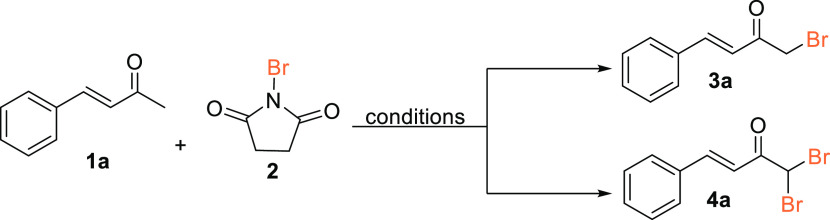
Abstract
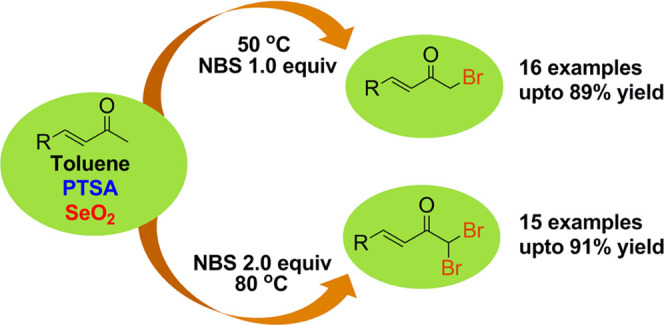
An efficient method for the synthesis of α,β-unsaturated α′-bromoketones and α,β-unsaturated α′,α′-dibromoketones is described using N-bromosuccinimide (NBS) as the brominating agent mediated by selenium dioxide (SeO2) in the presence of p-toluenesulfonic acid (PTSA) monohydrate in toluene. The method is simple, employing easily available shelf reagents to afford a wide range of products in good yields. The method highlighted that simple fine-tuning of the reaction conditions and molar equivalents of the reactants easily affords either mono- or dibrominated products in excellent yields. A number of these products have not been reported in the literature. All of the reactions were carried out in gram-scale quantities.
Introduction
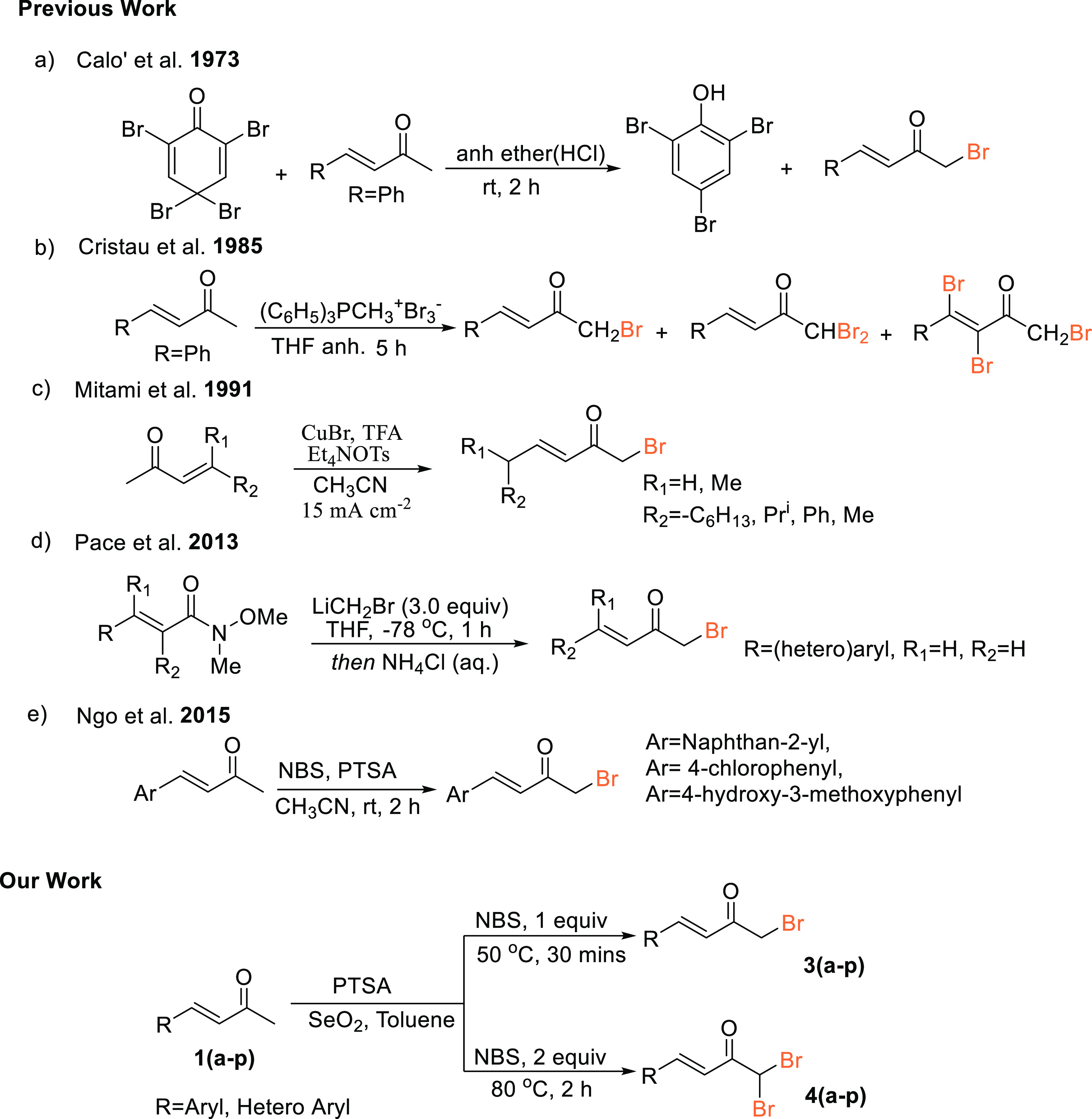
α-Haloketones are important intermediates in organic synthesis because of their high reactivity shown toward nucleophiles by both the electrophilic methylene and the carbonyl carbon atoms.1 They are key building blocks, valuable for the synthesis of a wide variety of biologically active heterocyclic compounds,2,3 natural products,1a pesticides, diagnostic aids, and surfactants.1a,2c,4 They serve as an important precursor for various transformations utilized in pharmaceutical synthesis.5 In particular, α-brominated carbonyl compounds are one of the most sought-after intermediates for further transformations both in industrial chemistry as well as in organic synthesis due to the wide range of utility of this bifunctional moiety.2c,6,7 Synthesis of α-bromoketones has been accomplished over the past decades by direct bromination of ketones8a−8d and using various available brominating agents.8e−8k α-Haloketones have also been achieved by hydration of haloalkynes employing expensive catalysts.9 However, literature survey revealed that not many procedures are available for the synthesis of α,β-unsaturated α′-bromoketones and α,β-unsaturated α′,α′-dibromoketones despite the fact that these moieties are commonly employed as an important intermediate in the synthesis of various pharmaceutical analogues.7 The few known synthetic routes of α,β-unsaturated α′-bromoketones and α,β-unsaturated α′,α′-dibromoketones are shown in Scheme 1. Earlier, Calo’ and co-workers reported the synthesis of α,β-unsaturated α′-bromoketones using 2,4,4,6-tetrabromocyclohexa-2,5-dienone (Scheme 1a),10 while Cristau et al. reported that a mixture of α,β-unsaturated α′-bromoketones and α,β-unsaturated α′,α′-dibromoketones were obtained when α,β-unsaturated ketones were treated with tribromo triphenylmethylphosphonium (Scheme 1b).11 In 1991, Mitami et al. reported a regioselective α′-bromination of α,β-unsaturated ketones by an electrochemical procedure (Scheme 1c).12 Recently, Pace et al. reported the synthesis of some α,β-unsaturated α′-haloketones through the chemoselective addition of halomethylithiums to Weinreb amides (Scheme 1d).13 Ngo and co-workers in their paper on the synthesis and antiproliferative activity study of new vinca alkaloids containing α,β-unsaturated aromatic side chains have reported the selective monobromination of the α′-methylketone of three α,β-unsaturated aryl ketones using N-bromosuccinimide (NBS) in the presence of PTSA (Scheme 1e).7 However, when we repeated the experiments under the same reaction conditions as described in the paper, except for naphtha-2-yl analogue, the other substituted benzylideneacetones yielded mixtures of mono- and disubstituted bromoketones. Unfortunately, these reported methodologies suffer from a series of drawbacks ranging from chemo- and regioselective issues to low proficiency and lack of generality.
Scheme 1. Synthesis of α,β-Unsaturated α′-Bromoketones and α,β-Unsaturated α′,α′-Dibromoketones.
Recently, we have shown the effective utilization of SeO2 as a proficient inorganic reagent, which, in the presence of a Lewis acid, facilitated the reaction between aromatic ketones and aromatic hydrocarbons to influence C–C coupling at the α-carbon atom;14a however, the same reaction catalyzed by an organic acid PTSA yielded unsymmetrical benzils.14b Also, aryl methyl ketones reacted efficiently with secondary amines in dimethyl sulfoxide (DMSO) in the presence of SeO2 to affect α-selenoamidation of aryl methyl ketones.14c Thus, in continuation of our study on the synthetic utility of SeO2 in organic synthesis, we wish to report herein an efficient protocol for the synthesis of α,β-unsaturated α′-bromoketones and α,β-unsaturated α′,α′-dibromoketones from α,β-unsaturated ketones with NBS in the presence of PTSA mediated by SeO2. Bromination of variously functionalized α,β-unsaturated α-ketones is highly emphasized here. We are employing NBS as the brominating agent, which has been widely used as an efficient and successful brominating agent15 in organic synthesis as it is user-friendly, easy to handle being solid at room temperature, inexpensive, and easily available.16
Results and Discussion
We began our investigation using benzylidene acetone (1a) and NBS (2) as the model substrates to determine the optimal reaction conditions (Table 1). Initially, a mixture of benzylidene acetone (1a, 1 equiv), NBS (2, 1 equiv), and SeO2 (0.5 equiv) was stirred with PTSA (0.5 equiv) in toluene (3 mL) at room temperature for 3 h when thin-layer chromatography (TLC) showed the selective formation of the monobrominated product 3a in 50% yield along with the unreacted starting ketone 1a (Table 1, entry 1). When the reaction was allowed to stir for a longer time (6 h), it was observed that 65% of the desired product 3a and a trace amount of the dibrominated product 4a were obtained (Table 1, entry 2). On further allowing the reaction to stir for a longer time (12 h), the starting material was completely consumed, affording 10% of compound 4a, while product 3a increased to 80% yields (Table 1, entry 3). When the reaction temperature of the reaction mixture was increased to 50 °C, surprisingly, we were able to obtain up to 84% of product 3a and only less than 10% of product 4a in just 30 min (Table 1, entry 4). On allowing the reaction to stir for a longer time (3 h), it was observed that 70% of the desired product 3a and 20% of the dibrominated product 4a was obtained (Table 1, entry 5). In the absence of selenium dioxide, when the reaction was heated at 50 °C for 30 min, 50% of product 3a and a trace amount of product 4a were obtained (Table 1, entry 6). In the absence of an acid, the formation of the desired product does not take place (Table 1, entry 7). Attempts to improve the efficiency of the reaction using different acids and solvents provided only mixtures of the two products in varying yields (Table 1, entries 8–11, 12–16). When the amount of PTSA was increased to 1 equiv, there was a marked decrease in the yield of product 3a (70%) with a corresponding increase of product 4a (25%; Table 1, entry 17). Interestingly, increasing the amount of NBS to 2 equiv showed a steady decrease in the formation of product 3a with increased reaction time and a corresponding increase in the formation of product 4a (Table 1, entries 18, 19). When the reaction was allowed to stir for 6 h, only product 4a was observed and was isolated in 83% yield (Table 1, entry 20). Increasing the amount of PTSA to 1 equiv increases the yield of product 4a to 70% with a corresponding decrease in the yield of product 3a to 20% (Table 1, entry 21). Similarly, increasing the amount of SeO2 to 1 equiv resulted in a marked decrease in the yield of product 3a (15%) with a corresponding increase of product 4a (78%; Table 1, entry 22). When the amount of SeO2 and PTSA was increased to 1 equiv, each resulted in 80% yield of product 4a and 10% of product 3a (Table 1, entry 23). Finally, when the reaction amount of SeO2 and PTSA was 1 equiv each and the temperature was increased to 80 °C, only product 4a (87%) was obtained, where the reaction time was appreciatively reduced to 2 h (Table 1, entry 24). In the absence of selenium dioxide under the same reaction condition, 25% of product 3a and 45% of product 4a were obtained (Table 1, entry 25). It was noted that there was no improvement in the yield of the products when the amount of SeO2 and PTSA was further increased, nor when the temperature was increased above 80 °C, which at 100 °C, causes the product to decompose (Table 1, entries 26–27).
Table 1. Optimization of the Reaction Conditionsa.
| yield
(%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| entry | NBS (2) (equiv) | SeO2 (equiv) | acid (equiv) | solvent | temp (°C) | time | 3a | 4a |
| 1 | 1 | 0.5 | PTSA (0.5) | toluene | rt | 3 h | 50 | |
| 2 | 1 | 0.5 | PTSA (0.5) | toluene | rt | 6 h | 65 | trace |
| 3 | 1 | 0.5 | PTSA (0.5) | toluene | rt | 12 h | 80 | 10 |
| 4 | 1 | 0.5 | PTSA (0.5) | toluene | 50 | 30 min | 84 | 5 |
| 5 | 1 | 0.5 | PTSA (0.5) | toluene | 50 | 3 h | 70 | 20 |
| 6 | 1 | PTSA (0.5) | toluene | 50 | 30 min | 50 | trace | |
| 7 | 1 | 0.5 | toluene | 50 | 30 min | |||
| 8 | 1 | 0.5 | BF3Et2O (0.5) | toluene | 50 | 30 min | 50 | 30 |
| 9 | 1 | 0.5 | TFA (0.5) | toluene | 50 | 30 min | 65 | 25 |
| 10 | 1 | 0.5 | AcOH (0.5) | toluene | 50 | 30 min | 30 | trace |
| 11 | 1 | 0.5 | AlCl3 (0.5) | toluene | 50 | 30 min | ||
| 12 | 1 | 0.5 | PTSA (0.5) | CH3CN | 50 | 30 min | 75 | 5 |
| 13 | 1 | 0.5 | PTSA (0.5) | DMSO | 50 | 30 min | 55 | 20 |
| 14 | 1 | 0.5 | PTSA (0.5) | H2O | 50 | 30 min | ||
| 15 | 1 | 0.5 | PTSA (0.5) | DCM | 50 | 30 min | 75 | 5 |
| 16 | 1 | 0.5 | PTSA (0.5) | THF | 50 | 30 min | 70 | 5 |
| 17 | 1 | 0.5 | PTSA(1) | toluene | 50 | 30 min | 70 | 25 |
| 18 | 2 | 0.5 | PTSA (0.5) | toluene | 50 | 30 min | 50 | 40 |
| 19 | 2 | 0.5 | PTSA (0.5) | toluene | 50 | 2 h | 25 | 60 |
| 20 | 2 | 0.5 | PTSA (0.5) | toluene | 50 | 6 h | 83 | |
| 21 | 2 | 0.5 | PTSA (1) | toluene | 50 | 2 h | 20 | 70 |
| 22 | 2 | 1 | PTSA (0.5) | toluene | 50 | 2 h | 15 | 78 |
| 23 | 2 | 1 | PTSA (1) | toluene | 50 | 2 h | 10 | 80 |
| 24 | 2 | 1 | PTSA (1) | toluene | 80 | 2 h | 87 | |
| 25 | 2 | PTSA (1) | toluene | 80 | 2 h | 25 | 45 | |
| 26 | 2 | 1 | PTSA (1) | toluene | 90 | 2 h | 87 | |
| 27 | 2 | 1 | PTSA (1) | toluene | 100 | 2 h | 5 | 75 |
Reaction conditions: α,β-unsaturated ketones (1a) (1.0 mmol, 1.0 equiv), solvent (3 mL).
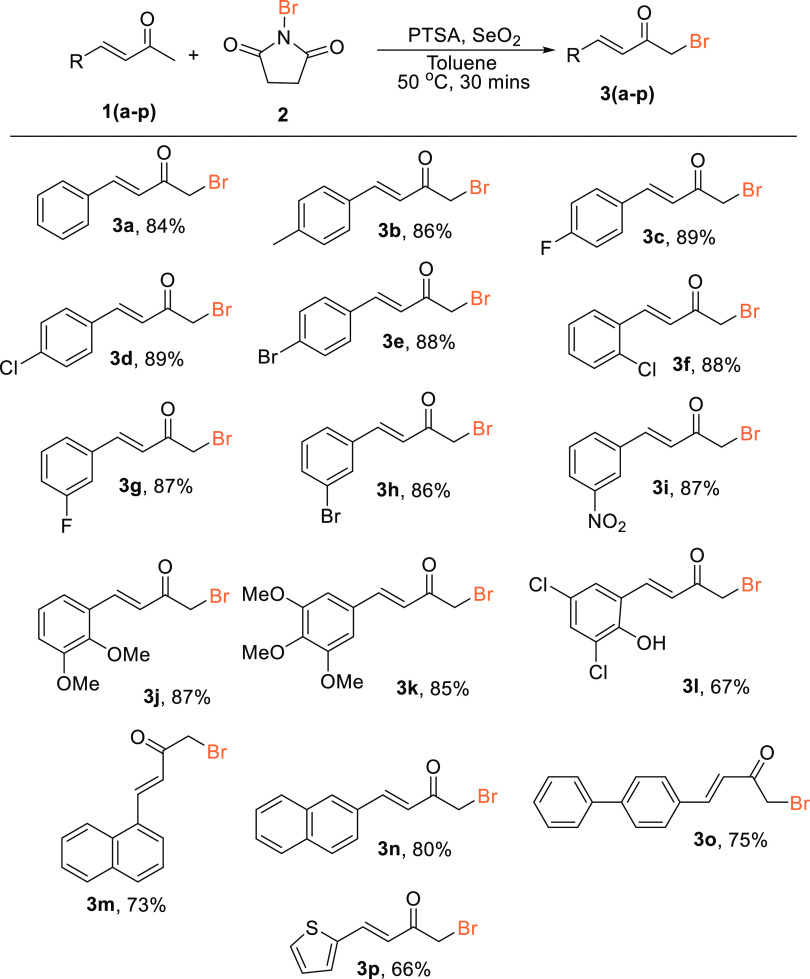
Having optimized the conditions for the monobromination of the α,β-unsaturated ketone (Table 1, entry 3), we proceeded to investigate the scope of the reactions to establish the generality of the methodology for the synthesis of α,β-unsaturated α′-bromoketones (Scheme 2). Various substituted α,β-unsaturated ketones, viz, 4-methylbenzylideneacetone (1b), 4-fluorobenzylideneacetone (1c), 4-chlorobenzylideneacetone (1d), 4-bromobenzylideneacetone (1e), and 2-chlorobenzylideneacetone (1f), readily reacted with NBS (2) in the presence of SeO2 and PTSA in toluene, affording the corresponding products 3b, 3c, 3d, 3e, and 3f in excellent yields (86, 89, 89, 88, and 88%, respectively). Other α,β-unsaturated ketones bearing electron-withdrawing groups substituted at the meta position, viz, 3-fluorobenzylideneacetone (1g), 3-bromorobenzylideneacetone (1h), and 3-nitrobenzylideneacetone (1i), similarly afforded the corresponding desired products 3g, 3h, and 3i in excellent yields (87, 86, and 87%, respectively). The scope of the reaction was further extended to di- and trisubstituted benzylideneacetones having an electron-donating group (−OMe) as the substituent. Thus, 2,3-dimethoxybenzylideneacetone (1j) and 3,4,5-trimethoxybenzylideneacetone (1k) were easily converted to the corresponding products 3j (87%) and 3k (85%) in satisfactory yields. It is worth mentioning that the reaction also proceeded smoothly with 3,5-dichloro(2-hydroxyphenyl)but-3-en-2-one (1l) to afford the desired product 3l in 67%. The generality of the method was further strengthened when α,β-unsaturated naphthylidene acetones (1m, 1n) reacted effortlessly to afford 3m and 3n in 73 and 80% yields, respectively. The reactions were also performed with α,β-unsaturated ketones containing biphenyl moiety (1o) and thiophene moiety (1p), which, to our delight readily afforded the desired products 3o and 3p in satisfactory yields (75 and 66%, respectively). Compound 3i was obtained as crystal, and the structure was confirmed by X-ray analysis (included in the Supporting Information).
Scheme 2. Substrate Scope of α,β-Unsaturated Ketones,
Reaction conditions: α,β-unsaturated ketones (1a) (6.84 mmol, 1.0 equiv), NBS (2) (6.84 mmol, 1.0 equiv), SeO2 (3.42 mmol, 0.5 equiv), PTSA (3.42 mmol, 0.5 equiv) toluene (3.0 mL), at 50 °C for 30 min.
Isolated yields after column chromatography.
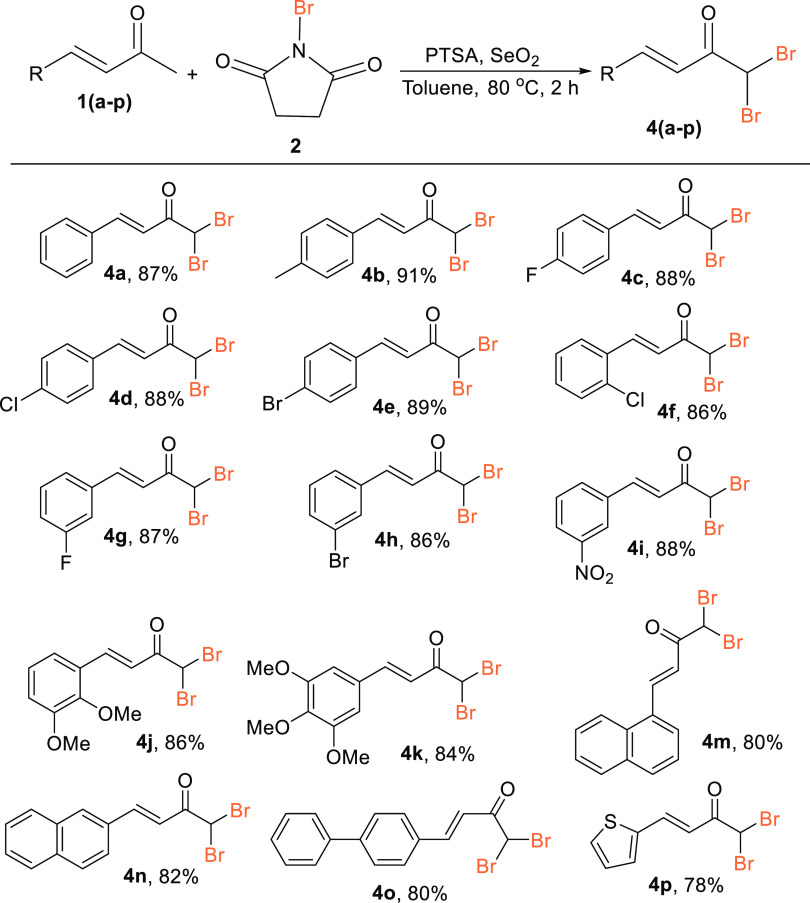
Second, with the conditions optimized for the synthesis of α,β-unsaturated ketone by dibromination (Table 1, entry 9), we next examined the scope of the reaction to establish the generality of the method for the synthesis of α,β-unsaturated α′,α′-dibromoketones (Scheme 3). Differently substituted α,β-unsaturated ketones, such as 4-methylbenzylideneacetone (1b), 4-fluorobenzylideneacetone (1c), 4-chlorobenzylideneacetone (1d), 4-bromobenzylideneacetone (1e), and 2-chlorobenzylideneacetone (1f), were selected and allowed to react as per the standardized procedure. All of the reactions afforded the corresponding dibrominated products 4b, 4c, 4d, 4e, and 4f in excellent yields (91, 88, 88, 89, and 86%, respectively). Here, the substrates exhibited a similar tolerance to the electron-withdrawing group and the electron-donating group. The substituted α,β-unsaturated ketones, at the meta position bearing electron-withdrawing substituents, viz, 3-fluorobenzylideneacetone (1g), 3-bromorobenzylideneacetone (1h), and 3-nitrobenzylideneacetone (1i), were successfully converted to the corresponding desired products 4g (87%), 4h (86%), and 4i (88%). The reaction worked equally well with the di- and trisubstituted benzylideneacetones having an electron-donating group (-OMe), viz, 2,3-dimethoxybenzylideneacetone (1j) and 3,4,5-trimethoxybenzylideneacetone (1k), to afford the corresponding products 4j and 4k in 89% and 84% overall yields, respectively. It may be noted that (3,5-dichloro 2-hydroxyphenyl)but-3-en-2-one did not react satisfactorily since only trace quantities of the required product were formed. The presence of the 2-OH group on the phenyl ring evidently hindered the attack of the next bromine atom on the α-carbon. The protocol was also found to be compatible with the extended aromatic ring system, such as α,β-unsaturated naphthylidene acetones (1m, 1n), which afforded products 4m and 4n in 85 and 88% overall yields, respectively. To further strengthen the generality of the method, reactions were also performed with α,β-unsaturated ketones containing the biphenyl moiety (1o) and thiophene moiety (1p), which furnished the desired products 4o and 4p in 83 and 81% overall yields. The structure of compound 4f was confirmed by X-ray analysis (included in the Supporting Information).
Scheme 3. Substrate Scope of α,β-Unsaturated Ketones,b.
Isolated yields after column chromatography.
Reaction conditions: α,β-unsaturated ketones (1a) (6.84 mmol, 1.0 equiv), NBS (2) (13.68 mmol, 2.0 equiv), SeO2 (6.84 mmol, 1.0 equiv), PTSA (6.84 mmol, 1.0 equiv), toluene (3.0 mL), at 80 °C for 2 h.
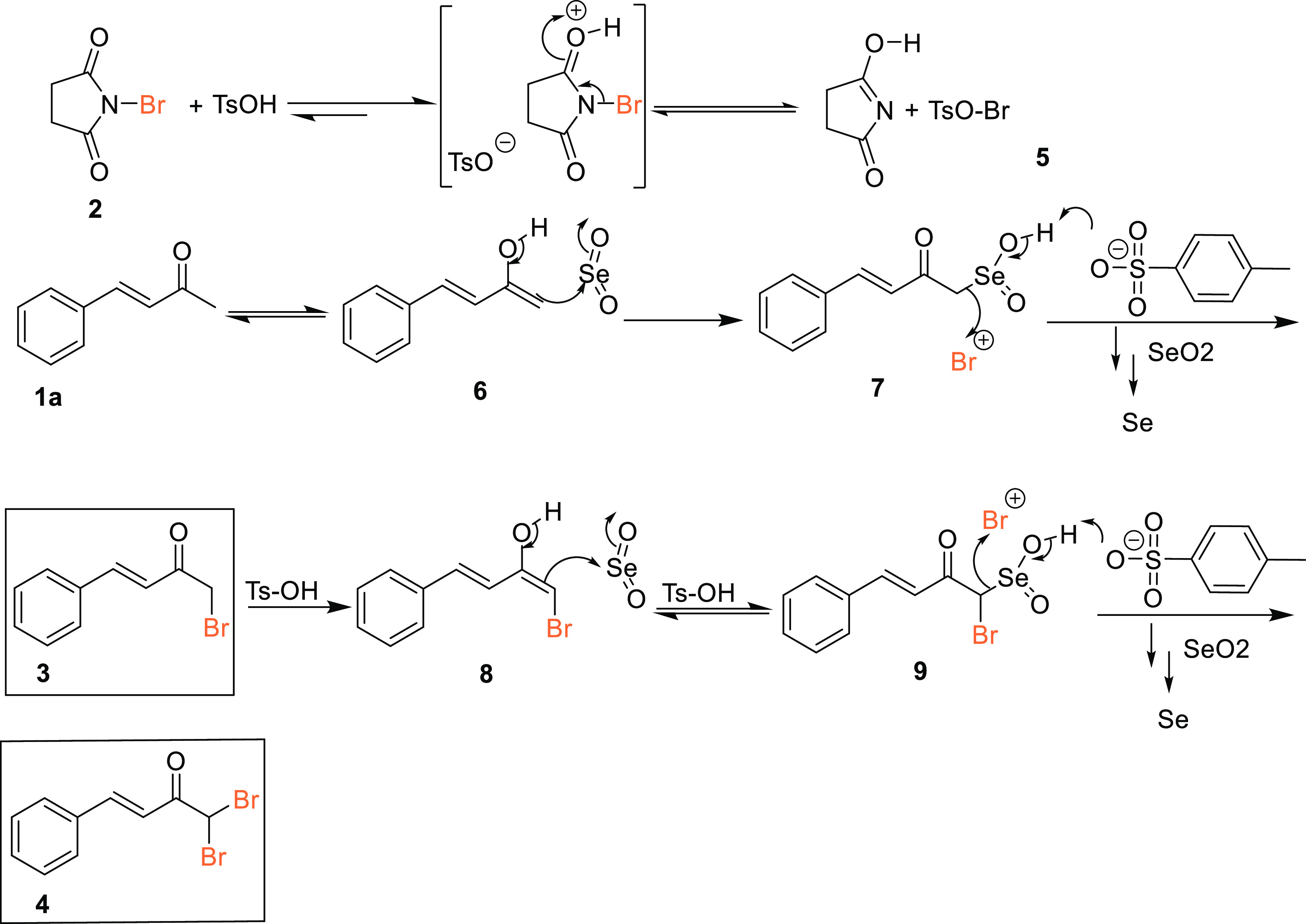
The plausible mechanism involved in the reaction of the α,β-unsaturated ketone 1 with NBS (2) in the presence of SeO2 and PTSA is depicted in Scheme 4. The initial reaction of the enol form 6 with SeO2 resulted in the formation of the organoselenium intermediate 7, which evidently underwent a PTSA-assisted electrophilic attack to the bromonium ion 5 generated from NBS17 to afford the α,β-unsaturated α′-bromoketone 3. In a similar fashion, in the presence of excess NBS, the enolate 8 presumably underwent a second electrophilic attack with the bromonium ion 5 to afford the α′,α′-dibromoketone 4. Although the role of SeO2 appears to be that of a catalyst, a little excess of it is always required since it is highly susceptible to reduction to elemental selenium as observed by red deposits at the end of each reaction.
Scheme 4. Plausible Mechanism.
Conclusions
In summary, this work reports an efficient protocol for the direct and quick access to α,β-unsaturated α′-bromoketones and α′,α′-dibromoketones from easily available α,β-unsaturated ketones. The method is general and allows a broad range of substrates with good product yields. It may be noted that most of the α′,α′-dibromoketones synthesized have not been reported so far except for compound 4a.11 The advantage of this method lies in the fact that the bromination methods have been improved and enhanced through the mediation of selenium dioxide.
Experimental Section
General Information
The arylidene methyl ketones were all prepared in the laboratory as per the standard procedure.18 Other reagents were purchased directly from commercial suppliers and used as such without any further purification unless otherwise mentioned. The reactions were monitored by thin-layer chromatography (TLC) using precoated aluminum sheets (silica gel 60 F254 0.2 mm thickness). The spots were visualized under UV (λ = 254 nm). Purification of the products was done by flash chromatography using silica gel (230–400 mesh). Characterization of compounds was done for 1H and 13C NMR spectra, which were recorded on a Bruker Avance II-400 spectrometer in CDCl3 using tetramethylsilane (TMS) as an internal standard; chemical shifts were reported in delta (δ) units, in parts per million (ppm), and coupling constants (J) in hertz (Hz). IR spectra were recorded on a PerkinElmer Spectrum 400 Fourier transform infrared (FTIR) instrument, and the frequencies are expressed in cm–1. Mass spectral data were obtained with a Waters ultraperformance liquid chromatography-TQD (UPLC-TQD) equipped with an electrospray ionization-mass spectrometer (ESI-MS). High-resolution mass spectra (ESI-HRMS) data were obtained using the accurate-mass Q-TOF LC/ME system (Agilent Technologies, Santa Clara, CS) equipped with an Agilent 1290 UPLC system and an ESI source. Melting points were recorded by the open capillary tube method and were uncorrected.
General Procedure A for the Synthesis of 1-Bromo-4-arylbut-3-en-2-one (3)
To a stirring mixture of α,β-unsaturated ketones (1) (6.84 mmol, 1 equiv), NBS (2) (6.84 mmol, 1 equiv), and SeO2 (3.42 mmol, 0.5 equiv) in toluene (3.0 mL) at 50 °C, PTSA (3.42 mmol, 0.5 equiv) was added portionwise for a period of 1 min. The reaction was allowed to stir for 30 min in an oil bath. On completion of the reaction, which was monitored by TLC, the reaction mixture was allowed to cool down to room temperature. The precipitate elemental selenium settled at the bottom of the flask, which was then filtered off and washed with ethyl acetate (2 × 10 mL), and the combined filtrate was transferred to a separating funnel and washed with conc. sodium bicarbonate solution (2 × 10 mL), followed by a wash with brine (2 × 10 mL). The organic layer was separated followed by the removal of water residual by filtering the organic layer over anhydrous Na2SO4, and the organic layer was concentrated in vacuum. The crude mass was purified by flash chromatography on silica gel (230–400 mesh) and using ethyl acetate/hexane as the eluent to obtain the corresponding products 3a–3p.
General Procedure B for the Synthesis of 1,1-Dibromo-4-arylbut-3-en-2-one (4)
To a stirring mixture of α,β-unsaturated ketones (1) (6.84 mmol, 1 equiv), NBS (2) (13.69 mmol, 2 equiv), and SeO2 (6.84 mmol, 1 equiv) in toluene (3.0 mL) at 80 °C, PTSA (6.84 mmol, 1 equiv) was added little by little for a short period of time. The reaction was allowed to stir for 2 h in an oil bath. On completion of the reaction, which was monitored by TLC, the precipitate elemental selenium settled at the bottom of the flask, which was then filtered off and washed with ethyl acetate (2 × 10 mL), and the combined filtrate was transferred to a separating funnel and washed with conc. sodium bicarbonate solution (2 × 10 mL), followed by a wash with brine (2 × 10 mL). The organic layer was separated, dried over anhydrous Na2SO4, and concentrated in vacuum. The crude mass was purified by flash chromatography on silica gel (230–400 mesh) and using ethyl acetate/hexane as the eluent to obtain the corresponding products 4a–4p.
1-Bromo-4-phenylbut-3-en-2-one (3a)5e,10−13,19
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.000 g, 6.84 mmol, 1 equiv), SeO2 (0.379 g, 3.420 mmol, 0.5 equiv), NBS (2) (1.217 g, 6.84 mmol, 1 equiv), and PTSA (0.650 g, 3.420 mmol, 0.5 equiv) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (1.293 g, 84% yield); mp 45 °C; IR (KBr film) 3058, 3017, 2934, 2376, 2254, 1945, 1876, 1811, 1687, 1618, 1560, 1471, 1420, 131, 1199, 1155, 1060, 975, 881, 857, 782, 739, 676, 651, 566 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.70 (d, J = 16.4 Hz, 1H), 7.59–7.57 (m, 2H), 7.43 (d, J = 1.6 Hz, 2H), 7.41 (d, J = 2 Hz, 1H), 6.96 (d, J = 16 Hz, 1H), 4.09 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 191.0, 145.4, 133.9, 131.1, 129.0, 128.6, 122.2, 33.1 ppm; HRMS (ESI) calcd for C10H10BrO [M + H]+ 224.9915; found 225.0000.
1-Bromo-4-(p-tolyl)but-3-en-2-one (3b)5e
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.095 g, 6.84 mmol, 1 equiv), SeO2 (0.379 g, 3.420 mmol, 0.5 equiv), NBS (2) (1.217 g, 6.84 mmol, 1 equiv), and PTSA (0.650 g, 3.420 mmol, 0.5 equiv) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): white solid (1.406 g, 86% yield); mp 88 °C; IR (KBr film) 3027, 2983, 2934, 2381, 2354, 2306, 1922, 1689, 1667, 1614, 1598, 1564, 1510, 1449, 1411, 1386, 1327, 1308, 1285, 1184, 1154, 1114, 1069, 980, 891, 841, 798, 712, 642, 590, 542, 516 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.61 (d, J = 16 Hz, 1H), 7.41 (d, J = 8 Hz, 2H), 7.15 (d, J = 8 Hz, 2H), 6.83 (d, J = 16.4 Hz, 1H), 4.02 (s, 2H), 2.32 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 191.1, 145.5, 141.8, 131.2, 129.8, 129.8, 128.7, 128.7, 121.2, 33.2, 21.6 ppm; MS (ES+) calcd for C11H14BrO [M + H]+ 239.00; found 239.00.
1-Bromo-4-(4-fluorophenyl)but-3-en-2-one (3c)5e
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.123 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (1.479 g, 89% yield); mp 100 °C; IR (KBr film) 3105, 3068, 2984, 2937, 1698, 1674, 1618, 1592, 1506, 1382, 1227, 1158, 1066, 986, 848, 810, 646 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.60 (d, J = 16 Hz, 1H), 7.53 (q, J = 3.2 Hz, 2H), 7.04 (t, J = 8.4 Hz, 2H), 6.82 (d, J = 16 Hz, 1H), 4.01 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 189.8, 163.3 (J = 251.3 Hz), 143.0, 129.6 (J = 8.7 Hz), 129.2 (J = 3.4 Hz), 120.8 (J = 2.4 Hz), 115.3 (J = 21.9 Hz), 33.1 ppm; HRMS (ESI) calcd for C10H9BrFO [M + H]+ 242.9821; found 242.9824.
1-Bromo-4-(4-chlorophenyl)but-3-en-2-one (3d)5e,7
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.235 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (1.579 g, 89% yield); mp 115 °C; IR (KBr film) 3096, 3033, 2994, 2852, 1695, 1581, 1483, 1400, 1260, 1129, 1073, 1047, 745 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.59 (d, J = 15.6 Hz, 1H), 7.45 (d, J = 8.4 Hz, 2H), 7.32 (d, J = 8 Hz, 2H), 6.86 (d, J = 16 Hz, 2H), 4.01 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 187.3, 140.0, 136.9, 131.6, 128.9, 118.6, 32.5 ppm; HRMS (ESI) calcd for C10H9BrClO [M + H]+ 258.9525; found 258.9615.
1-Bromo-4-(4-bromophenyl)but-3-en-2-one (3e)5e
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.539 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (1.829 g, 88% yield); mp 120 °C; IR (KBr film) 3014, 1672, 1600, 1327, 1142, 1067, 585 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.57 (d, J = 16.4 Hz, 1H), 7.48 (d, J = 8 Hz, 2H), 7.38 (d, J = 8 Hz, 2H), 6.80 (d, J = 16 Hz, 1H), 4.01 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 189.8, 142.9, 131.7, 131.3, 128.9, 124.5, 121.5, 32.1 ppm; MS (ES+) calcd for C10H9Br2O [M + H]+ 304.90; found 304.90.
1-Bromo-4-(2-chlorophenyl)but-3-en-2-one (3f)
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.235 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (1.562 g, 88% yield); mp 62 °C; IR (KBr film) 3061, 2854, 1695, 1607, 1388, 1129, 1073, 1047, 745 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.05 (d, J = 16 Hz, 1H), 7.61 (dd, J = 6 Hz, 1H), 7.37 (dt, J = 1.6 Hz, 1H), 7.29–7.22 (m, J = 2H), 6.85 (d, J = 16.4 Hz, 1H), 4.06 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 190.8, 141.1, 135.6, 132.2, 131.8, 130.3, 127.7, 127.2, 124.7, 32.8 ppm; HRMS (ESI) calcd for C10H9BrClO [M + H]+ 258.9525; found 258.9615.
1-Bromo-4-(3-fluorophenyl)but-3-en-2-one (3g)
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.123 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): yellow solid (1.446 g, 87% yield); mp 78 °C; IR (KBr film) 3059, 2985, 1698, 1616, 1582, 1485, 1386, 1327, 1270, 1145, 991, 971, 913, 866, 713 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.59 (d, J = 16 Hz, 1H), 7.30 (q, J = 6.8 Hz, 2H), 7.23 (s, 1H), 7.06 (t, J = 8.4 Hz, 1H), 6.88 (d, J = 15.6 Hz, 1H), 4.01 (s, 2H), ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 190.7, 162.9 (J = 246.9 Hz), 143.8 (J = 3.2 Hz), 136.2 (J = 7.9 Hz), 130.6 (J = 7.6 Hz), 124.7 (J = 3.4 Hz), 123.3, 117.9 (J = 21 Hz), 114.7 (J = 21.7 Hz), 33.1 ppm; HRMS (ESI) calcd for C10H9BrFO [M + H]+ 242.9821; found 242.9824.
1-Bromo-4-(3-bromophenyl)but-3-en-2-one (3h)5e
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.539 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brownish white solid (1.788 g, 86% yield); mp 60 °C; IR (KBr film) 3059, 3025, 2981, 2934, 1695, 1615, 1492, 1446, 1385, 1332, 1182, 1069, 991, 890, 746, 687, 554 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.64 (d, J = 16 Hz, 1H), 7.52 (t, J = 5.2 Hz, 2H), 7.36 (d, J = 1.2 Hz, 1H), 7.35 (s, 1H), 6.89 (d, J = 16.4 Hz, 1H), 4.03 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 191.1, 145.4, 133.9, 131.2, 129.1, 128.6, 122.2, 33.1 ppm; MS (ES+) calcd for C10H9Br2O [M + H]+ 304.90; found 305.00.
1-Bromo-4-(3-nitrophenyl)but-3-en-2-one (3i)
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.307 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (1.607 g, 87% yield); mp 130 °C; IR (KBr film) 3043, 2927, 2393, 1688, 1614, 1351, 1139, 994, 727, 680 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.38 (s, 1H), 8.21 (dd, J = 7.2 Hz, 1H), 7.82 (d, J = 7.6 Hz, 1H), 7.68 (d, J = 15.6, 1H), 7.56 (t, J = 8, 1H), 7.02 (d, J = 16 Hz, 1H), 4.04 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 190.4, 148.7, 142.2, 135.7, 134.3, 130.2, 125.2, 124.6, 122.7, 33.1 ppm; HRMS (ESI) calcd for C10H9BrNO3 [M + H]+ 269. 9766; found 269.9772.
1-Bromo-4-(2,3-dimethoxyphenyl)but-3-en-2-one (3j)
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.410 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): green solid (1.696 g, 87% yield); mp 78 °C; IR (KBr film) 3065, 2999, 2937, 2830, 1696, 1606, 1496, 1386, 1218, 1047, 991, 801, 717 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.01 (d, J = 16 Hz, 1H), 7.09 (d, J = 3.2 Hz, 1H), 6.98–6.94 (m, 2H), 6.87 (d, J = 9.2 Hz, 1H), 4.14 (s, 2H), 3.87 (s, 3H), 3.81 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 191.5, 153.5, 153.3, 140.5, 123.3, 123.1, 118.2, 113.2, 112.5, 56.1, 55.8, 33.1 ppm; HRMS (ESI) calcd for C12H14BrO3 [M + H]+ 285.0126; found 285.0000.
1-Bromo-4-(3,4,5-trimethoxyphenyl)but-3-en-2-one (3k)
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.616 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): yellow solid (1.832 g, 85% yield); mp 86 °C; IR (KBr film) 3043, 2998, 2971, 2834, 1680, 1594, 1481, 1424, 1247, 1201, 1109, 1000, 854 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.01 (d, J = 16 Hz, 1H), 7.20 (s, 1H), 6.91 (s, 1H), 6.68 (d, J = 16 Hz, 1H), 4.08 (s, 1H), 3.87 (s, 3H), 3.85 (s, 3H), 3.83 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 190.8, 152.9, 151.3, 145.6, 143.9, 129.2, 124.26, 113.9, 106.0, 61.3, 61.1, 56.3, 32.5 ppm; HRMS (ESI) calcd for C13H16BrO4 [M + H]+ 315.0232; found 315.0227.
1-Bromo-4-(3,5-dichloro-2-hydroxyphenyl)but-3-en-2-one (3l)
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.580 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): dark orange solid (1.420 g, 67% yield); mp 102 °C; IR (KBr film) 3073, 2982, 2930, 1687, 1606, 1250, 1165, 1077, 861, 753, 559 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.81 (d, J = 16 Hz, 1H), 7.37 (d, J = 2.4 Hz, 1H), 7.33 (d, J = 2 Hz, 1H), 7.07 (d, J = 16 Hz, 1H), 4.78 (s, 1H), 4.03 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 191.1, 149.5, 138.2, 130.3, 127.6, 125.8, 124.3, 123.4, 121.6, 33.1 ppm; HRMS (ESI) calcd for C10H7BrCl2O2 [M + H]+ 308.9085; found 308.9101.
1-Bromo-4-(naphthalen-1-yl)but-3-en-2-one (3m)5g
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.342 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): dark solid (1.373 g, 73% yield); mp 110 °C; IR (KBr film) 3049, 2937, 1689, 1600, 1381, 1346, 1098, 1063, 977, 789, 770, 585, 550 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.52 (d, J = 15.6 Hz, 1H), 8.13 (d, J = 8.4Hz, 1H), 7.87 (d, J = 8 Hz, 1H), 7.87 (d, J = 8 Hz, 1H), 7.82 (d, J = 7.6 Hz, 1H), 7.77 (d, J = 7.2 Hz, 1H), 7.55–7.42 (m, 3H), 6.99 (d, J = 15.6 Hz, 1H), 4.07 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 190.9, 142.2, 133.7, 131.7, 131.4, 131.2, 128.9, 127.2, 126.4, 125.5, 124.5, 123.2, 33.3 ppm; MS (ES+) calcd for C14H12BrO [M + H]+ 275.00; found 275.23.
1-Bromo-4-(naphthalen-2-yl)but-3-en-2-one (3n)5g,7
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.342 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (1.505 g, 80% yield); mp 78 °C; IR (KBr film) 3054, 2982, 2932, 1687, 1606, 1360, 1072, 978, 814, 745, 648 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.93 (s, 1H), 7.82–7.77 (m, 4H), 7.64 (dd, J = 8.8 Hz, 1H), 7.47 (t, J = 7.6 Hz, 2H), 6.99 (d, J = 16 Hz, 1H), 4.06 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 190.0, 144.5, 133.6, 133.2, 130.4, 130.2, 127.9, 126.8, 126.7, 125.9, 122.4, 121.2, 32.2 ppm; MS (ES+) calcd for C14H12BrO [M + H]+ 275.00; found 275.23.
4-([1,1′-Biphenyl]-4-yl)-1-bromobut-3-en-2-one (3o)5f,5g
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.520 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (1.545 g, 75% yield); mp 120 °C; IR (KBr film) 3085, 3029, 2985, 2963, 1695, 1606, 1381, 1483, 1385, 1068, 983, 761, 654 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.74 (d, J = 16 Hz, 1H), 7.62 (d, J = 17.2 Hz, 5H), 7.45 (d, J = 6 Hz, 2H), 7.39 (d, J = 6.8 Hz, 2H), 6.91 (d, J = 15.6 Hz, 1H), 4.09 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 190.9, 144.9, 143.9, 139.9, 132.9, 129.2, 128.9, 128.1, 127.7, 127.1, 122.0, 33.1 ppm; MS (ES+) calcd for C16H14BrO [M + H]+ 301.02; found 301.02.
1-Bromo-4-(thiophen-2-yl)but-3-en-2-one (3p)
The title compound was prepared via the general procedure A starting from 4-phenylbut-3-en-2-one (1.041 g, 6.84 mmol), SeO2 (0.379 g, 3.420 mmol), NBS (2) (1.217 g, 6.84 mmol), and PTSA (0.650 g, 3.420 mmol) in toluene (3.0 mL) at 50 °C. The product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): dark brown solid (1.043 g, 66% yield); mp 60–70 °C; IR (KBr film) 3005, 2946, 2856, 1674, 1590, 1416, 1154, 1002, 714 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.76 (d, J = 15.6 Hz, 1H), 7.39 (d, J = 5.2 Hz, 1H), 7.29 (d, J = 3.6 Hz, 1H), 7.03 (t, J = 4.8 Hz, 1H), 6.68 (d, J = 15.6 Hz, 1H), 3.98 (s, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 190.1, 137.2, 132.4, 129.4, 128.0, 126.9, 120.3, 32.7 ppm; HRMS (ESI) calcd for C8H1BrOS [M + H]+ 230.9479; found 230.9488.
1,1-Dibromo-4-phenylbut-3-en-2-one (4a)11
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.000 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): reddish brown semisolid, (1.808 g, 87% yield); IR (KBr film) 3058, 3022, 1686, 2922, 2852, 1686, 1608, 1494, 1448, 1331, 1135, 1062, 980, 728 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.80 (d, J = 15.6 Hz, 1H), 7.58 (q, J = 1.2 Hz, 3H), 7.37 (q, J = 2.8 Hz, 1H), 7.22 (d, J = 16 Hz, 1H), 5.86 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.5, 147.6, 133.8, 131.4, 139.0, 128.8, 117.7, 42.6 ppm; HRMS (ESI) calcd for C10H9Br2O [M + H]+ 304.9000; found 304.8990.
1,1-Dibromo-4-(p-tolyl)but-3-en-2-one (4b)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.095 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (1.979 g, 91% yield); mp 80 °C; IR (KBr film) 3027, 2964, 2918, 1671, 1591, 1564, 1329, 1138, 809, 750, 586 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.85 (d, J = 15.6 Hz, 1H), 7.54 (d, J = 8 Hz, 2H), 7.24 (d, J = 8 Hz, 1H), 7.24 (d, J = 15.6 Hz, 1H), 5.93 (s, 1H), 2.41 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.7, 147.8, 142.2, 131.1, 129.8, 128.9, 116.7, 42.7, 21.6 ppm; HRMS (ESI) calcd for C11H11Br2O [M + H]+ 318.9156; found 318.9155.
1,1-Dibromo-4-(4-fluorophenyl)but-3-en-2-one (4c)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.123 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid, (1.938 g, 88% yield); mp 85–90 °C; IR (KBr film) 3336, 3252, 3172, 3103, 3075, 3024, 1831, 1842, 1765, 1725, 1673, 1600, 1499, 1408, 1353, 1227, 940, 919, 775, 752, 399, 635, 586, 514 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.84 (d, J = 16 Hz, 1H), 7.65 (q, J = 2.8 Hz, 2H), 7.26 (d, J = 6 Hz, 2H), 7.14 (s, 1H), 7.14 (d, J = 16.8 Hz, 1H), 5.92 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.4, 164.5, (J = 251.9 Hz), 146.3, 130.9 (d, J = 8.7 Hz), 130.1 (d, J = 2.3 Hz), 117.4 (d, J = 2.5 Hz), 116.3 (d, J = 21.9 Hz), 42.5 ppm; HRMS (ESI) calcd for C10H7Br2FO [M + H]+ 322.8905; found 322.8878.
1,1-Dibromo-4-(4-chlorophenyl)but-3-en-2-one (4d)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.235 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (2.037 g, 88% yield); mp 110 °C; IR (KBr film) 3093, 3019, 1672, 1597, 1561, 1401, 1351, 1109, 1087 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.82 (d, J = 15.6 Hz, 1H), 7.57 (d, J = 8.4 Hz, 2H), 7.41 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 15.6. Hz, 2H), 5.92 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.3, 146.1, 137.4, 132.2, 130.0, 129.3, 118.1, 42.5 ppm; HRMS (ESI) calcd for C10H8Br2ClO [M + H]+ 338.8610; found 338.8575.
1,1-Dibromo-4-(4-bromophenyl)but-3-en-2-one (4e)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.539 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): yellow solid (2.330 g, 89% yield); mp 138 °C; IR (KBr film) 3093, 3054, 3015, 1672, 1600, 1581, 1558, 1481, 1326, 1109, 1065, 944, 711, 563 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.73 (d, J = 15.6 Hz, 1H), 7.51 (d, J = 8.8 Hz, 2H), 7.43 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 16 Hz, 1H), 5.85 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.3, 146.2, 132.7, 132.3, 130.2, 125.9, 118.3, 42.5 ppm; HRMS (ESI) calcd for C10H8Br3O [M + H]+ 382.8105; found 382.8098.
1,1-Dibromo-4-(2-chlorophenyl)but-3-en-2-one (4f)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.235 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired obtained product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): light yellow crystalline solid (1.990 g, 86% yield); mp 100–105 °C; IR (KBr film) 3018, 1760, 1674, 1466, 1437, 1324, 1266, 1199, 1142, 1043, 973, 921, 741, 681, 616, 586, 565 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.23 (d, J = 16 Hz, 1H), 7.70 (q, J = 5.6 Hz, 1H), 7.40 (dd, J = 8 Hz, 1H), 7.30–7.27 (m, 2H), 7.23 (d, J = 16 Hz, 1H), 5.88 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.2, 143.2, 135.9, 132.1, 130.4, 128.1, 127.2, 120.123, 42.4 ppm; HRMS (ESI) calcd for C10H8Br2ClO [M + H]+ 338.8610; found 338.3410.
1,1-Dibromo-4-(3-fluorophenyl)but-3-en-2-one (4g)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.123 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired obtained product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid, (1.915 g, 87% yield); mp 70 °C; IR (KBr film) 3060, 3027, 2964, 1685, 1612, 1577, 1486, 1444, 1323, 1238, 1163, 943, 790, 749, 699, 566, 561 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.82 (d, J = 15.6 Hz, 1H), 7.41 (t, J = 4 Hz, 2H), 7.34 (d, J = 9.6 Hz, 1H), 7.30 (d, J = 16 Hz, 1H), 7.18–7.13 (m, 1H), 5.93 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.3, 162.9 (J = 246.1 Hz), 146.1 (d, J = 2.9 Hz), 136.0 (d, J = 7.7 Hz), 130.6 (d, J = 8.1 Hz), 124.9 (d, J = 2.9 Hz), 119.0, 118.3 (d, J = 21.3 Hz), 114.9 (d, J = 21.8 Hz), 42.4 ppm; HRMS (ESI) calcd for C10H7Br2FO [M + H]+ 322.8905; found 322.8878.
1,1-Dibromo-4-(3-bromophenyl)but-3-en-2-one (4h)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.539 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): yellow solid (2.252 g, 86% yield); mp 56–60 °C; IR (KBr film) 3018, 2922, 1689, 1611, 1562, 1471, 1315, 1135, 786 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.72 (d, J = 16 Hz, 1H), 7.71 (s, 1H), 7.49 (t, J = 9.2 Hz, 2H), 7.26 (d, J = 8 Hz, 1H), 7.23 (d, J = 2.8 Hz, 1H), 5.85 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.2, 145.8, 135.9, 134.1, 131.3, 130.6, 127.6, 123.2, 119.1, 42.4 ppm; HRMS (ESI) calcd for C10H8Br3O [M + H]+ 382.8105; found 382.8098.
1,1-Dibromo-4-(3-nitrophenyl)but-3-en-2-one (4i)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.307 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid (2.100 g, 88% yield); mp 140 °C; IR (KBr film) 3093, 3044, 1686, 1614, 1524, 1351, 1140, 992, 814, 725 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.430 (s, 1H), 8.24 (d, J = 1.2 Hz, 1H), 8.23 (d, J = 1.2 Hz, 1H), 7.86 (q, J = 3.2 Hz, 1H), 7.59 (t, J = 8 Hz, 1H), 7.36 (d, J = 15.6 Hz, 1H), 5.87 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 183.8, 147.7, 143.4, 133.5, 129.2, 124.4, 121.9, 119.6, 41.2 ppm; HRMS (ESI) calcd for C10H8Br2NO3 [M + H]+ 349.8850; found 349.8838.
1,1-Dibromo-4-(2,3-dimethoxyphenyl)but-3-en-2-one (4j)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.410 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): green solid (2.141 g, 86% yield); mp 78 °C; IR (KBr film) 3005, 2964, 2936, 2835, 1687, 1605, 1576, 1476, 1267, 1000, 802, 749 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.11 (d, J = 16 Hz, 1H), 7.29 (d, J = 16 Hz, 1H), 7.20 (d, J = 6.8 Hz, 1H), 7.04 (t, J = 8 Hz, 1H), 6.93 (d, J = 8.4 Hz, 1H), 5.88 (s, 1H), 3.83 (s, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 184.8, 152.2, 148.3, 141.4, 126.9, 123.3, 118.8, 118.1, 113.9, 60.5, 54.9, 41.7 ppm; HRMS (ESI) calcd for C12H13B2O3 [M + H]+ 364.9211; found 364.9223.
1,1-Dibromo-4-(3,4,5-trimethoxyphenyl)but-3-en-2-one (4k)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.616 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired obtained product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): light yellow solid, (2.264 g, 84% yield); mp 100–105 °C; IR (KBr film) 3000, 2935, 1690, 1599, 1553, 1479, 1420, 1390, 1349, 1290, 1252, 1200, 1162, 1105, 1072, 1043, 1004, 975, 923, 856, 813, 745, 724, 679, 649, 608, 586, 565 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.3 (d, J = 15.6 Hz, 1H), 7.41 (s, 1H), 7.15 (d, J = 15.6 Hz, 1H), 7.04 (s, 1H), 5.98 (s, 1H), 3.95 (s, 6H), 3.91 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.1, 152.9, 146.0, 129.1, 119.3, 106.4, 61.3, 61.0, 56.4, 42.3 ppm; HRMS (ESI) calcd for C13H15Br2O4 [M + H]+ 394.9317; found 394.9281
1,1-Dibromo-4-(naphthalen-1-yl)but-3-en-2-one (4m)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.342 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired obtained product was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): yellow solid, (1.937 g, 80% yield); mp 30–35 °C; IR (KBr film) 3047, 2924, 2853, 1667, 1595, 1574, 1246, 1198, 1144, 108, 1030, 848, 743, 699, 581 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.75 (d, J = 15.2 Hz, 1H), 8.24 (d, J = 8.4 Hz, 1H), 7.97–7.89 (m, 3H), 7.64–7.52 (m, 3H), 7.41 (d, J = 15.2 Hz, 1H), 5.98 (s,1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.6, 144.4, 133.7, 131.8, 131.7, 131.1, 128.9, 127.3, 126.5, 125.8, 125.4, 123.2, 120.1, 42.7 ppm; HRMS (ESI) calcd for C14H11Br2O [M + H]+ 354.9156; found 354.9200.
1,1-Dibromo-4-(naphthalen-2-yl)but-3-en-2-one (4n)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.342 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 °C. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): yellow solid, (1.985 g, 82% yield); mp 75 °C; IR (KBr film) 3052, 3010, 2962, 2926, 1679, 1598, 1359, 1129, 960, 812, 750, 578 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 8.04–8.00 (m, 2H), 7.87 (d, J = 8 Hz, 3H), 7.76 (d, J = 8 Hz, 2H), 7.57 (d, J = 3.2 Hz, 2H), 7.39 (d, J = 15.6 Hz, 1H), 5.97 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.6, 147.8, 134.8, 133.2, 131.5 (d, J = 9.3 Hz), 128.9 (d, J = 6.0 Hz), 127.9 (d, J = 2.4 Hz), 126.9, 123.7, 117.9, 42.7 ppm; HRMS (ESI) calcd for C14H11Br2O [M + H]+ 354.9156; found 354.9200.
4-([1,1′-Biphenyl]-4-yl)-1,1-dibromobut-3-en-2-one (4o)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.520 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 oC. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): yellow solid, (2.079 g, 80% yield); mp 76 oC; IR (KBr film) 3081, 3014, 2962, 2854, 1680, 1604, 1325, 1141, 951, 804, 747, 689, 561 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.91 (d, J = 15.6 Hz, 1H), 7.72–7.61 (m, J = 9.6 Hz, 5H), 7.47 (t, J = 7.2 Hz, 2H), 7.40 (d, J = 6.8 Hz, 2H), 7.32 (d, J = 15.6 Hz, 1H), 5.95 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.5, 147.2, 144.247, 139.9, 132.8, 129.4, 128.9, 128.1, 127.7, 127.1, 117.6, 42.6 ppm; HRMS (ESI) calcd for C16H13Br2O [M + H]+ 380.9313; found 380.9327.
1,1-Dibromo-4-(thiophen-2-yl)but-3-en-2-one (4p)
The title compound was prepared via the general procedure B starting from 4-phenylbut-3-en-2-one (1.041 g, 6.840 mmol), SeO2 (0.759 g, 6.840 mmol), NBS (2) (2.435 g, 13.680 mmol), and PTSA (1.301 g, 6.840 mmol) in toluene (2.5 mL) at 80 oC. The desired product obtained was isolated by flash chromatography using hexane/ethyl acetate as an eluent (9:1): brown solid, (1.653 g, 78% yield); mp 58 oC; IR (KBr film) 3009, 2948, 1674, 1592, 1155, 713 cm–1; 1H NMR (400 MHz, CDCl3, with 1% v/v TMS) δ 7.96 (d, J = 15.2 Hz, 1H), 7.48 (d, J = 5.2 Hz 1H), 7.39 (d, J = 3.6 Hz, 1H), 7.10 (t, J = 4.2 Hz, 1H), 7.00 (d, J = 15.2 Hz, 1H), 5.89 (s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.4, 139.9, 139.3, 133.2, 130.4, 128.5, 116.4, 42.4 ppm; HRMS (ESI) calcd for C8H7Br2OS [M + H]+ 310.8564; found 310.8557.
Acknowledgments
The authors thank SAIF-NEHU, SAIF-CDRI Lucknow, and SAIF-IISC Bangalore for various analyses. T.M.L. thanks the University Grants Commission (UGC), India, for the financial assistance under the Junior/Senior Research Fellowship (JRF/SRF). B.M. acknowledges financial assistance from SERB, DST (SB/EMEQ-006/3013).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04352.
The authors declare no competing financial interest.
Supplementary Material
References
- a De Kimpe N.; Verhe R. In Chemistry of α-Haloketones, α-Haloaldehydes and α-Haloimines. Patai S., Ed, Wiley, Chichester, 1988, pp 1–119.; b Hoyle J.Patai’s Chemistry of Functional Groups, Some Synthetic Uses of halides, Willey, 2009, pp 1–77. [Google Scholar]
- a Erian A.; Sherif S.; Gaber H. The Chemistry of α-Haloketones and Their Utility in Heterocyclic Synthesis. Molecules 2003, 8, 793–865. 10.3390/81100793. [DOI] [Google Scholar]; b Jiang J.; Zou H.; Dong Q.; Wang R.; Lu L.; Zhu Y.; He W. Synthesis of 2-Keto(hetero)aryl Benzox(thio)azoles through Base Promoted Cyclization of 2-Amino(thio)phenols with α,α-Dihaloketones. J. Org. Chem. 2016, 81, 51–56. 10.1021/acs.joc.5b02093. [DOI] [PubMed] [Google Scholar]; c Talegaonkar J.; Mukhija S.; Boparai K. S. Determination of thiosemicarbazones by reaction with omega-bromoacetophenone. Talanta 1982, 29, 327–328. 10.1016/0039-9140(82)80119-7. [DOI] [PubMed] [Google Scholar]; d Marek A.; Kulhanek J.; Ludwig M.; Bures F. Facile Synthesis of Optically Active Imidazole Derivatives. Molecules 2007, 12, 1183–1190. 10.3390/12051183. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Loughlin W. A. A Heterocyclic Chemist’s Perspective. Aust. J. Chem. 1998, 51, 875–894. 10.1071/C98066. [DOI] [Google Scholar]; f Cho J.; Kim K. Reaction of Tetrasulfur Tetranitride with Bromomethyl Ketones. J. Heterocycl. Chem. 1992, 29, 1433–1439. 10.1002/jhet.5570290611. [DOI] [Google Scholar]; g Fülöpovaá V.; Soural M. Solid-Phase Synthesis of Heterocycles with α-Haloketones as the Key Building Blocks. Synthesis 2016, 48, 3684–3695. 10.1055/s-0035-1562519. [DOI] [Google Scholar]; h Alajarín M.; Cabrera J.; Pastor A.; Sánchez-Andrada P.; Bautista D. Diels-Alder Reactions of 4-Alkenylthiazoles: A New Approach to Thiazole Functionalization. J. Org. Chem. 2007, 72, 2097–2105. 10.1021/jo062417e. [DOI] [PubMed] [Google Scholar]
- Li X.-C.; Ferreira D.; Jacob M. R.; Zhang Q.; Khan S. I.; ElSohly H. N.; Nagle D. G.; Smillie T. J.; Khan I. A.; Walker L. A.; Clark A. M. Antifungal Cyclopentenediones from Piper coruscans. J. Am. Chem. Soc. 2004, 126, 6872–6873. 10.1021/ja048081c. [DOI] [PubMed] [Google Scholar]
- a Conde S.; Pérez D. I.; Martínez A.; Perez C.; Moreno F. J. Thienyl and Phenyl α-Halomethyl Ketones: New Inhibitors of Glycogen Synthase Kinase (GSK-3â) from a Library of Compound Searching. J. Med. Chem. 2003, 46, 4631–4633. 10.1021/jm034108b. [DOI] [PubMed] [Google Scholar]; b Arabaci G.; Guo X.-C.; Beebe K. D.; Coggeshall K. M.; Pei D. α-Haloacetophenone Derivatives As Photoreversible Covalent Inhibitors of Protein Tyrosine Phosphatases. J. Am. Chem. Soc. 1999, 121, 5085–5086. 10.1021/ja9906756. [DOI] [Google Scholar]
- a Larock R. C.A Guide to Functional Group Preparations. In Comprehensive Organic Transformations, 2nd ed., VCH-New York, Wiley, 1999, pp 715–719. [Google Scholar]; b Yunus U.; Winterfeldt E. Studies on α-Bromination of Ketone in Hydrindane Ring System. J. Chin. Chem. Soc. 2007, 54, 1087–1092. 10.1002/jccs.200700155. [DOI] [Google Scholar]; c Dagani M. J.; Barda H. J.; Benya T. J.; Sanders D. C.. Ullmann’s Encyclopedia of Industrial Chemistry, In Bromine Compounds, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2002, Vol 6, pp 331–358. [Google Scholar]; d Qin N.; Li C.-B.; Jin M.-N.; Shi L.-H.; Duan H.-Q.; Niu W.-Y. Synthesis and biological activity of novel tiliroside derivants. Eur. J. Med. Chem. 2011, 46, 5189–5195. 10.1016/j.ejmech.2011.07.059. [DOI] [PubMed] [Google Scholar]; e Musso L.; Cincinelli R.; Zuco V.; Zunino F.; Nurisso A.; Cuendet M.; Giannini G.; Vesci L.; Pisano C.; Dallavalle S. Investigation on the ZBG-functionality of phenyl-4-yl-acrylohydroxamic acid derivatives as histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 4457–4460. 10.1016/j.bmcl.2015.09.006. [DOI] [PubMed] [Google Scholar]; f Revuelto A.; Ruiz-Santaquiteria M.; de Lucio H.; Gamo A.; Carriles A. A.; Gutiérrez K. J.; Sanchez-Murcia P. A.; Hermoso J. A.; Gago F.; Camarasa M.-J.; Jiménez-Ruiz A.; Velazquez S. Pyrrolopyrimidine vs Imidazole-Phenyl-Thiazole Scaffolds in Nonpeptidic Dimerization Inhibitors ofLeishmania infantum Trypanothione Reductase. ACS Infect. Dis. 2019, 5, 873–891. 10.1021/acsinfecdis.8b00355. [DOI] [PubMed] [Google Scholar]; g Sadhukan S.; Santhi J.; Baire B. The a,a-Dihalocarbonyl Building Blocks: An Avenue for New Reaction Development in Organic Synthesis. Chem. - Eur. J. 2020, 26, 7145–7175. 10.1002/chem.201905475. [DOI] [PubMed] [Google Scholar]; h Imperiali B.; Abeles R. H. Inhibition of Serine Proteases by Peptidyl Fluoromethyl Ketones. Biochemistry 1986, 25, 3760–3767. 10.1021/bi00361a005. [DOI] [PubMed] [Google Scholar]; i Fäh C.; Hardegger L. A.; Baitsch L.; Schweizer W. B.; Meyer S.; Bur D.; Diederich F. New organofluorine building blocks: inhibition of the malarial aspartic proteases plasmepsin II and IV by alicyclic α,α-difluoroketone hydrates. Org. Biomol. Chem. 2009, 7, 3947–3957. 10.1039/b908489d. [DOI] [PubMed] [Google Scholar]; j Babu K. S.; Li X.-C.; Jacob M. R.; Zhang Q.; Khan S. I.; Ferreira D.; Clark A. M. Synthesis, Antifungal Activity, and Structure-Activity Relationships of Coruscanone A Analogues. J. Med. Chem. 2006, 49, 7877–7886. 10.1021/jm061123i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zhang W.; Curran D. P.; Chen C. H.-T. Use of fluorous silica gel to separate fluorous thiol quenching derivatives in solutions-phase parallel synthesis. Tetrahedron 2002, 58, 3871–3875. 10.1016/S0040-4020(02)00209-0. [DOI] [Google Scholar]; b Harwood H. Reactions of the Hydrocarbon chain of fatty acids. Chem. Rev. 1962, 62, 99–154. 10.1021/cr60216a002. [DOI] [Google Scholar]
- Ngo Q. A.; Nguyen L. A.; Vo N. B.; Nguyen T. H.; Roussi F.; Nguyen T. H.; Nguyen V. T. Synthesis and antiproliferative activity of new vinca alkaloids containing an a,ß-unsaturated aromatic side chain. Bioorg. Med. Chem. Lett. 2015, 25, 5597–5600. 10.1016/j.bmcl.2015.10.040. [DOI] [PubMed] [Google Scholar]
- a Curran D. P.; Chang C.-T. Atom Transfer Cyclization Reactions of α-Iodo Esters, Ketones, and Malonates: Examples of selectives 5-Exo, 6-Endo, 6-Exo, and 7-Endo Ring Closures. J. Org. Chem. 1989, 54, 3140–3157. 10.1021/jo00274a034. [DOI] [Google Scholar]; b Karimi S.; Grohmann K. G. Intramolecular Ring-Opening of Cyclopopranone by Enolate Anions. J. Org. Chem. 1995, 60, 554–559. 10.1021/jo00108a016. [DOI] [Google Scholar]; c Kumar R. S.; Kulangiappar K.; Kulandainathan M. A. Convenient Electrochemical Method for the Synthesis of α-Bromo Alkyl Aryl Ketones. Synth. Commun. 2010, 40, 1736–1742. 10.1080/00397910903161710. [DOI] [Google Scholar]; d Sultan A.; Abbas M.; Raza A. R.; Tahir M. N. Optimization of Conditions for the Facile, Efficient & Selective α-Bromination of Methyl And Methylene Ketones. Sci. Int. 2017, 29, 875–882. [Google Scholar]; e Tanemura K.; Suzuki T.; Nishida Y.; Satsumabayashi K.; Horaguchi T. A mild and efficient procedure for a-bromination of ketones using N-bromosuccinimide catalysed by ammonium acetate. Chem. Commun 2004, 4, 470–471. 10.1039/B315340A. [DOI] [PubMed] [Google Scholar]; f Vražič D.; Jereb M.; Laali K. K.; Stavber S. Brønsted Acidic Ionic Liquid Accelerated Halogenation of Organic Compounds with N-Halosuccinimides (NXS). Molecules 2013, 18, 74–96. 10.3390/molecules18010074. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Terent’ev A. O.; Khodykin S. V.; Krylov I. B.; Ogibin Y. N.; Nikishin G. I. A Convenient Synthesis of 2,2-Dibromo-1-arylethanones by Bromination of 1-Arylethanones with the H2O2–HBr System. Synthesis 2006, 1087–1092. 10.1055/s-2006-926386. [DOI] [Google Scholar]; h Jagatheesan R.; Raj K. J. S.; Lawrence S.; Christopher C. Electroselective α-bromination of acetophenone using in situ bromonium ions from ammonium bromide. RSC Adv. 2016, 6, 35602–35608. 10.1039/C6RA04541C. [DOI] [Google Scholar]; i Nobuta T.; Hirashima S.; Tada N.; Miura T.; Itoh A. Facile aerobic photo-oxidative synthesis of α,α-dibromoacetophenones from alkynes with 48% aq HBr. Tetrahedron Lett. 2010, 51, 4576–4578. 10.1016/j.tetlet.2010.06.120. [DOI] [Google Scholar]; j Madabhushi S.; Jillella R.; Mallu K. K. R.; Godala K. R.; Vangipuram V. S. A new and efficient method for the synthesis of α,α-dihaloketones by oxyhalogenation of alkynes using oxone-KX. Tetrahedron Lett. 2013, 54, 3993–3996. 10.1016/j.tetlet.2013.05.072. [DOI] [Google Scholar]; k Chawla R.; Singh A. K.; Yadav L. D. S. Catalyst- and Metal-Free Rapid Functionalizations of Alkynes Using TsNBr2. Synlett 2013, 24, 1558–1562. 10.1055/s-0033-1339194. [DOI] [Google Scholar]
- a Xie L.; Wu Y.; Yi W.; Zhu L.; Xiang J.; He W. Gold-Catalyzed Hydration of Haloalkynes to α-Halomethyl Ketones. J. Org. Chem. 2013, 78, 9190–9195. 10.1021/jo401437w. [DOI] [PubMed] [Google Scholar]; b Chen Z.-W.; Ye D.-N.; Ye M.; Zhou Z.-G.; Li S.-H.; Liu L.-X. AgF/TFA-promoted highly efficient synthesis of α-haloketones from haloalkynes. Tetrahedron Lett. 2014, 55, 1373–1375. 10.1016/j.tetlet.2014.01.027. [DOI] [Google Scholar]
- Calo’ V.; Lopez L.; Pesce G.; Todesco P. E. Contribution to the Bromination of Conjugate Unsaturated Ketones Synthesis of a,ß-Unsaturated Bromo Ketones. Tetrahedron 1973, 29, 1625–1628. 10.1016/S0040-4020(01)83408-6. [DOI] [Google Scholar]
- Cristau H.-J.; Toreiles E.; Morand P.; Christol H. Tribromures De Phosphoniums. Agents De Bromation De Substrats Organiques. Phosphorus Sulfur Relat. Elem. 1985, 25, 357–367. 10.1080/03086648508072751. [DOI] [Google Scholar]
- Mitani M.; Kobayashi T.; Koyama K. α′-Bromination of α,β-Unsaturated Ketones by an Electrochemical Procedure. J. Chem. Soc., Chem. Commun. 1991, 20, 1418–1419. 10.1039/C39910001418. [DOI] [Google Scholar]
- Pace V.; Castoldi L.; Holzer W. Synthesis of α,β-Unsaturated α′-Haloketones through the Chemoselective Addition of Halomethyllithiums to Weinreb Amides. J. Org. Chem. 2013, 78, 7764–7770. 10.1021/jo401236t. [DOI] [PubMed] [Google Scholar]
- a Laloo B. M.; Mecadon H.; Rohman M. R.; Kharbangar I.; Kharkongor I.; Rajbangshi M.; Nongkhlaw R.; Myrboh B. Reaction of Selenium Dioxide with Aromatic Ketones in the presence of Boron Trifluoride Etherate: A Protocol for the Synthesis of Triarylethanones. J. Org. Chem. 2012, 77, 707–712. 10.1021/jo201985n. [DOI] [PubMed] [Google Scholar]; b Rohman M. R.; Kharkongor I.; Rajbangshi M.; Mecadon H.; Laloo B. M.; Sahu P. R.; Kharbangar I.; Myrboh B. One-Pot Synthesis of Unsymmetrical Benzils by Oxidative Coupling Using Selenium Dioxide and p-Toluenesulfonic Acid Monohydrate. Eur. J. Org. Chem. 2012, 2012, 320–328. 10.1002/ejoc.201101012. [DOI] [Google Scholar]; c Shangpliang O. R.; Kshiar B.; Wanniang K.; Marpna I. D.; Lipon T. M.; Laloo B. M.; Myrboh B. Selenium Dioxide As an Alternative Reagent for the Direct α-Selenoamidation of Aryl Methyl Ketones.. J. Org. Chem. 2018, 83, 5829–5835. 10.1021/acs.joc.8b00558. [DOI] [PubMed] [Google Scholar]
- a Wohl A. Bromination of Unsaturated Compounds with N-Bromoacetamide. A Contribution to the Study of the Course of Chemical Processes. Ber. Dtsch. Chem. Ges. A/B 1919, 52, 51–63. 10.1002/cber.19190520109. [DOI] [Google Scholar]; b Koval I. V. N-Halo Reagents. N-Halosuccinimides in Organic Synthesis and in Chemistry of Natural Compounds. Russ. J. Org. Chem. 2002, 38, 301–337. 10.1023/A:1016390721218. [DOI] [Google Scholar]
- a Lee J. C.; Bae Y. H.; Chang S.-K. Efficient α-Halogenation of Carbonyl Compounds by N-Bromosuccinimide and N-Chlorosuccinimde. Bull. Korean Chem. Soc. 2003, 24, 407–408. 10.5012/bkcs.2003.24.4.407. [DOI] [Google Scholar]; b Das B.; Venkateswarlu K.; Mahender G.; Mahender I. A simple and efficient method for α-bromination of carbonyl compounds using N-bromosuccinimide in the presence of silica-supported sodium hydrogen sulfate as a heterogeneous catalyst. Tetrahedron Lett. 2005, 46, 3041–3044. 10.1016/j.tetlet.2005.03.020. [DOI] [Google Scholar]
- Adhikari M. V.; Samant S. D. Sonochemical bromination of Acetophenones usings p-toluenesulfonic acid-N-bromosuccinimide. Ultrason. Sonochem. 2002, 9, 107–111. 10.1016/S1350-4177(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Furniss B. S.; Hannaford A. J.; Rogers V.; Smith P. W. G.; Tatchell A. R.. Vogel’s Textbook of Practical Organic Chemistry, 4th ed., Longman, London, 1979, p 796. [Google Scholar]
- Awang D. V. C.; Wolfe S. Pyrrolidone hydrotribrornide: a brominating agent with selectivity for ketones. Can. J. Chem. 1969, 47, 706–709. 10.1139/v69-113. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.