polar oxidation products (sulfoxides and sulfones). One of the approaches used is to conduct the reaction at 80 °C and above, at which the desorption of the oxidation products from the catalyst pores increases. However, carrying out oxidation at an elevated temperature leads to a decrease in the catalysts’ selectivity. This can impair the fuel quality after the oxidation reaction when natural raw materials are desulfurized and not model media, such as those used in the experiments.
There are alternative approaches to reduce the sorption of oxidation products in the catalyst pores. One of them uses hydrophobic materials as carriers of oxidative desulfurization catalysts such as polymer matrices, nitride, carbon, porous aromatic frameworks, etc.12−15 Despite such systems’ effectiveness, they are expensive and can be partially destroyed during the oxidation process. Therefore, the synthesis of hydrophobized silicates (which can be free from such drawbacks) as carriers of oxidative desulfurization catalysts is of interest.
The literature describes examples of the successful synthesis of hydrophobized silicates such as MCM-41 and SBA-15.16−18 The described methods of hydrophobization of the surface of mesoporous silicates are mainly based on the interaction of surface silanol groups with silanes containing alkyl tails or with ionic liquids, most often based on imidazole. The use of alkyl-containing silanes19 allowed not only to minimize the filling of the pores with oxidation products but also to reduce the oxidation temperature. Thus, the oxidation of sulfides (methyl phenyl sulfide) peaked at 30 °C. Although this method helps to increase the hydrophobicity of the surface, another stage of surface functionalization is necessary for the chemical immobilization of metals. The use of ionic liquids avoids this stage since the ionic liquid can act both as a hydrophobizing agent and as a molybdenum-retaining component. Thus, the authors of the study9 used imidazole to modify the surface and further bind to phosphoric acid. SBA-15 unmodified by an ionic liquid was used as a control in that work. It was shown in that paper that modification with an ionic liquid facilitates the interaction at the interface of the model mixture, thus enhancing the oxidation degree. However, imidazole is not available for industrial-scale synthesis, and there are examples of the use of amino-containing catalysts in the literature.20,21 At the same time, in these studies, expensive heteropolyacids were used as the active phase. In the present study, we wanted to compare the catalysts immobilized with the available ammonium salts and the industrially available triethylamine. Note that there are no studies in the literature on the influence of the nature of the functional groups on the activity and the resource of the mesoporous catalysts. Therefore, we synthesized mesoporous materials containing methyl and amino groups for the subsequent chemical cross-linking of molybdenum compounds. In contrast to the impregnation technique, the chemical bonding of molybdenum allows one to increase the service life of the catalyst since the leaching of the active phase from the surface of the carrier is minimized.
2. Results and Discussion
2.1. Characterization of Supports and Catalysts
Many studies of oxidative desulfurization using catalysts based on the mesoporous carrier SBA-15 have been reported.22−25 Compounds containing transition metals, in particular, molybdenum and tungsten, were found to be the most promising as the active phase. However, the researchers face the problem of minimizing the leaching of the catalyst from the surface during the reaction and regeneration. One way to solve this problem can be the chemical immobilization of metals on the surface of the carrier.
The literature describes examples of chemical immobilization of molybdenum compounds on the surface of the carrier, for example, using imidazole and other ionic liquids.26 However, the functionalizing agent can act both as a molybdenum-retaining component and as a surface hydrophobizer. Since the oxidized sulfur-containing compounds have a sufficiently high polarity to be retained in the carrier pores, their formation during the reaction leads to a gradual deactivation of the catalyst and requires constant regeneration. Despite the known possibility of hydrophobization of the surface, there is no data in the literature on the effect of the functional groups attached to the surface of the carrier on the catalyst service life. To address this issue, we have chosen N+H3 and N+Et3 as functional groups in this work because of their similar structures but different hydrophobic properties. These groups were deposited on the surface of the carriers by the modification of SBA-15 with the corresponding silanes (Figure 1). After functionalization, the surface was subjected to an ion-exchange reaction with ammonium heptamolybdate to produce an active catalyst.
Figure 1.
Scheme for obtaining catalysts.
In this work, the mesoporous material SBA-15 was used as a carrier for forming heterogeneous catalysts, the synthesis of which was carried out by the template method similar to a previous work.7 For the chemical grafting of molybdenum on SBA-15, the surface of the latter had to be modified. The modification was carried out, according to the scheme shown in Figure 1. H3N-SBA-15 was obtained by reacting 3-aminopropyltriethoxysilane with hydroxyl groups on the surface of SBA-15 followed by the protonation of the surface. The Et3N-SBA-15 carrier was prepared by reacting 3-chloropropyl triethoxysilane with hydroxyl groups on the surface of SBA-15 followed by quaternization with triethylamine. Two samples with different organic groups were obtained for each carrier for subsequent application of 5 and 10 wt % of molybdenum. Carriers with a smaller number of functional groups are designated (I), and those with a higher number are designated (II).
2.1.1. N2 Adsorption/Desorption
The structure and texture of all the synthesized carriers and catalysts were characterized by low-temperature nitrogen adsorption/desorption (Figure 2 and Table 1).
Figure 2.

Nitrogen adsorption/desorption isotherms of obtained supports and catalysts.
Table 1. Texture Characteristics and Molybdenum Content in Various Samples.
| texture
characteristics |
||||
|---|---|---|---|---|
| sample | SBET (m2/g) | Vpore (cm3/g) | Dpore (Å) | the actual content of molybdenum (wt %) |
| SBA-15 | 720 | 0.70 | 65 | |
| Cl-SBA-15 | 646 | 0.61 | 62 | |
| Et3N-SBA-15 (I) | 492 | 0.50 | 58 | |
| 5%Mo/Et3N-SBA-15 | 366 | 0.42 | 53 | 4.7 |
| Et3N-SBA-15 (II) | 395 | 0.44 | 55 | |
| 10%Mo/Et3N-SBA-15 | 307 | 0.37 | 49 | 9.5 |
| H3N-SBA-15 (I) | 482 | 0.48 | 61 | |
| 5%Mo/H3N-SBA-15 | 429 | 0.45 | 56 | 4.8 |
| H3N-SBA-15 (II) | 348 | 0.40 | 59 | |
| 10%Mo/H3N-SBA-15 | 319 | 0.38 | 52 | 9.7 |
All synthesized samples exhibit a type IV isotherm typical of mesoporous materials. The synthesized SBA-15 has a high surface area (720 m2/g) and texture parameters (Table 1), characteristic of a pure mesoporous material of the SBA-15 type.27 Modification of the surface of SBA-15 by different functional groups leads to a decrease in the surface area. There is also a decrease in the pore volume, which indicates the functionalization of the inner surface of the pores by the corresponding groups.
The immobilization of molybdenum also leads to a reduction in the surface area and pore size, while a similar nitrogen adsorption/desorption isotherm is well observed. This trend indicates the attachment of metal inside the pores of the material. Note that, despite the significant reduction in the pore volume, the pore diameter decreases slightly, which allows the substrates to quickly diffuse into the pores to the active centers of the catalyst in the future.
2.1.2. Low-Angle XRD
To confirm that the modification of the SBA-15 by various functional groups will not lead to its destruction, low-angle X-ray diffraction (XRD) was carried out (Figure 3). The d100 peak, d110 peak, and d200 peak are characteristic of hexagonal mesoporous SBA-15. These results indicate that the uniform porous structure of SBA-15 is retained. According to the data obtained, it can be concluded that the modification of SBA-15 with silanes followed by ion exchange does not lead to distortion of the carrier structure.
Figure 3.

Low-angle XRD patterns of 10%Mo/Et3N-SBA-15.
2.1.3. FTIR Spectra
The SBA-15 support and all the catalysts were studied by IR spectroscopy (Figure 4). The infrared peaks located at 1065 and 800 cm–1 can be attributed to the asymmetric vibrations of free oxygen atoms in the Si–OH bonds and symmetric valence vibration Si–O–Si group. Also, a typical band related to the valence oscillation of the Si–OH group appears at 950 cm–1. From the obtained dependencies, it can be seen that the intensity of all the described peaks practically does not change after surface modification.28
Figure 4.

Fourier-transform infrared (FTIR) spectra of the obtained supports and catalysts.
2.1.4. TEM EDX
The presence of molybdenum and functional groups was also determined using the transmission electron microscopy energy-dispersive X-ray (TEM EDX) method. The elemental mapping images for catalyst 10%Mo/Et3N-SBA-15 are shown in Figure 5. The obtained data show a uniform distribution of silicon, molybdenum, nitrogen, oxygen, and carbon, which indicates a successful modification of the surface with triethylamine and subsequent ion exchange with the molybdate anion.
Figure 5.
Elemental mapping images of 10%Mo/Et3N-SBA-15.
The S/N ratio of the scanning TEM (STEM)-EDX mapping of Mo is too poor, which we associate with the finding of molybdenum atoms inside the pores of the catalyst since, according to the elemental analysis (Table 1), the content of molybdenum in the catalyst is 10% and this amount should be sufficient for detection by the EDX method.
2.1.5. TEM
The SBA-15, the Et3N-SBA-15, and the 10%Mo/Et3N-SBA-15 were examined by transmission electron microscopy (Figure 6).
Figure 6.
TEM micrographs of the SBA-15 (a, b), the Et3N-SBA-15 carrier (c, d), and the 10%Mo/Et3N-SBA-15 catalyst (e, f).
Figure 6a,b shows that the synthesized SBA-15 has clearly ordered hexagonal pores and a network of parallel channels. Functionalizing the surface by organic groups and subsequent application of the molybdenum anion does not lead to a distortion of the precise structure of the material (Figure 6c–f).
2.2. Investigation of the Activity of the Synthesized Catalysts
The activity of the synthesized catalytic systems was studied on a model mixture of dibenzothiophene (the initial total sulfur content was 500 ppm). Dibenzothiophene was chosen because it is the most difficult to remove among the sulfur-containing compounds of petroleum origin. Therefore, the greater part of the work on the oxidation of sulfur-containing compounds was carried out on a model mixture of dibenzothiophene. The oxidation of dibenzothiophene proceeds in two stages (Figure 7). At the first stage, sulfoxide is formed, an unstable compound in an oxidizing medium, which is almost immediately converted to sulfone.
Figure 7.
Dibenzothiophene oxidation scheme.
2.2.1. Comparison of the Activity of the Catalysts
At the first stage, the synthesized catalytic systems were compared in the oxidation of a model dibenzothiophene mixture (Figure 8). To justify the need for chemical sewing of the heptamolybdate anion on the surface of the catalyst, a catalyst was obtained by impregnating unmodified SBA-15 with ammonium heptamolybdate. This catalyst is designated as 10%Mo/SBA-15. As shown in Figure 8, catalysts modified with triethylamine show better results than those modified with ammonium groups, and the catalyst without preliminary modification of SBA-15 has a similar conversion with 10%Mo/H3N-SBA-15; catalyst activity steadily decreases in the series: 10%Mo/Et3N-SBA-15 > 5%Mo/Et3N-SBA-15 > 10%Mo/SBA-15 ≈ 10%Mo/H3N-SBA-15 > 5%Mo/H3N-SBA-15. This dependence can be due to the greater hydrophobicity of the triethylamine catalysts. Consequently, the diffusion of the substrate (DBT) into the pores of the catalyst is easier in this case than in that of ammonium catalysts.
Figure 8.

Dependence of DBT conversion on oxidation time. Oxidation conditions: H2O2:S = 4:1, w. (cat.) = 0.5%, 80 °C.
Catalysts containing 10% molybdenum are more active than their 5% counterparts. This is because a higher number of active peroxocomplexes are formed simultaneously, which enter into the oxidation reaction of DBT. From the above kinetic dependence (Figure 8), it can be seen that, in 2 h of the reaction, with a fourfold excess of hydrogen peroxide in the presence of 10%Mo/Et3N-SBA-15, it is possible to achieve almost 100% DBT conversion. At the same time, catalysts 5%Mo/Et3N-SBA-15 and 10%Mo/H3N-SBA-15 show a similar conversion in 4 h of oxidation. Catalyst 5%Mo/H3N-SBA-15 showed a low conversion rate (in 4 h, the DBT conversion rate was 64%), and therefore, it was not used in further experiments.
2.2.2. Effect of Temperature on DBT Conversion
For all catalysts, a decrease in temperature leads to a reduction in conversion (Figure 9). It is important to note that, for 5%Mo/Et3N-SBA-15 and 10%Mo/H3N-SBA-15 catalysts, the conversion degree falls more strongly upon a decrease in temperature from 80 to 60 °C than for the 10%Mo/Et3N-SBA-15 catalyst, for which the conversion drop is insignificant. This effect can be attributed to the fact that the adsorption of sulfones into the pores of the ammonium catalyst is easier than the ethyl-containing analog, and consequently, when the temperature decreases, the conversion is affected not only by the rate of oxidation of DBT but also by the desorption of oxidation products from the pores of the catalyst. The sulfones remaining inside the pores can make it difficult for DBT to diffuse into the catalyst pores. It should be noted that, at each temperature, the catalyst 10%Mo/Et3N-SBA-15 with a high molybdenum content shows better results in DBT conversion than the catalyst 5%Mo/Et3N-SBA-15, which, as indicated above, is associated with a large number of simultaneously formed peroxocomplexes. In further experiments, the oxidation reactions were carried out at 60 °C, at which the difference in the activity of the catalysts manifests itself better.
Figure 9.
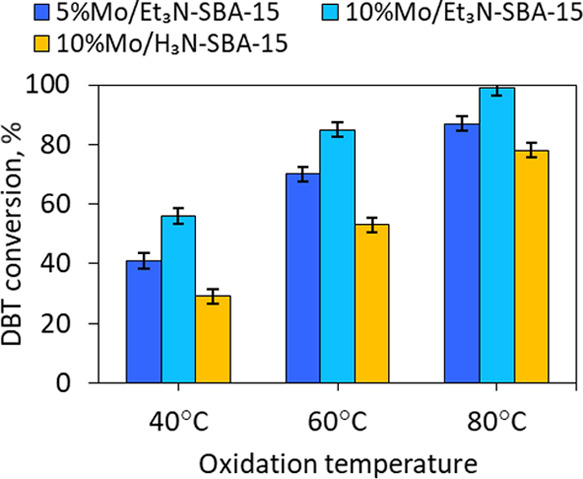
Effect of temperature on DBT conversion. Oxidation conditions: H2O2:S = 4:1,w. (cat.) = 0.5%, 120 min.
2.2.3. Effect of Catalyst Dosage on DBT Conversion
To study the dependence of the conversion of dibenzothiophene on the dosage of the catalyst, the amount of the latter was varied in the range from 0.05 to 1% by mass (Figure 10). This amount of catalyst is taken relative to the mass of the model fuel (in all experiments, 5 mL of the model mixture was used, and the mass (5 mL of fuel) was 3.75 g).
Figure 10.

Effect of catalyst dosage on DBT conversion. Oxidation conditions: H2O2:S = 4:1, 120 min, 60 °C.
An increase in the amount of the catalyst leads to a monotonous increase in the DBT conversion. This trend is observed for all the catalysts. An increase in the amount of the catalyst in the reaction mixture leads to an increase in the number of the active centers, contributing to an increase in the number of peroxocomplexes formed, which act as a source of active oxygen. Using a 1.0 wt % catalyst dosage ensures 100% DBT conversion for catalyst 10%Mo/ Et3N-SBA-15.
2.2.4. Effect of the Hydrogen Peroxide Amount on DBT Conversion
An increase in the amount of the hydrogen peroxide-to-sulfur ratio from 2:1 to 10:1 leads to an increase in the DBT conversion for Et3N-SBA-15-based catalysts (Figure 11). For catalyst H3N-SBA-15, an extremum is observed in the dependence of the DBT conversion on the amount of hydrogen peroxide. When the molar ratio increases from 2:1 to 6:1, the conversion rate increases, consistent with the literature data. A further increase in the hydrogen peroxide amount leads to a decrease in the conversion degree, which can be due to the observed aggregation of catalyst particles in the aqueous phase of hydrogen peroxide (Figure 12). Note that, for catalysts based on Et3N-SBA-15, there is no decrease in the conversion degree with an increase in the amount of hydrogen peroxide, which is probably due to their greater hydrophobicity, which, in turn, facilitates the diffusion of the hydrophobic substrate—dibenzothiophene. Despite the small dosage of the catalyst (0.25 wt %), for both thiethylammonium-containing catalysts, it is possible to achieve 100% DBT conversion in 1 h at 60 °C in the presence of an eightfold excess of hydrogen peroxide.
Figure 11.

Effect of the hydrogen peroxide amount on DBT conversion. Oxidation conditions: w. (cat.) = 0.25%, 60 min, 60 °C.
Figure 12.

Photos of the reactors during oxidation (on the left, there was an aggregation of the catalyst 10%Mo/H3N-SBA-15 particles with an excess of the water phase, and on the right, the catalyst 10%Mo/Et3N-SBA-15 is located in the entire reaction volume).
To confirm the different hydrophobicity of the synthesized catalysts, the wetting edge angles for 10%Mo/H3N-SBA-15 and 10%Mo/Et3N-SBA-15 were measured. For this purpose, images of a water drop dropped on pre-compressed catalyst tablets were taken (Figure 13).
Figure 13.

Contact angle for a water droplet on the surface of 10%Mo/H3N-SBA-15 (left) and 10%Mo/Et3N-SBA-15 (right).
As shown in Figure 13, the wettability of the surface in the case of an amino-containing catalyst is higher than in an ethyl-containing one. The calculated wetting edge angles are 10 and 38° for 10%Mo/H3N-SBA-15 and 10%Mo/Et3N-SBA-15, respectively. The data obtained suggest that the catalyst 10%Mo/Et3N-SBA-15 is more hydrophobic, making it the most promising one for the oxidation of DBT with hydrogen peroxide compared to the catalyst 10%Mo/H3N-SBA-15.
2.2.5. Effect of the Initial Sulfur Content on DBT Conversion
The presence of the alkyl substituents in Et3N-SBA-15-based catalysts affects the adsorption of hydrogen peroxide and water and minimizes the undesirable adsorption of the oxidation product—dibenzothiophene sulfone, which in turn increases the service life of the catalyst before regeneration. Previously, we have shown that an increase in the sulfur content in the initial fuel leads to a sharp decrease in the DBT conversion, which is associated with the sorption of oxidation products in the catalyst pores.7 To address this issue, a similar study of the dependence of the DBT conversion on the initial sulfur content was carried out for the catalysts 10%Mo/Et3N-SBA-15 and 10%Mo/H3N-SBA-15 (Figure 14). Thus, with an increase in the initial sulfur content from 500 to 2000 ppm, the DBT conversion for the 10%Mo/H3N-SBA-15 catalyst decreases from 53 to 21%, which is probably due to the adsorption of dibenzothiophene sulfone in the catalyst pores and the difficulty of diffusion of the hydrophobic DBT. At the same time, for the 10%Mo/Et3N-SBA-15 catalyst, an increase in the initial sulfur content only slightly suppresses the DBT conversion. This can be explained by the insignificant adsorption of DBT sulfone in the catalyst pores.
Figure 14.

Effect of the initial sulfur content on DBT conversion. Oxidation conditions: H2O2:S = 4:1, w. (cat.) = 0.5%, 60 min, 60 °C. The asterisk (*) marks the catalyst synthesized in an earlier work.7
2.2.6. Oxidation of Different Classes of Sulfur-Containing Compounds
Since natural fuels are a complex mixture of hydrocarbons that include sulfur-containing compounds of different structures, it was essential to evaluate the possibility of oxidation of various classes of sulfur-containing compounds. For this purpose, model mixtures of the following sulfur-containing compounds were prepared: thioanisole, benzothiophene, methylbenzothiophene, dibenzothiophene, methylbenzothiophene, and dimethyldibenzothiophene. The oxidation was carried out in the presence of the catalyst 10%Mo/Et3N-SBA-15, which proved to be the most active one.
According to the data obtained (Figure 15), the catalyst activity concerning sulfur compounds falls in the series sulfides > dibenzothiophenes > benzothiophenes. At the same time, the presence of a methyl substituent in benzo- and dibenzothiophene sharply reduces the conversion degree, which can be explained by a decrease in the electron density on the sulfur atom as well as possible steric difficulties.
Figure 15.
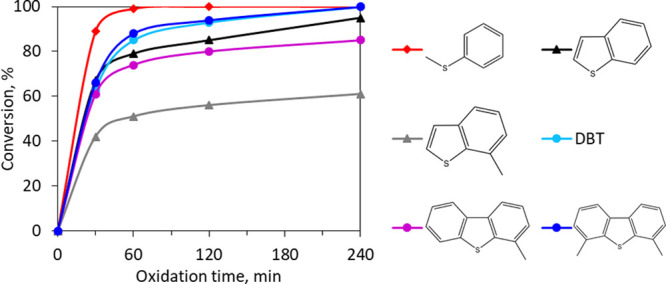
Oxidation of different classes of sulfur-containing compounds. Oxidation conditions: H2O2:S = 4:1, w. (10%Mo/Et3N-SBA-15) = 0.5%, 60 °C.
2.2.7. Catalyst Recycling
Reusability is an essential characteristic of heterogeneous catalysts. Since it was shown that the triethylamine-based catalyst is not clogged with sulfones, the catalyst was recycled without its regeneration. To do this, after the end of the reaction, the catalyst was separated from the reaction mixture by centrifugation, dried on a rotary evaporator until completely dry, and placed in a new portion of the model mixture with the oxidant. The experiments were made for two catalysts: 10%Mo/Et3N-SBA-15 and 10%Mo/H3N-SBA-15.
The catalyst 10%Mo/Et3N-SBA-15 retains its activity for six cycles, whereas the catalyst 10%Mo/H3N-SBA-15 loses much of its activity (Figure 16). This trend can be explained by the fact that the sulfones formed during the oxidation process are adsorbed in the pores of the catalyst 10%Mo/H3N-SBA-15 and do not allow the active centers to enter into a new oxidation reaction. Therefore, to maintain the activity of the catalyst, it is necessary to constantly regenerate it by washing with acetone from the oxidation products.
Figure 16.

Catalyst recycling. Oxidation conditions: H2O2:S = 4:1, w. (cat.) = 0.25%, 120 min, 60 °C.
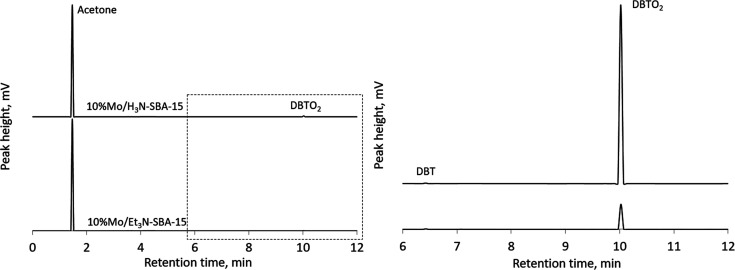
An experiment was also conducted to analyze the amount of sulfone adsorbed in the pores for catalysts containing 10% molybdenum. The presence of sulfone was determined by gas chromatography (Figure 17). To do this, the catalyst after the sixth reaction cycle was separated from the reaction mixture by centrifugation and washed with 1 mL of acetone. Next, acetone was analyzed by gas chromatography.
Figure 17.
Chromatogram of acetone after washing the catalyst (left); enlarged chromatogram area (right). The catalysts were washed with acetone after six recycles.
The obtained data show that the amount of adsorbed sulfone in the pores of the amino-containing catalyst is much more significant than that in the triethyl-containing catalysts. We conclude therefore that triethyl-containing catalysts are more hydrophobic and most promising for the oxidation of sulfur-containing compounds of petroleum origin due to their stability and long service life.
Another advantage of the synthesized catalysts is the absence of metal leaching from the surface of the catalysts. To confirm this fact, elemental analysis of the catalysts was performed after six cycles. To do this, the catalysts were pre-washed with acetone to wash out the oxidation products and dried in the air. A comparison of the molybdenum content in the freshly prepared and spent catalysts according to the elemental analysis data suggests that the proposed method of immobilization minimizes the leaching of the active phase from the surface (Table 2). Elemental analysis data also confirm the assumption that the decrease in the activity of 10%Mo/H3N-SBA-15 after six cycles is due to the adsorption of sulfones rather than to the leaching of the active phase of the catalyst.
Table 2. Texture Characteristics and Molybdenum Content in Catalysts before and after Oxidation.
| texture
characteristics |
||||
|---|---|---|---|---|
| sample | SBET (m2/g) | Vpore (cm3/g) | Dpore (Å) | the actual content of molybdenum (wt %) |
| 10%Mo/Et3N-SBA-15 before oxidation | 307 | 0.37 | 49 | 9.5 |
| 10%Mo/Et3N-SBA-15 after oxidation | 301 | 0.36 | 48 | 9.5 |
| 10%Mo/H3N-SBA-15 before oxidation | 319 | 0.38 | 52 | 9.7 |
| 10%Mo/H3N-SBA-15 after oxidation | 310 | 0.36 | 51 | 9.6 |
The catalysts after oxidation were also examined by low-temperature nitrogen adsorption/desorption to confirm the preservation of the structure of the catalysts after six oxidation cycles. According to the data presented in Table 2, the oxidation process practically does not affect the texture characteristics of the synthesized catalysts, which allows them to be used repeatedly without a loss of activity.
3. Conclusions
In this work, molybdenum-containing catalysts based on SBA-15 modified with various cations: Et3N-SBA-15 and H3N-SBA-15 were synthesized. A complex of physicochemical analysis methods (RSFA, low-temperature nitrogen adsorption/desorption, TEM, and IR spectroscopy) confirmed the composition and the structure of the obtained catalysts. It is shown that the different nature of the cations in the design of SBA-15 affects the hydrophobicity of the surface, which further has a strong effect on the possibility of using a catalyst in the oxidation of DBT with hydrogen peroxide. So, the edge angles for 10%Mo/H3N-SBA-15 and 10%Mo/Et3N-SBA-15 are 10 and 38°, respectively. The strong hydrophilicity of the surface of the catalyst 10%Mo/H3N-SBA-15 leads to the aggregation of the catalyst particles in the aqueous phase as well as to the clogging of its pores with oxidation products—sulfones, which dramatically reduces the service life of the catalyst. In contrast to 10%Mo/H3N-SBA-15, the catalyst 10%Mo/Et3N-SBA-15 practically does not adsorb sulfones, allowing it to be used for six cycles without an additional stage of regeneration (purification from oxidation products). In 1 h of the reaction at 60 °C and a small dosage of the catalyst (0.25 wt %), DBT was completely oxidized with an eightfold excess of hydrogen peroxide. It is also shown that, in 4 h, with a fourfold excess of hydrogen peroxide, thioanisole, dibenzothiophene, and dimethyldibenzothiophene can be removed entirely, and the most difficult-to-remove benzothiophene can be removed by 80%.
4. Experimental Section
4.1. Materials and Reagents
Tetraethoxysilane (TEOS) (Sigma-Aldrich), symmetric triblock copolymer Pluronic P123 (Sigma-Aldrich), toluene (99.5%), acetonitrile (ECOS-1), carbon tetrachloride (ECOS-1), acetone (reagent component), 3-aminopropyltrimethoxysilane (97%) (Sigma-Aldrich), triethylamine (Engineering), 3-chloropropyltrimethoxysilane (97%) (Sigma-Aldrich), and hydrochloric acid (HCl) (Sigma-Tech) were used for the synthesis of carriers. Ammonium heptamolybdate (NH4)6Mo7O24 (Prime Chemicals Group) was used as a source of molybdenum.
The model mixture consisted of sulfur-containing compounds (500 ppm sulfur): methylphenylsulfude (MeSPh, 99%, Acros Organics), benzothiophene (BT, 98%, Sigma-Aldrich), 3-methylbenzothiophene (MeBT, 97%, Sigma-Aldrich), dibenzothiophene (DBT, 98%, Sigma-Aldrich), 4-methyldibenzothiophene (MeDBT, 96%, Sigma-Aldrich), or 4,6-dimethyldibenzothiophene (Me2DBT, 95%, Sigma-Aldrich) in dodecane (99%, Acros Organics).
4.2. Synthesis of Catalysts and Their Characterization
4.2.1. SBA-15 Synthesis
The synthesis of SBA-15 was carried out similarly to a previous work:7 12.9 Pluronic P123 was dissolved in 486 mL of 1.6 M hydrochloric acid solution at a temperature of 40 °C, and then, 34.72 g of tetraethoxysilane was added dropwise for 2 h with constant stirring. After stirring at 40 °C for 24 h, the mixture was kept at 100 °C in a drying cabinet for 24 h. The white precipitate was then washed with distilled water to a neutral pH, filtered, and dried at 80–110 °C, increasing the temperature by 10 °C every 4 h. The final SBA-15 was obtained by calcining the material at 550 °C for 5 h at a heating rate of 5 °C/min. Further, the surface of SBA-15 was modified in two ways.
4.2.2. Et3N-SBA-15 Synthesis
A mixture of SBA-15 and 3-chloropropyltriethoxysilane in dry toluene was boiled with a reverse refrigerator at 95–105 °C for 15 h. After that, the mixture was filtered on a glass filter, washed twice with 20 mL of carbon tetrachloride, and then dried on a rotor evaporator under vacuum at 80 °C. Then, triethylamine quaternization was performed for 48 h: a mixture of the resulting carrier with triethylamine was boiled in a round-bottomed flask with a reverse refrigerator at 90–95 °C; after the reaction, it was filtered on a glass filter, washed two times with 20 mL of carbon tetrachloride, and dried on a rotor evaporator.
4.2.3. H3N-SBA-15 Synthesis
A mixture of 3-aminopropyltriethoxysilane and SBA-15 was boiled in pure toluene with a reverse refrigerator for 15 h at 90–95 °C. After that, the mixture was filtered on a glass filter, washed twice with 20 mL of carbon tetrachloride, and two times with acetonitrile. Then, the substance was dried on a rotor evaporator under vacuum at 80 °C. The surface was then protonated by adding 0.05 M hydrochloric acid to the mixture while stirring for 3 h. The substance was filtered on a glass filter and washed with water until a neutral reaction.
4.2.4. Synthesis of Molybdenum-Containing Catalysts
The calculated amount of ammonium heptamolybdate (NH4)6Mo7O24 was dissolved in 5 mL of water. Next, 15 mL of distilled water was added to the carrier and stirred in a water bath at 60 °C for 20 min. A solution of ammonium heptamolybdate was added, and the carrier was soaked for 24 h with stirring. After that, they were dried on a glass filter, washed twice with 20 mL of carbon tetrachloride, two times with 20 mL of acetonitrile, and then with water until the residues of ammonium heptamolybdate, physically adsorbed, were removed entirely. After that, they were dried in the air at room temperature for a day, and then in a drying cabinet at 80–110 °C, the temperature was increased by 10 °C every 4 h. The total calcination time was 16 h.
4.2.5. Synthesis of 10%Mo/SBA-15
The calculated amount of ammonium heptamolybdate (NH4)6Mo7O24 was dissolved in 5 mL of water. Then, unmodified SBA-15 was added to the resulting solution and kept at 60 °C with constant stirring until water was completely removed. After the catalyst was dried in a drying cabinet at 80–110 °C, the temperature was increased by 10 °C every 4 h.
An investigation of the elemental composition by X-ray spectral fluorescence analysis (RSFA) was carried out on an XRF fluorescence wave spectrometer ARL Perform’X (Thermo Fisher Scientific, New Wave).
The characteristics of the porous structure of the samples were determined on a Micromeritics Gemini VII 2390 (V1.02t) analyzer according to the standard procedure. Before analysis, the samples were evacuated at 350 °C for 12 h to a pressure of 3 × 10–3 Pa. The nitrogen adsorption/desorption isotherms were performed at 77 K. The characteristics of the porous structure were calculated using standard software. The specific surface area was calculated from the Brunauer–Emmett–Teller (BET) model at a relative partial pressure of P/P0 = 0.2. The total pore volume was calculated from the Barrett–Joyner–Halenda (BJH) model at a relative pressure of P/P0 = 0.95.
X-ray phase analysis was performed on a Rigaku Rotaflex D/max-RC instrument using copper Kα radiation (λ = 0.154 nm). The diffraction pattern from the sample was registered at a low angle in the range 2θ = 0.5–5° with a step of 0.01° and a recording rate of 2° min–1.
Transmission electron microscopy (TEM) investigations were performed using a JEOL JEM-2100 UHR (Japan) microscope operated at a 200 kV acceleration voltage. The microphotographs were acquired in bright-field mode using an Olympus Quemesa 11 megapixel CCD camera. The EDX mapping was performed in STEM mode with the help of an EX-24065JGT energy-dispersive X-ray analyzer. Fine powders of samples were dispersed in hexane and dropped onto a formvar/C Lacey TEM Cu grid (300 mesh, Ted Pella, Inc.).
FTIR spectra were recorded using a Nicolet IR2000 (Thermo Fisher Scientific) spectrometer equipped with multiple horizontal reflections attenuated total reflection accessory with a ZnSe crystal. The spectra were obtained using multiple distortions of the total internal reflection in the range of 4000–500 cm–1 with a resolution of 4 cm–1. All spectra were taken by averaging 100 scans.
4.3. Experimental and Analytical Method
The oxidation reaction was carried out in a 10 mL glass reactor with a temperature-controlled jacket (temperature control accuracy of 0.01°) with constant stirring. To obtain the model mixture with an initial sulfur concentration of 500 ppm, 0.413 g of DBT was used to dissolve into 200 mL of n-dodecane; solutions of other sulfur-containing compounds were prepared similarly. The oxidation reactions of the model mixtures of sulfur-containing compounds were carried out according to the following procedure: 0.0018–0.0375 g of a heterogeneous catalyst and 0.0065–0.05 mL of an oxidant (hydrogen peroxide) were added to 5 mL of a model mixture of sulfur compounds in dodecane. The oxidation was carried out for 0.1–4 h at a temperature of 40–80 °C. At different intervals, around a 0.1 mL sample was taken out, and the oil phase was collected to analyze by gas chromatography (Crystal-2000 M with a flame ionization detector, column—Zebron L = 30 m, d = 0.32 mm, liquid phase ZB-1 while programming the temperature from 150 to 250 °C). The conversion of sulfur compounds was calculated using the following formula:
| 1 |
Each experiment was made repeatedly to obtain a minimum of three convergent results (the convergent result is a result that differs from the average value by no more than 5%). An average of performed experiments was reported in the manuscript. The measurement error is not more than 5%.
Acknowledgments
The work was supported by the Russian Foundation for Basic Research (grant no. 18-29-05064).
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Houda S.; Lancelot C.; Blanchard P.; Poinel L.; Lamonier C. Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review. Catalysts 2018, 8, 344–369. 10.3390/catal8090344. [DOI] [Google Scholar]
- Akopyan A. V.; Fedorov R. A.; Andreev B. V.; Tarakanova A. V.; Anisimov A. V.; Karakhanov E. A. Oxidative Desulfurization of Hydrocarbon Feedstock. Russ. J. Appl. Chem. 2018, 91, 529–542. 10.1134/s1070427218040018. [DOI] [Google Scholar]
- Babich I. V.; Moulijn J. A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. 10.1016/s0016-2361(02)00324-1. [DOI] [Google Scholar]
- Akopyan A. V.; Grigoriev D. A.; Polikarpova P. L.; Eseva E. A.; Litvinova V. V.; Anisimov A. V. Ozone-assisted oxidative desulfurization of light oil fractions. Pet. Chem. 2017, 57, 904–907. 10.1134/s0965544117100024. [DOI] [Google Scholar]
- Julião D.; Mirante F.; Ribeiro S. O.; Gomes A. C.; Valença R.; Ribeiro J. C.; Pillinger M.; de Castro B.; Gonçalves I. S.; Balula S. S. Deep oxidative desulfurization of diesel fuels using homogeneous and SBA-15-supported peroxophosphotungstate catalysts. Fuel 2019, 241, 616–624. 10.1016/j.fuel.2018.11.095. [DOI] [Google Scholar]
- Lv G.; Deng S.; Yi Z.; Zhang X.; Wang F.; Li H.; Zhu Y. One-pot synthesis of framework W-doped TS-1 zeolite with robust Lewis acidity for effective oxidative desulfurization. Chem. Commun. 2019, 55, 4885–4888. 10.1039/c9cc00715f. [DOI] [PubMed] [Google Scholar]
- Akopyan A.; Polikarpova P.; Gul O.; Anisimov A.; Karakhanov E. Catalysts based on acidic SBA-15 for deep oxidative desulfurization of model fuels. Energy Fuels 2020, 34, 14611–14619. 10.1021/acs.energyfuels.0c02008. [DOI] [Google Scholar]
- Wang B.; Dai B.; Zhu M. Application of Fumed Silica as a Support during Oxidative Desulfurization. ACS Omega 2020, 5, 378–385. 10.1021/acsomega.9b02802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J.; Zhu W.; Ding W.; Yang L.; Chao Y.; Li H.; Zhu F.; Li H. Phosphotungstic Acid Immobilized on Ionic Liquid-Modified SBA-15: Efficient Hydrophobic Heterogeneous Catalyst for Oxidative Desulfurization in Fuel. Ind. Eng. Chem. Res. 2014, 53, 19895–19904. 10.1021/ie503322a. [DOI] [Google Scholar]
- Sikarwar P.; Kumar U. K. A.; Gosu V.; Subbaramaiah V. Catalytic oxidative desulfurization of DBT using green catalyst (Mo/MCM-41) derived from coal fly ash. J. Environ. Chem. Eng. 2018, 6, 1736–1744. 10.1016/j.jece.2018.02.021. [DOI] [Google Scholar]
- Akbari A.; Omidkhah M.; Darian J. T. Facilitated and selective oxidation of thiophenic sulfur compounds using MoOx/Al2O3-H2O2 system under ultrasonic irradiation. Ultrason. Sonochem. 2015, 23, 231–237. 10.1016/j.ultsonch.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Yang H.; Jiang B.; Sun Y.; Zhang L.; Huang Z.; Sun Z.; Yang N. Heterogeneous oxidative desulfurization of diesel fuel catalyzed by mesoporous polyoxometallate-based polymeric hybrid. J. Hazard. Mater. 2017, 333, 63–72. 10.1016/j.jhazmat.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Ghubayra R.; Nuttall C.; Hodgkiss S.; Craven M.; Kozhevnikova E. F.; Kozhevnikov I. V. Oxidative desulfurization of model diesel fuel catalyzed by carbon-supported heteropoly acids. Appl. Catal., B 2019, 253, 309–316. 10.1016/j.apcatb.2019.04.063. [DOI] [Google Scholar]
- Kulikov L. A.; Akopyan A. V.; Polikarpova P. D.; Zolotukhina A. V.; Maximov A. L.; Anisimov A. V.; Karakhanov E. A. Catalysts Based on Porous Polyaromatic Frameworks for Deep Oxidative Desulfurization of Model Fuel in Biphasic Conditions. Ind. Eng. Chem. Res. 2019, 58, 20562–20572. 10.1021/acs.iecr.9b04076. [DOI] [Google Scholar]
- Ahmed I.; Yu K.; Puthiaraj P.; Ahn W. S. Metal-free oxidative desulfurization over a microporous triazine polymer catalyst under ambient conditions. Fuel Process. Technol. 2020, 207, 106469. 10.1016/j.fuproc.2020.106469. [DOI] [Google Scholar]
- Bernardoni F.; Fadeev A. Y. Adsorption and wetting characterization of hydrophobic SBA-15 silicas. J. Colloid Interface Sci. 2011, 356, 690–698. 10.1016/j.jcis.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Veisi H.; Sedrpoushan A.; Faraji A. R.; Heydari M.; Hemmati S.; Fatahi B. A mesoporous SBA-15 silica catalyst functionalized with phenylsulfonic acid groups (SBA-15-Ph-SO3H) as a novel hydrophobic nanoreactor solid acid catalyst for a one-pot three-component synthesis of 2H-indazolo[2,1-b] phthalazine-triones and triazolo[1,2-a] indazole-triones. RSC Adv. 2015, 5, 68523–68530. 10.1039/c5ra04949k. [DOI] [Google Scholar]
- Cruz P.; Granados E. A.; Fajardo M.; del Hierro I.; Perez Y. Heterogeneous oxidative desulfurization catalysed by titanium grafted mesoporous silica nanoparticles containing tethered hydrophobic ionic liquid: A dual activation mechanism. Appl. Catal., A 2019, 587, 117241. 10.1016/j.apcata.2019.117241. [DOI] [Google Scholar]
- Karimi B.; Khorasani M. Selectivity Adjustment of SBA-15 Based Tungstate Catalyst in Oxidation of Sulfides by Incorporating a Hydrophobic Organic Group inside the Mesochannels. ACS Catal. 2013, 3, 1657–1664. 10.1021/cs4003029. [DOI] [Google Scholar]
- Li J.; Hu B.; Tan J.; Zhuang J. Deep oxidative desulfurization of fuels catalyzed by molybdovanadophosphoric acid on amino-functionalized SBA-15 using hydrogen peroxide as oxidant. Transition Met. Chem. 2013, 38, 495–501. 10.1007/s11243-013-9716-6. [DOI] [Google Scholar]
- Luo G.; Kang L.; Zhu M.; Dai B. Highly active phosphotungstic acid immobilized on amino functionalized MCM-41 for the oxidesulfurization of dibenzothiophene. Fuel Process. Technol. 2014, 118, 20–27. 10.1016/j.fuproc.2013.08.001. [DOI] [Google Scholar]
- Li X.; Huang S.; Xu Q.; Yang Y. Preparation of WO3-SBA-15 mesoporous molecular sieve and its performance as an oxidative desulfurization catalyst. Transition Met. Chem. 2009, 34, 943–947. 10.1007/s11243-009-9285-x. [DOI] [Google Scholar]
- Ding W.; Zhu W.; Xiong J.; Yang L.; Wei A.; Zhang M.; Li H. Novel heterogeneous iron-based redox ionic liquid supported on SBA-15 for deep oxidative desulfurization of fuels. Chem. Eng. J. 2015, 266, 213–221. 10.1016/j.cej.2014.12.040. [DOI] [Google Scholar]
- Wang C.; Chen Z.; Yao X.; Jiang W.; Zhang M.; Li H.; Liu H.; Zhu W.; Li H. One-pot extraction and aerobic oxidative desulfurization with highly dispersed V2O5/SBA-15 catalyst in ionic liquids. RSC Adv. 2017, 7, 39383–39390. 10.1039/c7ra07286d. [DOI] [Google Scholar]
- Estephane G.; Lancelot C.; Blanchard P.; Dufaud V.; Chambrey S.; Nuns N.; Toufaily J.; Hamiye T.; Lamonier C. W-SBA based materials as efficient catalysts for the ODS of model and real feeds: Improvement of their lifetime through active phase encapsulation. Appl. Catal., A 2019, 571, 42–50. 10.1016/j.apcata.2018.12.007. [DOI] [Google Scholar]
- Xiong J.; Zhu W.; Ding W.; Yang L.; Zhang M.; Jiang W.; Zhao Z.; Li H. Hydrophobic mesoporous silica-supported heteropolyacid induced by ionic liquid as a high efficiency catalyst for the oxidative desulfurization of fuel. RSC Adv. 2015, 5, 16847–16855. 10.1039/c4ra14382e. [DOI] [Google Scholar]
- Crucianelli M.; Bizzarri B. M.; Saladino R. SBA-15 Anchored Metal Containing Catalysts in the Oxidative Desulfurization Process. Catalysts 2019, 9, 984–1013. 10.3390/catal9120984. [DOI] [Google Scholar]
- Nejad N. F.; Shams E.; Amini M. K.; Choolaei M. Efficient desulfurization of fuel with functionalized mesoporous carbon CMK-3-O and comparison its performance with mesoporous carbon CMK-3. Fullerenes, Nanotubes, Carbon Nanostruct. 2016, 24, 786–795. 10.1080/1536383x.2016.1242484. [DOI] [Google Scholar]