Abstract
OBJECTIVE:
To present recent evidence on the prevalence, incidence, costs, activity limitations, and work limitations of common conditions requiring rehabilitation.
METHODS:
This was a systematic review. Medline (PubMed), SCOPUS, Web of Science, and the grey literature were searched for relevant articles about amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury. Two investigators independently reviewed articles and selected those for inclusion. Quality grading was performed using the Methodological Evaluation of Observational Research Checklist and Newcastle-Ottawa Quality Assessment Form.
RESULTS:
110 articles were included. The prevalence of back pain in the past 3 months is 33.9% among community-dwelling adults, and patients with back pain contribute $365 billion in all-cause medical costs. Osteoarthritis is the next most prevalent condition (approximately 10.4%), and patients with this condition contribute $460 billion in all-cause medical costs. These two conditions are the most prevalent and costliest (medically) of the illnesses explored here. Stroke follows these conditions in both prevalence (2.5–3.7%) and medical costs ($28 billion). Other conditions may have a lower prevalence but are associated with relatively higher per capita effects.
CONCLUSION:
Consistent with previous findings, back pain and osteoarthritis are the most prevalent conditions with large aggregate medical costs. By contrast, other conditions have a lower prevalence or cost but relatively higher per capita costs and effects on activity and work. The data are extremely heterogeneous which makes anything beyond broad comparisons challenging: additional information is needed to determine the relative impact of each condition.
Keywords: Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, Traumatic Brain Injury, Prevalence, Incidence, Cost, Activity Limitations, Work Limitations, Function, Disability, Rehabilitation
Disability significantly impacts individuals and society. The Centers for Disease Control and Prevention estimates that 61 million adults (1 in 4) in the United States (US) have some type of disability.1 In 2006, individuals with disabilities accounted for about one-quarter of all healthcare expenditures among adults, totaling $397.8 billion.2 Disability also affects individuals’ work abilities. In 2018, the Social Security Administration estimated that nearly 10 million individuals received disability payments; the majority of whom (86.1%) were disabled workers.3 In the same year, Social Security Disability Insurance provided $144 billion in benefits to elderly and disabled beneficiaries.4,5
Given the enormous impact of disability, Ma, Chan, and Carruthers5 performed a review that compared the prevalence, incidence, costs, and activity/work limitations of eight conditions commonly seen by rehabilitative professionals. Now, five years later, we wanted to explore how this information has changed or what trends have emerged given more recent data and updated methodologies. Our goal is to conduct a systematic review that builds on this work by: (1) providing updated evidence on the prevalence, incidence, costs, activity limitations, and work limitations of these conditions; and (2) grading the quality of available evidence to present the most relevant, nationally-representative data. In doing so, we hope that our work will serve as a launching point for further research that may inform policymakers, researchers, and clinicians.
METHODS
We examined the same eight conditions that Ma, Chan, and Carruthers identified in their review.5,6 These eight conditions are amputation, back pain, osteoarthritis (OA), rheumatoid arthritis (RA), multiple sclerosis (MS), spinal cord injury (SCI), stroke, and traumatic brain injury (TBI). We also investigated seven outcomes in relation to the conditions specified by this review. These outcomes include prevalence, incidence, direct costs, indirect costs, total costs, activity limitations, and work limitations.
We searched PubMed, Web of Science, SCOPUS, and the grey literature on July 26th, 2019 for articles published since the inclusion date (April 1st, 2013) used by Ma, Chan, and Carruthers.5 Details of the search terms are included in Supplemental Appendix 1. Search results were then exported to DistillerSR, an online tool for screening. DistillerSR de-duplicates articles based on titles and abstracts and allows investigators to review inclusion/exclusion criteria to decide which articles to pass onto further review.
Two investigators (JL, SF) independently screened articles by reading titles and abstracts. Conflicts were resolved through conversation and consensus between these two researchers. Those deemed eligible by both reviewers were then assessed via full-text review: each researcher (JL, SF) abstracted data from a portion of the total articles then decided which to include in the study. Inclusion criteria were: 1) published within date range of interest (April 1st, 2013 to July 26th, 2019); 2) relevant condition; and 3) relevant outcome measure. Exclusion criteria were: 1) non-English language; 2) non-US population; 3) pediatrics (<18 years old); and 4) inappropriate sample size. Only articles with primary data and full-text availability were included. Systematic reviews’ references were scanned for relevant articles. Those with primary data were included irrespective of publication date when no more recent data were available. This procedure allowed the inclusion of relevant information, despite being published outside our date range of interest.
An “appropriate” sample size was defined by-condition for prevalence studies using the following formula, where n is the minimum required sample size, z is the confidence level (set to 0.95), p is the prevalence estimate, and d is the precision:7
The prevalence estimate was derived from the nationally-representative survey that yielded the largest sample size for each condition, using US census data when necessary.8 A precision of 0.05 was used for conditions with a prevalence estimate between 0.1 and 0.9; a precision of p/2 was used otherwise.9
There is no gold standard for grading the quality of evidence across all conditions and outcomes.10 However, there are some validated tools. The Methodological Evaluation of Observational Research (MORE) Checklist for Observational Studies of Incidence or Prevalence of Chronic Diseases11 was modified and applied to studies investigating incidence and prevalence. The Newcastle-Ottawa Quality Assessment Form for Cost-of-Illness Studies12,13 was modified and applied to cost studies. Details about these forms can be found in Supplemental Appendix 2. These forms were only administered to journal articles (not website results or other grey literature) because the tools have only been validated on published literature.13,14 No quality grading rubric was used on work and activity limitations studies because they varied in their methods and outcomes.
Two investigators (JL, SF) trained on a subset of articles identified by this study to reach sufficient agreement on both the quality grading forms. Then, they independently reviewed all articles for quality. Cohen’s Kappa (κ) was calculated to assess agreement on an independent quality review of 10 prevalence/incidence articles and 10 cost articles.
The MORE makes no strict demarcations between high and low quality studies. Rather, it identifies “major” and “minor” flaws as well as “poor reporting.” By contrast, the Newcastle-Ottawa has a point system to grade articles as “good,” “fair,” and “poor.” All prevalence, incidence, and costs studies that were included in this study were graded according to these guidelines.
We tailored the grading tools to suit the particular aim of this study. Quality ratings assessed both internal, methodological rigor and generalizability of the data to the broader US population. In some cases, this means that studies were deemed of moderate quality because they utilized non-generalizable data. Although these studies’ may not have aimed to produce nationally-generalizable estimates, they were rated as lower quality than other studies for our purposes.
All costs were adjusted to 2019 dollars using the Consumer Price Index.15 The Medical Care Index was applied to studies of direct costs (medical). The All-Items Index was applied to studies of indirect (nonmedical) and total (medical and nonmedical) costs. Where possible, costs are further specified as: 1) incremental costs: additional costs that a patient has compared to a matched control without the condition; 2) condition-related costs: costs specifically associated with the condition; or 3) all-cause costs: any medicals costs that a patient has, whether or not they are related to the condition. Costs converted to 2019 dollars are shown in parentheses next to relevant data.
RESULTS
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart16 of the search results and selection criteria is presented in Figure 1. A total of 110 sources were utilized in this study. Out of 36 prevalence/incidence studies, 18 were free of major flaws. Out of 36 cost studies, 9 cost studies were good quality, 8 were fair quality, and 19 were poor quality.
Figure 1:
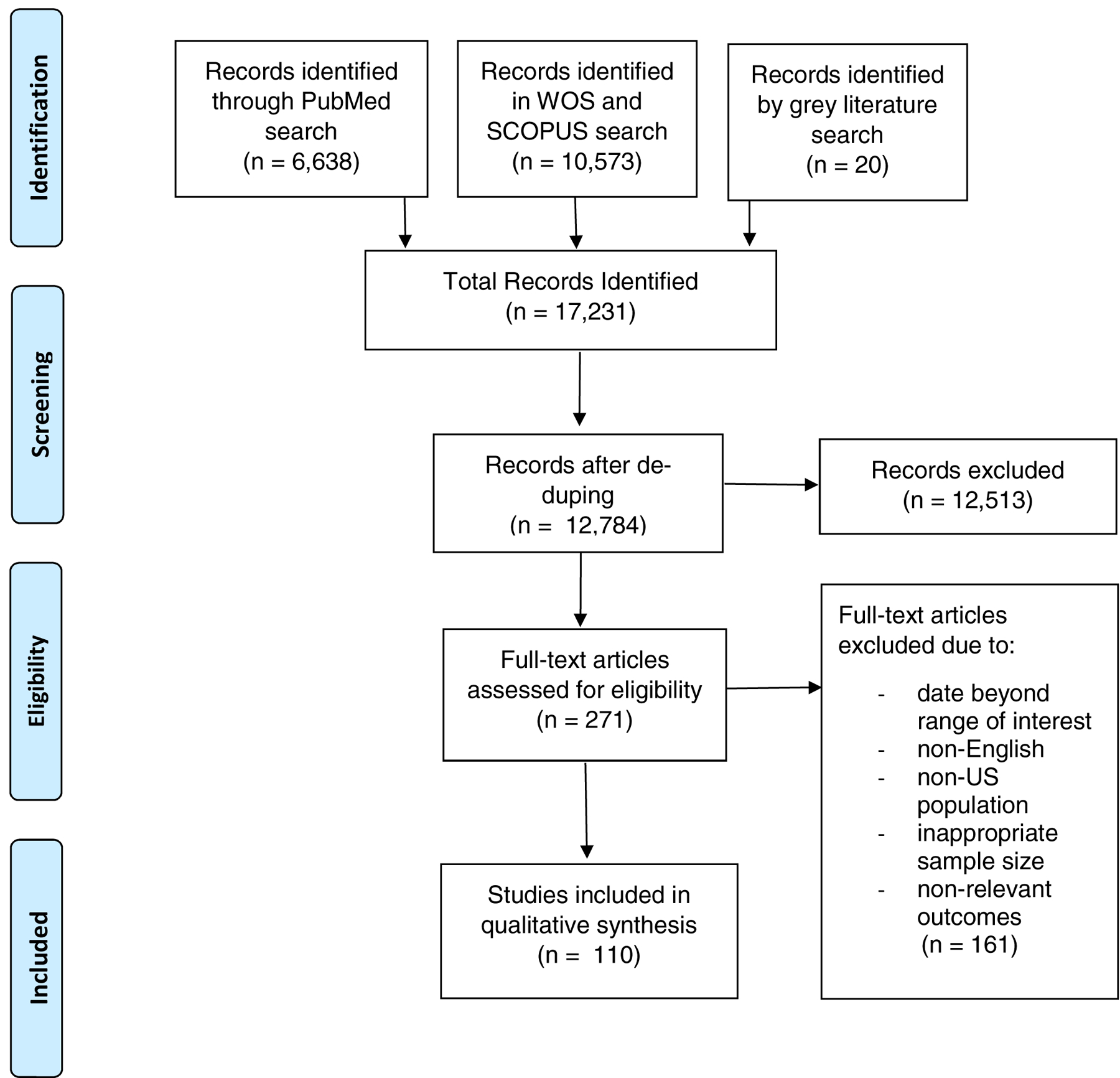
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Each question on the MORE and Newcastle-Ottawa checklists was assigned a κ score. The individual κ scores per question are reported in Supplemental Appendix 3. For the MORE, a raw agreement of 89% and a κ=0.77 (95% CI: 0.69–0.84) were obtained. For the Newcastle-Ottawa, a raw agreement of 92% and a κ=0.84 (95% CI: 0.70–0.98) were obtained. All results can be found in Table 1. Of the epidemiological and cost studies, those of higher quality are marked with double asterisks (**) or a single asterisk (*). The text body below only discusses studies that were assessed as fair to good quality and all grey literature results. When no fair or good quality evidence was available, all results were provided.
Table 1:
Overview of Prevalence, Incidence, Costs, and Activity/Work Limitations of Common Rehabilitation Conditions
| Condition | Prevalence | Incidence | Annual Direct Costs (2019 Values in Parentheses) | Annual Indirect Cost (2019 Values in Parentheses) | Annual Total Cost (2019 Values in Parentheses) | Activity Limitations | Work Limitations |
|---|---|---|---|---|---|---|---|
| Amputation | - 1.6 million (Ziegler-Graham et al17**; Ma et al5) |
General Population - 3.0 per 10,000 non-traumatic LEA (Gregg etal18**; Narres et al19) Diabetes-related - 46.2 per 10,000 LEA (Geiss et al20**) - 28.4 per 10,000 LEA (Gregg et al18**; Narres et al19) - 23 per 10,000 LEA above ankle, adults ≥65 (Newhall et al107) - 45 per 10,000 LEA Medicare population (Margolis et al108) |
- $509,275 ($878,927) total projected lifetime healthcare costs after LEA (MacKenzie et al21; Ma et al5) - $14,088 ($20,207) mean cost of LEA procedure due to peripheral vascular disease; $22,405 ($32,136) total inpatient cost year prior to amputation + amputation, adults ≥66 (Goodney et al22) - $17,103 ($24,010) mean cost per inpatient stay for diabetes-related LEA (Yin et al23) |
No new information identified. | Wo new information identified. | - 53.9% non-ambulatory at approximately 1 yr after major LEA (Chopra et al24) - 42.2% and 28.6% of military individuals with traumatic transfemoral and through-knee amputation, respectively, reported being fully disabled and unable to return to duty (Tennent et al25) |
- 42% report being unable to work 7 years after traumatic LEA; of those who could work, 20–25% reported work limitations (MacKenzie et al;26 Ma et al5) |
| Back Pain | - 28.6% LBP among community-dwelling adults aged 18–85 (Yang and Haldeman44**) - 25.7% LBP among employed adults (Yang et al45**) - 13.1% cLBP adults aged 20–69 (Shmagel et al43**) - 33.9% back pain among community-dwelling adults (BMUS 4th ed.30†) - 15.0% neck pain among community-dwelling adults (BMUS 4th ed.30†) - 12.9% prevalence of LBP among all ages; peak prevalence is 25.6% adults aged 80–84 (IHMEGBDTool 201732†) |
- 67% of men and 61 % of women aged ≥65 (Marshall et al, 201749; Marshall et al, 201650) - 12–14% of all adults have back pain annually; 57.1 million annual patient visits related to back pain, giving a rate of 18.1 persons in 100 seeking care (BMUS 4th ed30†) - 5,213 per 100,000 incidence of LBP among all ages; peak incidence is 10,425 per 100,000 among adults aged 75–79 (IHMEGBD Tool 201732†) |
- $315 ($365) billion annual all-cause medical costs from 2012–2014; $9,035 all-cause per patient direct costs; $1,615 ($1,873) per patient incremental costs (BMUS 4th ed.30†) - $56.5 ($62.3) billion national spine condition-related costs (AHRQ46†) - $6,892 ($10,104) LBP vs $2,091 ($3,066) for employee matched controls ages 18–64 (Ivanovaet al47**; Tymecka-Woszczerowicz et al48) - $8,296 ($11,231) uncomplicated LBP medical cost per case for working adults ≥18 (Shraim et al109) |
- $2,606 ($3,289) LBP-related cost per employed patient (private health insurance) (Ivanova et al47**; Tymecka-Woszczerowicz et al48) | - $9,498 ($11,986) LBP-related cost per employed patient on private health insurance (Ivanova, et al47**; Tymecka-Woszczerowicz et al48) | - 30% of men and 22% of women ≥65 reported limited activity (Marshall et al, 201749; Marshall et al, 2016;50) - 3,180,600 years lived with disability from LBP (Murray et al52) - 347% greater likelihood of difficulty performing ADLS among adults ≥70 with restricting back pain vs matched controls (Markis et al51) |
- 98 days mean length of disability among adults in 12 months after uncomplicated LBP diagnosis (Shraim et al109) - 12.8% of those with cLBP received disability; those with cLBP were less likely to work (Shmagel et al43) - 8.4 days of medically-related absenteeism on average for working adults (Ivanova et al47) - 25.8% (4.9 million) of adults reporting a health condition that precludes work are unable/limited in work due to chronic back or neck problems (BMUS 4th ed.30†) - Almost 264 million work days lost in one year related to back pain (BMUS 4th ed.30†) - 385,000 self-reported lost work days due to back pain (BMUS 4th ed.30†) |
| Multiple Sclerosis | - 727,344 (Wallin et al53**) - 150 per 100,000 (Dilokthornsakul et al;54) - 0.13% prevalence among all ages; peak prevalence is 0.21 % among adults aged 50–54 (IHME GBD Tool 201732†) |
- 3 per 100,000 incidence among all ages; 11.7 per 100,000 peak incidence in adults 25–29 (IHME GBD Tool 201732†) | - $4.3 ($5.0) billion national inpatient charges w/o professional fees (Chen et al110) - $4.0 ($4.4) billion Medicare Part D expenditure for MS-drugs (Hartung et al111) - $51,825-$67,116 ($57,172-$74,041 Jail-cause cost per pt depending on severity of disability (Jones et al55*) - $26,520 ($32,655) MS-related costs per pt on average vs $39,948 ($49,189) per pt with malaise/fatigue (Carroll et al112) - $17,545–41,969 ($21,604–51,678) all-cause, first yr since dx; $8,803–29,355 ($10,839–36,146) MS-related, first yr since dx (Parisé et al56*) |
- $4,146–9,226 ($4,690–10,437) all-cause; $1613–6939 ($1,825–7850) MS-related (Parise et al56) | - $53,438–74,055 ($58,997–81,890) per patient (aggregating Parisé et al56 and Jones et al55) | - 51.2% of working age adults reported using a mobility device; 68.2% reported having some level of mobility limitation (Bishop et al57) - 14.1% and 7.5% of patients on DMDs report malaise/fatigue and gait abnormality, respectively (Carroll et al112) |
- 40.7–48.1% employment post-diagnosis (full- or part-time), compared to 88.2% at time of diagnosis (Bishop et al57; Krause et al, 2019;59 Krause et al, 201858) |
| Osteoarthritis | - 13.4% (30.8 million) (Cisternas et al33**) - 15.1 million adults ≥25 with symptomatic knee OA (Deshpande et al35**) - 19.6% age-standardized prevalence of radiographic hip OA; 4.2% age-standardized prevalence of symptomatic hip OA (Kim et al113) - 10.5% all-ages (IHME GBD Tool 201732t) - 32.5 million adults from 2008–2014 (BMUS 4th ed.30†) - 10.5% prevalence among all ages; peak prevalence is 47.8% aged 90–94 (IHME GBD Tool 201732†) |
- 13.8% lifetime risk of diagnosed symptomatic knee OA (Losina et al36**) - 12.9% symptomatic hand OA over 12 years (Snyder et al114) - 1,300 per 100,000 for symptomatic hip OA(Moss et al115) - 460 per 100,000 among all ages; peak incidence is 1,216 per 100,000 among adults aged 60–64 (IHME GBD Tool 201732†) |
- $373.2 ($459.5) billion national all-cause; $65.5 billion ($80.6) OA-related; $11,502 ($14,163) per pt all-cause; $2,018 OA-related (BMUS 4th ed.30†) - $16.5 ($19.2) billion in national inpatient spending (Torio et al116) - $14,521 ($15,435) per pt all-cause general OA in a working-age population; $10,892 ($11,578) incremental; $23,272 ($24,737) all-cause hipOA; $19,551 ($20,782) all-cause spine OA; $15,599 ($16,581) all-cause knee OA; and $10,112 ($10,749) all-cause hand OA (Wang et al34**) - $19,600 ($22,726) knee OA-related lifetime cost (Losina et al37**) - $1,229 ($1274) per pt knee OA-related (Ong et al117) |
- $113.2 ($128.0) billion all-cause earnings lost; $71.3 ($80.7) billion OA-related earnings lost; $6,783 ($7,673) per pt earnings lost; $4,274 ($4,835) OA-related per pt earnings lost (BMUS 4th ed.30†) | - $486.4 ($550.2) billion total direct and indirect costs of OA and allied disorders; $136.8 ($154.8) billion total OA-related costs (BMUS 4th ed.30†) | - 43.5% of individuals with arthritis (23.7 million) had arthritis-attributable activity limitations, the majority of which are likely attributed to osteoarthritis) (Barbour et al27) | - 180.9 million total lost work days reported by adults with arthritis between 2013–2015; the majority likely attributed to osteoarthritis (BMUS 4th ed.30†) |
| Rheumatoid Arthritis | - 0.5% (1.3–1.4 million adults) (Hunter et al38**) - 0.8% (1.7 million adults on average from 2008–2012) (BMUS 4th ed.30†) - 2.0% among Medicare beneficiaries (Li et al118) - 0.63% (Crane et al119) - 0.6% prevalence among all ages’ peak prevalence is 1.7% among adults aged 70–74 (IHME GBD Tool32†) |
- 71 per 100,000 age- and sex-adjusted rate for persons at risk (Crane etal119) - 30.2 per 100,000 incidence among all ages; 77 per 100,000 peak incidence among adults aged 65–69 (IHME GBD Tool 201732†) |
- $32.9 ($40.5) billion national all-cause; $13.8 ($17.0) billion RA-related (BMUS 4th ed.30†) - $20,919 ($24,255) all-cause RA in Medicare population;$13,722 ($15,911) incremental; $11,587 ($13,435) RA-related (Chen I Chieh et al39**) - $12,509 ($13,800) per pt all-cause; $3,723 ($4,107) per pt RA-related costs (Hresko et al14*) - $14,158 ($14,682) per pt RA-related (Li et al120) - $5,317 ($6,020) RA-related costs for pts on targeted immunomodulators (Strand et al42) |
- $13.1 ($14.8) billion national earning loss and $14,542 ($16,450) per pt earning losses; RA-related earning losses were $7.9 ($8.9) billion in aggregate and $8,748 ($9,896) per pt (BMUS 4th ed.30†) - $596 ($704) per pt incremental; $252 ($298) million national cost due to RA-related absenteeism (Gunnarsson et al40**) - $3,324 ($3,573) RA-related per pt on targeted immunomodulators (Strand et al42) |
- $46 ($52) billion national all-cause; $21.6 ($24.4) billion RA-related costs (BMUS 4th ed.30†) - $8,641 ($9,593) per pt for RA-related, targeted immunomodulators (Strand et al42) |
- 236% higher relative prevalence of functional disability compared to age-matched controls (Myasoedova et al41) | - 20.3 days of work lost per yr for pts on targeted immunomodulators (Strand et al42) - 13.7 workdays missed per yr (about 4 days more than those without condition) (Gunnarsson et al40) |
| Spinal Cord Injury | - 2.6 million (James et al61†) - 291,000 (National Spinal Cord Injury Statistical Center60†) - 1.5 million (Armour et al62†) |
- 52–54 per 1,000,000 traumatic (Jain et al63**, National Spinal Cord Injury Center60†) - 56.4 per 1,000,000 traumatic (Selvarajah et al65**) - 260 per 1000,000 (James et al61†) |
Overall: - $1.7 ($2.2) billion cost of hospital izations (Mahabaleshwarkar and Khanna66**) - $1.6 ($2.1) billion acute treatment (Selvarajah et al65*) - $42,323 ($50,280) and $35,883 ($42,629) all-cause costs for patients with and without neuropathic pain, respectively (Margolis et al121) Cervical: - $1,129,302 ($1,148,407) in first yr then $196,107 (199,425) per yr after in SCI-related costs (DeVivo et al;68 National Spinal Cord Injury Center60†) -$160,000-$180,000 ($166,000–187000) in hospitalization costs per pt ≥65 (Asemota et al122) |
- $76,327 ($77,334) per person (National Spinal Cord Injury Center, 201960†) | - $9.7 ($15.7) billion (Berkowitz et al69 p. 81; Ma et al5) |
Overall: - Average spasticity was mild and persists in roughly 85% of pts (DiPiro et al71) Cervical: - Of those with CSCI, roughly 60% incomplete tetraplegia vs 40% complete tetraplegia. Of all DALYs associated with SCI, cervical SCIs account for roughly 66% (Hall et al67) |
- 35% employed after SCI (Trenaman et al;72 Ottomanelli et al73) |
| Stroke | - 2.5% or 7.0 million adults ≥20 yrs (Benjamin et al74†) - 2.9–3.7% of adults (Blackwell and Villarroel;76†CDC106†) - 2.6% among all ages; peak prevalence is 17.3% among adults aged 90–94 (IHME GBD Tool 201732†) |
- 192 per 100,000 men; 198 per 100,000 women (Madsen et al79**) - 127 per 100,000 acute ischemic stroke (Alqahtani et al123) - 373 per 100,000 for total stroke; 329 per 100,000 for ischemic; 49 per 100,000 for hemorrhagic stroke (Koton et al124) - 639 per 100,000 first stroke in pts ≥65 years old (Fang 2014125) - 795,000 cases of new/recurrent stroke based on 1999 data (Benjamin et al74†) - 185 per 100,000 incidence among all ages; peak incidence is 2,085 per 100,000 among adults aged 90–94 (IHME GBD Tool 201732†) |
- $21,916 ($32103) mean hospital charges for acute ischemic stroke in 2016 (Yacoub et al82**) - $28 ($30.9) billion (Benjamin et al74†) - $2.34 ($2.78) billion national hospitalization costs due to subarachnoid hemorrhage, $2.52 ($2.99) billion due to ischemic hemorrhage, and $12.55 ($14.9) billion due to acute ischemic stroke (Tong etal83*) - $18,796 ($22,330) per pt all-cause medical costs (Chinthammit et al89**) - $24 ($25.5) billion incremental among patients with hypertension who have stroke (Lekoubou et al80*) - $46,518 ($61,046) per admission (Stepanova et al81*) - $46,850 ($51,684) all-cause medical costs in first year post-injury (Mu et al87) - All-cause costs were $61,354 ($71,139) per pt on commercial health insurance vs $44,929 ($52,095) per pt on Medicare in first year (Johnson et al;126 Katan and Luft127) - $20,396 ($27,611) per admission for primary and secondary diagnosis of stroke (Wang et al128) |
- $30 ($31.1) billion stroke-related lost productivity (RTI International129) - $33.65 ($39.25) billion lost productivity (Ovbiagele et al84**) - $8,211 ($9,703) per pt annual cost due to informal caregiving (Joo et al85*) |
- $45.5 ($48.8) billion stroke-related (Benjamin et al74†) | - 3% of males and 2% of females attribute disability to stroke (Benjamin et al74†) - 60% reported at least some difficulty (w/ or w/o assistive device) in completing ADLs (≥65 years old) (Brenner et al90) - Majority reported severe to extreme difficulty with standing for long periods and walking a long distance (64.5%). Pts were restricted in self-care activities including bathing (40.8%) and performing household tasks (40.2%) (older Americans, most commonly 60–69 yrs old) (Arowoiya et al91) - 22% were discharged with disability (mean age of 64 years old) (Mu et al87) - Significantly higher percentage of patients with stroke (>65 yrs) report balance/coordination problems (52.4%) and use of a mobility device (22.8%) compared to controls without stroke (Wing etal92) - 23.1% reported ADL limitation (>50 yrs) (Chinthammit et al89) - 65–121% more likely to require help in self-care, mobility, and household activities than matched controls (≥65 years old) (Skolarus et al88) |
- 45.6% of participants with stroke in a community stated that they were unemployed as a result of their stroke (60–69 yrs) (Arowoiya et al91) |
| TBI | - Age-standardized rate of 605 per 100,000 (James et al61†) - 21.7% of state-based population report at least 1 TBI with LOC in lifetime (Corrigan et al130) - 42.5% of state-based population report lifetime history of TBI, the most common of which was mild TBI (Whiteneck et al102) |
- 2.8 million annual TBI-related emergency department visits, hospitalizations, and deaths among all ages (Taylor et at al93**) - 807.9 mild TBI per 100,000 ED visits (Cancelliere et al98**) - 1.6% of all injury- or illness-related ED visits in one state contained diagnosis code for TBI (Kerr et al131) - 2.9 million TBI-related ED visits, hosp., deaths among all ages (CDC94†) - Age-standardized rate of 333 per 100,000 (James et al61†) |
- $21.4 ($27.2) billion TBI-related admissions and $8.2 ($10.4) billion discharges/transports (Marin et al99*) - $9.2 ($17.4) billion injury-related (Finkelstein et al100 p. 68; Ma et al5) |
- $51.2 ($75.6) billion (Finkelstein et al100 p. 104; Ma et al5) | - $60.4 ($93.0) billion in lifetime costs (Finkelstein et al100 p. 136; Ma et al5) | - Roughly 50% do not return to pre-injury level in 1 year (Nelson et al101) - Based on state data, 41% of hospitalized pts with TBI and 33% of non-hospitalized pts with TBI report activity limitations. Prevalence of activity limitations increased by up to 307% compared to healthy controls without TBI, in accordance with injury severity (Whiteneck et al102) - Of those with moderate to severe TBI at 1 year post-injury, FIM scores were lowest in the domains of stair climbing, memory, and problem solving (Brooks et al132) |
- More than half of moderate/severe TBI were unemployed at 2 and 5 years post-injury (Cuthbert et al;103DiSanto et al104) - Roughly 80% of veterans with severe TBI were unemployed 1 year post-injury (Dillahunt-Aspillaga et al105) |
fair quality;
good quality OR no major flaws;
grey literature
Abbreviations:ADL – activities of daily living; cLBP – chronic low back pain; CSCI – cervical spinal cord injury; DALY – disability-adjusted life year; DMD – disease modifying drug; ED – emergency department; LBP – low back pain; LEA – lower extremity amputation; LOC – loss of consciousness; MS – multiple sclerosis; OA – osteoarthritis; PT – patient; RA – rheumatoid arthritis; SCI – spinal cord injury; TBI – traumatic brain injury; YR – year
Amputation
According to an estimate employing the National Inpatient Sample (NIS), 1.6 million people lived with an amputation in 2005.17 Lower extremity amputations (LEAs) constitute the majority of amputations and are most commonly attributed to diabetes and peripheral vascular disease.17 The age-standardized incidence of non-traumatic LEA was estimated at 3 per 10,00018,19 among the general population in 2010 and 28.4 per 10,000 people18,19 to 46.2 per 10,000 people20 among individuals with diabetes in 2015.
Lifetime, all-cause direct costs for LEA was estimated at $509,275 ($878,927) in 2005.5,21 There are more recent figures for sub-categories of patients. Based on Medicare claims, the mean inpatient cost of LEAs due to peripheral vascular diseases in the year preceding operation is $22,405 ($32,136) per patient, of which $14,088 ($20,207) is attributable to the procedure itself.22 The mean cost per inpatient stay due to diabetes-related LEA is $17,103 ($24,010).23 Drawing on prevalence estimates, these figures roughly translate to an overall cost of $35.8 ($51.3) billion for LEAs due to peripheral vascular disease, of which $22.5 ($32.3) billion is attributable to the procedure itself.17,22 Meanwhile, the overall cost of inpatient care following diabetes-related LEA is approximately $27.4 ($38.5) billion.17,23 No appropriate literature on the indirect or total costs of amputation was identified.
Impact on activity level depends on the location of amputation. After a major LEA, 53.9% of patients report being non-ambulatory at follow-up roughly half a year after an operation.24 Meanwhile, 42.2% and 28.6% of military individuals with transfemoral and through-knee amputation, respectively, report being fully disabled.25 Likewise, work limitations vary depending on the location of amputation. One study published in 2006 found that, of those with traumatic LEAs, 42% reported being unable to work 7 years after their procedure.5,26 This information stands to be updated with more recent data.
Arthritis
54 million27,28 and 66 million29 individuals have doctor-diagnosed and self-reported arthritis, respectively. The annual, all-cause medical cost of arthritis is $9,554 per patient30 while the incremental cost is $1,352 per patient.30 The total cost of arthritis is $303.5 ($331.4) billion.29 OA is the most common form of arthritis.31
Osteoarthritis
The Institute of Health Metrics Evaluation (IHME)’s Global Burden of Disease (GBD) Tool estimates that 10.5% of the US population has OA and that this figure increases with age.32 A study based on Medical Expenditure Panel Survey (MEPS) data calculates that 32.5 million adults have OA.30 Consistent with these findings, a separate study by Cisternas estimates that 30.8 million US adults, translating to 13.4% of the US adult population,33 has OA. OA commonly affects the back, hands, knees, and hips.31,34 15.1 million people have symptomatic knee OA,35 and Losina et al found that the lifetime risk of diagnosed symptomatic knee OA is 13.8%.36 The IHME GBD Tool estimates that the peak incidence of OA overall is 1,216 per 100,000 among those 60–64 years old.32
Estimates of the cost of OA vary by body location. Wang et al report that the mean all-cause healthcare utilization of working-age patients with OA is $14,521 ($15,435) per year, and they provide estimates of medical costs by body location: $23,272 ($24,737) per year for hip; $19,551 ($20,782) per year for spine; $15,599 ($16,581) per year for knee; and $10,122 ($10,749) per year for hand.34 Losina et al estimate that the OA-related lifetime cost is $19,600 ($22,726) per capita.37
At the population-level, the all-cause, direct cost of OA patients is $373.2 ($359.5) billion with an OA-related cost of $65.5 ($80.6) billion.30 Patients with OA lose $6,783 each year in earnings for an aggregate indirect cost of $113.2 ($128.0) billion.30 Of these indirect loses, $4,274 ($4,835) per person and $71.3 ($80.7) billion in aggregate are OA-related.30 Based on MEPS data, the all-cause total cost of OA is $486.4 ($550.2) billion while the OA-related cost is $136.8 ($154.8) billion, making it an extremely costly condition.30
There are few studies of OA-related activity and work limitations. One study found that 43.5% of individuals with arthritis generally experience arthritis-attributable activity limitations.27 Similarly, between 2013–2015, 180.9 million work days were lost due to arthritis broadly.30 Because OA constitutes the vast majority of all arthritis cases,31 it is likely that the bulk of these limitations are due to OA.
Rheumatoid Arthritis
Prevalence estimates of RA range from 0.5%38 to 0.8%30 of the US population. The IHME GBD Tool estimates that RA prevalence peaks at 1.7% among adults aged 70–7432 and that RA incidence peaks at 77 per 100,000 in adults aged 65–69,14 making it far less common than OA.
The mean all-cause direct cost of RA ranges from $12,509 ($13,800)14 to $20,919 ($24,255)39 per patient annually. By contrast, the mean RA-related direct cost ranges from $3,723 ($4,107)14 to $11,587 ($13,435) per patient annually. The indirect cost of absenteeism is $596 ($704) per capita annually.40 RA-related earnings loss is $8,748 ($9,896) per year.30 At the population level, the all-cause direct cost incurred by patients with RA is $32.9 ($40.5) billion.30 $252 ($298) million40 and $13.1 ($14.8) billion30 are lost per year due to absenteeism and in earnings, respectively. $7.9 ($8.9) billion in earnings losses were specifically RA-related.30 All-cause total costs are $46 ($52) billion per year40, and RA-related total costs are $21.6 ($24.4) billion.40
Followed over time, RA patients have at least a 236% higher relative prevalence of functional disability compared to age-matched controls without the condition.41 The estimated work days lost per patient ranges from 13.7 days per year40 to 20.3 days per year.42
Back Pain
Prevalence estimates of back pain have stayed roughly constant since 2005.30 The IHME GBD Tool estimates that the prevalence of low back pain among all ages is 12.9% and that the peak prevalence is 25.6% among adults aged 80–84.32 The point prevalence of chronic low back pain among adults aged 20–69 was 13.1% in the 2009 to 2010 National Health and Nutrition Examination Survey (NHANES).43 A study using the National Health Interview Survey recently found that 28.6% of adults44 (and 25.6% of employed adults)45 report low back pain in the past last 3 months. In addition, 15.0% of adults30 reported neck pain in the last 3 months. In all, an average of 33.9% of adults reported back pain broadly within the last 3 months.30 According to the National Hospital Ambulatory Medical Care Survey, more than 57.1 million patients visited physicians for back pain,30 and the IHME GBD Tool estimates low back pain prevalence as 5,213 per 100,000 among all ages.32
A 2015 analysis of MEPS data found that back pain-related costs averaged $56.5 ($62.3) billion annually, an increase of 112% from the late 1990s.46 All-cause direct healthcare costs for individuals with back pain totaled $315.0 ($365.2) billion annually over the same period.30 Using private health insurance data from 2004 to 2006 to generate incremental estimates of annual per patient costs, the direct cost of low back pain among working adults was $4,801 ($7,039) and the indirect cost was $1,856 ($2,342): generating an incremental total cost of $6,657 ($9,381).47,48 A more recent MEPS analysis estimates that costs attributable to back pain are $1,615 ($1,873) per patient, per year.30
Back pain imposes significant work and activity limitations. Approximately 264 million work days are lost annually due to back pain.30 In addition, back pain leads to 182 million bed days among the workforce age population.30 About 25.8% of working age adults who are unable to work reported to the National Health Interview Survey that their disability is due to back or neck problems.30 Employees with low back pain have 6.2 more days of medically-related absenteeism compared to controls without the condition.47,48 30% percent of men and 22% of women older than 65 report activity limitations due to back pain.49,50 Older adults with back pain report a 347% greater likelihood of difficulty performing activities of daily living (ADL).51 The GBD 2010 Study found that low back pain led to 3,180,600 years lived with disability in the US, the highest disability burden among the conditions that they studied.52
Multiple Sclerosis
Prevalence estimates of MS have increased. Previously, the National Multiple Sclerosis Society calculated a prevalence of 400,000 by extrapolating figures from the 1980s with Census data.5 However, a more recent estimate drawing on a combination of public- and private- health insurance claims places the prevalence at 727,344 individuals.53 Other prevalence estimates range from 120 to 150 per 100,000.32,54 The IHME GBD Tool estimates peak prevalence is 0.21% among adults aged 50–54.32 These figures represent an increase in MS prevalence relative to what was reported previously,5 though this may be attributable to different methodologies in sampling and detection. The IHME GBD Tool reports that the peak incidence of MS is 11.7 per 100,000 among adults age 25–29.32 Incidence rates have remained relatively stable over time.5
Costs attributed to MS vary depending on severity and relapses. Direct, all-cause costs range from $51,825 to $67,116 ($57,172 to $74,041) per year.55 Similarly, patients with MS have higher indirect costs than those without the condition. All-cause indirect costs for MS patients are $4,146 to $9,226 ($4,690 to $10,437), and MS-related indirect costs are $1,613 to $6,939 ($1,825 to 7,850).56 Because no single study identified a total cost of MS, we aggregated the best available data on direct costs55 and indirect costs56 to arrive at a rough estimate of the total cost, $53,438 to $74,055 ($58,997 to $81,890) per patient annually.
Mobility limitations are a key concern for patients with MS. Bishop reports that 68.2% of individuals with MS in their study cohort had some level of mobility limitation.57 Of the total group of MS individuals in that same study, 51.2% reported using a mobility device.57 The most common symptom expressed by MS patients is fatigue (81.8%).57 MS often interferes with work. In one study’s sample,58 88.2% of participants reported being employed at the time of diagnosis whereas only 40.7% were employed when the survey was conducted. This is consistent with other findings that employment rates among MS patients range from 44.6%58 to 48.1%.59
Spinal Cord Injury
Estimates of SCI prevalence range widely. The National Spinal Cord Injury Statistical Center estimates that the prevalence of SCI is approximately 291,000 people.60 By contrast, a systematic analysis using GBD data estimates that the prevalence of SCI is 2.6 million individuals in the US.61 Another study by the CDC estimates that there are 1.5 million individuals with SCI.62 These large discrepancies in estimates of SCI prevalence are due to varying methodologies and suggest that further reconciliation of the research is needed.
Although prevalence estimates differ, incidence rates have been fairly consistent across multiple sources. Jain et al estimate that the incidence rate was between 52 and 54 cases per 1,000,000 based on NIS data between 1993 and 2012.63 Using the Nationwide Emergency Department Sample, Selvarajah et al estimate that the incidence is 56.4 per 1,000,000. These figures fall within the range that Bernhard provided in 2005.64 Men have a higher incidence of SCI: 78% of new cases of SCI are male.60 Interestingly, the average age of SCI has become older (43 years old in 2019 versus 29 years old in the 1970s60), perhaps due to an increase in the proportion of SCIs caused by falls among a growing elderly population.
Estimates of direct cost range from $1.6 billion65 to $1.7 billion66 ($2.1–2.2 billion) per year in hospitalization costs, captured in the Nationwide Emergency Department Sample and NIS data respectively. Cervical SCIs bear a disproportionate impact on cost due to their high potential to cause disability.60,67,61 Based on data from 2000 to 2006, it was estimated that a person with a cervical-level injury will have $1.13 ($1.15) million in direct costs in the first year post-injury followed by $196,107 ($199,425) in expenses each year of their life thereafter.60,68 Annual indirect costs average $76,327 ($77,334) per person.60 In 1998, Berkowitz et al estimated that the total annual cost of SCI was $9.7 ($15.7) billion.5,69 This figure stands to be updated with newer data.
The impact of SCI on activity and work varies depending on the level of injury.67,70 85% of patients with any type of SCI report mild, persistent spasticity.71 For cervical injuries specifically, 60% of cases are incomplete tetraplegia while 40% are complete tetraplegia—the latter being associated with a greater functional burden.67 Cervical SCIs account for more than half (66%) of all disability-adjusted life years attributable to SCI.67 One review in 2009 found that the average rate of employment among individuals with SCI was 35%, roughly half that of the US population without disability.72,73
Stroke
The American Heart Association (AHA) estimates that 7 million adults have had a stroke.74 This, along with the IHME GBD Tool’s estimate (2.6%32), is slightly lower than what the AHA reported in 2013 (2.8%).75 Other estimates suggest that the prevalence of stroke is either unchanged (2.9%)76 or even higher than was previously estimated (3.7%).77 The vast majority of strokes (87%) are ischemic.77,78 The AHA has not updated its statistic based on 1999 data that there are 795,000 cases of new or recurrent stroke each year,74 the IHME GBD Tool has more recently quantified stroke incidence as 185 per 100,000, or 600,000 new cases each year.32 One interpretation of this is that the incidence of stroke is decreasing, though researchers have emphasized that the demographics of stroke are also changing. While there once was a gap in stroke incidence between men and women, this is no longer the case: in 2010, the incidence of all types of strokes among men was 192 per 100,000 and 198 per 100,000 among women.79
The AHA reports that stroke-related medical costs are $28.0 ($30.9) billion.74 Hypertension is a major predictor of stroke risk.74,77 One study reports that the direct incremental cost contributed by stroke patients with hypertension is $24 ($25.5) billion,80 accounting for a large share of the direct cost as a whole. NIS data from 2005 to 2009 suggested that the average cost per admission where stroke was the primary diagnosis was $46,518 ($61,046).81 Yacoub et al found that these costs have increased overtime: the mean hospital charges for acute ischemic stroke essentially doubled over roughly a ten-year window.82 Aggregated, annual stroke hospitalization costs by type are: $2.34 (2.78) billion for subarachnoid hemorrhage; $2.52 ($2.99) billion for ischemic hemorrhage; and $12.55 (14.91) billion for acute ischemic stroke.83 Annual indirect costs for stroke are $33.7 ($39.3) billion.84 Joo et al estimate that annually $8,211 ($9,703) per patient in indirect costs are lost due to informal caregiving.85 The AHA estimates that the annual total cost of stroke was $45.5 ($48.8) billion.74
According to the Survey of Income and Program Participation, 3% of men and 2% of women in the US attribute their disability to stroke.74 The majority of individuals with stroke are elderly77,86 with greater activity limitations than individuals without stroke, thus the following literature applies to elderly patients (typically 65 years or older). 22% of patients were discharged from the hospital with a disability.87 Individuals with stroke were 65–121% more likely to require help in self-care, mobility, and household activities than their matched controls without stroke.88 In one study, 23.1% of patients reported limitations in performing ADLs.89 Another study found that as many as 60% of their sample had difficulty completing ADLs with or without an assistive device.90 The majority (64.5%) of patients reported severe to extreme difficulty standing for long periods and walking long distances;91 in a separate cohort, nearly one-fifth of patients relied on a mobility device.92 Other common ADL restrictions were self-care activities such as bathing (40.8%) and performing household tasks (40.2%).91
Because stroke primarily occurs among elderly patients, there is limited data on work limitations since many individuals have already retired. One study referenced stroke and work: Arowoiya et al found that, in a cohort whose dominant age group was between 60–69 years old, 45.6% stated that they were unemployed as a result of their stroke.91
Traumatic Brain Injury
TBI is a heterogeneous condition. 2016 GBD data suggest that the prevalence of TBI is 605 per 100,000.61 Other data estimate that there are between 2.8 million93 and 2.9 million94,95 TBI-related emergency-departments visits, hospitalizations, and deaths among all ages in the US. Another estimate using GBD data states that the incidence of TBI is 333 cases per 100,000 individuals each year.61
Discrepancies in definitions and assessments of TBI make it difficult to acquire accurate measures of prevalence or incidence. In addition, many figures—especially those drawing on hospitalization data—are likely underestimates because they do not account for outpatient data or individuals who did not seek medical attention. The majority of TBI cases are mild.96,97 Cancelliere et al estimate that there are 807.9 mild TBI cases per 100,000 visits to the emergency department.98
TBI-related admissions cost $21.4 ($27.2) billion annually with an additional $8.2 ($10.4) billion for discharges and transports.99 Finkelstein, Corso, and Miller estimated in 2006 that TBI-related direct costs were $9.2 ($17.4) billion.5,100 They also estimated that the indirect cost of TBI was $51.2 ($75.6) billion, and total cost of TBI was $60.4 ($93.0) billion.5,100 These figures draw on data from over ten years ago and stand to be updated given the increasing interest in TBI.
Impact on activity and work depends on the nature of injury. A TRACK-TBI study on patients with mild TBI presenting to Level I trauma centers found that roughly 50% of individuals did not return to pre-injury levels of functioning one year post-injury.101 In a statewide survey where participants self-reported a lifetime history of any TBI, 44% of individuals who had a TBI requiring hospitalization and 33% of those without hospitalization stated that they experienced activity limitations.102 The majority (60.4%) of individuals with moderate and severe TBIs were found to be unemployed at two years post injury.103 At five years post-injury, 53.3% of individuals with moderate and severe TBIs were unemployed, and 9.3% had unstable employment.104 Among veterans with severe TBIs, approximately 80% were unemployed one year post-injury.105
DISCUSSION
Back pain and OA are the most prevalent conditions with the greatest direct costs. The United States Bone and Joint Initiative reports that the prevalence of back pain in the previous 3 months is 33.9% and that of OA is 10.4%.30 The all-cause medical costs associated with back pain and OA are $315 ($365) billion and $373 ($460) billion, respectively.30 These exact figures vary depending on the criteria utilized to define the condition and the data sources.43,44 Despite these variations, these conditions remain the most prevalent on the whole, and their costs have risen:5 suggesting that improvement is required in the prevention and management of these conditions.
Stroke is often perceived as one of the most debilitating conditions, and it follows back pain and OA in both prevalence and medical costs. Estimates of stroke prevalence range from 2.5–3.7%,74,76,106 and the direct medical costs have been estimated as $28 ($30.9) billion.74 This is not to suggest that stroke is less important than back pain or osteoarthritis. On the individual level, stroke can be a hugely impairing event.
Prevalence and aggregated medical costs alone provide only part of the information that would be needed to make specific, actionable recommendations. For example, although other conditions have a lower prevalence, they are associated with larger per capita costs. RA may only affect 0.5–0.8% of the population,38,30 but its associated medical costs are estimated at $32.9 billion.30 Essentially, although RA is roughly one-twentieth as prevalent as OA,30 the per capita medical costs associated with RA are approximately 1.5 times that of OA.
In some cases, estimates of prevalence varied widely. TBI and SCI are both prominent examples. Estimates of the prevalence of TBI range considerably based on differing methodologies, and only one nationally-representative study was identified.61 Among SCI figures, prevalence estimates ranged from 291,000 individuals60 to 2.6 million.61 Altogether, these examples suggest that epidemiological data present in the literature vary widely and perhaps indicates that further investigation is needed.
We caution readers against drawing too many comparisons between the conditions and crafting conclusions based solely on the data presented here. There is considerable methodological variability and subtlety in the information that we have provided. Indirect costs—and as a result, total costs—had variable methodologies. Likewise, studies of activity and work limitations could not be reviewed for quality because they differ in their methodologies and outcomes measures. Because of this, there is also no singular, validated tool to assess the quality of these types of studies. Making comparisons along any of these metrics is thus challenging.
This paper ultimately builds on findings by Ma, Chan, and Carruthers5 by implementing a quality review stage. Among studies assessed for quality, only about half were generalizable to the US population and were conducted with sufficient rigor to be included here, which complicates all but very broad comparisons between conditions. Thus, there is a need for more robust, standardized studies in order to guide policymakers’ and researchers’ approach to conditions frequently requiring rehabilitation.
Study Limitations
In this article, the conditions are treated as if they occur in isolation. In reality, individuals often have multiple comorbidities that may impact costs and functional limitations. Indeed, some of the conditions here may overlap: it is possible that an individual sustains both a SCI and TBI, and OA is a common cause of back pain. We were unable to fully address these interactions. Another limitation of this review is that, while the majority of sources were published within the last 5 years, the data used were sometimes older. More recent data are needed to produce more up to date analyses. A final limitation of this study is that studies varied widely in methodology, disease definitions, and data availability. While we performed quality grading of the papers included, we urge the reader to be cautious in making direct comparisons among these conditions. We have included only the broadest trends where possible while emphasizing that doing so does not imply that certain conditions ought to be prioritized over others. Our purpose is to present these data to serve as a springboard for further investigation, not to suggest which conditions are more important than others. These outcomes are only one part of the more complex analysis that needed to make specific recommendations. Other measures that were not considered here either systematically or at all (e.g. cost-efficacy of different interventions, years lived with disability, and so forth) may be relevant and enlightening.
CONCLUSIONS
Back pain and OA have the greatest overall prevalence and medical costs, followed by stroke. Other conditions such as TBI, SCI, MS, RA, or amputation may have a lower prevalence but carry a large per patient effect. Further research is needed to determine how the outcomes examined in this review should be weighed to determine their relative impact. Standardizing research methods across conditions would yield more comparable data which could be informative for further action.
Supplementary Material
Supplemental Appendix 1: Search Strategy and Data Abstraction
Supplemental Appendix 2: Quality Grading Forms: Modified MORE Checklist and Modified Newcastle-Ottawa Quality Assessment for Cost-of-Illness Studies
Supplemental Appendix 3: Results of Quality Grading
ACKNOWLEDGEMENTS
We are extremely grateful to the many people who assisted in the conception and execution of this paper. Dr. Elizabeth K. Rasch (PhD, PT) contributed her expertise on the epidemiology of disability by suggesting data sources to explore, providing input on the search strategy, and offering considerations for quality grading. Dr. Rasch also reviewed the final draft. We are thankful for the input that Brian Moore (PA-C, MPAS, MPH) provided on the design and execution of this project. Mr. Moore provided advice on the methodology of this paper, which helped to frame its goals and to shape the conclusions reached here. He also provided edits for the final draft. Finally, we would like to warmly thank Diane Cooper (MSLS) for helping to develop the search strategy for this paper.
ACKNOWLEDGEMENT OF FINANCIAL SUPPORT
This project was supported by resources from the National Institutes of Health Intramural Research Program.
Glossary
- ADL
Activities of Daily Living
- AHA
American Heart Association
- GBD
Global Burden of Disease
- IHME
Institute of Health Metrics Evaluation
- LEA
Lower Extremity Amputation
- MEPS
Medical Expenditure Panel Survey
- MORE
Methodological Evaluation of Observational Research Checklist
- MS
Multiple Sclerosis
- NHANES
National Health and Nutrition Examination Survey
- NIS
National Inpatient Sample
- OA
Osteoarthritis
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RA
Rheumatoid Arthritis
- SCI
Spinal Cord Injury
- TBI
Traumatic Brain Injury
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACKNOWLEDGEMENT OF PRESENTATION OF MATERIAL
This material has not been presented elsewhere.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
REPRINTS
Reprints will not be available.
REFERENCES
- 1.Centers for Disease Control and Prevention. Disability impacts all of us. https://www.cdc.gov/ncbddd/disabilityandhealth/infographic-disability-impacts-all.html. Accessed December 6, 2019.
- 2.Centers for Disease Control and Prevention. Disability and health healthcare cost data. https://www.cdc.gov/ncbddd/disabilityandhealth/data-highlights.html. Accessed December 6, 2019.
- 3.Social Security Administration. Annual statistical report on the social security disability insurance program, 2018. October 2019; https://www.ssa.gov/policy/docs/statcomps/di_asr/2018/index.html. Accessed December 6, 2019.
- 4.Social Security Administration. Summary: Actuarial Status of the Social Security Trust Funds. April 2019; https://www.ssa.gov/policy/trust-funds-summary.html. Accessed December 6, 2019.
- 5.Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan L, Koepsell TD, Deyo RA, et al. The effect of Medicare’s payment system for rehabilitation hospitals on length of stay, charges, and total payments. N Engl J Med. 1997;337(14):978–985. [DOI] [PubMed] [Google Scholar]
- 7.Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6(1):14–17. [PMC free article] [PubMed] [Google Scholar]
- 8.United States Census Bureau. https://data.census.gov/cedsci/.
- 9.Arya R, Antonisamy B, Kumar S. Sample size estimation in prevalence studies. Indian J Pediatr. 2012;79(11):1482–1488. [DOI] [PubMed] [Google Scholar]
- 10.Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol. 2010;63(10):1061–1070. [DOI] [PubMed] [Google Scholar]
- 11.Shamliyan TA, Kane RL, Ansari MT, et al. AHRQ Methods for Effective Health Care. In: Development of Quality Criteria To Evaluate Nontherapeutic Studies of Incidence, Prevalence, or Risk Factors of Chronic Diseases: Pilot Study of New Checklists. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011. [PubMed] [Google Scholar]
- 12.Deeks JD J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG. Evaluating non-randomised intervention studies. Vol 7. Tubridge Wells: Gray Publishing; 2003. [DOI] [PubMed] [Google Scholar]
- 13.Wells BS GA, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 10, 2019.
- 14.Hresko A, Lin TC, Solomon DH. Medical Care Costs Associated With Rheumatoid Arthritis in the US: A Systematic Literature Review and Meta-Analysis. Arthritis Care Res (Hoboken). 2018;70(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Department of Labor: Bureau of Labor Statistics. Consumer Price Index (CPI) Databases. https://www.bls.gov/cpi/data.htm. Accessed December 4, 2019.
- 16.Moher D LA, Tetzlaff J, Altman DG, The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–429. [DOI] [PubMed] [Google Scholar]
- 18.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–1523. [DOI] [PubMed] [Google Scholar]
- 19.Narres M, Kvitkina T, Claessen H, et al. Incidence of lower extremity amputations in the diabetic compared with the non-diabetic population: A systematic review. PLoS One. 2017;12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiss LS, Li Y, Hora I, Albright A, Rolka D, Gregg EW. Resurgence of Diabetes-Related Nontraumatic Lower-Extremity Amputation in the Young and Middle-Aged Adult U.S. Population. Diabetes Care. 2019;42(1):50–54. [DOI] [PubMed] [Google Scholar]
- 21.MacKenzie EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am. 2007;89(8):1685–1692. [DOI] [PubMed] [Google Scholar]
- 22.Goodney PP, Travis LL, Brooke BS, et al. Relationship between regional spending on vascular care and amputation rate. JAMA surgery. 2014;149(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin H, Radican L, Kong SX. A study of regional variation in the inpatient cost of lower extremity amputation among patients with diabetes in the United States. J Med Econ. 2013;16(6). [DOI] [PubMed] [Google Scholar]
- 24.Chopra A, Azarbal AF, Jung E, et al. Ambulation and functional outcome after major lower extremity amputation. J Vasc Surg. 2018;67(5). [DOI] [PubMed] [Google Scholar]
- 25.Tennent DJ, Polfer EM, Sgromolo NM, Krueger CA, Potter BK. Characterization of disability following traumatic through knee and transfemoral amputations. Injury. 2018;49(6). [DOI] [PubMed] [Google Scholar]
- 26.MacKenzie EJ, Bosse MJ, Kellam JF, et al. Early predictors of long-term work disability after major limb trauma. J Trauma. 2006;61(3):688–694. [DOI] [PubMed] [Google Scholar]
- 27.Barbour KE, Helmick CG, Boring M, Brady TJ. Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation - United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66(9):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbour KE, Moss S, Croft JB, et al. Geographic Variations in Arthritis Prevalence, Health-Related Characteristics, and Management - United States, 2015. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2018;67(4):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy LB, Cisternas MG, Pasta DJ, Helmick CG, Yelin EH. Medical Expenditures and Earnings Losses Among US Adults With Arthritis in 2013. Arthritis Care Res (Hoboken). 2018;70(6):869–876. [DOI] [PubMed] [Google Scholar]
- 30.United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States (BMUS) Fourth Edition. Forthcoming; 4th:http://www.boneandjointburden.org. Accessed December 20, 2019.
- 31.Centers for Disease Control and Prevention. Osteoarthritis (OA). January 10, 2019; https://www.cdc.gov/arthritis/basics/osteoarthritis.htm. Accessed December 3, 2019.
- 32.Institute for Health Metrics and Evaluation. GBD Results Tool. http://ghdx.healthdata.org/gbd-results-tool. Accessed December 9, 2019.
- 33.Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey. Arthritis Care Res (Hoboken). 2016;68(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SX, Ganguli AX, Bodhani A, Medema JK, Reichmann WM, Macaulay D. Healthcare resource utilization and costs by age and joint location among osteoarthritis patients in a privately insured population. J Med Econ. 2017;20(12). [DOI] [PubMed] [Google Scholar]
- 35.Deshpande BR, Katz JN, Solomon DH, et al. Number of Persons With Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res (Hoboken). 2016;68(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Losina E, Weinstein AM, Reichmann WM, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res (Hoboken). 2013;65(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken). 2015;67(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37(9). [DOI] [PubMed] [Google Scholar]
- 39.Chen CI, Wang L, Wei W, Yuce H, Phillips K. Burden of rheumatoid arthritis among US Medicare population: co-morbidities, health-care resource utilization and costs. Rheumatology advances in practice. 2018;2(1):rky005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunnarsson C, Chen J, Rizzo JA, Ladapo JA, Naim A, Lofland JH. The Employee Absenteeism Costs of Rheumatoid Arthritis: Evidence From US National Survey Data. J Occup Environ Med. 2015;57(6). [DOI] [PubMed] [Google Scholar]
- 41.Myasoedova E, Davis JM, Achenbach SJ, Matteson EL, Crowson CS. RISING PREVALENCE OF FUNCTIONAL DISABILITY IN PATIENTS WITH RHEUMATOID ARTHRITIS OVER 20 YEARS. Ann Rheum Dis. 2018;77:54–54. [Google Scholar]
- 42.Strand V, Tundia N, Song Y, Macaulay D, Fuldeore M. Economic Burden of Patients with Inadequate Response to Targeted Immunomodulators for Rheumatoid Arthritis. Journal of managed care & specialty pharmacy. 2018;24(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shmagel A, Foley R, Ibrahim H. Epidemiology of Chronic Low Back Pain in US Adults: Data From the 2009–2010 National Health and Nutrition Examination Survey. Arthritis Care Res (Hoboken). 2016;68(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H, Haldeman S. Behavior-Related Factors Associated With Low Back Pain in the US Adult Population. Spine (Phila Pa 1976). 2018;43(1). [DOI] [PubMed] [Google Scholar]
- 45.Yang H, Haldeman S, Lu ML, Baker D. Low Back Pain Prevalence and Related Workplace Psychosocial Risk Factors: A Study Using Data From the 2010 National Health Interview Survey. J Manipulative Physiol Ther. 2016;39(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agency for Healthcare Research and Quality. Total expenditures in millions by condition, United States, 2015. Medical Expenditure Panel Survey. Accessed December 13, 2019.
- 47.Ivanova JI, Birnbaum HG, Schiller M, Kantor E, Johnstone BM, Swindle RW. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. The spine journal : official journal of the North American Spine Society. 2011;11(7):622–632. [DOI] [PubMed] [Google Scholar]
- 48.Tymecka-Woszczerowicz A, Wrona W, Kowalski PM, Hermanowski T. Indirect costs of back pain - Review. Polish Annals of Medicine. 2015;22(2):143. [Google Scholar]
- 49.Marshall LM, Litwack-Harrison S, Makris UE, et al. A Prospective Study of Back Pain and Risk of Falls Among Older Community-dwelling Men. The journals of gerontology Series A, Biological sciences and medical sciences. 2017;72(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marshall LM, Litwack-Harrison S, Cawthon PM, et al. A Prospective Study of Back Pain and Risk of Falls Among Older Community-dwelling Women. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makris UE, Weinreich MA, Fraenkel L, Han L, Leo-Summers L, Gill TM. Restricting Back Pain and Subsequent Disability in Activities of Daily Living Among Community-Living Older Adults. J Aging Health. 2018;30(9):1482–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dilokthornsakul P, Valuck RJ, Nair KV, Corboy JR, Allen RR, Campbell JD. Multiple sclerosis prevalence in the United States commercially insured population. Neurology. 2016;86(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones E, Pike J, Marshall T, Ye X. Quantifying the relationship between increased disability and health care resource utilization, quality of life, work productivity, health care costs in patients with multiple sclerosis in the US. BMC Health Serv Res. 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parisé H, Laliberté F, Lefebvre P, et al. Direct and indirect cost burden associated with multiple sclerosis relapses: excess costs of persons with MS and their spouse caregivers. J Neurol Sci. 2013;330(1–2). [DOI] [PubMed] [Google Scholar]
- 57.Bishop M, Chan F, Rumrill PD, et al. Employment Among Working-Age Adults With Multiple Sclerosis: A Data-Mining Approach to Identifying Employment Interventions. Rehabilitation Research Policy and Education. 2015;29(2):135–152. [Google Scholar]
- 58.Krause JS, Rumrill P, Dismuke-Greer CE, Jarnecke M. Quality employment outcomes after multiple sclerosis: A comparison of participants from a specialty hospital and the National MS Society. Journal of Vocational Rehabilitation. 2018;48(2):177–186. [Google Scholar]
- 59.Krause JS, Dismuke-Greer CE, Jarnecke M, Li C, Reed KS, Rumrill P. Employment and Gainful Earnings Among Those With Multiple Sclerosis. Arch Phys Med Rehabil. 2019;100(5):931–937. [DOI] [PubMed] [Google Scholar]
- 60.National Spinal Cord Injury Center. Spinal Cord Injury: Facts and Figures at a Glance. 2019; https://www.nscisc.uab.edu/. Accessed December 4, 2019.
- 61.Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armour BS, Courtney-Long EA, Fox MH, Fredine H, Cahill A. Prevalence and Causes of Paralysis—United States, 2013. 2016;106(10):1855–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain NB, Ayers GD, Peterson EN, et al. Traumatic spinal cord injury in the United States, 1993–2012. JAMA. 2015;313(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernhard M, Gries A, Kremer P, Böttiger BW. Spinal cord injury (SCI)—Prehospital management. Resuscitation. 2005;66(2):127–139. [DOI] [PubMed] [Google Scholar]
- 65.Selvarajah S, Hammond ER, Haider AH, et al. The burden of acute traumatic spinal cord injury among adults in the united states: an update. J Neurotrauma. 2014;31(3). [DOI] [PubMed] [Google Scholar]
- 66.Mahabaleshwarkar R, Khanna R. National hospitalization burden associated with spinal cord injuries in the United States. Spinal Cord. 2014;52(2). [DOI] [PubMed] [Google Scholar]
- 67.Hall OT, McGrath RP, Peterson MD, et al. The Burden of Traumatic Spinal Cord Injury in the United States: Disability-Adjusted Life Years. Arch Phys Med Rehabil. 2019;100(1):95–100. [DOI] [PubMed] [Google Scholar]
- 68.DeVivo M, Chen Y, Mennemeyer S, Deutsch A. Costs of Care Following Spinal Cord Injury. Topics in Spinal Cord Injury Rehabilitation. 2011;16(4):1–9. [Google Scholar]
- 69.Berkowitz M, O’Leary PK, Kruse DL, Harvey C. Spinal Cord Injury: An Analysis of Medical and Social Costs. New York, NY, US: Demos Medical; 1998. [Google Scholar]
- 70.Pretz CR, Kozlowski AJ, Chen Y, Charlifue S, Heinemann AW. Trajectories of Life Satisfaction After Spinal Cord Injury. Arch Phys Med Rehabil. 2016;97(10):1706–1713.e1701. [DOI] [PubMed] [Google Scholar]
- 71.DiPiro ND, Li C, Krause JS. A longitudinal study of self-reported spasticity among individuals with chronic spinal cord injury. Spinal Cord. 2018;56(3). [DOI] [PubMed] [Google Scholar]
- 72.Trenaman L, Miller WC, Querée M, Escorpizo R. Modifiable and non-modifiable factors associated with employment outcomes following spinal cord injury: A systematic review. J Spinal Cord Med. 2015;38(4):422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ottomanelli L, Lind L. Review of critical factors related to employment after spinal cord injury: implications for research and vocational services. The journal of spinal cord medicine. 2009;32(5):503–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 75.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blackwell DL VM. Tables of Summary Health Statistics for U.S. Adults: 2017 National Health Interview Survey. National Center for Health Statistics. 2018; http://www.cdc.gov/nchs/nhis/SHS/tables.htm. Accessed July 22, 2019. [Google Scholar]
- 77.Centers for Disease Control and Prevention. Stroke Facts. September 6, 2017; https://www.cdc.gov/stroke/facts.htm. Accessed December 4, 2019.
- 78.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madsen TE, Khoury J, Alwell K, et al. Sex-specific stroke incidence over time in the Greater Cincinnati/Northern Kentucky Stroke Study. Neurology. 2017;89(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lekoubou A, Bishu KG, Ovbiagele B. Nationwide Healthcare Expenditures among Hypertensive Individuals with Stroke: 2003–2014. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2018;27(7). [DOI] [PubMed] [Google Scholar]
- 81.Stepanova M, Venkatesan C, Altaweel L, Mishra A, Younossi ZM. Recent trends in inpatient mortality and resource utilization for patients with stroke in the United States: 2005–2009. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013;22(4). [DOI] [PubMed] [Google Scholar]
- 82.Yacoub HA, Al-Qudah ZA, Khan HM, Farhad K, Ji AB, Souayah N. Trends in Outcome and Hospitalization Cost among Adult Patients with Acute Ischemic Stroke in the United States. J Vasc Interv Neurol. 2015;8(2):19–23. [PMC free article] [PubMed] [Google Scholar]
- 83.Tong X, George MG, Gillespie C, Merritt R. Trends in hospitalizations and cost associated with stroke by age, United States 2003–2012. International journal of stroke : official journal of the International Stroke Society. 2016;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ovbiagele B, Goldstein LB, Higashida RT, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44(8). [DOI] [PubMed] [Google Scholar]
- 85.Joo H, Dunet DO, Fang J, Wang G. Cost of informal caregiving associated with stroke among the elderly in the United States. Neurology. 2014;83(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in U.S. hospitals, 1989–2009. NCHS data brief. 2012(95):1–8. [PubMed] [Google Scholar]
- 87.Mu F, Hurley D, Betts KA, et al. Real-world costs of ischemic stroke by discharge status. Curr Med Res Opin. 2017;33(2). [DOI] [PubMed] [Google Scholar]
- 88.Skolarus LE, Burke JF, Brown DL, Freedman VA. Understanding stroke survivorship: expanding the concept of poststroke disability. Stroke. 2014;45(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chinthammit C, Coull BM, Nimworapan M, Bhattacharjee S. Co-occurring Chronic Conditions and Economic Burden among Stroke Survivors in the United States: A Propensity Score-Matched Analysis. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2017;26(2). [DOI] [PubMed] [Google Scholar]
- 90.Brenner AB, Burke JF, Skolarus LE. Moving Toward an Understanding of Disability in Older US Stroke Survivors. J Aging Health. 2018;30(1):75–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arowoiya AI, Elloker T, Karachi F, Mlenzana N, Lajn K, Rhoda A. Using the World Health Organization’s Disability Assessment Schedule (2) to assess disability in community-dwelling stroke patients. South African Journal of Physiotherapy. 2017;73(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wing JJ, Burke JF, Clarke PJ, Feng C, Skolarus LE. The role of the environment in falls among stroke survivors. Arch Gerontol Geriatr. 2017;72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2017;66(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Centers for Disease Control and Prevention. Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths. 2014; https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf. Accessed July 22, 2019.
- 95.Prevention CfDCa. TBI-related Emergency Department Visits, Hospitalizations, and Deaths (EDHDs). 2019.
- 96.Centers for Disease Control and Prevention. TBI: Get the Facts. March 11, 2019; https://www.cdc.gov/traumaticbraininjury/get_the_facts.html. Accessed December 5, 2019.
- 97.Centers for Disease Control and Prevention. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem September 2003; https://www.cdc.gov/traumaticbraininjury/pdf/mtbireport-a.pdf. Accessed December 5, 2019.
- 98.Cancelliere C, Coronado VG, Taylor CA, Xu L. Epidemiology of Isolated Versus Nonisolated Mild Traumatic Brain Injury Treated in Emergency Departments in the United States, 2006–2012: Sociodemographic Characteristics. The Journal of head trauma rehabilitation.32(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marin JR, Weaver MD, Mannix RC. Burden of USA hospital charges for traumatic brain injury. Brain Inj. 2017;31(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Finkelstein EA, Corso PS, Miller TR. Incidence and Economic Burden of Injuries in the United States. New York, NY, USA: Oxford University Press; 2006. [Google Scholar]
- 101.Nelson LD, Temkin NR, Dikmen S, et al. Recovery After Mild Traumatic Brain Injury in Patients Presenting to US Level I Trauma Centers: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Study. JAMA neurology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Whiteneck GG, Cuthbert JP, Corrigan JD, Bogner JA. Prevalence of Self-Reported Lifetime History of Traumatic Brain Injury and Associated Disability: A Statewide Population-Based Survey. J Head Trauma Rehabil. 2016;31(1):E55–E62. [DOI] [PubMed] [Google Scholar]
- 103.Cuthbert JP, Harrison-Felix C, Corrigan JD, Bell JM, Haarbauer-Krupa JK, Miller AC. Unemployment in the United States After Traumatic Brain Injury for Working-Age Individuals: Prevalence and Associated Factors 2 Years Postinjury. J Head Trauma Rehabil. 2015;30(3):160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DiSanto D, Kumar RG, Juengst SB, et al. Employment Stability in the First 5 Years After Moderate-to-Severe Traumatic Brain Injury. Arch Phys Med Rehabil. 2019;100(3):412. [DOI] [PubMed] [Google Scholar]
- 105.Dillahunt-Aspillaga C, Nakase-Richardson R, Hart T, et al. Predictors of Employment Outcomes in Veterans With Traumatic Brain Injury: A VA Traumatic Brain Injury Model Systems Study. J Head Trauma Rehabil. 2017;32(4):271–282. [DOI] [PubMed] [Google Scholar]
- 106.Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data 2015–2016. 2017; https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/MCQ_I.htm#MCQ160f. Accessed July 22, 2019.
- 107.Newhall K, Spangler E, Dzebisashvili N, Goodman DC, Goodney P. Amputation Rates for Patients with Diabetes and Peripheral Arterial Disease: The Effects of Race and Region. Ann Vasc Surg. 2016;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Margolis DJ, Hoffstad O, Weibe DJ. Lower-extremity amputation risk is associated with variation in behavioral risk factor surveillance system responses. Diabetes Care. 2014;37(8). [DOI] [PubMed] [Google Scholar]
- 109.Shraim M, Cifuentes M, Willetts JL, Marucci-Wellman HR, Pransky G. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen AY, Chonghasawat AO, Leadholm KL. Multiple sclerosis: Frequency, cost, and economic burden in the United States. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2017;45. [DOI] [PubMed] [Google Scholar]
- 111.Hartung DM. Economics and Cost-Effectiveness of Multiple Sclerosis Therapies in the USA. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2017;14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carroll CA, Fairman KA, Lage MJ. Updated cost-of-care estimates for commercially insured patients with multiple sclerosis: retrospective observational analysis of medical and pharmacy claims data. BMC Health Serv Res. 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim C, Linsenmeyer KD, Vlad SC, et al. Prevalence of radiographic and symptomatic hip osteoarthritis in an urban United States community: the Framingham osteoarthritis study. Arthritis & rheumatology (Hoboken, NJ). 2014;66(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Snyder EA, Alvarez C, Golightly YM, Renner JB, Jordan JM, Nelson AE. INCIDENCE AND PROGRESSION OF HAND OSTEOARTHRITIS IN THE JOHNSTON COUNTY OSTEOARTHRITIS PROJECT. Osteoarthritis Cartilage. 2019;27:S257–S258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moss AS, Murphy LB, Helmick CG, et al. Annual incidence rates of hip symptoms and three hip OA outcomes from a US population-based cohort study: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2016;24(9):1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Torio CM, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. [PubMed] [Google Scholar]
- 117.Ong KL, Runa M, Lau E, Altman RD. Cost-of-illness of knee osteoarthritis: potential cost savings by not undergoing arthroplasty within the first 2 years. Clinicoeconomics and Outcomes Research. 2019;11:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li SY, Molony JT, Peng Y, Nieman KM, Gilbertson DT. Prevalence of Rheumatoid Arthritis and Associated Comorbidities in the 2011–2015 Medicare Population. Arthritis & Rheumatology. 2017;69. [Google Scholar]
- 119.Crane MM, Juneja M, Allen J, et al. Epidemiology and Treatment of New-Onset and Established Rheumatoid Arthritis in an Insured US Population. Arthritis Care Res (Hoboken). 2015;67(12). [DOI] [PubMed] [Google Scholar]
- 120.Li N, Chan E, Peterson S. The economic burden of depression among adults with rheumatoid arthritis in the United States. J Med Econ. 2019;22(4). [DOI] [PubMed] [Google Scholar]
- 121.Margolis JM, Juneau P, Sadosky A, Cappelleri JC, Bryce TN, Nieshoff EC. Health care resource utilization and medical costs of spinal cord injury with neuropathic pain in a commercially insured population in the United States. Arch Phys Med Rehabil. 2014;95(12). [DOI] [PubMed] [Google Scholar]
- 122.Asemota AO, Ahmed AK, Purvis TE, Passias PG, Goodwin CR, Sciubba DM. Analysis of Cervical Spine Injuries in Elderly Patients from 2001 to 2010 Using a Nationwide Database: Increasing Incidence, Overall Mortality, and Inpatient Hospital Charges. World Neurosurg. 2018;120. [DOI] [PubMed] [Google Scholar]
- 123.Alqahtani F, Aljohani S, Busu T, et al. Temporal Trends in the Incidence and Outcomes of Acute Ischemic Stroke in the United States. Circulation. 2017;136. [Google Scholar]
- 124.Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312(3). [DOI] [PubMed] [Google Scholar]
- 125.Fang MC, Perraillon MC, Ghosh K, Cutler DM, Rosen AB. Trends in stroke rates, risk, and outcomes in the United States, 1988 to 2008. The American journal of medicine. 2014;127(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnson BH, Bonafede MM, Watson C. Short- and longer-term health-care resource utilization and costs associated with acute ischemic stroke. ClinicoEconomics and outcomes research : CEOR. 2016;8:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Katan M, Luft A. Global Burden of Stroke. Semin Neurol. 2018;38(2). [DOI] [PubMed] [Google Scholar]
- 128.Wang G, Zhang Z, Ayala C, Dunet DO, Fang J, George MG. Costs of hospitalization for stroke patients aged 18–64 years in the United States. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association.23(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.RTI International. Cardiovascular Disease: A Costly Burden for America - Projections through 2035. 2017; https://healthmetrics.heart.org/wp-content/uploads/2017/10/Cardiovascular-Disease-A-Costly-Burden.pdf. Accessed July 22, 2019.
- 130.Corrigan JD, Yang J, Singichetti B, Manchester K, Bogner J. Lifetime prevalence of traumatic brain injury with loss of consciousness. Injury prevention : journal of the International Society for Child and Adolescent Injury Prevention. 2018;24(6). [DOI] [PubMed] [Google Scholar]
- 131.Kerr ZY, Harmon KJ, Marshall SW, Proescholdbell SK, Waller AE. The epidemiology of traumatic brain injuries treated in emergency departments in North Carolina, 2010–2011. N C Med J. 2014;75(1):8. [DOI] [PubMed] [Google Scholar]
- 132.Brooks JC, Strauss DJ, Shavelle RM, Paculdo DR, Hammond FM, Harrison-Felix CL. Long-term disability and survival in traumatic brain injury: results from the National Institute on Disability and Rehabilitation Research Model Systems. Arch Phys Med Rehabil. 2013;94(11):2203–2209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Appendix 1: Search Strategy and Data Abstraction
Supplemental Appendix 2: Quality Grading Forms: Modified MORE Checklist and Modified Newcastle-Ottawa Quality Assessment for Cost-of-Illness Studies
Supplemental Appendix 3: Results of Quality Grading


