Abstract
Introduction
Approximately half of patients with cancer use some form of complementary medicine alongside conventional cancer treatment. The topic of complementary medicine often remains undiscussed in consultations between patients with cancer and their healthcare providers. This results in increased risks for adverse or interaction effects and decreased access to the benefits of evidence-based complementary medicine for patients with cancer. This paper describes the design of patient participatory study titled ‘COMMON’ that aims to explore and enhance open and effective communication about complementary medicine in oncology. The study is carried out in collaboration with 12 (former) patients with breast cancer as coresearchers.
Methods and analysis
The study complies with the six steps of the intervention mapping framework. Three non-academic hospitals recruit participants (patients with cancer, oncology healthcare providers and managers) for interviews about the organisation, experiences and needs regarding complementary medicine. To assess communication about complementary medicine, recorded oncology consultations are analysed. For an overview of evidence-based complementary medicine available to patients with cancer, a review of reviews is conducted on the evidence on cancer patient-reported outcomes of complementary medicine frequently used by patients with cancer, supplemented with an online search and survey among organisations and persons providing complementary medicine to patients with cancer. Together, these steps generate input for the development of a toolbox that supports an open and effective discussion on complementary medicine in oncology. In a pilot study, acceptability and usability of the toolbox are assessed among patients with cancer and oncology healthcare providers. Dissemination of the toolbox is covered by the commitment of stakeholder parties.
Ethics and dissemination
The Medical Ethics Committee Arnhem-Nijmegen declared the study was exempted from formal approval under the Dutch Medical Research Involving Human Subjects Act. The results will be disseminated through open-access, peer-reviewed publications, stakeholder-reporting and presentations at relevant conferences.
Keywords: complementary medicine, oncology, qualitative research, health services administration & management
Strengths and limitations of this study.
The COMMON study is the first to develop a toolbox for improving communication about complementary medicine for patients with cancer and oncology healthcare providers in the Netherlands.
The participation of patients with breast cancer as coresearchers enhances the validity and relevance of the findings.
The study data collection can be carried out entirely online and will not be delayed due to COVID-19 restrictions.
The study specifically involves patients with breast cancer, decreasing the generalisability of the results and the toolbox to the entire population of patients with cancer.
The success of the toolbox depends on the support from national stakeholders in the implementation process and on the willingness of healthcare providers and patients to use the toolbox.
Introduction
The use of complementary medicine among patients with cancer has become increasingly common over the last decades.1 Nowadays, approximately half of all oncology patients use complementary medicine alongside conventional cancer treatment.1–3 However, the topic of complementary medicine remains undiscussed in the majority of oncology consultations.4–6
Complementary medicine in oncology entails health approaches that are not typically part of conventional cancer treatments, but are used to complement them.7 8 This contrasts with alternative medicine replacing conventional cancer treatment. Together, they are often referred to as CAM (Complementary and Alternative Medicine). The current study focuses solely on complementary medicine and adopts a broad definition, encompassing all approaches that complement biomedical treatment of the oncological disease and that aim to contribute to the physical, mental or social well-being of the patient. The definition includes approaches that were previously considered complementary, but are now regularly incorporated in conventional supportive care (eg, exercise and psychological therapies).
Complementary medicine approaches frequently used by patients with cancer in Western countries are mind-body therapies, massage, nutrition counselling and acupuncture.9 Patients with cancer state several reasons for using complementary medicine, such as improving physical and emotional well-being, quality of life or reducing side effects from conventional treatment.10 In the field of conventional medicine, the added value of complementary medicine is still hotly debated. Complementary medicine is a heterogeneous field and for many approaches neither effectiveness nor safety have been sufficiently proven. Some supplements or herbs can have adverse effects or interact with conventional cancer treatment.11 12 On the other hand, a growing body of evidence shows that particular types of complementary medicine can be efficacious and safe to use for patients with cancer, such as acupressure for reducing chemotherapy-induced nausea and vomiting.13 A few complementary medicine approaches receive recommendation in oncology guidelines,14 implying sufficient evidence.
However, 20%–77% of the patients with cancer do not disclose their use of complementary medicine to conventional healthcare providers.15 In 2017, the Dutch Breast Cancer Association administered a survey among 750 members showing that 65% of the patients use complementary medicine. Of those patients, 29% did not discuss their complementary medicine use with their healthcare provider.16 A similar survey among 229 Dutch patients with haematological cancer reported that 43% of the respondents use complementary medicine, of which 38% did not discuss this with their healthcare provider.17 The main reasons for nondisclosure stated by patients with cancer are related to healthcare providers’ disapproval, disinterest, lack of inquiry or inability to provide information.15 Conventional healthcare providers often feel uncomfortable discussing complementary medicine due to limited education and knowledge on the topic.3 18 In view of the frequent use of complementary medicine among patients with cancer, not discussing the topic in oncology is problematic. It increases risks for patients’ exposure to misleading information, adverse effects due to inappropriate use and interactions with conventional cancer treatment.12 19 Additionally, some patients may remain devoid of evidence-based complementary medicine that could potentially support them.
It is well known that effective communication, the cornerstone of patient-centred care, can positively influence physical and psychosocial aspects of a patients’ health.20–22 An effective discussion about complementary medicine consists of exchanging adequate information, responding to emotional needs and managing uncertainty of patients.19 Furthermore, the healthcare provider can play a significant role in informed decision-making about complementary medicine use. In an open dialogue on the topic, both benefits and risks or the lack of evidence thereof, can be acknowledged.23 Open communication about complementary medicine fosters mutual trust between patients and healthcare providers and encourages patients to discuss their (interest in) complementary medicine use. Both patients and clinicians are found to report higher satisfaction with the oncology consultation when complementary medicine was discussed.6
It seems evident that communication about complementary medicine decreases risks and potentially maximises positive outcomes for patients with cancer. In some countries, tools have been developed to support oncology healthcare providers in discussing complementary medicine, such as educational courses or decision aids.24–27 A recent study reported on a complementary therapy education seminar for patients with cancer in Canada.28 These supporting tools cannot simply be generalised across countries, given differences in language, culture and healthcare systems. To the best of our knowledge, there are no nationwide tools available for healthcare providers or patients with cancer and there is no scientific literature reporting on communication about complementary medicine in oncology in the Netherlands.
Aim
This paper describes the design of this study titled COMMON, in which patients participate as coresearchers. The study aims to explore communication about complementary medicine in oncology and to enhance an open and effective dialogue on the topic by the development of a toolbox for patients with cancer and oncology healthcare providers. This project seeks to answer the following research questions:
What organisational and process factors hinder or contribute to communication and implementation of complementary medicine in oncology?
How is complementary medicine currently being discussed in consultations between healthcare providers and patients with cancer?
What are the experiences, needs and expectations regarding communication about and access to complementary medicine in patients with (breast) cancer and oncology healthcare providers?
What is the evidence on patient-reported outcomes on complementary medicine frequently used by patients with (breast) cancer?
What is the acceptability and usability of the developed toolbox?
Methods and analysis
Setting and participants
This patient participatory multicentre study is conducted in the Netherlands. Three non-academic hospitals with an oncology department have committed to recruit participants for the study. We deliberately selected hospitals that differ in the extent to which they implemented initiatives regarding complementary medicine in standard oncology care. This contributes to the diversity of the study participants and provides opportunities to learn from fellow hospitals’ experiences. The study specifically focuses on patients with breast cancer, the most commonly diagnosed cancer in women worldwide.29 Patients with breast cancer are found to be the most frequent users of complementary medicine compared with patients with other cancers.30 31 In total six categories of study participants are included (see table 1).
Table 1.
Participant categories
| Participant category | Recruitment | Data collection | Expected numbers |
| Patients with (breast) cancer | Participating hospitals | Interviews about needs | 16–20 |
| Pilot study toolbox | 90 | ||
| Healthcare providers | Participating hospitals | Interviews about organisation | 6–9 |
| Interviews about needs | 16–20 | ||
| Pilot study toolbox | 30 | ||
| Healthcare managers | Participating hospitals | Interviews about organisation | 6–9 |
| Complementary medicine providers | Researchers from Nivel | Online survey | Unknown |
| Patients with advanced breast cancer and their oncologist | Secondary analysis | Observation of consultations | 45 patients and 12 oncologists |
| Patients with cancer and their healthcare provider | Secondary analysis | Observation of consultations | 26 patients and 16 healthcare providers |
The first three participant categories are recruited by a designated nurse coordinator in each participating hospital:
Patients with (breast) cancer, currently or during the last 6 months in active treatment, older than 18 years of age, Dutch-speaking.
Healthcare providers working with patients with cancer, such as oncologists and oncology nurses.
-
Healthcare managers responsible for or connected to an oncology department.
The nurse coordinator in the hospital informs eligible participants about study aims and methods and provides them with a participant information letter. When a participant is interested in participating, the nurse coordinator asks for consent to share their contact details with the researcher team. Before study data collection, written informed consent is obtained.
The fourth category of participants is recruited by the research team from Nivel:
-
Persons and organisations providing complementary medicine to patients with cancer, recruited through professional organisations, stakeholder parties and researchers’ networks.
Data derived from two observational studies in 2018 is used for secondary analysis. Two categories of participants were recruited from different hospitals in the Netherlands:
Patients with incurable breast cancer, female, >18 years of age, with sufficient command of Dutch language, scheduled for a test-result consultation. Patients were approached by the participating hospital via phone and when interested by the research team. Information was sent by post. Written informed consent was obtained preceding the consultation.32
Patients with incurable cancer, >18 years of age, with sufficient command of Dutch, with limited health literacy (based on a vocational level education or lower and/or screening questions for health literacy and/or clinician’s views). Patients were approached by the hospital/research team by phone and when interested met by the research team, prior to the consultation when written consent was obtained.33
Patient and public involvement
Before submission, this research protocol was reviewed by the Patient Advocacy Group, a joint initiative of the Breast Cancer Research Group (BOOG) and National Breast Cancer Society (BVN) in the Netherlands. Furthermore, several other stakeholder parties in the Netherlands (Dutch Nursing Society (V&VN), Netherlands Comprehensive Cancer Organisation (IKNL) and the online information platform for Dutch patients with cancer (Kanker.nl) are involved since the beginning of the study to provide feedback on interim findings and advise on dissemination of results and output of the study.
Patients as coresearchers
For the cancer system to become more responsive to the needs of patients, involvement of patients with cancer in research is vital. As healthcare users with their own experiential knowledge, they can more easily extract relevant themes or interpret experiences from fellow patients. This unique patient perspective enhances the relevance of the research findings.34 Therefore, coresearchers prominently participate in the current study. We aim to recruit twelve Dutch-speaking patients aged at least 18 years that are diagnosed with breast cancer in the last 2 years with a fairly stable health and emotional situation. The recruitment of the coresearchers is performed by the nurse coordinator of each involved hospital and by means of an online advertisement on the website of the BVN.
In research, patients can provide input by means of (1) consultation, where patients are seen as objects of research (2) collaboration, where researchers and patients work in a partnership and make joint decisions (3) control, where patients have complete decision-making power.35 In this study, patients acting as coresearchers collaborate with the researchers. They are involved in formulating interview questions, conducting interviews, interpreting the research findings and designing the toolbox. To ensure the privacy of study participants, the coresearchers sign a confidentiality agreement. Two important conditions for participatory research were yielded by a previous multiple case study36: a good working environment and good collaboration. These conditions can be achieved by the organisation of training sessions, availability of the researcher, (financial) appreciation and a clear division of roles. In the current study, an introductory meeting and three half-day training sessions are organised at the start of the project to prepare the coresearchers for their role. A follow-up training is provided after 1 year, next to several evaluation moments during the study. One of the researchers (MM) is continuously available as contact person and keeps the coresearchers up to date by means of a quarterly newsletter. We aim to create an open, safe working environment in which coresearchers feel free to ask questions and discuss their needs. The coresearchers are reimbursed for their participation. The nature of participation as a coresearcher is voluntary and withdrawal is possible at any time.
Data collection and analysis
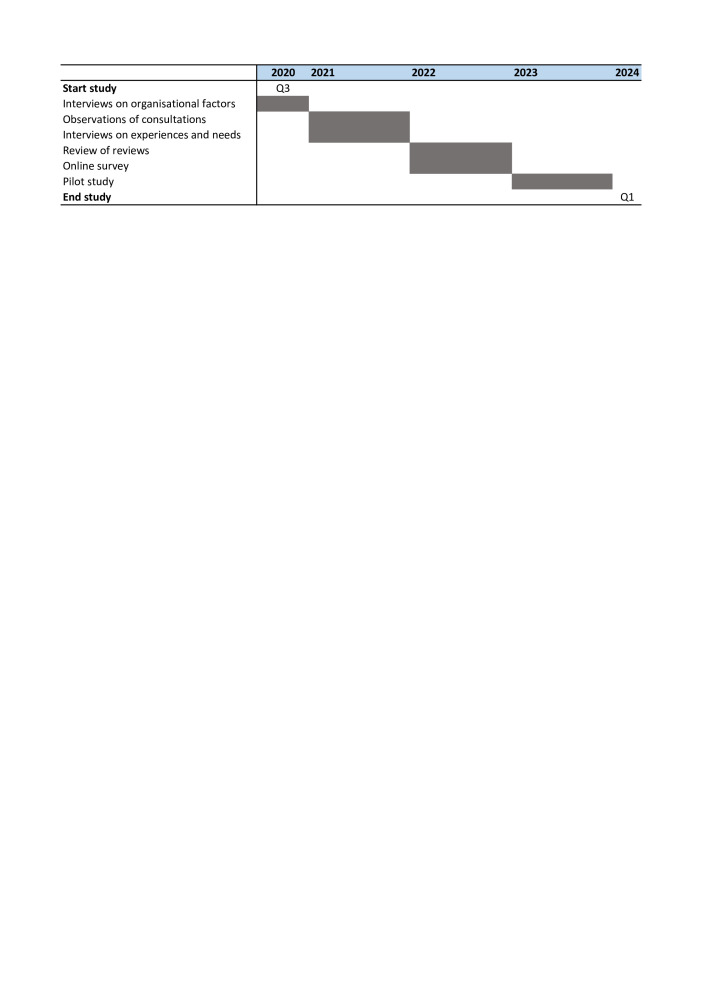
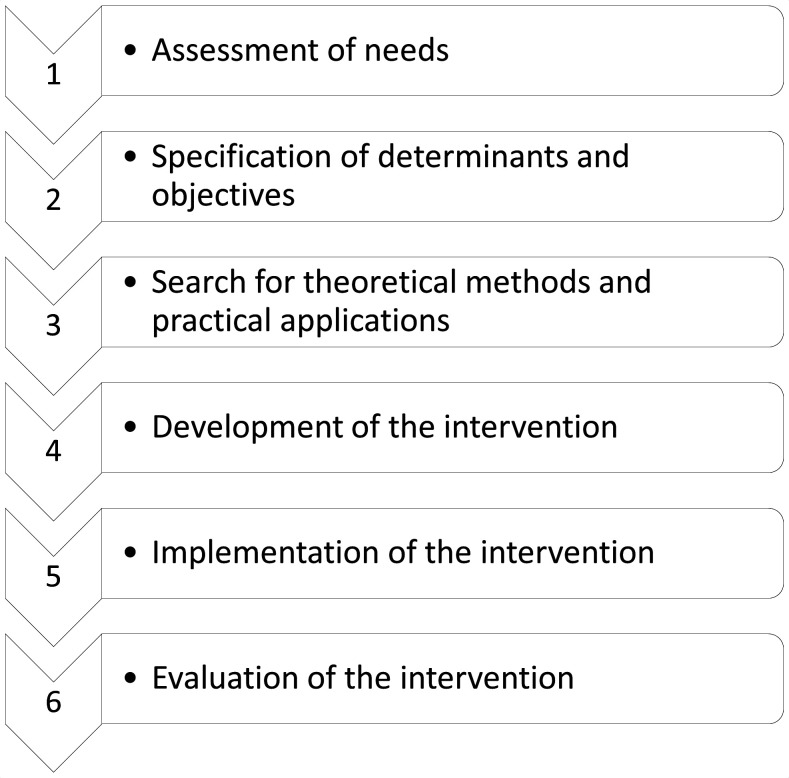
The data collection takes place during the period 2020–2024 (see figure 1). For development of the toolbox, the six steps of the intervention mapping (IM) framework are followed: (1) assessing the needs of the target group, (2) specifying the problem and its determinants into change objectives, (3) selecting theoretical intervention methods and practical applications for change, (4) designing and developing the intervention, (5) implementing the intervention and (6) evaluating the intervention37 (see figure 2). The IM framework supports health promotion programme planners in systematically developing an evidence-based intervention. Several effective interventions in oncology have been developed using the IM framework.38
Figure 1.
Time frame of the study titled ‘COMMON’.
Figure 2.
The six steps of the intervention mapping framework.
Step 1: needs assessment
In this first step, the needs of the target group regarding communication and access to complementary medicine are assessed to specify the goals for the intervention. The needs assessment of this study consists of interviewing healthcare managers and healthcare providers in oncology and observing previously recorded consultations32 33 between patients with cancer and healthcare providers.
Interviews on organisational factors
By means of semistructured interviews with healthcare managers and healthcare providers working in oncology, insight is sought in the organisational factors that hinder or facilitate communication and implementation of complementary medicine in oncology (research question 1). The interview guide will be developed in close collaboration with the coresearchers. Data saturation39 is expected to be achieved after interviewing 2–3 healthcare managers and 2–3 healthcare providers in each participating hospital (12–18 interviews in total). Each interview is conducted by one of the researchers together with a coresearcher, preceded by a one-on-one meeting to prepare the coresearcher for the interview. The interviews have a planned duration of 45 min and are held at a time and location convenient for the interviewees (online interviews are an option given COVID-19 restrictions). With permission of the participants, the interviews are audiorecorded. The recordings are transcribed verbatim and coded thematically using MAXQDA. For the analysis of the interview data, we make use of a framework on implementation of innovations in healthcare40 and an indicative method for identifying, analysing and reporting patterns within data.41
Observation of consultations
By secondary analysis of recorded consultations from two previous observational studies, we observe how complementary medicine is currently discussed in oncology consultations between patients and healthcare providers (research question 2). In the first study, consultations of 45 patients with incurable breast cancer and 12 oncologists in two hospitals were audio-recorded between August and December 2018. Postconsultation, the participants completed a self-created questionnaire on sociodemographic and disease characteristics.32 42 43 Given the fact that curative treatment was not a topic of discussion, complementary medicine is expected to be discussed relatively often, like previously found in an Australian study.5 In the second study, consultations between 26 patients with incurable cancer and 16 healthcare providers (medical specialists and nurses) were videorecorded between April and October 2018. Preconsultation, background characteristics were assessed by the research team.33
An observation scheme to code communication about complementary medicine during the consultations will be developed based on insights from literature on categorisations of complementary medicine and guidelines on grading of complementary medicine interventions.44–46 Using the observation scheme, one observer codes all recordings. For inter-rater agreement, a second observer independently codes 10% of the recordings and discrepancies will be discussed among the observers until consensus is reached. Descriptive statistics (Stata V.14.0) are employed for analysing the data.
Interviews on experiences and needs
We investigate experiences and needs (eg, timing in treatment programme) regarding communication about and access to complementary medicine by conducting semistructured interviews with patients with breast cancer and healthcare providers (research question 3). Interview questions are formulated in close collaboration with the coresearchers. The recommendations of Francis et al47 for sample size in qualitative studies are followed. The initial analysis sample consists of 16 interviews with patients with breast cancer and the same number of healthcare providers. The stopping criterion is three consecutive interviews without additional emerging themes in each of the two groups. The interviews last approximately 45 min and are held at a time and location convenient for the interviewees (online interviews are a possibility given COVID-19 restrictions). The interviews are conducted by one of the researchers together with one coresearcher. After permission, the interviews are audiorecorded. The audiorecordings are transcribed verbatim and then thematically coded using MAXQDA.
Steps 2 and 3: specifying determinants, objectives, theoretical methods and practical application
The goal of step 2 is specifying what or who changes as a result of the toolbox. By analysing the input from the needs assessment in step 1, behavioural and environmental determinants that are changeable are translated into a list of intervention objectives. In step 3, we seek theory-based methods and practical applications to change the determinants of behaviour and environment to meet the intervention objectives. The stakeholder’s parties will be involved in the selection of strategies. The main criterion is the possibility to integrate the toolbox in daily clinical practice without interfering in conventional cancer treatment.
Step 4: development of the intervention
The goal of this step is to develop the toolbox supporting patients with cancer and oncology healthcare providers in discussing complementary medicine. The coresearchers are involved in designing the structure and lay-out of the toolbox. The content is based on the information gathered in steps 1–3. The toolbox will at least consist of a communication guideline to support patients and healthcare providers in discussing complementary medicine, supplemented with a list of available evidence-based complementary medicine frequently used by patients with cancer in the Netherlands. This list will be based on a review of systematic reviews on the evidence of complementary medicine on patient-reported outcomes and an online survey among persons and organisations providing complementary medicine.
Review of reviews
A review of reviews is conducted on the evidence on patient-reported outcomes (eg, quality of life, coping skills, general well-being, perceived psychological and physical symptoms) of complementary medicine that is frequently used by patients with cancer (research question 4). The search on review studies is initially developed in PubMed/MEDLINE and adapted to other databases such as Cochrane library, PsycINFO, CINAHL and Embase. For this search, we make use of medical subject heading terms and/or keywords frequently used in literature on complementary medicine. The search is limited to systematic reviews and meta-analyses published after 2000, written in English. Two researchers will independently screen titles and abstracts. Subsequently, a full-text screening is conducted by two researchers. In case of inconsistencies between the two researchers, consensus will be reached by discussion. If necessary, a third researcher can be involved. For inclusion in the review of reviews, articles should meet the criteria of having a methods section that describes a search strategy and an a priori approach to synthesising the data. Then, methodological quality is assessed by means of quality criteria adapted from the Quality of Reporting of Meta-analyses (QUOROM)48 and the Assessment of Multiple Systematic Reviews.49 A comprehensive, detailed protocol of the review of reviews will be registered in PROSPERO.
Online survey
To gain an overview on what types of evidence-based complementary medicine are currently available in the Netherlands, an online search accompanied by an online survey is performed among persons and organisations providing complementary medicine approaches that are widely used by patients with cancer (research question 4). Survey questions are formulated in close collaboration with the coresearchers, but minimally covers what kind of complementary medicine is being offered, to whom, with what goal and what the outcomes are.
Step 5: implementation of the intervention
Planning the implementation of the toolbox begins as soon as the needs assessment has been performed and is continuously revisited during the study by the involvement of coresearchers and the stakeholder parties.
Pilot study
Following the development of the toolbox, we conduct a pilot study for which 90 patients with cancer (half of them complementary medicine users) and 30 oncology healthcare providers are recruited from the three hospitals. If relevant, informal caregivers are also recruited. As recommended by the Medical Research Council guidance,50 the exact selection of instruments and dimensions evaluated in the pilot study will be adjusted to the eventual design of the toolbox. The toolbox will be at least assessed on acceptability, usability, layout and transparency using descriptive statistics. The coresearchers are involved in the design of the pilot study. No risks are expected to be associated with participation in the pilot study, because the toolbox will not interfere with conventional cancer treatment.
The results of the pilot study are used to optimise the toolbox before shifting to the phase where it is made publicly available. We plan to organise an invitational conference to guarantee proper use and uptake of the toolbox and to report on its development and evaluation. Dissemination in the intended population is covered by the commitment of the stakeholder parties. Naturally, the content of the toolbox changes as new insights into effectiveness and safety of complementary medicine becomes available. To ensure continuation of the intervention, budget is reserved for biennial updates of the toolbox for at least 4 years after dissemination.
Step 6: evaluation of the intervention
As described in step 5, a preliminary evaluation of the toolbox by means of a pilot study will take place. In the future, we intend to recruit funding for an additional, larger trial to measure the impact of the toolbox on aspects of communication about complementary medicine (eg, initiation, satisfaction) between healthcare providers and patients with cancer. A description of the trial design for an effect evaluation of the toolbox is beyond the scope of the current protocol paper that describes the development of the toolbox.
In conclusion
To the best of our knowledge, this is the first patient participatory study that aims to explore and enhance communication about complementary medicine in oncology. Despite the frequent use of complementary medicine among patients with cancer, the topic often remains undiscussed in consultation with the healthcare provider.4–6 This results in risks for adverse or interaction effects and decreases access to evidence-based complementary medicine for patients with cancer. To date, it is unknown to what extent complementary medicine is discussed and implemented in oncology in the Netherlands and what the experiences and needs of patients with cancer and oncology healthcare providers are in this area. This study will fill these information gaps by conducting interviews, observations, a survey and a review of reviews on complementary medicine in oncology. Perspectives of patients with cancer, oncology healthcare providers, healthcare managers and complementary medicine providers are included. The collected data are used for the systematic development37 of an unique toolbox supporting the dialogue on complementary medicine in oncology. Preceded by a pilot-test, the toolbox is disseminated with the support of national stakeholders to the intended population: patients with cancer and oncology healthcare providers. The toolbox aims to provide (1) tips and tricks on how to conduct an open and effective discussion about the use of complementary medicine in oncology and (2) evidence-based complementary medicine interventions which patients with cancer can use safely alongside their conventional cancer treatment. Thereby, we want to minimise the risks and maximise the benefits of evidence-based complementary medicine for patients with cancer.
Ethics and dissemination
The Medical Ethics Committee Arnhem-Nijmegen declared the study was exempted from formal approval under the Dutch Medical Research Involving Human Subjects Act (case number 2020-6917). No risks are expected to be associated with participation in the study. Results of this study will be disseminated through open-access, peer-reviewed publications, stakeholder-reporting and presentations at relevant conferences.
Supplementary Material
Footnotes
Contributors: All authors contributed to, reviewed and approved the article drafts and final manuscript. SvD, JN, MB and LVV designed the study protocol and raised funding, led by SvD. MM was responsible for writing the manuscript. SvD, JN and JT-B read several versions of the manuscript and provided their feedback and suggestions regularly.
Funding: This work was supported by the Dutch Cancer Society grant number 12 566.
Disclaimer: The funding organisation played no role in designing the study or writing the manuscript for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Horneber M, Bueschel G, Dennert G, et al. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther 2012;11:187–203. 10.1177/1534735411423920 [DOI] [PubMed] [Google Scholar]
- 2.Keene MR, Heslop IM, Sabesan SS, et al. Complementary and alternative medicine use in cancer: a systematic review. Complement Ther Clin Pract 2019;35:33–47. 10.1016/j.ctcp.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 3.King N, Balneaves LG, Levin GT, et al. Surveys of cancer patients and cancer health care providers regarding complementary therapy use, communication, and information needs. Integr Cancer Ther 2015;14:515–24. 10.1177/1534735415589984 [DOI] [PubMed] [Google Scholar]
- 4.Juraskova I, Hegedus L, Butow P, et al. Discussing complementary therapy use with early-stage breast cancer patients: exploring the communication gap. Integr Cancer Ther 2010;9:168–76. 10.1177/1534735410365712 [DOI] [PubMed] [Google Scholar]
- 5.Schofield PE, Juraskova I, Butow PN. How oncologists discuss complementary therapy use with their patients: an audio-tape audit. Support Care Cancer 2003;11:348–55. 10.1007/s00520-002-0420-x [DOI] [PubMed] [Google Scholar]
- 6.Roter DL, Yost KJ, O'Byrne T, et al. Communication predictors and consequences of complementary and alternative medicine (cam) discussions in oncology visits. Patient Educ Couns 2016;99:1519–25. 10.1016/j.pec.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West HJ. Complementary and alternative medicine in cancer care. JAMA Oncol 2018;4:139. 10.1001/jamaoncol.2017.3120 [DOI] [PubMed] [Google Scholar]
- 8.National Center for Complementary and Integrative Health . Complementary, Alternative, or Integrative Health: What’s In a Name? 2018. Available: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name [Accessed 21 Jan 2021].
- 9.Seely DM, Weeks LC, Young S. A systematic review of integrative oncology programs. Curr Oncol 2012;19:436–61. 10.3747/co.19.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Callaghan V. Patients' perceptions of complementary and alternative medicine. Cancer Forum 2011;35:44–7. [Google Scholar]
- 11.Ambrosone CB, Zirpoli GR, Hutson AD, et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J Clin Oncol 2020;38:804–14. 10.1200/JCO.19.01203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee RT, Kwon N, Wu J, et al. Prevalence of potential interactions of medications, including herbs and supplements, before, during, and after chemotherapy in patients with breast and prostate cancer. Cancer 2021;127:1827–35. 10.1002/cncr.33324 [DOI] [PubMed] [Google Scholar]
- 13.Dibble SL, Luce J, Cooper BA, et al. Acupressure for chemotherapy-induced nausea and vomiting: a randomized clinical trial. Oncol Nurs Forum 2007;34:1–8. 10.1188/07.ONF.813-820 [DOI] [PubMed] [Google Scholar]
- 14.Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol 2018;36:2647–55. 10.1200/JCO.2018.79.2721 [DOI] [PubMed] [Google Scholar]
- 15.Davis EL, Oh B, Butow PN, et al. Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: a systematic review. Oncologist 2012;17:1475–81. 10.1634/theoncologist.2012-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borstkanker Vereniging Nederland . Maak(te) jij gebruik van 'complementaire zorg'? 2017. Available: https://bforce.nl/maakte-jij-gebruik-van-complementaire-zorg [Accessed 21 Jan 2021].
- 17.Hematon . Grote groep patiënten maakt gebruikt van complementaire zorg, 2018. Available: https://www.hematon.nl/nieuwsberichten/grote+groep+patienten+maakt+gebruik+van+complementaire+zorg [Accessed 1 Mar 2021].
- 18.Corbin Winslow L, Shapiro H. Physicians want education about complementary and alternative medicine to enhance communication with their patients. Arch Intern Med 2002;162:1176–81. 10.1001/archinte.162.10.1176 [DOI] [PubMed] [Google Scholar]
- 19.Frenkel M, Ben-Arye E, Cohen L. Communication in cancer care: discussing complementary and alternative medicine. Integr Cancer Ther 2010;9:177–85. 10.1177/1534735410363706 [DOI] [PubMed] [Google Scholar]
- 20.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ 1995;152:1423–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Street RL, Makoul G, Arora NK, et al. How does communication heal? pathways linking clinician-patient communication to health outcomes. Patient Educ Couns 2009;74:295–301. 10.1016/j.pec.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 22.Epstein RM, Street Jr RL. Patient-centered communication in cancer care: promoting healing and reducing suffering. Bethesda, MD: National Institutes of Health, 2007: 1–17. [Google Scholar]
- 23.Evans M, Shaw A, Thompson EA, et al. Decisions to use complementary and alternative medicine (cam) by male cancer patients: information-seeking roles and types of evidence used. BMC Complement Altern Med 2007;7:25. 10.1186/1472-6882-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balneaves LG, Truant TLO, Verhoef MJ, et al. The complementary medicine education and outcomes (CAMEO) program: a foundation for patient and health professional education and decision support programs. Patient Educ Couns 2012;89:461–6. 10.1016/j.pec.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 25.Ben-Arye E, Frenkel M, Bar-Sela G, et al. Teaching complementary medicine at an academic oncology department. J Cancer Educ 2008;23:46–50. 10.1080/08858190701821261 [DOI] [PubMed] [Google Scholar]
- 26.Witt CM, Helmer SM, Schofield P, et al. Training oncology physicians to advise their patients on complementary and integrative medicine: an implementation study for a manual-guided consultation. Cancer 2020;126:3031–41. 10.1002/cncr.32823 [DOI] [PubMed] [Google Scholar]
- 27.Chong W-Q, Mogro MJ, Arsad A, et al. Use of decision aid to improve informed decision-making and communication with physicians on the use of oral complementary and alternative medicine (cam) among cancer patients on chemotherapy treatment: a randomised controlled trial. Support Care Cancer 2021;29:3689–96. 10.1007/s00520-020-05872-5 [DOI] [PubMed] [Google Scholar]
- 28.Allen Searson N, Balneaves LG, Thorne SE, et al. The effect of a complementary therapy education seminar on support persons of individuals with cancer. J Altern Complement Med 2021;27:365–72. 10.1089/acm.2020.0443 [DOI] [PubMed] [Google Scholar]
- 29.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 30.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol 2008;26:665–73. 10.1200/JCO.2007.13.5905 [DOI] [PubMed] [Google Scholar]
- 31.Morris KT, Johnson N, Homer L, et al. A comparison of complementary therapy use between breast cancer patients and patients with other primary tumor sites. Am J Surg 2000;179:407–11. 10.1016/S0002-9610(00)00358-5 [DOI] [PubMed] [Google Scholar]
- 32.van Vliet LM, Francke AL, Meijers MC, et al. The use of expectancy and empathy when communicating with patients with advanced breast cancer; an observational study of Clinician-Patient consultations. Front Psychiatry 2019;10:464. 10.3389/fpsyt.2019.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noordman J, Schulze L, Roodbeen R, et al. Instrumental and affective communication with patients with limited health literacy in the palliative phase of cancer or COPD. BMC Palliat Care 2020;19:152. 10.1186/s12904-020-00658-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright D, Corner J, Hopkinson J, et al. Listening to the views of people affected by cancer about cancer research: an example of participatory research in setting the cancer research agenda. Health Expect 2006;9:3–12. 10.1111/j.1369-7625.2006.00353.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.INVOLVE . Briefing note seven: approaches to public involvement in research, 2012. Available: http://www.invo.org.uk/posttyperesource/ [Accessed 19 Nov 2020].
- 36.Scheffelaar A, Bos N, de Jong M, et al. Lessons learned from participatory research to enhance client participation in long-term care research: a multiple case study. Res Involv Engagem 2020;6:27. 10.1186/s40900-020-00187-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartholomew L, Parcel G, Kok G. Planning health promotion program: an intervention mapping approach. San Francisco, CA: Jossey-Bass, 2011. [Google Scholar]
- 38.Lamort-Bouché M, Sarnin P, Kok G, et al. Interventions developed with the intervention mapping protocol in the field of cancer: a systematic review. Psychooncology 2018;27:1138–49. 10.1002/pon.4611 [DOI] [PubMed] [Google Scholar]
- 39.Given LM. 100 questions (and answers) about qualitative research. thousand oaks. CA: SAGE Publications, 2015. [Google Scholar]
- 40.Herzlinger RE. Why innovation in health care is so hard. Harv Bus Rev 2006;84:58–66. [PubMed] [Google Scholar]
- 41.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 42.Westendorp J, Stouthard J, Meijers MC, et al. The power of clinician-expressed empathy to increase information recall in advanced breast cancer care: an observational study in clinical care, exploring the mediating role of anxiety. Patient Educ Couns 2021;104:1109–15. 10.1016/j.pec.2020.10.025 [DOI] [PubMed] [Google Scholar]
- 43.Hoffstädt H, Stouthard J, Meijers MC, et al. Patients' and clinicians' perceptions of Clinician-Expressed empathy in advanced cancer consultations and associations with patient outcomes. Palliat Med Rep 2020;1:76–83. 10.1089/pmr.2020.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng GE, Rausch SM, Jones LW, et al. Complementary therapies and integrative medicine in lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of chest physicians evidence-based clinical practice guidelines. Chest 2013;143:e420S–36. 10.1378/chest.12-2364 [DOI] [PubMed] [Google Scholar]
- 45.Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin 2017;67:194–232. 10.3322/caac.21397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng GE, Frenkel M, Cohen L, et al. Evidence-based clinical practice guidelines for integrative oncology: complementary therapies and botanicals. J Soc Integr Oncol 2009;7:85–120. [PubMed] [Google Scholar]
- 47.Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25:1229–45. 10.1080/08870440903194015 [DOI] [PubMed] [Google Scholar]
- 48.Moher D, Cook DJ, Eastwood S, et al. [Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM Statement]. Rev Esp Salud Publica 2000;74:107–18. [PubMed] [Google Scholar]
- 49.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10. 10.1186/1471-2288-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical Research Council guidance. Int J Nurs Stud 2013;50:587–92. 10.1016/j.ijnurstu.2012.09.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.