Abstract
Both dogs and humans can be coinfected with various Ehrlichia, Bartonella, Rickettsia, and Babesia species. We investigated a kennel of sick Walker Hounds and their owners in southeastern North Carolina for evidence of tick-borne infections and associated risk factors. A high degree of coinfection was documented in the dog population. Of the 27 dogs, 26 were seroreactive to an Ehrlichia sp., 16 to Babesia canis, and 25 to Bartonella vinsonii, and 22 seroconverted to Rickettsia rickettsii antigens. According to PCR results, 15 dogs were infected with Ehrlichia canis, 9 with Ehrlichia chaffeensis, 8 with Ehrlichia ewingii, 3 with Ehrlichia equi, 9 with Ehrlichia platys, 20 with a Rickettsia species, 16 with a Bartonella species, and 7 with B. canis. The detection of DNA from any Ehrlichia species was associated with clinical illness and with concurrent B. canis infection (by PCR). Both E. canis and an uncharacterized Rickettsia species appeared to result in chronic or recurrent infection. Death in the dog population was associated with living in a dirt lot rather than the concrete kennel. Of 23 people on whom serologic testing was conducted, eight were seroreactive to Bartonella henselae, one to E. chaffeensis, and one to R. rickettsii antigen; however, none had clinical or hematologic abnormalities consistent with illness caused by these organisms. We conclude that kennel dogs with heavy tick exposure can be infected at a high rate with multiple, potentially zoonotic, tick-borne pathogens. In addition, our findings further illustrate the utility of PCR for documenting coinfection with tick-transmitted pathogens.
Infection with tick-borne disease agents, including several Ehrlichia and Rickettsia species, has been described in humans and dogs in North Carolina (2, 8, 9, 13, 16, 18, 22, 42, 48). Other tick-borne organisms, including some Babesia and Bartonella spp., have also been shown to cause disease in animals and people (6, 20, 28–31, 41). Both humans and dogs infected with these agents can experience a wide range of clinical manifestations (2, 6, 7, 16, 18, 20–24, 26, 29, 30). Recently, case reports of coinfection with multiple tick-borne organisms in humans and dogs have been published (4, 9, 15, 19, 32, 36, 38, 42, 46). However, the consequences of coinfection have not been well established in either species, compared with infection with a single organism. Simultaneous infection with multiple agents may account for some of the diversity observed among clinical cases when only one tick-transmitted pathogen is considered.
Simultaneous infection with tick-borne organisms can occur as a result of the transmission of multiple organisms by the same tick vector or as a result of the independent transmission of chronic infections by different ticks at different times. Dogs would logically be at a greater risk of coinfection with tick-borne diseases than humans, due to the increased likelihood of dogs being simultaneously infested with numerous ticks, concurrently infested with different tick species, and exposed to a wider range of tick species than humans, making dogs potential sentinels for tick-borne diseases in humans.
Organisms from this study that have the same known tick vector and are found in the United States include the following: Ehrlichia canis, Babesia canis, some Rickettsia species, and potentially Bartonella vinsonii (41) and Ehrlichia platys (7) (Rhipicephalus sanguineus); Ehrlichia chaffeensis (1), Ehrlichia ewingii, and Rickettsia species (Amblyomma americanum); and Ehrlichia equi (37, 40) and Rickettsia spp. (47) (Ixodes scapularis). Rickettsia rickettsii is the only organism from this study thought to be transmitted by Dermacentor variabilis.
During May 1997, a veterinarian practicing in southeastern North Carolina contacted the North Carolina State University Veterinary Teaching Hospital (NCSU-VTH) for assistance with diagnostic and treatment issues involving possible tick-borne illness in a Walker Hound kennel under his care. Additionally, members of the family that owned the dogs, all of whom were living in close proximity to the kennel, stated that they had experienced symptoms consistent with infection with tick-borne pathogens.
The primary purpose of this investigation was to characterize the degree of coinfection with multiple tick-borne organisms in this cohort of dogs. Additional objectives were to determine if dogs with serologic and/or molecular evidence of multiple tick-borne infections experienced adverse clinical outcomes and if dogs with evidence of coinfection with multiple organisms differed in outcome from dogs infected with a single organism. Subsequently, we attempted to identify risk factors associated with patterns of coinfection and to characterize the degree of exposure to these tick-borne pathogens in the dogs’ owners.
(This work was presented in part at the International Conference on Emerging Infectious Diseases, 8 to 11 March 1998, Atlanta, Ga.)
MATERIALS AND METHODS
Investigation.
A team composed of veterinarians and veterinary students visited the kennel during May 1997 to perform clinical and ophthalmologic examinations and to collect blood samples from all dogs in the kennel for hematologic, serologic, and molecular analysis. During additional visits in June 1997, clinical exams were performed and blood samples were collected from those dogs that appeared ill at the time of the visit. In August, the North Carolina Department of Environment, Health and Natural Resources (DEHNR) was contacted for assistance in assessing illness in the human population living near the dog kennel. A team composed of NCSU-VTH and DEHNR investigators subsequently made a series of visits to the kennel site during the next 3 months. Blood samples were collected from both humans and dogs; questionnaires concerning signs, symptoms, and exposure information for both humans and dogs were administered; and ticks and fleas were collected from dogs, vegetation, and kennel structures.
Hematology.
For the dogs, complete blood counts were performed on samples collected on 19 May, 5 June, and 6 September 1997. Anemia was defined as a hematocrit value of <38%, thrombocytopenia was defined as <200,000/μl, an elevated leukocyte count was defined as >17,000/μl, and neutrophilia was defined as >12,000/μl. The detection of immature or toxic neutrophils, nucleated erythrocytes, or other erythrocyte or leukocyte abnormalities was noted.
For each human subject with serologic evidence of infection, a physical examination and a complete blood count with a differential leukocyte count were performed and liver enzyme activities were measured.
Serology.
Canine blood specimens collected on 19 May, 5 June, 17 June, and 6 September were used for serologic testing. A microimmunofluorescence test was used at NCSU-VTH to detect antibodies to E. canis Florida, E. chaffeensis Ark (human origin), E. equi NY (human origin), R. rickettsii Domino (canine origin), B. canis, and B. vinsonii subspecies berkhoffii 93-CO-1 in dog sera on 30-well Teflon-coated slides (9, 41). Serial twofold dilutions of sera from dogs were reacted with fluorescein isothiocyanate anti-canine immunoglobulin G conjugate (Cappel; ICN Pharmaceuticals, Inc., Costa Mesa, Calif.). Endpoint titers were determined as the last dilution at which brightly stained organisms could be detected on a fluorescence microscope with exciter and barrier filters.
Serologic analysis for humans was performed at the North Carolina State Laboratory of Public Health on specimens collected on 19 August, 28 August, 12 September, and 6 October 1997. The indirect fluorescent-antibody technique was used as described above, with E. chaffeensis, R. rickettsii, Rickettsia typhi, Coxiella burnetii, E. equi, Bartonella henselae, and B. vinsonii antigens. An affinity-purified fluorescein-labeled goat anti-human polyvalent globulin (Kirkegaard and Perry, Gaithersburg, Md.) conjugate was used.
DNA extraction.
EDTA-anticoagulant–blood specimens collected from the dogs on 19 May and 6 September were studied. DNA was extracted from 300 μl of stored blood samples as previously described (9). Positive and negative controls for each species were run for each assay.
Ehrlichia genus amplification.
A one-tube nested PCR was performed by using outer primers EHR-OUT1 and EHR-OUT2 and inner primers GE2f and EHRL3-IP2 to amplify a 122-bp product as previously described (9).
Ehrlichia species amplification.
A one-tube nested PCR amplification was performed as previously described (9) by using outer primers EHR-OUT1 and EHR-OUT2, inner primer HE3-R paired with E. canis (14), HE3-R paired with E. chaffeensis (14), HE3-R paired with E. ewingii (14), HE3-R paired with E. equi (3), or E. platys primers (5′-GAT TTT TGT CGT AGC TTG CTA-3′) paired with Ehrl3-IP2 (5′-TCA TCT AAT AGC GAT AAA TC-3′). For the specific identification of E. platys, primers were designed to produce a 151-bp product from the 16S rRNA gene. Cycling conditions are similar to those described for other Ehrlichia species. All PCR products were electrophoresed through 1 to 2% agarose gels in Tris-boric acid-EDTA buffer, and the DNA fragments were visualized by ethidium bromide staining under UV fluorescence.
Babesia amplification.
B. canis-specific primers were derived from a variable region of the 16S-like rRNA gene. PCR amplification was performed in a 50-μl reaction volume containing 1 μg of DNA template, a 200 μM concentration of each deoxynucleoside triphosphate, 2 mM MgCl2, 50 pmol of each primer (B. canis [5′-GCA TTT AGC GAT GGA CCA TTC AAG-3′] and Babesia common [5′-CCT GTA TTG TTA TTT CTT GTC ACT ACC TC-3′]), and 1.25 U of Taq DNA polymerase in a 1× reaction buffer. Amplification cycles included denaturation at 95°C for 45 s, annealing at 60°C for 45 s, and chain extension at 72°C for 1 min. This was repeated for 35 cycles followed by a final chain extension at 72°C for 5 min.
Rickettsia amplification.
A one-tube nested PCR analysis was used for the detection of both spotted fever and typhus group Rickettsia spp. As previously described (11), 0.5 pmol of outer primers Rr-out1 and Rr-out2 and 50 pmol of inner primers Rr-prim3 and Rr-prim4 were added to a standard 50-μl reaction mixture. First-round amplification included 20 cycles at 94°C for 1 min, 70°C for 2 min, and 72°C for 3 min. This was followed immediately by the second round of amplification, which included 40 cycles at 94°C for 1 min, 45°C for 1 min, and 72°C for 2 min. This was followed by a final extension of 5 min at 72°C.
Bartonella amplification.
PCR amplification was performed without modifications as previously described (5) by using primers Bh16SF and Bh16SR to obtain a 185-bp product.
Sequencing.
Three amplicons derived from EDTA-blood samples (one E. canis, one E. chaffeensis, and one E. equi sample) that were PCR positive were prepared for DNA sequencing. Forty-microliter samples were electrophoresed through a 2% low-melting-point agarose gel (FMC BioProducts, Rockland, Maine). The appropriate-size band was cut from the gel, and DNA was extracted with a QIAquick gel extraction kit (Qiagen, Valencia, Calif.). Purified samples were sequenced by using a Perkin-Elmer ABI Prism 377 apparatus at an on-site sequencing facility. Nucleotide sequence comparison searches were made through the National Center for Biotechnology Information BLAST network service.
Questionnaires.
Questionnaires related to the dogs were administered to their primary handlers by telephone. Information collected in questionnaires included age, sex, type of housing, current flea and tick loads, past medical history, and prior treatment of the individual dog for ectoparasites. In addition, a list of descriptive terms for signs consistent with tick-borne illness was read, and the owner was asked to note which abnormalities had been observed in each dog between May and September 1997.
Questionnaires related to human subjects were administered in person. Information requested included demographics, extent of risk factors for tick exposure, observed flea contact and tick attachment, preventive measures taken against ectoparasites, extent of contact with dogs, and history of symptoms consistent with vector-borne illness between May and October 1997.
Entomology.
Ticks and fleas were collected from a sample of dogs on 19 May and 6 June and randomly from dogs, the kennel, and the surrounding environment on 19 August.
Case definitions and data analysis.
Serologic data were used as the basis for diagnostic inclusion (i.e., a case) in this study. For dogs, a single titer of ≥1:80 was required for implicating infection with any Ehrlichia spp., ≥1:40 was required for B. canis infection, and ≥1:32 was required for B. vinsonii infection. A fourfold or greater increase or decrease in titer was required for inclusion as a Rickettsia sp. infection. All 27 dogs had at least two serum samples, obtained 2 or more weeks apart, tested for R. rickettsii antibodies. All dogs surviving to the end of the study had at least three serum samples tested against R. rickettsii antigens. Among human subjects, a case of E. chaffeensis, B. henselae, or R. rickettsii infection was defined as a single titer of ≥1:128.
A binomial variable for clinical illness, created on the basis of signs observed by the owners, was used to categorize dogs as clinically ill (those with at least two signs of illness reported) or not ill (dogs with only one sign or no signs). Descriptive statistics and univariate associations for dog data were performed by using EpiInfo (version 6.04b; Centers for Disease Control and Prevention, Atlanta, Ga.). When providing P values for dog data, Fisher’s exact test with two-tailed comparisons was used. Statistically significant results (P < 0.05) are presented. Human data were analyzed by using SAS statistical software, with results included only if significant (P < 0.05).
RESULTS
Kennel investigation.
Included in the investigation were 27 dogs: 11 adult males, 14 adult females, and 2 juveniles less than 12 months old (one male and one female). The ages of the dogs ranged from 6 months to 9 years with a median age of 2 years. Thirteen adult female Walker Hounds lived in a wire-enclosed shelter with cement runs, whereas 10 adult male Walker Hounds lived in an adjacent dirt and brush pen. The remaining four dogs, which were nonhunting dogs, lived in the yards of the houses adjacent to the kennel area.
All 27 dogs lived in or around the kennel, which had been in existence for approximately 7 years. The kennel area consisted of two types of adjacent enclosures. The first type was a dirt and brush pen, approximately 20 by 30 feet, that was enclosed by an electrified wire fence and contained one wooden dog house, one camper shell, and a large amount of underbrush and tree coverage for shelter. Next to the pen was a cement-floored kennel subdivided into three runs (10 by 10 feet each), separated by chain-link fencing and covered with a tin roof. Multiple rodent burrows were observed along the edge of the kennels and pen. The human subjects resided in a group of houses located near the kennel on approximately 2 acres of sandy soil, most of which was overgrown with brushy vegetation.
From mid-October through the first day of January, the Walker Hounds were primarily used for deer hunting in a neighboring North Carolina county with a similar topography. During the hunting season, the dogs hunted approximately once per week, with extra hunts during Thanksgiving and Christmas holiday periods. Between seasons, the dogs were exercised by allowing them to run at large around the family home and surrounding area with a range of several acres.
All 27 dogs were chronically infested with both fleas and ticks. In 1997, the infestation problem was reported to be worse than usual, despite periodic treatment of the premises with an unspecified type of pelleted pesticide during previous years and with diazinon spray beginning in 1997. Permethrin and pyrethrin dips and sprays were applied to the dogs two to three times each summer. No insecticides were used in the owners’ house.
The dogs had historically been dewormed with a fenbendazole product. Beginning in April 1997, pyrantel pamoate was administered to all puppies, and ivermectin was administered to all adult dogs on a monthly basis for heartworm (Dirofilaria immitis) prophylaxis. All vaccinations, except for that for rabies, were administered by the owners. In late April, dogs with signs of illness were treated with doxycycline and cephalexin (no records identified which dogs were treated), and on 19 May all dogs in the kennel were treated with doxycycline. On 27 June and 11 July, all adult dogs were treated for ehrlichiosis and babesiosis with imidocarb diproprionate (Imizol) (Schering Plough) at a volume of 1.5 ml (approximately 180 mg/dog) administered intramuscularly.
The number of signs of illness observed by the owners in each dog ranged from 0 to 13, of a possible 26 reportable abnormal signs. The median number of signs observed by the owner was five. Seventeen dogs (63%) were included in the ill category with a median of nine signs. A complete listing of signs observed in the dogs is provided in Table 1. Ophthalmologic examinations identified ocular abnormalities in 13 dogs (47%). Twelve dogs had retinal hemorrhages, and one had uveitis and glaucoma. During the course of the investigation six dogs (22%) died.
TABLE 1.
Signs of illness observed in kennel dogs by their owners, May to September 1997, and selected outcomes (n = 27)
| Observation | % of dogs |
|---|---|
| Conditionsa | |
| Weight loss | 74 |
| Pale gums (anemia) | 52 |
| Anorexia | 52 |
| Lethargy | 48 |
| Cloudy eyes (uveitis) | 48 |
| Decreased stamina | 44 |
| Ocular discharge | 44 |
| Cough | 37 |
| Stumbling or dizziness (neurologic dysfunction) | 33 |
| Difficulty breathing | 22 |
| Difficulty conceiving or stillbirths (n = 10) (reproductive problems) | 20 |
| Swollen glands (lymphadenopathy) (n = 25) | 12 |
| Scrotal swelling or atrophy (n = 12) | 8 |
| Diarrhea | 7 |
| Redness around eyes (conjunctivitis) | 4 |
| Vomiting | 4 |
| Swelling in limbs (edema) | 4 |
| Fresh blood in stool | 4 |
| Yellow membranes or eyes (jaundice) | 4 |
| Outcomes | |
| Ophthalmic disease | 47 |
| Categorized as ill | 63 |
| Died | 22 |
Signs not observed in any dogs include hyphema, ecchymoses or petechiae, hematuria or hemoglobinuria, lameness, swollen joints, nasal discharge, melena, and epistaxis.
Hematologic results for specimens collected 19 May and 6 September 1997 are provided in Table 2. By 6 September, six dogs had died and one dog was not available for blood sample collection.
TABLE 2.
Hematology results for kennel dogs in 1997
| Characteristic | 19 May (n = 27)
|
6 September (n = 20)
|
||||
|---|---|---|---|---|---|---|
| Median | Range | % of dogs with abnormality | Median | Range | % of dogs with abnormality | |
| Platelet counta | 118,000 | 37,000–320,000 | 78 | 129,000 | 61,000–300,000 | 70 |
| Hematocrit (%)b | 35 | 18–52 | 59 | 33 | 19–45 | 63 (n = 19) |
| Leukocyte countc | 20,185 | 8,360–56,980 | 63 | 19,843 | 9,681–34,975 | 65 |
| Neutrophil countd | 11,179 | 3,760–37,785 | 46 (n = 26) | 8,930 | 4,066–23,172 | 33 (n = 15) |
| Immature neutrophil counte | 0 | 0–3,527 | 19 | 0 | 0–28 | 10 (n = 15) |
| Nucleated erythrocyte countf | 0 | 0–3 | 39 | 0 | 0 | 0 (n = 15) |
A count of <200,000 platelets/μl is considered abnormal.
A hematocrit value of <38% is considered abnormal.
A count of >17,000 leukocytes/μl is considered abnormal.
A count of >12,000 neutrophils/μl is considered abnormal.
The presence of any immature neutrophils is considered abnormal.
The presence of any nucleated erythrocytes is considered abnormal.
All dogs had serologic evidence of infection with at least one organism for which testing was performed (Table 3). Of 27 total dogs, 26 (96%) were seroreactive to a species of Ehrlichia, 16 (59%) to B. canis, and 25 (93%) to B. vinsonii, and 22 (81%) seroconverted to R. rickettsii antigens. Eleven dogs (41%) were seroreactive to at least one antigen in each of the four genera (Rickettsia, Bartonella, Babesia, and Ehrlichia). Fourteen dogs (52%) were seroreactive to antigens derived from three genera, with each of the fourteen serologically reactive to at least one Ehrlichia species.
TABLE 3.
Serologic results for kennel dogs in 1997
| Antigen | 19 May
|
5 June
|
19 June
|
6 September
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of dogs tested | % Seroreactive | No. of dogs tested | % Seroreactive | % Seroconversion | No. of dogs tested | % Seroconversion | No. of dogs tested | % Seroreactive | % Seroconversion | |
| E. canis | 25 | 92 | 27 | 85 | 20 | 85 | ||||
| E. chaffeensis | 23 | 91 | 27 | 89 | 20 | 80 | ||||
| E. equi | 25 | 80 | 9 | 89 | 20 | 80 | ||||
| Ehrlichia spp. | 25 | 92 | 27 | 89 | 20 | 90 | ||||
| B. vinsonii | 25 | 72 | 11a | 91 | 21 | 100 | ||||
| B. canis | 25 | 44 | 0 | NAb | 20 | 45 | ||||
| R. rickettsii | 25c | 56 | 25 | 60 | 20 | 95 | ||||
Two of 11 samples were drawn on 19 August.
NA, not available.
The number of dogs still alive from which at least two samples had been drawn by the specified date.
According to the results of PCR testing, every dog in this investigation was infected with at least one tick-transmitted organism (Table 4). Two dogs were coinfected with four different Ehrlichia species (E. canis, E. chaffeensis, E. ewingii, and E. platys), and one dog was found to be concurrently infected with six different species. Blood from nearly half of the dogs contained DNA amplicons representing four or more species or genera. DNA from a single species was detected in only two dogs. Figures 1 and 2 illustrate representative PCR gels from dogs infected with single or multiple species. The concordance between indirect fluorescent-antibody and PCR results, achieved at the genus level, was 73.1% for Ehrlichia, 63.0% for Rickettsia, 58.3% for Bartonella, and 48.1% for Babesia. For all four organisms, dogs were more likely to be seroreactive than PCR positive. When the results of serologic and PCR testing were combined, all dogs had evidence of infection with organisms from at least two genera and three dogs had evidence of infection with at least seven different species. Serologic and PCR test results for individual dogs are shown in Table 5.
TABLE 4.
Individual serologic titers and PCR results for kennel dogsa
| Dog no. | E. canis titer | E. canis PCR result | E. chaffeensis PCR result | E. ewingii PCR | E. equi titer | E. equi PCR result | E. platys PCR result | R. rickettsii titer | Rickettsia PCR result | B. canis titer | B. canis PCR result | B. vinsonii titer | Bartonella PCR result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ≥10,240 | + | − | − | 640 | − | − | 32 | + | 80 | + | 64 | + |
| 2 | ≥10,240 | − | + | − | 320 | + | − | 512† | + | 320 | − | 512 | + |
| 3 | ≥10,240 | + | + | + | 160 | − | − | 256† | + | 40 | + | 256 | − |
| 4 | ≥10,240 | + | − | − | 1,280 | − | − | 64† | + | 80 | + | 64 | + |
| 5 | 640 | + | + | − | 640 | − | + | 512† | + | <40 | − | 64 | + |
| 6 | 5,120 | − | + | − | 320 | − | + | 64† | + | <40 | − | 32 | + |
| 7 | ≥10,240 | + | + | + | 5,120 | − | − | 64† | + | <40 | − | 512 | + |
| 8 | ≥10,240 | − | − | − | 1,280 | − | − | 32 | − | 160 | − | <16 | + |
| 9 | ≥10,240 | + | + | − | 320 | − | − | 512† | + | 320 | − | 128 | + |
| 10 | ≥10,240 | + | + | + | 320 | − | + | 256 | + | 320 | + | 128 | − |
| 11 | ≥10,240 | − | − | + | 10,240 | + | − | 32 | + | 160 | − | 128 | + |
| 12 | ≥10,240 | − | − | − | 320 | − | − | 128 | + | 160 | − | 256 | ND |
| 13 | ≥10,240 | + | + | + | 80 | − | − | 2,048† | − | 40 | − | <16 | − |
| 14 | ≥10,240 | + | + | + | 320 | − | + | 128† | − | <40 | + | 256 | − |
| 15 | ≥10,240 | + | − | − | 2,560 | − | + | 128† | − | 80 | + | 512 | − |
| 16 | ≥10,240 | + | − | + | 1,280 | − | + | 128† | + | 40 | − | 256 | − |
| 17 | 1,280 | − | − | − | 640 | − | + | 64† | − | <40 | + | 512 | ND |
| 18 | 5,120 | − | − | − | 2,560 | − | − | 256† | + | <40 | − | 2,048 | + |
| 19 | 1,280 | + | − | − | 320 | − | − | 128† | − | <40 | − | 256 | + |
| 20 | ≥10,240 | − | − | − | 1,280 | − | − | 128† | + | 40 | − | 128 | + |
| 21 | ≥10,240 | + | − | − | 640 | − | + | 256† | + | 40 | − | 32 | + |
| 22 | ≥10,240 | − | − | − | 1,280 | − | − | 256† | + | 40 | − | 128 | + |
| 23 | ≥10,240 | + | − | − | 1,280 | − | − | 128† | + | <40 | − | 256 | + |
| 24 | 320 | − | − | − | <80 | − | − | 256† | + | <40 | − | 128 | + |
| 25 | <80 | − | − | − | <80 | + | + | 128† | − | <40 | − | 64 | ND |
| 26 | <80 | − | − | − | <80 | − | − | 128† | + | <40 | − | 512 | + |
| 27 | ≥10,240 | + | − | + | 1,280 | − | − | 256† | + | 40 | − | 256 | + |
Cumulative results for all dates for which diagnostic information is available. The highest titers are reported. †, a diagnostic fourfold change in titer to R. rickettsii antigen. ND, not done.
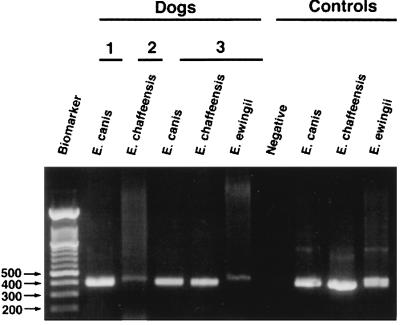
FIG. 1.
PCR detection of Ehrlichia spp. infection in EDTA-blood samples from three representative dogs, obtained by using species-specific E. canis, E. chaffeensis, or E. ewingii primers. Controls include uninfected EDTA-dog blood and culture-grown E. canis, E. chaffeensis, and E. ewingii DNA from a naturally infected dog with numerous granulocytic inclusions. Numbers to the left of the gel are molecular sizes (in base pairs).
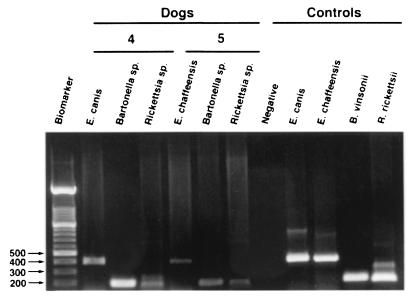
FIG. 2.
PCR detection of coinfection in EDTA-blood samples from two representative dogs, obtained with E. canis or E. chaffeensis species-specific primers or with Bartonella or Rickettsia genus primers. Controls include uninfected EDTA-dog blood and culture-grown E. canis, E. chaffeensis, B. vinsonii, and R. rickettsii DNA. Numbers to the left of the gel are molecular sizes (in base pairs).
TABLE 5.
PCR results for dog blood taken in 1997
| Organism | 19 May
|
6 September
|
19 May or 6 September
|
|||
|---|---|---|---|---|---|---|
| No. of dogs tested | % Positivea | No. of dogs tested | % Positivea | No. of dogs tested | % Positivea | |
| E. canis | 24 | 38 | 20 | 50 | 27 | 56 |
| E. chaffeensis | 24 | 38 | 21 | 0 | 27 | 33 |
| E. ewingii | 24 | 33 | 21 | 0 | 27 | 30 |
| E. equi | 24 | 8 | 21 | 5 | 27 | 11 |
| E. platys | 24 | 13 | 21 | 29 | 27 | 33 |
| B. canis | 24 | 17 | 21 | 14 | 27 | 26 |
| Rickettsia spp. | 24 | 63 | 21 | 43 | 27 | 74 |
| Bartonella spp. | 24 | 75 | 0 | NAb | 24 | 75 |
Percentage of blood containing DNA of the organism indicated.
NA, not available.
Compared to sequences available in GenBank, the 233-bp sequence derived from dog 7 was 100% identical to E. canis (accession no. U26740). The amplification of DNA from the blood of dog 6 yielded a 234-bp sequence that was 100% identical to the GenBank sequence for E. chaffeensis (accession no. U23503 and U60476). The amplification of DNA from the blood of dog 11 yielded a 239-bp sequence that was 100% identical to the GenBank sequence for the agent that causes human granulocytic ehrlichiosis (HGE) (accession no. U02521).
There were no statistical associations between the age and origin of the dog, the number of hunting seasons, and clinical, serologic, or molecular evidence of illness. The detection of DNA from any Ehrlichia species in blood samples collected on the first visit was associated with anemia (P = 0.024) and clinical illness (P = 0.032). Blood samples containing E. canis DNA, on the first date of collection, were more likely to also contain E. platys (P = 0.042) and B. canis DNA (P = 0.012), and these dogs were reported as clinically ill (P = 0.033). The detection of E. ewingii DNA on the first test date was strongly correlated with anemia (P = 0.0095).
Dogs that died (all were male) were not more likely to have been classified as ill according to signs reported by the owners. However, the owners did report a higher median number of signs in dogs that died than in dogs that did not die during the study (nine and two signs, respectively). Dogs that died were more likely to have been kept in the dirt lot (P = 0.00028), as were dogs that were seroreactive to B. canis antigens (P = 0.00028). There were no associations between death and any pattern of infection with the tick-transmitted pathogens for which we tested.
All dogs had molecular evidence of infection with at least one organism for which testing was performed (Table 5). Table 6 provides an indication of the chronology and the chronicity of infection with these organisms. Only dogs with blood samples available for PCR analysis on both specimen collection dates are included to examine the apparent persistence, clearance, or acquisition of new infection. E. canis and an organism within the genus Rickettsia were the only two organisms that appeared, through PCR testing, to cause either chronic or recurrent infection in these dogs. Some dogs appeared to acquire infection with E. canis, E. platys, or B. canis during the course of the study. Although blood samples from a few dogs contained E. equi DNA on at least one test date, none of these dogs had an alternate sample available for testing and therefore could not be included in this analysis. Treatment with imidocarb may or may not have resulted in the elimination of E. canis from these dogs, as many dogs appeared to become rapidly reinfected following treatment, whereas most dogs remained PCR negative for E. chaffeensis, E. ewingii, E. platys, and B. canis organisms following treatment.
TABLE 6.
Chronology of PCR results in dog blood taken in 1997 (n = 18)a
| PCR result on:
|
No. (%) of dogs positive for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 19 May | 6 September | E. canis | E. chaffeensis | E. ewingii | E. equi | E. platys | B. canis | Rickettsia spp. |
| Negative | Negative | 5 (29) | 12 (67) | 13 (72) | 18 (100) | 11 (61) | 14 (78) | 3 (17) |
| Positive | Negative | 2 (12) | 6 (33) | 5 (28) | 0 (0) | 4 (22) | 2 (11) | 7 (39) |
| Negative | Positive | 6 (35) | 0 (0) | 0 (0) | 0 (0) | 3 (17) | 2 (11) | 4 (22) |
| Positive | Positive | 4 (24) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (22) |
Only dogs with PCR results for both 19 May and 6 September 1997 are included.
Human investigation.
Seventeen people, ranging in age from 4 to 71 years and living in a cluster of houses surrounding the kennels, consented to participate in the investigation. All were relatives of the household that owned the kennel. Ten (59%) participants were male. Sixteen (94%) had occupations that were classified as indoor, and one (6%) was classified as working outdoors. Eight (47%), all of whom were male, were active participants in hunting activities, but all 17 people had some degree of contact with the dogs. Of those who were hunters, the median number of times hunting in the preceding season (October 1996 to January 1997) was 46 (range, 8 to 66).
Eleven (65%) people reported flea bites since the beginning of the spring season; five (29%) reported tick attachment and two (12%) reported at least one tick found crawling on the body with no known attachment. Of those with tick attachment, the median number of ticks found during the season was four (range, 2 to 15). Of those with ticks found crawling on the skin, counts ranged from 3 to 4.
There were no associations between age, sex, length of time in the area, number of historical symptoms, history of tick contact or attachment, history of flea bites, or presence of rash and clinical illness. Sixteen (94%) of the human participants reported at least one symptom compatible with vector-borne illness during the spring season. The median number of symptoms experienced was 6.5, and the range was 0 to 16. Eight (47%) people had serologic evidence of prior infection with an Ehrlichia, Rickettsia, or Bartonella species. All eight were seroreactive to B. henselae antigen, with one person additionally reactive to E. chaffeensis and one to R. rickettsii. No one was seroreactive to E. canis, E. equi, B. vinsonii, C. burnetii, or R. typhi. None of the human participants were symptomatic at the time specimens were collected. The two family members with serologic evidence of Ehrlichia or Rickettsia had normal physical examinations, normal complete blood counts, and normal liver enzyme studies. Both patients had histories of symptomatic illness consistent with a tick-borne disease, although neither was experiencing any signs or symptoms at the time of physical examination. People who spent only 1 to 2 h outside during weekdays were more likely than those who spent 3 h or more outside to have been seroreactive to B. henselae antigens. There were no other serologic associations.
Entomological observations.
Four R. sanguineus (brown dog tick) adults were collected from dogs during the 19 May visit. On 5 June, one A. americanum (Lone Star tick) was collected from a child, and seven R. sanguineus adults were collected from dogs at the kennel. It was noted at this time that there had been a marked reduction in the number of ticks and fleas observed on the dogs because of recently intensified pest control. On 19 August, both ticks and fleas were collected from the environment and R. sanguineus ticks (all three stages) were found on 7 of the 11 dogs (64%). Ctenocephalides felis (common cat flea) fleas were also found on 10 dogs (91%), Echidnophaga gallinacea (sticktight flea) fleas were collected from two juvenile dogs, and Pulex irritans (human flea) was found on one of the juvenile dogs. A tick drag recovered eight C. felis fleas and one R. sanguineus nymph. The presence of ticks and fleas inside the house was not evaluated.
DISCUSSION
An unprecedented degree of concurrent infection with multiple tick-borne pathogens was documented by serologic and molecular methods in this Walker Hound kennel. Approximately 40% of the 27 dogs had serologic evidence of infection with organisms from four genera, and an additional 52% had serologic evidence of infection with organisms from a combination of three genera. Seroprevalence studies in stray or hospitalized dogs from North Carolina have estimated exposure rates at 2.5% for E. canis, 1.2% for E. equi (45), 5% for R. rickettsii (8), 3.6% for B. vinsonii (41), and 3.8% for B. canis (34). Almost half of the dogs had evidence of infection with four or more species or genera by PCR.
The relationship between the detection of E. canis DNA and clinical illness indicates that infection with this organism, although not found to be a risk factor for death in this study, likely contributed to illness and may have had a contributory effect on death as an outcome. The detection of E. canis DNA was also associated with the detection of B. canis and E. platys DNA, all of which may share R. sanguineus as a tick vector, presumptively in the case of E. platys (7). Signs of illness associated with E. canis infection, as observed in the study dogs, are consistent with signs reported with E. canis, E. platys, or B. canis. The natural or experimental infection of dogs with any of these organisms causes a wide range of clinical and pathological abnormalities, ranging in severity from asymptomatic infection to death (7, 24). Experimentally, concurrent infection with E. canis and B. canis can result in the potentiation of disease manifestations, which may account for some of the variability in clinical presentation attributed to these organisms, particularly when coinfections are overlooked (18, 33).
The detection of E. equi DNA in three dogs and E. chaffeensis DNA in nine dogs provides additional molecular evidence that E. equi, the presumptive cause of HGE, and E. chaffeensis, the cause of human monocytic ehrlichiosis, may be found in dogs in North Carolina (9). The DNA sequence obtained from the blood of dog 8 was 100% identical to the typical HGE strain (12). Similarly, the DNA sequence for the E. chaffeensis amplicon derived from dog 6 was identical to the sequence derived from a human isolate from Arkansas (17).
In this study, seroconversion to R. rickettsii antigen did not correlate with reported disease manifestations or death. Because this observation is inconsistent with the expected disease manifestations associated with clinical and experimental studies of canine Rocky Mountain spotted fever, we were suspicious that antibody reactivity was not related to exposure to R. rickettsii. However, Rickettsia species DNA was detected in the blood samples of most of the seroconverting dogs. Because our primers amplify spotted fever or typhus group rickettsiae, the PCR results were not specific for R. rickettsii. Ongoing studies in our laboratory indicate that these dogs may have been infected with an as yet unidentified rickettsial organism. Persistent detection of rickettsial DNA, as well as the relatively small number of uninfected dogs at either sample date, was not expected, due to our failure to find D. variabilis (American dog tick), the recognized vector for R. rickettsii in the southeastern United States. Infection with R. rickettsii causes acute disease manifestations followed by rapid immunologic clearance of the organisms. Collectively, this information provides evidence that another rickettsia was responsible for the seroreactivity to R. rickettsii detected in these dogs.
The association between seroreactivity to B. canis antigens and residence in the dirt lot would seem to support the hypothesis that dogs kept in the open lot had a greater likelihood of infestation with R. sanguineus. Although R. sanguineus is a vector for both E. canis and B. canis, residence in the dirt lot did not correlate with PCR detection of E. canis, potentially due to prior treatment with doxycycline. Interventions to control ticks and fleas occurred before and during the study period. As a consequence, we were unable to quantify the tick numbers found on the two groups of dogs.
By sampling on two occasions, approximately 3 months apart, our PCR data provide limited insights regarding the persistence of infection, the potential for acute infection or reexposure, and the influence of therapeutic interventions accompanied by enhanced efforts to eliminate tick exposure. The chronicity of documented infection with E. canis in this group of dogs, along with the rate of newly acquired infections, is not unexpected given the lack of protective immunity following E. canis infection (10) and the persistent R. sanguineus infestation. As documented, we would expect a similar pattern of infection with B. canis, reflecting the common tick vector. Although apparently new infections with B. canis occurred during the study period, treatment with imidocarb diproprionate appeared to clear the B. canis infection or, alternatively, decreased the number of organisms below the level of detection of our PCR assay. Imidocarb diproprionate also appeared efficacious for the treatment of E. chaffeensis and E. ewingii infections. As doxycycline did not appear to eliminate E. chaffeensis in naturally infected dogs (9), imidocarb diproprionate may offer an effective form of therapy. Either imidocarb diproprionate appears to be less effective in eliminating E. canis infection or the dogs were rapidly reinfected.
Infection with E. platys appeared to be acquired during the study period, supporting that R. sanguineus may be a vector for this organism, as previously hypothesized (43). The cyclic nature of ehrlichiemia associated with this organism may, however, also have been responsible for the appearance and disappearance of E. platys DNA. The absence of newly acquired infection with E. chaffeensis and E. ewingii in these dogs indicates that there was not a persistent vector for transmitting infection. Our inability to find A. americanum during follow-up visits to the kennel supports this assumption, particularly for E. chaffeensis and possibly for E. ewingii. As E. equi results in an acute self-limiting infection, recent exposure to a vector-competent tick was expected. However, we were not able to find any I. scapularis (black-legged tick) ticks, a documented vector for E. equi, during our visits.
The only significant associations with death in this population of dogs included living in the dirt and brush lot and male gender. Because only males were kept in the dirt lot and only females were kept in the kennel, we were unable to differentiate between the effect of gender and that of location within the kennel. Males and females were exposed to identical training regimens, parasite control measures, and food rations, with their only ambient difference being type of housing. The statistical association between residence in the dirt lot with pale gums, decreased hematocrit values, and seroreactivity to babesia antigens suggests either an effect of chronic babesia infection (anemia frequently accompanies acute B. canis infection) or the possibility of an interaction with other individual or combined, documented or undocumented organisms. As infections with Babesia, Ehrlichia, and Bartonella species are reported to induce immunosuppression, secondary opportunistic infections may have played a role in the disease manifestations reported in this kennel (27, 39). Conditions in the dirt lot were more conducive to the transmission of intestinal parasites and other urine- or fecally transmitted organisms, since the fenced kennel had a cement floor which was hosed off approximately daily. Although not expected to be a primary cause of death in adult dogs, fecally transmitted organisms may have contributed to death in dogs coinfected with multiple blood-borne pathogens. However, this would seem unlikely due to the historical use of antihelminthics and the infrequency of gastrointestinal manifestations.
Although tick collection efforts associated with this study were limited, only A. americanum and R. sanguineus ticks were found on the dogs and in the environment. Tick collection results were likely influenced by extensive tick control efforts undertaken by the owners of the kennel during the course of the study to try to halt the progression of illness in existing dogs. The only A. americanum tick collected during the study was from an early visit. It is unlikely that an A. americanum infestation was present at the site of the kennel since so few were collected. It is therefore unlikely that dogs were continually infected with organisms transmitted by this tick. Presumably, exposure to A. americanum and I. scapularis ticks occurred during exercise periods in the spring and summer months, and while deer hunting in the fall and winter months. The continued presence of R. sanguineus ticks, both on the dogs and in the kennel environment, indicates that dogs could have been continually reinfected with organisms transmitted by this tick throughout the study period.
Considering the severity of tick-borne infection in this kennel, our results indicate that these dogs posed a minimal risk of contributing to human infection, particularly with E. chaffeensis. Although some of the people with dog contact had antibodies to tick-borne pathogens, most had antibodies only to B. henselae, an organism for which dogs have not been implicated as a reservoir. In clinical reports, ticks have been implicated in the transmission of Bartonella spp. (25, 35, 44). The species of bartonella causing infection in these dogs was not determined, as current PCR techniques in our laboratory did not allow for the species characterization of the bartonella amplicons derived from EDTA-blood samples. Due to substantial cross-reactivity among Bartonella species, serologic differentiation was not attempted. The association of B. henselae infection with people who spent fewer hours outside may be related to increased exposure to cats and cat fleas on the premises. Although flea bites were reported frequently by the respondents, we did not attempt to collect fleas from inside the house and did not collect any specimens from the cats living around the neighborhood. The two patients with antibodies to E. chaffeensis or R. rickettsii antigens did not participate in hunting activities but did have regular contact with at least one dog. Although they complained of ongoing symptoms, neither had hematologic or biochemical abnormalities detected, supporting the hypothesis of prior exposure rather than active infection.
In summary, this study illustrates that kennel dogs with heavy tick exposure can be simultaneously infected with multiple tick-borne pathogens of potential clinical importance to both veterinary and human medicine. Dogs seroreactive to E. canis antigen were shown to be simultaneously infected with multiple Ehrlichia species when blood samples were assessed by PCR amplification techniques. Public and private health providers, particularly veterinarians and physicians, should be aware of the increased potential for vector-borne illnesses in both dogs and humans, especially when large numbers of dogs with extensive tick exposure are maintained for recreational purposes.
ACKNOWLEDGMENTS
This research was supported by the State of North Carolina, through a grant from Mallinckrodt Veterinary, Inc., currently named Schering-Plough Animal Health Corporation, and through salary support for Barbara Hegarty from Heska Corporation and Intervet.
We thank the kennel owners for their cooperation, Bill Oglesby for facilitating treatments and sample collection from the dogs, and Barry Engber and Andy Fox for assistance with ectoparasite collection and identification.
REFERENCES
- 1.Anderson B E, Sims K G, Olson J G, et al. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1994;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 2.Archibald L K, Sexton D J. Long-term sequelae of Rocky Mountain spotted fever. Clin Infect Dis. 1995;20:1122–1125. doi: 10.1093/clinids/20.5.1122. [DOI] [PubMed] [Google Scholar]
- 3.Barlough J E, Madigan J E, DeRock E, Bigornia L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus) Vet Parasitol. 1996;63:319–329. doi: 10.1016/0304-4017(95)00904-3. [DOI] [PubMed] [Google Scholar]
- 4.Barton L L, Dawson J E, Letson G W, Luisiri A, Scalzo A J. Simultaneous ehrlichiosis and Lyme disease. Pediatr Infect Dis. 1990;9:127–129. doi: 10.1097/00006454-199002000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Bergmans A M, Schellekens J F, van Embden J D, Schouls L M. Predominance of two Bartonella henselae variants among cat-scratch disease patients in The Netherlands. J Clin Microbiol. 1996;34:254–260. doi: 10.1128/jcm.34.2.254-260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boustani M R, Gelfand J A. Babesiosis. Clin Infect Dis. 1996;22:611–615. doi: 10.1093/clinids/22.4.611. [DOI] [PubMed] [Google Scholar]
- 7.Bradfield J F, Vore S J, Pryor W H. Ehrlichia platys infection in dogs. Lab Anim Sci. 1996;46:565–568. [PubMed] [Google Scholar]
- 8.Breitschwerdt E B, Moncol D J, Corbett W T, MacCormack J N, Burgdorfer W, Ford R B, Levy M G. Antibodies to spotted fever-group rickettsiae in dogs in North Carolina. Am J Vet Res. 1987;48:1436–1440. [PubMed] [Google Scholar]
- 9.Breitschwerdt E B, Hegarty B C, Hancock S I. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–2651. doi: 10.1128/jcm.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitschwerdt E B, Hegarty B C, Hancock S I. Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation with two Ehrlichia canis strains. Antimicrob Agents Chemother. 1998;42:362–368. doi: 10.1128/aac.42.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitschwerdt E B, Papich M G, Hegarty B C, Gilger B, Hancock S I, Davidson M G. Efficacy of doxycycline, azithromycin, or trovafloxacin for treatment of experimental Rocky Mountain spotted fever in dogs. Antimicrob Agents Chemother. 1999;43:813–821. doi: 10.1128/aac.43.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton M J, Clarke M J, Holman R C, Krebs J W, Fishbein D B, Olson J G, Childs J E. National surveillance for Rocky Mountain spotted fever, 1981–1992, epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg. 1995;52:405–413. doi: 10.4269/ajtmh.1995.52.405. [DOI] [PubMed] [Google Scholar]
- 14.Dawson J E, Biggie K L, Warner C K, Cookson K, Jenkins S, Levine J F, Olson J G. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res. 1996;57:1175–1179. [PubMed] [Google Scholar]
- 15.Duffy J, Pittlekow M R, Kolbert C P, Rutledge B J, Persing D H. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Lancet. 1997;349:399. doi: 10.1016/S0140-6736(97)80017-7. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 16.Dumler J S, Bakken J S. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 17.Dumler J S, Chen S M, Asanovich K, Trigiani E, Popov V L, Walker D H. Isolation and characterization of a new strain of Ehrlichia chaffeensis from a patient with nearly fatal monocytic ehrlichiosis. J Clin Microbiol. 1995;33:1704–1711. doi: 10.1128/jcm.33.7.1704-1711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eng T R, Harkess J R, Fishbein D B, Dawson J E, Greene C N, Redus M A, Satalowich F T. Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. JAMA. 1990;264:2251–2258. [PubMed] [Google Scholar]
- 19.Ewing S A, Buckner R G. Manifestations of babesiosis, ehrlichiosis, and combined infections in the dog. Am J Vet Res. 1965;26:815–828. [PubMed] [Google Scholar]
- 20.Farwell G E, LeGrand E K, Cobb C C. Clinical observations on Babesia gibsoni and Babesia canis infections in dogs. J Am Vet Med Assoc. 1982;180:507–511. [PubMed] [Google Scholar]
- 21.Fishbein D B, Dawson J E, Robinson L E. Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med. 1994;120:736–743. doi: 10.7326/0003-4819-120-9-199405010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Goldman E E, Breitschwerdt E B, Grindem C B, Hegarty B C, Walls J J, Dumler J S. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J Vet Intern Med. 1998;12:61–70. doi: 10.1111/j.1939-1676.1998.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 23.Greig B, Asanovich K M, Armstrong P J, Dumler J S. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J Clin Microbiol. 1996;34:44–48. doi: 10.1128/jcm.34.1.44-48.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrus S, Aroch I, Lavy E, Bark H. Clinical manifestations of infectious canine cyclic thrombocytopenia. Vet Rec. 1997;141:247–250. doi: 10.1136/vr.141.10.247. [DOI] [PubMed] [Google Scholar]
- 25.Hofmeister E K, Kolbert C P, Abdulkarim A S, Magera J M, Hopkins M K, Uhl J R, Ambyaye A, Telford III S R, Cockerill III F R, Persing D H. Cosegregation of a novel Bartonella species with Borrelia burgdorferi and Babesia microti in Peromyscus leucopus. J Infect Dis. 1998;177:409–416. doi: 10.1086/514201. [DOI] [PubMed] [Google Scholar]
- 26.Johansson K E, Pettersson B, Uhlen M, Gunnarsson A, Malmqvist M, Olsson E. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horses by direct solid phase sequencing of PCR products from 16S rRNA gene. Res Vet Sci. 1995;58:109–112. doi: 10.1016/0034-5288(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura M, Maede Y, Namioka S. Mitogenic responsibilities of lymphocytes in canine babesiosis and the effects of splenectomy on it. Jpn J Vet Res. 1987;35:1–10. [PubMed] [Google Scholar]
- 28.Kordick D L, Wilson K H, Sexton D J, Hadfield T L, Berkhoff H A, Breitschwerdt E B. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol. 1995;33:3245–3251. doi: 10.1128/jcm.33.12.3245-3251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kordick D L, Swaminathan B, Greene C E, Wilson K H, Whitney A M, O’Connor S, Hollis D G, Matar G M, Steigerwalt A G, Malcolm G B, Hayes P S, Hadfield T L, Breitschwerdt E B, Brenner D J. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol. 1996;46:704–709. doi: 10.1099/00207713-46-3-704. [DOI] [PubMed] [Google Scholar]
- 30.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kordick D L, Brown T T, Shin K, Breitschwerdt E B. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J Clin Microbiol. 1999;37:1536–1547. doi: 10.1128/jcm.37.5.1536-1547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krause P J, Telford S R, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing D H. Concurrent Lyme disease and babesiosis. JAMA. 1996;275:1657–1660. [PubMed] [Google Scholar]
- 33.Krause P J, Speilman A, Telford III S R, Sikand V K, McKay K, Christianson D, Pollack R J, Brassard P, Magera J, Ryan R, Persing D H. Persistent parasitemia after acute babesiosis. N Engl J Med. 1998;339:160–165. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 34.Levy M G, Breitschwerdt E B, Moncol D J. Antibody activity in Babesia canis in dogs in North Carolina. Am J Vet Res. 1987;48:339–341. [PubMed] [Google Scholar]
- 35.Lucey D M, Dolan J, Moss C W, Garcia M, Hollis D G, Wegner S, Moran G, Almeida R, Leong D, Greisen K S, Welch D F, Slater L N. Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: implication for therapy and new epidemiological associations. Clin Infect Dis. 1992;14:683–688. doi: 10.1093/clinids/14.3.683. [DOI] [PubMed] [Google Scholar]
- 36.Magnarelli L A, Dumler J S, Anderson J F, Johnson R C, Fikrig E. Coexistence of antibodies to tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in human sera. J Clin Microbiol. 1995;33:3054–3057. doi: 10.1128/jcm.33.11.3054-3057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magnarelli L A, Ijdo J W, Anderson J F, Madigan J E, Dumler J S, Fikrig E. Antibodies to Ehrlichia equi in dogs from the northeastern United States. J Am Vet Med Assoc. 1997;211:1134–1137. [PubMed] [Google Scholar]
- 38.Mitchell P D, Reed K D, Hofkes J M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34:724–727. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyindo M, Huxsoll D L, Ristic M, Kakoma I, Brown J L, Carson C A, Stephenson E H. Cell-mediated and humoral immune responses of German shepherd dogs and beagles to experimental infection with Ehrlichia canis. Am J Vet Res. 1979;41:250–255. [PubMed] [Google Scholar]
- 40.Pancholi P, Kolbert C P, Mitchell P D, et al. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 41.Pappalardo B L, Correa M T, York C C, Peat C Y, Breitschwerdt E B. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am J Vet Res. 1997;58:467–471. [PubMed] [Google Scholar]
- 42.Sexton D J, Corey G R, Carpenter C, Kong L Q, Gandhi T, Breitschwerdt E, Hegarty B, Chen S M, Feng H M, Yu X J, Olano J, Walker D H, Dumler S J. Dual infection with Ehrlichia chaffeensis and a spotted fever group rickettsia: a case report. Emerg Infect Dis. 1998;4:311–316. doi: 10.3201/eid0402.980222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson R M, Gaunt S D, Hair J A, Kocan K M, Henk W G, Casey H W. Evaluation of Rhipicephalus sanguineus as a potential biologic vector of Ehrlichia platys. Am J Vet Res. 1991;52:1537–1541. [PubMed] [Google Scholar]
- 44.Staub-Schmidt T, Jaulhac B, Christmann D. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Epidemiology of cat scratch disease: a new case transmitted by a tick bite, abstr. K35; p. 294. [Google Scholar]
- 45.Suksawat, J., B. C. Hegarty, and E. B. Breitschwerdt. Seroprevalence of Ehrlichia canis, Ehrlichia equi and Ehrlichia risticii in sick dogs from North Carolina and Virginia. Submitted for publication. [DOI] [PubMed]
- 46.Walker D H, Barbour A G, Oliver J H, Lane R S, Dumler S J, Dennis D T, Persing D H, Azad A F, McSweegan E. Emerging bacterial zoonotic and vector-borne diseases. JAMA. 1996;275:463–469. [PubMed] [Google Scholar]
- 47.Weller S J, Baldridge G D, Munderloch U G, Noda H, Simser J, Kurtti T J. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J Clin Microbiol. 1998;36:1305–1317. doi: 10.1128/jcm.36.5.1305-1317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilfert C M, MacCormack J N, Kleeman K, Philip R N, Austin E, Dickinson V, Turner L. The prevalence of antibodies to Rickettsia rickettsii in an area endemic for Rocky Mountain spotted fever. J Infect Dis. 1985;151:823–831. doi: 10.1093/infdis/151.5.823. [DOI] [PubMed] [Google Scholar]