Abstract
A modular and flexible three-step synthetic strategy has been developed for the synthesis of acridone natural products of biological significance. The tetracyclic core of acridone derivatives has been achieved efficiently in high yield from commercially available anthranilic acid and phenol derivatives via condensation reaction, followed by regioselective annulation. Acridone alkaloids acronycine and noracronycine are synthesized in improved overall yields in fewer steps than the previously reported approaches. The method has further been used for the synthesis of atalaphyllidine and 5-hydroxynoracronycine in excellent yields for the first time. Moreover, the synthetic utility of the present strategy has been showcased by the synthesis of oxa and thia analogues of acronycine alkaloid.
Introduction
Acridone alkaloids represent a large panel of biologically active compounds1 and exhibit a wide spectrum of biological activities ranging from antitumor, anticancer2 to antiviral3 and antimalarial properties.4 Among these alkaloids, acronycine, a pyranoacridone alkaloid (Figure 1) isolated from the Australian scrub ash Baurella simplicifolia (Endl.) Hartley (Rutaceae),5 exhibits the broadest spectrum of in vivo antineoplastic activity.1c,6 Noracronycine was isolated from Medicosma subsessilis (Figure 1).7 Recently, two new polycyclic acridone alkaloids, chlorospermine A and B, along with atalaphyllidine and acrifoline have been isolated from the stem bark of Glycosmis Chlorosperma (Figure 1).8 Among them, chlorospermine B possesses significant inhibitory property against dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A).8 Although several approaches have been developed over the years for the synthesis of various acridone alkaloids and their analogues;9−12 implementation of an efficient and modular synthetic route to acridone derivatives holds a high significance. Especially, the reported approaches do not provide the central core with a fully functionalized A ring, and a structure–activity study using various functional groups to access ring A is still missing.
Figure 1.
Some representative acridone alkaloids.
The reported synthesis of various acridone derivatives is mainly based on Friedel–Crafts reactions of electron-rich arenes under strongly acidic conditions.9,10 The Claisen rearrangement-based approach has been reported using 3-chloro-3-methylbut-1-yne to synthesize tetracyclic acridone derivatives from tricyclic dihydroxy acridones.11 In a different method developed by Kolokythas and co-workers, the reaction of methyl 3,5-dihydroxybenzoate with 3-chloro-3-methyl-1-butyne provided the corresponding chromene derivative, which on treatment with 2-chloronicotinic acid followed by cyclization furnished the desired acridone derivative.12a Recently, Maji and co-workes developed a cobalt-catalyzed amidation protocol that gives access to acridone-based natural products efficiently.12b Zheng et al. developed a one-pot synthesis of 1-hydroxyacridones via the DBU-mediated reaction of quinols and ortho-methoxycarbonylaryl isocyanates following a sequence of intramolecular condensation, tautomerization, and decarboxylation.12c Zyryanov and co-workers developed a new method for the synthesis of cytotoxic tetracyclic acridone derivatives and further evaluated their bioactivity and protein-binding properties by biological and biophysical studies.12d
Results and Discussion
We envisioned developing a linear yet efficient synthetic route for the preparation of tetracyclic acridone alkaloids. We hypothesized that tetracyclic acridone alkaloids 1 can be synthesized by selective protection and deprotection of the cyclic precursor 2. The D ring could be installed via titanium isopropoxide-mediated regioselective annulation of 1,3-dihydroxyacridone derivative 4 with prenal 3.13 The dihydroxy intermediate 4 could be easily accessed by condensation of 5 and 6 (Scheme 1).14
Scheme 1. Our Retrosynthetic Analysis.
We have first set our goal towards the synthesis of acronycine 1a and noracronycine 1b (Scheme 2). The condensation of anthranilic acid 5a and phloroglucinol 6 in the presence of the catalytic amount of TsOH in n-hexanol provided the 1,3-dihydroxyacridone derivative 4a in excellent yield.14 The product was obtained in pure form by filtration without chromatographic purification. As per our plan, the Ti(OiPr)4-mediated regioselective annulation of 4a with prenal 3 was next carried out to obtain the desired tetracyclic compound 2a in excellent yield (Scheme 2).13
Scheme 2. Synthesis of Acronycine and Noracronycine.
The regioselective cyclization involves the sole participation of the C3-OH group of 4a, which may be attributed to the strong intramolecular H-bonding between the carbonyl group and the C5-OH group (Figure 2). The treatment of 2a with excess of methyl iodide provided acronycine 1a in almost quantitative yield. A subsequent BBr3-mediated demethylation resulted in noracronycine 1b in excellent overall yield (Scheme 2).
Figure 2.

X-ray crystal structure of compound 2b.
The synthesis of acronycine and noracronycine was first reported by Beck and co-workers via the formation of dihydro-noracronycine, which upon oxidation followed by methylation provided noracronycine and acronycine, respectively.15 Reisch et al. synthesized noracronycine analogues by the reaction of the N-methyl-1,3-dihydroxyacridone derivative with propinols under Mitsunobu condition.16 Recently, Lee and co-workers reported the synthesis of acronycine and noracronycine via ethylenediamine diacetate-mediated cyclization of the dihydroxyacridone derivative followed by selective methylation.17 In our approach, we have successfully accomplished the synthesis of acronycine and noracronycine in a comparatively lesser number of steps with an improvement in overall yields.
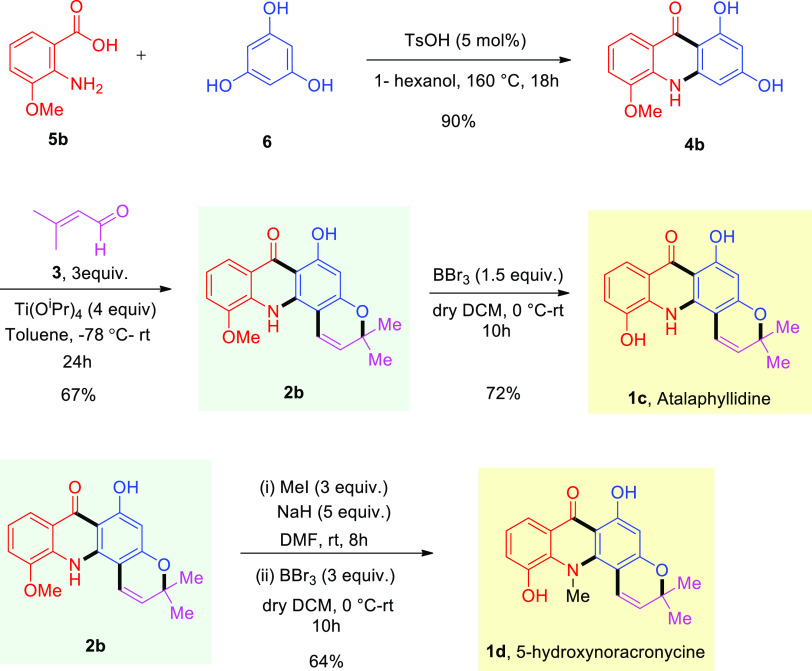
With the success of our designed protocol, our goal was to validate our strategy for a concise total synthesis of atalaphyllidine 1c and 5-hydroxynoracronycine 1d. To date, no synthetic routes have been reported for the synthesis of these two acridone alkaloids. In order to obtain these acridone derivatives, we started our synthetic journey with 2-amino-3-methoxybenzoic acid 5b instead of anthranillic acid 5a as used previously. The reaction of 5b with 6, under refluxing conditions, provided the desired dihydroxyacridone derivative 4b in high yield (Scheme 3). The regioselective annulation of 4b using titanium isopropoxide and prenal 3 gave rise to the requisite tetracyclic acridone derivative 2b, a suitable precursor for the synthesis of atalaphyllidine 1c and 5-hydroxynoracronycine 1d (Scheme 3). The treatment of 2b with BBr3 in dichloromethane successfully afforded atalaphyllidine 1c in excellent yield. Compound 2b could also be converted to 5-hydroxynoracronycine 1d via a two-step process as shown in Scheme 3. The reaction of 2b with excess methyl iodide provided the N- and O-methyl protected acridone intermediate, which was directly treated with BBr3 to obtain the desired product 1d in good isolated yield (Scheme 3). The 1H and 13C NMR spectra of 1c and 1d match with the reported spectra.18,19
Scheme 3. Synthesis of Atalaphyllidine and 5-Hydroxynoracronycine.
It is intriguing to mention here that the intramolecular hydrogen bonding plays a key role in the construction of the tetracyclic acridone core. The strong intramolecular hydrogen bonding between the C5-OH group and the carbonyl moiety promotes the efficient and regioselective mono-annulation. We could obtain a product like 7 (Scheme 4) but not the pentacyclic acridone core 8, as the C5-OH group does not participate in annulation due to this hydrogen bonding. The single-crystal X-ray analysis of compound 2b also clearly demonstrates this hydrogen bonding (Figure 2, CCDC 2063223; Figure S1, Supporting Information (S.I.)).20
Scheme 4. Intramolecular Hydrogen Bonding-Directed Annulation.
Next, we planned to expand this synthetic route for the preparation of oxa and thia analogues of tetracyclic acridones. The condensation of salicylic acid and thiosalicylic acid (9a and 9b, respectively) with phloroglucinol 6 generated the dihydroxy oxa and thia acridone derivatives 10a and 10b in excellent yields (Scheme 4).21 The regioselective annulation of 10a and 10b under the developed reaction conditions furnished the desired oxa and thia tetracyclic acridone derivatives 11a and 11b in 73 and 70% yields, respectively (Scheme 5).
Scheme 5. Synthesis of Oxa and Thia Analogues of Acronycine.
Conclusions
In conclusion, we have demonstrated a new and efficient synthetic route for the construction of different acridone alkaloids. The synthetic route is feasible, enabling the target compounds in excellent yields. Titanium isopropoxide-mediated intramolecular hydrogen bonding-directed regioselective mono-annulation is employed for the construction of the tetracyclic core of acridone natural products. Acronycine and noracronycine are synthesized in high overall yields than those already reported. Two new acridone alkaloids, atalaphyllidine and 5-hydroxynoracronycine, are successfully and efficiently synthesized. Moreover, we have extended this synthetic pathway for constructing tetracyclic oxa and thia acridone alkaloids.
Experimental Section
General Information
All experiments were carried out under an inert atmosphere of argon in flame-dried flasks. Solvents were dried using standard procedures. All starting materials were obtained from commercial suppliers and used as received. Products were purified by flash chromatography on silica gel (100–200 mesh, Merck). Unless otherwise stated, yields refer to analytical pure samples. NMR spectra were recorded in dimethyl sulfoxide (DMSO)-d6 unless otherwise stated. 1H NMR spectra were recorded using Brüker AVANCE 500 MHz and JEOL-400 MHz instruments at 298K. Signals are quoted as δ values in ppm using the residual protonated solvent signal as internal standard (DMSO-d6: δ 2.50 ppm). Data are reported as follows: chemical shift, integration, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, br = broad, m = multiplet), and coupling constants (Hz). 13C NMR spectra were recorded on either a JEOL-400 (100 MHz) or a Brüker ADVANCE 500 MHz (125 MHz) with complete proton decoupling. Chemical shifts (δ) are reported in ppm downfield from tetramethylsilane with the solvent as the internal reference (DMSO-d6: δ 39.50 ppm). High-resolution mass spectrometry (HRMS) analyses were performed with Q-TOF YA263 high-resolution (Water Corporation) instruments by +ve mode electrospray ionization.
General Procedure for the Synthesis of the Tricyclic Acridone Derivative (GP-1)
To a solution of anthranilic acid derivative 5 (1.0 equiv) and phloroglucinol 6 (1.0 equiv) in 1-hexanol, p-toluenesulfonic acid (TsOH, 0.05 equiv) was added and the reaction mixture was refluxed at 160 °C for 18 h. After 30 min, the color of the homogeneous solution became deep orange and after the reaction was over, a greenish yellow precipitate was formed. After cooling the reaction mixture to room temperature, n-hexane was added. The resulting precipitate was filtered and washed with hexane and dichloromethane until the odor of 1-hexanol diminished. Finally, the residue was dried in vacuum to provide the 1,3-dihydroxyacridone derivative 4, which was used directly for the next step without further purification (Scheme S1, S.I.).
Synthesis of 1,3-Dihydroxyxanthone and 1,3-Dihydroxythioxanthone (GP-2)
A mixture of phosphorus pentoxide (5 equiv) and methanesulfonic acid (25 mL) was heated on a steam bath (80 °C) for 15 min until a clear solution was obtained. To this solution, phloroglucinol (1.0 equiv) and the corresponding heteroaromatic benzoic acid (1.0 equiv) were added and the reaction mixture was allowed to stir for 15 min at this temperature. After completion of the reaction, the mixture was poured into ice-cold water and formation of the precipitate was observed. The resulting precipitate was collected by filtration, washed with water, and dried in air to afford acridone derivative 10 (Scheme S2, S.I.).
General Procedure for Ti(OiPr)4-Mediated Regioselective Cyclization (GP-3)
To a stirred solution of dihydroxyacridone derivatives 4 and 10 (1.0 equiv) and prenal 3 (3.0 equiv) in dry toluene at −78 °C was added Ti(OiPr)4 (4.0 equiv) dropwise. The reaction mixture was allowed to stir vigorously for 24 h. After completion of the reaction as monitored by thin layer chromatography (TLC), the reaction mixture was quenched with saturated aqueous NaHCO3 solution and extracted with ethyl acetate. The combined organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuum. The crude residue was then purified by column chromatography on silica gel with EtOAc-hexane to give compounds 2 and 11 (Scheme S3, S.I.).
Synthesis of Acronycine 1a
To a solution of 2a (1.0 equiv, 3.21 mmol) in dimethylformamide (DMF) was added NaH (3 equiv, 10.23 mmol) portionwise at 0 °C, and the reaction mixture was allowed to stir at 0 °C for 30 min. After that, MeI (3 equiv, 10.23 mmol) was added to the reaction mixture and the reaction was allowed to stir at room temperature for 8 h. After completion of the reaction as monitored by TLC, the reaction was quenched with addition of ice-cold saturated ammonium chloride solution. The aqueous layer was extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, and the solvent was removed under reduced pressure. It was then purified by column chromatography on silica gel with EtOAc-hexane to provide acronycine (967 mg, 90%) 1a as a greenish yellow solid.
Synthesis of Noracronycine 1b
To a solution of 1a (1.0 equiv, 3.11 mmol) in dichloromethane was added BBr3 (1.5 equiv, 1 M in DCM) dropwise at 0 °C, and the reaction mixture was allowed to stir for 10 h at room temperature. After completion of the reaction, the reaction mixture was quenched with saturated aqueous NaHCO3 solution and extracted with dichloromethane. The combined organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuum. The crude residue was then purified by column chromatography on silica gel with EtOAc-hexane to give the target demethylated product noracronycine 1b (745 mg, 78%) as a greenish yellow solid.
Synthesis of Atalaphyllidine 1c
To a solution of 2b (1.0 equiv, 3.09 mmol) in dichloromethane was added BBr3 (1.5 equiv, 1 M in DCM) dropwise at 0 °C, and the reaction mixture was allowed to stir for 10 h at room temperature. After completion of the reaction, the reaction mixture was quenched with saturated aqueous NaHCO3 solution and extracted with dichloromethane. The combined organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuum. The crude residue was then purified by column chromatography on silica gel with EtOAc-hexane to provide atalaphyllidine 1c (688 mg, 72%) as a greenish yellow solid.
Synthesis of 5-Hydroxynoracronycine 1d
To a solution of compound 2b (1.0 g, 3.09 mmol) in DMF was added NaH (3 equiv, 9.27 mmol) portionwise at 0 °C, and the reaction mixture was allowed to stir at 0 °C for 30 min. After that, MeI (3 equiv, 9.27 mmol) was added to the reaction mixture and the reaction was allowed to stir at room temperature for 8 h. After completion of the reaction as monitored by TLC, the reaction was quenched with addition of ice-cold saturated ammonium chloride solution. The aqueous layer was extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, and the solvent was removed under reduced pressure to obtain the crude dimethylated intermediate. The crude compound was then directly taken in dichloromethane, BBr3 (1.5 equiv, 1 M in DCM) was added dropwise at 0 °C, and the reaction mixture was allowed to stir for 10 h at room temperature. After completion of the reaction, the reaction mixture was quenched with saturated aqueous NaHCO3 solution and extracted with dichloromethane. The combined organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuum. The crude residue was then purified by column chromatography on silica gel with EtOAc-hexane to provide 5-hydroxynoracronycine 1d (639 mg, 64%) as a greenish yellow solid.
Analytical Data of Compounds
1,3-Dihydroxyacridinone (4a)13
Using the general procedure GP-1, anthranilic acid 5a (2.0 g, 14.59 mmol) and phloroglucinol 6 (1.84 g, 14.59 mmol) were used to provide compound 4a (2.91 g, 88%) as a yellowish green amorphous powder. m.p.: 344–346 °C; 1H NMR (500 MHz, DMSO-d6): 14.23 (1H, s), 11.77 (1H, s), 10.52 (1H, s), 8.14 (1H, d, J = 8.4 Hz), 7.70 (1H, t, J = 7.6 Hz), 7.46 (1H, d, J = 8.4 Hz), 7.24 (1H, t, J = 6.7 Hz), 6.30 (1H, s), 6.00 (1H, s); 13C (100 MHz, DMSO-d6): 180.0, 164.2, 163.7, 143.3, 140.7, 133.7, 125.0, 121.1, 118.8, 116.8, 103.2, 95.6, 90.8; HRMS (ESI) calcd for C13H10NO3 [M + H]+: 228.0661; Found: 228.0657.
1,3-Dihydroxy-5-methoxyacridinone (4b)13
Using the general procedure GP-1, 3-methoxy anthranilic acid 5b (2.0 g, 11.97 mmol) and phloroglucinol 6 (1.5 g, 11.97 mmol) were used to provide compound 4b (2.76 g, 90%) as a yellowish green amorphous powder. m.p.: 350–352 °C; 1H NMR (400 MHz, DMSO-d6): 14.26 (1H, sbr), 11.23 (1H, s), 10.45 (1H, sbr), 7.71 (1H, d, J = 8.0 Hz), 7.30 (1H, d, J = 8.0 Hz), 7.17 (1H, t, J = 8.0 Hz), 6.68 (1H, s), 6.00 (1H, s), 4.01 (3H, s); 13C (100 MHz, DMSO-d6): 179.8, 163.9, 163.4, 147.3, 143.1, 131.7, 120.7, 119.4, 116.1, 112.7, 103.4, 95.8, 92.0, 56.2; HRMS (ESI) calcd for C14H12NO4 [M + H]+: 258.0766; Found: 258.0764.
1,3-Dihydroxyxanthone (10a)21
Using the procedure GP-2, salicylic acid 9a (2.0 g, 14.49 mmol) and phloroglucinol 6 (1.82 mg, 14.49 mmol) were used to provide compound 10a (2.77 g, 84%) as a reddish brown solid. 1H NMR (400 MHz, DMSO-d6): 12.87 (1H, sbr), 11.12 (1H, sbr), 8.14 (1H, d, J = 8.1 Hz), 7.86 (1H, t, J = 7.9 Hz), 7.60 (1H, d, J = 8.5 Hz), 7.47 (1H, t, J = 7.4 Hz), 6.41 (1H, s), 6.22 (1H, s); 13C (100 MHz, DMSO-d6): 179.7, 165.9, 162.8, 157.4, 155.3, 135.6, 125.2, 124.4, 119.8, 117.7, 102.2, 98.1, 94.0; HRMS (ESI) calcd for C13H9O4 [M + H]+: 229.0501; Found: 229.0093.
1,3-Dihydroxythioxanthone (10b)21
Using the procedure GP-2, 2-mercaptobenzoic acid 9b (2.0 g, 12.98 mmol) and phloroglucinol 6 (1.63 g, 12.98 mmol) were used to provide compound 10b (2.69 g, 85%) as a deep red solid. 1H NMR (400 MHz, DMSO-d6): 14.37 (1H, s), 11.08 (1H, sbr), 8.43 (1H, d, J = 7.9 Hz), 7.77–7.75 (2H, m), 7.59–7.55 (1H, m), 6.65 (1H, d, J = 2.2 Hz), 6.31 (1H, d, J = 2.2 Hz); 13C (100 MHz, DMSO-d6): 183.1, 166.6, 163.9, 140.0, 136.3, 133.2, 128.4, 127.3, 126.7, 125.8, 107.5, 103.0, 101.0; HRMS (ESI) calcd for C13H9SO3 [M + H]+: 245.0272; Found: 245.0269.
6-Hydroxy-3,3-dimethyl-3H-pyrano[2,3-c]acridin-7(12H)-one (2a)15
Using the general procedure GP-3, 1,3-dihydroxyacridinone 4a (2.0 g, 8.81 mmol), prenal 3 (2.5 mL, 26.43 mmol), and Ti(OiPr)4 (10.4 mL, 35.24 mmol) were used to yield compound 2a (1.8 g, 70%) as a greenish yellow solid. m.p.: 216–218 °C; 1H NMR (400 MHz, DMSO-d6): 14.65 (1H, s), 11.15 (1H, s), 8.16 (1H, d, J = 8.7 Hz), 7.80–7.73 (2H, m), 7.32–7.28 (1H, m), 7.00 (1H, d, J = 9.9 Hz), 6.04 (1H, s), 5.73 (1H, d, J = 10.0 Hz), 1.43 (6H, s); 13C (100 MHz, DMSO-d6): 180.5, 163.8, 159.2, 140.8, 137.7, 133.9, 125.7, 124.8, 121.8, 118.8, 117.5, 116.0, 103.9, 98.0, 96.2, 77.0, 27.4; HRMS (ESI) calcd for C18H16NO3 [M + H]+: 294.1130; Found: 294.1127.
6-Hydroxy-11-methoxy-3,3-dimethyl-3H-pyrano[2,3-c]acridin-7(12H)-one (2b)
Using the general procedure GP-3, 1,3-dihydroxy-5-methoxyacridinone 4b (2.0 g, 7.78 mmol), prenal 3 (2.3 mL, 23.3 mmol), and Ti(OiPr)4 (9.21 mL, 31.12 mmol) were used to yield compound 2b (1.68 g, 67%) as a greenish yellow amorphous solid. m.p.: 200–202 °C; 1H NMR (400 MHz, DMSO-d6): 14.53 (1H, s), 9.71 (1H, s), 7.73 (1H, dd, J = 8.1 Hz, 1.1 Hz), 7.37 (1H, dd, J = 7.8 Hz, 1.0 Hz), 7.25 (1H, t, J = 8.0 Hz), 7.00 (1H, d, J = 10.0 Hz), 6.07 (1H, s), 5.70 (1H, d, J = 10.0 Hz), 4.02 (3H, s), 1.42 (6H, s); 13C (125 MHz, DMSO-d6): 180.5, 163.3, 159.1, 147.3, 136.8, 131.2, 125.6, 121.7, 119.5, 115.9, 115.8, 113.2, 104.1, 98.3, 96.5, 77.1, 56.3, 27.4HRMS (ESI) calcd for C19H18NO4 [M + H]+: 324.1236; Found: 324.1232.
6-Hydroxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (11a)
Using the general procedure GP-3, 1,3-dihydroxyxanthone 10a (2.0 g, 8.77 mmol), prenal 3 (2.51 mL, 26.31 mmol), and Ti(OiPr)4 (10.38 mL, 35.08 mmol) were used to yield compound 11a (1.88 g, 73%) as a deep red solid. m.p.: 176–178 °C; 1H NMR (400 MHz, CDCl3): 12.96 (1H, s), 8.25 (1H, d, J = 7.9 Hz), 7.71 (1H, t, J = 7.7 Hz), 7.46 (1H, d, J = 8.4 Hz), 7.38 (1H, t, J = 7.5 Hz), 6.84 (1H, d, J = 10.0 Hz), 6.27 (1H, s), 5.61 (1H, d, J = 10.0 Hz), 1.49 (6H, s); 13C (100 MHz, CDCl3): 181.0, 163.4, 161.1, 156.0, 151.9, 135.0, 127.3, 126.1, 124.2, 120.8, 117.7, 115.1, 103.9, 101.2, 99.5, 78.4, 28.4; HRMS (ESI) calcd for C18H15O4 [M + H]+: 295.0970; Found: 295.0966.
6-Hydroxy-3,3-dimethylthiochromeno[2,3-f]chromen-7(3H)-one (11b)
Using the general procedure GP-3, 1,3-dihydroxythioxanthone 10b (2.0 g, 8.19 mmol), prenal 3 (2.35 mL, 24.59 mmol), and Ti(OiPr)4 (9.69 mL, 32.76 mmol) were used to yield compound 11b (1.77 g, 70%) as a deep red solid. m.p.: 234–236 °C; 1H NMR (400 MHz, CDCl3): 14.71 (1H, s), 8.55 (1H, d, J = 8.0 Hz), 7.61 (1H, t, J = 8.1 Hz), 7.55 (1H, d, J = 7.9 Hz), 7.47 (1H, t, J = 8.1 Hz), 6.63 (1H, d, J = 10.0 Hz), 6.39 (1H, s), 5.70 (1H, d, J = 10.0 Hz), 1.43 (6H, s); 13C (100 MHz, CDCl3): 132.6, 129.3, 128.4, 128.2, 126.3, 125.5, 116.0, 103.4, 78.3, 28.6; HRMS (ESI) calcd for C18H15SO3 [M + H]+: 311.0742; Found: 311.0741.
Acronycine 1a(15)
m.p.: 176–178 °C; 1H NMR (500 MHz, DMSO-d6): 8.08 (1H, dd, J = 1.9, 6.3 Hz), 7.72–7.69 (1H, m), 7.54 (1H, d, J = 8.2 Hz), 7.26 (1H, t, J = 7.6 Hz), 6.68 (1H, d, J = 9.5 Hz), 6.38 (1H, s), 5.61 (1H, d, J = 10.1 Hz), 3.83 (3H, s), 3.79 (3H, s), 1.49 (6H, s); 13C (100 MHz, DMSO-d6): 175.3, 162.1, 158.6, 146.1, 144.1, 132.7, 125.8, 124.6, 123.0, 121.5, 117.0, 109.6, 102.7, 94.1, 76.2, 55.9, 43.9, 26.4; HRMS (ESI) calcd for C20H20NO3 [M + H]+: 322.1443; Found: 322.1436.
Noracronycine 1b(15)
m.p.: 200–202 °C; 1H NMR (400 MHz, DMSO-d6): 14.86 (1H, s), 8.24 (1H, dd, J = 8.0 Hz, 1.5 Hz), 7.90–7.85 (1H, m), 7.71 (1H, d J = 8.6 Hz), 7.40 (1H, t, J = 7.4 Hz), 6.77 (1H, d, J = 9.6 Hz), 6.16 (1H, s), 5.63 (1H, d, J = 9.6 Hz), 3.93 (3H, s), 1.49 (6H, s); 13C (100 MHz, DMSO-d6): 180.3, 164.2, 160.9, 144.5, 143.9, 134.5, 125.1, 122.9, 122.3, 121.3, 120.8, 117.4, 101.0, 96.6, 76.5, 43.4, 26.5; HRMS (ESI) calcd for C19H18NO3 [M + H]+: 308.1287; Found: 308.1294.
Atalaphyllidine 1c
m.p.: 274–276 °C; 1H NMR (400 MHz, DMSO-d6): 14.64 (1H, s), 10.86 (1H, sbr), 9.62 (1H, sbr), 7.63 (1H, d, J = 8.0 Hz), 7.20 (1H, dd, J = 1.4, 7.6 Hz), 7.15 (1H, t, J = 7.8 Hz), 7.00 (1H, d, J = 10.0 Hz), 6.06 (1H, s), 5.70 (1H, d, J = 10.0 Hz), 1.43 (6H, s); 13C (100 MHz, DMSO-d6): 180.7, 163.6, 159.1, 145.3, 136.8, 130.8, 125.7, 121.9, 119.9, 116.8, 115.6, 114.8, 103.9, 98.1, 96.3, 77.1, 27.4; HRMS (ESI) calcd for C18H16NO4 [M + H]+: 310.1079; Found: 310.1078.
5-Hydroxynoracronycine 1d
m.p.: 252–254 °C; 1H NMR (400 MHz, DMSO-d6): 14.43 (1H, s), 7.94 (1H, d, J = 7.4 Hz), 7.17–7.09 (3H, m), 6.66 (1H, d, J = 9.3 Hz), 5.84 (1H, sbr), 5.55 (1H, d, J = 9.8 Hz), 3.75 (3H, s), 1.52 (6H, s); 13C (100 MHz, DMSO-d6): 182.0, 161.9, 159.6, 146.6, 123.9, 123.1, 121.4, 121.0, 120.0, 118.6, 107.0, 106.8, 102.3, 98.5, 48.7, 27.; HRMS (ESI) calcd for C19H18NO4 [M + H]+: 324.1236; Found: 324.1237.
(E)-6-Hydroxy-11-methoxy-3,3-dimethyl-5-(3-methylbuta-1,3-dien-1-yl)-12,12a-dihydro-3H-pyrano[2,3-c]acridin-7(6aH)-one (7)
Obtained as a yellow solid; 1H NMR (500 MHz, DMSO-d6): 14.85 (1H, s), 9.44 (1H, s), 7.71 (1H, d, J = 6.5 Hz), 7.36 (1H, d, J = 6.1 Hz), 7.25 (1H, t, J = 6.4 Hz), 6.92 (1H, d, J = 13.4 Hz), 6.65–6.60 (2H, m), 5.74 (1H, d, J = 7.9 Hz), 5.16 (2H, s), 4.00 (3H, s), 2.03 (2H, s), 1.45 (6H, s); 13C NMR (100 MHz, DMSO-d6): 180.7, 157.1, 156.3, 146.8, 141.7, 137.8, 134.5, 130.5, 127.2, 121.8, 119.1, 119.0, 117.2, 116.0, 115.0, 113.0, 103.6, 101.6, 101.4, 78.0, 56.7, 27.9, 18.0; HRMS (ESI) calcd for C24H24NO4 [M + H]+: 390.1705; Found: 390.1703.
Acknowledgments
J.D. thanks the Wellcome Trust–DBT India Alliance [Grant Number, IA/S/18/2/503986] and CSIR for funding. T.M. thanks IACS, Kolkata, for the research fellowship. S.K. thanks CSIR-India for the research fellowship. The authors thank Partha Mitra and Manish Jana, IACS, for helping with single-crystal X-ray analysis. We thank Technical Research Center, IACS for funding.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03629.
Author Present Address
† School of Natural Sciences, Shivnadar University, Uttar Pradesh 201314, India
Author Contributions
‡ T.M. and S.K. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- For recent reviews, see; a Michael J. P. Quinoline, quinazoline and acridonealkaloids. Nat. Prod. Rep. 2008, 25, 166. 10.1039/B612168N. [DOI] [PubMed] [Google Scholar]; b Gensicka-Kowalewska M.; Cholewi′nski G.; Dzierzbicka K. Recent developments in the synthesis and biological activity of acridine/acridone analogues. RSC Adv. 2017, 7, 15776. 10.1039/C7RA01026E. [DOI] [Google Scholar]; for other references see; c Kawaii S.; Tomono Y.; Katase E.; Ogawa K.; Yano M.; Takemura Y.; Ju-ichi M.; Ito C.; Furukawa H. The Antiproliferative Effect of Acridone Alkaloids on Several Cancer Cell Lines. J. Nat. Prod. 1999, 62, 587. 10.1021/np980504z. [DOI] [PubMed] [Google Scholar]
- a Banerjee J.; Kundu I.; Zhang S.; Wen S.; Chattopadhyay S. K. Synthesis and Preliminary Biophysical and Cellular Evaluation of Some Ring-Enlarged Analogues of the Anti-Tumor Plant Alkaloid Acronycine. ACS Omega 2019, 4, 6106. 10.1021/acsomega.8b03673. [DOI] [Google Scholar]; b Nguyen Q. C.; Nguyen T. T.; Yougnia R.; Gaslonde T.; Dufat H.; Michel S.; Tillequin F. Acronycine Derivatives: A Promising Series of Anti-Cancer Agents. Anti-Cancer Agents Med. Chem. 2009, 9, 804. 10.2174/187152009789056921. [DOI] [PubMed] [Google Scholar]; c Viola A.; Mannoni P.; Chanon M.; Julliard M.; Mehta G.; Maiya B. G.; Muthusamy S.; Sambaiah T. Phototoxicity of some novel porphyrin hybrids against the human leukemic cell line TF-1. J. Photochem. Photobiol., B 1997, 40, 263. 10.1016/S1011-1344(97)00067-5. [DOI] [PubMed] [Google Scholar]; d Drexler C.; Hosseini M. W.; Pratviel G.; Meunier B.; et al. Design, synthesis and cleaving activity of an abiotic nuclease based on a manganese(III) porphyrin complex bearing two acridine moieties. Chem. Commun. 1998, 1343. 10.1039/a801095a. [DOI] [Google Scholar]; e Gamage S. A.; Spicer J. A.; Atwell G. J.; Finlay G. J.; Baguley B. C.; Denny W. A. Structure–Activity Relationships for Substituted Bis(acridine-4-carboxamides): A New Class of Anticancer Agents. J. Med. Chem. 1999, 42, 2383. 10.1021/jm980687m. [DOI] [PubMed] [Google Scholar]
- a Yamamoto N.; Furukawa H.; Ito Y.; Yoshida S.; Maeno K.; Nishiyama Y. Anti-herpesvirus activity of citrusinine-I, a new acridone alkaloid, and related compounds. Antiviral Res. 1989, 12, 21. 10.1016/0166-3542(89)90065-X. [DOI] [PubMed] [Google Scholar]; b Kramer M. J.; Cleeland R.; Grunberg E. Antiviral activity of 10-carboxymethyl-9-acridanone. Antimicrob. Agents Chemother. 1976, 9, 233. 10.1128/AAC.9.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shen Y. C.; Wang L. T.; Khalil A. T.; Chiang L. C.; Cheng P. W. Bioactive Pyranoxanthones from the Roots of Calophyllum blancoi. Chem. Pharm. Bull. 2005, 53, 244. 10.1248/cpb.53.244. [DOI] [PubMed] [Google Scholar]; b Kumar A.; Srivastava K.; Kumar S. R.; Puriand S. K.; Chauhan M. S. Synthesis of 9-anilinoacridine triazines as new class of hybrid antimalarial agents. Bioorg. Med. Chem. Lett. 2009, 19, 6996. 10.1016/j.bmcl.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Hughes G. K.; Lahey F. N.; Price J. R.; Webb L. J. Alkaloids of the Australian Rutaceæ. Nature 1948, 162, 223. 10.1038/162223a0. [DOI] [PubMed] [Google Scholar]
- Gout P. W.; Dunn B. P.; Beer C. T. Effects of acronycine on nucleic acid synthesis and population growth in mammalian tumor cell cultures. J. Cell. Physiol. 1971, 78, 127. 10.1002/jcp.1040780116. [DOI] [PubMed] [Google Scholar]
- Minh N. T.; Michel S.; Tillequin F.; Litaudon M.; Sevenet T.; Lallemand M. -C. A New Pyranoacridone Alkaloid from the Bark of Medicosma subsessilis (Rutaceae). Z. Naturforsch., B: J. Chem. Sci. 2003, 58, 1234. 10.1515/znb-2003-1213. [DOI] [Google Scholar]
- Beniddir M. A.; Borgne E. L.; Iorga B. I.; Loaëc N.; Lozach O.; Meijer L.; Awang K.; Litaudon M. Acridone Alkaloids from Glycosmis chlorosperma as DYRK1A Inhibitors. J. Nat. Prod. 2014, 77, 1117. 10.1021/np400856h. [DOI] [PubMed] [Google Scholar]
- a Loughhead D. G. Synthesis of des-N-methylacronycine and acronycine. J. Org. Chem. 1990, 55, 2245. 10.1021/jo00294a052. [DOI] [Google Scholar]; b Elomri A.; Michel S.; Tillequin F.; Koch M. A Novel Synthesis of 6-Demethoxyacronycine. Heterocycles 1992, 34, 799. 10.3987/COM-92-5981. [DOI] [Google Scholar]; c Anand R. C.; Selvapalam N. A practical regiospecific approach towards acronycine and related alkaloids. Chem. Commun. 1996, 199. 10.1039/cc9960000199. [DOI] [Google Scholar]
- a Kostakis I. K.; Magiatis P.; Pouli N.; Marakos P.; Skaltsounis A. −L.; Pratsinis H.; Leoncé S.; Pierré A. Design, Synthesis, and Antiproliferative Activity of Some New Pyrazole-Fused Amino Derivatives of the Pyranoxanthenone, Pyranothioxanthenone, and Pyranoacridone Ring Systems: A New Class of Cytotoxic Agents. J. Med. Chem. 2002, 45, 2599. 10.1021/jm011117g. [DOI] [PubMed] [Google Scholar]; b MacNeil S. L.; Wilson B. J.; Snieckus V. Anionic N-Fries Rearrangement of N-Carbamoyl Diarylamines to Anthranilamides. Methodology and Application to Acridone and Pyranoacridone Alkaloids. Org. Lett. 2006, 8, 1133. 10.1021/ol053162e. [DOI] [PubMed] [Google Scholar]
- Cholewiński G.; Dzierzbicka K.; Ko3odziejczyk A. M. Natural and synthetic acridines/acridones as antitumor agents: their biological activities and methods of synthesis. Pharmacol. Rep. 2011, 63, 305. 10.1016/S1734-1140(11)70499-6. [DOI] [PubMed] [Google Scholar]
- a Kolokythas G.; Pouli N.; Marakos P.; Pratsinis H.; Kletsas D. Design, synthesis and antiproliferative activity of some new azapyranoxanthenone aminoderivatives. Eur. J. Med. Chem. 2006, 41, 71. 10.1016/j.ejmech.2005.10.011. [DOI] [PubMed] [Google Scholar]; b Bera S. S.; Sk M. R.; Maji M. S. Weakly Coordinating, Ketone-Directed (η5-Pentamethylcyclopentadienyl)cobalt(III)- and (η5-Pentamethylcyclopentadienyl)rhodium(III)-Catalyzed C–H Amidation of Arenes: A Route to Acridone Alkaloids. Chem. Eur. J. 2019, 25, 1806. 10.1002/chem.201805376. [DOI] [PubMed] [Google Scholar]; c Wu J.; Zhang J.; Soto-Acosta R.; Mao L.; Lian J.; Chen K.; Pillon G.; Zhang G.; Geraghty R. J.; Zheng S. One-Pot Synthesis of 1-Hydroxyacridones from para-Quinols and ortho-Methoxycarbonylaryl Isocyanates. J. Org. Chem. 2020, 85, 4515. 10.1021/acs.joc.9b03307. [DOI] [PubMed] [Google Scholar]; d Veligeti R.; Madhu R. B.; Anireddy J.; Pasupuleti V. R.; Avula V. K. R.; Ethiraj K. S.; Uppalanchi S.; Kasturi S.; Perumal Y.; Anantaraju H. S.; Polkam N.; Guda M. R.; Vallela S.; Zyryanov G. V. Synthesis of novel cytotoxic tetracyclic acridone derivatives and study of their molecular docking, ADMET, QSAR, bioactivity and protein binding properties. Sci Rep. 2020, 10, 20720 10.1038/s41598-020-77590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sartori G.; Casiraghi G.; Bolzoni L.; Casnati G. General synthesis of 2H-benzo[b]pyrans (chrom-3-enes) from metal phenoxides and.alpha.,.beta.-unsaturated carbonyl compounds. J. Org. Chem. 1979, 44, 803. 10.1021/jo01319a030. [DOI] [Google Scholar]; b Chakraborti G.; Paladhi S.; Mandal T.; Dash J. “On Water’’ Promoted Ullmann-Type C–N Bond-Forming Reactions: Application to Carbazole Alkaloids by Selective N-Arylation of Aminophenols. J. Org. Chem. 2018, 83, 7347. 10.1021/acs.joc.7b03020. [DOI] [PubMed] [Google Scholar]; c Mandal T.; Chakraborti G.; Karmakar S.; Dash J. Divergent and Orthogonal Approach to Carbazoles and Pyridoindoles from Oxindoles via Indole Intermediates. Org. Lett. 2018, 20, 4759. 10.1021/acs.orglett.8b01827. [DOI] [PubMed] [Google Scholar]
- a Smolders R. R.; Hanuise J.; Voglet N. Synthesis of Some Hydroxylated 9-Acridanones. Bull. Soc. Chim. Belg. 1984, 93, 239. 10.1002/bscb.19840930312. [DOI] [Google Scholar]; b Reisch J.; Herath H. M. T. B.; Kumar N. S. Synthesis of some new acridones. Liebigs Ann. Chem. 1990, 1990, 1047. 10.1002/jlac.1990199001189. [DOI] [Google Scholar]; c Costes N.; Le Deit H.; Michel S.; Tillequin F.; Koch M.; Pfeiffer B.; Renard P.; Léonce S.; Guilbaud N.; Kraus-Berthier L.; Pierré A.; Atassi G. Synthesis and Cytotoxic and Antitumor Activity of Benzo[b]pyrano[3,2-h]acridin-7-one Analogues of Acronycine. J. Med. Chem. 2000, 43, 2395. 10.1021/jm990972l. [DOI] [PubMed] [Google Scholar]; d Jolivet C.; Rivalle C.; Bisagni E. Reaction of noracronycine and 1-hydroxy-3-methoxy-10-methylacridone with alkyl- and aryl-lithiums: formation of quinone methides. J. Chem. Soc., Perkin Trans. 1 1995, 511. 10.1039/p19950000511. [DOI] [Google Scholar]; e Putic A.; Stecher L.; Prinz H.; Muller K. Structure–activity relationship studies of acridones as potential antipsoriatic agents. 1. Synthesis and antiproliferative activity of simple N-unsubstituted 10H-acridin-9-ones against human keratinocyte growth. Eur. J. Med. Chem. 2010, 45, 3299. 10.1016/j.ejmech.2010.04.013. [DOI] [PubMed] [Google Scholar]; f Commandeur C.; Florent J. −C.; Rousselle P.; Bertounesque E. Easy Access to Pyranoacridines, Pyranoxanthenes, and Arylchromenes Through a Domino Reaction. Eur. J. Org. Chem. 2011, 1447. 10.1002/ejoc.201001598. [DOI] [Google Scholar]
- a Beck J. R.; Kwok R.; Booher R. N.; Brown A. C.; Patterson L. E.; Pranc P.; Rockey B.; Pohland A. Synthesis of acronycine. J. Am. Chem. Soc. 1967, 89, 3934. 10.1021/ja00991a066. [DOI] [PubMed] [Google Scholar]; b Beck J. R.; Kwok R.; Booher R. N.; Brown A. C.; Patterson L. E.; Pranc P.; Rockey B.; Pohland A. Synthesis of acronycine. J. Am. Chem. Soc. 1968, 90, 4706. 10.1021/ja01019a036. [DOI] [PubMed] [Google Scholar]
- Reisch J.; Voerste A. A. W.; Top M.; Dziemba P. Acetylene chemistry, part XXII [1, 2]: Synthesis of noracronycine and some of its analogs via Mitsunobu-reaction. Monatsh. Chem. 1992, 123, 473. 10.1007/BF00817605. [DOI] [Google Scholar]
- Hari G. S.; Lee Y. R.; Wang X.; Lyoo W. S.; Kim S. H. New Synthetic Routes to Acronycine, Noracronycine, and Their Analogues. Bull. Korean Chem. Soc. 2010, 31, 2406. 10.5012/bkcs.2010.31.8.2406. [DOI] [Google Scholar]
- Basa S. C. Extractives of rutaceae: Atalaphyllidine, a new acridone base. Experientia 1975, 31, 1387. 10.1007/BF01923199. [DOI] [Google Scholar]
- Fraser A. W.; Lewis J. R. Rutaceous constitutents. Part II. Two acridone alkaloids from Atalantia ceylanica (Rutaceae). J. Chem. Soc., Perkin Trans. 1 1973, 1173. 10.1039/p19730001173. [DOI] [PubMed] [Google Scholar]
- CCDC 2063223 contains the supplementary crystallographic data for compound 2b. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre (see also S.I.).
- a Pillai R. K. M.; Naiksatam P.; Johnson F.; Rajagopalan R.; Watts P. C.; Cricchio R.; Borras S. Thermorubin II. 1,3-Dihydroxy-9H-xanthones and 1,3-dihydroxy-9H-xanthenes. New methods of synthesis. J. Org. Chem. 1986, 51, 717. 10.1021/jo00355a024. [DOI] [Google Scholar]; b Verbanac D.; Jain S. C.; Jain N.; Chand M.; Palijetak H. Č.; Matijašič M.; Perič M.; Stepanič V.; Saso L. An efficient and convenient microwave-assisted chemical synthesis of (thio)xanthones with additional in vitro and in silico characterization. Bioorg. Med. Chem. 2012, 20, 3180. 10.1016/j.bmc.2012.03.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.