Abstract
Aim
To provide a contemporary analysis of incidence trends of infective endocarditis (IE) with its changing epidemiology over the past two decades in Europe.
Methods
A systematic review was conducted at the Mayo Clinic, Rochester. Ovid EBM Reviews, Ovid Embase, Ovid Medline, Scopus and Web of Science were searched for studies published between 1 January 2000 and 30 November 2020. All studies were independently reviewed by four referees and those that included a population-based incidence of IE in patients, irrespective of age, in Europe were included. Least squares regression was used to estimate pooled temporal trends in IE incidence.
Results
Of 9138 articles screened, 18 studies were included in the review. Elderly men predominated in all studies. IE incidence increased 4.1% per year (95% CI 1.8% to 6.4%) in the pooled regression analysis of eight studies that included comprehensive and consistent trends data. When trends data were weighted according to population size of individual countries, an increase in yearly incidence of 0.27 cases per 100 000 people was observed. Staphylococci and streptococci were the most common pathogens identified. The rate of surgical intervention ranged from 10.2% to 60.0%, and the rate of inpatient mortality ranged from 14.3% to 17.5%. In six studies that examined the rate of injection drug use, five of them reported a rate of less than 10%.
Conclusion
Based on findings from our systematic review, IE incidence in Europe has doubled over the past two decades in Europe. Multiple factors are likely responsible for this striking increase.
Trial registeration number
CRD42020191196.
Keywords: endocarditis, systematic reviews as topic, epidemiology
Key questions.
What is already known about this subject?
Infective endocarditis (IE) is an uncommon life-threatening infection that, despite aggressive medical and surgical interventions, remains associated with high morbidity and mortality. Recent studies, however, have reported mixed results regarding temporal changes in trends of IE incidence.
What does this study add?
This study pooled data from all nationwide population-based registries in Europe and demonstrated an alarming increase in IE incidence over the past two decades.
How might this impact on clinical practice?
Our findings alert clinicians as to the increasing incidence of IE and the need to include it in the differential diagnosis of patients who present with systemic complaints of infection. The study also highlights the inefficiency in coding practices, which can compromise accurate IE incidence trends determinations.
Introduction
Infective endocarditis (IE) is one of the most lethal infection syndromes. Despite almost universal hospitalisation for initial treatment and the availability of multiple recent advances in diagnosis and management, it is characterised by a 1-year mortality rate that exceeds 30%.1 Due to the rarity of the syndrome, both the management of, and investigation into the disease can be difficult. For the former, an IE diagnosis may be delayed, and treatment may be suboptimal resulting in worse outcomes. For the latter, generation of clinical trial data with adequate enrolment of patients can be challenging.
Fortunately, multiple European countries systematically record nationwide hospital admissions data and code the reasons for those admissions, which permit population-based investigations of the epidemiology of diseases.2–4 Incidence trends, in particular, have been of keen interest and have been prompted, in part, by changes in the 2008 National Institute for Health and Care Excellence (NICE) guidelines5 and the 2009 European Society of Cardiology (ESC) guidelines,6 that either called for a total elimination of antibiotic prophylaxis (AP) use in the dental and other settings (NICE) or a marked reduction in this practice (ESC). As a result, concerns that an increase in IE incidence due to viridans group streptococci (VGS) would occur following publication of these recommendations prompted investigations of nationwide data.
There are several other factors including ageing populations, increased comorbid conditions, increased placement of medical devices and injection drug use (IDU) that impact IE incidence trends and epidemiology. These factors have been addressed, to some degree, in most of the European national investigations.
Based on the availability of trend data of IE incidence and epidemiology from several countries in Europe, we conducted a contemporary systematic review of European population-based investigations of IE during the 21st century.
Methods
A literature search was performed in December 2020 with a focus on the incidence and epidemiology of IE using Ovid EBM Reviews, Ovid Embase, Ovid Medline, Scopus and Web of Science to identify articles published between 1 January 2000 and 30 November 2020. The search was limited to the English language and search strategies are outlined in online supplemental material. All results were exported to Endnote where duplicates were deleted. Two authors (KMT and LMB) performed the literature review and any disagreements were solved by discussion with two authors (MJD and DCD). Corresponding authors of studies were contacted via email in cases where queries existed.
openhrt-2021-001846supp001.pdf (135.9KB, pdf)
Inclusion and exclusion criteria
All publications that provided information on population-based trends of IE in European populations from the year 2000 onwards were included in the review. Single-centre and multicentre studies, clinical trials, case reports, abstracts, systematic reviews and animal studies were excluded. Investigations that examined IE incidence specific to infecting pathogens or unique patient populations (eg, HIV, congenital heart disease) were excluded. Studies that included data from the COVID-19 pandemic period (January 2020 onwards) and those that reported data for less than 6 months each year for two or more years were excluded.
Data extraction
Data that described authors, publication year, study location, population covered, mean/median age, sex distribution, incidence, microbiology, mortality, IDU and rates of surgical valvular intervention were extracted from all studies. Four authors (MD, KMT, VA and WT) worked independently to extract data from studies and contacted study investigators if additional data was required.
Study definition and outcomes
The primary outcome was the trend of IE incidence; secondary outcomes included temporal trends of pathogen prevalence, age, sex, prosthetic valve placement, IDU and mortality (in-patient, 30 days, 6 months and 1 year).
Risk of bias
Two reviewers (KMT and WT) independently rated the methodological quality of each study. The quality of each population-based survey was assessed, based on four key features: adequacy of population definition, sampling techniques, disease definition and completeness of case ascertainment (online supplemental table 1).7 A population definition was deemed to be inadequate if the residency status of all IE patients was not ascertained. Since all studies used population data from national or regional registries, the population definition was deemed adequate for all studies. Optimal sampling techniques included complete enumeration or random sampling techniques. Disease definition was defined as adequate if studies used Duke/modified Duke criteria for a diagnosis of IE. Adequacy of case ascertainment to include all cases in a given country was assessed based on case-finding procedures, inclusion of postmortem diagnoses and number of hospitals serving the population under study that participated in the study. Author statements about shortfall in case ascertainment were also considered an indication of inadequate case ascertainment. Based on these criteria, studies were excluded that had considerable shortfalls in case ascertainment and/or lacked a case definition. Reviewer disagreements were resolved by consensus after rereview of the article.
The study was registered with the International Prospective Register of Systematic Reviews, which is an international database of prospectively registered systematic reviews in health and social care (Registration ID: CRD42020191196).8
Statistical analysis
Studies included in the trend analysis had cases from more than two time points, at least 100 observed cases in each time period, and estimates of population size from which cases were observed. For each time point within a study, the incidence per 100 000 population was calculated by taking the observed cases divided by the population estimate multiplied by 100 000. Least squares regression was done on both the incidence per 100 000 and the log transformed incidence per 100 000. Interpretation of the regression results for the untransformed incidence yields an annual increase in cases per 100 000 while interpretation of the log transformed incidence per 100 000 resulted in an estimate of yearly percent increase in incidence rates. SEs and 95% confidence limits were calculated.
The pooled regression estimate from the primary analysis to the simple unweighted average of the individual regression estimates were compared and were similar. This suggested that the pooled regression was a reasonable estimate of the overall trend.
Patient and public involvement
No patients or public were involved at any stage of the synthesis of this study.
Results
Study selection
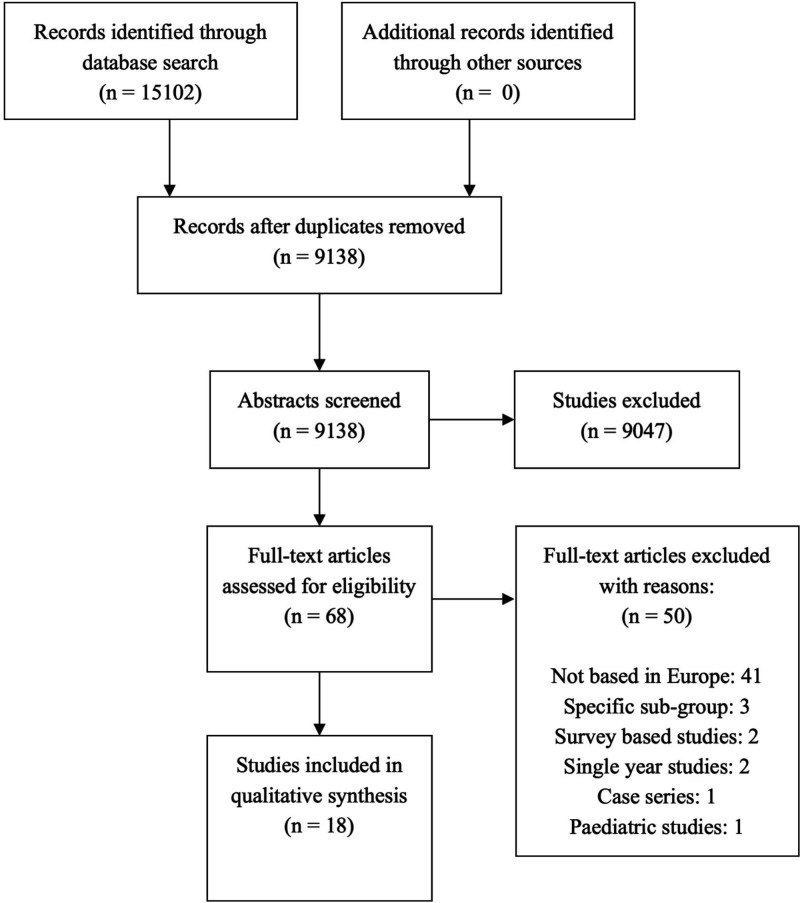
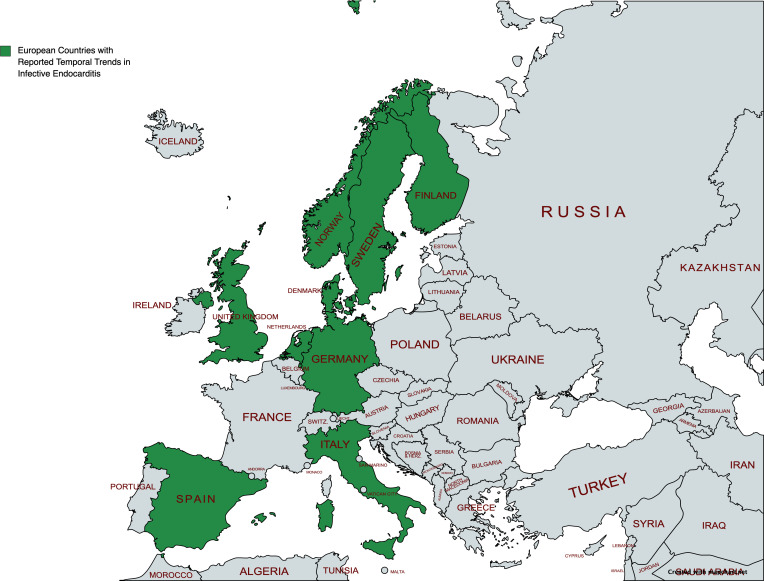
A total of 9138 studies were identified from the search engines after deduplication. The study abstracts were screened, and 91 were identified for full text review. Eighteen studies met our inclusion criteria. A schematic representation of studies included using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines9 is included in figure 1. England had the highest number of studies included (4); the remainder of geographical distribution of included studies is illustrated in figure 2. A detailed profile of studies is presented in table 1. A total of 15 studies defined IE using a primary or secondary diagnosis that was based on International Classification of Diseases (ICD), ninth revision (ICD 9) and tenth revision (ICD 10) (table 2).
Figure 1.
Schematic representation of study selection using PRISMA checklist. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Figure 2.
Visual representation of European countries included in the systematic review.
Table 1.
Clinical features of patient populations in included studies
| # | Author | Total cases (N) | Years included | Country | AGE | Male sex % | Microbiology (%) | Prosthetic valve % | Cardiovascular device % | INJECTION DRUG USE % | Mortality % | Required surgery % |
| 1 | Scudeller 200918 | 254 | 2004–2008 | Italy | Mean: 67.0 (SD 14.0) | 66.5 | Enterococci 21.6 S. aureus 17.5 VGS 14.0 |
32.3 | NP | 2.0 | 3 months 20.5 | 40.2 |
| 2 | Fedeli 201126 | 1863 | 2000–2008 | Italy | Median 68.0 (IQR: 57.0–77.0) | 63.0 | Blood culture data S. aureus 29.5 CoNS 6.7 Enterococcus faecalis 12.4 Streptococci 28.6 Secondary code data Streptococci 36.7 S. aureus 17.4 CoNS 6.0 E. faecalis 5.3 |
6.2 | NP | NP | Overall 14.3 | In-patient 37.0 1 year 38.0 |
| 3 | Thornhill 201114 | 2000–2010 | England | NP | NP | NP | NP | NP | NP | NP | NP | |
| 4 | Ternhag 201316 | 7609 | 1997–2007 | Sweden | Mean 65.7 (IQR: 55–79) | 59.2 | NP | 11.7 | NP | 4.7 | 30 days—33.7 5 years—14.7 |
5 years follow-up 13.0 |
| 5 | Dayer 20152 | 19 804 | 2000–2013 | England | Mean 59.1 59.0 (SD: 20.3) before 2008, 59.3 (SD 20.8) after 2008 |
68.5 | NP | NP | NP | NP | NP | NP |
| 6 | Cresti 20161 | 170 | 1998–2014 | Italy | Mean 65.7(SD: 16.0) | 60.9 | S. aureus 25.0 CoNS 22.0 Streptococcus viridans 15.0 Enterococci 14.0 |
30.0 | NP | 4.0 | 1 year 31.8 In-patient 24.0 | 1 year 46.5; Urgent/emergent 31.8; Operated within 10 days 29.0 |
| 7 | Erichsen 201624 | 5486 | 1994–2011 | Denmark | Mean: 62.7 (no SD provided) | 64.4 | NP | NP | NP | NP | NP | NP |
| 8 | Keller 20163 | 94 364 | 2005–2014 | Germany | NP | NP | Streptococci 20.8 Staphylococci 21.9 |
NP | NP | NP | Overall 17.0 | NP |
| 9 | van den Brink 201631 | 5213 | 2005–2011 | The Netherlands | Mean 67.5 (range: 22.0–97.0) | 69.9. | Staphylococci 36.1 S. aureus 30.1 Streptococci 37.4 |
30.1 | 7.9 | NP | All-cause 36.1% (Median follow-up 4.2 years) | 38.9 |
| 10 | Olmos 201717 | 16 867 | 2003–2014 | Spain | Mean 63.8 (SD: 17.5) | 66.3 | Streptococci 20.4 S. aureus 17.1 Enterococci 13.1 CoNS 12.2 |
18.0 | 1.1 | 2.6 | In-patient 20.4 | 23.0 |
| 11 | Ahtela 201811 | 2611 | 2005–2014 | Finland | Mean 60.0 (SD: 18.3) | 68.2 | NP | 5.5 | 1.5 | NP | 30 days 11.3 | NP |
| 12 | Jordal 201812 | 706 | 1996–2015 | Norway | Mean 59.2 (need to calculate combined SD) | 69.1 |
S. aureus 31.1 VGS 23.1 CoNS 11.5 Enterococci 9.5 |
29.9 | NP | 20.7 | 30 days 12.7 1 year 21.4 |
34.1 |
| 13 | Ortega 201923 | 25 952 | 1997–2014 | Spain | Overall mean: 62.2 (18.6) | 65.9 | Staphylococci 32.7 S. aureus 19.6 Streptococci 2.5 |
9.6 | 6.5; CIED related IE 21.2 | 6.8 | 90 days 26.2 | NP |
| 14 | Jensen 20204 | 7669 | 1997–2017 | Denmark | Median 70.2 (IQR: 58.3–78.8) | 65.2 | NP | 15.9 | 10.9 | NP | NP | NP |
| 15 | Quan 202025 | 1998–2017 | England | NP | NP | Streptococci 49.0 Staphylococci 43.0 |
NP | NP | NP | NP | NP | |
| 16 | Shah 202013 | 7638 | 1990–2014 | Scotland | Mean 65.0 (SD: 17.0) | 49.0 | Staphylococci 42.4 S. aureus 31.7 Streptococci 35.5 Enterococci 8.9 |
NP | NP | NP | 1 year 32.0 30 days 14.7% |
30 days 4.8 1 year 10.6 |
| 17 | Thornhill 202015 | 1998–2018 | England | NP | NP | NP | NP | NP | NP | NP | NP | |
| 18 | Vähäsarja10 | 4647 | 2008–2017 | Sweden | Mean 64.9 (range: 17.0–100.0) | 68.0 |
S. aureus 37.0 VGS 24.7% |
NP | NP | NP | NP | In-patient 10.2 |
*The genus and species of the pathogens have been listed as presented in the individual studies. Since the pathogens were grouped differently in each study, it was not possible for us to standardise them.
CIED, cardiac implantable electronic device; CoNS, coagulase-negative staphylococci; IE, infective endocarditis; NP, not provided; VGS, viridans group streptococci.
Table 2.
List of ICD codes used in included studies
| Study | ICD codes | Primary/secondary/tertiary |
| Scudeller 200918 | No ICD code data available in study | – |
| Fedeli 201126 | ICD 9: 421, 98.84 or 112.81 | P+S |
| Thornhill 201114 | ICD 10: I33 | P+S |
| Ternhag 201316 | ICD 10: I33, I38 or I39 | Unspecified |
| Dayer 20152 | ICD 10: I33 | P+S |
| Cresti 20161 | ICD 9: 421 .x | P+S |
| Erichsen 201624 | ICD 10: I33 or I38.9 | Unspecified (first-time code of incident IE recorded) |
| Keller 20163 | ICD 10: I33 | Unspecified |
| van den Brink 201631 | Insurance-based codes used for case extraction | – |
| Olmos 201717 | ICD 9: 421.0, 421.1, 421.9 or 424.99 | P+S |
| Ahtela 201811 | ICD 10: I33, I38 or I39 (specificity 96.8%) | P (66.3%)+S (24.1%)+T (9.6%) |
| Jordal 201812 | ICD 10: I33.0, I38, or I39.0 (after year 1999) | Unspecified |
| Ortega 201928 | ICD 9: 421.0, 421.1, 421.9, 112.81, 115.04, 115.14, or 115.94 | P+S |
| Jensen 20204 | ICD 10: I33.x, I38.x or I39.8 ICD 8: 421 |
P+S |
| Quan 202025 | ICD 10: I33.0, I33.9, I39.0, I39.8, I01.1, B37.6, or T82.6 | P+S |
| Shah 202013 | ICD 10: I33, I38 or I39 ICD 9: 421.1, 424.91, 424.90 or 424.99 |
Unspecified |
| Thornhill 202015 | ICD 10: I33 | P+S |
| Vähäsarja 202010 | No ICD code data available in study | – |
ICD, International Classification of Diseases.
Patient demographics
IE was predominately seen in older patients with the lowest mean age (59.1 years) recorded by Dayer2 and the highest median age (70.2) recorded by Jensen.4 Fourteen studies included investigations included all patient age groups. Vähäsarja et al10 included patients aged ≥17 years, Ahtela et al11 and Jordal et al12 included patients aged ≥18 years and Shah13 included patients aged ≥20 years. IE predominantly affected men, as reported in all but one study.13
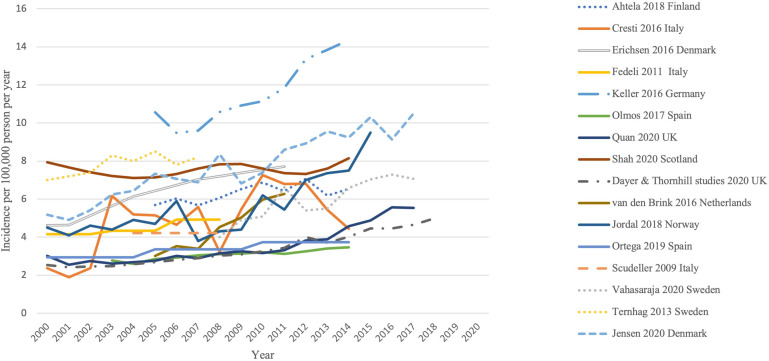
Overall incidence
All studies reported temporal trends in IE incidence as illustrated in figure 3. Results of three studies from England2 14 15 were combined into one line graph since there was duplication of data owing to similar methodology and overlapping time periods. Overall, an appreciable increase in the incidence of IE was demonstrated.
Figure 3.
Temporal trends of crude incidence of IE across all studies from 2000 to 2020. The y-axis denotes number of cases per 100 000 people while the x-axis denotes years 2000–2020. IE, infective endocarditis.
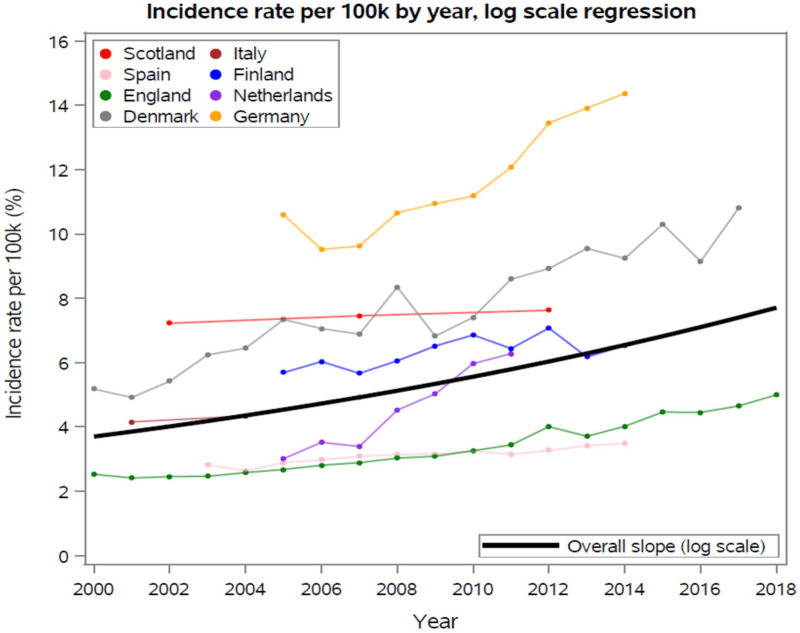
Figure 4 displays estimates from each country with the pooled regression slope overlaid on individually observed data. Table 3 shows the least squares regression slopes for each study included in pooled log scale regression analysis for temporal trends of IE incidence and the average of individual studies. The pooled regression estimate was 4.1%±1.2% per year increase in IE incidence, amounting to a compound increase in incidence of 106% over 18 years. The two Swedish studies10 16 used different databases and differed in methodology; hence, they were excluded from the primary analysis. They were included in online supplemental table 2 and figure 1, which demonstrated an overall estimated percent annual increase of 2.8%±1.0%. The Norway study by Jordal et al. was also excluded as it only provided two data points of decade-wise estimates of IE cases and did not include yearly population estimates used to calculate annual incidence.
Figure 4.
Individual and pooled incidence rate per 100 000 /year, log scale regression. The y-axis denotes incidence rate per 100 000 (%), while the x-axis denotes years 2000–2018.
Table 3.
Least squares regression slopes for each study included in the pooled log scale regression analysis for temporal trends of IE incidence and the average of individual studies
| Studies included | Country | Incidence per 100 k | Log incidence | ||
| Estimated annual increase (per 100 k) | 95% CI | Estimated annual increase (%) | 95% CI | ||
| Shah 202013 | Scotland | 0.040 | 0.033 to 0.047 | 0.54 | 0.44 to 0.64 |
| Olmos 201717 | Spain | 0.064 | 0.050 to 0.078 | 2.09 | 1.61 to 2.57 |
| Dayer and Thornhill 2011–20202 | England | 0.144 | 0.126 to 0.162 | 4.23 | 3.82 to 4.64 |
| Jensen 20204 | Denmark | 0.312 | 0.263 to 0.361 | 4.17 | 3.50 to 4.84 |
| Fedeli 201126 | Italy | 0.128 | 0.052 to 0.204 | 2.83 | 1.58 to 4.08 |
| Ahtela 201911 | Finland | 0.103 | 0.024 to 0.182 | 1.66 | 0.42 to 2.89 |
| van den Brink 201631 | Netherlands | 0.583 | 0.470 to 0.696 | 13.05 | 10.59 to 15.51 |
| Keller 20163 | Germany | 0.535 | 0.387 to 0.683 | 4.52 | 1.77 to 6.39 |
| Average of individual studies | 0.239 | 0.060 to 0.418 | 4.14 | 0.90 to 7.37 | |
| Pooled regression | 0.238 | 0.096 to 0.380 | 4.08 | 1.77 to 6.39 | |
IE, infective endocarditis.
Finally, we investigated the degree to which the pooled trend might be altered if we weighted regression estimates based on the underlying country populations. This was done on original scale, incidence per 100 000. Because all studies had estimates from 2008, the population in 2008 was used as the weight in this calculation. As shown in table 3, the estimated yearly increase in IE incidence per 100 000 was 0.24 IE cases per 100 000 per annum without weighting. When weights were applied this increased to 0.27 IE cases per 100 000 per annum. This is likely due to the German study3 having the highest estimated yearly increase as well as the highest population. If Germany data were excluded, the unweighted average was 0.20 IE cases per 100 000 per annum and the weighted average was 0.16 IE cases per 100 000 per annum.
Injection drug use
Six studies reported percentages of patients with IDU and IE (table 1). Trends in IDU over study periods were reported by two other studies. Jordal et al12 reported an overall increase in IDU from 16.5% to 23.5% from 1996 to 2015. In contrast, Olmos et al17 reported an overall decrease from 4.4% to 1.1% from 2003 to 2014 in Spain.
Microbiology
Details of microbiological data were provided in 11 studies (table 1). Seven studies reported staphylococcal species as the most common pathogens, of which three specified Staphylococcus aureus as the most common organism. Streptococcal species were identified as the most common pathogens in three studies. Scudeller et al18 reported enterococci as the most common causative pathogens. Olmos et al17 demonstrated a significant rise in yearly percentage prevalence of enterococci from 10.4% in 2003 to 16.6% in 2014.
Outcomes
Details of the proportion who underwent surgery are listed in table 1 (range, 10.2%– 60.0%). Follow-up duration among these patients was highly variable (inpatient to 5 year post-IE diagnosis). Other outcomes examined included inpatient, 30 days, 3 months and 1-year mortality rates (table 1).
Discussion
This is the first systematic review that has included pooled trends of incidence of IE across multiple nationwide population-based studies in Europe. The current investigation has highlighted a 4% per year rise in incidence of IE which, when compounded, is an alarming doubling in incidence between 2000 and 2018. Based on findings of our extensive systematic review that involved multiple countries with nationwide databases, support the notion that the IE incidence escalation seen is valid and is likely due to multiple factors operative in the 21st century. Factors that need to be considered include (1) improvements in diagnosis; (2) changes in epidemiology and associated risk factors; (3) restrictions in AP use promulgated by updated versions of guidelines and (4) improvements in coding practice.
Diagnostic advances in IE have characterised the past two decades and, while not examined specifically in our review, these advances likely contributed to the increasing incidence of IE. Li et al19 modified the Duke criteria that included echocardiography as a pivotal tool in establishing an IE diagnosis in patients who do not undergo valve surgery or autopsy for diagnosis confirmation. Inpatient data available through the Nationwide Inpatient Sample for the first decade of this century in the USA supports the notion that echocardiography use has indeed increased among hospitalised patients20 21 and anecdotally the use of echocardiography is far more widespread in Europe too. Echocardiography, both transthoracic and transoesophageal has become a ‘mainstay’ of cardiovascular evaluation in suspect cases of IE and its complications. The increase in use of echocardiography, particularly the transoesophageal approach is expected to continue which is a potential factor in the increase in IE incidence due to enhanced diagnostic features.
Additional tools, in particular multislice CT, 18fluorodeoxyglucose positron emission tomography/CT, and single-photon emission CT, have also been helpful in securing an IE diagnosis when transoesophageal echocardiography has been insufficient or unavailable. Such methods were incorporated in the latest ESC guidelines.6 In addition, there have been significant laboratory advances in performing blood cultures, which are also key in the diagnosis of IE using the modified Duke criteria.22
Studies included in this review did not provide an analysis of factors that could have accounted for the increase in IE incidence and a temporal sequence of prevalence of epidemiological variables. It is, however, intuitive that contemporary aspects of healthcare that include invasive procedures and placement of indwelling cardiovascular devices have increased over the past two decades and likely impacted both IE incidence and its clinical features. Nevertheless, four investigations documented an increase in prosthetic valve placement.4 17 23 24
Although some studies have reported increasing IE incidence after guideline changes,23 this was not found universally. It would be premature to link a rise in IE incidence in Europe to restriction of AP use. Quan et al reported, for example, increasing trends of IE in England which neither correlated with the change in recommendations by NICE in 2008, nor to a rise in prevalence of VGS as a causative pathogen.25 Moreover, most of the included studies in this systematic review identified staphylococci and streptococci as the most common pathogens of IE over the past two decades (table 1). Hence, an increase in IE incidence cannot be explained simply by trends in pathogen prevalence, which has its own array of pitfalls. This includes the fact that not all patient records included secondary designated microbiology codes, which provides a partial profile of microbiology. Fedeli et al noted a shockingly low percentage (~21%) of secondary code designations of IE-related pathogens in Italy.26 Dayer et al reported that ~50% of cases in England were designated secondary codes for microbiology, which increased towards the end of the study period in 2013.2 Similarly, Quan et al reported that only 67% of the total cases were designated codes for microbiology.25 Perhaps the most important inherent limitation in all studies was the absence of a specific ICD-10 code for VGS. This has greatly hampered accuracy of reporting microbiological data as we strive to define the impact, if any, of changes in AP use in the prevention of IE.
The impact of ICD coding on IE incidence is often underappreciated and it is critical to emphasise because many of the investigations included in our review were dependent on ICD coding to identify IE admissions (table 2). Cresti et al, for example, found that 28% of IE cases extracted using ICD-9 codes were false positive and failed to pick up 14% of confirmed cases, which raised questions on the validity of current code designations.1 The potential impact of coding was also highlighted by Fawcett et al27 who reported a sensitivity of IE of 76% for specific codes in ICD-10 with more than half of cases coded by using ICD-10 as IE were not confirmed cases. The code I33 had a positive predictive value (PPV) of 82%–85%; in contrast, and the code I38 had a PPV of <6% and accounted for many of the false-positive cases and was used in seven investigations in the current systematic review. Although such coding practices may not have a major impact on estimating burden of disease for more prevalent diseases, for IE, an uncommon malady, consistent coding practices to accurately estimate temporal trends is essential. Because 12 of 23 studies that were included in our systematic review used ICD-10 coding and all included I33, the most specific code for IE, significant trend variation in IE incidence across investigations was not observed (figure 2).
The two Spanish investigations17 23 included in the systematic review reported incidence data for a similar time period. Ortega-Loubon et al included a larger sample size and a higher annual incidence but reported a similar trend to the Olmos study. However, Ortega-Loubon et al reported a higher prevalence (16%) of Gram-negative rods as compared with streptococcal species (2.5%), while Olmos et al identified streptococcal species as the most common causative pathogen of IE (20.4%). Two similar contemporary studies with the same methodology from the authors of the Ortega study reported that causative pathogens were ‘unspecified’ in ~86% of cases,28 29 while the 2019 study reported microbiological findings in 52.2% of cases. This suggests that microbiological data from the Ortega study may not be reliable and Olmos et al likely provided a more accurate representation of the pathogen distribution in Spain.
Williams et al recently published a systematic review detailing contemporary epidemiological changes in IE following major guideline changes.30 They included studies from North America and Europe and concluded that although there was no appreciable increase in IE incidence in North America following pertinent guideline changes, there was a potential rise in incidence in Europe. They included just five European studies in their review, whereas our current investigation includes 18 studies, where the incidence trends were examined statistically over 18 years. Furthermore, Williams et al specifically studied the impact of guideline changes for AP use on the incidence of IE, while we focused on trends, irrespective of AP guideline changes, to assess all factors responsible for increasing IE trends.
Despite the thoroughness of this review, there were some limitations to the current study. Data for patient demographics, microbiology, mortality and surgery were provided for most studies as a single percentage over the study period, and not as yearly trends. Hence, a risk factor meta-regression analysis could not be performed, which would have helped quantify the contribution of specific risk factors associated with the rising trends of IE. Furthermore, these studies are only observational and by design, unable to determine aetiological factors associated increasing incidence.
Conclusion
This study highlights a greater than twofold increase in incidence of IE in Europe between 2000 and 2018. This is likely due to a combination of factors including an increasingly elderly population, a rise in cardiovascular device implantation procedures, increased use of multiple imaging modalities for diagnosis, improved coding and possibly a restriction of AP use. However, it is difficult to ascertain a single factor for this change, since there is great heterogeneity in how data are reported across studies and inadequacy in coding of microbiological data.
Footnotes
Contributors: We confirm that all authors of this study contributed significantly to the synthesis of the manuscript, meet the authorship criteria and have agreed to report all potential conflicts of interest in the manuscript. The authors also agree to release the copyright should the manuscript be accepted for publication. KMT is the guarantor of the manuscript.
Funding: The authors are extremely grateful for the philanthropic support provided by a gift from Eva and Gene Lane (LMB), which was paramount in our work to advance the science of cardiovascular infections and has been an ongoing focus of investigation at Mayo Clinic for over 60 years. We also recognise the unique expertise of Danielle J. Gerberi, M.L.S., AHIP for conducting the literature search of the systematic review and Barbara A. Abbott for data retrieval from the Rochester Epidemiology Project (REP).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: LMB, M.D. reports Boston Scientific, consultant duties; UpToDate, royalty payments (authorship duties); Botanix Pharmaceuticals, consulting duties; Roivant Sciences, consultant duties. MRS, M.D. reports receiving funds from TYRX and Medtronic for prior research unrelated to this study administered according to a sponsored research agreement between Mayo Clinic and study sponsor that prospectively defined the scope of the research effort and corresponding budget; and honoraria/consulting fees from Medtronic, Philips and Aziyo Biologics. Research Grant: Medtronic. MJD, M.B.B.S. reports payments from Biotronik unrelated to this study. IMT, M.D. reports UpToDate, royalty payments (authorship duties). The remaining authors have nothing to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Cresti A, Chiavarelli M, Scalese M, et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther 2017;7:27–35. 10.21037/cdt.2016.08.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet 2015;385:1219–28. 10.1016/S0140-6736(14)62007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller K, von Bardeleben RS, Ostad MA, et al. Temporal trends in the prevalence of infective endocarditis in Germany between 2005 and 2014. Am J Cardiol 2017;119:317–22. 10.1016/j.amjcard.2016.09.035 [DOI] [PubMed] [Google Scholar]
- 4.Jensen AD, Bundgaard H, Butt JH, et al. Temporal changes in the incidence of infective endocarditis in Denmark 1997-2017: a nationwide study. Int J Cardiol 2021;326:145–52. 10.1016/j.ijcard.2020.10.029 [DOI] [PubMed] [Google Scholar]
- 5.Richey R, Wray D, Stokes T, et al. Prophylaxis against infective endocarditis: summary of NICE guidance. BMJ 2008;336:770–1. 10.1136/bmj.39510.423148.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–128. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 7.Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest 2007;132:1025–35. 10.1378/chest.06-2048 [DOI] [PubMed] [Google Scholar]
- 8.Page MJ, Shamseer L, Tricco AC. Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst Rev 2018;7:018–699. 10.1186/s13643-018-0699-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 2021;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vähäsarja N, Lund B, Ternhag A, et al. Incidence of infective endocarditis caused by viridans group streptococci in Sweden - effect of cessation of antibiotic prophylaxis in dentistry for risk individuals. J Oral Microbiol 2020;12:1768342. 10.1080/20002297.2020.1768342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahtela E, Oksi J, Porela P, et al. Trends in occurrence and 30-day mortality of infective endocarditis in adults: population-based registry study in Finland. BMJ Open 2019;9:e026811. 10.1136/bmjopen-2018-026811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordal S, Kittang BR, Salminen P-R, et al. Infective endocarditis in Western Norway: a 20-year retrospective survey. Infect Dis 2018;50:757–63. 10.1080/23744235.2018.1482419 [DOI] [PubMed] [Google Scholar]
- 13.Shah ASV, McAllister DA, Gallacher P, et al. Incidence, microbiology, and outcomes in patients hospitalized with infective endocarditis. Circulation 2020;141:2067–77. 10.1161/CIRCULATIONAHA.119.044913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ 2011;342:d2392. 10.1136/bmj.d2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornhill MH, Dayer MJ, Nicholl J, et al. An alarming rise in incidence of infective endocarditis in England since 2009: why? Lancet 2020;395:1325–7. 10.1016/S0140-6736(20)30530-4 [DOI] [PubMed] [Google Scholar]
- 16.Ternhag A, Cederström A, Törner A, et al. A nationwide cohort study of mortality risk and long-term prognosis in infective endocarditis in Sweden. PLoS One 2013;8:e67519. 10.1371/journal.pone.0067519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olmos C, Vilacosta I, Fernández-Pérez C, et al. The Evolving Nature of Infective Endocarditis in Spain: A Population-Based Study (2003 to 2014). J Am Coll Cardiol 2017;70:2795–804. 10.1016/j.jacc.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 18.Scudeller L, Badano L, Crapis M, et al. Population-based surveillance of infectious endocarditis in an Italian region. Arch Intern Med 2009;169:1718–3. 10.1001/archinternmed.2009.307 [DOI] [PubMed] [Google Scholar]
- 19.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 20.Papolos A, Narula J, Bavishi C, et al. U.S hospital use of echocardiography: insights from the nationwide inpatient sample. J Am Coll Cardiol 2016;67:502–11. 10.1016/j.jacc.2015.10.090 [DOI] [PubMed] [Google Scholar]
- 21.Virnig BA, Shippee ND, O'Donnell B. Trends in the use of echocardiography, 2007 to 2011: Data Points #20. [PubMed]
- 22.Liesman RM, Pritt BS, Maleszewski JJ, et al. Laboratory diagnosis of infective endocarditis. J Clin Microbiol 2017;55:2599–608. 10.1128/JCM.00635-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortega-Loubon C, Muñoz-Moreno MF, Andrés-García I, et al. Nosocomial vs. community-acquired infective endocarditis in Spain: location, trends, clinical presentation, etiology, and survival in the 21st century. J Clin Med 2019;8. 10.3390/jcm8101755. [Epub ahead of print: 22 10 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erichsen P, Gislason GH, Bruun NE. The increasing incidence of infective endocarditis in Denmark, 1994-2011. Eur J Intern Med 2016;35:95–9. 10.1016/j.ejim.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 25.Quan TP, Muller-Pebody B, Fawcett N, et al. Investigation of the impact of the NICE guidelines regarding antibiotic prophylaxis during invasive dental procedures on the incidence of infective endocarditis in England: an electronic health records study. BMC Med 2020;18:020–1531. 10.1186/s12916-020-01531-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedeli U, Schievano E, Buonfrate D, et al. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis 2011;11:1471–2334. 10.1186/1471-2334-11-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fawcett N, Young B, Peto L, et al. 'Caveat emptor': the cautionary tale of endocarditis and the potential pitfalls of clinical coding data-an electronic health records study. BMC Med 2019;17:019–1390. 10.1186/s12916-019-1390-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortega-Loubon C. Ruiz López del Prado G, Muñoz-Moreno MF, et al. impact of the economic crisis on endocarditis mortality in Spain: a nationwide study. Int J Health Serv 2021;29:00207314211012357. [DOI] [PubMed] [Google Scholar]
- 29.Heredia-Rodríguez M, Hernández A, Bustamante-Munguira J, et al. Evolution of the incidence, mortality, and cost of infective endocarditis in Spain between 1997 and 2014. J Gen Intern Med 2018;33:1610–3. 10.1007/s11606-018-4514-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams ML, Doyle MP, McNamara N, et al. Epidemiology of infective endocarditis before versus after change of international guidelines: a systematic review. Ther Adv Cardiovasc Dis 2021;15:17539447211002687. 10.1177/17539447211002687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Brink FS, Swaans MJ, Hoogendijk MG, et al. Increased incidence of infective endocarditis after the 2009 European Society of Cardiology guideline update: a nationwide study in the Netherlands. Eur Heart J Qual Care Clin Outcomes 2017;3:141–7. 10.1093/ehjqcco/qcw039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2021-001846supp001.pdf (135.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.