Currently, four COVID-19 vaccines are authorized for use in the European Union by the European Medicines Agency (EMA), i.e., Ad26.COV2.S (1), ChAdOx1 nCoV-19 (2), mRNA1273 (3), and BNT162b2 (4). A boost (second vaccination) after the prime (first vaccination) is given for the latter three, to induce a strong and durable immune response (5). The prime and boost vaccines are administered in a so-called “homologous” vaccination regimen, meaning that both given vaccinations are identical.
Despite the unprecedented speed of vaccine development, vaccination programs have been impacted by unforeseen delays in production, logistic challenges, and the need for adaptation of vaccination schedules in response to emergence of more transmissible variants. Combining different vaccines in “heterologous” vaccination regimens would enable more flexible vaccination programs in the future, which would facilitate fast-track implementation and reduce the impact of any supply-chain disruptions (5). Several studies in mice have shown that combining different vaccines (two-dose heterologous vaccination strategy) elicits a broader immune response (neutralizing antibodies and T-cell responses) (6, 7). This is in line with initial COVID-19 trial results in humans, which found that a heterologous prime-boost induces a broader immune response (8, 9).
From an immunological point of view, heterologous vaccination regimens could provide a broader and more robust immune response. Several studies in humans investigate the reactogenicity and immunogenicity of such regimens, combining ChAdOx1 nCoV-19 with BNT162b2 vaccines, in Great Britain [Com-COV 1 and 2 (10)], Germany (11), and Spain [CombiVacS (12)]. The Com-COV 1 study showed that heterologous regimens with a 4-week prime-boost interval, combining ChAdOx1 nCoV-19 and BNT162b2 vaccines, induced superior immune responses compared to homologous regimens. The study reported significantly higher neutralizing antibody titers and higher T cell responses. The results of the Com-COV2 study, combining ChAdOx1 nCoV-19 with either mRNA1273 or NVX-CoV2373 are yet to be published (13). In addition, a heterologous ChAdOx1 nCoV-19/BNT162b2 immunization regimen with a 10-12 week vaccine interval was shown to be as least as immunogenic compared to homologous BNT162b2 vaccination with a three week prime-boost interval (11). The CombiVacS (12) study shows that a second dose of BNT162b2 administered 8-12 weeks after a prime with ChAdOx1 nCoV-19 elicited a robust immune response. As for reactogenicity in heterologous regimens, an increase in reactogenicity after the booster dose was observed in the ChAdOx1 nCoV-19/BNT162b2 immunization regimen (14). In contrast, a prospective study reported comparable reactogenicity between heterologous (ChAdOx1 nCoV-19/BNT162b2) and homologous (BNT162b2/BNT162b2) vaccine regimens after a 12-week dose interval (15).
Taken together, these data support flexibility in the use of heterologous prime-boost regimens with ChAdOx1 nCoV-19 and BNT162b2. Whether combining other approved vaccines in heterologous prime-boost vaccination strategies also elicit a robust immune response and has a similar reactogenicity profile remains of relevance for immunology and public health policy (14).
The promising data on heterologous prime boost vaccination regimens prompted us to initiate the SWITCH trial. The SWITCH trial is a multi-center, single-blind, randomized controlled trial among Health Care Workers (HCWs) primed with the Ad26.COV2.S vaccine without previous SARS-CoV-2 infection to be executed in four academic hospitals in the Netherlands (ClinicalTrial.gov identifier NCT04927936). The key objective of the SWITCH trial is to compare the effect of a homologous boost with Ad26.COV2.S with a heterologous boost with BNT162.b2 or mRNA1273. To date, Ad26.COV2.S is the only authorized vaccine intended for single vaccination. However, at this moment the safety and efficacy of a two-dose vaccination regimens with a 57-day prime-boost interval is investigated in the ENSEMBLE2 trial (ClinicalTrial.gov identifier NCT04614948). One other heterologous vaccine regimen trial with Ad26.COV2.S is performed by the National Institute of Allergy and Infectious Diseases (NIAID). In this trial, mRNA1273 is used as a boost 12-20 weeks after prime and the effect on SARS-CoV-2 specific antibodies will be evaluated (ClinicalTrial.gov identifier NCT04889209). Two recent studies showed that although there are durable immune responses elicited by a single dose of Ad26.COV2.S (16, 17), boosting may be required to rapidly increase humoral immune responses.
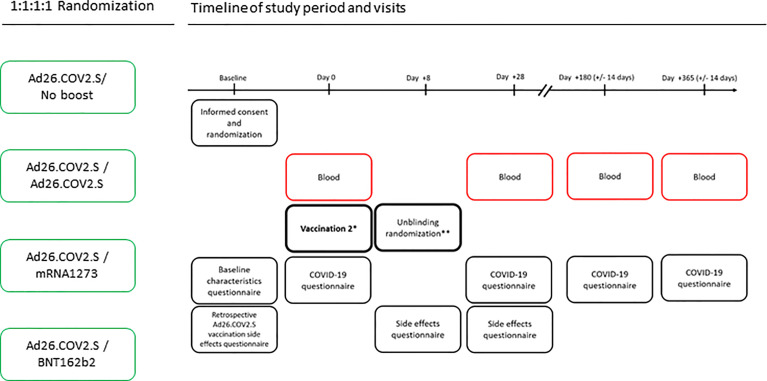
The SWITCH trial ( Figure 1 ) started recruitment at the end of June 2021 and will be completed at the end of 2022. HCWs are randomized in one of the four arms: Ad26.COV2.S/no boost; Ad26.COV2.S/Ad26.COV2.S; Ad26.COV2.S/mRNA1273; and Ad26.COV2.S/BNT162b2. The boost will be given 84 days (-7days/+21days) after the prime with Ad26.COV2.S. In total, 432 participants (108 HCWs in each arm) will be randomized. We will analyze the data per protocol, with three pre-defined contrasts, (i) Ad26.COV2.S/no boost vs. Ad26.COV2.S/Ad26.COV2.S; (ii) Ad26.COV2.S/Ad26.COV2.S vs. Ad26.COV2.S/mRNA1273; and (iii) Ad26.COV2.S/Ad26.COV2.S vs. Ad26.COV2.S/BNT162b2. The primary outcome is the detection of SARS-CoV-2 specific antibodies as measured by a quantitative IgG assay 28 days after the boost. Binding antibodies will be measured using the LIAISON SARS-CoV-2 TrimericS IgG assay in combination with the NIBSC/WHO COVID-19 reference serum 20/136 allowing for standardized results (18). Secondary outcomes are reactogenicity and extensive characterization of immunogenicity, including neutralizing antibody titers and SARS-CoV-2-specific T-cell responses against different SARS-CoV-2 variants (also see, ( Supplementary File 1 ), page 13). The SWITCH trial was designed in line with the other COVID-19 vaccination randomized controlled trials (10–13) and was approved by the local medical ethics committee (MEC 2021-0132).
Figure 1.
SWITCH trial design. The SWITCH trial includes four arms that receive a booster vaccination 84 days after prime with Ad26.COV2.S. Blood samples will be collected at indicated timepoints by venepunctures. Questionnaires will be performed to collect reactogenicity data and to determine whether participants had breakthrough infections despite vaccinations. *One of the four arms or 25% of the randomized participants will not receive a second vaccination; **Unblinding the randomization of participants will be done after the side effects (i.e., reactogenicity) questionnaire is completed.
This trial will give insight into the immunogenicity and safety of heterologous prime-boost vaccination regimens starting with Ad26.COV2.S, and specifically addresses this knowledge gap in a side-by-side comparison with the homologous counterpart. If booster vaccinations are eventually indicated for individuals who received a single-dose of Ad26.COV2.S, the SWITCH trial will also provide information on which vaccine is best suited as a booster. Reactogenicity data will address the safety profile of heterologous COVID-19 vaccine regimens starting with the Ad26.COV2.S vaccine. In addition to the strength and durability of the immune response, this trial addresses the breadth of the induced immune response by measuring reactivity to circulating SARS-CoV-2 variants. In order to adequately disseminate the results of the SWITCH trial, we will publish the results as soon as possible to enable implementation in vaccination campaign strategy and comparison with other trials.
Author Contributions
All authors were involved in the design and execution of the study and writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The trial is funded by ZonMW in the COVID-19 Vaccine program (project grantnumber: 10430072110001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.753319/full#supplementary-material
Protocol for the SWITCH trial approved by the medical ethics committee.
References
- 1. Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med (2021) 384(19):1824–35. doi: 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and Efficacy of the Chadox1 Ncov-19 Vaccine (AZD1222) Against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet (2021) 397:99–111. doi: 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med (2021) 384(5):403–16. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med (2020) 383(27):2603–15. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ledford H. Could Mixing COVID Vaccines Boost Immune Response? Nature (2021) 590:375–6. doi: 10.1038/d41586-021-00315-5 [DOI] [PubMed] [Google Scholar]
- 6. He Q, Mao Q, An C, Zhang J, Gao F, Bian L, et al. Heterologous Prime-Boost: Breaking the Protective Immune Response Bottleneck of COVID-19 Vaccine Candidates. Emerg Microbes Infect (2021) 10(1):629–37. doi: 10.1080/22221751.2021.1902245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spencer AJ, McKay PF, Belij-Rammerstorfer S, Ulaszewska M, Bissett CD, Hu K, et al. Heterologous Vaccination Regimens With Self-Amplifying RNA and Adenoviral COVID Vaccines Induce Robust Immune Responses in Mice. Nat Commun (2021) 12(1):2893. doi: 10.1038/s41467-021-23173-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and Reactogenicity of a Heterologous COVID-19 Prime-Boost Vaccination Compard With Homologous Vaccine Regimens. medRxiv (2021). [Google Scholar]
- 9. Groß R, Zanoni M, Seidel A, Conzelmann C, Gilg A, Krnavek D, et al. Heterologous Chadox1 Ncov-19 and BNT162b2 Prime-Boost Vaccination Elicits Potent Neutralizing Antibody Responses and T Cell Reactivity. medRxiv (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. Safety and Immunogenicity of Heterologous Versus Homologous Prime-Boost Schedules With an Adenoviral Vectored and mRNA COVID-19 Vaccine (Com-COV): A Single-Blind, Randomised, Non-Inferiority Trial. Lancet (2021) 398(10303):856–69. doi: 10.1016/S0140-6736(21)01694-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hillus D, Schwarz T, Tober-Lau P, Hastor H, Vanshylla H, Thibeault C, Hastor H, et al. Safety, Reactogenicity, and Immunogenicity of Homologous and Heterologous Prime-Boost Immunisation With Chadox1-Ncov19 and BNT162b2: A Prospective Cohort Study. Lancet Respir Med (2021) 12:S2213–2600(21):00357–X. doi: 10.1016/S2213-2600(21)00357-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al. Immunogenicity and Reactogenicity of BNT162b2 Booster in Chadox1-s-Primed Participants (Combivacs): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet (2021) 398(10295):121–30. doi: 10.1016/S0140-6736(21)01420-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Snape MD, Ramasamy M, Heath P, Green C, Turner D, Collins A, et al. Protocol Com-COV2: A Single-Blind, Randomised, Phase II UK Multi-Centre Study to Determine Reactogenicity and Immunogenicity of Heterologous Prime/Boost COVID-19 Vaccine Schedules – Stage 2. (2021). Accessed from: com-cov2protocolv2123-apr-2021cleanpdf(comcovstudy.org.uk). [Google Scholar]
- 14. Shaw RH, Stuart A, Greenland M, Liu X, Nguyen-Van-Tam JS, Snape MD. Heterologous Prime-Boost COVID-19 Vaccination: Initial Reactogenicity Data. Lancet (2021) 397(10289):2043–6. doi: 10.1016/S0140-6736(21)01115-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hillus D, Tober-Lau P, Hastor H, Helbig ET, Lippert LJ, Thibeault C, et al. Reactogenicity of Homologous and Heterologous Prime-Boost Immunisation With BNT162b2 and Chadox1-nCoV19: A Prospective Cohort Study. medRxiv (2021). doi: 10.1101/2021.05.19.21257334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadoff J, Le Gars M, Cardenas V, Shukarev G, Vaissiere N, Heerwegh D, et al. Durability of Antibody Responses Elicited by a Single Dose of Ad26.COV2.s and Substantial Increase Following Late Boosting. medRxiv (2021). doi: 10.1101/2021.08.25.21262569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barouch DH, Stephenson KE, Sadoff J, Yu J, Chang A, Gebre M, et al. Durable Humoral and Cellular Immune Responses 8 Months After Ad26.COV2.S Vaccination. NEJM (2021) 385:951–3. doi: 10.1056/NEJMc2108829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, et al. SARS-CoV-2 Variants of Concern Partially Escape Humoral But Not T-Cell Responses in COVID-19 Convalescent Donors and Vaccinees. Sci Immunol (2021) 6(59):eabj1750. doi: 10.1126/sciimmunol.abj1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the SWITCH trial approved by the medical ethics committee.