Abstract
Central vasopressin (AVP) has been implicated in the control of multiple behaviors, including social behavior, anxiety-like behavior, and sickness behavior. The extent to which the different AVP-producing cell groups contribute to regulating these behaviors has not been extensively investigated. Here we test the role of AVP cells in the suprachiasmatic nucleus (SCN) in these behaviors by ablating these cells using viral-mediated, Cre-dependent caspase in male and female AVP-Cre+ mice and Cre− controls. We compared anxiety and social behaviors, as well as sickness behaviors (lethargy, anhedonia (indexed by sucrose consumption), and changes in anxiety-like- and social behavior) induced via injection of bacterial lipopolysaccharide (LPS). We found that SCN AVP cell ablation increased anxiety-like behavior and sucrose consumption in both sexes, as well as increased urine marking by males in a non-social context, but did not alter behavioral responses to sickness. Our data suggest that SCN AVP does not strongly affect LPS-induced behavioral changes, but may contribute to anxiety-like behavior, and may play a role in ingestive reward/motivation and fluid intake.
Keywords: Vasopressin, suprachiasmatic nucleus, anxiety, sickness, sucrose preference, LPS
Introduction
Animals do not live in isolation; they must respond to both internal and external contexts in order to thrive and reproduce. These needs are met by a combination of homeostatic physiological mechanisms and behavioral interactions. For many organisms, including humans, social behavior is critical for survival, and must be tuned to multiple contexts. One neuromodulatory system heavily involved in regulating both physiology and behavioral outputs is arginine vasopressin (AVP), a nonapeptide neuromodulator and hormone. AVP from various sources, such as the paraventricular nucleus of the hypothalamus (PVN), the bed nucleus of the stria terminalis (BNST), the supraoptic nucleus (SON), and the suprachiasmatic nucleus (SCN) (Rood and De Vries, 2011), is important for regulating stress responses, anxiety, social behaviors, and water balance in rats and mice (Caldwell, 2017; Caldwell et al., 2008; Dumais and Veenema, 2016; Gizowski et al., 2017; Neumann and Landgraf, 2012).
Additionally, AVP contributes to sickness: the body’s response to infection and inflammation. Activation of the immune system causes multiple physiological and behavioral changes, such as fever, altered metabolic processes, reduced activity, social withdrawal, anxiety-like behavior, and anhedonia (Dantzer et al., 2008; Hart, 1988; Kelley et al., 2003). AVP modulates some of these changes; for example, AVP from the BNST and medial amygdala reduces fever in male rats (Federico et al., 1992a, 1992b; Pittman et al., 1998b, 1998a; Sens et al., 2017). Deletion of AVP cells in the PVN of mice alters motivated behaviors, such as increasing anhedonia during sickness caused by lipopolysaccharide (LPS), a gram-negative bacterial coat protein (Whylings et al., 2020). AVP is, therefore, well-positioned to act in concert with other inflammatory-responding systems to generate physiological and behavioral responses to sickness.
The sources of AVP that regulate social and anxiety-like behaviors, in both healthy and sick contexts, are thought to be primarily AVP-expressing cells in the PVN and BNST (Caldwell, 2017; Caldwell et al., 2008; Dumais and Veenema, 2016; Kelly and Goodson, 2014). However, another major source of centrally-projecting AVP, the SCN (Hoorneman and Buijs, 1982; Rood et al., 2013), may also contribute to these behaviors. SCN AVP cells project to multiple midline nuclei, such as the PVN, paraventricular thalamus, dorsomedial hypothalamus, and preoptic area (Novak et al., 2000; Rood et al., 2013), several of which overlap with the social behavior neural network (Newman, 1999; O’Connell and Hofmann, 2012). SCN AVP cells are critical for coordinating biological clock function (Edwards et al., 2016; Kalsbeek et al., 2010) and behavioral circadian rhythms (Mieda et al., 2015; Yamaguchi et al., 2013), and have been implicated in regulating food intake (Santoso et al., 2017) and circadian thirst (Gizowski et al., 2018, 2016; Reghunandanan et al., 1992). However, the role of SCN AVP cells in regulating emotional and social behavior has not been directly studied.
In addition to circadian rhythms, the SCN as a whole modulates inflammatory response. Circadian rhythms alter responses to immune stimuli (Marpegan et al., 2009; Narasimamurthy et al., 2012), and immune challenges can disrupt circadian rhythms (Duhart et al., 2013; Marpegán et al., 2005; Palomba and Bentivoglio, 2008). Disruptions of the SCN, ranging from dim light exposure during the dark phase (Fonken et al., 2013) to ablation of the entire SCN (Guerrero-Vargas et al., 2014), increase the inflammatory response to endotoxin challenges. Although LPS has been shown to increase AVP release from SCN cells (Nava et al., 2000), it is unknown whether SCN AVP is involved in the changes in behaviors noted during sickness. If AVP in mice abates sickness behavior as it does in rats (Dantzer et al., 1991) and AVP cells in the SCN contribute to this effect, we predict that ablation of these cells will enhance sickness behavior.
Here, we directly test the role of SCN AVP cells in social and emotional behavior during health and in sickness in mice across two experiments. In the first experiment, we measured the effects of selectively ablating SCN AVP cells on anxiety-like and social behaviors in healthy mice. In the second experiment, we examined whether deletion of SCN AVP cells would alter behaviors known to change during LPS-induced sickness.
Methods
Animals
All mice were maintained at 22°C on a 12:12 reverse light cycle with food and water available ad libitum, housed in individually ventilated cages (Animal Care Systems, Centennial, CO, USA), and provided with corncob bedding, a nestlet square, and a housing tube. All animal procedures were approved by the Georgia State University Institutional Animal Care and Use Committee (IACUC) and were in accordance with the regulations and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Subjects
AVP-IRES2-Cre-D (AVP-Cre) mice were obtained from The Jackson Laboratory (Stock No: 023530; Bar Harbor, Maine, USA). AVP-Cre knockin mice have Cre recombinase expression directed to vasopressin-expressing cells that are restricted to populations within the hypothalamus. Subjects were derived by crossing heterozygous Cre+ mutants to wildtype C57Bl/6J mice and genotyped (ear punch) by polymerase chain reaction (PCR) at 21–24 days of age (Transnetyx, Cordova, TN, USA). Both Cre+ and Cre− littermates were used in behavioral experiments. A total of 54 adult experimental mice, from 12 different litters, were tested: 14 Cre− males, 11 Cre− females, 16 Cre+ males, and 13 Cre+ females.
Stimulus Animals
Stimulus animals, adult C57B6/J and CD-1 mice of both sexes, were housed in same-sex groups. CD-1 female stimulus mice (25–27g) were ovariectomized and implanted subcutaneously with a Silastic implant of estradiol (0.7 cm active length; 1.02 mm inner diameter, 2.16 mm outer diameter, Dow Corning, Midland, MI, USA; 1:1 estradiol benzoate:cholesterol). Before any testing for sex behavior (social experience and copulatory behavior, as described below), female stimulus mice were injected with 0.1mL progesterone (500 μg dissolved in sesame oil, Sigma, St. Louis, USA) to induce behavioral estrus. Male CD-1 stimulus (28–31g) mice were gonadectomized, and group-housed. A subset of male CD-1 stimulus mice, receiving subcutaneous implants of testosterone (0.7 cm active length; 1.02 mm inner diameter, 2.16 mm outer diameter, Dow Corning, Midland, MI, USA; crystalline testosterone, Sigma, St. Louis, USA) and singly-housed, were used for providing social experience and copulatory behavior. Surgery and implant procedures were performed as described previously in detail in Rigney et al., 2020, 2019.
Social Experience
All subjects received social experience (described in detail in Rigney et al., 2020, 2019) prior to viral vector injections, as sexual and aggressive experiences promote communication behaviors in mice (Lumley et al., 1999; Roullet et al., 2011). Briefly, this experience consisted of two separate sexual experiences and two separate aggressive experiences, where each sexual experience was followed by an aggressive experience the subsequent day with at least one day between the first and second sets of sex-aggression experiences. Female subjects were given sexual experiences on days of estrus, as determined by visual analysis of epithelial cells collected via vaginal lavage two hours before sexual encounters.
Sexual experience was provided by placing a sexually-experienced, hormone-implanted, opposite-sex CD-1 stimulus mouse overnight in the subjects’ home cage (first experience) or for ninety minutes (second experience). For aggressive experience, subjects were exposed to a same-sex, non-territorial (gonadectomized and group-housed), CD-1 mouse as an intruder in the subject’s home cage. The stimulus animal was removed after the subject’s first offensive attack (biting) or after ten minutes if no fighting occurred. Female subjects were exposed to female intruders; however, this did not elicit attacks from subject or intruder. Male subjects that did not copulate or attack during testing and female subjects that did not copulate were removed from the study.
Viral Vectors
AVP driven-, Cre-expressing SCN neurons were ablated using an adeno-associated virus (AAV; serotype 2/1 (3×1012 IIU/mL) AAV-flex-taCasp3-TEVp; UNC Vector Core) that encodes, in a Cre-dependent fashion, a mutated pro-caspase-3 and its activator (TEVp). This system activates an apoptotic signaling cascade, cleaving multiple structural and regulatory proteins critical for cell survival and maintenance (Unger et al., 2015; Yang et al., 2013) and killing cells with far less collateral inflammation than other lesion approaches (Morgan et al., 2014; Rigney et al., 2020).
Stereotaxic surgery
All surgeries were carried out using 1.5–3% isoflurane gas anesthesia in 100% oxygen; 3 mg/kg of carprofen was given before surgery to reduce pain. Mice were positioned in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) with ear and incisor bars holding bregma and lambda level. After a midline scalp incision, a hand operated drill was used to make holes in the skull, exposing the dura. For all subjects, 250 nl of AAV-flex-taCasp3-TEVp was delivered bilaterally to the SCN (coordinates: −0.2 mm AP; ± 1.73 mm ML; −5.74 mm DV, 15 degree angle) (Paxinos and Franklin, 2012) at a rate of 100 nl/min using a 5 μl Hamilton syringe with a 30-gauge beveled needle mounted on a stereotaxic injector. Following virus delivery, the syringe was left in place for 15 minutes and then slowly withdrawn from the brain. After surgery, subjects recovered for three weeks prior to behavioral testing.
Experiment 1: Effects of ablation of SCN AVP cells on social and emotional behaviors
All testing occurred within the first eight hours of the dark cycle under red light illumination. All tests were scored by an experimenter blind to the genotype of the subject. On experimental days, subjects were adapted to the experimental room for fifteen minutes prior to testing. First, we tested mice on an elevated plus maze to test for anxiety-related behavior. Mice were then tested in the three-chamber apparatus over five days. Lastly, copulatory and aggressive behavior were measured sequentially in the subject’s home cage. Female subjects were tested irrespective of estrous cycle day, except during copulation testing, when they were in behavioral estrus, confirmed by analysis of vaginal cell morphology following vaginal lavage (see above).
Elevated-Plus Maze
The elevated plus maze (EPM) consisted of two open arms (30 × 5 × 0 cm) and two closed arms (30 × 5 × 25 cm) crossed perpendicularly and raised 60 cm above the floor. Subjects were placed in the intersecting center zone and were habituated to the apparatus for one minute; subjects’ behavior was scored over the following five minutes. Due to software failure, results from a subset of subjects could not be reliably quantified; behaviors from the remaining subjects (9 Cre− males, 5 Cre− females, 8 Cre+ males, 5 Cre+ females, all of which met inclusion criteria based on cell reduction) were recorded with a digital video recorder, and scored by an observer using Noldus Observer XT 11 software (Leesburg, VA, USA). Behaviors measured include time spent in open and closed arms, open arm entries, and the number of risk assessment behaviors (stretch-attend posture, head-dips; Rodgers and Cole, 1993). Three subjects (2 Cre− males, 1 Cre+ male) were removed from EPM data analysis because they fell off the maze during testing.
Social Behavior and Communication
Ultrasonic vocalizations (USV), urine marking, and social investigation were recorded in an acrylic three-chamber apparatus (Harvard Apparatus, Holliston, MA, USA; dimensions: 20.3 × 42 × 22 cm). Subjects were all acclimated to the apparatus over two days of exposure (2 mins) to the testing area and 3-chamber apparatus. During the three days of behavior testing, mice were first exposed to the apparatus with no stimuli in either chamber; the following two testing days subjects were exposed to male and female CD-1 stimulus animals in separate tests. CD-1 mice were used as stimulus animals because strain differences in mice increase social investigation (Gheusi et al., 1994). Instead of a solid floor, the apparatus was placed on absorbent paper (Nalgene Versi-dry paper, Thermo Fisher Scientific, Waltham, MA, USA) so as to accurately measure urine marking. During testing with stimulus animals, subjects had access to either a CD-1 stimulus animal in a cylindrical cage (8 cm (D), 18 cm (H); 3 mm diameter steel bars, 7.4 mm spacing) or an empty cage placed in the outer chambers of the apparatus. Subjects and stimulus animals had limited ability to directly contact each other; they were able to pass extremities (e.g. paws, tail) through the smaller cage bars during investigation. The location of stimulus and the “clean” cage were counterbalanced across subjects. After placing the subject in the center of the middle chamber, we measured across a 5-minute trial close investigation of clean and stimulus cages as well as USV and urine marking, as described below. After testing, the apparatus and cages were thoroughly cleaned with 70% ethanol and allowed to dry before further testing. The order of male and female stimuli presentation was counterbalanced across subjects.
Social Investigation and Ultrasonic Vocalizations
Close investigation of stimulus cages was defined as time spent sniffing within 2 cm of each cage. USV emanating from mice within the apparatus were detected using a condenser microphone connected to an amplifier (UltraSoundGate CM16/CMPA, 10 kHz – 200 kHz, frequency range) placed 4 cm inside the apparatus and directly above the center compartment. USV were sampled at 200 kHz (16-bit) with target frequency set to 70 kHz (UltraSoundGate 116Hb, Avisoft Bioacoustics, Berlin, Germany). Recordings were then analyzed using a MATLAB (MATLAB, Mathworks, RRID:SCR_001622) plug-in that automates USV analysis (MUPET, Van Segbroeck et al., 2017). Using this program, sonograms were generated by calculating the power spectrum on Hamming-windowed data and then transformed into compact acoustic feature representations (Gammatone Filterbank). Each 200-millisecond window containing the maximum USV syllable duration was then clustered, via machine learning algorithms, into USV syllable types (repertoire units) based on time-frequency USV shape. Repertoire units that appeared as background noise were discarded. As the number of USV syllables produced is low in this strain, we limited our analysis to the total number of USV syllables produced during each test.
Urine Marking
Following testing, each substrate sheet was allowed to dry and was then sprayed with ninhydrin fixative (LC-NIN-16; Tritech Forensics Inc., Southport, NC, USA) to visualize urine marks. After twenty-four hours, sheets were imaged with a digital camera and then analyzed using imaging software (ImageJ, RRID:SCR_003070). Visualized marking was outlined and areas measured and summed. Urine marking was measured as the total area (pixels) of visualized ninhydrin urine marks in the entire arena. Areas of urination that were larger than 6 cm2 and directed toward corners were counted as elimination ‘pools’, not urine marks, and were removed from analysis (Bishop and Chevins, 1987).
Copulatory and Aggressive Behavior
To measure copulatory behavior, the stimulus mouse was placed in the subject’s home cage for ninety minutes. The latency to mount and intromit were recorded; if neither occurred, they were scored as maximum time (90 minutes). To measure territorial aggression, stimulus animals were placed in the subject’s home cage and then removed after the subject’s first offensive attack (biting) within a ten-minute period; the latency to this attack was recorded. If subjects did not attack, this was scored as maximum time (10 minutes).
Odor Discrimination
At the end of experiment 1, a subset of subjects (9 Cre−, 5 Cre+) were tested for their olfactory ability using a habituation-discrimination approach that measured whether subjects could distinguish between social and non-social odors,; this was done to confirm that cell ablation did not cause olfactory deficits. Subjects were repeatedly exposed (five times; two minute trials; 1 minute intertrial intervals) in their home cage to filter paper containing one odorant. Afterwards, the stimulus was replaced with a different odor which was then also repeatedly presented. Subjects were first exposed to distilled water, then to one of three non-social odors (lemon extract, almond extract, coconut extract, Kroger, Cincinnati OH, USA), then to a second non-social odors (counterbalanced), followed by a social odor (male or female urine), and then by the other social odor (female or male urine, counterbalanced). Time investigating each odor was recorded, and the time investigating both the first and last exposure to each odor were used to analyze habituation and discrimination.
Experiment 2: LPS-induced sickness behavior
At least one week after the behavioral tests in Experiment 1, subjects were tested for their behavioral changes following LPS-induced sickness. Subjects were weighed and injected intraperitoneally with either 0.5 mg/kg LPS (from E. coli 0111:B4, Sigma, St. Louis, MO, USA) or sterile saline one hour before dark phase (ZT11). As described in previous experiments (Whylings et al., 2020, 2019), the open field test (OFT) was conducted three hours following LPS injections, and the elevated zero maze (EZM) test was conducted immediately following the OFT. Sucrose preference was then assessed in the home cage during the following 24-hour period. The day after LPS or saline injections, subjects were tested during the dark phase in the three-chamber apparatus for social preference and novelty detection. Subjects were tested twice, first within ~24 hours after LPS/Saline, then again one week later after Saline/LPS in a counterbalanced manner. In all cases, subjects were acclimated to the behavior testing suite for at least one hour before testing, and tests were done during the dark phase under dim red lighting.
Open-Field and Elevated Zero Maze
Three hours after LPS injections, subjects were placed in an open field chamber (43 cm × 43 cm × 30 cm) for 10 min and behavior was automatically tracked via breakage of infrared beam (Med Associates, Fairfax, VT, USA). Distance traveled and time spent in the (anxiogenic) center area were analyzed as measures of locomotion and anxiety-like behavior, respectively (Gould et al., 2009; Walsh and Cummins, 1976). Immediately after OFT, subjects were tested on an elevated zero maze (EZM). This apparatus consists of a 5.5 cm wide circular platform (internal diameter 35 cm) raised 50 cm off the ground, with two equally spaced enclosed compartments covering half of the platform. Video was manually scored (Noldus Observer) by an observer blind to subject genotype for time spent in both open and closed arms as a measure of anxiety-like behavior, and for zone crosses (subject crossing from open to closed arm and vice versa) as a measure of activity.
Sucrose Preference
For at least 2 days before LPS/saline injections, subjects were acclimated to having two water bottles placed in their home cage. After OFT/EZM assessment, subjects were returned to their home cage, and bottles were replaced with pre-weighed bottles, one containing sucrose solution (2.5% in tap water) and the other tap water. Subjects had access to both sucrose solution and water for the next 20 hours, until the start of the next day’s testing. The bottles were then removed and weighed to measure consumption. A control water bottle in a nearby empty cage showed a <1 g loss over the same period and room conditions. Sucrose consumption, water consumption and preference, calculated as the percentage of sucrose consumed of total consumption (Sucrose / (Sucrose + Water) *100%), were analyzed.
Social Preference and Social Novelty Preference
To measure social preference and social novelty, subjects were tested in the three-chamber apparatus as described above, 26 hours after LPS injection. Subjects were placed in the apparatus for 5 min before testing to habituate the subjects to the environment and were then temporarily removed while stimulus animals/objects, contained within smaller cages (8 cm diameter, 18 cm height, 3-mm diameter bars, 7.4 mm spacing) were placed in the center of each of the two outer chambers. First, to test for social preference, a novel toy object (either a mouse, robot, or small car figurine) and a novel same-sex C57 stimulus animal were placed in opposite cages. Subjects and stimulus animals had limited ability to directly contact each other; they were able to pass extremities (e.g. paws, tail) through the smaller cage bars during investigation. The subjects were then returned to the apparatus and allowed 10 min to explore the apparatus. At the end of this test, the subjects were removed again, and the toy object replaced with a novel stimulus animal (from a different cage from the first stimulus) to test for recognition of social novelty. The subject was then placed into the center chamber again and given 10 min to explore the apparatus. The position of object and original stimulus animal was counterbalanced across trials but did not change between social preference and social novelty preference tests. Videos were manually scored (Noldus Observer XT 11, Leesburg, VA, USA) by an observer blind to subject genotype for: outer chamber entries as a measure of activity, time spent in each chamber, and close investigation, defined by the subject’s snout within 2 cm of the stimulus cage. The percentage of the total time spent directly investigating the stimulus animal or the novel stimulus animal was used as a measure of preference for social interaction and social novelty, respectively.
Histology and In Situ Hybridization
Following all testing, subjects were killed via CO2 asphyxiation. Brains were extracted and flash-frozen via submersion in 2-methyl-2-butanol (Sigma, St. Louis, MO, USA) for 10–20 s and stored at −80°C until sectioned into 3 series of 20 μm. Tissue was sectioned and labeled for AVP mRNA (accession number NM_027106.4) via fluorescent in situ hybridization as described in detail in previous work (Rigney et al., 2020, 2019). Bilateral images of the SCN were taken at 20x magnification using a Zeiss Axio Imager.M2 microscope (Carl Zeiss Microimaging, Göttingen, Germany), and analyzed using ImageJ (Schindelin et al., 2012, National Institute of Health, Bethesda, MD, USA). The SCN was outlined, and fluorescent cells were manually counted in each hemisphere of the SCN. Cell counts and SCN area were summed and averaged across each subject to generate an overall SCN cell/area metric which we then compared across ablated and control subjects. Ablated subjects were included in the analysis if they had > 30% SCN AVP-expressing cell ablation overall and >50% deletion within at least one hemisphere. Additionally, AVP cells were counted and analyzed in a similar way for the PVN to ensure there was no off-target cell ablation.
Data Analysis
In Experiment 1, the number of remaining AVP cells per area and EPM data were analyzed for effects of genotype and sex using a two-way ANOVA. Social investigation was analyzed using a mixed-model, four-way ANOVA, using stimulus identity (animal or empty cage) and stimulus sex (male or female trials) as within-subject variables, and genotype (Cre+, Cre−) and subject sex (male, female) as between-subject variables. Due to substantial baseline sex differences, as seen in this and previous studies (Rigney et al., 2020, 2019), urine marking, USV, sex behavior, and aggressive behavior were analyzed separately by sex to focus solely on effects of genotype. Data that met requirements for parametric testing were analyzed using independent t-tests. Urine marking and USV data did not meet normality or sphericity requirements for repeated-measures testing, and thus were analyzed separately by trial (control, male stimulus, female stimulus). Urine marking data were transformed using a Box-Cox procedure followed by t-tests, and USV data was analyzed using a nonparametric Kolmgorov-Smirnoff test.
For odor habituation and discrimination tests, we compared the amount of time Cre+ and Cre− subjects spent investigating each odor on the first and last presentation of each odor (habituation) and then between the last presentation of the familiar odor and the first presentation of the novel odor (discrimination), using mixed-model ANOVA (investigation time as a within-subjects factor, genotype and sex as between-subjects factors).
In Experiment 2, data from OFT, EZM, sucrose preference, and social preference percentage were analyzed using a mixed-model, three-way ANOVA, using treatment (LPS, Saline) as within-subjects variable and both subject sex and genotype as between-subject variables.
Results with p-values equal to or less than 0.05 were considered statistically significant, and partial eta squared used as a measure of effect size. Degrees of freedom were not adjusted in our mixed model ANOVA analyses as all data sets maintained sphericity (Mauchly’s test of sphericity). Post-hoc t-tests were used to analyze genotype differences following significant ANOVA main effects and interactions. In addition, we calculated Pearson’s correlation coefficients to analyze the relationship between SCN AVP cell loss and behavior, using the number of remaining SCN AVP cells/mm2 in Cre+ subjects. All statistical analysis was conducted using SPSS 27 (IBM).
Results
Fluorescent in situ hybridization and cell ablation
Viral expression of Cre-dependent caspase caused a marked reduction in AVP cells in the SCN. Cre+ subjects were kept in the analysis if there was at least a 30% reduction of cell count/area as compared to Cre− controls, and 50% reduction in at least one hemisphere. 4 Cre+ males and 6 Cre+ females were removed from analyses for not meeting these criteria. The average reduction in ablated Cre+ mice was 53.55 ± 3.75 % of controls. There was a significant difference between the cell count/area of Cre− and Cre+ subjects (F 1, 37 = 51.33, p < 0.001, ηp2 = 0.58), with no effect of sex (F 1, 37 = 3.30, p = 0.82, ηp2 = 0.082). Cell reductions occurred throughout the anterior-posterior axis of the SCN, with no clear directional bias. Figure 2 shows representative photomicrographs of AVP labeling in a Cre− (control) and Cre+ (ablated) subject. We detected no difference in the number of AVP cells in the PVN Cre+ and Cre− mice (F 1, 37 = 0.10, p = 0.75, ηp2 = 0.003), confirming that cell ablation did not affect AVP cells in nearby areas. Most behavioral measures were not significantly correlated with the number of remaining SCN AVP cells (all p < 0.05, see supplementary table), with one exception. Preference for social novelty after saline treatment was positively correlated with the number of remaining AVP cells (R = 0.54, p = 0.016).
Figure 2.
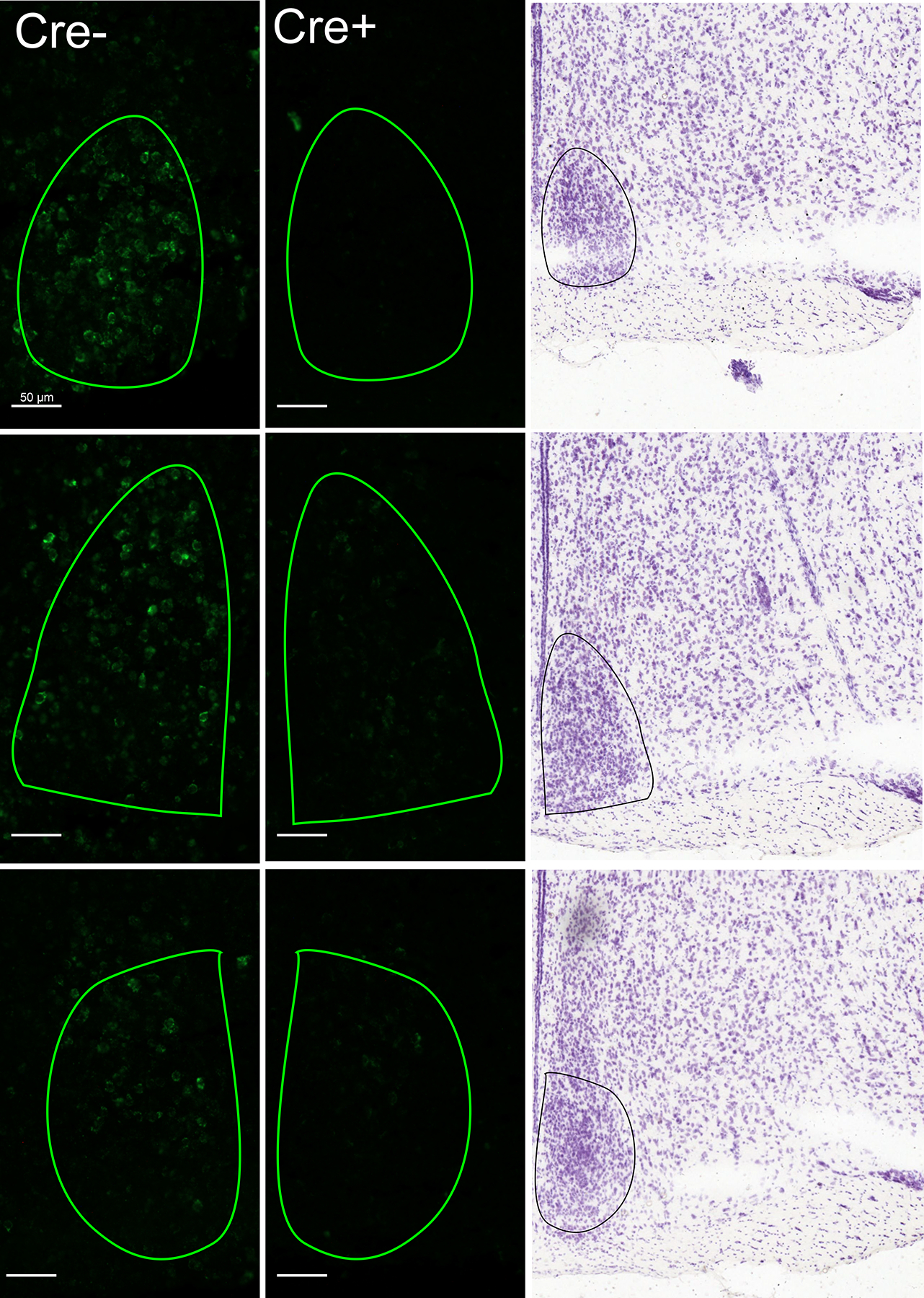
AVP fluorescent in situ hybridization. Example photomicrographs of unilateral SCN (outlined) in both a Cre− control (left) mouse and a Cre+ (right) mouse. Reference images adapted from Allen Mouse Brain Reference Atlas (2011; Lein et al., 2007).
Experiment 1: Effects of ablation of SCN AVP cells on social and emotional behaviors
Elevated Plus Maze
SCN AVP cell ablation increased anxiety-like behavior in the EPM. Cre+ mice spent significantly less time in the open arms of the elevated plus maze (F 1, 20 = 5.95, p = 0.024, ηp2 = 0.23; Fig 3), and more time in the closed arms of the maze (F 1,20 = 5.11, p = 0.035, ηp2 = 0.2), with no effect of sex. Cre+ mice produced fewer head dips (F 1,20 = 6.430, p = 0.02, ηp2 = 0.24; Fig 2C), but did not change the amount of stretch-attends compared to controls (F 1, 20 = 0.045, p = 0.83, ηp2 = 0.055). Activity, as measured by the total number of entries into all zones, did not differ between genotypes (F 1,20 = 0.27, p = 0.61, ηp2 = 0.013).
Figure 3.
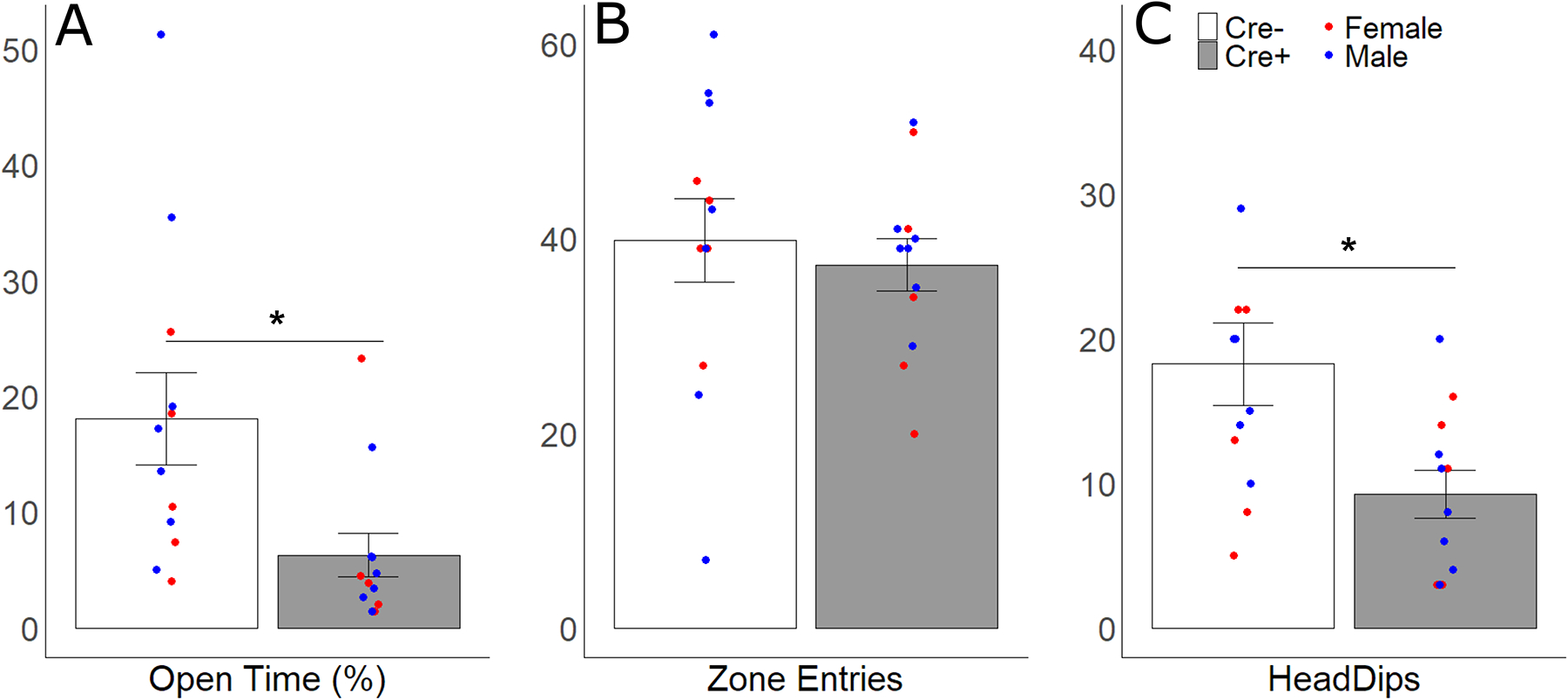
Anxiety-like behaviors in the elevated plus maze. Mean ± SEM of (A) percentage of total time that was spent in the open arms, (B) number of entries into the open and closed arms, and (C) number of head dips for Cre− (white bars) and Cre+ (gray bars) subjects. Points represent individual data from males (blue points) and females (red points); asterisks represent significant (p < 0.05) genotype differences.
Social Investigation and Communication
For all subjects, there was a significant difference between investigation of the empty cage and the stimulus cage. All subjects spent more time investigating social stimuli than the empty chamber (F 1, 40 = 45.40, p < 0.001, ηp2 = 0.53; Fig 4). There were no other significant main effects or interactions of subject genotype and other factors. Neither sex (F 1,40 = 0.64, p = 0.43, ηp2 = 0.017) nor genotype (F 1,40 = 1.14, p = 0.29, ηp2 = 0.029) altered total investigation time of empty chambers during the initial clean control trial.
Figure 4.
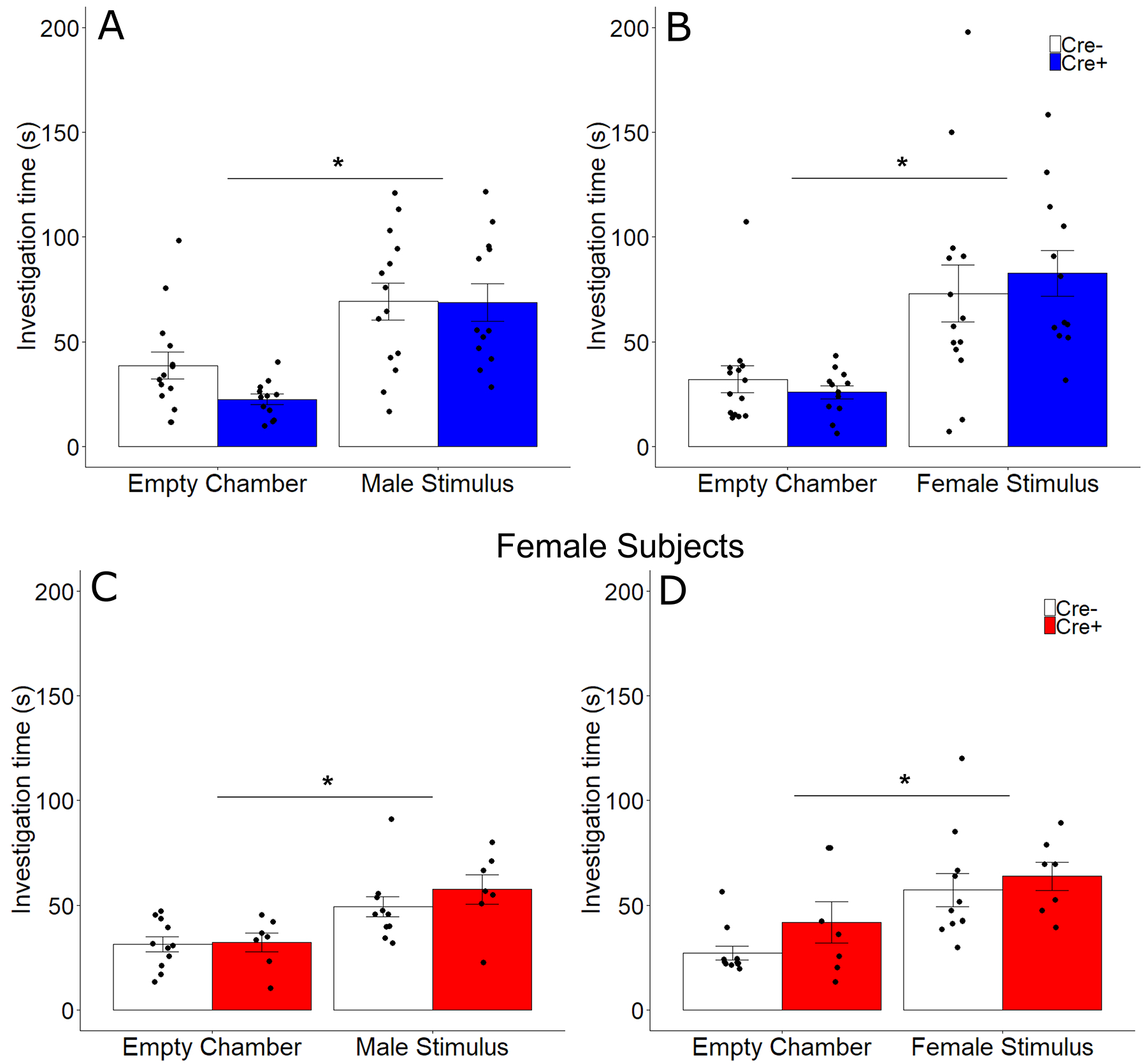
Investigation of male and female stimuli. Mean ± SEM of investigation time of male (A, C) and female (B, D) stimulus animals compared to a clean cage by male (A, B) and female (C, D) subjects in both Cre− (white bars) and Cre+ (filled bars) subjects. Points represent individual data and asterisks represent significant (p < 0.05) investigation of stimulus animals.
As mouse communication behavior varies significantly based on the sex of both the subject and stimulus (Rigney et al 2019, 2020), urine marking and USV were analyzed seperately for both sexes and each trial (clean control, male stimulus, female stimulus). In response to the clean environment, Cre+ (ablated) males deposited more urine marks than Cre− (control) males (t 24 = 2.58, p = 0.02, d = 1.07; Fig 5); there was, however, no genotype differences between female subjects in urine mark deposition (t 24 = 0.31, p = 0.76). Urine marking to social cues (male or female stimuli) did not differ according to genotype in male or female subjects. Genotype did not alter urine marking in either sex during investigation of male (Male subjects: t 24 = 0.41, p = 0.69; Female subjects: t 16 = 0.008, p = 0.99) or female stimulus animals (Male subjects: t 24 = 1.12, p = 0.27; Female subjects: t 16 = 0.57, p = 0.78). There were no differences between genotypes in USV production during any trial for involving male subjects (clean: K-S D =0.76, p = 0.62; male stimulus: K-S D = 0.42, p = 0.94; female stimulus: K-S D = 0.94, p = 0.34) or female subjects (clean: K-S D =0.45, p = 0.99; male stimulus: K-S D = 0.43, p = 0.99; female stimulus: K-S D = 0.99, p = 0.28).
Figure 5.
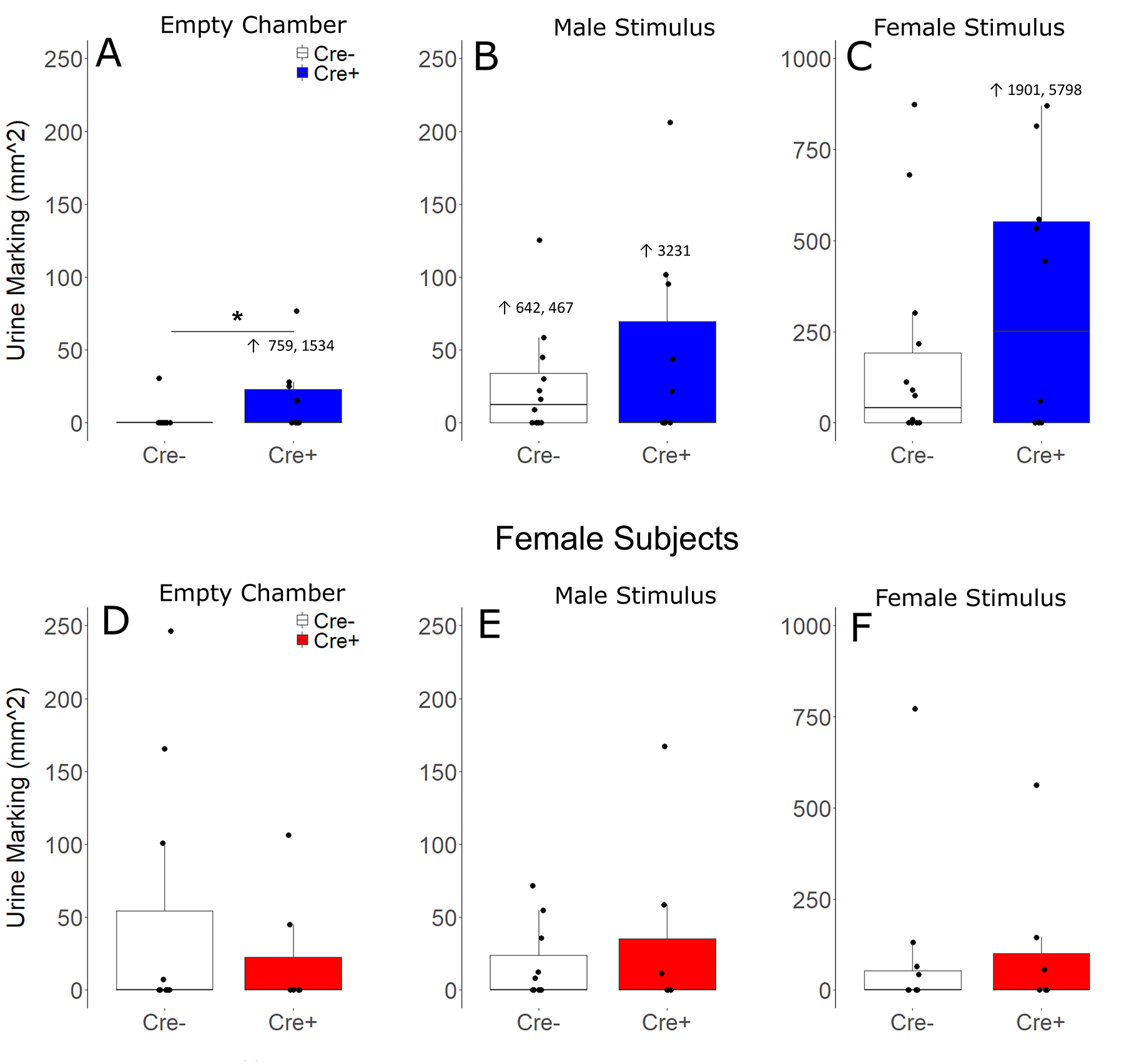
Urine marking. Median and interquartile range of urine marked area (mm2) when subjects were presented with empty chambers (A, D), male stimuli (B, E), and female stimuli (C, F), for both male (A, B, C) and Female (D, E, F) subjects. Points represent individual subjects. Outliers that are greater than the scale of boxplots are represented as numbers rather than data points.
Sex, and Aggression
There were no significant differences between genotypes of either sex in mount latency (Males: t 24 = 2.00, p = 0.068; Females: t 16 = 1.32, p = 0.19), intromission latency (Males: t 24 = 1.7, p = 0.099; Females: t 16 = 0.51, p = 0.62), or attack latency (Males: t 24 = 0.054, p = 0.96; Females: t 16 = 0.95, p = 0.36).
Odor Habituation and Discrimination
Of the subset of subjects tested, all showed habituation in their investigation to repeated exposures to both non-social (first non-social odor test: F 1,10 = 14.376, p = 0.0004, ηp2 = 0.59; second non-social odor test: F 1,10 = 37.917, p < 0.001, ηp2 = 0.34) and social odors (first social odor test: F 1,10 = 116.696, p < 0.01, ηp2 = 0.921; second social odor test: F 1,10 = 51.54, p < 0.001, ηp2 = 0.837). Subjects also discriminated between non-social odors (F 1,10 = 14.218, p = 0.004, ηp2 = 0.59), social odors (F 1,10 = 50.65, p < 0.001, ηp2 = 0.84), and between a non-social and social odor (F 1,10 = 204.7, p < 0.001, ηp2 = 0.953).
Experiment 2: LPS-induced sickness behavior
Open Field (OFT) and Elevated Zero Maze (EZM)
In the OFT, treatment with LPS reduced time spent in the center zone by all subjects (F 1,38 = 17.79, p < 0.001, ηp2 = 0.32; Fig 6); two subjects did not move from their initial placement in the testing environment and so were removed as outliers (both were > 3 standard deviations above the mean). No other main effects or interactions were detected in the OFT. LPS treatment also reduced distance traveled, a measure of overall activity (F 1,38 = 731.53, p < 0.001, ηp2 = 0.95), with no other significant main effects or interactions. Unexpectedly, treatment with LPS did not alter time spent in the open arms in the EZM; however, LPS treatment did decrease the number of entries into the open arm (F 1, 40 = 90.81, p < 0.001, ηp2 = 0.69), as well as decreasing the total number of head-dips (F 1, 40 = 110.52, p < 0.001, ηp2 = 0.73) and stretch attends (F 1, 40 = 556.83, p < 0.001, ηp2 = 0.93). There were no other significant main effects or interactions in EZM test metrics.
Figure 6.
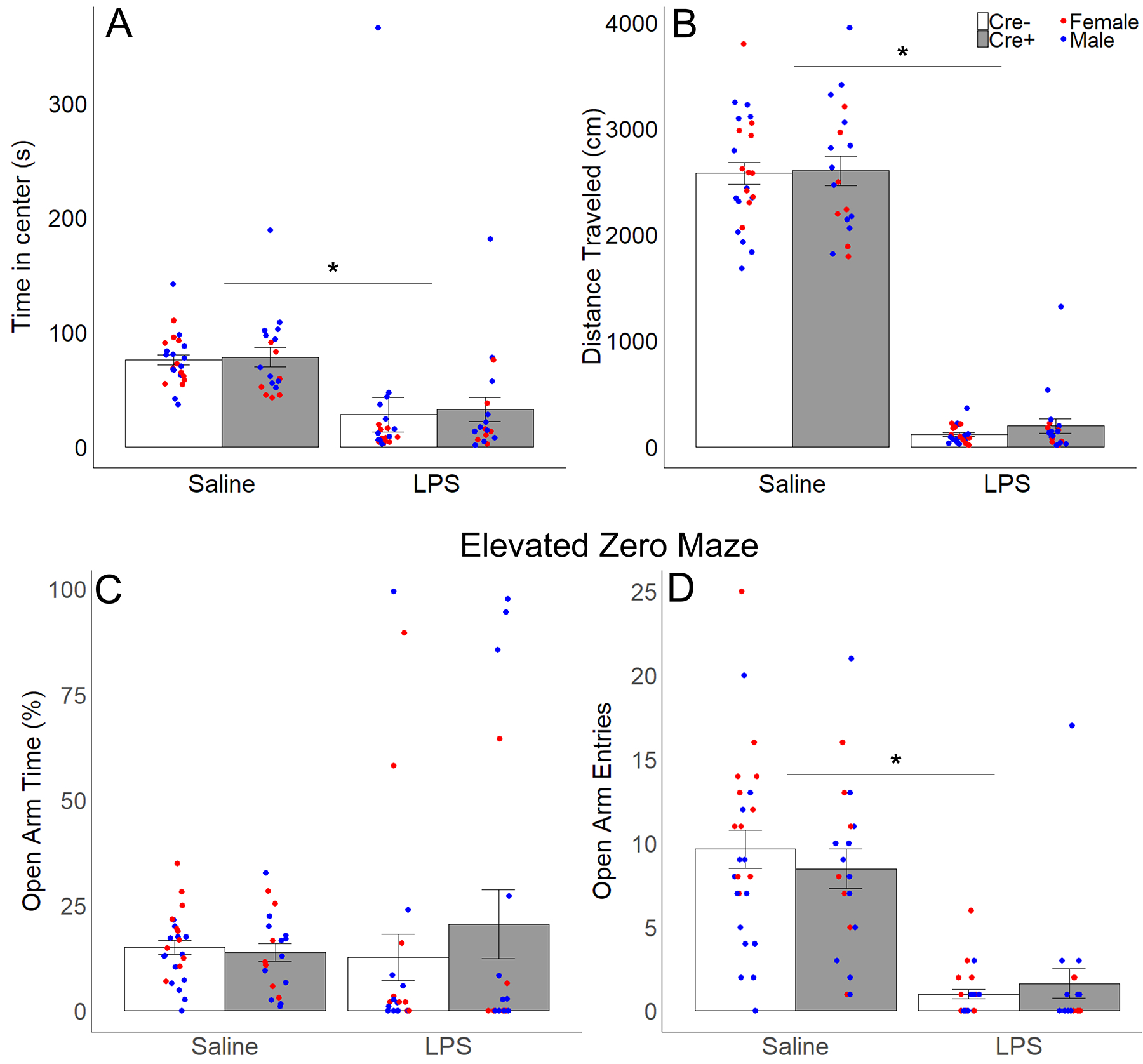
Anxiety-like behavior and locomotion in the open field test (OFT) and elevated zero maze (EZM). Mean ± SEM of (A) time in the center zone and (B) distance traveled in the OFT; (C) percentage of total time that was spent in the open arms and (D) number of entries into the open arms in the EZM for Cre− (white bars) and Cre+ (gray bars) subjects after LPS and saline treatment. Points represent individual data from males (blue points) and females (red points); asterisks represent significant (p < 0.05) treatment differences (repeated measures).
Sucrose Preference Test
Treatment with LPS reduced preference for sucrose compared to water (F 1,40 = 31.81, p < 0.001, ηp2 = 0.44; Fig 7), with no effect of genotype or sex. Both sucrose consumption (F 1,40 = 210.62, p < 0.001, ηp2 = 0.84) and water consumption (F 1,40 = 12.62, p = 0.001, ηp2 =0.24) were reduced by LPS injections. However, only the consumption of sucrose showed a significant effect of genotype (F 1,40 = 5.54, p = 0.024, ηp2 = 0.12). Mice with AVP cell ablations in the SCN (Cre+) consumed more sucrose than Cre− controls, although this did not affect the overall preference (percentage of sucrose consumed). There were no significant effects of genotype on water consumption, and no main effects or interactions with sex on any measure. Additionally, there was no genotype effect on body weight (F 1,40 = 0.001, p = 0.97, ηp2 < 0.001), but an expected sex difference as males weighed more than females (F 1,40 = 47.29, p < 0.001, ηp2 =0.54).
Figure 7.
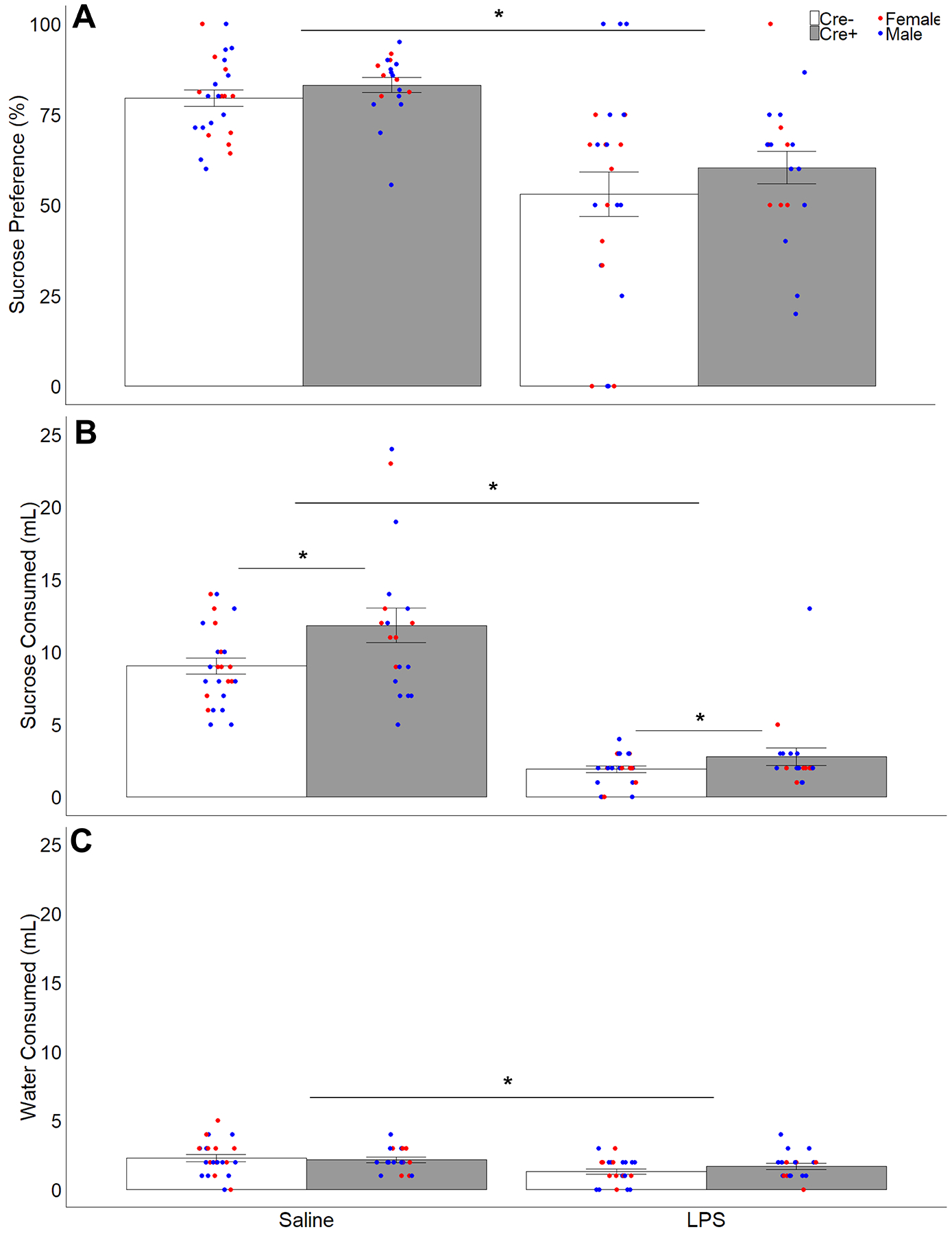
Fluid consumption and sucrose preference. Mean ± SEM of (A) sucrose preference (percentage of sucrose solution consumed of total fluid consumption), (B) sucrose solution consumed, and (C) water consumed during the sucrose preference test for Cre− (white bars) and Cre+ (gray bars) subjects after LPS or saline treatment. Points represent individual data from male (blue points) and female (red points) subjects; asterisks represent significant treatment differences (repeated measures) in all three measures, and significant genotype differences in sucrose consumption (p < 0.05).
Social Preference tests
During the social preference phase of testing, subjects showed a preference for investigating the animal stimulus over the object stimulus, spending more than 50% of their investigation time with the animal stimulus (Figure 8). LPS treatment of subjects increased their percent investigation of the animal stimulus (F 1,39 = 4.196, p = 0.047, ηp2 = 0.097), with no effect of sex or genotype. In the social novelty phase of testing, subjects spent greater than 50% of their investigation time with the novel animal stimulus compared to their investigation of the original stimulus animal, with no significant effects of treatment, sex, or genotype. In saline-treated Cre+ subjects, a positive correlation between the number of AVP cells remaining in SCN and their preference to investigate the novel stimulus animal was observed (R = 0.542, p = 0.016). LPS treatment did significantly reduce the number of chamber entries, a measure of general activity, across tests (F 1,39 = 153.82, p < 0.001, ηp2 = 0.80), with no effects of sex or genotype.
Figure 8.
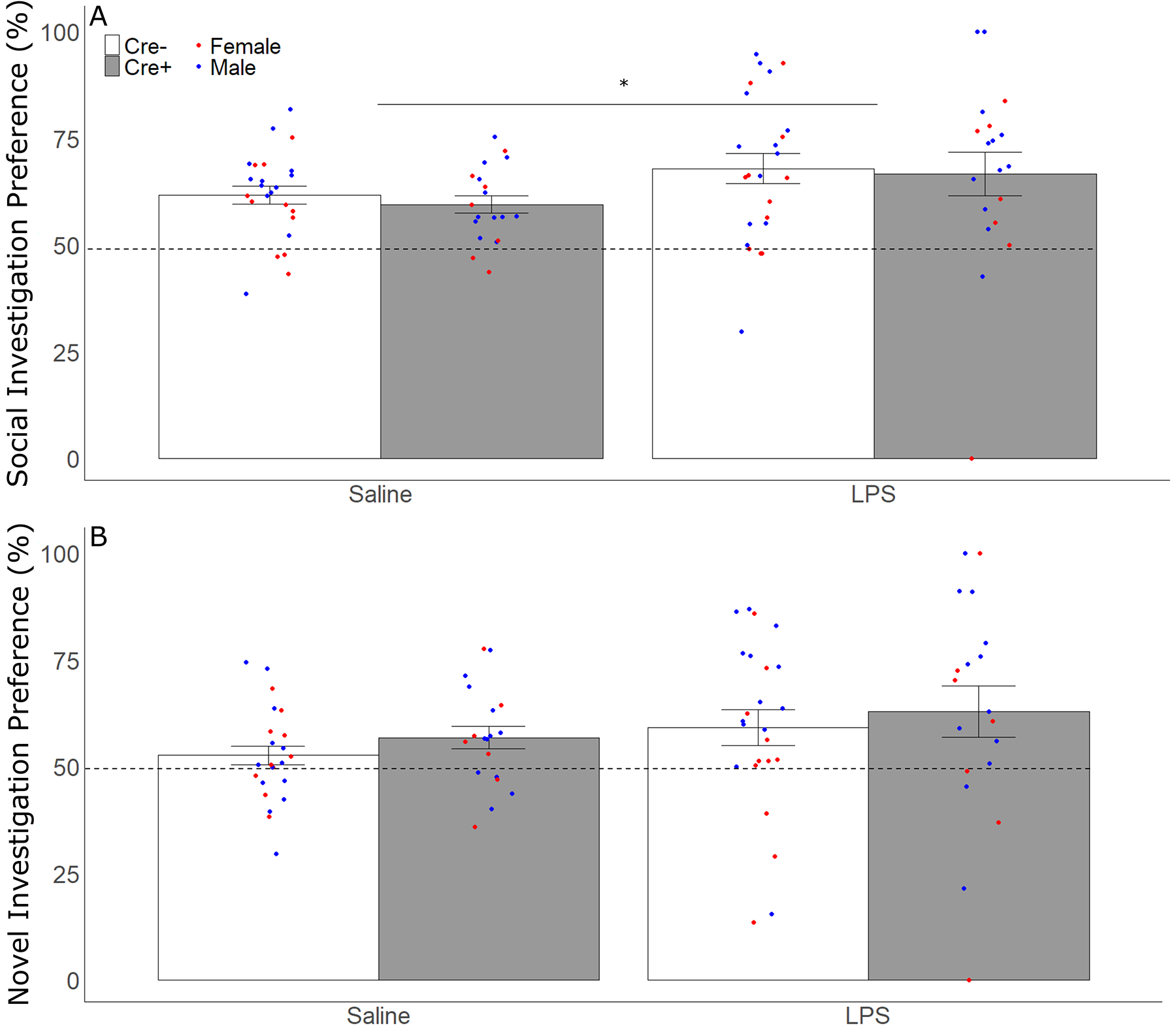
Social and Social Novelty Preference. (A) Mean ± SEM of the percentage of time subjects spent investigating the stimulus animal compared to total time investigating both animal and object stimuli in the social preference test for both Cre− (white bars) and Cre+ (gray bars) subjects after LPS and saline treatment (repeated measures). (B) Mean ± SEM of percentage of time spent investigating the novel stimulus animal over total time investigating both novel and original stimulus animal in the social novelty preference test for both Cre− (white bars) and Cre+ (gray bars) subjects after LPS and saline treatment (repeated measures). Points represent individual data from male (blue points) and female (red points) subjects; asterisks represent significant (p < 0.05) differences in stimulus investigation.
Discussion
Ablation of SCN AVP cells increased anxiety-like behavior and sucrose consumption in both sexes. It also specifically increased urine marking by males, but only in a non-social context. Reductions of AVP cells in the SCN did not significantly affect other social behaviors, social communication, odor discrimination, or the intensity of sickness behaviors. These results suggest a specific role for this cell population in modulating anxiety-like behavior and perhaps fluid intake.
AVP cells within the SCN were significantly reduced by our manipulation, but not entirely eliminated. As remaining AVP cells may have been sufficient for maintaining functions of this cell population, we cannot completely discount possible contributions of SCN AVP to social or sickness behaviors not affected by SCN AVP cell ablation. As SCN AVP cells express GABA (Kalsbeek et al., 2006; Mieda, 2020; Moore and Speh, 1993) and possibly other neuropeptides, it is possible that the effects we observed after SCN AVP cell reduction may be due to loss of other neuropeptides and GABA co-released from these cells rather than the loss of AVP signaling; more specific targeting of AVP production would be required to confirm its function within the SCN.
Reduction of SCN AVP cells did cause a significant increase in anxiety-like behaviors (decreased time in open arm, reduced exploratory head dips) in the elevated plus maze (EPM) in Experiment 1, but not when tested later in the open-field test (OFT) or the elevated zero-maze (EZM) in Experiment 2. As handling, injections, and repeated testing in anxiogenic environments can all reduce subsequent measures of anxiety-like behavior (Lapin, 1995; Tucker and McCabe, 2017; von Kortzfleisch et al., 2019), it is possible that the amount of handling and testing that took place in Experiment 1 reduced our ability to detect changes in anxiety-like behavior in Experiment 2.
The increase in anxiety-like behavior after SCN AVP ablation may be driven by its projections to nuclei such as the dorsomedial hypothalamus (DMH), midline thalamic nuclei, and the PVN (Kalsbeek et al., 2010; Rood et al., 2013), which are all implicated in anxiety-like behavior (Canteras et al., 2010; Kirouac, 2015). For example, AVP derived from the PVN regulates stress and anxiety-like behaviors (Bunck et al., 2009; Hernández et al., 2016) and AVP expression in the PVN is affected by AVP in the SCN. Reducing AVP expression in the SCN can both increase or decrease AVP content within the PVN, depending on PVN cell type: reducing AVP expression in the SCN by injecting monoclonal antibodies against AVP conjugated to neurotoxins increased AVP expression in magnocellular neurons but reduced it in the centrally projecting parvocellular neurons (Gomez et al., 1997). Consequently, ablating SCN AVP cells may have increased anxiety-like behavior by altering AVP expression in the PVN. Consistent with this idea, Cre-dependent ablations of AVP cells in the PVN caused similar increases in anxiety-like behavior in the EPM as observed in the present study (Rigney et al., 2020; Whylings et al., 2020).
Our experiments do not support a strong role for SCN AVP cells in abating sickness behavior. Although LPS treatment affected anxiety-like and reward-seeking behavior in the predicted direction (increased or reduced it, respectively), ablation of AVP cells did not affect the level of these changes. However, given the strong behavioral effects of LPS, we cannot fully eliminate a potential role for these cells in regulating these behaviors. It is possible that LPS-induced behavioral changes were at a ceiling and obscured more subtle effects due to AVP cell loss. A milder immune stimulus could reveal a role for these cells in regulating anxiety-like behaviors during sickness that was not seen in this study.
Although ablation of SCN AVP cells did not affect sucrose preference (all subjects preferred sucrose solution over plain water), it did increase the consumption of sucrose solution without changing contemporaneous plain water consumption in LPS as well as saline-treated mice. This suggests that ablating SCN AVP cells may have increased the motivation to consume sucrose (Meyerolbersleben et al., 2020). While these ablations increased anxiety-like behavior, the increase in sucrose consumption suggests that they did not cause depressive-like anhedonia, but rather the opposite. Increased sucrose consumption after SCN AVP cell ablation may also reflect an increase in caloric drive or fluid intake, as SCN AVP projections influence food and water intake (Gizowski et al., 2017, 2016; Santoso et al., 2017). However, mice did not gain weight after ablation of AVP cells in SCN, which would be expected if food intake was increased overall. Alternatively, ablation of SCN AVP cells may have altered thirst, thereby increased drinking. This could drive the increased consumption of the already greatly-preferred sucrose solution, instead of indicating a change in motivation. Future studies would be needed to better understand the role for SCN AVP in drinking and reward-related motivation.
The increased urine marking by males within the clean chamber after ablation of AVP cells in SCN may indicate alterations in the fluid balance that resulted in excessive urine production and elimination. However, this is unlikely as we did not detect changes in urine marking or eliminative pools in our other testing conditions, as would be expected by a general change in fluid balance. Moreover, increased consumption of sucrose solution was observed in both sexes, whereas females did not increase urine marking. Instead, the male-specific increase in urine marking may reflect a response to spatial novelty as mice, particularly males, show increased marking in clean environments (Hurst, 1987; Maruniak et al., 1974).
AVP within the SCN serves to coordinate its cellular clocks (Li et al., 2009; Mieda et al., 2016, 2015), and so the behavioral effects of reducing AVP in the SCN in this study may be due to altered circadian rhythms, despite animals being maintained on a consistent light cycle (12:12) throughout testing. Circadian phase influences activity in the EPM and alters sucrose consumption. In the EPM, general activity is greater during the active (dark) phase than the inactive (light) phase, but light phase does not change time spent in the open or closed arms (Beeler et al., 2006; Jones and King, 2001). In the present study, however, ablation of SCN AVP cells did not alter overall activity but did affect the time spent in open versus closed arms, suggesting that the observed effects on anxiety-like behavior are independent from general activity rhythms. Sucrose consumption is also higher during the active phase (Bainier et al., 2017), and potential circadian disruption could contribute to the increased sucrose solution intake in this study. However, the sucrose preference test as a measure of anhedonia was conducted over a 24-hour period, encompassing both active and inactive phases. Nevertheless, the effects of SCN AVP cell ablation on functions that show circadian rhythmicity, such as sucrose consumption, suggests that these cells might contribute to this rhythmicity.
Conclusion
Central AVP has been implicated in anxiety-related and social behaviors as well as in dampening sickness. Although our results did not indicate that SCN AVP cells may influence sickness behavior, they do suggest that SCN AVP cells regulate anxiety-like behaviors and sucrose consumption. The specific role of AVP in this, and the possibility that these cells may directly influence circadian rhythms in these behaviors, warrant further studies.
Supplementary Material
Figure 1.
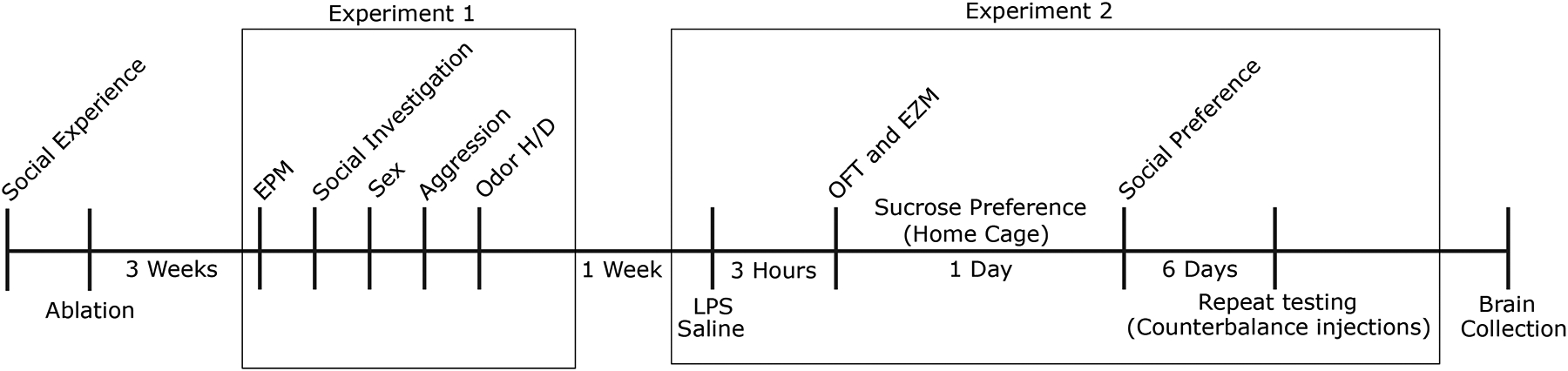
Timeline of experimental procedures and behavioral tests.
Table 1.
Sex and Aggressive behavior. Means ± SEM for male and female copulatory and aggressive behaviors.
| Cre− | Cre+ | ||
|---|---|---|---|
| Males | Mount Latency (s) | 519.79 ± 129.8 | 1825.75 ± 637.84 |
| Intromission Latency (s) | 1124.29 ± 370.94 | 2257.17 ± 568.756 | |
| Attack Latency (s) | 271.64 ± 68.236 | 266.36 ± 68.103 | |
| Females | Mount Latency (s) | 1897.27 ± 546.09 | 3204.43 ± 828.744 |
| Intromission Latency (s) | 2960.73 ± 610.191 | 3485.43 ± 773.823 | |
| Attack Latency (s) | 500.3 ± 66.75 | 372.6 ± 139.31 |
Table 2.
Odor Habituation and Discrimination. Mean ± SEM for investigation times (seconds) of both the first and last exposure to each non-social and social odor for subjects of both sexes and genotypes.
| Cre− Males | Cre− Females | Cre+ Male | Cre+ Females | ||
|---|---|---|---|---|---|
| Non-social Odor 1 | First Exposure | 6.53 ± 2.33 | 4.12 ± 1.4 | 3.81 ± 1.95 | 9.52 ± 0.05 |
| Last Exposure | 4.66 ± 1.79 | 1.25 ± 0.46 | 2.9 ± 2.66 | 1.33 ± 0.33 | |
| Non-social Odor 2 | First Exposure | 4.57 ± 1.1 | 3.5 ± 1.27 | 7.44 ± 0.81 | 8.77 ± 5.54 |
| Last Exposure | 0.51 ± 0.24 | 1.1 ± 0.41 | 1.96 ± 1.31 | 0.31 ± 0.31 | |
| Social Odor 1 | First Exposure | 32.33 ± 3.42 | 22.91 ± 1.6 | 31.17 ± 4.27 | 23.01 ± 1.08 |
| Last Exposure | 2.16 ± 0.63 | 5.83 ± 4.17 | 0.69 ± 0.44 | 3.41 ± 0.59 | |
| Social Odor 2 | First Exposure | 31.46 ± 6.74 | 18.27 ± 3.58 | 26.49 ± 3.96 | 36.36 ± 0.76 |
| Last Exposure | 4.39 ± 2.51 | 3.03 ± 1.63 | 1.46 ± 1.46 | 16.09 ± 3.88 |
Acknowledgements
We would like to thank Rachael Beaumont, Selma Belkasim, Jade Christman, and Adam Zbib for assistance in behavioral data collection and analysis.
This work was supported by NIH grants R01 MH121603 and R21 MH111104.
References
- Allen Institute for Brain Science. Allen Mouse Brain Reference Atlas [WWW Document], 2011. URL https://mouse.brain-map.org/static/atlas
- Bainier C, Mateo M, Felder-Schmittbuhl MP, Mendoza J, 2017. Circadian rhythms of hedonic drinking behavior in mice. Neuroscience 349, 229–238. 10.1016/j.neuroscience.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Beeler JA, Prendergast B, Zhuang X, 2006. Low amplitude entrainment of mice and the impact of circadian phase on behavior tests. Physiol. Behav 87, 870–880. 10.1016/j.physbeh.2006.01.037 [DOI] [PubMed] [Google Scholar]
- Bishop MJ, Chevins P, 1987. Urine Odours and Marking Patterns in Territorial Laboratory Mice (Mus Musculus). Behav. Processes 15, 233–248. 10.1016/0376-6357(87)90009-X [DOI] [PubMed] [Google Scholar]
- Bunck M, Czibere L, Horvath C, Graf C, Frank E, Keßler MS, Murgatroyd C, Müller-Myhsok B, Gonik M, Weber P, Pütz B, Muigg P, Panhuysen M, Singewald N, Bettecken T, Deussing JM, Holsboer F, Spengler D, Landgraf R, 2009. A hypomorphic vasopressin allele prevents anxiety-related behavior. PLoS One 4. 10.1371/journal.pone.0005129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, 2017. Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. Neuroscientist 23, 517–528. 10.1177/1073858417708284 [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS, 2008. Vasopressin: Behavioral roles of an “original” neuropeptide. Prog. Neurobiol 84, 1–24. 10.1016/j.pneurobio.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Resstel LB, Bertoglio LJ, de Carobrez AP, Guimaraes FS, 2010. Neuroanatomy of Anxiety. Curr. Top. Behav. Neurosci 2, 77–96. 10.1007/7854_2009_7 [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Kelley KW, 1991. Androgen-dependent vasopressinergic neurotransmission attenuates interleukin-1-induced sickness behavior. Brain Res 557, 115–120. 10.1016/0006-8993(91)90123-D [DOI] [PubMed] [Google Scholar]
- Dantzer R, Connor JCO, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. 10.1038/nrn2297.From [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhart JM, Leone MJ, Paladino N, Evans JA, Castanon-Cervantes O, Davidson AJ, Golombek DA, 2013. Suprachiasmatic astrocytes modulate the circadian clock in response to TNF-α. J. Immunol 191, 4656–64. 10.4049/jimmunol.1300450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH, 2016. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol 40, 1–23. 10.1016/j.yfrne.2015.04.003.Vasopressin [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MD, Brancaccio M, Chesham JE, Maywood ES, Hastings MH, 2016. Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling. Proc. Natl. Acad. Sci 113, 201519044. 10.1073/pnas.1519044113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico P, Malkinson TJ, Cooper KE, Pittman QJ, Veale WL, 1992a. Vasopressin perfusion within the medial amygdaloid nucleus attenuates prostaglandin fever in the urethane-anaesthetized rat. Brain Res 587, 319–326. 10.1016/0006-8993(92)91014-6 [DOI] [PubMed] [Google Scholar]
- Federico P, Veale WL, Pittman QJ, 1992b. Vasopressin-induced antipyresis in the medial amygdaloid nucleus of consious rats. Am. J. Physiol 262, 901–908. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Weil ZM, Nelson RJ, 2013. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain. Behav. Immun 34, 159–163. 10.1016/j.bbi.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Gheusi G, Bluthé RM, Goodall G, Dantzer R, 1994. Social and individual recognition in rodents: Methodological aspects and neurobiological bases. Behav. Processes 33, 59–87. 10.1016/0376-6357(94)90060-4 [DOI] [PubMed] [Google Scholar]
- Gizowski C, Trudel E, Bourque CW, 2017. Central and peripheral roles of vasopressin in the circadian defense of body hydration. Best Pract. Res. Clin. Endocrinol. Metab 31, 535–546. 10.1016/j.beem.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Gizowski C, Zaelzer C, Bourque CW, 2018. Activation of organum vasculosum neurones and water intake in mice by vasopressin neurones in the suprachiasmatic nucleus. J. Neuroendocrinol 30, 1–7. 10.1111/jne.12577 [DOI] [PubMed] [Google Scholar]
- Gizowski C, Zaelzer C, Bourque CW, 2016. Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature 537, 685–688. 10.1038/nature19756 [DOI] [PubMed] [Google Scholar]
- Gomez F, Chapleur M, Fernette B, Burlet C, Nicolas JP, Burlet A, 1997. Arginine vasopressin (AVP) depletion in neurons of the suprachiasmatic nuclei affects the AVP content of the paraventricular neurons and stimulates adrenocorticotrophic hormone release. J. Neurosci. Res 50, 565–574. [DOI] [PubMed] [Google Scholar]
- Gould TD, Dao DT, Kovacsics CE, 2009. The Open Field Test, in: Mood and Anxiety Related Phenotypes in Mice pp. 1–20. 10.1007/978-1-61779-313-4 [DOI] [Google Scholar]
- Guerrero-Vargas NN, Salgado-Delgado R, Basualdo MDC, García J, Guzmán-Ruiz M, Carrero JC, Escobar C, Buijs RM, 2014. Reciprocal interaction between the suprachiasmatic nucleus and the immune system tunes down the inflammatory response to lipopolysaccharide. J. Neuroimmunol 273, 22–30. 10.1016/j.jneuroim.2014.05.012 [DOI] [PubMed] [Google Scholar]
- Hart B, 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev 12, 123–137. [DOI] [PubMed] [Google Scholar]
- Hernández VS, Hernández OR, de La Mora MP, Gómora MJ, Fuxe K, Eiden LE, Zhang L, 2016. Hypothalamic vasopressinergic projections innervate central amygdala GABAergic neurons: Implications for anxiety and stress coping. Front. Neural Circuits 10, 1–19. 10.3389/fncir.2016.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorneman EMD, Buijs RM, 1982. Vasopressin fiber pathways in the rat brain following suprachiasmatic nucleus lesioning. Brain Res 243, 235–241. 10.1016/0006-8993(82)90246-3 [DOI] [PubMed] [Google Scholar]
- Hurst JL, 1987. The functions of urine marking in a free-living population of house mice, Mus domesticus Rutty. Anim. Behav 35, 1433–1442. 10.1016/S0003-3472(87)80016-7 [DOI] [Google Scholar]
- Jones N, King SM, 2001. Influence of circadian phase and test illumination on pre-clinical models of anxiety. Physiol. Behav 72, 99–106. 10.1016/S0031-9384(00)00388-7 [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Hofman M. a., Swaab DF, Buijs RM, 2010. Vasopressin and the output of the hypothalamic biological clock. J. Neuroendocrinol 22, 362–372. 10.1111/j.1365-2826.2010.01956.x [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FAJL, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM, 2006. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms 21, 458–469. 10.1177/0748730406293854 [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR, 2003. Cytokine-induced sickness behavior. Brain. Behav. Immun 17, 112–118. 10.1016/S0889-1591(02)00077-6 [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL, 2014. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: What do we really know? Front. Neuroendocrinol 35, 512–529. 10.1016/j.yfrne.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, 2015. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci. Biobehav. Rev 56, 315–329. 10.1016/j.neubiorev.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Lapin IP, 1995. Only controls: Effect of handling, sham injection, and intraperitoneal injection of saline on behavior of mice in an elevated plus-maze. J. Pharmacol. Toxicol. Methods 34, 73–77. 10.1016/1056-8719(95)00025-D [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen Lin, Chen Li, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR, 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- Da Li J, Burton KJ, Zhang C, Hu SB, Zhou QY, 2009. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am. J. Physiol. - Regul. Integr. Comp. Physiol 296, 824–830. 10.1152/ajpregu.90463.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL, 1999. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol. Behav 67, 769–775. 10.1016/S0031-9384(99)00131-6 [DOI] [PubMed] [Google Scholar]
- Marpegán L, Bekinschtein TA, Costas MA, Golombek DA, 2005. Circadian responses to endotoxin treatment in mice. J. Neuroimmunol 160, 102–109. 10.1016/j.jneuroim.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA, 2009. Diurnal Variation in Endotoxin-Induced Mortality in Mice: Correlation With Proinflammatory Factors. Chronobiol. Int 26, 1430–1442. 10.3109/07420520903408358 [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Owen K, Bronson FH, Desjardins C, 1974. Urinary marking in male house mice: Responses to novel environmental and social stimuli. Physiol. Behav 12, 1035–1039. 10.1016/0031-9384(74)90151-6 [DOI] [PubMed] [Google Scholar]
- Meyerolbersleben L, Winter C, Bernhardt N, 2020. Dissociation of wanting and liking in the sucrose preference test in dopamine transporter overexpressing rats. Behav. Brain Res 378, 74–77. 10.1016/j.bbr.2019.112244 [DOI] [PubMed] [Google Scholar]
- Mieda M, 2020. The central circadian clock of the suprachiasmatic nucleus as an ensemble of multiple oscillatory neurons. Neurosci. Res 156, 24–31. 10.1016/j.neures.2019.08.003 [DOI] [PubMed] [Google Scholar]
- Mieda M, Okamoto H, Sakurai T, 2016. Manipulating the Cellular Circadian Period of Arginine Vasopressin Neurons Alters the Behavioral Circadian Period. Curr. Biol 26, 2535–2542. 10.1016/j.cub.2016.07.022 [DOI] [PubMed] [Google Scholar]
- Mieda M, Ono D, Hasegawa E, Okamoto H, ichi Honma K, Honma S, Sakurai T, 2015. Cellular clocks in AVP neurons of the scn are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron 85, 1103–1116. 10.1016/j.neuron.2015.02.005 [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, 1993. GABA is the principal neurotransmitter of the circadian system. Neurosci. Lett 150, 112–116. 10.1016/0304-3940(93)90120-A [DOI] [PubMed] [Google Scholar]
- Morgan CW, Julien O, Unger EK, Shah NM, Wells JA, 2014. Turning ON Caspases with Genetics and Small Molecules. Methods Enzym 544, 179–213. 10.1038/cdd.2010.172.MicroRNAs [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM, 2012. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A 109, 12662–12667. 10.1073/pnas.1209965109/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1209965109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava F, Carta G, Haynes LW, 2000. Lipopolysaccharide increases arginine-vasopressin release from rat suprachiasmatic nucleus slice cultures. Neurosci. Lett 288, 228–230. 10.1016/S0304-3940(00)01199-X [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R, 2012. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci 35, 649–659. 10.1016/j.tins.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Newman S, 1999. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci 887, 242–257. 10.1111/j.1749-6632.1999.tb09271.x [DOI] [PubMed] [Google Scholar]
- Novak CM, Harris J. a., Smale L, Nunez A. a., 2000. Suprachiasmatic nucleus projections to the paraventricular thalamic nucleus in nocturnal rats (Rattus norvegicus) and diurnal nile grass rats (Arviacanthis niloticus). Brain Res 874, 147–157. 10.1016/S0006-8993(00)02572-5 [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA, 2012. Evolution of a Vertebrate Social Decision-Making Network. Science (80-. ) 336, 1154–1157. [DOI] [PubMed] [Google Scholar]
- Palomba M, Bentivoglio M, 2008. Chronic inflammation affects the photic response of the suprachiasmatic nucleus. J. Neuroimmunol 193, 24–27. 10.1016/j.jneuroim.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K, 2012. The mouse brain in stereotaxic coordinates, 4th ed. Academic Press, San Diego, CA. [Google Scholar]
- Pittman QJ, Chen X, Mouihate A, Hirasawa M, Martin S, 1998a. Arginine vasopressin, fever and temperature regulation. Prog. Brain Res 119, 383–392. 10.1016/S0079-6123(08)61582-4 [DOI] [PubMed] [Google Scholar]
- Pittman QJ, Chen X, Mouihate A, Martin S, 1998b. Vasopressin-Induced Antipyresis: Sex- and Experience-Dependent Febriles Responses. Ann. New York Acad. Sci 53–61. [DOI] [PubMed] [Google Scholar]
- Reghunandanan V, Reghunandanan R, Marya RK, Singh PI, 1992. Vasopressin antagonist disrupts the circadian rhythm of water intake on suprachiasmatic injection. Chronobiol. Int 9, 356–361. 10.3109/07420529209064547 [DOI] [PubMed] [Google Scholar]
- Rigney N, Whylings J, de Vries GJ, Petrulis A, 2020. Sex Difference in the Control of Social Investigation and Anxiety by Vasopressin Cells of the Paraventricular Nucleus of the Hypothalamus. Neuroendocrinology 10.1159/000509421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigney N, Whylings J, Mieda M, De Vries GJ, Petrulis A, 2019. Sexually Dimorphic Vasopressin Cells Modulate Social Investigation and Communication in Sex-Specific Ways. eNeuro 6, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC, 1993. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiol. Behav 54, 729–736. 10.1016/0031-9384(93)90084-S [DOI] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ, 2011. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J. Comp. Neurol 519, 2434–2474. 10.1002/cne.22635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJW, Woodbury ME, De Vries GJ, 2013. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J. Comp. Neurol 521, 2321–2358. 10.1002/cne.23288 [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wöhr M, Crawley JN, 2011. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav. Brain Res 216, 19–28. 10.1016/j.bbr.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso P, Nakata M, Ueta Y, Yada T, 2017. Suprachiasmatic Vasopressin to Paraventricular Oxytocin Neurocircuit in the Hypothalamus Relays Light Reception to Inhibition of Feeding Behavior 2 3 Running title: Light inhibits feeding via SCN AVP to PVN Oxt circuit. Am J Physiol Endocrinol Metab 10.1152/ajpendo.00338.2016 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sens J, Schneider E, Mauch J, Schaffstein A, Mohamed S, Fasoli K, Saurine J, Britzolaki A, Thelen C, Pitychoutis PM, 2017. Lipopolysaccharide administration induces sex-dependent behavioural and serotonergic neurochemical signatures in mice. Pharmacol. Biochem. Behav 153, 168–181. 10.1016/j.pbb.2016.12.016 [DOI] [PubMed] [Google Scholar]
- Tucker LB, McCabe JT, 2017. Behavior of Male and Female C57BL/6J Mice Is More Consistent with Repeated Trials in the Elevated Zero Maze than in the Elevated Plus Maze. Front. Behav. Neurosci 11, 1–8. 10.3389/fnbeh.2017.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger EK, Burke KJJ, Yang CF, Bender KJ, Fuller PM, Shah NM, 2015. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep 10, 453–462. 10.1016/j.celrep.2014.12.040.Medial [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Segbroeck M, Knoll AT, Levitt P, Narayanan S, 2017. MUPET—Mouse Ultrasonic Profile ExTraction: A Signal Processing Tool for Rapid and Unsupervised Analysis of Ultrasonic Vocalizations. Neuron 94, 465–485.e5. 10.1016/j.neuron.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kortzfleisch VT, Kästner N, Prange L, Kaiser S, Sachser N, Richter SH, 2019. Have I been here before? Complex interactions of age and test experience modulate the results of behavioural tests. Behav. Brain Res 367, 143–148. 10.1016/j.bbr.2019.03.042 [DOI] [PubMed] [Google Scholar]
- Walsh R, Cummins R, 1976. The Open-Field Test: a critical review. Psychol. Bull 83, 482–504. 10.1037/0033-2909.83.3.482 [DOI] [PubMed] [Google Scholar]
- Whylings J, Rigney N, de Vries GJ, Petrulis A, 2020. Removal of Vasopressin cells from the Paraventricular Nucleus of the Hypothalamus enhances LPS-induced sickness behaviour in mice. J. Neuroendocrinol 10.1111/jne.12915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whylings J, Rigney N, Peters NV, de Vries GJ, Petrulis A, 2019. Sexually dimorphic role of BNST vasopressin cells in sickness and social behavior in male and female mice. Brain. Behav. Immun 83, 68–77. 10.1016/j.bbi.2019.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, Fustin J-M, Yamazaki F, Mizuguchi N, Zhang J, Dong X, Tsujimoto G, Okuno Y, Doi M, Okamura H, 2013. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science 342, 85–90. 10.1126/science.1238599 [DOI] [PubMed] [Google Scholar]
- Yang CF, Chiang M, Gray DC, Prabhakaran M, Juntti SA, Unger EK, Wells JA, Shah NM, 2013. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909. 10.1016/j.cell.2013.04.017.Sexually [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


