Abstract
The dissolution behavior of cellulose in the mixtures of dimethyl sulfoxide (DMSO) and different ionic liquids (ILs) at 25 °C was studied. High solubility of cellulose was reached in the mixtures of ILs and DMSO at mole fractions of 1:2, 1:2, and 1:1 for 1-butyl-3-methylimidazolium acetate, 1-propyl-3-methylimidazolium acetate, and 1-ethyl-3-methylimidazolium acetate, respectively. At high DMSO/IL molar ratios (10:1–2:1), a longer alkyl chain of the IL cation led to higher cellulose solubility. However, shorter cation alkyl chains favored cellulose dissolution at 1:1. Rheological, Fourier transform infrared spectroscopy (FTIR), and nuclear magnetic resonance (NMR) measurements were used to understand cellulose dissolution. It was found out that the increase of the DMSO ratio in binary mixtures caused higher cellulose solubility by decreasing the viscosity of systems. For cations with longer alkyl chains, stronger interaction between the IL and cellulose and higher viscosity of DMSO/IL mixtures were observed. The new knowledge obtained here could be useful to the development of cost-effective solvent systems for biopolymers.
Introduction
Due to the excessive exploitation of nonrenewable resources and increasing environmental concerns, the processing and application of cellulose as the most abundant natural polymer have gained wide interest considering its wide availability, low cost, biocompatibility, and biodegradability.1,2 Cellulose is a homopolysaccharide consisting of numerous d-glucose units linked through β(1–4) glycosidic bonds, which is mainly present in cell walls, green plants, cotton (90%), and wood (50%).3 Cellulose is traditionally used in textile and paper-making industries, and recently, it has been considered to be a promising feedstock for biobased products and fuels.4 However, the highly ordered crystalline structure and its dense chain interactions in cellulose make this biopolymer hardly soluble in common solvents, limiting its wide applications.5 Traditional cellulose solvent systems such as sodium hydroxide/carbon disulfide mixtures, N-methylmorpholine N-oxide (NMMO), and aqueous solutions of metal complexes suffer drawbacks like insufficient solvation capability, high energy cost, volatility, toxicity, poisonous gas pollution, and poor solvent recovery.1,5,6 Therefore, it is in great demand to develop alternative “green” solvents to overcome these issues.
Ionic liquids (ILs) have been widely recognized as promising “green solvents” to replace traditional biopolymer solvents due to their excellent dissolving abilities and desirable properties such as negligible vapor pressure, low toxicity, high thermal and chemical stability, nonflammability, structural designability, and recyclability.1,7−9 ILs are salts made up of an organic cation and an organic or inorganic anion and have a melting point below 100 °C.7 The dissolution of cellulose in ILs is mainly determined by the IL cation and anion structures (e.g., ion type, the length and symmetry of substituent groups).5,10 The length of the side alkyl chain, as well as the symmetry of ILs, are also investigated in a number of other scientific areas.11−13 Over the past few years, ILs have been increasingly demonstrated to serve as excellent media for cellulose dissolution,5,14 which also allows for the chemical modification of cellulose with high degrees of substitution (DS),15,16 the pretreatment of biomass for the subsequent enzymatic conversion into sugars or ethanol,17 and the development of various cellulose-based materials such as cellulose films,18 solid polymer electrolytes,19 and drug carriers.20 Nonetheless, the strong association between cations and anions makes ILs a highly viscous medium,21 leading to slow and high dissolution temperatures for cellulose in ILs.4,16,22
Recently, the mixed solvents of ILs and polar aprotic co-solvents (e.g., dimethyl sulfoxide (DMSO), dimethylacetamide (DMA), and dimethylformamide (DMF)) have gained substantial attention due to their higher dissolving rates and lower costs and dissolving temperatures compared to pure ILs.23,24 Xu et al. found that mixtures of 1-butyl-3-methylimidazolium acetate ([C4mim][CH3COO]) and DMSO, DMF, or DMA could effectively dissolve cellulose at ambient temperature,25−27 while DMSO was considered as a most suitable co-solvent to combine with ILs for cellulose dissolution.26 Some researchers investigated the influence of the IL structure on the solubility of cellulose in DMSO/IL mixtures and found that cellulose solubility was related to the Kamlet–Taft hydrogen-bond basicity (β) of ILs.16,22,28,29
The dissolution behavior of cellulose in DMSO/IL mixtures was assumed to be affected by the viscosity of the mixture solvents and the interactions between IL and DMSO and between IL and cellulose.1,30−33 However, this assumption is yet to be verified by direct experimental evidence and, particularly, the relationship between cellulose dissolution and the interactions between different ILs and cellulose at different DMSO/IL ratios have rarely been studied. Imidazolium acetate is one kind of ILs and is most commonly used to dissolve cellulose due to its desirable properties such as high dissolving capacity, easy availability, low toxicity, low corrosiveness, low melting point, and favorable biodegradability.3 In this study, we investigate the effect of the DMSO/IL ratio and the alkyl chain length of the IL cation on the dissolution of cellulose in DMSO/IL mixtures by characterizing the rheological properties of DMSO/IL mixtures and the interactions between IL and DMSO and between IL and cellulose using steady-shear rheological measurement, attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy.
Results and Discussion
Dissolution of Cellulose in Different DMSO/IL Mixtures
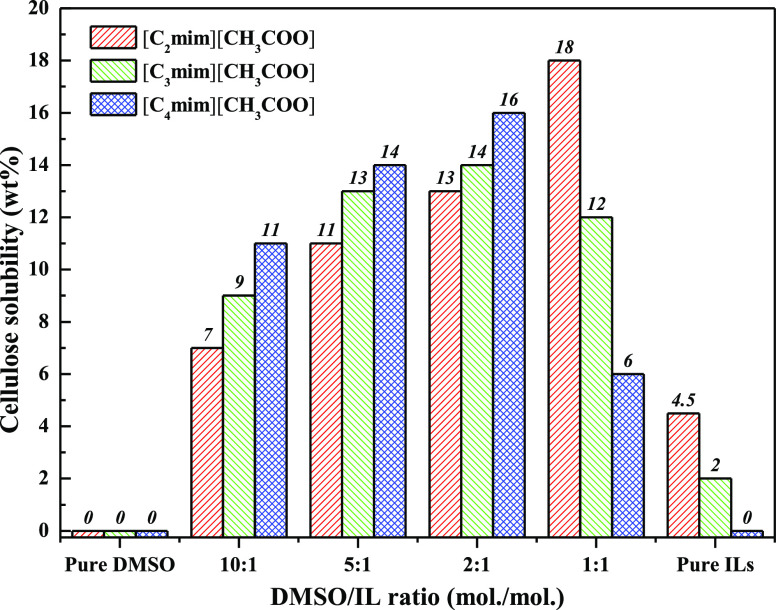
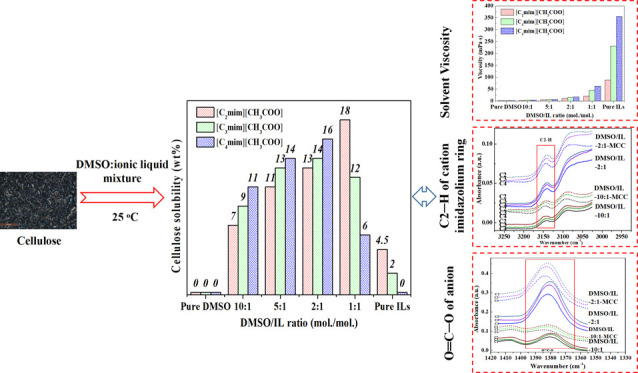
The solubility of MCC in pure DMSO, pure ILs, and DMSO/IL mixtures of different molar ratios at 25 °C is shown in Figure 1. At ambient temperature, cellulose was insoluble in pure DMSO and insoluble or slightly soluble in pure ILs. The solubility of MCC in DMSO/IL mixtures depends on the DMSO/IL ratio. In DMSO/[C4mim][CH3COO] and DMSO/[C3mim][CH3COO] mixtures, with the DMSO/IL molar ratio decreasing from 10:1 to 2:1, the cellulose solubility increased from 9 to 16 wt % and from 11 to 14 wt %, respectively. However, in the DMSO/[C2mim][CH3COO] mixture, the cellulose solubility increased from 7 wt % to the maximum (18 wt %) as the DMSO/IL molar ratio decreased from 10:1 to 1:1. It was found that compared to the cellulose solubility in pure [C4mim][CH3COO] at 70 °C (15.5 wt %),34 a higher solubility of cellulose in the DMSO/IL mixtures at 25 °C was achieved with significantly reduced cost and energy consumption. For all of these solvent systems, a further decrease in the DMSO/IL ratio reduced the cellulose solubility. These observations indicate that a low content of ILs in DMSO/IL mixtures facilitates the dissolution of cellulose compared with a high concentration of ILs.
Figure 1.
Solubility of cellulose in DMSO/IL mixtures of different molar ratios at 25 °C.
The alkyl chain length of the IL cation also affected the cellulose solubility (Figure 1). The solubility of MCC increased with an increasing alkyl chain length of the IL cation from C2 to C4 at DMSO/IL molar ratios of 10:1, 5:1, and 2:1, whereas at higher IL concentrations (1:1 (mol/mol) DMSO/IL mixtures or pure ILs), the cellulose solubility followed the order [C2mim][CH3COO] > [C3mim][CH3COO] > [C4mim][CH3COO]. These results suggest that at high DMSO/IL molar ratios (10:1–2:1), a longer cation alkyl chain of ILs is favorable for cellulose dissolution, whereas at a low DMSO/IL molar ratio (1:1), cellulose solubility increases with decreasing alkyl chain length.
Rheological Properties of DMSO/IL Mixtures
The steady-shear viscosities of pure DMSO, pure ILs, and DMSO/IL mixtures are compared (Table 1). For the three types of DMSO/IL mixtures, increasing the DMSO ratio resulted in a significant decrease in the mixture viscosity (p < 0.05), probably due to the disruption of IL clusters by DMSO.33 As shown in Table 1, the viscosity of DMSO/IL mixtures increased with a higher IL content in the DMSO/IL mixture and a longer cation alkyl chain (p < 0.05), which is in general agreement with our previous results for water/IL mixtures.35,36 Irrespective of the IL type, the viscosities of 1:1 (mol/mol) DMSO/IL mixtures were two to four times higher than those of the 2:1 (mol/mol) mixtures (p < 0.05), while 10:1 and 5:1 (mol/mol) DMSO/IL mixtures exhibited relatively low viscosities (p > 0.05). Interestingly, as the DMSO/IL molar ratio decreased from 2:1 to 1:1, the DMSO/[C2mim][CH3COO] mixture showed a smaller viscosity increase (9.7 mPa·s) than the DMSO/[C3mim][CH3COO] mixture (30.2 mPa·s) and the DMSO/[C4mim][CH3COO] mixture (44.6 mPa·s).
Table 1. Viscosities of DMSO/IL Mixtures at Different Molar Ratios.
| samples | viscosity (mPa·s) |
|---|---|
| pure DMSO | 2.06 ± 0.19a |
| DMSO/[C2mim][CH3COO]-10:1 | 3.64 ± 0.13b |
| DMSO/[C2mim][CH3COO]-5:1 | 5.38 ± 0.11cd |
| DMSO/[C2mim][CH3COO]-2:1 | 11.88 ± 0.19e |
| DMSO/[C2mim][CH3COO]-1:1 | 21.58 ± 0.25h |
| pure [C2mim][CH3COO] | 88.58 ± 0.67k |
| DMSO/[C3mim][CH3COO]-10:1 | 3.92 ± 0.15bc |
| DMSO/[C3mim][CH3COO]-5:1 | 6.33 ± 0.19d |
| DMSO/[C3mim][CH3COO]-2:1 | 16.10 ± 0.18f |
| DMSO/[C3mim][CH3COO]-1:1 | 46.31 ± 0.21i |
| pure [C3mim][CH3COO] | 231.28 ± 2.85m |
| DMSO/[C4mim][CH3COO]-10:1 | 4.19 ± 0.04bc |
| DMSO/[C4mim][CH3COO]-5:1 | 6.72 ± 0.22d |
| DMSO/[C4mim][CH3COO]-2:1 | 18.31 ± 0.25g |
| DMSO/[C4mim][CH3COO]-1:1 | 62.90 ± 0.42j |
| pure [C4mim][CH3COO] | 355.38 ± 1.67n |
Values are mean ± standard deviation, and values with the same lowercase letters in the same column are not significantly different (p < 0.05).
ATR-FTIR Analysis of DMSO/IL Mixtures and DMSO/IL-MCC Mixtures
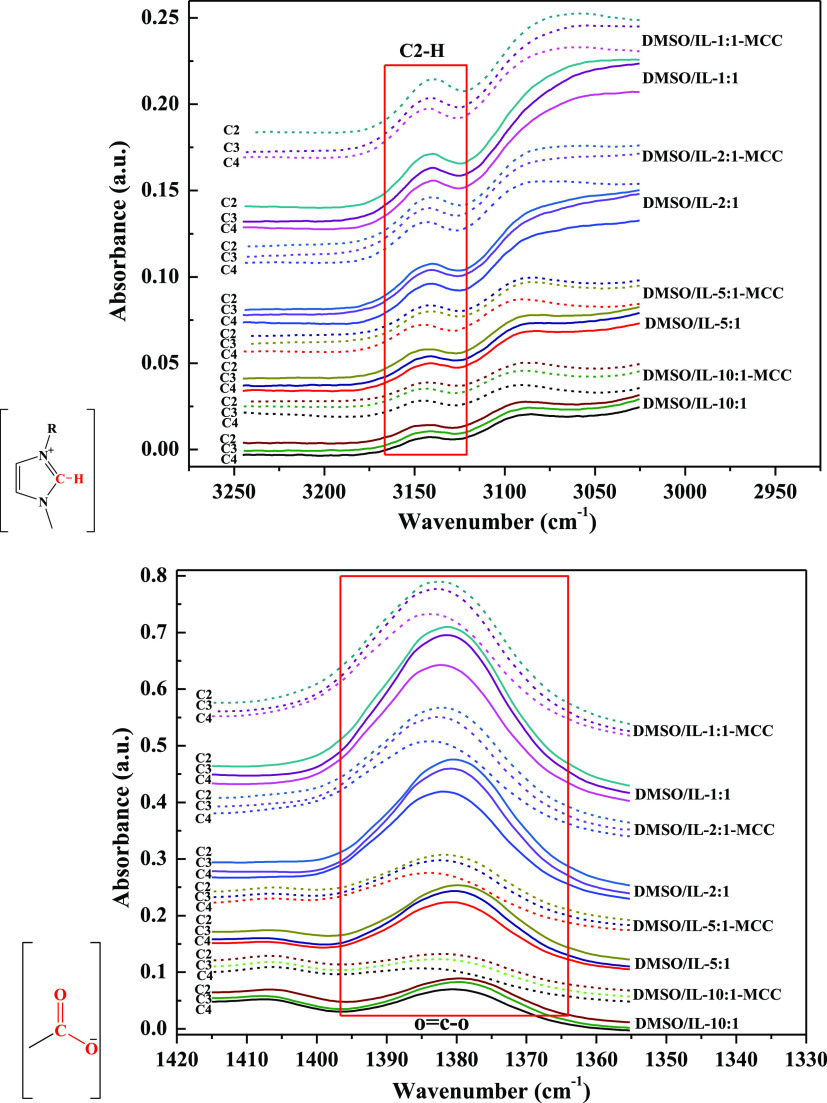
Figure 2 shows the ATR-FTIR spectra for DMSO/IL and DMSO/IL-MCC mixtures (MCC concentration: 5 wt %). Among them, C2–H on the cationic imidazole ring and O=C–O on the anions of ILs are the main sites for ILs to interact with DMSO and cellulose via hydrogen bonding.16,37Table 2 lists the infrared wavenumbers corresponding to C2–H (υC2–H) and O=C–O (υO=C–O) for DMSO/IL mixtures, and these mixtures contain 5 wt % cellulose with different DMSO/IL molar ratios. As shown in Figure 2 and Table 2, for the mixture solvents without cellulose, with decreasing DMSO/IL molar ratio from 10:1 to 1:1, there was a red shift for C2–H of IL cations (υC2–H decreased) and a blue shift for O=C–O of the IL anion (acetate) (υO=C–O increased). This indicates that with a higher IL content in the mixtures, the interaction between the IL cation and DMSO weakens while that between IL ions becomes stronger.37,38 For all of these three DMSO/IL mixtures added with cellulose, the υC2–H and υO=C–O values increased (blue shift), indicating that the ILs interacted with cellulose. For all of these DMSO/IL-MCC mixtures, the extent of the blue shift for C2–H of IL cations (ΔυC2–H) and O=C–O of the IL anion (ΔυO=C–O) increased with increasing DMSO/IL molar ratio from 1:1 to 10:1, indicating that a lower IL content in DMSO/IL mixtures is conducive to the interaction between ILs and cellulose. Moreover, ΔυO=C–O was always greater than ΔυC2–H, suggesting that the anions of ILs play a predominant role in the interaction between ILs and cellulose with interactions involving cations being much weaker. At the same DMSO/IL ratio, both ΔυC2–H and ΔυO=C–O increased with an increasing alkyl chain length of the IL cation, indicating stronger interactions between cellulose and ILs with longer alkyl chains.
Figure 2.
ATR-FTIR spectra for different DMSO/IL mixtures before (real line) and after (dash line) addition of 5 wt % cellulose at 25 °C for 2 h.
Table 2. Wavenumbers Associated with C2–H of the Cation Imidazolium Ring and O=C–O of the Anion for DMSO/IL Mixtures Before and After the Addition of 5 wt % Cellulose at 25 °C for 2 h.
| DMSO/IL |
DMSO/IL-MCC |
|||||
|---|---|---|---|---|---|---|
| samples | υC2–H | υO=C–O | υC2–H | υO=C–O | ΔυC2–H | ΔυO=C–O |
| DMSO/[C4mim][CH3COO]-10:1 | 3141.4 | 1380.5 | 3145.0 | 1385.2 | 3.6 | 4.7 |
| DMSO/[C4mim][CH3COO]-5:1 | 3141.2 | 1380.9 | 3144.0 | 1384.8 | 2.8 | 3.9 |
| DMSO/[C4mim][CH3COO]-2:1 | 3140.7 | 1381.9 | 3143.3 | 1383.6 | 2.6 | 2.7 |
| DMSO/[C4mim][CH3COO]-1:1 | 3140.3 | 1382.2 | 3141.8 | 1383.6 | 1.5 | 1.4 |
| DMSO/[C3mim][CH3COO]-10:1 | 3141.1 | 1379.8 | 3143.8 | 1382.6 | 2.7 | 2.8 |
| DMSO/[C3mim][CH3COO]-5:1 | 3140.9 | 1380.3 | 3142.6 | 1382.3 | 1.7 | 2.0 |
| DMSO/[C3mim][CH3COO]-2:1 | 3140.7 | 1380.9 | 3141.9 | 1382.7 | 1.2 | 1.8 |
| DMSO/[C3mim][CH3COO]-1:1 | 3139.9 | 1381.5 | 3140.7 | 1382.7 | 0.8 | 1.2 |
| DMSO/[C2mim][CH3COO]-10:1 | 3140.6 | 1379.6 | 3142.6 | 1382.1 | 2.0 | 2.5 |
| DMSO/[C2mim][CH3COO]-5:1 | 3140.2 | 1379.8 | 3141.7 | 1381.7 | 1.5 | 1.9 |
| DMSO/[C2mim][CH3COO]-2:1 | 3139.9 | 1380.4 | 3140.6 | 1382.1 | 0.7 | 1.7 |
| DMSO/[C2mim][CH3COO]-1:1 | 3139.5 | 1381.3 | 3139.9 | 1382.4 | 0.4 | 1.1 |
NMR Analysis of DMSO/IL and DMSO/IL-MCC Mixtures
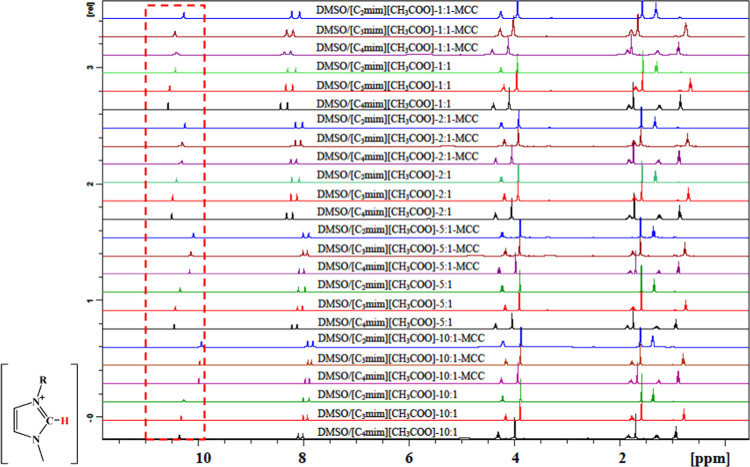
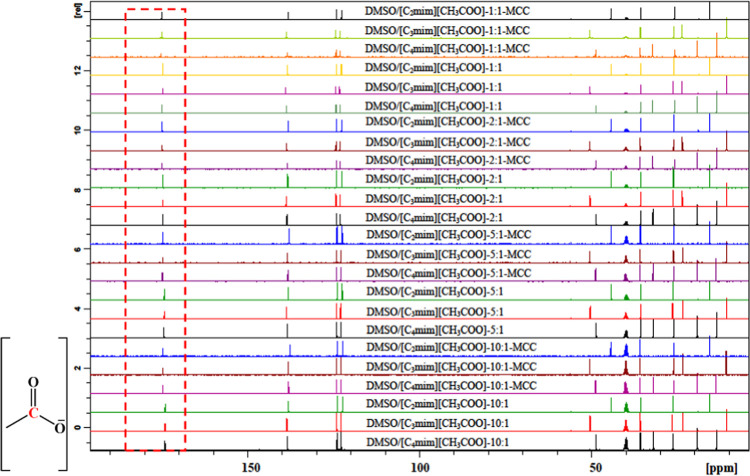
The 1H NMR and 13C NMR spectra for DMSO/IL and DMSO/IL-MCC mixtures (MCC concentration: 5 wt %) are shown in Figures 3 and 4, respectively. The chemical shifts for hydrogen tethered at the C(2) of the imidazolium ring and for the carbon atom of O=C–O of the IL anion (designated as δC2–H and δO=C–O, respectively) are listed in Table 3. The changes in the chemical shifts for these atoms are indicative of interactions between IL and DMSO33,37 or between IL ions and cellulose molecules.16,26,32 For DMSO/IL solvents without cellulose, δC2–H and δO=C–O increased with a decrease in the DMSO/IL molar ratio from 10:1 to 1:1, indicating enhanced cation–anion interactions.33,39 For the mixture solvents added with cellulose, the signal for the C(2) hydrogen of the imidazolium ring moved upfield (a decrease in δC2–H), whereas that for the carboxyl carbon atom moved downfield (an increase in δO=C–O), which might be due to the interaction of ILs with the hydroxyl groups of cellulose.32 With decrease in the DMSO/IL molar ratio from 10:1 to 1:1, the extent of shifting for δC2–H and δO=C–O increased, suggesting that the addition of DMSO to ILs facilitates the interaction between cellulose and ILs. At the same DMSO/IL ratio, the shifting extent for δC2–H and δO=C–O followed the order [C4mim][CH3COO] > [C3mim][CH3COO] > [C2mim][CH3COO], indicating that the interactions between IL and cellulose increased with a longer alkyl chain of the IL cation. The NMR results here are consistent with the ATR-FTIR analysis.
Figure 3.
1H NMR spectra for different DMSO/IL mixtures before and after the addition of 5 wt % cellulose at 25 °C for 2 h.
Figure 4.
13C NMR spectra for different DMSO/IL mixtures before and after the addition of 5 wt % cellulose at 25 °C for 2 h.
Table 3. Chemical Shift (C2–H of the Cation Imidazolium Ring in 1H NMR and O=C–O of the Anion in 13C NMR) of DMSO/IL Mixtures Before and After the Addition of 5 wt % Cellulose at 25 °C for 2 h.
| DMSO/IL |
DMSO/IL-MCC |
|||||
|---|---|---|---|---|---|---|
| samples | δC2-H | δO=C–O | δC2–H | δO=C–O | ΔδC2–H | ΔδO=C–O |
| DMSO/[C4mim][CH3COO]-10:1 | 10.36 | 174.07 | 9.99 | 174.72 | –0.37 | 0.65 |
| DMSO/[C4mim][CH3COO]-5:1 | 10.46 | 174.39 | 10.15 | 174.84 | –0.31 | 0.45 |
| DMSO/[C4mim][CH3COO]-2:1 | 10.52 | 174.69 | 10.32 | 175.06 | –0.20 | 0.37 |
| DMSO/[C4mim][CH3COO]-1:1 | 10.57 | 174.98 | 10.40 | 175.23 | –0.17 | 0.25 |
| DMSO/[C3mim][CH3COO]-10:1 | 10.33 | 174.05 | 9.98 | 174.64 | –0.35 | 0.64 |
| DMSO/[C3mim][CH3COO]-5:1 | 10.43 | 174.28 | 10.15 | 174.68 | –0.28 | 0.40 |
| DMSO/[C3mim][CH3COO]-2:1 | 10.48 | 174.64 | 10.31 | 174.92 | –0.17 | 0.28 |
| DMSO/[C3mim][CH3COO]-1:1 | 10.55 | 174.84 | 10.38 | 175.09 | –0.17 | 0.25 |
| DMSO/[C2mim][CH3COO]-10:1 | 10.27 | 174.04 | 9.94 | 174.67 | –0.33 | 0.63 |
| DMSO/[C2mim][CH3COO]-5:1 | 10.35 | 174.27 | 10.10 | 174.65 | –0.25 | 0.38 |
| DMSO/[C2mim][CH3COO]-2:1 | 10.42 | 174.62 | 10.26 | 174.90 | –0.16 | 0.28 |
| DMSO/[C2mim][CH3COO]-1:1 | 10.44 | 174.81 | 10.28 | 175.06 | –0.16 | 0.25 |
General Discussion
Rheological, ATR-FTIR, and NMR spectroscopy analyses clearly indicate that the dissolution of cellulose in DMSO/IL mixture solvents depends on the viscosity of these solvents and IL–DMSO and IL–cellulose interactions. Specifically, an increasing DMSO/IL ratio (from 1:1 to 10:1) means increased interactions between DMSO and ILs and reduced interactions between the cation and anion of ILs (Figures 2–44) and also results in a lower viscosity (Table 1). All these can facilitate interactions between the IL and cellulose (as shown by the increase in Δυ and Δδ in Tables 2 and 3, respectively), and thus, the dissolution of cellulose in DMSO/IL mixtures at ambient temperature. However, when the DMSO/IL molar ratio is high enough (2:1 DMSO/[C4mim][CH3COO], 2:1 DMSO/[C3mim][CH3COO], and 1:1 DMSO/[C2mim][CH2COO]), there is not enough IL to disrupt the hydrogen bonds in cellulose, resulting in lower cellulose solubility. On the other hand, a higher IL content in the mixture solvent increases the solvent viscosity and decreases the IL ion mobility, which can limit the penetration of IL into the cellulose structure for the disruption of hydrogen bonds. Therefore, a certain DMSO/IL ratio is required for the maximum dissolution of cellulose in DMSO/IL mixtures.
The ATR-FTIR and NMR spectroscopy results indicate that the interactions between ILs and cellulose strengthen with an increasing alkyl chain length of the IL cation (as shown by the increase in Δυ and Δδ in Tables 2 and 3, respectively). When the DMSO/IL molar ratio is high (10:1, 5:1, and 2:1), longer alkyl chains lead to stronger interactions between ILs and cellulose, which result in more effective disruption of the hydrogen bonds in cellulose and a higher solubility of cellulose. On the other hand, when the DMSO/IL ratio is low (DMSO/IL 1:1 (mol/mol) mixtures or pure ILs), DMSO/[C4mim][CH3COO] and DMSO/[C3mim][CH3COO] mixtures (with higher viscosity) are less effective at dissolving cellulose than the DMSO/[C2mim][CH3COO] mixture (with lower viscosity). This can be linked to the effect of the viscosity of different ILs on the viscosity of the mixture solvents, and the latter affects the ion mobility of ILs and their hydrogen-bond disruption capability.
Conclusions
Our results show that the room-temperature dissolution behavior of cellulose in DMSO/IL mixtures was affected by the DMSO/IL ratio and the alkyl chain length of the IL cation and, more importantly, there is an interplay between these two factors. Specifically, for DMSO/[C4mim][CH3COO] and DMSO/[C3mim][CH3COO] mixtures, the maximum cellulose solubility occurred at a DMSO/IL molar ratio of 2:1, while for the DMSO/[C2mim][CH3COO] mixture, it was shown at 1:1 (mol/mol). Cellulose dissolution can be facilitated by a longer cation alkyl chain of the IL cation at high DMSO/IL molar ratios (10:1, 5:1, and 2:1) but impeded at a low DMSO/IL molar ratio (1:1). Besides, it is demonstrated in this work that the DMSO/IL ratio and the alkyl chain length of the IL cation determine the viscosity and hydrogen-bonding capacity of DMSO/IL mixtures, which is the major determinant for the dissolution of cellulose in these solvents. At high DMSO/IL molar ratios (10:1, 5:1, and 2:1), the interactions between IL and DMSO and between IL and cellulose are key to determining cellulose solubility, whereas at a low DMSO/IL ratio (1:1), the viscosity of the DMSO/IL mixtures was the predominant factor governing cellulose dissolution. The findings from this work not only enrich our knowledge of the dissolution mechanisms of cellulose in DMSO/IL mixtures but also benefit the design of cost-effective solvents for natural biopolymers, facilitating the development of high-value-added processes for cellulose such as cellulose derivatization, biomass conversion, and degradable material fabrication.
Materials and Methods
Materials
Microcrystalline cellulose powder (MCC) with a viscosity-average degree of polymerization (DP) of 270 (purity ≥95%, water content <5%) and DMSO (99.5% pure, CAS 67-68-5) were purchased from Sigma Chemical Co. (St. Louis, MO). Three ILs used in this work were 1-butyl-3-methylimidazolium acetate ([C4mim][CH3COO], CAS 284049-75-8), 1-propyl-3-methylimidazolium acetate ([C3mim][CH3COO]), and 1-ethyl-3-methylimidazolium acetate ([C2mim][CH3COO], CAS 143314-17-4) (Figure 5), which were supplied by Nuowei Chemistry Co., Ltd. (Wuhu, Anhui, China). All of these ILs were ≥95% pure (water content <0.5%) and used without further purification. The deuterated DMSO (DMSO-d6, CAS 2206-27-1) (>99.0% pure) used for NMR samples was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
Figure 5.
Structures of the ionic liquids used.
Dissolution of Cellulose
The DMSO/IL mixtures with different DMSO/IL molar ratios (10:1, 5:1, 2:1, and 1:1) were prepared at ambient temperature. In a typical dissolution experiment, 0.02 g of MCC was dispersed in 2.0 g of the DMSO/IL mixture, and the resulting DMSO/IL-MCC mixture was stirred magnetically at 25 ± 0.5 °C.26 More MCC (0.02 or 0.01 g each time) was added and the solution was continuously stirred. If the added cellulose could not be dissolved within another 2 h, that is, the solution became unclear under a polarized light microscope (DM-4000M-LED, Leica, Germany), the solution was considered to be saturated with cellulose. The cellulose solubility is calculated from eq 1
| 1 |
where Mcellulose is the total mass of the dissolved cellulose and Msolvent is the initial mass of the solvent. In the following text, abbreviations “DMSO/IL-n:m-MCC” are used, where “n:m” indicates the molar ratio of DMSO/IL.
Rheology
The rheological properties of DMSO/IL mixtures were analyzed according to the previous method described elsewhere.36 An Anton Paar MCR302 rheometer (Anton Paar, GmbH., Austria) with a Peltier temperature-control system and a cone-plate geometry (4° angle and 40 mm diameter) was used to record the steady-state shear viscosity of 0.7 mL of samples over a shear rate range of 10–500 s–1 at 25 °C. To prevent the absorption and evaporation of water vapor, a small amount of silicone oil was placed at the edge of the measuring cell.
Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
The DMSO/IL-MCC mixture with an MCC concentration of 5.0 wt % was prepared by dissolving MCC in the respective DMSO/IL mixture at 25 °C for about 2 h until complete dissolution of cellulose. ATR-FTIR spectra for DMSO/IL and DMSO/IL-MCC mixtures were acquired using a Thermo Scientific Nicolet IS50 spectrometer (Thermo Fisher Scientific) with a diamond crystal at ambient temperature. The samples were scanned between 4000 and 400 cm–1 at a resolution of 4 cm–1 against the air as the background.37
Nuclear Magnetic Resonance (NMR) Spectroscopy
Mixtures of DMSO-d6/IL at different molar ratios (10:1, 5:1, 2:1, and 1:1) were prepared. DMSO-d6/IL-MCC mixtures with an MCC concentration of 5.0 wt % were obtained by dissolving MCC in respective DMSO-d6/IL mixtures at 25 °C for about 2 h until complete dissolution of cellulose. 1H NMR and 13C NMR spectra for DMSO-d6/IL mixtures and DMSO-d6/IL-MCC mixtures were acquired using a DMX 300 NMR spectrometer (300 MHz) (Bruker) at ambient temperature.16
Statistical Analysis
Rheological analysis was performed at least in triplicates for each sample and the results are reported as mean ± standard deviation.35 For ATR-FTIR and NMR, only one measurement was performed. One-way analysis of variance (ANOVA) followed by post hoc Duncan’s multiple range tests (p < 0.05) was conducted to determine the significant differences between mean values using SPSS 17.0 statistical software (SPSS Inc. Chicago, IL).
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (32030084 and 31871796) and the Natural Science Foundation of Tianjin Municipal Science and Technology Commission (20ZYJDJC00040 and 17JCJQJC45600).
Author Contributions
S.W. and F.X. conceived and designed the study. F.R. conducted the experiments and data analysis. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Verma C.; Mishra A.; Chauhan S.; Verma P.; Srivastava V.; Quraishi M. A.; Ebenso E. E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustainable Chem. Pharm. 2019, 13, 100162 10.1016/j.scp.2019.100162. [DOI] [Google Scholar]
- Mahmood H.; Moniruzzaman M.; Yusup S.; Welton T. Ionic liquids assisted processing of renewable resources for the fabrication of biodegradable composite materials. Green Chem. 2017, 19, 2051–2075. 10.1039/C7GC00318H. [DOI] [Google Scholar]
- Wang H.; Gurau G.; Rogers R. D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. 10.1039/c2cs15311d. [DOI] [PubMed] [Google Scholar]
- Kasprzak D.; Krystkowiak E.; Stępniak I.; Gali M. Dissolution of cellulose in novel carboxylate-based ionic liquids and dimethyl sulfoxide mixed solvents. Eur. Polym. J. 2019, 113, 89–97. 10.1016/j.eurpolymj.2019.01.053. [DOI] [Google Scholar]
- Li Y.; Wang J.; Liu X.; Zhang S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. 10.1039/C7SC05392D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A.; Wang H.; et al. Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem. 2010, 12, 268–275. 10.1039/B916882F. [DOI] [Google Scholar]
- Ren F.; Wang J.; Xie F.; Zan K.; Wang S.; Wang S. Applications of ionic liquids in starch chemistry: A review. Green Chem. 2020, 22, 2162–2183. 10.1039/C9GC03738A. [DOI] [Google Scholar]
- Pinkert A.; Marsh K. N.; Pang S.; Staiger M. P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. 10.1021/cr9001947. [DOI] [PubMed] [Google Scholar]
- Mäki-Arvela P.; Anugwom I.; Virtanen P.; Sjöholm R.; Mikkola J. P. Dissolution of lignocellulosic materials and its constituents using ionic liquids-a review. Ind. Crops Prod. 2010, 32, 175–201. 10.1016/j.indcrop.2010.04.005. [DOI] [Google Scholar]
- Uto T.; Yamamoto K.; Kadokawa J. Cellulose crystal dissolution in imidazolium-based ionic liquids: A theoretical study. J. Phys. Chem. B 2018, 122, 258–266. 10.1021/acs.jpcb.7b09525. [DOI] [PubMed] [Google Scholar]
- Papović S.; Bešter-Rogač M.; Vraneš M.; Gadžurić S. The effect of the alkyl chain length on physicochemical features of (ionic liquids+γ-butyrolactone) binary mixtures. J. Chem. Thermodyn. 2016, 99, 1–10. 10.1016/j.jct.2016.03.034. [DOI] [Google Scholar]
- Rocha M. A. A.; Neves C. M. S. S.; Freire M. G.; Russina O.; Triolo A.; Coutinho J. A. P.; Santos L. M. N. B. F. Alkylimidazolium based ionic liquids: Impact of cation symmetry on their nanoscale structural organization. J. Phys. Chem. B 2013, 117, 10889–10897. 10.1021/jp406374a. [DOI] [PubMed] [Google Scholar]
- Papović S.; Gadžurić S.; Bešter-Rogač M.; Jović B.; Vraneš M. A Systematic study on physicochemical and transport properties of imidazolium-based ionic liquids with γ-butyrolactone. J. Chem. Thermodyn. 2018, 116, 330–340. 10.1016/j.jct.2017.10.004. [DOI] [Google Scholar]
- Salama A.; Hesemann P. Recent trends in elaboration, processing, and derivatization of cellulosic materials using ionic liquids. ACS Sustainable Chem. Eng. 2020, 8, 17893–17907. 10.1021/acssuschemeng.0c06913. [DOI] [Google Scholar]
- Wu J.; Jun Z.; Hao Z.; Jiasong H.; Qiang R.; Guo M. Homogeneus acetylation of cellulosa in a new ionic liquid. Biomacromolecules 2004, 5, 266–268. 10.1021/bm034398d. [DOI] [PubMed] [Google Scholar]
- Ferreira D. C.; Oliveira M. L.; Bioni T. A.; Nawaz H.; King A. W. T.; Kilpeläinen I.; Hummel M.; Sixta H.; El O. A. Binary mixtures of ionic liquids-DMSO as solvents for the dissolution and derivatization of cellulose: Effects of alkyl and alkoxy side chains. Carbohydr. Polym. 2019, 212, 206–214. 10.1016/j.carbpol.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Sáez F.; Ballesteros M.; Ballesteros I.; Manzanares P.; Oliva J. M.; Negro M. J. Enzymatic hydrolysis from carbohydrates of barley straw pretreated by ionic liquids. J. Chem. Technol. Biotechnol. 2013, 88, 937–941. 10.1002/jctb.3925. [DOI] [Google Scholar]
- Li L.; Zhang Y.; Sun Y.; Sun S.; Shen G.; Zhao P.; Cui J.; Qiao H.; Wang Y.; Zhou H. Manufacturing pure cellulose films by recycling ionic liquids as plasticizers. Green Chem. 2020, 22, 3835–3841. 10.1039/D0GC00046A. [DOI] [Google Scholar]
- Chong M. Y.; Liew C.-W.; Numan A.; Yugal K.; Ramesh K.; Ng H. M.; Chong T. V.; Ramesh S. Effects of ionic liquid on the hydroxylpropylmethyl cellulose (HPMC) solid polymer electrolyte. Ionics 2016, 22, 2421–2430. 10.1007/s11581-016-1768-0. [DOI] [Google Scholar]
- Chen J.; Xie F.; Li X.; Chen L. Ionic liquids for the preparation of biopolymer materials for drug/gene delivery: A review. Green Chem. 2018, 20, 4169–4200. 10.1039/C8GC01120F. [DOI] [Google Scholar]
- Fumino K.; Peppel T.; Geppert-Rybczyn M.; Zaitsau D. H.; Lehmann J. K.; Verevkin S. P.; Ludwig R.; Ko M. The influence of hydrogen bonding on the physical properties of ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 14064–14075. 10.1039/c1cp20732f. [DOI] [PubMed] [Google Scholar]
- Tomimatsu Y.; Suetsugu H.; Yoshimura Y.; Shimizu A. The solubility of cellulose in binary mixtures of ionic liquids and dimethyl sulfoxide: Influence of the anion. J. Mol. Liq. 2019, 279, 120–126. 10.1016/j.molliq.2019.01.093. [DOI] [Google Scholar]
- Rinaldi R. Instantaneous dissolution of cellulose in organic electrolyte solutions. Chem. Commun. 2011, 47, 511–513. 10.1039/C0CC02421J. [DOI] [PubMed] [Google Scholar]
- Xu A.; Wang F. Carboxylate ionic liquid solvent systems from 2006 to 2020: Thermal properties and application in cellulose processing. Green Chem. 2020, 22, 7622–7664. 10.1039/D0GC02840A. [DOI] [Google Scholar]
- Xu A.; Cao L.; Wang B. Facile cellulose dissolution without heating in [C4mim][CH3COO]/DMF solvent. Carbohydr. Polym. 2015, 125, 249–254. 10.1016/j.carbpol.2015.02.045. [DOI] [PubMed] [Google Scholar]
- Xu A.; Zhang Y.; Zhao Y.; Wang J. Cellulose dissolution at ambient temperature: role of preferential solvation of cations of ionic liquids by a cosolvent. Carbohydr. Polym. 2013, 92, 540–544. 10.1016/j.carbpol.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Xu A.; Guo X.; Xu R. Understanding the dissolution of cellulose in 1-butyl-3-methylimidazolium acetate+DMAc solvent. Int. J. Biol. Macromol. 2015, 81, 1000–1004. 10.1016/j.ijbiomac.2015.09.058. [DOI] [PubMed] [Google Scholar]
- Tomimatsu Y.; Yoshimura Y.; Shimizu A. Solubility of cellulose in binary mixtures of 1-alkyl-3-methylimidazolium acetate and dimethyl sulfoxide: Influence of alkyl chain length in the cation. Aust. J. Chem. 2019, 72, 669–673. 10.1071/CH19047. [DOI] [Google Scholar]
- Aryafard M.; Jahanshahi M.; Harifi-Mood A. R.; Minofar B.; Smatanova I. K. Experimental and theoretical studies of preferential solvation of 4-nitroaniline and 4-nitroanisole in an amino acid ionic liquid with molecular solvents. J. Chem. Eng. Data 2019, 64, 5755–5764. 10.1021/acs.jced.9b00719. [DOI] [Google Scholar]
- Zhao Y.; Liu X.; Wang J.; Zhang S. Insight into the cosolvent effect of cellulose dissolution in imidazolium-based ionic liquid systems. J. Phys. Chem. B 2013, 117, 9042–9049. 10.1021/jp4038039. [DOI] [PubMed] [Google Scholar]
- Huo F.; Liu Z.; Wang W. Cosolvent or antisolvent? A molecular view of the interface between ionic liquids and cellulose upon addition of another molecular solvent. J. Phys. Chem. B 2013, 117, 11780–11792. 10.1021/jp407480b. [DOI] [PubMed] [Google Scholar]
- Xu A.; Zhang Y. Insight into dissolution mechanism of cellulose in [C4mim][CH3COO]/DMSO solvent by 13C NMR Spectra. J. Mol. Struct. 2015, 1088, 101–104. 10.1016/j.molstruc.2015.02.031. [DOI] [Google Scholar]
- Radhi A.; Le K. A.; Ries M. E.; Budtova T. Macroscopic and microscopic study of 1-ethyl-3-methyl-imidazolium acetate-DMSO mixtures. J. Phys. Chem. B 2015, 119, 1633–1640. 10.1021/jp5112108. [DOI] [PubMed] [Google Scholar]
- Lu B.; Xu A.; Wang J. Cation does matter: How cationic structure affects the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 1326–1335. 10.1039/C3GC41733F. [DOI] [Google Scholar]
- Ren F.; Xie F.; Luan H.; Wang S.; Wang S. Phase transition of maize starch in aqueous ionic liquids: Effects of water:ionic liquid ratio and cation alkyl chain length. Ind. Crops Prod. 2020, 144, 112043 10.1016/j.indcrop.2019.112043. [DOI] [Google Scholar]
- Ren F.; Wang J.; Yu J.; Xiang F.; Wang S.; Wang S.; Copeland L. Dissolution of maize starch in aqueous ionic liquid: The role of alkyl chain length of cation and ratio of water:ionic liquid. ACS Sustainable Chem. Eng. 2019, 7, 6898–6905. 10.1021/acssuschemeng.8b06432. [DOI] [Google Scholar]
- Chen Y.; Cao Y.; Sun X.; Mu T. Hydrogen bonding interaction between acetate-based ionic liquid 1-ethyl-3-methylimidazolium acetate and common solvents. J. Mol. Liq. 2014, 190, 151–158. 10.1016/j.molliq.2013.11.010. [DOI] [Google Scholar]
- Jiang J.-C.; Lin K.-H.; Li S.-C.; Shih P.-M.; Hung K.-C.; Lin S. H.; Chang H.-C. Association structures of ionic liquid/DMSO mixtures studied by high-pressure infrared spectroscopy. J. Chem. Phys. 2011, 134, 044506 10.1063/1.3526485. [DOI] [PubMed] [Google Scholar]
- Remsing R. C.; Liu Z.; Sergeyev I.; Moyna G. Solvation and aggregation of N,N′-dialkylimidazolium ionic liquids: A multinuclear NMR spectroscopy and molecular dynamics simulation study. J. Phys. Chem. B 2008, 112, 7363–7369. 10.1021/jp800769u. [DOI] [PubMed] [Google Scholar]