Abstract
Volumetric muscle loss (VML) following a severe trauma or injury is beyond the intrinsic regenerative capacity of muscle tissues, and hence interventional therapy is required. Extensive muscle loss concomitant with damage to neuromuscular components overwhelms the muscles’ remarkable regenerative capacity. The loss of nervous and vascular tissue leads to further damage and atrophy, so a combined treatment for neuromuscular junction (NMJ) along with the volumetric muscle regeneration is important. There have been immense advances in the field of tissue engineering for skeletal muscle tissue and peripheral nerve regeneration, but very few address the interdependence of the tissues and the need for combined therapies to repair and regenerate fully functional muscle tissue. This review addresses the problem and presents an overview of the biomaterials that have been studied for tissue engineering neuromuscular tissues associated with skeletal muscles.
Keywords: Neuromuscular junction, Nerve regeneration, Muscle regeneration, Tissue engineering
I. Introduction
Explosion or blast trauma, bullet wounds, road traffic accidents, contusion injury (as in sports), or even debridement or removal of dead muscle tissue in compartment syndrome (CS) can cause penetrating soft tissue injuries leading to VML injuries, which leads to functional loss and cosmetic deformities[1–4]. VML can be defined as traumatic loss or surgical removal of the skeletal muscle with functional impairment and is often associated with neuromusculoskeletal damage [5–7]. Various neural components (Figure 1) such as peripheral and intramuscular nerves, NMJ, innervation to muscle fibers, and motoneurons-muscle fiber signaling can be damaged or lost from the muscle tissue due to VML[8,9]. Without prompt surgical intervention, profound consequences beyond the frank loss of muscle can occur. This includes extensive fibrosis, loss of muscle contractility, range of movement, strength, necrosis, amputation, and a marked loss of quality of life of the patient[5,10–12]. Moreover, traumatic bone fractures and osteosynthesis are the main contributors for soft tissue damage, including approximately 70% of peripheral nerve lesions[13]. Furthermore, vascular lesions due to musculoskeletal intervention operations affect up to 8% of cases and lead to 17.5% of traumatic nerve injuries. Long-distance nerve lesions and axon damage fail to regrow and reinnervate target muscles. Nerve defects longer than 8mm in size could not regenerate, motor functional recovery is virtually nonexistent, and necessitate a nerve transplantation[13]. If the regenerating axons do not reach the targeted muscle within a specific time period, neuromuscular and motor functions will certainly fail[14].
Figure 1:
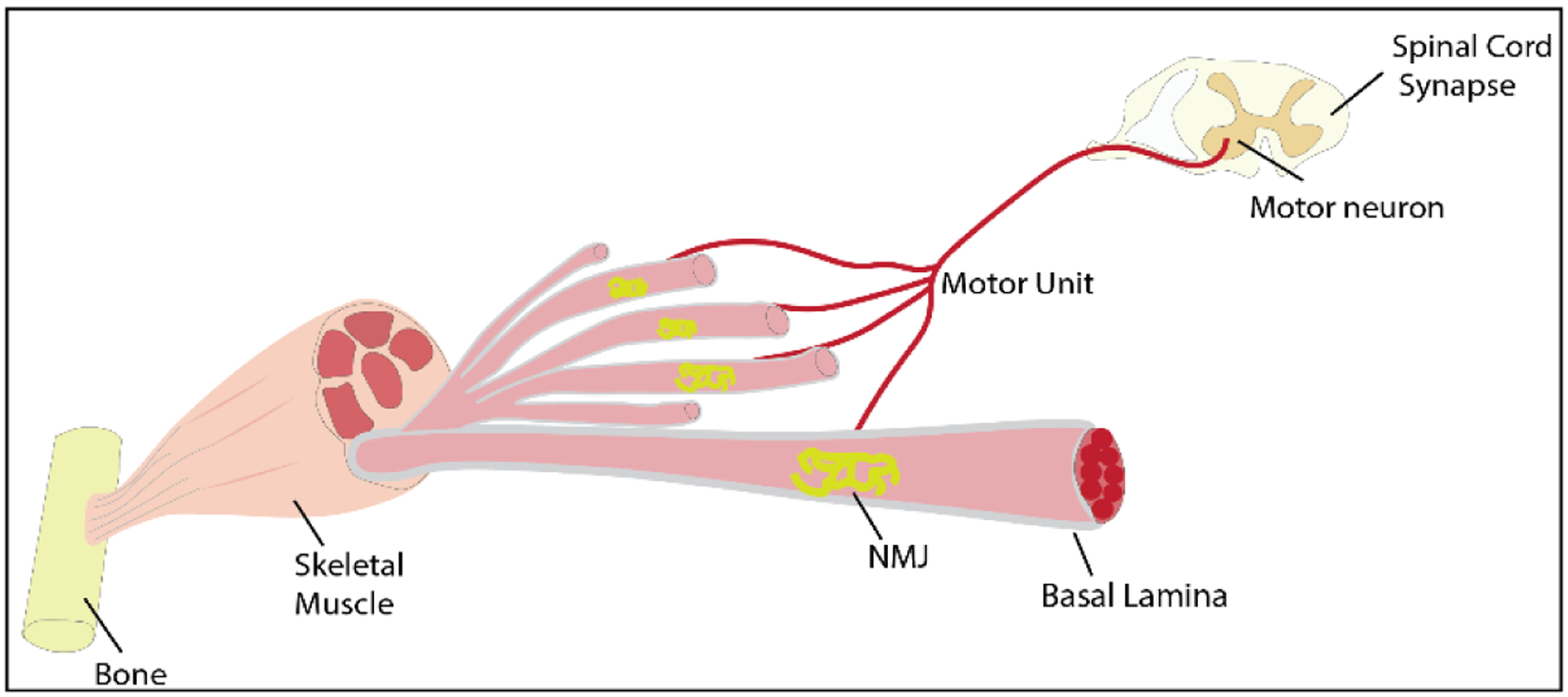
Illustration of the Neuromuscular Junction in Skeletal Muscle
Compounding this problem, nearly 6 million fractures occur in the US annually with more than 3% consisting of open fractures, increasing the possibility of CS and VML[15]. Global MSK disorders (including VML and fractures) afflict >1.7 billion people worldwide, including >130 million Americans and up to $873.8B in medical care costs[16,17]. This will likely rise by 400% by 2050 as life-expectancy increases[18]. Thus, there is a great need to treat MSK, CS, and VML disorders to improve patient’s quality of life, reduce societal burden, and escalating healthcare costs.
Thus, repair and regeneration of tissues lost from VML injuries require activation, proliferation, and differentiation of a resident pool of stem cells known as satellite cells[19–21]. These cells are activated for myofiber regeneration following inflammation or hematoma formation following muscle injury. There is either maturation of regenerated muscle fibers with muscle function recovery or fibrous tissue formation and impaired muscle function in cases of severe trauma (VML)[12,20,22]. Fibrous tissue should be surgically debrided for regeneration of functional tissue, debridement further leads to loss of the muscle increasing the compartment volume (As seen in Figure 2 by Corona et al.) [12]. In such cases, vascularization and innervation are vital for functional muscle regeneration. Therefore, there is not only an urgent need for therapeutic modalities that promote satellite cells for muscle tissue regeneration, but also promote neuronal and endothelial cells for inducing peripheral nerve repair for functional tissue regeneration.
Figure 2:
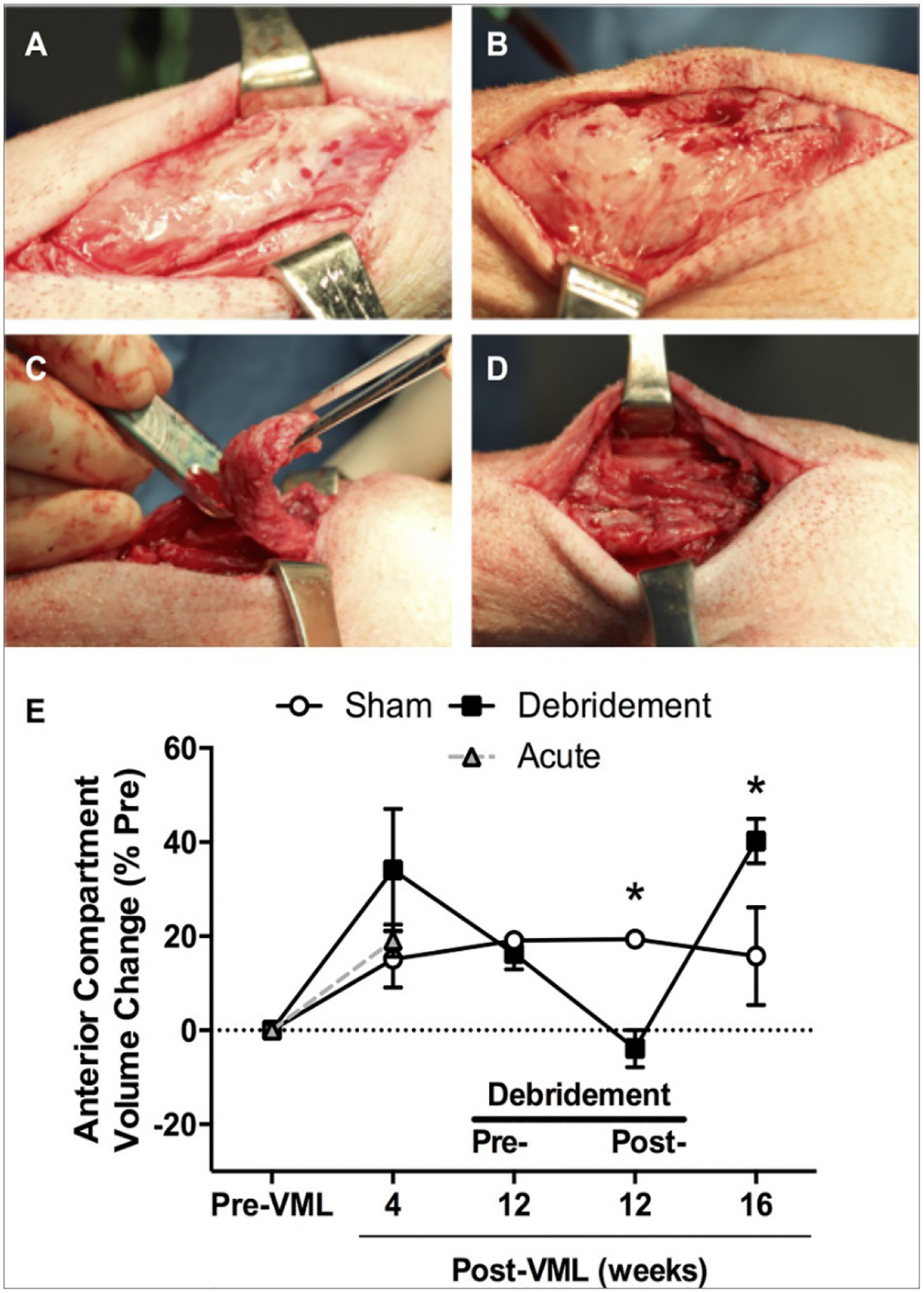
Debridement induces increase in compartment volume. Peroneus tertius muscles underwent volumetric muscle loss (VML) injury. (a & b) Twelve weeks after VML injury, fibrous tissue enveloped the anterior compartment (c & d) at which time the overlaying fibrous tissue was surgically debrided. (e) Lower limb anterior compartment volume was measured using computed tomography imaging at the times specified. Values are means ± SE. Image taken from Corona, B.T., J.C. Rivera, and S.M. Greising, Inflammatory and Physiological Consequences of Debridement of Fibrous Tissue after Volumetric Muscle Loss Injury. Clinical and translational science, 2018. 11(2): p. 208–217.
II. Pathophysiology associated with VML
The pathophysiology of sudden loss of muscle in VML injuries is highly ordered[22]. Primary order effects of VML are particularly challenging because skeletal muscle is incapable of extensive fiber regeneration after VML injuries[3,7]. Satellite cells and the basal lamina are the main components for effective muscle healing, that must remain intact[23–25], otherwise, VML leads to ablation of these critical components and the remaining musculature is unable to heal the damaged tissue. Improving the strength of the VML-injured muscle depends on: (1) number of remaining muscle fibers, (2) activation of survived fibers, and (3) promotion of new muscle fiber formation and density[22]. Furthermore, inflammation must be optimally resolved or regeneration deteriorates into fibrosis[26]. VML injuries result in exacerbation of the inflammatory phase of fracture healing[27], indicated by elevation in a cluster of markers (CD3+) lymphocytes and CD68+ macrophages in the fracture callus at 3 and 14 days post-injury in rats, respectively[28]. A common treatment for inflammatory response includes surgical debridement. Surgical debridement not only leads to increase in compartment volume (Figure 2 E), but also results in upregulation of inflammatory and fibrotic transcriptional pathways for at least one month after surgery in-vivo[12]. The current clinical care strategy for VML involves the use of free muscle transfer (i.e., muscle flaps) for bone coverage in animal models followed by extensive physical rehabilitation[22,29]. Yet, these procedures are not intended to restore muscle function clinically[30] and experience donor site morbidity and secondary surgery that adds to surgical complexity and cost[30].
While the general homeostasis and regenerative capacity of skeletal muscle depends on the presence of neural and vascular influence, the innervation of neuromuscular junctions depends on the sympathetic neurons which are of crucial importance for the integrity and function of nerve–muscle contact. A recent study presented the distribution and functions of sympathetic neurons in mouse skeletal muscle as shown in Figure 3. It is also controlled by various regulatory factors along with extracellular matrix protein synthesis and degradation[31–33]. VML can affect or damage the related peripheral nerves, intramuscular nerves and innervation at NMJs[8,29].The functional loss caused by VML can deteriorate over time due to the limited regenerative capacity of these large defects affecting muscle fiber and its neural components. This leads to the loss of the contractile proteins and the failure of excitation-contraction coupling, resulting in neuromuscular strength deficits[8].
Figure 3:
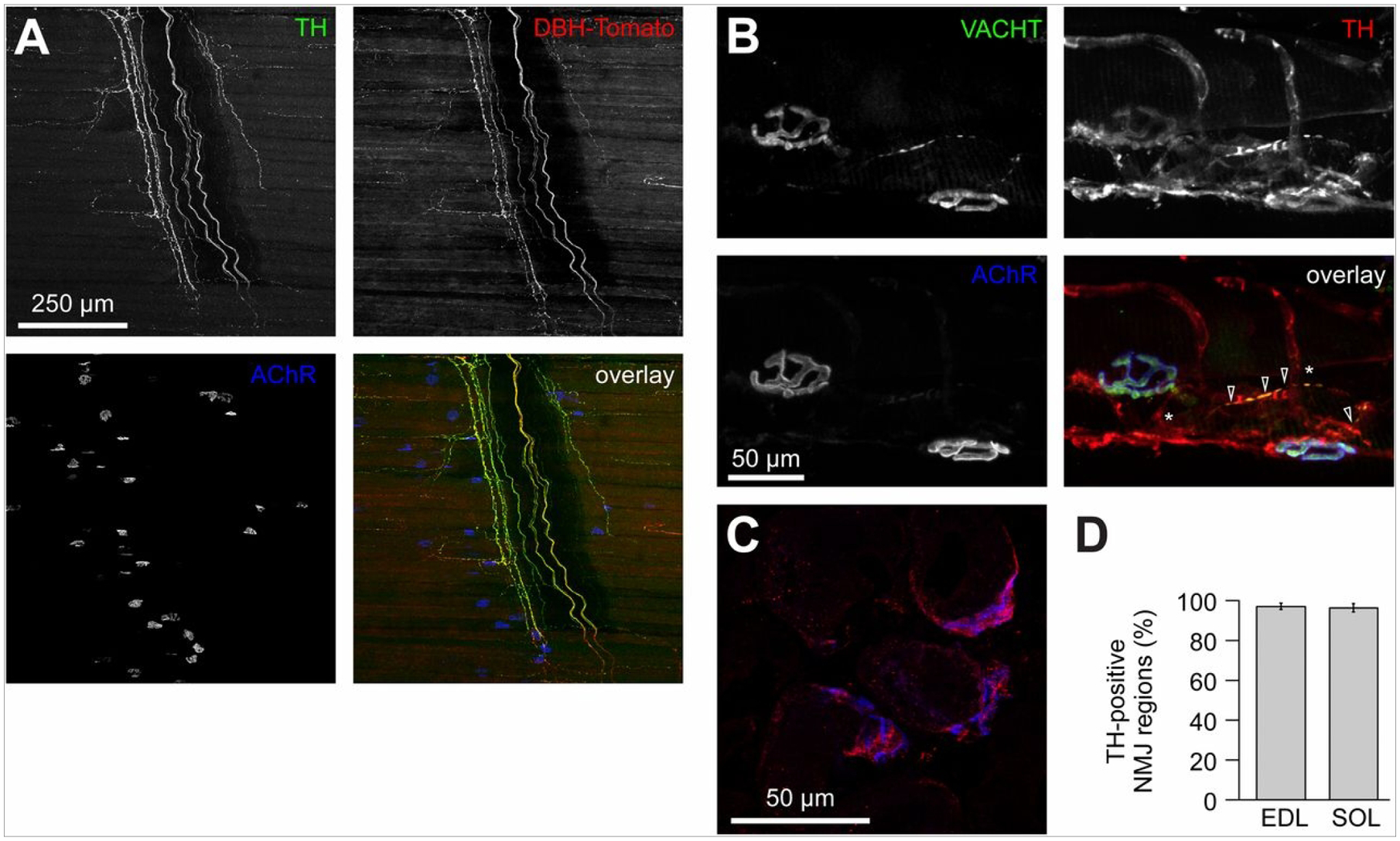
Distribution of sympathetic neurons in skeletal muscle. (A) Diaphragm muscle of a DBH-Tomato mouse expressing Tomato protein in sympathetic neurons was co-stained with anti-TH antibody and BGT-AF647 (AChR). Signals from TH, Tomato, and BGT are depicted in the overlay in green, red, and blue, respectively. Three-dimensional maximum projection of a confocal z stack of a representative region is shown. All channels were brightness/contrast-enhanced. (B) Longitudinal sections of wild-type EDL muscles were labeled against VACHT, TH, and BGTAF647. Signals from these markers are depicted in the overlay in green, red, and blue, respectively. Three-dimensional maximum projection of a confocal z stack of a representative region is shown. All channels were brightness/contrast-enhanced. (C and D) EDL and soleus muscles were sectioned transversally, stained with BGT-AF555 (blue in overlay) and anti-TH antibody (red in overlay), and then imaged with confocal microscopy. (C) Representative confocal brightness/contrast-enhanced optical section from EDL. (D) Quantification of TH-positive NMJ regions from EDL and soleus (SOL) muscles. Mean ± SEM (n = 4 muscles each). Negative controls lacking primary antibodies showed 0.7 ± 0.7% (mean ± SEM, n = 4 muscles) in EDL and 0.0% (mean ± SEM, n = 4 muscles) in soleus of TH-positive NMJ regions. Image taken from Khan, M.M., et al., Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proceedings of the National Academy of Sciences, 2016.
The peripheral nerve injury causes cascade of physiologic events including Wallerian degeneration at the distal ends[34] and retrograde degeneration as well as initiation of the regenerative process at the proximal ends of the peripheral nerve[35]. The peripheral nerve can regenerate by itself to some extent, but in cases of severe injury (as expected in VML) there is a retraction of the axonal terminals and initiation of a significant inflammatory response which leads to either limiting or complicating (scar formation) the regenerative capacity of the nerves[35]. Chronic axotomy of the motoneurons, and denervation of the Schwann cells and muscle fibers can further affect the functional capacity of the muscle[8]. The denervation also activates protein degradation pathways that lead to muscle atrophy by exceeding the protein synthesis[9,31,36,37]. Due to the aggravated muscle atrophy and complicated intrinsic regenerative capacity, surgical intervention or tissue engineering strategies become necessary for full recovery in these cases.
III. Molecular Pathways Associated with Neuromuscular Degeneration in VML
VML injury promotes degeneration and loss of function progressively after initial site injury due to acute tissue loss. Several molecular pathways are involved in the progression of muscle degeneration post VML injury. The degeneration of muscle tissue appears to have a similar pathophysiology as Duchene’s Muscular Dystrophy. The reperfusion of vasculature initiates the downward cycle of muscle tissue degeneration owed to the accumulation of reactive oxygen species (ROS). Upon injury, initial ischemia develops within 4–8 hours of ischemic shock inducing irreversible neuromuscular damage. Return of oxygen upon reperfusion of muscle produces a burst of oxygen free radicals, nitric oxide species, and cellular and inflammatory infiltrate which causes swelling[38,39]. This can induce oxidative damage to the varied cell types making up the muscles being re-vascularized. Oxidative damage and regulation of oxidative free radicals play a central role in regenerating the injured muscle.
Neuromuscular degeneration following severe injury revolves around the perpetual downward cycle of inflammation, oxidative stress, fibrosis, and resultant interruption of the neuromuscular regeneration machinery. Several key markers such as transforming growth factor signaling (TGF), WNT (Wingless-related integration site) signaling pathway, nuclear factor erythroid 2-related factor 2 (NRF2), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α), are interconnected in their role to regulate inflammation, fibrosis, and oxidative stress[40]. This comes down to an increase in WNT/TGF signaling that impedes myogenesis and neurogenesis. Wnt/β-catenin cascade activates TGFβ signaling which is a crucial step for proper muscle repair. TGF signaling pathway plays a molecular role in neuromuscular tissue degeneration and its expression level is a representation of various levels and stages of inflammation (Figure 4). TGFβ along with the Mitogen-activated protein kinase (MAPK) family can phosphorylate SMADs leading to activation of collagen transcription shown in Figure 4. Hence, the TGFβ and MAPK pathway lead to the synthesis of extracellular matrix and lead to fibrosis[41]. WNT signaling is one of the central signal pathways that is involved in the perpetuation of fibrosis[41].
Figure 4.
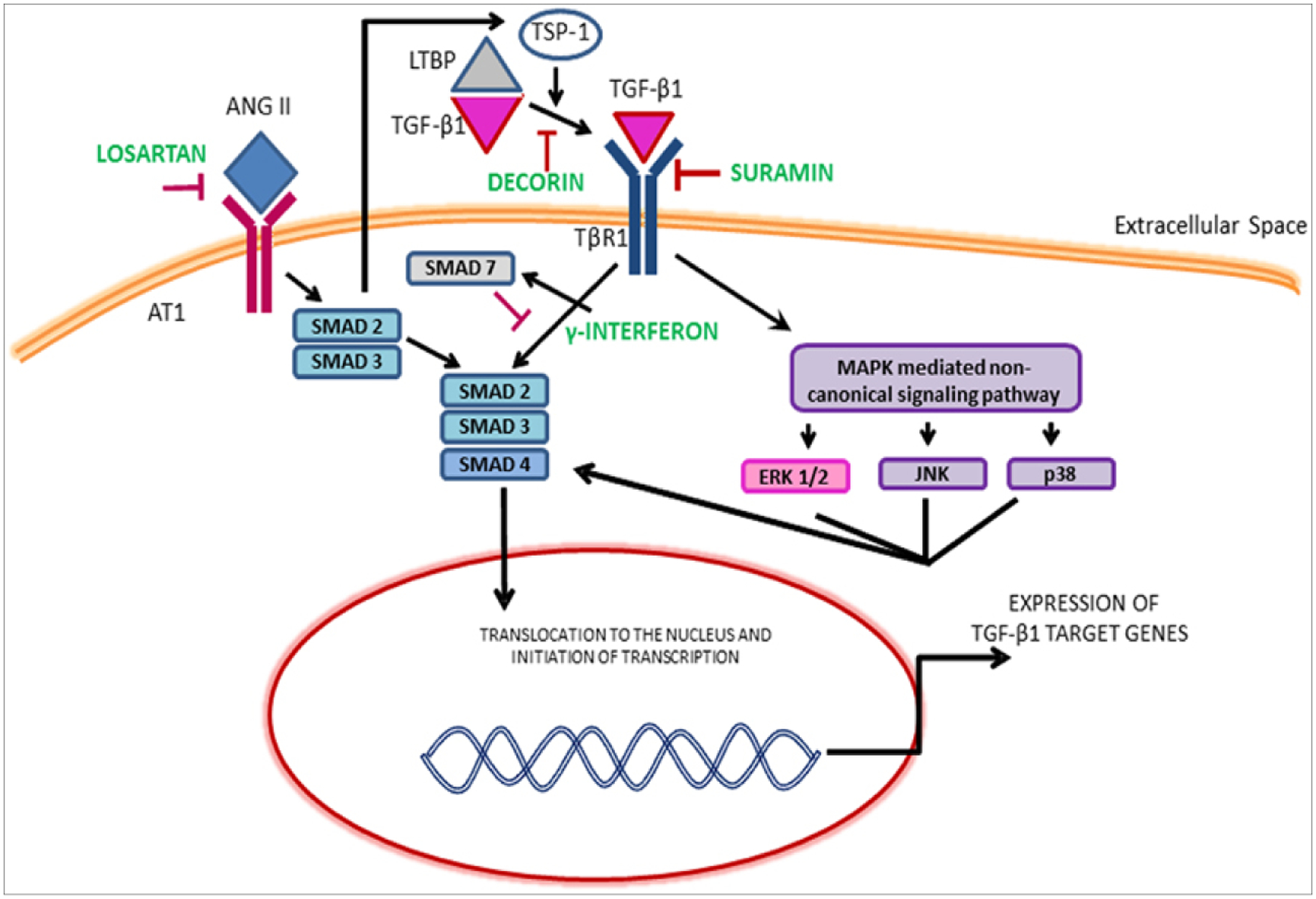
(Garg, 2015): Illustration of the TGF-β1 signaling pathways and the mechanism of therapeutics. ERK, Extracellular signal regulated kinase; JNK, c-Jun N-terminal kinase; LTBP, Latent transforming growth factor binding proteins; MAPKs, Mitogen-activated protein kinase; TSP-1, Thrombospondin-1. Image taken from K. Garg, B.T. Corona, T.J. Walters, Therapeutic strategies for preventing skeletal muscle fibrosis after injury, Front. Pharmacol. 6 (2015) 87. https://doi.org/10.3389/fphar.2015.00087.
Oxygen free radicals and inflammatory signaling (Figure 5) trigger the expression of NRF2 signaling to regulate the impact of oxidative stress[42]. This centers on improving mitochondrial function under these conditions. NRF2 has also been implicated in mitochondrial health when it triggers the PGC1-α pathway[42,43]. The keap1-NRF2 pathway (Figure 5) is critical for promoting antioxidant expression to mediate pro-oxidant metabolites while being a key anti-inflammatory signaling molecule after traumatic tissue injury. In the NMJ of skeletal muscle of wild type and aged rats, NRF2 deficiency[44] was associated with reduced mitochondrial oxygen consumption, increased mitochondrial ROS production, increased protein nitrosylation, cellular redox dysregulation, and reduced acetylcholine receptor expression. On the other hand, PGC1-α is a key receptor of several myokines in myoblasts that play several roles in bone and neuronal tissue health and offer protection from oxidative damage. Furthermore, it regulates NRF2 transcription in a regulatory feedback loop and plays a central role on the expression of myokines needed to stimulate muscle and neuronal cell formation of NMJ. In addition, PGC1-α rescue overcame oxidative damage during VML injury and rescued the pathophysiology of the induction of persistent loss of muscle tissue[45].
Figure 5:
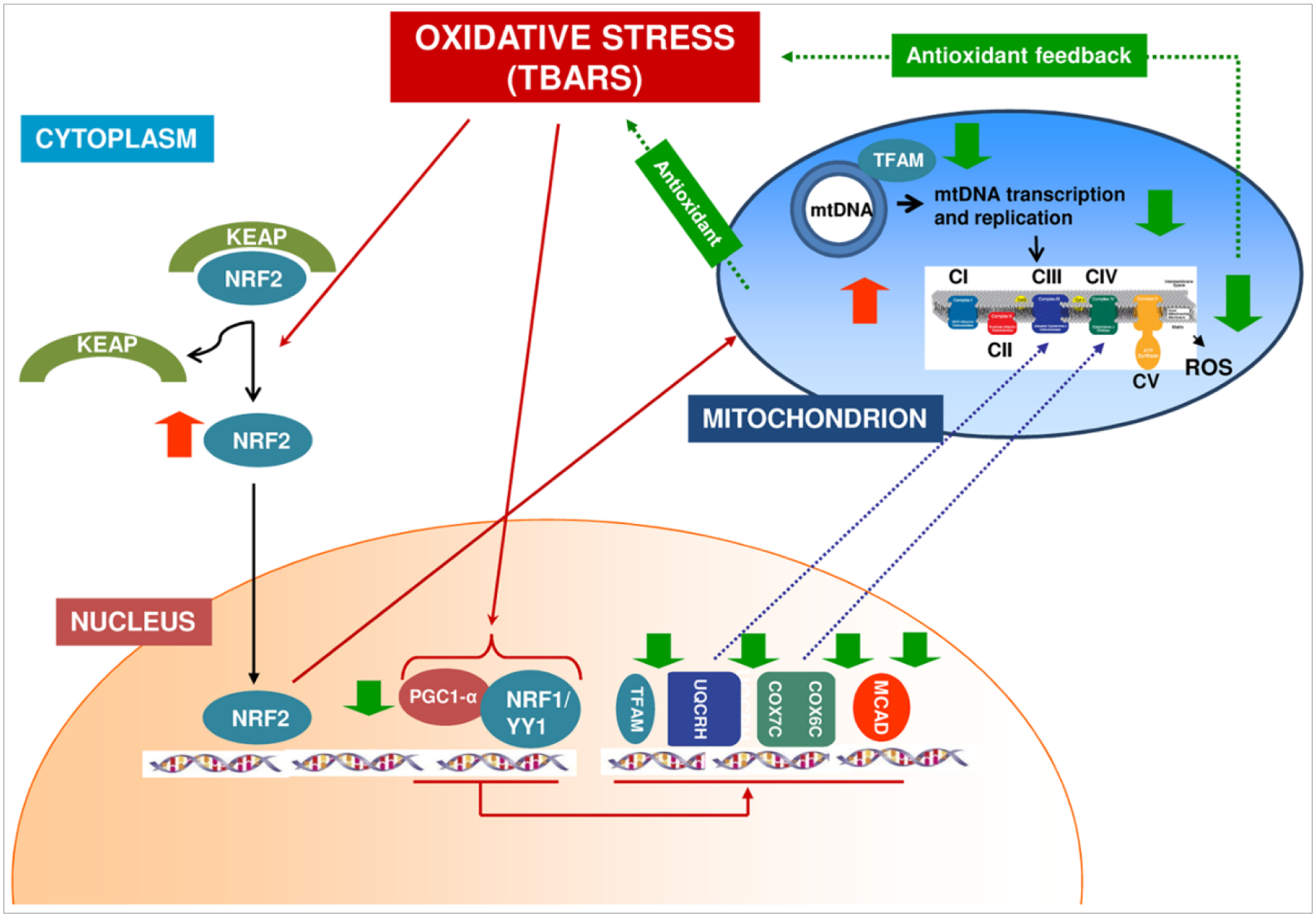
Supposed mechanism of PGC1-α/NRF1-NRF2 pathway anti-oxidative stress cellular defense in chronic kidney disease patients in peritoneal dialysis treatment. Oxidative stress alters the interaction of Kelch-like ECH-associated protein 1 (Keap1) and Nuclear factor erythroid-derived 2-like 2 (Nrf2), thereby liberating Nrf2 activity from repression by Keap1. NRF2 migrates into the nucleus where it activates the transcription of Superoxide dismutase 2, mitochondrial (SOD2). At the same time, oxidative stress causes the down-regulation of Peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1-α) and Nuclear respiratory factor-1 (NRF-1) with the consequent down-regulation of PGC-1α downstream target genes (TFAM, COX6C, COX7C, UQCRH and MCAD). The reduced TFAM expression causes a decrease in mitochondrial transcription and replication. The downregulation of all these factors suggests the decrease in mitochondrial OXPHOS activity to reduce ROS accumulation and creating an antioxidant feedback. Image taken from Zaza, G., et al., Downregulation of Nuclear-Encoded Genes of Oxidative Metabolism in Dialyzed Chronic Kidney Disease Patients. PLOS ONE, 2013. 8(10): p. e77847.
IV. Current Strategies for Neuromuscular Repair and Regeneration Related to VML
There are several studies that focus on regeneration of VML, but very few investigated the NMJ and the peripheral nerve regeneration regarding muscle tissue. This section will focus on the current grafting and tissue engineering strategies for NMJ in VML and vascular tissue loss models. Autologous tissue grafts and physical therapy are the current gold standards for VML injury repair. A healthy muscle graft (i.e, Latissimus dorsi muscle or Gracilis muscle) is transplanted in clinical studies from the donor site with or without neurorrhaphy to the site of the injury to restore the lost muscle tissue[31,46]. Limited donor tissue, donor site morbidity, graft failure due to infection or necrosis, and need for multiple surgeries are the main limitations to the autologous tissue grafts[31,47,48].
For nerve injuries related to VML, skeletal muscle graft could work as a nerve conduit and enhance nerve tissue regeneration in short nerve gap injuries (sheep femoral nerve 5mm and rat sciatic nerve 2mm), and not for long nerve gap defects, as studied in-vivo[49–51]. Muscle transplantation with neurorrhaphy can have complications such as tubular collapse of the nerve, poor regeneration, and scar tissue proliferation. Several pre-clinical animal studies have used artery, vein, and tendon autografts for peripheral nerve regeneration which presented similar results to the muscle autografts[49,51–54]. Yet, nerve loss in VML is still a critical challenge clinically in functional muscle regeneration.
Physical therapy helps improve the function and strengthen the remaining muscle by releasing growth factors, modulating immune response, promoting vascularization, and reducing scar formation[31,53,55]. Manual as well as mechanical stimulation has been shown to induce peripheral nerve regeneration, improving the muscle fiber reinnervation along with better functionality in various studies[56–60]. Although, these studies should be very carefully planned and executed in a clinical setting to benefit the tissue rather than causing additional motor or sensory damage[56]. Also, exercise, physical therapy, and mechanical or manual stimulation may not be a viable option for certain severe injury cases and would need additional surgical manipulation for better tissue repair and regeneration.
V. Need for Tissue Engineering
Tissue engineering involves the utilization of scaffolds, cells, and bioactive molecules known as the tissue engineering triad for repair and regeneration of injured tissues. Several in-vitro studies showed that the myoblast survival, migration rate, and myogenic regeneration were greatly enhanced by the scaffold designing designing along with growth factor delivery[1,21,61]. Similar is the case for nerve regeneration, especially in VML where the defect is large, in-vivo[49,62,63]. It has been proven that scaffolds mimicking the extracellular matrix environment and microarchitecture of the tissue lead to favorable results. The combination of appropriate stem cells along with growth factors in a microenvironment mimicking the extracellular matrix of a muscle will result in improved regeneration of skeletal muscle tissue. Thus, tissue engineering is needed for muscle tissue along with the affected neuromuscular tissue in a VML injury for a fully functional tissue regeneration. But the combination of these three different aspects together has yet to be completely explored. Clinical studies show neural innervation of the muscles and reestablishment of the NMJ are important aspects in regeneration of the muscular tissue. This not only avoids atrophy in the newly regenerated muscle tissue at later stages[9,31,64], but can also influence muscle fiber growth, alignment and type[65]. The extent of reinnervation of the NMJs have not been studied in a VML injury model after treatment, but Wu et al indicated a weaker force output in such regenerated tissues[37]. This can be attributed to the loss of neural function as well as increased fibrous connective tissue growth and failure of complete regeneration of the muscle tissue[37,66].
Recent studies in-vitro use co-culture of neurons and muscle cells to study the mechanism and interrelation of these tissue types for completely functional muscle tissue regeneration in a large sized defect[31,67–69]. We will focus on the tissue engineering triad regarding cells, and bioactive molecules along with biomaterials that have been studied for muscle tissue regeneration and are also common with or support neuronal growth.
VI. Cell and Molecular Approaches For Muscle Tissue Regeneration Supporting Neuronal Growth
The incorporation of stem cells have been an important aspect of tissue engineering and has been widely studied for muscle and nerve tissue regeneration. For example, treatment of Tibialis Anterior (TA) using cardiac stromal cells have ameliorated muscle fibrosis and promoted endogenous regeneration of dystrophin-deficient muscle in mdx mice via exosome stimulation of satellite stem cells, a model of Duchenne Muscular Dystrophy (DMD)[70,71] which is currently under clinical trials[72,73]. Other studies illustrate the essential role that satellite cells play in myogenesis[74]. Satellite cells have been very popular for skeletal muscle tissue regeneration, also for neuromuscular tissue, satellite cells and Schwann cells are believed to support neuronal growth. Transplantation of satellite cells have shown to promote myoblast survival and proliferation in-vivo, but clinical studies have not been that successful, as mentioned earlier, VML produces tissue loss greater than the regenerative potential of the native tissue, preventing functional restoration to the limb[31]. Mesenchymal stem cells (MSCs) are multipotent stromal cells with the ability to divide into different tissue types. It is believed to be a source of trophic mediators[75–77] and can modulate various tissue functions including musculoskeletal tissue[78,79] and peripheral nerves[75,80,81]. MSCs promote angiogenesis which enhances the healing by increased vascularization to the regeneration site. MSCs have a stimulatory effect on Schwann cell population by increasing the neurotrophic expression which in turn increases myelinization and axonal regeneration[82–84]. Furthermore, MSCs can reduce scar tissue formation, while adipose derived stem cells have been used for nerve tissue regeneration[85–87]. However, mesenchymal stem cells have increased rejection due to culture time for high cell numbers, exposure to serum prions and peptides, low viability, and increased senescence[88].
Along with cellular approaches, the controlled release of growth factors along with the biomaterials has been explored and used in various tissue regenerative therapy for promoting faster growth. Vascular Endothelial Growth Factor (VEGF) has been proved to promote angiogenesis[61,89,90], while VEGF and Insulin like growth factor (IGF-1) have shown to enhance vascular tissue formation in ischemic muscle tissue enhancing muscle tissue regeneration[21,91,92]. VEGF has also been shown to promote axonal regeneration by upregulating expression of nerve growth factor (NGF) and glial-derived neurotrophic factor (GDNF)[89,93]. Basic fibroblast growth factor (bFGF) has shown an improvement in functional recovery and innervation of muscular tissue with minimum fibrotic tissue[94]. Several studies have shown that FGF increase in myelin axon, increased functional motor return, nerve amplitude and muscle action potential, and improved axonal regeneration in in-vivo models for up to 10mm of nerve repair[49,95–97]. Neurotrophic factors such as NGF and BDNF secreted from Schwann cells are naturally released in the nerve regeneration cascade[95,98]. NGF has shown to promote axonal regeneration by increasing the number of Schwann cells at the injured axon end, for improved nerve repair[49,99]. It has also been seen that improving the expression of neuromodulators like GDNF promotes angiogenic factors which improves muscular regeneration along with the NMJ restores[100].
Exogenous growth factors that target these conditions have severe side effects (ectopic bone formation, prolonged inflammation, and pain)[101] and poor efficacy (high dose to low cell density) clinically [102,103]. Finally, drug delivery (e.g., nitrates) or gene therapy vehicles (e.g., viral vectors) impede endothelial cell (EC) function owed to cytotoxicity, immune toxicity, nitrate tolerance, and altered gene expression[104–107]. Therefore, cellular approaches require added microenvironmental and structural support to improve the rate and quality of healed muscle. Various in-vitro and in-vivo studies show targeted markers including myokines[108]; such as the aminobutyric acid family (GABA, BAIBA)[109], interleukin family, neurturin[110], and Brain-Derived Neurotrophic Factor (BDNF), lipidomics of signaling lipid mediators[111]; including prostaglandins (PGs) and leukotrienes (LTs) families, and myogenic genes (especially MyoD, Myogenin, and MyH2) that play a critical role in muscle regeneration[112,113]. Neurturin is involved in motor nerve recruitment and NMJ remodeling, GABA is involved in metabolic regulation via brown adipose tissue markers, and BAIBA is a muscle-derived osteocyte survival factor also implicated in the reduction of insulin resistance and skeletal muscle inflammation. These myokines are thought to be released following activation of the PGC-1α receptor due to stressors, nutrient scarcity, degeneration due to age, injury, and disease and act via retrograde signaling[109,114,115].
VII. Decellularized ECM Approaches
Decellularized extracellular matrix (DECM) approaches (with or without bioprinting) have also been attempted in-vivo[26,31,116]. In a pig model of VML, DECM could not abate inflammatory signaling while fibrosis could not be averted[12]. Rodent VML studies have demonstrated virtually no Pax7 positive cells within the defect two weeks after implantation of various biomaterials, pointing to material-induced fibrous encapsulation and/or inadequate chemo-traction as primary deficiencies in host satellite migration[26]. This was further demonstrated by autologous muscle flap paste mixed with DECM and fixated using fascia, leading to scarring in some aging animals[117]. Moreover, pre-fabricated scaffolds or decellularized transplant muscle modifications during surgery to fit the defect dimensions and use of silk sutures between biomaterial/ transplant and surrounding muscle[118] can add surgical time or micro-structural mismatch with surrounding tissues in mouse animal models. Further disadvantages include mechanical properties and chemical composition (e.g., degradation rate, stiffness/rigidity) that are generally harder to control in ECM treatments[119]. Thus, control over the properties of the micro- structure and bioactivity of therapies are needed to improve the healing process.
VIII. Biomaterials Used in VML and Peripheral Nerve Regeneration
These properties can be more precisely controlled in natural and synthetic biomaterials. However, disadvantages include challenges in cellular attachment, the potential degradation into byproducts that impede regeneration, and the potential formation of fibrous capsules due to an inflammatory response have occurred[119,120].
Collagen is the most abundant extracellular matrix protein found in many tissues and can promote cellular proliferation and tissue healing[27,49]. Purified collagen is FDA approved and is used for many applications. Collagen has shown to promote muscle cell survival and differentiation. Studies have shown myoblast differentiation to myofibers and integration to the tissues[31,121]. Collagen has also shown good properties for peripheral nerve regeneration and is used as a nerve conduit (commercially available as NeuroGen1)[49]. Kroehne et al showed that collagen scaffolds enhanced myotube differentiation and when implanted in an excised muscle in a mice model, the myotubes aligned and integrated to the host tissue following better healing along with electrical stimulation to the muscle[122]. Collagen scaffolds still has drawbacks as they are easily degradable and lack mechanical stiffness. Other natural biopolymers such as fibrin and chitosan have also shown capabilities to promote myoblastic activity[123,124]. Fibrin microthread scaffolds were shown to support the growth of myofibrils in healing of a VML in a mouse model as compared to a nanoporous fibrin gel[124]. Fibrin nerve conduits also show to increase the axonal regeneration due to its porous structure that allows the neurotropic factors to penetrate through it and also its degradability avoids nerve compression[86]. Myotube differentiation along with acting as a nerve conduit suggests that these materials can induce neuromuscular regeneration in a VML. Engineered gelatin hydrogels promote cell growth, form Glycosaminoglycan (GAG)-like structure, and are biodegradable[125–133], For instance, placing an osteoactivin doped gelatin (without cross-linker) hydrogel scaffold was used in rat VML, however, no effect was observed on neuromuscular regeneration[134]. This was likely owed to the need for modification to structurally support and promote rapid new muscle and vasculature.
As compared to natural biopolymer, synthetic biopolymers such as poly-L-lactic acid (PLLA), poly (lactic-co-glycolic acid) (PLGA), and poly-caprolactone (PCL)[135–140] can be modified to improve the mechanical properties, degradation rate and controlled release of growth factors to induce neuromuscular and eventual myogenic regeneration. PLGA is FDA approved and has widely been used as suturing material for scaffolds. Studies have found out that PLGA improves cell filtration and promotes angiogenesis and hence a material of interest for VML injury cases[137,141–143]. Luis et al showed that PLGA proved to enhance peripheral nerve regeneration in-vitro but had limited capabilities when used in a large gap model (10mm) in a rat sciatic nerve[144]. PCL is an FDA approved biomaterial and has been explored for musculoskeletal tissue regeneration. PCL has been preferred for use in slow drug delivery applications and where more structural strength is needed because of its slow degradability rate[137,145–147]. To modify and control the degradability rate along with the mechanical properties, copolymers PCL along with PGA and PLA are widely used[148]. Electrospun PCL along with natural biopolymers like chitosan has been explored to improve the myoblast cell proliferation, differentiation, and alignment of cells to form myotubes in-vitro[137,149,150]. However, these synthetic polymers have poor bioactivity and cell affinity. These scaffolds can cause immune or foreign body response due to the polymer or result of its degradation products[31,151]. PCL and PLGA scaffolds are even fabricated using 3D printing or additive manufacturing techniques for different geometries which help modify the mechanical properties, porosity of the scaffold, degradation rates, and elasticity which can affect the cellular response to the surface in terms of bioactivity, biocompatibility, local cellular responses, immune or foreign body responses[137,152,153].
Carbon nanotubes & polypyrrole (PPy) have been widely explored for neural tissue regeneration due to their electroconductivity. These materials boosted axon regeneration and neuromuscular recovery for end to end nerve repair. The combination of nanotubes along with mesenchymal stem cells have been seen to enhance the expression of neuronal markers, and growth factors are important for neuronal growth[154,155]. Incorporation or doping of such electrically conducted material has shown to improve the properties of various biomaterials. For example, PPy along with poly-d,llactic acid (PDLLA) has shown to enhance nerve regeneration in a rat sciatic nerve in-vivo[156].
Further, biopolymer surface modification with single peptides lack multi-functionality to mimic ECM[157], have low half-lives and lack thermal stability[158], while mini-proteins limit angiogenesis via low VEGF activity, thereby limiting their use for tissue healing[159]. While therapies should attenuate fibrosis and inflammation for improving the regeneration of muscle[20,160], most of the above approaches have not adequately addressed this issue as an intrinsic property of the biomaterial or via adequate drug delivery.
IX. Conclusion and Future Perspectives
There are few studies which have combined different techniques to overcome the complex anatomy issue for the VML repair and regeneration. The study performed by Novakova et al mentions the use of tissue engineered skeletal muscle units to promote regeneration and better integration of tissues with the underlying muscle tissue in a VML using a sheep model[161]. In combination with the skeletal muscle units, they also use an engineered neural conduit for better bridging and innervation of the skeletal muscle units to the adjacent tissues. Sheep bone marrow stromal cells were used to create the engineered 3D grafts and conduits in-vitro and implanted in the sheep. The resultant led to proper linear alignment of newly regenerated muscle tissue fibers with no necrotic core[161]. It is not well understood if the newly generated muscle tissues can withstand similar forces as normal.
With the complexity of the tissue, it is well understood now that a single technique or a material may not mimic the intrinsic cascade of events for the regeneration of a tissue as seen in Figure 6. The tissue engineering triad involving scaffolds, cells, and bioactive molecules has changed with understanding of various aspects. The effect and role of the composition of biomaterials and its biomechanics play a very important role and can enhance the regenerative capacity for a certain type of cell to grow along with the growth factors. Along with that the tissue engineering architecture also plays an important role and with the development of 3D printers, complex geometries controlling various factors inside it is now possible to attempt to mimic these structure-function relationships.
Figure 6:
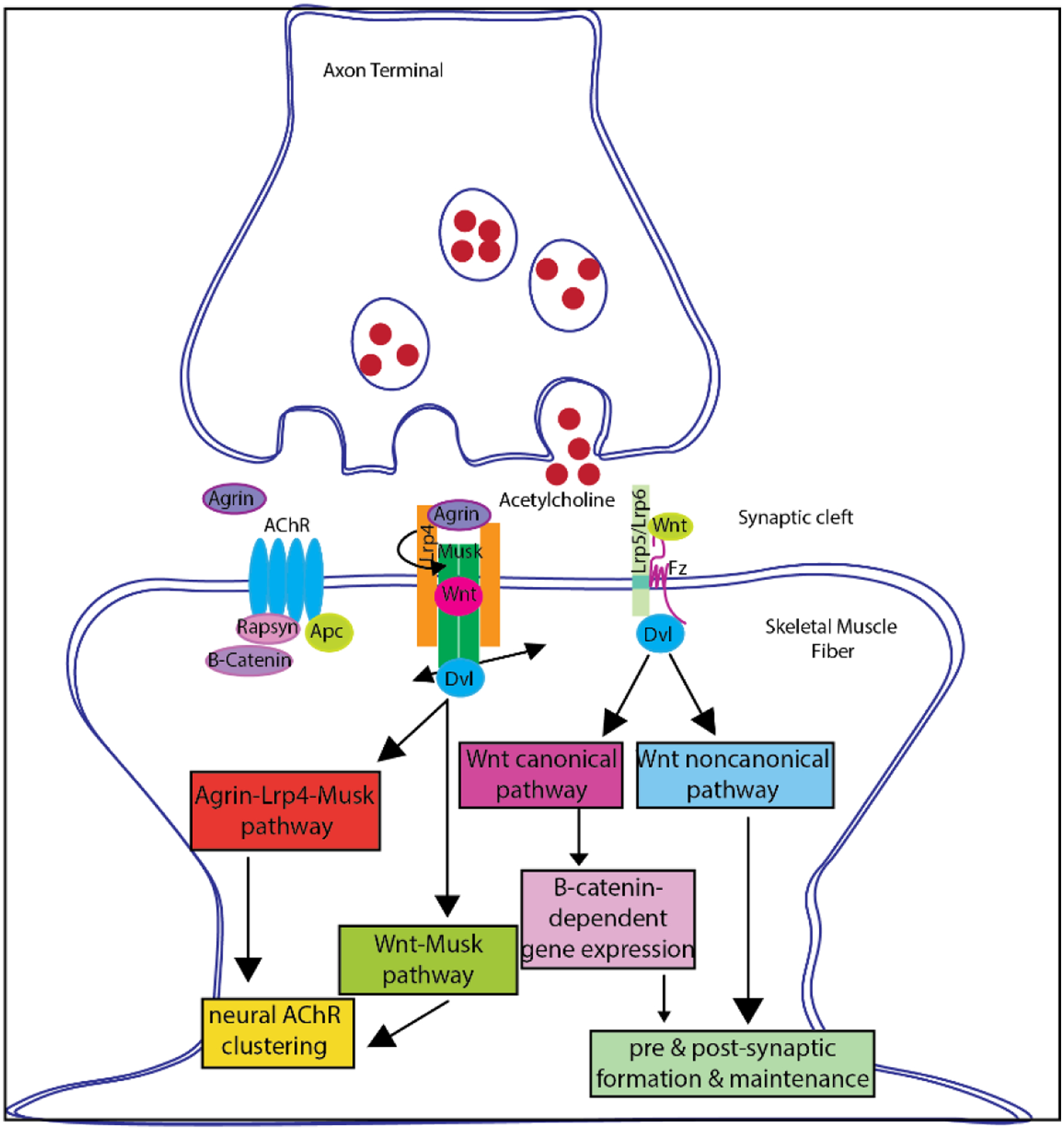
Illustration of cell signaling cascades involved in Neuromuscular junction regeneration & remodeling
Thus, there is a vital need for a new approach for improving the outcome of VML defect healing by novel scaffold chemistry, architecture, and method of intra-defect treatment that will yield improved autologous healing rates and quality of regenerated muscle tissue along with related endothelial and neuronal growth. The therapies we propose here are clearly needed to promote functional muscle tissue regeneration, vascular and nerve regeneration, reduce inflammation, and shorten recovery time from VML[22]. Our group and others have consistently demonstrated muscle strength to be the primary determinant of functional capacity in humans. Thus, providing structural support and promoting autologous healing will lead to muscle regeneration, functional restoration, and greatly improve the outcomes of patients afflicted with VML.
Investigation into tissue engineered constructs for neuromuscular repair has increased in recent years due to the discovery of the interdependence between muscle and neuronal tissues. Various studies tried different techniques for co-culture of skeletal muscle and neuronal cells to achieve this. Cvetkovic et al. upgraded the 2 dimensional co-culture by building a modular cellular system made by multi-layered tissue rings which contained differentiated skeletal muscle myotubes mixed with ECM for the first layer and motor neuron aggregates mixed with ECM proteins in another layer making a 3D construct (Figure 7)[27]. This co-culture was amenable for both cell types. The use of 3D printing provided flexibility as well as better integration to the co-culture design which helped in functional NMJ behaviour which can lead to physical, mechanical as well as functional muscle tissue repair and regeneration[27]. One method currently under investigation for repair of VML injuries involves using preinnervated tissue-engineered muscle constructs[162]. These constructs were created by plating mouse myoblasts on a PCL aligned nanofiber sheet to form a myocyte layer, then plating dissociated motor neurons, harvested from the spinal cord of Sprague Dawley rat pups, on top of the myocyte layer to form a co-culture. Co-cultures were differentiated for 14 days and then implanted into a 20% VML defect in the Tibialis Anterior of athymic rats for one and three weeks. The co-cultured scaffolds resulted in a significant increase in muscle cross sectional area after 3 weeks compared to non-innervated aligned myocyte scaffolds. While this method is useful for determining the relationship between muscle and peripheral motor neurons, it lacks clinical relevance as it involves harvesting motor neurons from the host and the pre-cultured scaffolds cannot be quickly modified in response to unforeseen changes in spatial distribution during surgical implantation. Additional Studies have been conducted over constructs composed of murine myoblasts seeded on an acellularized porcine bladder matrix and kept in a bioreactor for one week[163]. The constructs were then implanted into latissimus dorsi defects involving a 50% critical loss of the latissimus dorsi muscle in nude mice. This method had promising results for treating VML related injuries including increased myofiber formation, angiogenesis, and neurovascular bundle density two months after implantation. Despite these positive results, this method also involves the use of a bioreactor and the resultant scaffolds are not easily modifiable to match spatial requirements.
Figure 7:
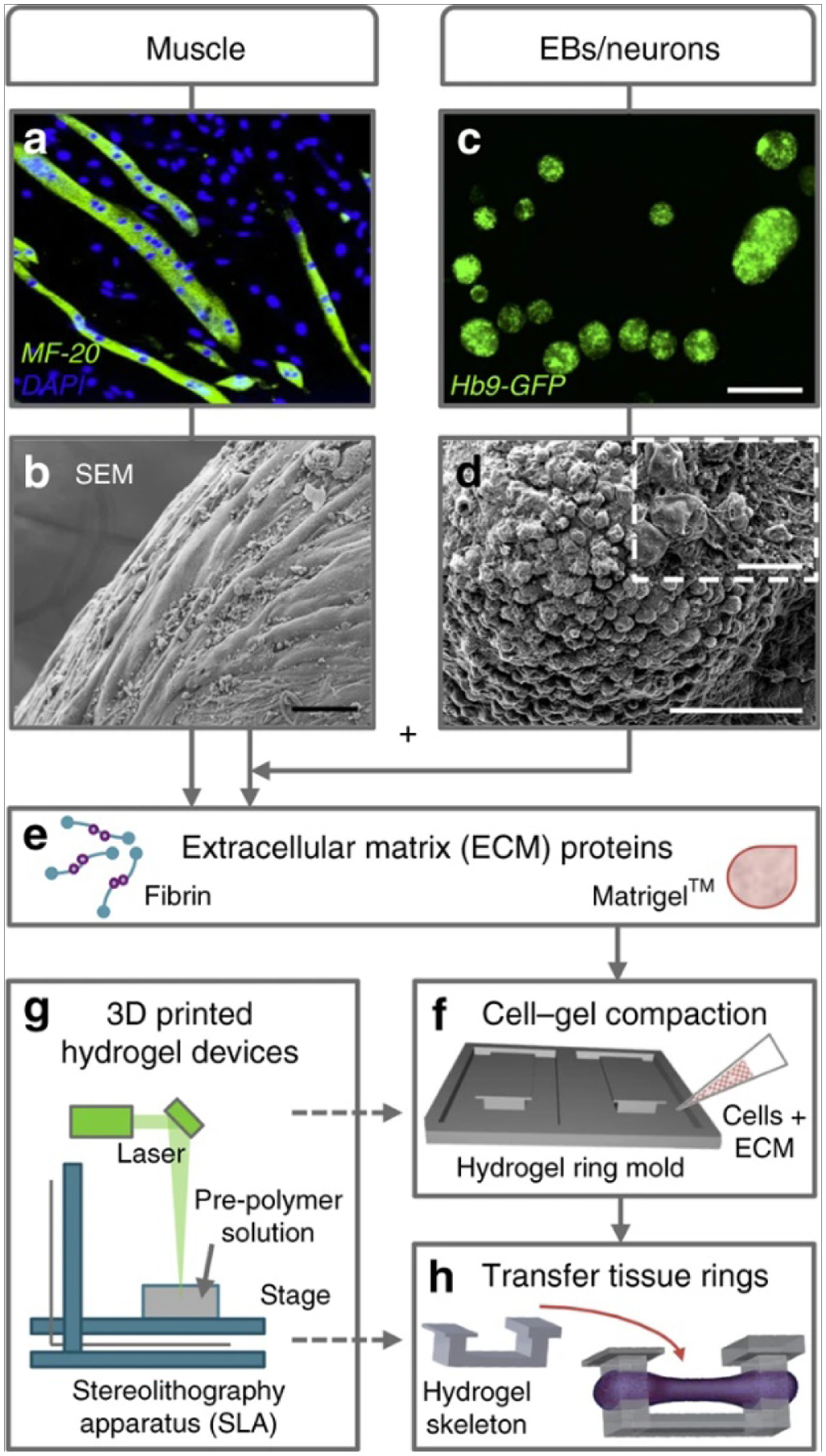
Skeletal muscle cells and motor neurons were combined into a fabricated 3D co-culture system. C2C12 myoblasts were differentiated into multinucleated myotubes (a) and combined with extracellular matrix (ECM) proteins to create an engineered muscle ring tissue (b). In parallel, mouse embryonic stem cells (HBG3 mESCs) were differentiated into motor neurons (MNs) through the formation of embryoid bodies (EBs) (c and d) and then combined with the engineered muscle tissue and ECM proteins (e) on 3D-printed hydrogel devices (f and g). Once the multi-layered rings sequentially compacted and fused together, they were then placed on a stationary hydrogel skeleton (h). Scale bars, 50 μm (b and d), 500 μm (c), and 10 μm (d, inset)[1]. Image taken from Cvetkovic, C., et al., A 3D-printed platform for modular neuromuscular motor units. Microsystems & Nanoengineering, 2017. 3(1): p. 17015.
There has been a lot of ongoing research in tissue engineering for muscle regeneration, for not only the use of cells but also the advancement in biomaterials and growth factors needed to enhance the cellular proliferation and differentiation in a VML model. With these advancements, one needs to also address the question of regeneration of the NMJ along with the muscle tissue to better mimic the natural tissue function and maintain the tissue morphology by avoiding atrophy. Incorporation of hybrid or combination of biomaterials and growth factors that direct the growth and support the regeneration of these two different tissue types which are closely interrelated is very important. The ongoing research in 3D bioprinting of tissues, using a variety of biomaterials, stem cells, in situ bioprinting, and bioactive molecules, can be designed to replicate the natural cascade of growth of various tissue types.
Highlights.
Volumetric muscle loss (VML) often leads to damage to neuromuscular components
Regenerative capacity of skeletal muscle is under neurogenic & vascular influence
Nerve loss still remains a critical challenge in functional muscle regeneration
Need for studying the extent of reinnervation of neuromuscular components in large defects
Tissue engineering use biomaterials to mimic structure-function relationship in VML
X. Acknowledgements
The authors would like to thank The University of Texas STARS award, UTA Departmental Start-up, the National Institutes of Health (Grant Number 1R03DE023872-01, 1R56DE027964-01A1-01, NIH S10OD025230), and the Osteo Science Foundation Peter L. Geistlich Research Grant for their support. We would also like to acknowledge Dr. Zui Pan for her motivation to write this manuscript and our lab member Thy Than Vo for her contribution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hill E, Boontheekul T, Mooney DJ, Designing scaffolds to enhance transplanted myoblast survival and migration., Tissue Eng. 12 (2006) 1295–1304. 10.1089/ten.2006.12.1295. [DOI] [PubMed] [Google Scholar]
- [2].Owens BD, Kragh JFJ, Wenke JC, Macaitis J, Wade CE, Holcomb JB, Combat wounds in operation Iraqi Freedom and operation Enduring Freedom., J. Trauma 64 (2008) 295–299. 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- [3].Mase VJJ, Hsu JR, Wolf SE, Wenke JC, Baer DG, Owens J, Badylak SF, Walters TJ, Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect., Orthopedics. 33 (2010) 511. 10.3928/01477447-20100526-24. [DOI] [PubMed] [Google Scholar]
- [4].Quintero AJ, Wright VJ, Fu FH, Huard J, Stem cells for the treatment of skeletal muscle injury., Clin. Sports Med 28 (2009) 1–11. 10.1016/j.csm.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Grogan BF, Hsu JR, Volumetric muscle loss., J. Am. Acad. Orthop. Surg 19 Suppl 1 (2011) S35–7. 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- [6].Wu X, Corona BT, Chen X, Walters TJ, A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies, Biores. Open Access 1 (2012) 280–290. 10.1089/biores.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Corona BT, Rivera JC, Owens JG, Wenke JC, Rathbone CR, Volumetric muscle loss leads to permanent disability following extremity trauma., J. Rehabil. Res. Dev 52 (2015) 785–792. 10.1682/JRRD.2014.07.0165. [DOI] [PubMed] [Google Scholar]
- [8].Corona BT, Flanagan KE, Brininger CM, Goldman SM, Call JA, Greising SM, Impact of volumetric muscle loss injury on persistent motoneuron axotomy., Muscle Nerve. 57 (2018) 799–807. 10.1002/mus.26016. [DOI] [PubMed] [Google Scholar]
- [9].Guntinas-Lichius O, Irintchev A, Streppel M, Lenzen M, Grosheva M, Wewetzer K, Neiss WF, Angelov DN, Factors limiting motor recovery after facial nerve transection in the rat: combined structural and functional analyses., Eur. J. Neurosci 21 (2005) 391–402. 10.1111/j.1460-9568.2005.03877.x. [DOI] [PubMed] [Google Scholar]
- [10].Oprel PP, Eversdijk MG, Vlot J, Tuinebreijer WE, den Hartog D, The acute compartment syndrome of the lower leg: a difficult diagnosis?, Open Orthop. J 4 (2010) 115–119. 10.2174/1874325001004020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Corona BT, Wu X, Ward CL, McDaniel JS, Rathbone CR, Walters TJ, The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM., Biomaterials. 34 (2013) 3324–3335. 10.1016/j.biomaterials.2013.01.061. [DOI] [PubMed] [Google Scholar]
- [12].Corona BT, Rivera JC, Greising SM, Inflammatory and Physiological Consequences of Debridement of Fibrous Tissue after Volumetric Muscle Loss Injury, Clin. Transl. Sci 11 (2018) 208–217. 10.1111/cts.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Neubauer T, Brand J, Hartmann A, [Neuromuscular complications of fractures of the extremities, part 2 : Nerve injuries and iatrogenic damage]., Unfallchirurg. 123 (2020) 225–237. 10.1007/s00113-020-00768-9. [DOI] [PubMed] [Google Scholar]
- [14].Asthana P, Zhang G, Sheikh KA, Him Eddie Ma C, Heat shock protein is a key therapeutic target for nerve repair in autoimmune peripheral neuropathy and severe peripheral nerve injury., Brain. Behav. Immun 91 (2021) 48–64. 10.1016/j.bbi.2020.08.020. [DOI] [PubMed] [Google Scholar]
- [15].Cross WW 3rd, Swiontkowski MF, Treatment principles in the management of open fractures, Indian J. Orthop 42 (2008) 377–386. 10.4103/0019-5413.43373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F, N.A.D. Workgroup, Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II, Arthritis Rheum. 58 (2008) 26–35. 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Watkins-Castillo S, Andersson G, United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States (BMUS), 2014. http://www.boneandjointburden.org.
- [18].Dhanwal DK, Dennison EM, Harvey NC, Cooper C, Epidemiology of hip fracture: Worldwide geographic variation, Indian J. Orthop 45 (2011) 15–22. 10.4103/0019-5413.73656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Scharner J, Zammit PS, The muscle satellite cell at 50: the formative years., Skelet. Muscle 1 (2011) 28. 10.1186/2044-5040-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aguilar CA, Greising SM, Watts A, Goldman SM, Peragallo C, Zook C, Larouche J, Corona BT, Multiscale analysis of a regenerative therapy for treatment of volumetric muscle loss injury, Cell Death Discov. 4 (2018) 33. 10.1038/s41420-018-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Laumonier T, Menetrey J, Muscle injuries and strategies for improving their repair., J. Exp. Orthop 3 (2016) 15. 10.1186/s40634-016-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Greising SM, Dearth CL, Corona BT, Regenerative and Rehabilitative Medicine: A Necessary Synergy for Functional Recovery from Volumetric Muscle Loss Injury., Cells. Tissues. Organs 202 (2016) 237–249. 10.1159/000444673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chang NC, Chevalier FP, Rudnicki MA, Satellite Cells in Muscular Dystrophy - Lost in Polarity., Trends Mol. Med 22 (2016) 479–496. 10.1016/j.molmed.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G, Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration., Development. 138 (2011) 3625–3637. 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lepper C, Partridge TA, Fan C-M, An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration., Development. 138 (2011) 3639–3646. 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Greising SM, Rivera JC, Goldman SM, Watts A, Aguilar CA, Corona BT, Unwavering Pathobiology of Volumetric Muscle Loss Injury, Sci. Rep 7 (2017) 13179. 10.1038/s41598-017-13306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cvetkovic C, Rich MH, Raman R, Kong H, Bashir R, A 3D-printed platform for modular neuromuscular motor units, Microsystems Nanoeng. 3 (2017) 17015. 10.1038/micronano.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hurtgen BJ, Ward CL, Garg K, Pollot BE, Goldman SM, McKinley TO, Wenke JC, Corona BT, Severe muscle trauma triggers heightened and prolonged local musculoskeletal inflammation and impairs adjacent tibia fracture healing, J. Musculoskelet. Neuronal Interact 16 (2016) 122–134. https://pubmed.ncbi.nlm.nih.gov/27282456. [PMC free article] [PubMed] [Google Scholar]
- [29].Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ, Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury., Tissue Eng. Part A 20 (2014) 705–715. 10.1089/ten.TEA.2012.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hallock GG, The Role of Muscle Flaps for Salvage of Failed Perforator Free Flaps, Plast. Reconstr. Surgery. Glob. Open 3 (2015) e564–e564. 10.1097/GOX.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu J, Saul D, Böker KO, Ernst J, Lehman W, Schilling AF, Current Methods for Skeletal Muscle Tissue Repair and Regeneration, Biomed Res. Int 2018 (2018) 1984879. 10.1155/2018/1984879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pannérec A, Marazzi G, Sassoon D, Stem cells in the hood: the skeletal muscle niche., Trends Mol. Med 18 (2012) 599–606. 10.1016/j.molmed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- [33].Gordon BS, Kelleher AR, Kimball SR, Regulation of muscle protein synthesis and the effects of catabolic states., Int. J. Biochem. Cell Biol 45 (2013) 2147–2157. 10.1016/j.biocel.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lindborg JA, Mack M, Zigmond RE, Neutrophils Are Critical for Myelin Removal in a Peripheral Nerve Injury Model of Wallerian Degeneration., J. Neurosci 37 (2017) 10258–10277. 10.1523/JNEUROSCI.2085-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hussain G, Wang J, Rasul A, Anwar H, Qasim M, Zafar S, Aziz N, Razzaq A, Hussain R, de Aguilar J-LG, Sun T, Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery, Int. J. Biol. Sci 16 (2020) 116–134. 10.7150/ijbs.35653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M, Mechanisms regulating skeletal muscle growth and atrophy., FEBS J. 280 (2013) 4294–4314. 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- [37].Wu P, Chawla A, Spinner RJ, Yu C, Yaszemski MJ, Windebank AJ, Wang H, Key changes in denervated muscles and their impact on regeneration and reinnervation., Neural Regen. Res 9 (2014) 1796–1809. 10.4103/1673-5374.143424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fitridge R, Thompson M, Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists, Adelaide (AU): University of Adelaide Press;, Adelaide (AU), 2011. [PubMed] [Google Scholar]
- [39].Awad K, Ahuja N, Fiedler M, Peper S, Wang Z, Aswath P, Brotto M, Varanasi V, Ionic Silicon Protects Oxidative Damage and Promotes Skeletal Muscle Cell Regeneration, Int. J. Mol. Sci 22 (2021). 10.3390/ijms22020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Girardi F, Le Grand F, Wnt Signaling in Skeletal Muscle Development and Regeneration., Prog. Mol. Biol. Transl. Sci 153 (2018) 157–179. 10.1016/bs.pmbts.2017.11.026. [DOI] [PubMed] [Google Scholar]
- [41].Garg K, Corona BT, Walters TJ, Therapeutic strategies for preventing skeletal muscle fibrosis after injury, Front. Pharmacol 6 (2015) 87. 10.3389/fphar.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zaza G, Granata S, Masola V, Rugiu C, Fantin F, Gesualdo L, Schena FP, Lupo A, Downregulation of Nuclear-Encoded Genes of Oxidative Metabolism in Dialyzed Chronic Kidney Disease Patients, PLoS One. 8 (2013) e77847. 10.1371/journal.pone.0077847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gureev AP, Shaforostova EA, Popov VN, Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways., Front. Genet 10 (2019) 435. 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ahn B, Pharaoh G, Premkumar P, Huseman K, Ranjit R, Kinter M, Szweda L, Kiss T, Fulop G, Tarantini S, Csiszar A, Ungvari Z, Van Remmen H, Nrf2 deficiency exacerbates age-related contractile dysfunction and loss of skeletal muscle mass, Redox Biol. 17 (2018) 47–58. 10.1016/j.redox.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Southern WM, Nichenko AS, Tehrani KF, McGranahan MJ, Krishnan L, Qualls AE, Jenkins NT, Mortensen LJ, Yin H, Yin A, Guldberg RE, Greising SM, Call JA, PGC-1α overexpression partially rescues impaired oxidative and contractile pathophysiology following volumetric muscle loss injury, Sci. Rep 9 (2019) 4079. 10.1038/s41598-019-40606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Estrella EP, Montales TD, Functioning free muscle transfer for the restoration of elbow flexion in brachial plexus injury patients., Injury. 47 (2016) 2525–2533. 10.1016/j.injury.2016.08.011. [DOI] [PubMed] [Google Scholar]
- [47].Lin C-H, Lin Y-T, Yeh J-T, Chen C-T, Free functioning muscle transfer for lower extremity posttraumatic composite structure and functional defect., Plast. Reconstr. Surg 119 (2007) 2118–2126. 10.1097/01.prs.0000260595.85557.41. [DOI] [PubMed] [Google Scholar]
- [48].V Stevanovic M, Cuéllar VG, Ghiassi A, Sharpe F, Single-stage Reconstruction of Elbow Flexion Associated with Massive Soft-Tissue Defect Using the Latissimus Dorsi Muscle Bipolar Rotational Transfer., Plast. Reconstr. Surgery. Glob. Open 4 (2016) e1066. 10.1097/GOX.0000000000001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Siemionow M, Bozkurt M, Zor F, Regeneration and repair of peripheral nerves with different biomaterials: review., Microsurgery. 30 (2010) 574–588. 10.1002/micr.20799. [DOI] [PubMed] [Google Scholar]
- [50].Chen LE, V Seaber A, Urbaniak JR, Murrell GA, Denatured muscle as a nerve conduit: a functional, morphologic, and electrophysiologic evaluation., J. Reconstr. Microsurg 10 (1994) 137–144. 10.1055/s-2007-1006579. [DOI] [PubMed] [Google Scholar]
- [51].Geuna S, Tos P, Battiston B, Guglielmone R, Giacobini-Robecchi MG, A stereological study of long-term regeneration of rat severed sciatic nerve repaired by means of muscle-vein-combined grafts., Ital. J. Anat. Embryol. = Arch. Ital. Di Anat. Ed Embriol 105 (2000) 65–73. [PubMed] [Google Scholar]
- [52].Nishiura Y, Brandt J, Nilsson A, Kanje M, Dahlin LB, Addition of cultured Schwann cells to tendon autografts and freeze-thawed muscle grafts improves peripheral nerve regeneration., Tissue Eng. 10 (2004) 157–164. 10.1089/107632704322791808. [DOI] [PubMed] [Google Scholar]
- [53].Gregory TM, Heckmann RA, Francis RS, The effect of exercise on the presence of leukocytes, erythrocytes and collagen fibers in skeletal muscle after contusion., J. Manipulative Physiol. Ther 18 (1995) 72–78. [PubMed] [Google Scholar]
- [54].Zhu Y, Zhang Y-J, Liu W-W, Shi A-W, Gu N, Salidroside Suppresses HUVECs Cell Injury Induced by Oxidative Stress through Activating the Nrf2 Signaling Pathway., Molecules. 21 (2016). 10.3390/molecules21081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Faria FET, Ferrari RJ, Distefano G, Ducatti AC, Soares KF, Montebelo MIL, Minamoto VB, The onset and duration of mobilization affect the regeneration in the rat muscle., Histol. Histopathol 23 (2008) 565–571. 10.14670/HH-23.565. [DOI] [PubMed] [Google Scholar]
- [56].Pavlov SP, Grosheva M, Streppel M, Guntinas-Lichius O, Irintchev A, Skouras E, Angelova SK, Kuerten S, Sinis N, Dunlop SA, Angelov DN, Manually-stimulated recovery of motor function after facial nerve injury requires intact sensory input., Exp. Neurol 211 (2008) 292–300. 10.1016/j.expneurol.2008.02.019. [DOI] [PubMed] [Google Scholar]
- [57].Evgenieva E, Schweigert P, Guntinas-Lichius O, Pavlov S, Grosheva M, Angelova S, Streppel M, Irintchev A, Skouras E, Kuerten S, Sinis N, Dunlop S, Radeva V, Angelov DN, Manual stimulation of the suprahyoid-sublingual region diminishes polynnervation of the motor endplates and improves recovery of function after hypoglossal nerve injury in rats., Neurorehabil. Neural Repair 22 (2008) 754–768. 10.1177/1545968308316387. [DOI] [PubMed] [Google Scholar]
- [58].Bischoff A, Grosheva M, Irintchev A, Skouras E, Kaidoglou K, Michael J, Angelova SK, Kuerten S, Sinis N, Dunlop SA, Angelov DN, Manual stimulation of the orbicularis oculi muscle improves eyelid closure after facial nerve injury in adult rats., Muscle Nerve. 39 (2009) 197–205. 10.1002/mus.21126. [DOI] [PubMed] [Google Scholar]
- [59].Guntinas-Lichius O, Hundeshagen G, Paling T, Streppel M, Grosheva M, Irintchev A, Skouras E, Alvanou A, Angelova SK, Kuerten S, Sinis N, Dunlop SA, Angelov DN, Manual stimulation of facial muscles improves functional recovery after hypoglossal-facial anastomosis and interpositional nerve grafting of the facial nerve in adult rats., Neurobiol. Dis 28 (2007) 101–112. 10.1016/j.nbd.2007.07.006. [DOI] [PubMed] [Google Scholar]
- [60].Angelov DN, Ceynowa M, Guntinas-Lichius O, Streppel M, Grosheva M, Kiryakova SI, Skouras E, Maegele M, Irintchev A, Neiss WF, Sinis N, Alvanou A, Dunlop SA, Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking., Neurobiol. Dis 26 (2007) 229–242. 10.1016/j.nbd.2006.12.016. [DOI] [PubMed] [Google Scholar]
- [61].Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ, The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration., Biomaterials. 32 (2011) 8905–8914. 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Doolabh VB, Hertl MC, Mackinnon SE, The role of conduits in nerve repair: a review., Rev. Neurosci 7 (1996) 47–84. 10.1515/revneuro.1996.7.1.47. [DOI] [PubMed] [Google Scholar]
- [63].Nakamura T, Inada Y, Fukuda S, Yoshitani M, Nakada A, Itoi S, Kanemaru S, Endo K, Shimizu Y, Experimental study on the regeneration of peripheral nerve gaps through a polyglycolic acid-collagen (PGA-collagen) tube., Brain Res. 1027 (2004) 18–29. 10.1016/j.brainres.2004.08.040. [DOI] [PubMed] [Google Scholar]
- [64].Järvinen TAH, Järvinen TLN, Kääriäinen M, Aärimaa V, Vaittinen S, Kalimo H, Järvinen M, Muscle injuries: optimising recovery., Best Pract. Res. Clin. Rheumatol 21 (2007) 317–331. 10.1016/j.berh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [65].Donghui C, Shicai C, Wei W, Fei L, Jianjun J, Gang C, Hongliang Z, Functional modulation of satellite cells in long-term denervated human laryngeal muscle., Laryngoscope. 120 (2010) 353–358. 10.1002/lary.20796. [DOI] [PubMed] [Google Scholar]
- [66].Degreef I, Debeer P, Van Herck B, Van Den Eeden E, Peers K, De Smet L, Treatment of irreparable rotator cuff tears by latissimus dorsi muscle transfer., Acta Orthop. Belg 71 (2005) 667–671. [PubMed] [Google Scholar]
- [67].Larkin LM, van der Meulen JH, Dennis RG, Kennedy JB, Functional evaluation of nerve-skeletal muscle constructs engineered in vitro, Vitr. Cell. Dev. Biol. - Anim 42 (2006) 75–82. 10.1290/0509064.1. [DOI] [PubMed] [Google Scholar]
- [68].Das M, Rumsey JW, Gregory CA, Bhargava N, Kang J-F, Molnar P, Riedel L, Guo X, Hickman JJ, Embryonic motoneuron-skeletal muscle co-culture in a defined system., Neuroscience. 146 (2007) 481–488. 10.1016/j.neuroscience.2007.01.068. [DOI] [PubMed] [Google Scholar]
- [69].Khan MM, Lustrino D, Silveira WA, Wild F, Straka T, Issop Y, O’Connor E, Cox D, Reischl M, Marquardt T, Labeit D, Labeit S, Benoit E, Molgó J, Lochmüller H, Witzemann V, Kettelhut IC, Navegantes LCC, Pozzan T, Rudolf R, Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease., Proc. Natl. Acad. Sci. U. S. A 113 (2016) 746–750. 10.1073/pnas.1524272113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rogers RG, Fournier M, Sanchez L, Ibrahim AG, Aminzadeh MA, Lewis MI, Marbán E, Disease-modifying bioactivity of intravenous cardiosphere-derived cells and exosomes in mdx mice, JCI Insight. 4 (2019). 10.1172/jci.insight.125754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Boldrin L, Zammit PS, Morgan JE, Satellite cells from dystrophic muscle retain regenerative capacity., Stem Cell Res. 14 (2015) 20–29. 10.1016/j.scr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hao M, Wang R, Wang W, Cell Therapies in Cardiomyopathy: Current Status of Clinical Trials., Anal. Cell. Pathol. (Amst) 2017 (2017) 9404057. 10.1155/2017/9404057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chakravarty T, Makkar RR, Ascheim DD, Traverse JH, Schatz R, DeMaria A, Francis GS, Povsic TJ, Smith RR, Lima JA, Pogoda JM, Marbán L, Henry TD, ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) Trial: Rationale and Design., Cell Transplant. 26 (2017) 205–214. 10.3727/096368916X692933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Berberoglu MA, Gallagher TL, Morrow ZT, Talbot JC, Hromowyk KJ, Tenente IM, Langenau DM, Amacher SL, Satellite-like cells contribute to pax7-dependent skeletal muscle repair in adult zebrafish., Dev. Biol 424 (2017) 162–180. 10.1016/j.ydbio.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Caseiro AR, Pereira T, Ivanova G, Luís AL, Maurício AC, Neuromuscular Regeneration: Perspective on the Application of Mesenchymal Stem Cells and Their Secretion Products., Stem Cells Int. 2016 (2016) 9756973. 10.1155/2016/9756973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Caplan AI, Dennis JE, Mesenchymal stem cells as trophic mediators., J. Cell. Biochem 98 (2006) 1076–1084. 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- [77].da S. Meirelles L, Fontes AM, Covas DT, Caplan AI, Mechanisms involved in the therapeutic properties of mesenchymal stem cells., Cytokine Growth Factor Rev. 20 (2009) 419–427. 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [78].Caseiro AR, Pereira T, Bártolo PJ, Santos JD, Luís AL, Maurício AC, Trends in Mesenchymal Stem Cells’ Applications for Skeletal Muscle Repair and Regeneration, Prog. Stem Cell Transplant (2015). 10.5772/60919. [DOI] [Google Scholar]
- [79].Pereira T, Armada-da Silva PAS, Amorim I, Rêma A, Caseiro AR, Gärtner A, Rodrigues M, Lopes MA, Bártolo PJ, Santos JD, Luís AL, Maurício AC, Effects of Human Mesenchymal Stem Cells Isolated from Wharton’s Jelly of the Umbilical Cord and Conditioned Media on Skeletal Muscle Regeneration Using a Myectomy Model., Stem Cells Int. 2014 (2014) 376918. 10.1155/2014/376918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V, Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo., PLoS One. 6 (2011) e17899. 10.1371/journal.pone.0017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gärtner A, Pereira T, Gomes R, Luís AL, França ML, Geuna S, Armada-da-silva P, Maurício AC, Mesenchymal Stem Cells from Extra-Embryonic Tissues for Tissue Engineering – Regeneration of the Peripheral Nerve, (n.d.).
- [82].Cartarozzi LP, Spejo AB, Ferreira RSJ, Barraviera B, Duek E, Carvalho JL, Góes AM, Oliveira ALR, Mesenchymal stem cells engrafted in a fibrin scaffold stimulate Schwann cell reactivity and axonal regeneration following sciatic nerve tubulization., Brain Res. Bull 112 (2015) 14–24. 10.1016/j.brainresbull.2015.01.005. [DOI] [PubMed] [Google Scholar]
- [83].Gärtner A, Pereira T, Alves MG, Armada-da-Silva PAS, Amorim I, Gomes R, Ribeiro J, França ML, Lopes C, Carvalho RA, Socorro S, Oliveira PF, Porto B, Sousa R, Bombaci A, Ronchi G, Fregnan F, Varejão ASP, Luís AL, Geuna S, Maurício AC, Use of poly(DL-lactide-ε-caprolactone) membranes and mesenchymal stem cells from the Wharton’s jelly of the umbilical cord for promoting nerve regeneration in axonotmesis: in vitro and in vivo analysis., Differentiation. 84 (2012) 355–365. 10.1016/j.diff.2012.10.001. [DOI] [PubMed] [Google Scholar]
- [84].Gärtner A, Pereira T, Armada-da-Silva P, Amado S, Veloso A, Amorim I, Ribeiro J, Santos J, Bárcia R, Cruz P, Cruz H, Luís A, Santos J, Geuna S, Maurício A, Effects of umbilical cord tissue mesenchymal stem cells (UCX®) on rat sciatic nerve regeneration after neurotmesis injuries, J. Stem Cells Regen. Med 10 (2014) 14–26. 10.46582/jsrm.1001004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sun F, Zhou K, Mi W, Qiu J, Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats., Biomaterials. 32 (2011) 8118–8128. 10.1016/j.biomaterials.2011.07.031. [DOI] [PubMed] [Google Scholar]
- [86].di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF, Adipose-derived stem cells enhance peripheral nerve regeneration., J. Plast. Reconstr. Aesthet. Surg 63 (2010) 1544–1552. 10.1016/j.bjps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- [87].Liu G, Cheng Y, Guo S, Feng Y, Li Q, Jia H, Wang Y, Tong L, Tong X, Transplantation of adipose-derived stem cells for peripheral nerve repair., Int. J. Mol. Med 28 (2011) 565–572. 10.3892/ijmm.2011.725. [DOI] [PubMed] [Google Scholar]
- [88].Undale AH, Westendorf JJ, Yaszemski MJ, Khosla S, Mesenchymal Stem Cells for Bone Repair and Metabolic Bone Diseases, Mayo Clin. Proc 84 (2009) 893–902. 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kwee BJ, Mooney DJ, Biomaterials for skeletal muscle tissue engineering, Curr. Opin. Biotechnol 47 (2017) 16–22. 10.1016/j.copbio.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H, Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold., Mol. Ther 22 (2014) 1441–1449. 10.1038/mt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ, Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors., Proc. Natl. Acad. Sci. U. S. A 107 (2010) 3287–3292. 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Schiaffino S, Mammucari C, Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models, Skelet. Muscle 1 (2011) 4. 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shvartsman D, Storrie-White H, Lee K, Kearney C, Brudno Y, Ho N, Cezar C, McCann C, Anderson E, Koullias J, Tapia JC, Vandenburgh H, Lichtman JW, Mooney DJ, Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling., Mol. Ther 22 (2014) 1243–1253. 10.1038/mt.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hwang JH, Kim IG, Piao S, Jung AR, Lee JY, Park KD, Lee JY, Combination therapy of human adipose-derived stem cells and basic fibroblast growth factor hydrogel in muscle regeneration., Biomaterials. 34 (2013) 6037–6045. 10.1016/j.biomaterials.2013.04.049. [DOI] [PubMed] [Google Scholar]
- [95].Hudson TW, Evans GR, Schmidt CE, Engineering strategies for peripheral nerve repair., Clin. Plast. Surg 26 (1999) 617–28, ix. [PubMed] [Google Scholar]
- [96].Midha R, Munro CA, Dalton PD, Tator CH, Shoichet MS, Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube., J. Neurosurg 99 (2003) 555–565. 10.3171/jns.2003.99.3.0555. [DOI] [PubMed] [Google Scholar]
- [97].Cordeiro PG, Seckel BR, Lipton SA, D’Amore PA, Wagner J, Madison R, Acidic fibroblast growth factor enhances peripheral nerve regeneration in vivo., Plast. Reconstr. Surg 83 (1989) 1011–1013. [PubMed] [Google Scholar]
- [98].Huang Y-C, Huang Y-Y, Biomaterials and strategies for nerve regeneration., Artif. Organs 30 (2006) 514–522. 10.1111/j.1525-1594.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- [99].Ciardelli G, Chiono V, Materials for peripheral nerve regeneration., Macromol. Biosci 6 (2006) 13–26. 10.1002/mabi.200500151. [DOI] [PubMed] [Google Scholar]
- [100].Han JW, Choi D, Lee MY, Huh YH, Yoon Y, Bone Marrow-Derived Mesenchymal Stem Cells Improve Diabetic Neuropathy by Direct Modulation of Both Angiogenesis and Myelination in Peripheral Nerves., Cell Transplant. 25 (2016) 313–326. 10.3727/096368915X688209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Carragee EJ, Hurwitz EL, Weiner BK, A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned., Spine J. 11 (2011) 471–491. 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- [102].Vo TN, Kasper FK, Mikos AG, Strategies for controlled delivery of growth factors and cells for bone regeneration, Adv. Drug Deliv. Rev 64 (2012) 1292–1309. 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Baumgartner I, Therapeutic angiogenesis: Theoretic problems using vascular endothelial growth factor, Curr. Cardiol. Rep 2 (2000) 24–28. 10.1007/s11886-000-0021-6. [DOI] [PubMed] [Google Scholar]
- [104].Papageorgiou N, Tousoulis D, Katsargyris A, Charakida M, Androulakis E, Siasos G, Tentolouris C, Stefanadis C, Antioxidant treatment and endothelial dysfunction: is it time for flavonoids?, Recent Pat. Cardiovasc. Drug Discov 8 (2013) 81–92. 10.2174/15748901113089990018. [DOI] [PubMed] [Google Scholar]
- [105].Su JB, Vascular endothelial dysfunction and pharmacological treatment, World J. Cardiol 7 (2015) 719–741. 10.4330/wjc.v7.i11.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Duvall WL, Endothelial dysfunction and antioxidants., Mt. Sinai J. Med 72 (2005) 71–80. [PubMed] [Google Scholar]
- [107].Daiber A, Steven S, Weber A, V Shuvaev V, Muzykantov VR, Laher I, Li H, Lamas S, Münzel T, Targeting vascular (endothelial) dysfunction, Br. J. Pharmacol 174 (2017) 1591–1619. 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Pedersen BK, Exercise-induced myokines and their role in chronic diseases., Brain. Behav. Immun 25 (2011) 811–816. 10.1016/j.bbi.2011.02.010. [DOI] [PubMed] [Google Scholar]
- [109].Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jähn K, Yi J, Zhou J, Brotto M, Bonewald LF, β-aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor, Cell Rep. 22 (2018) 1531–1544. 10.1016/j.celrep.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mills R, Taylor-Weiner H, Correia JC, Agudelo LZ, Allodi I, Kolonelou C, Martinez-Redondo V, Ferreira DMS, Nichterwitz S, Comley LH, Lundin V, Hedlund E, Ruas JL, Teixeira AI, Neurturin is a PGC-1α1-controlled myokine that promotes motor neuron recruitment and neuromuscular junction formation., Mol. Metab 7 (2018) 12–22. 10.1016/j.molmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang Z, Bian L, Mo C, Kukula M, Schug KA, Brotto M, Targeted quantification of lipid mediators in skeletal muscles using restricted access media-based trap-and-elute liquid chromatography-mass spectrometry., Anal. Chim. Acta 984 (2017) 151–161. 10.1016/j.aca.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Delgado I, Huang X, Jones S, Zhang L, Hatcher R, Gao B, Zhang P, Dynamic gene expression during the onset of myoblast differentiation in vitro., Genomics. 82 (2003) 109–121. 10.1016/s0888-7543(03)00104-6. [DOI] [PubMed] [Google Scholar]
- [113].Willkomm L, Schubert S, Jung R, Elsen M, Borde J, Gehlert S, Suhr F, Bloch W, Lactate regulates myogenesis in C2C12 myoblasts in vitro., Stem Cell Res. 12 (2014) 742–753. 10.1016/j.scr.2014.03.004. [DOI] [PubMed] [Google Scholar]
- [114].Pedersen BK, Muscles and their myokines., J. Exp. Biol 214 (2011) 337–346. 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- [115].Rai M, Demontis F, Systemic Nutrient and Stress Signaling via Myokines and Myometabolites., Annu. Rev. Physiol 78 (2016) 85–107. 10.1146/annurev-physiol-021115-105305. [DOI] [PubMed] [Google Scholar]
- [116].Choi Y-J, Jun Y-J, Kim DY, Yi H-G, Chae S-H, Kang J, Lee J, Gao G, Kong J-S, Jang J, Chung WK, Rhie J-W, Cho D-W, A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss., Biomaterials. 206 (2019) 160–169. 10.1016/j.biomaterials.2019.03.036. [DOI] [PubMed] [Google Scholar]
- [117].Kim JT, Kasukonis B, Dunlap G, Perry R, Washington T, Wolchok JC, Regenerative Repair of Volumetric Muscle Loss Injury is Sensitive to Age., Tissue Eng. Part A 26 (2020) 3–14. 10.1089/ten.TEA.2019.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Egozi D, Shandalov Y, Freiman A, Rosenfeld D, Ben-Shimol D, Levenberg S, Engineered Vascularized Muscle Flap, J. Vis. Exp (2016) 52984. 10.3791/52984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Nakayama KH, Shayan M, Huang NF, Engineering Biomimetic Materials for Skeletal Muscle Repair and Regeneration, Adv. Healthc. Mater 8 (2019) 1801168. 10.1002/adhm.201801168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Maleiner B, Tomasch J, Heher P, Spadiut O, Rünzler D, Fuchs C, The Importance of Biophysical and Biochemical Stimuli in Dynamic Skeletal Muscle Models, Front. Physiol 9 (2018) 1130. 10.3389/fphys.2018.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ju YM, Atala A, Yoo JJ, Lee SJ, In situ regeneration of skeletal muscle tissue through host cell recruitment., Acta Biomater. 10 (2014) 4332–4339. 10.1016/j.actbio.2014.06.022. [DOI] [PubMed] [Google Scholar]
- [122].Kroehne V, Heschel I, Schügner F, Lasrich D, Bartsch JW, Jockusch H, Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts., J. Cell. Mol. Med 12 (2008) 1640–1648. 10.1111/j.1582-4934.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Beier JP, Kneser U, Stern-Sträter J, Stark GB, Bach AD, Y chromosome detection of three-dimensional tissue-engineered skeletal muscle constructs in a syngeneic rat animal model., Cell Transplant. 13 (2004) 45–53. 10.3727/000000004772664888. [DOI] [PubMed] [Google Scholar]
- [124].Grasman JM, Do DM, Page RL, Pins GD, Rapid release of growth factors regenerates force output in volumetric muscle loss injuries., Biomaterials. 72 (2015) 49–60. 10.1016/j.biomaterials.2015.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].An J, Yuan X, Luo Q, Wang D, Preparation of chitosan-graft-(methyl methacrylate)/Ag nanocomposite with antimicrobial activity, Polym. Int 59 (2010) 62–70. 10.1002/pi.2689. [DOI] [Google Scholar]
- [126].Ragetly G, Griffon D, Lee H, Fredericks L, Gordon-Evans W, Chung Y, Effect of chitosan scaffold microstructure on mesenchymal stem cell chondrogenesis, Acta Biomater. 6 (2009) 1430–1436. 10.1016/j.actbio.2009.10.040. [DOI] [PubMed] [Google Scholar]
- [127].Ragetly GR, Griffon DJ, Lee H-B, Fredericks LP, Gordon-Evans W, Chung YS, Effect of chitosan scaffold microstructure on mesenchymal stem cell chondrogenesis, Acta Biomater. 6 (2010) 1430–1436. 10.1016/j.actbio.2009.10.040. [DOI] [PubMed] [Google Scholar]
- [128].Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E, Kaunas R, Gaharwar AK, Bioactive Nanoengineered Hydrogels for Bone Tissue Engineering: A Growth-Factor-Free Approach, ACS Nano. 9 (2015) 3109–3118. 10.1021/nn507488s. [DOI] [PubMed] [Google Scholar]
- [129].Angele P, Müller R, Schumann D, Englert C, Zellner J, Johnstone B, Yoo J, Hammer J, Fierlbeck J, Angele MK, Nerlich M, Kujat R, Characterization of esterified hyaluronan-gelatin polymer composites suitable for chondrogenic differentiation of mesenchymal stem cells., J. Biomed. Mater. Res. A 91 (2009) 416–427. 10.1002/jbm.a.32236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Salamon A, Van Vlierberghe S, Van Nieuwenhove I, Baudisch F, Graulus G-J, Benecke V, Alberti K, Neumann H-G, Rychly J, Martins JC, Dubruel P, Peters K, Gelatin-Based Hydrogels Promote Chondrogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells In Vitro, Materials (Basel). 7 (2014) 1342–1359. 10.3390/ma7021342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Chung C, Burdick JA, Influence of Three-Dimensional Hyaluronic Acid Microenvironments on Mesenchymal Stem Cell Chondrogenesis, Tissue Eng. Part A 15 (2009) 243–254. 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA, Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues, Biomaterials. 34 (2013) 5571–5580. 10.1016/j.biomaterials.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA, The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy., Biomaterials. 34 (2013) 413–421. 10.1016/j.biomaterials.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ma J, Baker AR, Calabro A, Derwin KA, Exploratory study on the effect of osteoactivin on muscle regeneration in a rat volumetric muscle loss model, PLoS One. 12 (2017) e0175853–e0175853. 10.1371/journal.pone.0175853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Green JJ, Elisseeff JH, Mimicking biological functionality with polymers for biomedical applications, Nature. 540 (2016) 386–394. 10.1038/nature21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ, The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes, Biomaterials. 29 (2008) 2899–2906. 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- [137].Wolf MT, Dearth CL, Sonnenberg SB, Loboa EG, Badylak SF, Naturally derived and synthetic scaffolds for skeletal muscle reconstruction, Adv. Drug Deliv. Rev 84 (2015) 208–221. 10.1016/j.addr.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kim JH, Seol Y-J, Ko IK, Kang H-W, Lee YK, Yoo JJ, Atala A, Lee SJ, 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration, Sci. Rep 8 (2018) 12307. 10.1038/s41598-018-29968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Ku SH, Lee SH, Park CB, Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation, Biomaterials. 33 (2012) 6098–6104. 10.1016/j.biomaterials.2012.05.018. [DOI] [PubMed] [Google Scholar]
- [140].Jun I, Jeong S, Shin H, The stimulation of myoblast differentiation by electrically conductive sub-micron fibers, Biomaterials. 30 (2009) 2038–2047. 10.1016/j.biomaterials.2008.12.063. [DOI] [PubMed] [Google Scholar]
- [141].Lesman A, Koffler J, Atlas R, Blinder YJ, Kam Z, Levenberg S, Engineering vessel-like networks within multicellular fibrin-based constructs, Biomaterials. 32 (2011) 7856–7869. 10.1016/j.biomaterials.2011.07.003. [DOI] [PubMed] [Google Scholar]
- [142].Peters MC, Polverini PJ, Mooney DJ, Engineering vascular networks in porous polymer matrices, J. Biomed. Mater. Res 60 (2002) 668–678. 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- [143].Smith MK, Peters MC, Richardson TP, Garbern JC, Mooney DJ, Locally Enhanced Angiogenesis Promotes Transplanted Cell Survival, Tissue Eng. 10 (2004) 63–71. 10.1089/107632704322791709. [DOI] [PubMed] [Google Scholar]
- [144].Luis A, Rodrigues J, Geuna S, Amado S, Shirosaki Y, Lee J, Fregnan F, Lopes M, Veloso A, Ferreira A, Santos J, Armada-da-Silva P, Varejão A, Mauricio AC, Use of PLGA 90:10 Scaffolds Enriched with In Vitro – Differentiated Neural Cells for Repairing Rat Sciatic Nerve Defects, Tissue Eng. Part A 14 (2008) 979–993. 10.1089/ten.tea.2007.0273. [DOI] [PubMed] [Google Scholar]
- [145].Grizzi I, Garreau H, Li S, Vert M, Hydrolytic degradation of devices based on poly(dl-lactic acid) size-dependence, Biomaterials. 16 (1995) 305–311. 10.1016/0142-9612(95)93258-F. [DOI] [PubMed] [Google Scholar]
- [146].Lam CXF, Hutmacher D, Schantz J, Woodruff M, Teoh SH, Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo., J. Biomed. Mater. Res. A 90 3 (2009) 906–919. [DOI] [PubMed] [Google Scholar]
- [147].Lam CXF, Savalani MM, Teoh S-H, Hutmacher DW, Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: accelerated versus simulated physiological conditions, Biomed. Mater 3 (2008) 34108. 10.1088/1748-6041/3/3/034108. [DOI] [PubMed] [Google Scholar]
- [148].Bellón J, Rodríguez M, García-Honduvilla N, Pascual G, Buján J, Partially absorbable meshes for hernia repair offer advantages over nonabsorbable meshes, Am. J. Surg 194 (2007) 68–74. 10.1016/j.amjsurg.2006.11.016. [DOI] [PubMed] [Google Scholar]
- [149].Jana S, Leung M, Chang J, Zhang M, Effect of nano- and micro-scale topological features on alignment of muscle cells and commitment of myogenic differentiation, Biofabrication. 6 (2014) 35012. 10.1088/1758-5082/6/3/035012. [DOI] [PubMed] [Google Scholar]
- [150].Leung M, Cooper A, Jana S, Tsao C-T, Petrie TA, Zhang M, Nanofiber-Based in Vitro System for High Myogenic Differentiation of Human Embryonic Stem Cells, Biomacromolecules. 14 (2013) 4207–4216. 10.1021/bm4009843. [DOI] [PubMed] [Google Scholar]
- [151].Yang J, Jao B, McNally A, Anderson J, In vivo quantitative and qualitative assessment of foreign body giant cell formation on biomaterials in mice deficient in natural killer lymphocyte subsets, mast cells, or the interleukin-4 receptorα and in severe combined immunodeficient mice, J. Biomed. Mater. Res. A 102 (2014). 10.1002/jbm.a.35152. [DOI] [PubMed] [Google Scholar]
- [152].Seyednejad H, Gawlitta D, V Kuiper R, de Bruin A, van Nostrum CF, Vermonden T, Dhert WJA, Hennink WE, In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxylfunctionalized poly(ε-caprolactone), Biomaterials. 33 (2012) 4309–4318. 10.1016/j.biomaterials.2012.03.002. [DOI] [PubMed] [Google Scholar]
- [153].Cei D, Malena A, De Maria C, Loro E, Sandri F, Moro G, Bettio S, Vergani L, Vozzi G, In vitro development of engineered muscle using a scaffold based on the pressure-activated microsyringe (PAM) technique, J. Tissue Eng. Regen. Med 11 (2014). 10.1002/term.1894. [DOI] [PubMed] [Google Scholar]
- [154].Ribeiro J, Pereira T, Caseiro A, Armada-da-Silva P, Pires I, Prada J, Amorim I, Amado S, França M, Gonçalves C, Lopes M, Santos J, Silva D, Geuna S, Luis A, Mauricio AC, Evaluation of biodegradable electric conductive tube-guides and mesenchymal stem cells, World J. Stem Cells 7 (2015) 956–975. 10.4252/wjsc.v7.i6.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Lee JH, Lee J-Y, Yang SH, Lee E-J, Kim H-W, Carbon nanotube-collagen three-dimensional culture of mesenchymal stem cells promotes expression of neural phenotypes and secretion of neurotrophic factors, Acta Biomater. 10 (2014) 4425–4436. 10.1016/j.actbio.2014.06.023. [DOI] [PubMed] [Google Scholar]
- [156].Xu H, Holzwarth J, Yan Y, Xu P, Zheng H, Yin Y, Li S, Ma P, Conductive PPY/PDLLA conduit for peripheral nerve regeneration, Biomaterials. 35 (2013). 10.1016/j.biomaterials.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Collier JH, Segura T, Evolving the use of peptides as components of biomaterials, Biomaterials. 32 (2011) 4198–4204. 10.1016/j.biomaterials.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Chow D, Nunalee ML, Lim DW, Simnick AJ, Chilkoti A, Peptide-based Biopolymers in Biomedicine and Biotechnology, Mater. Sci. Eng. R. Rep 62 (2008) 125–155. 10.1016/j.mser.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Treggiari D, Zoccatelli G, Molesini B, Degan M, Rotino GL, Sala T, Cavallini C, MacRae CA, Minuz P, Pandolfini T, A cystine-knot miniprotein from tomato fruit inhibits endothelial cell migration and angiogenesis by affecting vascular endothelial growth factor receptor (VEGFR) activation and nitric oxide production., Mol. Nutr. Food Res 59 (2015) 2255–2266. 10.1002/mnfr.201500267. [DOI] [PubMed] [Google Scholar]
- [160].Cittadella Vigodarzere G, Mantero S, Skeletal muscle tissue engineering: strategies for volumetric constructs., Front. Physiol 5 (2014) 362. 10.3389/fphys.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Novakova S, Nutter G, Larkin L, In Vitro Assessment of Scaffoldless Tissue-Engineered Skeletal Muscle for Volumetric Muscle Loss Repair, FASEB J. 33 (2019) 769.9–769.9. 10.1096/fasebj.2019.33.1\_supplement.769.9. [DOI] [Google Scholar]
- [162].Das S, Browne KD, Laimo FA, Maggiore JC, Hilman MC, Kaisaier H, Aguilar CA, Ali ZS, Mourkioti F, Cullen DK, Pre-innervated tissue-engineered muscle promotes a pro-regenerative microenvironment following volumetric muscle loss, Commun. Biol 3 (2020) 330. 10.1038/s42003-020-1056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, Dannahower A, Zhao W, Yoo JJ, Christ GJ, A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model., Tissue Eng. Part A 17 (2011) 2291–2303. 10.1089/ten.TEA.2010.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]


