Abstract
Cholangiocarcinoma and pancreatic cancer are the most common causes of malignant biliary obstruction. The majority of patients are diagnosed at a late stage when surgical resection is rarely possible. In these cases, palliative chemotherapy and radiotherapy provide only limited benefit and are associated with poor survival. Radiofrequency ablation (RFA) is a procedure for locoregional control of tumours, whereby a high-frequency alternating current turned into thermal energy causes coagulative necrosis of the tissue surrounding the catheter. The subsequent release of debris and tumour antigens by necrotic cells can stimulate local and systemic immunity. The development of endoluminal RFA catheters has led to the emergence of endoscopically delivered RFA, a treatment mainly used for malignant biliary strictures to prolong survival and/or stent patency. Other indications include recanalisation of occluded biliary stents and treatment of intraductal ampullary adenoma or benign biliary strictures. This article presents a comprehensive review of endobiliary RFA, mainly focusing on its use in patients with malignant biliary obstruction. The available data suggest that biliary RFA may be a promising modality, having positive impacts on survival and stent patency and boasting a reasonable safety profile. However, further studies with better characterised and stratified patient populations are needed before the method becomes accepted within routine clinical practice.
Keywords: Radiofrequency, Ablation, Biliary, Stenosis, Cholangiocarcinoma, Pancreatic cancer
Core Tip: Cholangiocarcinoma and pancreatic cancer are typically diagnosed at a late stage with poor prognosis. These conditions often cause biliary obstruction, which can be targeted by endoluminal radiofrequency ablation. Induced heat results in coagulative necrosis and the release of tumour antigens, in turn activating the systemic immune response. This review presents the available evidence for biliary radiofreqency ablation efficiency and safety. Although some of the data indicate positive impacts on survival and stent patency, further studies incorporating better defined patient populations and randomised settings are needed to confirm these promising results.
INTRODUCTION
Cholangiocarcinoma (CCC) and GB [pancreatic cancer (PC)] represent the main causes of malignant biliary obstruction. They are diagnosed in most cases at a late stage, with surgical resection only possible in a minority of cases. Palliative chemotherapy and radiotherapy are of limited efficiency and most patients generally do not survive beyond one year. Endoscopic or percutaneous stent placement for biliary drainage is an important part of palliative care. The use of self-expandable metal stents (SEMS) over plastic stents is recommended for longer patency. However, even metal stents become occluded over time, with a median-duration patency of 6-8 mo[1]. Therefore, novel therapies aimed at improving survival and stent patency are of pressing need.
Local ablative therapies are used to induce cell death in areas close to the application site. Radiofrequency or microwave ablation, cryoablation, ethanol injection and focused ultrasound are among the local ablative therapies available. Photodynamic therapy has been shown in some randomised trials to improve the survival of patients with hilar cholangiocarcinoma. However, this method is limited in availability, expensive, and also associated with induced photosensitivity[2-4].
Radiofrequency ablation (RFA) results from thermal damage created by a high-frequency alternating current released from an electrode into tissue. Temperatures greater than 50˚C lead to coagulative necrosis and cell death. Consequently, the release of some intracellular components can be immunogenic, activating local and systemic immunity.
Percutaneous RFA is routinely used for the treatment of solid liver tumours and has become an important part of the recommended treatment algorithm for hepatocellular cancer[5]. However, CCC and PC are not amenable to percutaneous interventions due to poor visualisation and the risk of damage to adjacent structures. Intraoperative RFA represents another treatment option, but even laparotomy can impose an unnecessary burden on at-risk patients.
Endoscopically delivered luminal RFA is the method most commonly used to treat invisible high-grade dysplasia and to eradicate the remaining Barrett’s mucosa after cancer resection. Using an over-the-wire endoluminal biliary catheter, RFA can be introduced to the tumour vicinity from the main bile ducts. The catheter is positioned endoscopically or percutaneously over a wire into the bile duct strictured by cancer, enabling accurate delivery of thermal energy to the surrounding tumour. Technical feasibility, safety and impact on survival, as well as stent patency have all been investigated by numerous studies. Although some research points to a beneficial effect on various parameters, most of the data are derived from retrospective series characterised by limited numbers of patients and a high heterogeneity of patients within and across these studies.
Despite the lack of controlled data, the commercial availability of biliary RFA catheters has led to the wide use of this technique. The aim of this review is to present the current evidence for the use of endoluminal biliary radiofrequency ablation.
ENDOSCOPIC RADIOFREQENCY ABLATION IN MALIGNANT BILIARY OBSTRUCTION
RFA
RFA is a method of mini-invasive treatment for local destruction of the tumour mass. This is achieved by sufficiently increasing temperature to induce irreversible cellular injury of the target tissue while minimising local and systemic complications.
RFA induces cell death via hyperthermic injury causing coagulative necrosis. The principle of RFA is based on the biophysical interaction between a high-frequency alternating current (within a radio-wave range of 400-500 kHz) and biological tissue. A simple electrical circuit is established using a generator, cabling, electrodes and biological tissue as the resistive element. The electrical current oscillates between the active and reference electrodes (grounding pad) or between two active electrodes in bipolar systems. This ionic oscillation induces friction that heats the tissue. Temperatures above 60°C cause protein denaturation and subsequent loss of intracellular fluid, resulting in coagulation necrosis. The field intensity dictates the frequency of oscillation[6-8].
The target temperature for immediate induction of coagulation necrosis with irreversible damage to mitochondrial and cytosolic enzymes and histones ranges between 60-100°C. As heating is most effective in areas of high current density, tissues nearest the electrode are heated the most while the more distant areas receive heat by thermal conduction. The central zone of ablation is encircled by a peripheral zone comprised of surrounding tissue into which heat is diffused rather via conduction, gradually decreasing from the central zone. The cells in this peripheral zone, defined as any area where cells are exposed to temperatures between 40-60°C, may not receive a lethal thermal dose but still undergo thermal distress[9]. Sublethal temperatures can induce mitochondrial damage or heat-mediated lysosomal activation leading to apoptotic cell death, although cell recovery can also occur. Temperatures above 100°C result in tissue charring, vaporisation and carbonisation. These processes lead to increased impedance, decreased electrical conductivity, isolated heat spread and reduced RFA effectiveness[6-8].
Thermal damage is dependent on the type, length and width of the delivery electrode, tissue electrical conductivity, the temperature achieved by the RFA device, and heating duration. Therefore, the correct setting of the generator is important. Animal studies that have investigated the effects of different settings and exposure times suggest that 7-10 wk for 30-120 s is optimal for inducing necrosis extending from the bile duct to the surrounding tissue[10-12]. In one study, an endoscopic bipolar catheter inserted into the porcine bile duct induced incomplete ablation at 5 wk, intramural ablation at 7 wk and transmural ablation at 10 wk[13]. Conductivity is influenced by tissue composition, fibrosis, calcifications and the adjacent bloodstream. The dissipation of thermal energy due to cooling of tissue by adjacent blood vessels is known as the heat sink effect. In experimental studies, vessels larger than 3 mm produce this phenomenon[14].
Necrosis instigates a loss of plasma membrane integrity. Necrotic cells release intracellular antigens, damage-associated proteins such as heat-shock proteins and high-mobility group protein B1, parts of intracellular organelles, and RNA and DNA fragments. This debris is a source of tumour antigens that can be recognised and targeted by the host immune system, leading to production of cytokines and activation of immunocompetent cells, thus stimulating local and systemic immune responses[15].
Generally, RFA can be performed percutaneously by laparotomy, laparoscopy, endoscopy or endoscopic ultrasound. It is also a validated method for treating malignant liver lesions, especially hepatocellular carcinoma and Barrett’s neoplasia. Emerging indications include pancreatic neuroendocrine tumours, pancreatic cystic neoplasia, gastric antral vascular ectasia and radiation proctitis.
Although RFA is a well-tolerated therapy, complications can occur. The first type of complication, caused by the thermal effect generated by RFA, involves flu-like syndrome, pain, skin burns at the grounding pad site and thermal injury to adjacent organs. The use of bipolar probes obviate the risk of skin burns. The most feared adverse events during pancreatobiliary RFA are pancreatitis, bile duct strictures, pleural effusions, biliary fistulas, cholangitis, cholecystitis and bleeding. One study reported three cases of bleeding, two resulting in death[16]. In another study, two severe adverse events were reported: One hepatic liver infarction and one hepatic coma[17]. Contraindications for RFA include cardiac pacemakers, cardioverter defibrillators, pregnancy and coagulopathy[18].
Technical aspects of endoscopic RFA
In endoluminal biliary RFA, a catheter is introduced over a wire into the bile duct during conventional endoscopic cholangiopancreaticography (ERCP). Once a contrast is injected, cholangiography is performed by localising the site of the bile duct stricture caused by the tumour. The dimensions of the ablation target are then determined. X-ray-visible markings enable the catheter to be correctly positioned within the stricture (Figures 1, 2). Power is applied at the recommended setting for 30-120 s. For longer strictures, the RFA catheter is repositioned to cover the whole length of the stricture with a small overlap to ensure no gaps remain. After ablation, some authors advocate removing necrotic debris with a balloon sweep before stents are introduced. Finally, plastic or metal stents are inserted to treat the obstruction (Figures 1, 2). Mechanical dilation is sometimes required prior to introducing the RFA catheter or stenting.
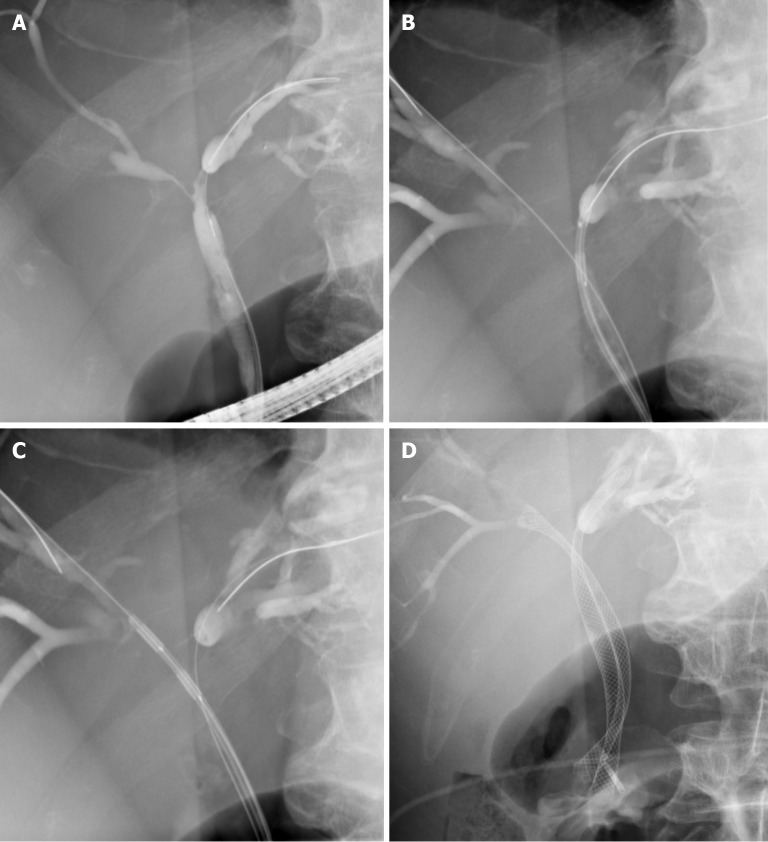
Figure 1.
Radiofrequency ablation of a hilar cholangiocarcinoma. A: Hilar stricture; B: Radiofrequency ablation (RFA) of the right hepatic duct; C: RFA of the left hepatic duct; D: Bilateral self-expandable metal stents drainage post RFA.
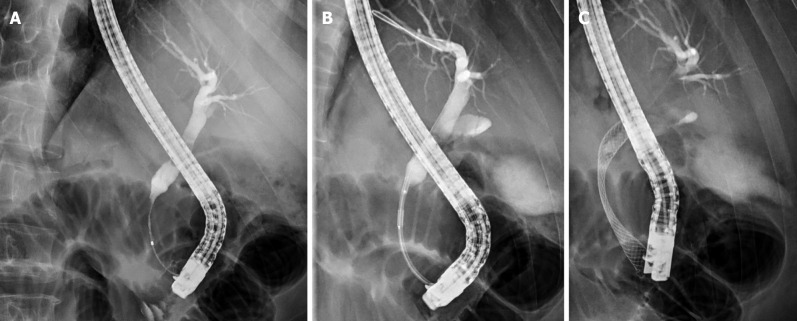
Figure 2.
Radiofrequency ablation of a pancreatic cancer. A: Distal common bile duct stricture; B: Radiofrequency ablation (RFA) of the bile duct stricture; C: Self-expandable metal stents drainage post RFA.
SEMS are generally favoured over plastic stents for palliative treatment in patients with malignant biliary obstruction. Advantages include longer stent patency, less need for re-intervention, increased survival and cost-effectiveness[19]. Interestingly, one study reported three cases of bleeding (two lethal) in patients where plastic stents were used after RFA[16].
Over the past ten years, two endoluminal biliary catheters have become commercially available. The Habib EndoHPB biliary RFA catheter (Boston Scientific, Massachusetts, United States) is a single-use bipolar device for delivering endoluminal RFA. Comprising an 8F (2.6 mm) catheter 180 cm in length, the device is passed down the endoscope with a working channel at least 3.2 mm in diameter. Two circumferential 8-mm electrodes separated by 8 mm of free space are located proximally from a 5-mm-long distal leading tip. Cylindrical ablation of a 24-mm area can be achieved. The Habib probe can be connected either to an ERBE electrosurgical generator (Surgical Technology Group, Hampshire, United Kingdom) or a RITA-1500X generator (Angiodynamics, Latham, NY, United States). The typical settings are effect 8 at 10 wk.
The second available endoluminal electrode is the ELRA™ (EndoLuminal Radiofrequency Ablation, Taewoong Medical, South Korea), a 7Fr (2.3 mm) 175-cm-long catheter with a 9 mm leading tip. Four bipolar electrodes facilitate linear ablation in four different lengths (11-33 mm). The catheter is only compatible with the VIVA Combo Generator (Taewoong Medical), the specific feature of this generator is its ability to control temperature and impedance. This generator can also be used with a specifically designed needle for EUS-guided RFA treatment.
Recently, a newly developed balloon-RFA catheter with an automatic temperature control system was developed and compared to a standard RFA catheter in a porcine model. The balloon RFA produced similar extent of ablation but with less variation in depth compared to the standard RFA (0.73 ± 0.31 mm vs 2.00 ± 0.62 mm; P < 0.001). Furthermore, excessive ablation was present only in conventional RFA (0% vs 67%; P = 0.005)[20].
No clear and universally accepted technical protocol for the use of endoluminal ablation exists which presents a critical problem. Different results between patients may thus represent not only differences that can be attributed to the anatomy and biology of the patients tumors, but also to differences between the generators and settings used. Table 1 provides details about technical protocols used in various studies.
Table 1.
Technical information on endoluminal radiofrequency ablation
|
Ref.
|
RFA device
|
Type of RFA generator
|
Frequency of electric energy
|
Power of RFA generator
|
Duration of RFA
|
Resting Period Duration
|
| Steel et al [24] | Habib EndoHPB | 1500 RF generator, RITA medical system, Fremont, Calif, United States | 400 Hz | 7-10 wk | 120 s | 60 s |
| Figueroa-Barojas et al[26] | Habib EndoHPB | RITA 1500X, angioDynamics, Latham, NY, United States | Not stated | 7-10 wk | 120 s | 60 s |
| Dolak et al[17] | Habib EndoHPB | Not stated | Standard high frequency generator (400-500 Hz) | 7-10 wk | up to 120 s | Not stated |
| Sharaiha et al[27] | Habib EndoHPB | RITA 1500X, angioDynamics, Latham, NY or ERBE, United States | Not stated | 7-10 wk | 90-120 s | 60-120 s |
| Strand et al[29] | Habib EndoHPB | Not stated | Not stated | 7 wk for intrahepatic strictures, 10 wk for extrahepatic strictures | 2 applications for 90 s | 60 s |
| Sharaiha et al[28] | Habib EndoHPB | RITA 1500X, angioDynamics, Latham, NY or ERBE, United States | Not stated | Not stated | Not stated | Not stated |
| Kallis et al[35] | Habib EndoHPB | 1500 RF generator, RITA medical system, Fremont, Calif or ERBE VIO200 D, ERBE medical United Kingdom, Ltd, Leeds, United Kingdom | Not stated | 10 wk | 120 s | Not stated |
| Liang et al[31] | Habib EndoHPB | RITA 1500X, angioDynamics, Latham, NY, United States | 400 Hz | 10 wk | 120 s | 60 s |
| Schmidt et al[30] | Habib EndoHPB | RITA 1500X, angioDynamics, Latham, NY or ERBE Vio 200, Electromedicie GmbH | 400 Hz | 7 wk; 7 wk | 90 s; 90 s | Not stated |
| Laleman et al[25] | ELRA | VIVA combo generator, Taewoong Medical, Korea | Not stated | 7-10 wk | 120 s | Not stated |
| Bokemayer et al[33] | Habib EndoHPB | Not stated | Not stated | 8-10 wk | Not stated | Not stated |
| Yang et al[32] | Habib EndoHPB | RITA 1500X, angioDynamics, Latham, NY, United States | 400 Hz | 7-10 wk | 90 s | 60 s |
| Inoue et al[36] | Habib EndoHPB | VIVA combo generator, Taewoong Medical, South Korea | Not stated | 7 wk | 90 s | Not stated |
RFA: Radiofrequency ablation; ELRA: EndoLuminal Radiofrequency Ablation.
Malignant biliary obstruction
Malignant biliary tract obstruction results from stenosis and blockage of the bile ducts in the biliary tree. Generally caused by local invasion or compression, the condition is associated with a wide range of cancers, including CCC and pancreatic (PC) and hepatocellular carcinomas as well as other malignancies such as gallbladder cancer and metastatic cancers. Patients with this condition are usually at an advanced non-resectable stage at the time of diagnosis and thus only eligible for palliative treatment. The main goal of endoscopic treatment is to improve quality of life by treating or preventing biliary obstruction, thus reducing related symptoms such as pruritus, jaundice and cholangitis.
Cholangiocarcinoma is a rare heterogenous malignant disease arising from the biliary epithelium. Classified into intrahepatic, perihilar and distal types according to anatomical location, the disease accounts for about 3% of all gastrointestinal malignancies. Late diagnosis is typically associated with poor prognosis. Most patients are not eligible for surgical resection in such a locally advanced stage, with the 5-year survival rate reported at below 10%. Hilar tumours (Klatskin) represent 60%-80% of all cholangiocarcinomas. Although patients with Klatskin tumours of limited extent may undergo surgical resection to improve survival, the procedure is associated with significant morbidity and mortality. The mean survival of patients with unresectable disease is between 7 and 9 mo[21].
Pancreatic ductal adenocarcinoma is the most frequent PC (around 90%) and the third main cause of cancer death worldwide, with a 5-year survival rate of 5%. Pancreatic resection improves survival, but only a minority of patients are eligible due to locally advanced disease, distant metastasis or comorbidities. Up to 70% of patients with PC manifestation exhibit malignant biliary obstruction[22].
It has been 10 years since the first reported case of RFA-treated cholangiocarcinoma involving a cholangioscopy view of the ablated bile duct[23]. This publication coincided with the first report on a series of patients with malignant biliary obstruction treated with endoscopic RFA[24]. Since then, several studies have documented the use of biliary endoscopic RFA, including retrospective, retrospective comparative and prospective studies, one randomised study and one meta-analysis. Our review reports on althogether 415 patients with RFA-treated malignant biliary obstruction, 228 of them reported in uncontrolled studies and 187 in controlled studies. Most of the studies involve a mixture of patients with malignant biliary obstruction of different aetiologies; some consist of only patients with CCC and one study includes patients with PC only.
The reported technical feasibility of endoscopic RFA is close to 100%. While some studies report only minor adverse events, others describe severe events including lethalities. Most studies have used the Habib probe, with only one study detailing use of the ELRA catheter[25].
Steel at al[24] documented 22 patients with malignant bile duct obstruction, 16 with PC and 6 with cholangiocarcinoma. Immediate 30 d complication rates and 90 d stent patency were evaluated as primary parameters. Intraductal RFA was followed by SEMS placement. One patient developed asymptomatic elevation of amylase, another rigour, and two cholecystitis requiring cholecystectomy. Except for 3 patients, all achieved 90 d stent patency.
Figueroa-Barojas et al[26] evaluated 20 patients with malignant biliary strictures, unresectable cholangiocarcinoma (n = 11), unresectable PC (n = 7), intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia (n = 1) and metastasis of gastric cancer in the bile duct (n = 1). Either SEMS (n = 14) or plastic stents (n = 6) were inserted and the mean stricture diameter was found to significantly increase (1.7 mm; range, 0.5–3.4 vs 5.2 mm; range, 2.6–9, respectively).
In an Austrian national retrospective study, 84 ablations were performed in 58 patients across 11 centres. Cholangiocarcinoma was detected in 45 patients. Technical feasibility was 100%, but multiple complications occurred (1 Liver infarct, 5 cholangitis, 3 haemobilia, 2 sepsis, 1 gallbladder empyema, 1 Liver coma). The median stent patency was 170 d (95%CI: 63-277), with longer duration in metal compared to plastic stents (218 vs 115 d, P = 0.051). The median survival post-RFA was 10.6 mo (95%CI: 6.9-14.4) [17].
In a retrospective comparative study by Sharaiha et al[27], 26 patients with pancreatic or bile duct cancer underwent RFA followed by plastic or metal stent placement. This group was compared to 40 matched controls who underwent only stenting. The technical success rate was 100%. Statistical analysis showed RFA to be an independent predictor of survival [HR 0.29 (0.11–0.76), P = 0.012] together with age and treatment by chemotherapy [HR 1.04 (1.01–1.07), P = 0.011; HR 0.26 (0.10–0.70), P = 0.007]. SEMS patency rates were the same across all groups.
One year later, the same group published a comparative study of 69 patients with malignant biliary obstruction, including unresectable cholangiocarcinoma (n = 45), PC (n = 19), gallbladder cancer (n = 2), gastric cancer (n = 1) and colon cancer liver metastasis (n = 3), representing a total of 98 RFA sessions. All patients received post-RFA stenting, either plastic or metal, and were subsequently compared to registry data. There was a statistically substantial enhancement in stricture diameter (P < 0.0001), a trend even more considerable in PC-related strictures. The median survival was significantly prolonged in both major groups (PC 14.6 vs 5.9 mo, P < 0.0001, cholangiocarcinoma 17.7 vs 6.2 mo)[28].
Strand et al[29] carried out a retrospective comparison of RFA and photodynamic therapy in patients with cholangiocarcinoma. Sixteen patients who underwent RFA had the same survival as 32 patients who underwent PDT (median survival 9.6 vs 7.5 mo, P = 0.779). A similar European comparison study carried out by Schmidt et al[30] compared 14 RFA patients with a historical cohort of 20 PDT patients. All individuals had hilar cholangiocarcinoma, undergoing a total of 31 RFA sessions or 36 PDT sessions. While the rate of premature stent failure (< 3 mo) was smaller in the RFA group (29% vs 65%, P < 0.01), the rate of complications was greater in the PDT group (21% vs 40%, P = 0.277).
The ELRA catheter was used in a prospective study of 18 patients with hilar cholangiocarcinoma (9), distal cholangiocarcinoma (2) and PC (7). Three- and six-month stent patency was achieved in 80% and 69% of patients still alive, respectively. Median stent patency was 110 d (16-374) and the median survival 227 d (16-374). No complications were reported[25].
A retrospective analysis -by Liang et al[31] compared stent patency and survival among 76 patients with unresectable extrahepatic CCC (27 patients with Bismuth type I and 47 patients with distal CCC). Metal stents were used in all patients. RFA was administered in 34 patients. Stent patency in the RFA group was longer than in the stent-only group (median 9.5 mo vs 8.4 mo, P = 0.024). Survival was also significantly prolonged in patients given RFA (P = 0.036).
To date, there has only been one randomised controlled study aimed at assessing survival of patients with an extrahepatic CCC after RFA therapy. This study included 65 patients with unresectable extrahepatic CCC (Bismuth type I/II). The RFA group (n = 32) had a mean survival of 13.2 mo compared to the stent-only group (n = 33), which had a median survival of 8.3 mo (P = 0.001). The mean stent patency was also longer in the RFA group (6.8 vs 3.4 mo, P = 0.02). There were no significant differences in adverse events between groups (6.3% vs 9.1%, P = 0.67)[32].
A small case control study of 32 CCC patients retrospectively evaluated benefits of combined RFA and stent application in patients with hilar CCC Bismuth type III and IV CCC (n = 20; 14 patients received repeat RFA) compared to controls (n = 22) treated with stents only. The study revealed longer survival time in the RFA group (342 d vs 221 d, P = 0.046)[33].
Kim et al[34] demonstrated encouraging results for the use of RFA treatment in malignant distal biliary obstruction. Forty-three patients (CCC 28, PC 11, gallbladder cancer 4) were treated with RFA and either covered/uncovered SEMS or plastic stents. The median stent patency time was 173 d for the uncovered SEMS group and 203 d for the covered SEMS group. The median survival was 449 d, with 630 d for biliary tract cancer and 191 d for PC.
Kallis et al[35] published a retrospective case-controlled study of 69 patients with unresectable pancreatic carcinoma only. RFA treatment was given to 23 patients. The median survival time in the RFA group was longer than in controls (226 d vs 135 d), with SEMS patency the same for both groups. The RFA procedure was associated with minimal complications and adverse effects.
A recent retrospective series of 41 patients with malignant hilar biliary obstruction (cholangiocarcinoma 65.9%, gallbladder cancer 22%) treated by RFA (Habib EndoHPB, 7 wk, 90 s) and two uncovered SEMS showed a technical success rate of 95.1%, acute complication rate of 2.4% (cholangitis), late complication rate of 7.7% (cholecystitis, cholangitis, liver abscess) and a rate of recurrent biliary obstruction of 38.5% after a median time of 230 d. The same authors previously reported a shorter median patency time of 140 d when no RFA was used[36,37].
The only meta-analysis on the topic evaluated 9 prospective and retrospective studies involving 505 patients with malignant biliary obstruction. Patients underwent RFA with metallic or plastic stent placement (n = 239) or biliary stent only (n = 266) using percutaneous transhepatic cholangiography or ERCP. The analysis demonstrated prolonged survival (285 d vs 248 d) and improved stent patency (pooled weighted mean difference 50.6 d; cholangiocarcinoma subgroup 42.7 d). However, RFA was associated with a higher rate of adverse events such as abdominal pain (31% vs 20%, P = 0.003)[38].
The above-mentioned studies are summarized in Table 2 (uncontrolled studies and Table 3 (controlled studies).
Table 2.
Uncontrolled radiofrequency ablation studies
|
Ref.
|
Number of patients
|
Etiology
|
Type of study design
|
Case control analysis
|
Method
|
RFA device
|
Aim
|
Results
|
| Steel et al[24] | 22 | CCC (n = 6) PC (n = 16) | Prospective | No | ERFA before SEMS | Habib EndoHPB | RFA catheter deployment, stent patency; adverse events (AE) | (1) 21/22 technical success; (2) 21/21 stent patency; 3/21 stent occlusion at 90 days; (3) AE 1 acute pancreatitis, 2 cholecystitis |
| Figueroa-Barojas et al[26] | 20 | CCC (n = 11) PC (n = 7) IPMN (n = 1) Gastric cancer (n = 1) | Prospective | No | ERFA before stenting (metallic or plastic) | Habib EndoHPB | Stricture diameter size; adverse events | (1) Significant increase of 3.5 mm duct diameter post RFA (P value < 0.0001); (2) 2 AE (1 mild pancreatitis, 1 cholecystitis) |
| Dolak et al[17] | 58 | MBO mainly CCC (n = 48) | Retrospective | No | ERFA + stenting, repeated ERFA for blocked SEMS, percutaneous RFA | Habib EndoHPB | Stent patency, survival adverse events, survival | (1) Median stent patency 170 d; Metal vs plastic stent (218 vs 115 d, P = 0.051); (2) Median survival 10.6 mo; (3) 12 AE (1 partial liver infarction, 5 Cholangitis, 2 hemobilia, 2 cholangiosepsis, 1 hepatic coma, 1 left bundle branch block) |
| Sharaiha et al[28] | 69 | CCC (n = 45) PC (n = 19) GB (n = 2) Gastric cancer (n = 1) Colon cancer liver metastasis (n = 3) | Retrospective (multicentric registry) | No | Mainly ERFA before placing metallic or plastic stent | Habib EndoHPB | Survival; stricture diameter; Adverse events | (1) Median survival 11.46 mo; (2) Significant improvement in stricture diameter post-ablation (P < 0.0001); (3) AE 10% (1 pancreatitis 2 cholecystitis, 1 hemobilia, 3 abdominal pain) |
| Laleman et al[25] | 18 | CCC, PC | Prospective | No | ERFA before stenting | ELRA | Feasibility, bilirubin level, survival and stent patency rate | (1) 6 AE (4 cholangitis, 2 pancreatitis); (2) Bilirubin level post-RFA decreased from 7.8 ± 1 mg/dL to 1.7 ± 0.4 mg/dL; P < 0.001; (3) Median survival of 227 d; (4) Stent patency 80% at 90 d and 69% at and 180 d respectively |
| Inoue et al[20] | 41 | MBO mainly CCC (n = 27) GB (n = 9) | Retrospective | No | ERFA before bilateral stenting (uncovered metallic) | Habib EndoHPB | Technical success; adverse effect; recurrent biliary obstruction (RBO) and stent patency rate | (1) Technical success was 95.1% (39/41); (2) 1 acute cholangitis, 1 cholecystitis, 1 nonocclusion cholangitis, 1 liver abcess; (3) RBO rate 38.5 % (15/39), and the median time to RBO was 230 d; (4) The median time to RBO was significantly longer in patients with strictures > 15 mm in length vs strictures ≤ 15 mm (314 vs 156 d; P = 0.02) |
CCC: Cholangiocarcinoma; PC: Pancreatic cancer; GB: Gallbladder cancer; IPMN: Intradu ctal papillary mucinous neoplasm; RBO: Recurrent biliary obstruction.
Table 3.
Controlled recurrent biliary obstruction studies
|
Ref.
|
Number of patients
|
Etiology
|
Type of study design
|
Case control analysis
|
Method
|
RFA device
|
Aim
|
Results
|
| Sharaiha et al[27] | 66 (26 RFA) | CCC (n = 37) PC (n = 29) | Retrospective case control study | Yes | ERFA before stenting (26pts) vs stenting alone (40 pts) | Habib EndoHPB | Survival; Stent patency; Adverse events (AE) | (1) The median survival was 5.9 mo in both groups; (2) SEMS patency rates were equivalent; (3) No differences in AE (2 RFA vs 3 no-RFA) |
| Strand et al[29] | 48 (16 RFA) | CCC | Retrospective case control study | Yes | ERFA (16 pts) vs PDT (32) | Habib EndoHPB | Survival, stent occlusion | (1) Median survival of 9.6 mo in RFA vs 7.5 mo in PDT group; (2) RFA group more frequent stent occlusion (0.06 vs 0.02, P = 0.008) and cholangitis (0.13 vs 0.05, P = 0.008) |
| Kallis et al[35] | 69 (23 RFA) | PC | Retrospective case control study | Yes | ERFA before stenting (23 pts) vs stenting alone (46 pts) | Habib EndoHPB | Survival, stent patency | (1) Survival time in RFA group 226 vs 123.5 da in controls (P < 0.01); (2) SEMS patency rate equivalent in both group |
| Liang et al[31] | 76 (34 RFA) | CCC | Retrospective case control study | Yes | ERFA before stenting (34 pts) vs stenting alone (42 pts) | Habib EndoHPB | Survival, stent patency, adverse events | (1) The median survival in the ERFA + SEMS group was significantly better vs SEMS only (P = 0.036); (2) ERFA+ SEMS patency rate 9.5 mo vs 8.4 mo; (P = 0.024); (3) AE equivalent |
| Sampath et al[51] | 25 (10 RFA) | CCC | Retrospective case control study | Yes | ERFA before stenting (10 pts) vs stenting alone (15 pts) | Habib EndoHPB | Survival | (1) Median survival 404 d vs 228 d in controls. (P < 0.001) |
| Schmidt et al[30] | 34 (14 RFA) | CCC | Retrospective case control study | Yes | Repeated ERFA (14 pts) vs repeated PDT (20) | Habib EndoHPB | Bilirubin levem Advere events, | (1) PDT group no significant decrease (P = 0.67) vs in RFA significant decrease (P = 0.046); (2) AE more frequently in PDT (n = 8; 40%) than with RFA (n=3; 14.21%) (P = 0.277). |
| Bokemayer et al[33] | 54 (32 RFA) | CCC (n = 45 + 1 intrahepatic); PC (n = 2); GB (n = 2); Other (n = 4) | Retrospective case control study | Yes | ERFA before stenting (32 pts) vs stenting alone (22 pts) | Habib EndoHPB | Survival | (1) Survival time in RFA group 342 ± 57 vs 221 ± 26 d in controls; (P = 0.046) |
| Yang et al[32] | 65 (32 RFA) | CC | Randomised controlled trial | Yes | ERFA before stenting (32 pts) vs stenting alone (33 pts) | Habib EndoHPB | Overall survival, stent patency; post-ERCP AE | (1) ERFA + stent vs the stent only (13.2 ± 0.6 vs 8.3 ± 0.5 mo, P < 0.001); (2) Stent patency (6.8 vs 3.4 mo, P = 0.02); (3) Similar AE 6.3% vs 9.1%, (P = 0.67) |
RFA: Recurrent biliary obstruction; CCC: Cholangiocarcinoma; PC: Pancreatic cancer; GB: Gallbladder cancer; pts: Patients; AE: Adverse events; SEMS: Self-expandable metal stents.
Occluded biliary stents
In 2010, a case was reported of a 91-year-old woman with cholangiocarcinoma whose uncovered metal stent became occluded due to tumour ingrowth 18 mo after placement. Intraductal RFA was successfully performed and necrotic tissue removed using a balloon sweep. However, since a plastic stent was also inserted, the real benefit of the ablation could not be verified[39].
In a more recent study, 25 patients with occluded stents treated by RFA (Habib RFA electrode) were matched and compared to 25 patients with occluded stents treated by stent placement. RFA was successful only in 14 out of the 25 patients (56%), with the remaining 11 also stented. The patency rate evaluated at 90 d was 56% in the RFA group and 24% in the control group (P = 0.04). Stent patency was significantly longer in the RFA group (119.5 d) compared to the stent group (65.3 d, P = 0.03). The groups did not differ with regard to 30 d mortality or 3 mo and 6 mo survival[40].
In another study on this topic, only 7 patients with occluded stents were treated with RFA but with a different catheter (ELRA). The treatment was sufficient to produce optimal drainage only in 2 patients (29%), with the rest requiring stent placement. Three patients died of their disease within 52 d of treatment[41].
The above results suggest that RFA should be cautiously used to protect against occluded SEMS. Interestingly, many of the treated patients could not be left without a stent, calling in to question the effect of the treatment. Furthermore, it is not known whether RFA of an occluded SEMS produces any additional effect on the tumour beyond the lumen of the stent. An experimental study performed on pigs and gel phantoms stented with uncovered and covered SEMS showed that ablation depth was markedly reduced in porcine bile ducts stented with SEMS. Additionally, RFA was terminated early when the coagulated area came into contact with the uncovered SEMS in polyacrylamide-gel phantoms. These results indicate that the effect of bipolar endobiliary RFA was attenuated by the presence of SEMS. It is unlikely, therefore, that the tumour tissue outside the SEMS was affected[42].
Ampullary cancer
Ampullary cancer (major duodenal papilla, ampulla of Vater) is treated by surgical resection, similarly to PC. Benign ampullary lesions such as adenomas can usually be resected endoscopically. However, intraductual growth of ampullary adenoma may be indication for surgery. Both duodenal resection and duodenopancreatectomy are complex procedures associated with significant morbidity and even mortality.
Following on from single case reports describing individual patients[43-45], Suarez et al[46] published a series on 4 patients in whom catheter-based RFA was applied to a remaining duct extension after ampullary resection. All patients underwent prophylactic placement of biliary and pancreatic stents. Three patients with adenoma experienced complete eradication, while in one patient adenocarcinoma recurred. There were no immediate adverse events, although one patient developed a post-procedural bile duct stricture requiring endoscopic therapy. The follow-up time, however, was unacceptably short (38 to 105 d only).
A retrospective multicentre study reported the outcomes of 14 patients with adenoma extensions into the common bile duct and pancreatic duct. Multiple ablations were performed (median 1, range 1-5). Additional modalities such as argon plasma coagulation, thermal probes and photodynamic therapy were also used in 7 patients. After a median follow-up of 16 mo (range 5-46), treatment success defined by negative intraductal biopsy was 92% and 100% for those treated solely by RFA. The rate of adverse events was 43%, with 5 ductal strictures and 1 retroduodenal abscess[47].
A prospective multicentre trial by Camus et al[48] produced data on 20 patients treated with intraductal RFA with post-ampullectomy residual adenoma extending into the bile duct. The patients were treated on average 1.4 years after the original ampullectomy. The electrode was positioned in the distal common bile duct and RFA applied at 10 wk for 30 s. All patients underwent biliary stent placement, with a quarter undergoing prophylactic pancreatic stent placement. Three patients developed mild pancreatitis (none having a pancreatic stent), with another three developing biliary strictures treated endoscopically. Residual neoplasia was detected in 15% of patients after 6 mo and in 30% of patients after one year, with some treated by repeat RFA. In summary, 70% efficiency was achieved at one year, with a 40% complication rate.
Benign strictures
Biliary strictures can also be benign, typically resulting from operative injury, chronic inflammation, chronic pancreatitis or liver transplantation. Endoscopic treatment with multiple plastic or self-expandable metal stents has become the method of choice for high efficiency. However, some strictures do not resolve or recur. There is limited experience with RFA in this indication. In one study, nine patients with benign strictures were treated by endoscopic RFA at 10 wk and 90 s followed by balloon dilation. While in three patients the stricture resolved immediately, stent placement was required in the remaining six patients, two of whom displayed proven stricture resolution during follow-up. One patient had mild pancreatitis[49]. In another study, RFA was applied using a percutaneous transhepatic approach in 18 patients with a benign hepaticojejunostomy stricture. RFA was followed by balloon dilation but not by stenting. Over a mean follow-up of 7.3 mo, ten patients had no stricture recurrence[50].
CONCLUSION
Extrahepatic cholangiocarcinoma and PC are malignancies with poor prognosis. Most patients diagnosed at an advance stage are not suitable for surgical resection, with malignancies typically resistant to current chemotherapy and radiation protocols. With the search for improved outcomes and the development of endoluminal RFA catheters, endoscopic RFA has become an emerging palliative treatment for extrahepatic cholangiocarcinoma and PCs. Although there is some evidence of improved survival and longer stent patency with the treatment, most of the available data come from retrospective and often uncontrolled studies. One randomised trial of limited cholangiocarcinoma and two metanalyses showed improved survival of 1-5 mo. However, the studies published thus far are highly heterogenous with regard to aetiology, stage of disease (cancers of different biology and a wide spectrum of stages, from disease limited to the bile duct to distant metastatic spread) and the type of treatment delivered (type of electrode/generator, power and duration of ablation, presence or absence of temperature control, single or repeat ablation, type and quantity of plastic/metal stents). Furthermore, the prognosis of patients with malignant biliary obstruction can be significantly influenced by the extent and quality of biliary drainage achieved and by concomitant therapies, factors often not accounted or controlled for in these studies. Reports also document a risk of complications, some of them fatal. Moreover, there are considerable costs associated with RFA. Only continued investigation in the form of well-designed randomised controlled studies will provide a definitive answer to the questions whether and to what extent endoluminal RFA benefits patients with malignant biliary obstruction.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: February 21, 2021
First decision: June 4, 2021
Article in press: August 24, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Czech Republic
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bauerfeind P S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
Contributor Information
Jana Jarosova, Department of Gastroenterology and Hepatology, Institute for Clinical and Experimental Medicine, Praha 14021, Czech Republic.
Peter Macinga, Department of Gastroenterology and Hepatology, Institute for Clinical and Experimental Medicine, Praha 14021, Czech Republic.
Alzbeta Hujova, Department of Gastroenterology and Hepatology, Institute for Clinical and Experimental Medicine, Praha 14021, Czech Republic.
Jan Kral, Department of Gastroenterology and Hepatology, Institute for Clinical and Experimental Medicine, Praha 14021, Czech Republic.
Ondrej Urban, Department of Internal Medicine II – Gastroenterology and Geriatrics, University Hospital Olomouc, Faculty of Medicine and Dentristry, Palacky University Olomouc, Olomouc 77900, Czech Republic.
Julius Spicak, Department of Gastroenterology and Hepatology, Institute for Clinical and Experimental Medicine, Praha 14021, Czech Republic.
Tomas Hucl, Department of Gastroenterology and Hepatology, Institute for Clinical and Experimental Medicine, Praha 14021, Czech Republic. tomas.hucl@ikem.cz.
References
- 1.Zorrón Pu L, de Moura EG, Bernardo WM, Baracat FI, Mendonça EQ, Kondo A, Luz GO, Furuya Júnior CK, Artifon EL. Endoscopic stenting for inoperable malignant biliary obstruction: A systematic review and meta-analysis. World J Gastroenterol. 2015;21:13374–13385. doi: 10.3748/wjg.v21.i47.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426–2430. doi: 10.1111/j.1572-0241.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 3.Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Pereira SP, Jitlal M, Duggan M, Lawrie E, Beare S, O'Donoghue P, Wasan HS, Valle JW, Bridgewater J, Ramage J, Przemioslo R, Hammonds R, Aithal G, Murphy F, Foster G, Sturgess R. PHOTOSTENT-02: porfimer sodium photodynamic therapy plus stenting versus stenting alone in patients with locally advanced or metastatic biliary tract cancer. ESMO Open. 2018;3:e000379. doi: 10.1136/esmoopen-2018-000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13:129–147. doi: 10.1016/s0929-8266(01)00126-4. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg SN, Gazelle GS. Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepatogastroenterology. 2001;48:359–367. [PubMed] [Google Scholar]
- 9.van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, Fütterer JJ, den Brok MH, Adema GJ. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother. 2017;66:247–258. doi: 10.1007/s00262-016-1891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoi T, Isayama H, Sofuni A, Itokawa F, Tamura M, Watanabe Y, Moriyasu F, Kahaleh M, Habib N, Nagao T, Yokoyama T, Kasuya K, Kawakami H. Evaluation of effects of a novel endoscopically applied radiofrequency ablation biliary catheter using an ex-vivo pig liver. J Hepatobiliary Pancreat Sci. 2012;19:543–547. doi: 10.1007/s00534-011-0465-7. [DOI] [PubMed] [Google Scholar]
- 11.Zacharoulis D, Lazoura O, Sioka E, Potamianos S, Tzovaras G, Nicholls J, Koukoulis G, Habib N. Habib EndoHPB: a novel endobiliary radiofrequency ablation device. An experimental study. J Invest Surg. 2013;26:6–10. doi: 10.3109/08941939.2012.681832. [DOI] [PubMed] [Google Scholar]
- 12.Atar M, Kadayifci A, Daglilar E, Hagen C, Fernandez-Del Castillo C, Brugge WR. Ex vivo human bile duct radiofrequency ablation with a bipolar catheter. Surg Endosc. 2018;32:2808–2813. doi: 10.1007/s00464-017-5984-0. [DOI] [PubMed] [Google Scholar]
- 13.Daglilar ES, Yoon WJ, Mino-Kenudson M, Brugge WR. Controlled swine bile duct ablation with a bipolar radiofrequency catheter. Gastrointest Endosc. 2013;77:815–819. doi: 10.1016/j.gie.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- 15.Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR, Fry TJ, Wood BJ. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251:58–66. doi: 10.1148/radiol.2511072175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tal AO, Vermehren J, Friedrich-Rust M, Bojunga J, Sarrazin C, Zeuzem S, Trojan J, Albert JG. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc. 2014;6:13–19. doi: 10.4253/wjge.v6.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolak W, Schreiber F, Schwaighofer H, Gschwantler M, Plieschnegger W, Ziachehabi A, Mayer A, Kramer L, Kopecky A, Schrutka-Kölbl C, Wolkersdörfer G, Madl C, Berr F, Trauner M, Püspök A Austrian Biliary RFA Study Group. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2014;28:854–860. doi: 10.1007/s00464-013-3232-9. [DOI] [PubMed] [Google Scholar]
- 18.Dunki-Jacobs EM, Philips P, Martin RC 2nd. Evaluation of resistance as a measure of successful tumor ablation during irreversible electroporation of the pancreas. J Am Coll Surg. 2014;218:179–187. doi: 10.1016/j.jamcollsurg.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910–930. doi: 10.1055/a-0659-9864. [DOI] [PubMed] [Google Scholar]
- 20.Inoue T, Ito K, Yoneda M. Novel balloon catheter-based endobiliary radiofrequency ablation system: Ex-vivo experimental study. Dig Endosc. 2020;32:974–978. doi: 10.1111/den.13622. [DOI] [PubMed] [Google Scholar]
- 21.Sharma P, Yadav S. Demographics, tumor characteristics, treatment, and survival of patients with Klatskin tumors. Ann Gastroenterol. 2018;31:231–236. doi: 10.20524/aog.2018.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, Reddy DN. Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos) Gastrointest Endosc. 2011;74:935–937. doi: 10.1016/j.gie.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149–153. doi: 10.1016/j.gie.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Laleman W, van der Merwe S, Verbeke L, Vanbeckevoort D, Aerts R, Prenen H, Van Cutsem E, Verslype C. A new intraductal radiofrequency ablation device for inoperable biliopancreatic tumors complicated by obstructive jaundice: the IGNITE-1 study. Endoscopy. 2017;49:977–982. doi: 10.1055/s-0043-113559. [DOI] [PubMed] [Google Scholar]
- 26.Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013;2013:910897. doi: 10.1155/2013/910897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharaiha RZ, Natov N, Glockenberg KS, Widmer J, Gaidhane M, Kahaleh M. Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: is there an added benefit? Dig Dis Sci. 2014;59:3099–3102. doi: 10.1007/s10620-014-3264-6. [DOI] [PubMed] [Google Scholar]
- 28.Sharaiha RZ, Sethi A, Weaver KR, Gonda TA, Shah RJ, Fukami N, Kedia P, Kumta NA, Clavo CM, Saunders MD, Cerecedo-Rodriguez J, Barojas PF, Widmer JL, Gaidhane M, Brugge WR, Kahaleh M. Impact of Radiofrequency Ablation on Malignant Biliary Strictures: Results of a Collaborative Registry. Dig Dis Sci. 2015;60:2164–2169. doi: 10.1007/s10620-015-3558-3. [DOI] [PubMed] [Google Scholar]
- 29.Strand DS, Cosgrove ND, Patrie JT, Cox DG, Bauer TW, Adams RB, Mann JA, Sauer BG, Shami VM, Wang AY. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014;80:794–804. doi: 10.1016/j.gie.2014.02.1030. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt A, Bloechinger M, Weber A, Siveke J, von Delius S, Prinz C, Schmitt W, Schmid RM, Neu B. Short-term effects and adverse events of endoscopically applied radiofrequency ablation appear to be comparable with photodynamic therapy in hilar cholangiocarcinoma. United European Gastroenterol J. 2016;4:570–579. doi: 10.1177/2050640615621235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang H, Peng Z, Cao L, Qian S, Shao Z. Metal Stenting with or without Endobiliary Radiofrequency Ablation for Unresectable Extrahepatic Cholangiocarcinoma. J Cancer Ther. 2015;6:981–992. [Google Scholar]
- 32.Yang J, Wang J, Zhou H, Zhou Y, Wang Y, Jin H, Lou Q, Zhang X. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy. 2018;50:751–760. doi: 10.1055/s-0043-124870. [DOI] [PubMed] [Google Scholar]
- 33.Bokemeyer A, Matern P, Bettenworth D, Cordes F, Nowacki TM, Heinzow H, Kabar I, Schmidt H, Ullerich H, Lenze F. Endoscopic Radiofrequency Ablation Prolongs Survival of Patients with Unresectable Hilar Cholangiocellular Carcinoma - A Case-Control Study. Sci Rep. 2019;9:13685. doi: 10.1038/s41598-019-50132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim EJ, Chung DH, Kim YJ, Kim YS, Park YH, Kim KK, Cho JH. Endobiliary radiofrequency ablation for distal extrahepatic cholangiocarcinoma: A clinicopathological study. PLoS One. 2018;13:e0206694. doi: 10.1371/journal.pone.0206694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallis Y, Phillips N, Steel A, Kaltsidis H, Vlavianos P, Habib N, Westaby D. Analysis of Endoscopic Radiofrequency Ablation of Biliary Malignant Strictures in Pancreatic Cancer Suggests Potential Survival Benefit. Dig Dis Sci. 2015;60:3449–3455. doi: 10.1007/s10620-015-3731-8. [DOI] [PubMed] [Google Scholar]
- 36.Inoue T, Ibusuki M, Kitano R, Kobayashi Y, Ohashi T, Nakade Y, Sumida Y, Ito K, Yoneda M. Endobiliary radiofrequency ablation combined with bilateral metal stent placement for malignant hilar biliary obstruction. Endoscopy. 2020;52:595–599. doi: 10.1055/a-1133-4448. [DOI] [PubMed] [Google Scholar]
- 37.Inoue T, Ishii N, Kobayashi Y, Kitano R, Sakamoto K, Ohashi T, Nakade Y, Sumida Y, Ito K, Nakao H, Yoneda M. Simultaneous Versus Sequential Side-by-Side Bilateral Metal Stent Placement for Malignant Hilar Biliary Obstructions. Dig Dis Sci. 2017;62:2542–2549. doi: 10.1007/s10620-017-4691-y. [DOI] [PubMed] [Google Scholar]
- 38.Sofi AA, Khan MA, Das A, Sachdev M, Khuder S, Nawras A, Lee W. Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87:944–951.e1. doi: 10.1016/j.gie.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Lui KL, Li KK. Intraductal radiofrequency ablation of tumour ingrowth into an uncovered metal stent used for inoperable cholangiocarcinoma. Hong Kong Med J. 2013;19:539–541. doi: 10.12809/hkmj133867. [DOI] [PubMed] [Google Scholar]
- 40.Kadayifci A, Atar M, Forcione DG, Casey BW, Kelsey PB, Brugge WR. Radiofrequency ablation for the management of occluded biliary metal stents. Endoscopy. 2016;48:1096–1101. doi: 10.1055/s-0042-115938. [DOI] [PubMed] [Google Scholar]
- 41.Nayar MK, Oppong KW, Bekkali NLH, Leeds JS. Novel temperature-controlled RFA probe for treatment of blocked metal biliary stents in patients with pancreaticobiliary cancers: initial experience. Endosc Int Open. 2018;6:E513–E517. doi: 10.1055/s-0044-102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon WJ, Daglilar ES, Kamionek M, Mino-Kenudson M, Brugge WR. Evaluation of radiofrequency ablation using the 1-Fr wire electrode in the porcine pancreas, liver, gallbladder, spleen, kidney, stomach, and lymph nodes: A pilot study. Dig Endosc. 2015 doi: 10.1111/den.12575. [DOI] [PubMed] [Google Scholar]
- 43.Mehendiratta V, Desilets DJ. Use of radiofrequency ablation probe for eradication of residual adenoma after ampullectomy. Gastrointest Endosc. 2015;81:1055–1056. doi: 10.1016/j.gie.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Tian Q, Wang G, Zhang Y, Jin Y, Cui Z, Sun X, Shen Z. Endoscopic radiofrequency ablation combined with fully covered self-expandable metal stent for inoperable periampullary carcinoma in a liver transplant patient: A case report. Medicine (Baltimore) 2017;96:e5790. doi: 10.1097/MD.0000000000005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valente R, Urban O, Del Chiaro M, Capurso G, Blomberg J, Löhr JM, Arnelo U. ERCP-directed radiofrequency ablation of ampullary adenomas: a knife-sparing alternative in patients unfit for surgery. Endoscopy. 2015;47 Suppl 1 UCTN:E515–E516. doi: 10.1055/s-0034-1392866. [DOI] [PubMed] [Google Scholar]
- 46.Suarez AL, Coté GA, Elmunzer BJ. Adjunctive radiofrequency ablation for the endoscopic treatment of ampullary lesions with intraductal extension (with video) Endosc Int Open. 2016;4:E748–E751. doi: 10.1055/s-0042-107665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rustagi T, Irani S, Reddy DN, Abu Dayyeh BK, Baron TH, Gostout CJ, Levy MJ, Martin J, Petersen BT, Ross A, Topazian MD. Radiofrequency ablation for intraductal extension of ampullary neoplasms. Gastrointest Endosc. 2017;86:170–176. doi: 10.1016/j.gie.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Camus M, Napoléon B, Vienne A, Le Rhun M, Leblanc S, Barret M, Chaussade S, Robin F, Kaddour N, Prat F. Efficacy and safety of endobiliary radiofrequency ablation for the eradication of residual neoplasia after endoscopic papillectomy: a multicenter prospective study. Gastrointest Endosc. 2018;88:511–518. doi: 10.1016/j.gie.2018.04.2332. [DOI] [PubMed] [Google Scholar]
- 49.Hu B, Gao DJ, Wu J, Wang TT, Yang XM, Ye X. Intraductal radiofrequency ablation for refractory benign biliary stricture: pilot feasibility study. Dig Endosc. 2014;26:581–585. doi: 10.1111/den.12225. [DOI] [PubMed] [Google Scholar]
- 50.Özdemir M, Küçükay F, Özdemir FAE, Acu R, Tola M, Yurdakul M. Percutaneous endobiliary radiofrequency ablation for refractory benign hepaticojejunostomy and biliary strictures. Diagn Interv Imaging. 2018;99:555–560. doi: 10.1016/j.diii.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Sampath K, Hyder SM, Gardner T, Gordon SR. Tu1526 The Effect of Endoscopic Radiofrequency Ablation on Survival in Patients with Unresectable Peri-Hilar Cholangiocarcinoma. Gastrointest Endosc. 2016;83:AB595. [Google Scholar]