Abstract
Goals:
Our aims were to describe the diagnostic and prognostic performance of transient elastography (TE) and magnetic resonance elastography (MRE) in patients with primary biliary cholangitis (PBC).
Background:
The diagnostic performance of TE and MRE in detecting advanced fibrosis in PBC and in predicting outcomes independent of existing serologic prognostic markers is incompletely understood.
Study:
Five hundred and thirty-eight consecutive patients with PBC at three centers with liver stiffness (LS) measurements by TE (n=286) or MRE (n=332) were reviewed. LS cut-offs for predicting fibrosis stages were determined by receiver operating characteristic curves among those with a liver biopsy (TE n=63, MRE n=98). Cox proportional hazard regression modeling was used to identify associations between covariates and hepatic decompensation.
Results:
The optimal LS thresholds for predicting histologic stage F4 were 14.40 kilopascals (kPa) [Area under the curve (AUC) 0.94] for TE and 4.60 kPa [AUC 0.82] for MRE. Both TE and MRE outperformed biochemical markers for prediction of histologic advanced fibrosis. Optimal LS thresholds to predict hepatic decompensation were 10.20 kPa on TE and 4.30 kPa on MRE. LS by TE and MRE (respectively) remained predictors of hepatic decompensation after adjusting for UDCA responsiveness [hazard ratio (HR), 1.14; 95% confidence interval (CI), 1.05–1.24 and HR, 1.68; 95% CI, 1.28–2.19] and the GLOBE score [HR, 1.13; 95% CI, 1.07–1.19 and HR, 2.09; 95% CI, 1.57–2.78].
Conclusions:
LS measurement with either TE or MRE is able to accurately detect advanced fibrosis and offers additional prognostic value beyond existing serologic predictive tools.
Keywords: Liver stiffness, magnetic resonance elastography, transient elastography, primary biliary cholangitis
Introduction
Primary biliary cholangitis (PBC) is an autoimmune chronic cholestatic liver disease which can lead to cirrhosis and complications related to portal hypertension (1). Indeed, prognosis is largely tied to the rate and extent of parenchymal extinction and fibrotic deposition (2). Understanding the natural history of this progression and how to optimally assess it is important in the management and risk stratification of patients with PBC (3, 4).
Liver biopsy is an imperfect gold standard for the assessment of fibrosis severity. It is limited by its invasiveness, cost, variability in interpretation amongst pathologists and the limited volume of liver sampled (5, 6). Liver stiffness (LS) has been increasingly used as a surrogate marker for hepatic fibrosis (7, 8). LS can be quantified through transient elastography (TE) and magnetic resonance elastography (MRE) (9–14). Several studies have shown correlation between fibrosis stage and LS by TE in PBC (15–21). An increased LS measured by TE has been shown to be associated with adverse outcomes in PBC (15). However, the diagnostic and prognostic role of MRE in PBC has been unexplored and it remains unclear if TE or MRE measured LS has prognostic value independent of ursodeoxycholic acid (UDCA) response status and the GLOBE PBC score.
To address these gaps in our knowledge, we aimed to characterize the diagnostic performances of TE and MRE for predicting fibrosis on liver biopsy and to probe the prognostic capabilities of LS by TE and MRE among those with PBC.
Materials and Methods
Patients
This study was approved by the Institutional Review Board of Mayo Clinic. Using the electronic medical record, a retrospective review was conducted across all Mayo Clinics in Minnesota (MN), Arizona (AZ) and Florida (FL). Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) initiative recommendations were applied for the manuscript design (22).
Inclusion criteria included adult patients diagnosed with PBC according to established criteria (elevated alkaline phosphatase (ALP), and positive anti-mitochondrial antibody or elevated ALP and compatible histologic features among those who tested negative for anti-mitochondrial antibodies) who underwent at least one TE or MRE between January 1st 2007 to January 1st 2019 (23, 24). Subjects with concurrent autoimmune hepatitis (AIH) were included and AIH-PBC overlap was established on the basis of histologic features in conjunction with an established diagnosis of PBC (23, 24). However, individuals with other causes of chronic liver diseases beyond AIH were excluded. Subjects with recurrent PBC post-transplant detected on liver biopsy were excluded from survival analyses if the only LS measurement occurred following liver transplantation (LT). However, they were able to remain in the analysis which correlated LS and fibrosis stage when recurrent PBC was confirmed on liver biopsy provided the LS was measured after transplant and within 1 year of the liver biopsy. TE-derived LS measurements with an interquartile range (IQR) to median ratio exceeding 30% were excluded, per the manufacturer’s recommendation. MRE was performed as described previously by authors of this study (25).
Data collection
LS was quantified as kilopascals (kPa) using TE (Echosens, Paris, France) or MRE as previously described (25–27). Serum biochemical values were collected within 6 months of the LS assessment. These values were used to calculate the aspartate aminotransferase (AST) to platelet ratio index (APRI), and GLOBE score. Alanine aminotransferase (ALT) & ALP were measured as ratios to their upper limits of normal (ULN). UDCA non-responders were defined using the Toronto criteria (28). Splenomegaly was defined as a splenic width measurement (largest anterior-posterior measurement) greater than 11cm (29). Among patients without hepatic decompensation, portal hypertension was defined as the presence of splenomegaly, varices, thrombocytopenia or portosystemic shunts.
Liver biopsy specimens were included in the analysis, provided they were obtained within 12 months of the LS assessment. The liver biopsy specimens were reviewed by expert liver pathologists and the fibrosis stage and inflammatory grade were determined by the Batts Ludwig criteria (30, 31). High grades of inflammation were described as grades 3&4 on biopsy or having an ALT/ULN >2 (32, 33).
If subjects had both MRE and TE, for baseline demographics, they were assigned to whichever LS assessment occurred first (Table 1). Otherwise, all TE and MRE data were included in subsequent analyses. If an individual had both a TE and MRE within 1 year of a liver biopsy they were counted twice (once in the TE cohort and once in the MRE cohort). Similarly, if a subject had both a TE and MRE they were used twice (once in the TE cohort and once in the MRE cohort) for survival analyses.
Table 1.
Baseline characteristics of the cohort at the time of liver stiffness measurement
| Variable | Transient elastography* (n = 222) | Magnetic resonance elastography* (n = 316) | Total (n = 538) | p value |
|---|---|---|---|---|
| Female | 198/222 (89.19) | 285/316 (90.19) | 483/538 (89.78) | 0.71 |
| Duration of PBC (years) | 6.06 (0.82–12.93) | 3.48 (0.41–9.30) | 4.21 (0.53–10.35) | 0.002 |
| Age (years) | 60.95 (53.63–67.52) | 60.21 (50.18–66.53) | 60.61 (51.48–66.90) | 0.18 |
| PBC-AIH overlap | 27/222 (12.16) | 52/316 (16.46) | 79/538 (14.68) | 0.16 |
| AMA positive | 195/222 (87.83) | 272/316 (86.08) | 467/538 (86.80) | 0.58 |
| Total bilirubin (mg/dL) | 0.50 (0.40–0.80) | 0.50 (0.40–0.90) | 0.50 (0.40–0.80) | 0.98 |
| ALP (U/L)/ULN | 1.22 (0.82–1.90) | 1.37(0.91–2.50) | 1.30 (0.88–2.20) | 0.02 |
| ALT (U/L)/ULN | 0.78 (0.56–1.18) | 0.91(0.60–1.72) | 0.83 (0.56–1.44) | 0.01 |
| Albumin (g/dL) | 4.20 (3.80–4.40) | 4.20 (3.90–4.40) | 4.20 (3.90–4.40) | 0.79 |
| INR | 1.00 (0.92–1.01) | 1.00 (0.95–1.10) | 1.00 (0.95–1.10) | 0.03 |
| Platelet count (x109/L) | 232.50 (180.75–285.50) | 230.00 (170.00–290.50) | 231.00 (173.50–287.50) | 0.41 |
| APRI | 0.33 (0.25–0.61) | 0.45 (0.28–0.80) | 0.39 (0.27–0.71) | 0.001 |
| GLOBE score | −0.40 (−0.96–0.19) | 0.29 (−0.75–1.77) | −0.20 (−0.86–1.22) | <0.001 |
| Taking UDCA † | 186/212 (84.91) | 249/307 (77.20) | 425/519 (80.35) | 0.03 |
| UDCA responder | 95/131 (72.52) | 128/181 (70.72) | 223/312 (71.47) | 0.73 |
| Fibrosis stage ‡ • F0 • F1 • F2 • F3 • F4 |
4/51 (7.84) 5/51 (9.80) 22/51 (43.14) 13/51 (25.49) 7/51 (13.73) |
8/93 (8.60) 12/93 (12.90) 32/93 (34.41) 32/93 (34.41) 9/93 (9.68) |
12/144 (8.33) 17/144 (11.81) 54/144 ( 37.50) 45/144 (31.25) 16/144 (11.11) |
0.68 |
| Inflammatory grade • Grade 0 • Grade 1 • Grade 2 • Grade 3 • Grade 4 |
12/51 (23.53) 12/51 (23.53) 15/51 (29.41) 12/51 (23.53) 0/51 (0.00) |
28/93 (30.11) 18/93 (19.35) 29/93 (31.18) 15/93 (16.13) 3/93 (3.23) |
40/144 (27.78) 30/144 (20.83) 44/144 (30.56) 27/144 (18.75) 3/144 (2.08) |
0.31 |
Continuous variables are expressed as median (interquartile range) and categorical variables are expressed as number/total (percentage)
Abbreviations: PBC, Primary Biliary Cholangitis; AIH, Autoimmune Hepatitis; AMA, Anti-mitochondrial Antibody; ALP, Alkaline Phosphatase level; ALT, Alanine Aminotransferase; ULN, Upper Limit of Normal; INR, International Normalized Ratio; APRI, Aspartate Aminotransferase to Platelet Ratio Index; UDCA, Ursodeoxycholic acid; F, Fibrosis stage.
Patients who had both transient elastography and magnetic resonance elastography were included in only one cohort in this table, depending on which scan was done first.
Patients who had a transient elastography or a magnetic resonance elastography and were taking UDCA for more than 1 year at the time of the scan are 131/212 (61.79%) and 181/307 (58.96%) p = 0.52, respectively. Patients who had either of the scans and were taking UDCA for more than 1 year at the time of the scan are 312/519 (60.12%).
Out of the 222 patients who had transient elastography, 36 patients were not on UDCA at the time of the elastography because of a recent PBC diagnosis, intolerance to UDCA or elected not to take UDCA
Out of the 316 patients who had magnetic resonance elastography, 67 patients were not on UDCA at the time of the elastography because of a recent PBC diagnosis, intolerance to UDCA or elected not to take UDCA.
A liver biopsy following transplant confirmed recurrent PBC in 12 subjects (TE n=5; MRE n=7).
Patients who had a biopsy done within a year of the time of the scan and had both transient elastography and magnetic resonance elastography were included in only one cohort in this table, depending on which scan was done first, to allow a comparison of the TE and MRE cohorts.
If we consider patients who had both scans, the distribution for the transient elastography would be: F0 6/63 (9.52%); F1 5/63 (7.94%); F2 28/63 (44.44%); F3 16/63 (25.40%); F4 8/63 (12.70%).
If we consider patients who had both scans, the distribution for the magnetic resonance elastography would be: F0 8/98 (8.16%); F1 12/98 (12.24%); F2 34/98 (34.69%); F3 34/98 (34.69%); F4 10/98 (10.20%).
Statistical Analysis
Statistical analysis was performed by using SAS v9.4 (SAS Institute Inc., Cary, NC), and some figures were made using R (34). Categorical data are presented as numbers (percentages), while continuous variables are expressed as median (IQR), unless otherwise stated. Categorical and continuous variables were compared by ChiSquare and non-parametric Wilcoxon Rank Sum tests, respectively. Statistical significance was determined by a p-value <0.05.
Spearman’s correlation was used to assess the relationship between the LS, and the fibrosis stage and covariates associated with increased LS. Diagnostic performances of TE and MRE to predict fibrosis stage were characterized by receiver operating characteristic (ROC) curves, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) with their respective 95% confidence intervals (CI). ROC curves were done only in subset of patients who had both a biopsy and biochemical variables of interest. LS thresholds were determined based on the cut-off that maximized the sum of sensitivity and specificity. The AUC for LS to detect advanced fibrosis was compared with the AUC of other laboratory tests among those with a liver biopsy.
Prognostic capabilities of TE and MRE were examined. Hepatic decompensation is directly related to fibrosis and portal hypertension. LS is a surrogate marker for both pathologies; thus, the primary endpoint was defined as hepatic decompensation (development of ascites, variceal hemorrhage, or hepatic encephalopathy). Secondary endpoint was defined as hepatic decompensation, LT, hepatocellular carcinoma (HCC) or death, whichever occurred earlier. Subjects were followed from the time of LS assessment (baseline) to the development of primary and secondary endpoints. Censoring occurred at the time of last follow up, LT, or when the primary endpoint was assessed (whichever was earlier) or the last follow up when the secondary endpoint was assessed. Patients with any of the endpoints prior to the time of the scan were excluded from the survival analyses. Cox proportional hazard regression was used to identify association between different covariates and the endpoints. Results were expressed as hazard ratio (HR) and 95% CI. Only 2 variables were allowed into the multivariable analyses to prevent instability in the model given the limited number of events. Hence LS plus either UDCA responsive status or covariates significant in the univariable analyses were included in a series of multivariable models. Cumulative incidence of outcomes was determined by the Kaplan-Meier method. The optimal LS cut-off to predict development of primary and secondary endpoints were determined as suggested by Contal and O’Quigley (35). The reporting of this study was done in accordance with the Standards for Reporting of Diagnostic Accuracy (STARD) guidelines (36).
Results
Patients
Five hundred and forty-four patients with PBC at three centers were identified. Six subjects were excluded for unreliable TE performance. Ultimately, 538 unique individuals were included across three Mayo Clinic sites (Rochester, MN, n=238; Jacksonville, FL, n=193; Scottsdale, AZ, n=107). Unique single LS measurement was performed using TE alone n=206; MRE alone n=252 and both TE and MRE n=80. Hence, there were 286 unique TE LS measurements and 332 unique MRE LS measurements used in the analyses (Supplementary Figure 1). There was a moderate correlation (r=0.51) between TE and MRE among the 18 subjects who had both studies within 12 months of each other. Baseline characteristics are shown in Table 1. The patients were followed for a median of 2.24 (0.88 – 4.29) years.
Markers of cholestasis and portal hypertension (as measured by the platelet count and APRI) correlated with increases in LS measured by both TE and MRE (Table 2). LS was lower among individuals who had a response to UDCA (Supplementary Figure 2).
Table 2.
Predictors of increased liver stiffness
| Variables | Transient elastography | Magnetic resonance elastography | ||
|---|---|---|---|---|
| r (95% CI) | p value | r (95% CI) | p value | |
| Female | 0.01 (−0.10–0.13) | 0.81 | −0.14 (−0.24- −0.03) | 0.02 |
| Age | 0.06 (−0.06–0.17) | 0.33 | 0.12 (0.02–0.23) | 0.01 |
| ALT/ULN | 0.33 (0.22–0.43) | <0.001 | 0.35 (0.25–0.45) | <0.001 |
| ALP / ULN | 0.35 (0.24–0.45) | <0.001 | 0.34 (0.24–0.43) | <0.001 |
| Total Bilirubin | 0.33 (0.22–0.43) | <0.001 | 0.52 (0.44–0.60) | <0.001 |
| Albumin | −0.26 (−0.37- −0.14) | <0.001 | −0.44 (−0.52- −0.34) | <0.001 |
| Platelet Count | −0.22 (−0.33- −0.10) | <0.001 | −0.41 (−0.50- −0.32) | <0.001 |
| INR | 0.08 (−0.04–0.20) | 0.20 | 0.16 (0.04–0.27) | 0.01 |
| APRI | 0.49 (0.39–0.58) | <0.001 | 0.59 (0.51–0.66) | <0.001 |
| GLOBE Score | 0.23 (0.09–0.37) | 0.002 | 0.46 (0.33–0.57) | <0.001 |
Abbreviations: r, Spearman’s Correlation Coefficient; CI, Confidence Interval; ALT, Alanine Aminotransferase; ULN, Upper Limit of Normal; ALP, Alkaline Phosphatase; INR, International Normalized Ratio; APRI, Aspartate Aminotransferase to Platelet Ratio Index.
Diagnostic performance of liver stiffness
Transient elastography
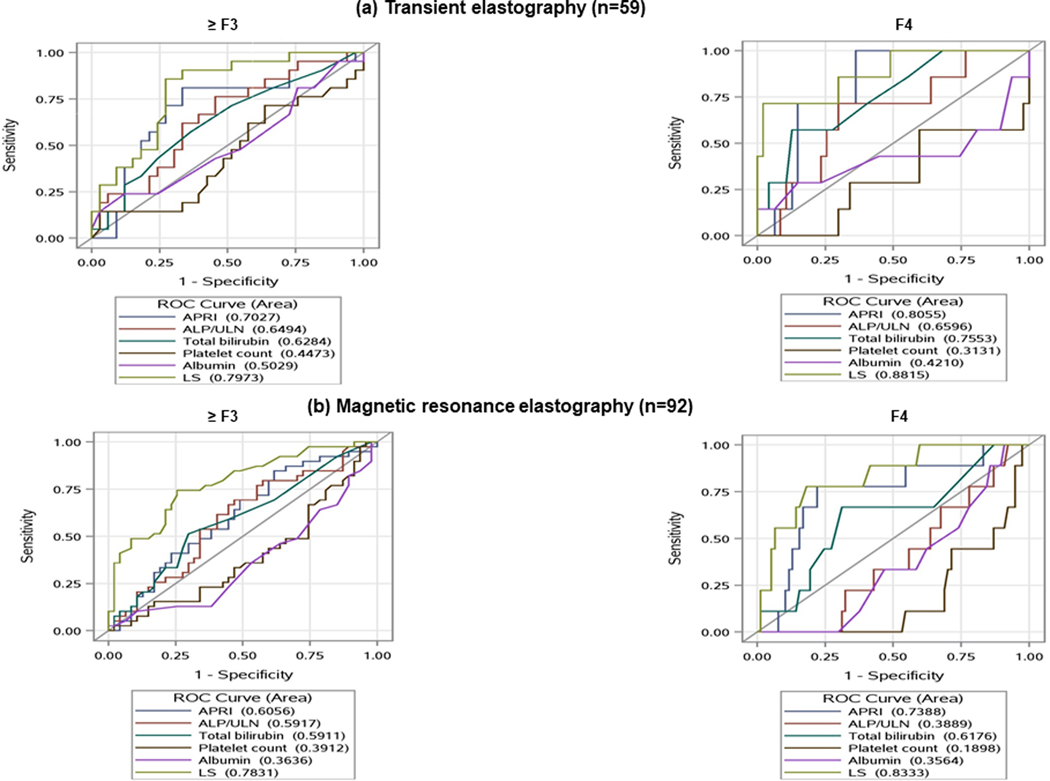
The length of time between biopsy and TE measurement was 82.00 (23.50–218.50) days. LS correlated with fibrosis stage (r = 0.51, p <0.001) (Supplementary Figure 3a). The optimal LS thresholds by TE for predicting histologic fibrosis stage ≥F1, ≥F2, ≥F3 and =F4 were 6.60 kPa (AUC 0.70), 7.00 kPa (AUC 0.65), 7.50 kPa (AUC 0.73), and 14.40 kPa (AUC 0.94), respectively (Table 3). Since performance of TE in PBC-AIH overlap is not validated we did a subgroup analysis after excluding patients with AIH overlap. These cut-offs were similar when subjects with concomitant AIH were excluded (Supplementary Table 1). LS measured by TE had an enhanced ability to predict advanced fibrosis and cirrhosis when compared to biochemical markers such as ALP/ULN, APRI, total bilirubin, platelet count and albumin (Figure 1a).
Table 3.
Diagnostic performance of liver stiffness to detect hepatic fibrosis
| Scan | Fibrosis Stage | n | Cut-off (kPa) | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|---|---|
| TE | ≥ F1 | 57 | 6.60 | 0.70 (0.57–0.81) | 0.70 (0.60–0.85) | 0.50 (0.04–0.78) | 0.91 (0.79–0.98) | 0.12 (0.02–0.36) |
| ≥ F2 | 52 | 7.00 | 0.65 (0.52–0.77) | 0.65 (0.51–0.78) | 0.73 (0.31–0.89) | 0.90 (0.75–0.97) | 0.28 (0.12–0.49) | |
| ≥ F3 | 24 | 7.50 | 0.73 (0.60–0.83) | 0.92 (0.73–0.99) | 0.67 (0.45–0.77) | 0.60 (0.42–0.75) | 0.92 (0.75, 0.99) | |
| F4 | 8 | 14.40 | 0.94 (0.85– 0.98 | 0.75 (0.35–0.97) | 0.98 (0.88–1.00) | 0.75 (0.35–0.97) | 0.96 (0.88, 1.00) |
|
| MRE | ≥ F1 | 90 | 3.80 | 0.50 (0.40–0.60) | 0.46 (0.37–0.59) | 0.88 (0.35–0.97) | 0.96 (0.85–1.00) | 0.11 (0.04–0.23) |
| ≥ F2 | 78 | 3.80 | 0.60 (0.50–0.70) | 0.51 (0.42–0.65) | 0.90 (0.62–0.97) | 0.93 (0.82–0.99) | 0.32 (0.12–0.46) | |
| ≥ F3 | 44 | 3.70 | 0.71 (0.61–0.80) | 0.75 (0.60–0.87) | 0.76 (0.54–0.81) | 0.66 (0.51–0.79) |
0.77 (0.63–0.88) | |
| F4 | 10 | 4.60 | 0.82 (0.73–0.89) | 0.80 (0.44–0.98) | 0.83 (0.72–0.89) | 0.33 (0.16–0.55) |
0.97 (0.91–1.00) |
Abbreviations: TE, Transient Elastography; MRE, Magnetic Resonance Elastography; AUC, Area Under the Curve; CI, Confidence Interval; PPV, Positive Predictive value; NPV, Negative Predictive Value; F, Fibrosis stage
Features of portal hypertension were present at the time of transient elastography amongst the following subgroups who underwent a liver biopsy: F0 2/6 (33.33%); F1 1/5 (20.00%); F2 11/28 (39.29%); F3 8/16 (50.00%); F4 4/8 (50.00%).
Features of portal hypertension were present at the time of magnetic resonance elastography amongst the following subgroups who underwent a liver biopsy: F0 3/8 (37.50%); F1 3/12 (25.00%); F2 5/34 (14.71%); F3 14/34 (41.18%); F4 8/10 (80.00%).
Figure 1.
Ability of liver stiffness and other biomarkers to predict the presence of advanced fibrosis
Abbreviations: ROC, Receiver Operating Characteristic; APRI, AST to Platelet Ratio Index; ALP, Alkaline Phosphatase; ULN, Upper limit normal; LS, Liver Stiffness
Magnetic resonance elastography
The length of time between biopsy and MRE measurement was 45.00 (11.50–112.00) days. Similar to the findings of the TE, there was a correlation between LS and fibrosis stage (r=0.51, p<0.001) (Supplementary Figure 3b). While MRE measured LS was able to detect the presence of cirrhosis, its ability to distinguish various fibrosis stages was sub-optimal in this cohort. For example, the optimal LS thresholds by MRE for predicting histologic fibrosis stage ≥F1, ≥F2, ≥F3 and =F4 were 3.80 kPa (AUC 0.50), 3.80 kPa (AUC 0.60), 3.70 kPa (AUC 0.71), and 4.60 kPa (AUC 0.82), respectively (Table 3). We investigated the following confounders that could have explained MRE’s inability to distinguish lower fibrosis stages in this cohort (Supplementary Table 1&2): presence of overlap with AIH; presence of high degree of inflammation defined by grade 3 or 4 on biopsy or an ALT/ULN >2 and variations across centers. However, these sensitivity analyses did not improve the diagnostic performance at earlier stages. Finally, there were a high proportion of individuals with portal hypertension who had F0-F2 fibrosis on their biopsy (Table 3, footnote). This may suggest either the presence of cirrhosis missed on the biopsy or pre-sinusoidal portal hypertension which can occur in PBC. However, a similar observation was noted in the TE cohort (Table 3, footnote) making sampling error as a confounder in the MRE cohort less likely.
LS measured by MRE also had an enhanced ability to predict advanced fibrosis and cirrhosis when compared to biochemical markers such as ALP/ULN, APRI, total bilirubin, platelet count and albumin (Figure 1b).
Prognostic performance of liver stiffness
Transient elastography
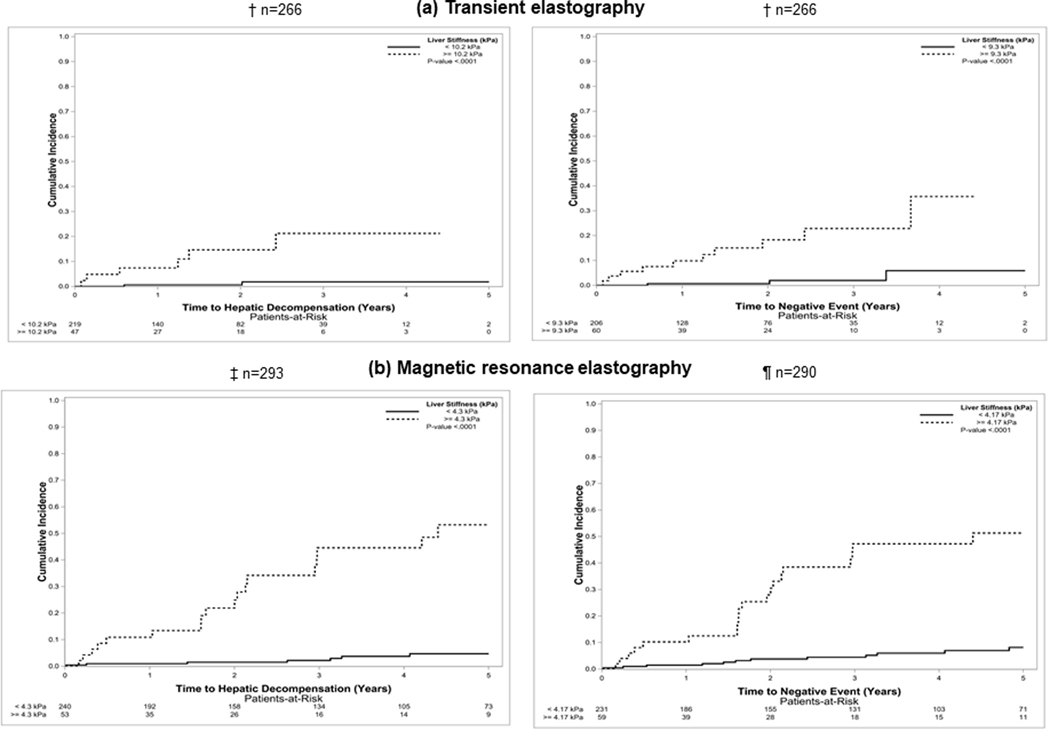
Of those who had TE assessment of LS, 8 patients developed the primary endpoint of hepatic decompensation (ascites, n=7; multiple, n=1). Thirteen developed at least one of the secondary endpoints (hepatic decompensation, n=7; LT alone, n=1; death, n=4; multiple, n=1). This includes a total of 2 subjects who required LT (1 individual developed decompensation before transplantation and were included in the multiple category). The causes of death were either liver related (n=3) or unknown (n=1). No patient developed HCC before the other outcomes in the secondary endpoint. LS was higher for those who developed hepatic decompensation compared to those who did not: 12.60 (8.96 – 32.85) kPa, vs 6.15 (4.60 – 8.73) kPa, p=0.001. Similarly, LS for those who developed at least one secondary endpoint was higher compared to those who did not: 11.30 (8.90 – 30.65) kPa vs 6.10 (4.60 – 8.60) kPa, p=0.001. Using TE, the optimal thresholds to predict hepatic decompensation was 10.20 kPa (HR, 13.73; 95% CI, 2.77 – 68.06) and 9.30 kPa (HR, 10.51; 95% CI, 2.89 – 38.20) to predict the secondary endpoint (Figure 2a).
Figure 2.
Optimal liver stiffness thresholds to predict adverse events
Abbreviation: kPa, kiloPascals
† Twenty patients developed hepatic decompensation before the liver stiffness measurement by transient elastography
‡ Thirty-nine patients developed hepatic decompensation before the liver stiffness measurement by magnetic resonance elastography
¶ Three patients developed hepatocellular carcinoma before the liver stiffness measurement by magnetic resonance elastography
Covariates associated with the primary and secondary endpoints are shown in Table 4. C-statistic for LS measured by TE in predicting hepatic decompensation was 0.85 (95% CI, 0.74 – 0.97) and in predicting secondary endpoints was 0.87 (95% CI, 0.78–0.95). LS measured by TE remained associated with adverse outcomes after adjusting for these other prognostic variables including UDCA response and the GLOBE score (Table 5).
Table 4.
Univariable analysis of prognostic factors associated with primary and secondary endpoints
| Variables | Transient elastography | Magnetic resonance elastography | ||||||
|---|---|---|---|---|---|---|---|---|
| Primary endpoint | Secondary endpoint | Primary endpoint | Secondary endpoint | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Male | 3.29 (0.66–16.39) | 0.15 | 1.96 (0.43–8.88) | 0.39 | 3.51 (1.40–8.81) | 0.01 | 2.19 (0.84–5.68) | 0.11 |
| Age | 0.73 (0.37–1.45) | 0.37 | 1.05 (0.59–1.88) | 0.87 | 1.12 (0.79–1.58) | 0.52 | 1.27 (0.93–1.72) | 0.13 |
| LS | 1.12 (1.07–1.18) | <0.001 | 1.12 (1.08–1.17) | <0.001 | 1.65 (1.40–1.94) | <0.001 | 1.58 (1.36–1.84) | <0.001 |
| AST / ULN | 2.37 (1.54–3.65) | <0.001 | 2.12 (1.49–3.00) | <0.001 | 1.23 (1.08–1.41) | 0.002 | 1.23 (1.10–1.38) | <0.001 |
| ALP / ULN | 1.48 (1.20–1.82) | <0.001 | 1.51 (1.29–1.77) | <0.001 | 1.19 (1.10–1.28) | <0.001 | 1.19 (1.11–1.27) | <0.001 |
| Total Bilirubin | 1.76 (1.05–2.93) | 0.03 | 2.02 (1.47–2.77) | <0.001 | 1.25 (1.10–1.42) | <0.001 | 1.29 (1.18–1.42) | <0.001 |
| Albumin | 0.09 (0.02–0.42) | 0.003 | 0.09 (0.03–0.32) | <0.001 | 0.18 (0.08–0.42) | <0.001 | 0.14 (0.07–0.28) | <0.001 |
| Sodium | 1.17 (0.83–1.65) | 0.38 | 1.37 (1.01–1.86) | 0.04 | 1.00 (0.84–1.18) | 0.97 | 0.91 (0.78–1.04) | 0.16 |
| Creatinine | 1.02 (0.24–4.27) | 0.98 | 1.34 (0.66–2.70) | 0.42 | 1.01 (0.22–4.53) | 0.99 | 0.38 (0.06–2.41) | 0.31 |
| Platelet Count | 0.99 (0.94–1.00) | 0.01 | 0.99 (0.98–1.00) | 0.05 | 0.99 (0.98–0.99) | <0.001 | 0.99 (0.99–1.00) | 0.002 |
| INR | 0.84 (0.03–22.83) | 0.92 | 1.13 (0.18–6.96) | 0.90 | 1.05 (0.68–1.62) | 0.82 | 1.05 (0.74–1.51) | 0.77 |
| APRI | 5.15 (2.74–9.69) | <0.001 | 3.90 (2.38–6.40) | <0.001 | 1.31 (1.15–1.50) | <0.001 | 1.27 (1.12–1.45) | <0.001 |
| GLOBE Score | 1.96 (1.05–3.65) | 0.03 | 1.96 (1.16–3.30) | 0.01 | 1.31 (0.99–1.74) | 0.06 | 1.49 (1.16–1.91) | 0.002 |
| UDCA Responder | 0.15 (0.02–1.40) | 0.10 | 0.33 (0.07–1.47) | 0.15 | 0.26 (0.08–0.84) | 0.03 | 0.28 (0.10–0.81) | 0.02 |
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval; LS, Liver Stiffness; AST, Aspartate aminotransferase; ULN, Upper Limit of Normal; ALP, Alkaline Phosphatase; INR, International Normalized Ratio; APRI, AST to Platelet Ratio Index; UDCA, Ursodeoxycholic acid.
Table 5.
Bivariable analysis of prognostic factors associated with primary and secondary endpoints
| Variables | Transient elastogtraphy | Magnetic resonance elastography | ||||||
|---|---|---|---|---|---|---|---|---|
| Primary endpoint | Secondary endpoint | Primary endpoint | Secondary endpoint | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| LS | 1.12 (1.05–1.19) | <0.001 | 1.12 (1.06–1.17) | <0.001 | 1.61 (1.36–1.90) | <0.001 | 1.56 (1.33–1.82) | <0.001 |
| AST/ULN | 1.73 (1.09–2.74) | 0.02 | 1.56 (1.07–2.26) | 0.02 | 1.15 (0.96–1.37) | 0.12 | 1.16 (1.00–1.34) | 0.05 |
| LS | 1.15 (1.08–1.22) | <0.001 | 1.15 (1.09–1.21) | <0.001 | 1.63 (1.37–1.94) | <0.001 | 1.55 (1.32–1.82) | <0.001 |
| ALP/ULN | 1.54 (1.22–1.95) | <0.001 | 1.56 (1.31–1.87) | <0.001 | 1.14 (1.05–1.25) | 0.002 | 1.15 (1.07–1.23) | <0.001 |
| LS | 1.13 (1.07–1.19) | <0.001 | 1.12 (1.07–1.18) | <0.001 | 1.62 (1.37–1.93) | <0.001 | 1.54 (1.31–1.81) | <0.001 |
| Total Bilirubin | 1.68 (0.86–3.27) | 0.13 | 2.05 (1.43–2.96) | <0.001 | 1.18 (1.02–1.36) | 0.03 | 1.23 (1.11–1.36) | <0.001 |
| LS | 1.11 (1.04–1.18) | 0.002 | 1.10 (1.04–1.16) | <0.001 | 1.54 (1.29–1.85) | 0.002 | 1.45 (1.22–1.72) | <0.001 |
| Albumin | 0.27 (0.05–1.46) | 0.13 | 0.25 (0.06–0.96) | 0.04 | 0.36 (0.14–0.94) | 0.04 | 0.24 (0.11–0.56) | 0.001 |
| LS | 1.11 (1.05–0.18) | <0.001 | 1.12 (1.07–1.17) | <0.001 | 1.55 (1.28–1.87) | <0.001 | 1.54 (1.30–1.82) | <0.001 |
| Platelet Count | 0.99 (0.98–1.00) | 0.08 | 0.99 (0.99–1.01) | 0.38 | 0.99 (0.99–1.00) | 0.08 | 1.00 (0.99–1.00) | 0.12 |
| LS | 1.10 (0.02–1.019) | 0.02 | 1.10 (1.04,1.17) | 0.001 | 1.64 (1.38–1.94) | 0.02 | 1.52 (1.35–1.85) | 0.001 |
| APRI | 3.36 (1.61–7.00) | 0.001 | 2.43 (1.37–4.31) | 0.002 | 1.25 (1.07–1.47) | 0.006 | 1.20 (1.03–1.40) | 0.02 |
| LS | 1.13 (1.07–1.19) | <0.001 | 1.13 (1.07–1.18) | <0.001 | 2.09 (1.57–2.78) | <0.001 | 1.98 (1.52–2.57) | <0.001 |
| GLOBE Score | 1.60 (0.81–3.18) | 0.18 | 1.64 (0.92–2.93) | 0.09 | 1.00 (0.71–1.41) | 0.99 | 1.18 (0.89–1.58) | 0.25 |
| LS | 1.14 (1.05–1.24) | 0.002 | 1.14 (1.07–1.21) | <0.001 | 1.68 (1.28–2.19) | <0.001 | 1.57 (1.22–2.00) | <0.001 |
| UDCA Responder | 0.15 (0.01–1.68) | 0.12 | 0.41 (0.09–1.93) | 0.26 | 0.72 (0.17–3.07) | 0.66 | 0.63 (0.18–2.22) | 0.47 |
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval; LS, Liver Stiffness; AST, Aspartate aminotransferase; ULN, Upper Limit of Normal; ALP, Alkaline Phosphatase; APRI, AST to Platelet Ratio Index; UDCA, Ursodeoxycholic acid.
Magnetic resonance elastography
Among those who had MRE assessment of LS, 25 patients developed hepatic decompensation (ascites, n=12; variceal hemorrhage, n=1; hepatic encephalopathy, n=3; multiple, n=9). Thirty-three patients developed at least one secondary endpoint before LS assessment by MRE (hepatic decompensation, n=18; LT alone n=1; death n=8; multiple, n=6).Three individuals underwent a LT (this includes 2 individuals who developed decompensation before LT and were included in the multiple category). The causes of death were liver related (n=5) and non-liver related (ischemic stroke, n=2; breast cancer, n=1). LS was higher for those who developed hepatic decompensation compared to those who did not: 4.60 (3.71 – 6.29) kPa vs 2.90 (2.40 – 3.70) kPa, p <0.001. Similarly, LS for those who developed at least one secondary endpoint was higher compared to those who did not: 4.30 (3.25 – 5.85) kPa vs 2.89 (2.40 – 3.60) kPa, p <0.001. Using MRE, the optimal thresholds to predict hepatic decompensation was 4.30 kPa (HR, 15.74; 95% CI, 6.55–37.83) and 4.17 kPa (HR, 8.33; 95% CI, 4.13–16.79) to predict the secondary endpoint (Figure 2b).
Covariates associated with the primary and secondary endpoints are shown in Table 4. C-statistic for LS measured by MRE in predicting hepatic decompensation was 0.83 (95% CI, 0.76 – 0.89) and in predicting secondary endpoints was 0.78 (95% CI, 0.70–0.85). Similar to TE, MRE measured LS remained associated with adverse outcomes after adjusting for these other prognostic variables including UDCA response and the GLOBE score (Table 5).
Discussion
This study presents a comprehensive appraisal of LS in subjects with PBC, as well as its predictors and clinical implications. To the best of our knowledge, this is the largest examination of TE and the first that evaluated MRE in PBC, to date. We have shown several findings that enhance the understanding of LS in PBC. First, LS measured by either TE or MRE have good diagnostic performance in PBC for predicting advanced stages of fibrosis that outperform existing biochemical predictors. Second, we describe the relationships between clinical features and LS. Third, LS is an independent predictor for poor clinical outcomes, even after adjustment for other prognostic variables including UDCA responsiveness and the GLOBE score.
Our findings validate the work of our European colleagues who previously examined the diagnostic and prognostic performance of TE-derived LS measurements. Corpechot et al showed optimal LS cut offs of 10.70, and 16.90 kPa for histologic fibrosis stages ≥F3, and F4, respectively while Floreani et al showed optimal diagnostic cut offs of 7.60, and 11.40 kPa for fibrosis stages ≥F3 and F4, respectively (15, 20). The LS cut-offs in this North American cohort reside approximately between these studies (Table 3 and Supplementary Table 1). Moreover, we reaffirmed that LS is superior at predicting liver fibrosis compared to biochemical parameters (Figure 1) (15). In addition, Corpechot et al observed that LS >9.60 kPa was associated with a five-fold increased risk of hepatic decompensation, liver transplant, and death (15). This is also consistent with our observations (Figure 2a).
This study represents the first effort to examine MRE in PBC. This is important as MRE may offer several advantages when quantifying LS over TE. First, MRE can characterize a larger volume of the liver when compared to TE (25, 37). Second, MRE has been shown to have a lower failure rate and better diagnostic performance in other liver diseases when compared to TE (11, 13, 38, 39). Last, unlike TE, MRE is not influenced by obesity (25). A cut-off of 4.60 kPa on MRE was able to accurately detect cirrhosis (Table 3). After using a conversion factor of 3 to approximate and compare shear based MRE measurements with the Young’s modulus TE measurements, this was similar to the TE-derived LS cutoff for cirrhosis in the present study and similar to other MRE-derived LS cut-offs to detect cirrhosis in other liver diseases (40, 41). Unlike TE, MRE had a surprisingly suboptimal performance to differentiate between earlier fibrosis stages. We did not observe confounding differences between the TE and MRE cohorts that explain this observation. The AUC of MRE-derived LS in predicting cirrhosis was high; coupled with a low PPV can indicate a possible role of MRE in excluding cirrhosis rather than predicting it (Table 3). Similar to TE, MRE-derived LS was able to predict adverse outcomes independent of other prognostic markers (Figure 2, Table 5). The limited number of subjects who had both TE and MRE prevent us from making a direct comparison between elastography modalities. However, our observations suggest that MRE is unlikely to offer a significant advantage over TE among most patients with PBC. Therefore, it may be reasonable to reserve the use of MRE among individuals with an elevated BMI, prior failed or unreliable LS measurements using TE and those who need cross-sectional imaging to screen for other liver related complications.
Several new observations in the present study add credence to the importance of routine LS measurement in clinical practice. First, an increased LS among UDCA non-responders further illustrates this subgroup of patients is at a higher risk for adverse events and should be considered for adjunctive therapies and closer monitoring (24). Second, while identifying UDCA non-responders and quantifying progression risk using the GLOBE score is important, LS assessment also adds prognostic value. Indeed, LS retains its prognostic value regardless of an individual’s response to UDCA responsiveness or GLOBE score (Table 5).
Our study has several limitations. First, it was a retrospective cohort study conducted at referral centers. Second, the small number of cases who had both TE and MRE performed prevented us from concluding if one technique is superior to the other among those with PBC. However, the side-by-side assessment presented here-in has relevance and provides reassurance to practitioners that either elastography method has diagnostic and prognostic value. Third, there were an insufficient number of patients who underwent serial LS measurements. Consequently, we are unable to comment on the natural history of LS changes and their prognostic relevance. Similarly, our ability to examine the impact of UDCA adjuvant therapies such as obeticholic acid on LS and outcomes was limited by the small sample size. Last, the follow-up duration was limited which prevents us from drawing conclusions regarding the role of LS and long-term outcomes. However, its ability to predict short term outcomes is robust.
In the largest evaluation of LS by TE in patients with PBC, as well as the first evaluation of LS by MRE in patients with PBC, this study has demonstrated that both TE and MRE have reliable diagnostic capabilities to predict advanced histologic fibrosis, outperforming biochemical tests. Moreover, both TE and MRE can predict hepatic decompensations and other clinically relevant endpoints in patients with PBC, independent of UDCA response status and the GLOBE risk score.
Supplementary Material
Supplementary Figure 1. Patients included
Supplementary Figure 2. Liver stiffness among those who did and did not respond to ursodeoxycholic acid after 1 year
Supplementary Figure 3. Liver stiffness and fibrosis stage
Supplementary Table 1. Diagnostic performance of liver stiffness to detect hepatic fibrosis excluding those with concomitant autoimmune hepatitis
Supplementary Table 2. Diagnostic performance of liver stiffness measured by magnetic resonance elastography to detect hepatic fibrosis after excluding possible confounding factors
Acknowledgments
All authors approved the final draft submitted.
Abbreviations:
- PBC
Primary biliary cholangitis
- LS
Liver stiffness
- TE
Transient elastography
- MRE
Magnetic resonance elastography
- UDCA
Ursodeoxycholic acid
- MN
Minnesota
- AZ
Arizona
- FL
Florida
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- ALP
Alkaline phosphatase
- AIH
Autoimmune hepatitis
- LT
Liver transplantation
- IQR
Interquartile range
- kPa
kiloPascals
- AST
Aspartate aminotransferase
- APRI
AST to platelet ratio index
- ALT
Alanine aminotransferase
- ULN
Upper limits of normal
- ROC
Receiver operating characteristic
- PPV
Positive predictive value
- NPV
Negative predictive value
- AUC
Area under the curve
- CI
Confidence interval
- HCC
Hepatocellular carcinoma
- HR
Hazard ratio
- STARD
Standards for Reporting of Diagnostic Accuracy
Footnotes
Guarantor of the article: John E. Eaton, MD.
Conflicts of Interests: The Mayo Clinic has intellectual property rights and a financial interest related to Magnetic Resonance Elastography, a technology used in this study. The coauthors have nothing else to disclose. This paper has not been previously published or submitted elsewhere.
References
- 1.Blanc J, Bioulac-Sage P, Balabaud C, et al. Investigation of liver fibrosis in clinical practice. Hepatology research. 2005;32(1):1–8. [DOI] [PubMed] [Google Scholar]
- 2.Poupon R, Corpechot C. Elastography-based assessment of primary biliary cirrhosis staging. Digestive and Liver Disease. 2011;43(11):839–40. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353(12):1261–73. [DOI] [PubMed] [Google Scholar]
- 4.Moreno-Otero R, Lisker-Melman M, Jones EA. Primary biliary cirrhosis. Medical Clinics of North America. 1989;73(4):911–29. [DOI] [PubMed] [Google Scholar]
- 5.Abdi W, Millan JC, Mezey E. Sampling variability on percutaneous liver biopsy. Archives of Internal Medicine. 1979;139(6):667–9. [PubMed] [Google Scholar]
- 6.Zein CO, Angulo P, Lindor KD. When is liver biopsy needed in the diagnosis of primary biliary cirrhosis? Clinical Gastroenterology and Hepatology. 2003;1(2):89–95. [DOI] [PubMed] [Google Scholar]
- 7.Corpechot C. Clinical Trials in PBC Going Forward. Seminars in liver disease; 2019. Apr 30. Thieme Medical Publishers. [DOI] [PubMed] [Google Scholar]
- 8.Corpechot C, Chazouillères O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. New England Journal of Medicine. 2018;378(23):2171–81. [DOI] [PubMed] [Google Scholar]
- 9.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. Journal of hepatology. 2008;48(5):835–47. [DOI] [PubMed] [Google Scholar]
- 10.Huwart L, Peeters F, Sinkus R, et al. Liver fibrosis: non-invasive assessment with MR elastography. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo. 2006;19(2):173–9. [DOI] [PubMed] [Google Scholar]
- 11.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135(1):32–40. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology. 2013;11(12):1573–84. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clinical Gastroenterology and Hepatology. 2015;13(3):440–51. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clinical Gastroenterology and Hepatology. 2007;5(10):1207–13. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56(1):198–208. [DOI] [PubMed] [Google Scholar]
- 16.Corpechot C, El Naggar A, Poujol-Robert A, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43(5):1118–24. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich-Rust M, Müller C, Winckler A, et al. Assessment of liver fibrosis and steatosis in PBC with FibroScan, MRI, MR-spectroscopy, and serum markers. Journal of clinical gastroenterology. 2010;44(1):58–65. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Dominguez E, Mendoza J, Garcia-Buey L, et al. Transient elastography to assess hepatic fibrosis in primary biliary cirrhosis. Alimentary pharmacology & therapeutics. 2008;27(5):441–7. [DOI] [PubMed] [Google Scholar]
- 19.Joshita S, Umemura T, Tanaka E. Clinical utility of FibroScan® as a noninvasive diagnostic test for primary biliary cholangitis. Ultrasound in Medicine & Biology. 2019;45:S98. [Google Scholar]
- 20.Floreani A, Cazzagon N, Martines D, et al. Performance and utility of transient elastography and noninvasive markers of liver fibrosis in primary biliary cirrhosis. Digestive and Liver Disease. 2011;43(11):887–92. [DOI] [PubMed] [Google Scholar]
- 21.Wu H-M, Sheng L, Wang Q, et al. Performance of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis-primary biliary cholangitis overlap syndrome. World journal of gastroenterology. 2018;24(6):737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Annals of internal medicine. 2007;147(8):573–7. [DOI] [PubMed] [Google Scholar]
- 23.Lindor KD, Bowlus CL, Boyer J, et al. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Clinical Liver Disease. 2020;15(1):1. [DOI] [PubMed] [Google Scholar]
- 24.Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69(1):394–419. [DOI] [PubMed] [Google Scholar]
- 25.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. Journal of Magnetic Resonance Imaging. 2013;37(3):544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41(1):48–54. [DOI] [PubMed] [Google Scholar]
- 27.Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–50. [DOI] [PubMed] [Google Scholar]
- 28.Kumagi T, Guindi M, Fischer SE, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. American Journal of Gastroenterology. 2010;105(10):2186–94. [DOI] [PubMed] [Google Scholar]
- 29.Frank K, Linhart P, Kortsik C, et al. Sonographic determination of spleen size: normal dimensions in adults with a healthy spleen. Ultraschall in der Medizin (Stuttgart, Germany: 1980). 1986;7(3):134–7. [DOI] [PubMed] [Google Scholar]
- 30.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. The American journal of surgical pathology. 1995;19(12):1409–17. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig J, Dickson E, McDonald GSA. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Archiv A. 1978;379(2):103–12. [DOI] [PubMed] [Google Scholar]
- 32.Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. American Journal of Gastroenterology. 2017;112(1):18–35. [DOI] [PubMed] [Google Scholar]
- 33.Ichikawa S, Motosugi U, Nakazawa T, et al. Hepatitis activity should be considered a confounder of liver stiffness measured with MR elastography. Journal of Magnetic Resonance Imaging. 2015;41(5):1203–8. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. [Google Scholar]
- 35.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational statistics & data analysis. 1999;30(3):253–70. [Google Scholar]
- 36.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Clinical chemistry. 2015;61(12):1446–52. [DOI] [PubMed] [Google Scholar]
- 37.Scheuer PJ. Pathologic features and evolution of primary biliary cirrhosis and primary sclerosing cholangitis. Mayo Clinic Proceedings; 1998;73(2):179–83. [DOI] [PubMed] [Google Scholar]
- 38.Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51(3):828–35. [DOI] [PubMed] [Google Scholar]
- 39.Ichikawa S, Motosugi U, Morisaka H, et al. Comparison of the diagnostic accuracies of magnetic resonance elastography and transient elastography for hepatic fibrosis. Magnetic resonance imaging. 2015;33(1):26–30. [DOI] [PubMed] [Google Scholar]
- 40.Eaton JE, Dzyubak B, Venkatesh SK, et al. Performance of magnetic resonance elastography in primary sclerosing cholangitis. Journal of gastroenterology and hepatology. 2016;31(6):1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eaton JE, Sen A, Hoodeshenas S, et al. Changes in Liver Stiffness, Measured by Magnetic Resonance Elastography, Associated with Hepatic Decompensation in Patients with Primary Sclerosing Cholangitis. Clinical Gastroenterology and Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Patients included
Supplementary Figure 2. Liver stiffness among those who did and did not respond to ursodeoxycholic acid after 1 year
Supplementary Figure 3. Liver stiffness and fibrosis stage
Supplementary Table 1. Diagnostic performance of liver stiffness to detect hepatic fibrosis excluding those with concomitant autoimmune hepatitis
Supplementary Table 2. Diagnostic performance of liver stiffness measured by magnetic resonance elastography to detect hepatic fibrosis after excluding possible confounding factors