Abstract
Background:
Clinical diagnosis of vesiculobullous disorders (VBD) is not always straightforward. It is a challenge for a dermatologist to make the right diagnosis noninvasively in a short time.
Objective:
To evaluate dermoscopic patterns associated with vesiculobullous disorders.
Methods:
A total of 230 patients, irrespective of age and gender, with a history and clinical presentation suggestive of VBD (including primarily infectious, inflammatory, genetic, antibody-mediated, mechanical, environmental, metabolic, and drug-related) were recruited into the study. Patients with secondarily infected lesions were excluded. Dermoscopic examination along with Tzanck smear/skin biopsy smear test was performed on the most representative lesions. Data were compiled and statistically analyzed using SPSS version 21.0.
Results:
Lesions with erythematous (vascular) and yellowish (serum) translucent background with regular margins were seen in most of the VBD studied. Chickenpox (CP) and herpes zoster (HZ) lesions evolved with the progress of their clinical stages. Follicular and eccrine openings were commonly seen, but the pigmentation around them was specific to pemphigus vulgaris. A distorted pigment network was noted in bullous pemphigoid. White rosettes (keratin blockage) were characteristic of epidermolysis bullosa, Wickham striae (orthokeratosis) of lichen planus, and crumpled fabric appearance (flaccidity) of Hailey-Hailey disease. Globules/dots (microvesicles) of different colors were also seen in various VBD. Blue/black color usually corresponded to retained melanin.
Conclusion:
Some dermoscopic patterns are observed consistently with certain diseases, and these can be used for their diagnosis, complementary to histopathological examination.
Key Words: Blister, dermatology, dermoscopy, diagnosis, skin diseases
Introduction
Vesiculobullous disorders (VBD) are a type of mucocutaneous diseases characterized by fluid-filled lesions called vesicles (<5–10 mm) and bullae (>5–10 mm), which may rupture, leaving behind erosions and ulcerations.[1] They usually have an underlying infectious, autoimmune, or genetic etiopathology, and are diagnosed on clinical, histopathological, and immunological grounds.[2] However, overlap in the clinical presentation of these pathologies as well as the time, expenditure, and morbidity associated with biopsy examination engenders the need for better alternatives.[2,3,4,5,6]
Dermoscopy is a non-invasive skin imaging technique performed using a handheld device called a dermatoscope that permits the visualization as well as recording of subsurface structures, colors, and patterns in skin lesions, often imperceptible to the naked eye. The working principle behind it lies in modifying the cutaneous air-tissue optical interface and providing magnification (typically 10X).[3,4,5,6] It is functionally similar to a magnifying lens but with the added features of an inbuilt illuminating system, a higher adjustable magnification, the ability to assess structures as deep as in the reticular dermis, immediate availability of the results, and the ability to record and store images. A rapid, efficient, and safe diagnostic tool—the dermatoscope is now considered as the dermatologists' stethoscope.[7]
Some dermoscopic patterns are observed consistently with certain diseases, which makes it a useful aid for differential diagnosis, prognostic evaluation, and treatment response monitoring.[8,9] Dermoscopy can identify both pigmented as well as non-pigmented subtle vascular structures like hemorrhagic areas, inflammations, infections/infestations, and amelanotic neoplasms. Thus, this in-office procedure may eventually obviate the need for skin biopsy for diagnosis and follow-up.[10,11] However, dermoscopic findings need to be correlated with the clinical and histological characteristics of these lesions in order to validate it as a competitive method or better alternative to biopsy and establish it as a link between microscopic and macroscopic features. Hence, this study aims to establish the differentiating dermoscopic patterns for various VBD and correlate them with their corresponding clinical and histopathological characteristics.
Materials and Methods
This hospital-based, cross-sectional study was conducted at a tertiary care teaching hospital, Maharashtra, India from November 2017 to October 2019, after obtaining ethical clearance from the Institutional Review Board. A total of 230 patients, irrespective of age and gender, reporting to the outpatient section of this hospital department with a history and clinical presentation suggestive of VBD (including primarily infectious, inflammatory, genetic, antibody-mediated, mechanical, environmental, metabolic, and drug-related VBD) were recruited into the study by non-probability purposive sampling technique, after obtaining written informed consent from them. Patients with secondarily infected lesions were excluded from the study. A complete history was recorded, and a thorough dermatological examination was performed. Variables such as age, gender, site, and duration of the lesions were documented. The VBD studied included infections like varicella/chickenpox (CP), herpes zoster (HZ), herpes labialis (HL), and hand-foot-and-mouth disease (HFMD) as well as immunological disorders like bullous pemphigoid (BP), pemphigus vulgaris (PV), pemphigus foliaceus (PF), pompholyx (PX), erythema multiforme (EM), linear IgA disease (LID), lichen planus pemphigoides (LPP), bullous lichen planus (BLP), epidermolysis bullosa (EB), bullous fixed drug eruptions (BFDE), contact dermatitis (CD), insect bite reaction (IBR), and Hailey-Hailey disease (HHD). The terminology and criteria used to describe the dermoscopic findings of these VBD followed the International Dermoscopy Society consensus.[12]
Dermoscopic examination: It was performed on the most representative lesions of the respective diseases using the DermLite DL4 dermatoscope (3Gen Inc., San Juan Capistrano, CA, USA) (10X magnification) with an attached mobile phone camera to record the images. Both polarized and non-polarized modes were used with ultrasound gel as an interface medium.
Histological examination: Tzanck smears and skin biopsies were also obtained from these representative lesions to confirm the diagnosis, whenever necessary. The dermoscopic findings were correlated with the histopathological findings.
Statistical analysis: Data were collected, compiled, and analyzed using SPSS software 21.0 version. Categorical variables were expressed in terms of frequencies and percentages. Chi-square test was employed to determine the association between the final diagnosis of VBD and other study parameters. P < 0.05 was considered statistically significant.
Results
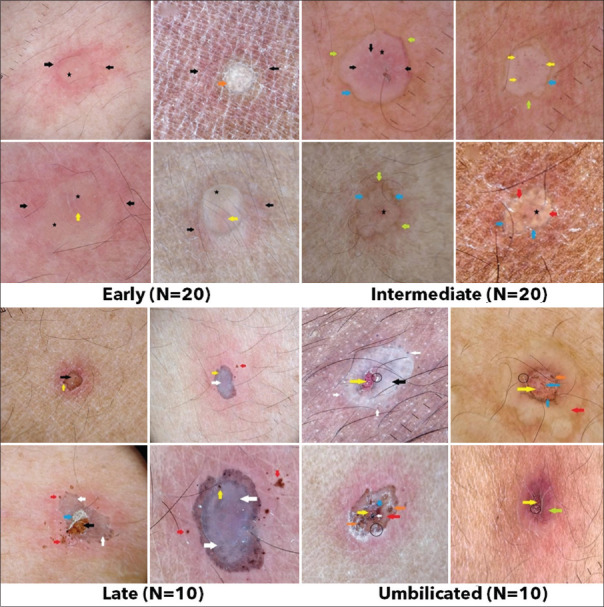
The mean age of the included participants was 35.33 ± 17.77 years. The mean duration of the lesions was 4 days (ranging from 1 to 21 days). The frequency distribution of the various characteristics of the study subjects is shown in Table 1. The dermoscopic findings in CP had a light erythematous background with white globules in early and intermediate stages [Figure 1]; whereas, late and umbilicated stages exhibited black and red dots, respectively. The lesion surfaces in different stages were observed to be pale yellow translucent structureless area, brownish rim, brown amorphous, crater surrounded by structureless grayish-white area for the early, intermediate, late, umbilicated consecutively. HZ infections were characterized by bright erythematous background [Figure 2]. The early stages of HZ lesions exhibited cloudy polylobular lacunae of purple, pale pink color with brown, red globules surrounded with white halos. The intermediate stage had pale pink (yellow stars), grayish-black structureless lesion surface with grayish-black globules, brownish-black dots, and irregular white globules. Furthermore, late-stage HZ was represented by grayish, bluish-gray, and brownish-gray structureless lesion surface with brown dots and globules in solar eclipse pattern. Table 2 and Figure 3 summarize the dermoscopic features observed in the various VBD. Correlation between the histopathological and the dermoscopic findings in these VBD is presented in Table 3.
Table 1.
Distribution of patients based on gender, type of vesiculobullous disorder, and dermoscopic findings in various cases (n=230)
| Variable | Subcategories | Frequency | Percentage (%) |
|---|---|---|---|
| Gender | Male | 120 | 52.17 |
| Female | 110 | 47.83 | |
| Type of vesiculobullous disorder | Varicella (Chickenpox) | 60 | 26.08 |
| Herpes Zoster | 50 | 21.73 | |
| Pemphigus Vulgaris | 30 | 13.04 | |
| Bullous Pemphigoid | 30 | 13.04 | |
| Herpes Labialis | 10 | 04.34 | |
| Pompholyx | 10 | 04.34 | |
| Insect Bite Reaction | 5 | 02.17 | |
| Contact Dermatitis | 5 | 02.17 | |
| Hand-Foot-and-Mouth Disease | 5 | 02.17 | |
| Pemphigus Foliaceus | 5 | 02.17 | |
| Erythema Multiforme | 5 | 02.17 | |
| Linear IgA Disease | 3 | 02.17 | |
| Bullous FDE | 3 | 01.30 | |
| Lichen Planus Pemphigoides | 3 | 01.30 | |
| Bullous Lichen Planus | 2 | 00.86 | |
| Epidermolysis Bullosa | 2 | 00.86 | |
| Hailey-Hailey disease | 2 | 00.86 | |
| Dermoscopic findings in various cases | Yellowish-pink translucent area | 220 | 95.65 |
| Surrounding Erythema | 210 | 91.30 | |
| Regular margins | 205 | 89.13 | |
| Absent pigment network | 184 | 80 | |
| Absent eccrine opening | 165 | 71.73 | |
| Absent follicular openings | 152 | 66.08 | |
| Black/Brown dots | 60 | 26.08 |
Figure 1.
Dermoscopic findings in various stages of chickenpox
Figure 2.
Dermoscopic findings in various stages of herpes zoster
Table 2.
Dermoscopic findings in various vesiculobullous disorders
| Vesiculobullous disorders | Lesion surface | Globules/Dots | Other features | Background/Periphery |
|---|---|---|---|---|
| Pemphigus vulgaris | Yellowish-pink translucent area | Linear white folds (white arrow) | Peripheral gray margin (blue arrow) | |
| Absent pigment network | White cloudy areas (hypopyon) (yellow arrow) | Erythematous background | ||
| Central brown area (brown arrow) | Follicular and eccrine openings | |||
| Bullous pemphigoid | Yellowish-pink translucent areas | Prominent follicular and eccrine openings | Erythematous | |
| Distorted pigment network (black arrow) | Perifollicular and perieccrine pigmentation | |||
| Herpes labialis | Yellowish-pink translucent areas | White globules | Reticular network (white arrow) | |
| Patchy peripheral pigment network. | Pink lacunae (star) | |||
| Crater | ||||
| Pompholyx | Yellow translucent areas | White globules | White reticular network (white arrows) | Gray-brown rim |
| White structureless areas | Yellowish-pink lacunae | |||
| Insect bite reaction | Yellow translucent areas (yellow star) | Crater | Erythematous background (red dots) | |
| Contact dermatitis | Yellowish-white translucent areas | Irregular, thick, white reticular network (white arrow) | Pink and blue background | |
| White structureless area (black arrow) | ||||
| Retained pigment network | ||||
| Hand-foot-and-mouth disease | Central bluish-gray globules | Black/gray dots | White halo (white arrow) | Peripheral erythema |
| Pemphigus foliaceus | Brownish-pink translucent area | Black/gray dots | White linear folds (white arrow), | Brown/gray rim (black arrow) |
| Follicular and eccrine openings preserved | Peripheral erythema | |||
| Erythema multiforme | Yellowish translucent area (star) | Red, blue, and black dots at the center (red arrow) | Peripheral erythema | |
| Bullous fixed drug eruptions | Dark brown pigment network | Multiple grouped black, brown, and steel blue dots and globules | Prominent eccrine openings | Irregular margin |
| Bullous lichen planus | Yellow structureless areas | Bluish-gray/black dots and globules | Leaf venation pattern of Wickham striae | Orange and bluish-pink background |
| Yellow structures | Prominent eccrine openings | |||
| Epidermolysis bullosa | Patchy pigment network | White globules | White shiny rosettes | Erythematous (red) |
| Dark brown dots and globules | White lacy network | |||
| Brown clods | ||||
| Hailey-Hailey disease (HHD) | Irregular pinkish-white areas separated by pink furrows | Irregular brownish-grayish-black crusting | Cloud-like white areas (crumpled fabric appearance) |
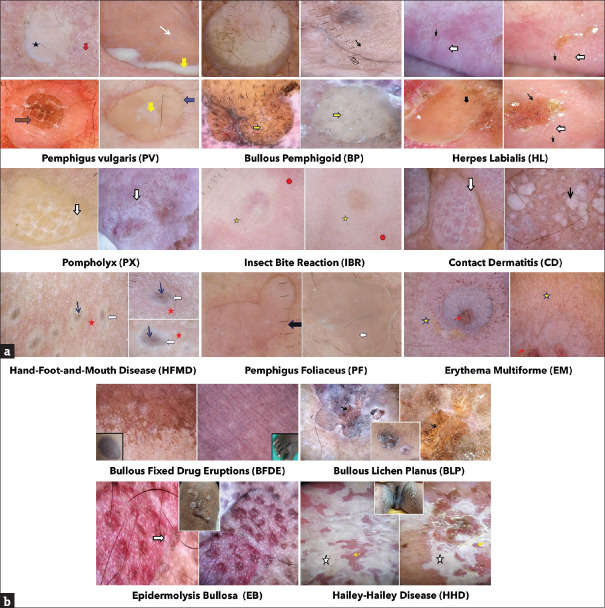
Figure 3.
(a): Dermoscopic findings in various vesiculobullous disorders. (b) Dermoscopic findings in various vesiculobullous disorders
Table 3.
Correlation between histopathological and dermoscopic findings in the various vesiculobullous disorders
| Dermoscopic patterns | Histopathological correlation |
|---|---|
| Yellowish translucent area | Serum |
| White globules | Spongiotic microvesicles within epidermis |
| Pink area | Dilated and tortuous blood vessels |
| Orange-yellow globules | Serum and red blood cells within blister cavity |
| Grayish area | Necrotic pigmented epithelium over regenerating non-pigmented epithelium |
| Brown amorphous area | Dried serum, necrotic keratinocytes, and melanin |
| Perilesional brown zone | Post-inflammatory pigment and hemosiderin at periphery |
| White lines | Ballooning degeneration of cells and water retention |
| Brown dots | Necrotic, ballooned, and degenerated cells with melanin retention |
| Scales | Hyperkeratosis |
| Blue-gray globules | Dermal melanin |
| White shiny rosettes | Narrowing of acrosyringeal infundibula or blockage by keratin |
| Wickham striae | Compact orthokeratosis and dermal fibrosis |
| Irregular white network | Spongiosis, papillodermal edema |
| White linear folds | Overlap of superficial epithelium due to flaccidity |
Dermoscopically, a yellowish translucent background, corresponding to the serum inside the vesicles, was seen in most of the VBD including CP (90%), BP (70%), PV (80%), HL (80%), PX (90%), IBR (100%), CD (100%), EM (80%), LID, LPP, and BLP. In the lesions of CP, an increase in turbidity of the vesicular fluid with the aging of the lesions gave rise to an opaque, structureless area. Multiple-grouped vesicles divided by white linear strands appeared as cloudy, polylobular lacunae in early HZ. An erythematous background or periphery, associated with increased vascularity secondary to inflammation, was also prevalent in almost all lesions of CP (100%), HZ (100%), HFMD (100%), PF (100%), IBR (100%), HHD (100%), and EM (100%). The appearance of the CP (70%), PF (100%), and BLP lesions evolved into a brownish-pink translucent area as the blood vessels became more dilated and tortuous. Grayish, amorphous, structureless areas were also seen in CP (50%), HZ (20%), and HHD lesions due to the presence of necrotic, pigmented epithelium over regenerating non-pigmented epithelium. A brownish hue to this amorphous area was imparted by dried serum, necrotic keratinocytes, and melanin in CP (50%) and PV (63.3%). White structureless areas similar to regenerating epidermal keratinocytes were seen in CP, PX (60%), and CD (100%). Some lesions like CP (100%), PF (60%), LID, PV (56.6%), and PX (30%) showed a brown or gray-brown rim around the lesions owing to post-inflammatory pigment and hemosiderin deposition. The margins were mostly regular in all lesions, except HHD and BFDE.
Neutrophilic spongiosis (at the periphery of the blister) gave rise to white cloudy areas (hypopyon) in PV lesions (26.6%). White lines and reticular networks were noted in HZ (90%), HL (70%), PX (80%), EB (80%), and CD (80%) as a dermoscopic sign of ballooning degeneration of cells and water retention. This looked like a white halo in HFMD (100% of cases). These were differentiated from the arborizing leaf venation pattern of Wickham striae seen in BLP due to compact orthokeratosis and dermal fibrosis. Overlap of the superficial epithelium due to flaccidity led to the white linear folds observed in PV (83.3%), PF (80%), and HHD (crumpled fabric appearance).
White globules corresponding histologically to spongiotic microvesicles within the epidermis were also recorded in CP (10–30%), HZ (20–80% I), HL (40%), PX (70%), and EB. In certain cases of CP (10%), HZ (10–50%), and EB, the serum and red blood cells (RBC) accumulated within the blister cavity were perceived as orange-yellow or brown/red globules. Dermal melanin accumulation imparted the globules a blue-gray hue in HZ (805) and HFMD (100%) lesions. Necrotic, ballooned, and degenerated keratinocytes with melanin retention presented as gray/brown/black dots in CP (10–70% L), HZ (50%), EB, PF (60%), EM (60%), BLP, and HFMD (80%). Comparative analysis showed a statistically significant difference (P < 0.001) in the appearance of white globules and brown dots in CP and HZ.
Craters were seen in CP (100% of umbilicate lesions), HL (30%), and IBR (60%). Pink to yellowish-pink lacunae were noted in HL (70%) and PX (70%). Further, CP lesions (late stages) and HHD also revealed white superficial scales representing underlying hyperkeratosis. Narrowing of acrosyringeal infundibula or blockage by keratin appeared as white shiny rosettes, a characteristic feature of EB.
Follicular openings were retained in BP (83.3%), PV (70%), LID, and LPP; whereas eccrine openings were prominent in BP (80%), PV (60%), LID, LPP, BFDE, BLP, and HHD (resembling milia-like cysts in chronic stage). Perifollicular and perieccrine pigmentation was characteristic of BP, seen in approximately 30% of the cases, which differentiated it from PV.
A distorted pigment network (due to subepidermal vesiculation, papillary dermal edema, and mild spongiosis) was observed in LID and BP (93.3%), which in case of BP, consisted of reticular lines (70%), curved lines (26.6%), and circles (30%), and was colored dark brown (73.3%) or light brown (20%), depending upon the amount and turbidity of the fluid. This differentiated BP from PV (P < 0.001). BFDE and CD (20%) also retained pigment networks, which showed a patchy peripheral distribution in HL (20%) and EB but were absent in PVV (93.3%) and LPP.
Discussion
This study was conducted to establish the differentiating dermoscopic patterns for various VBD and correlate them with their corresponding clinical and histopathological characteristics. There are limited studies available on this topic, especially those describing stage-wise evolution of these diseases. Hence, this study offers a unique perspective on dermatological diagnoses, utilizing a safe, efficient, rapid, and non-invasive diagnostic tool that correlates well with the respective clinicohistological features. Interestingly, the dermoscopic findings in the various stages on these VBD were consistent in all patients irrespective of age, gender, and site of involvement.
Lesional appearances of CP, HZ, HL, PV, BP, PF, EM, HFMD, and IBR, similar to the present study, were noted by Nayak et al.[13] The stage-wise evolution of CP lesions was in concordance with that noted by Bajaj et al.,[14] Celebi et al.,[15] Johr et al.,[16] Weismann et al.,[17] and Xu et al.[18] The 'cobblestone' appearance of early lesions (multiple grouped vesicles), 'reticulate' pattern of intermediate lesions (as lobules coalesced together), and 'solar eclipse' (necrotic grayish center surrounded by bright red inflammatory halo) type of manifestation of late stages of HZ were also noted by these researchers.[14,15,16,17,18]
A statistically significant difference (P < 0.001) was seen between CP and HZ in the appearance of (i) white globules (microvesicles) which were more pronounced in HZ, and (ii) brown dots which were seen earlier in the course of HZ. Multilobulated structures of HZ were not noted in CP. Also, the erythematous background was lighter in CP (milder inflammation) and brighter in HZ (acute and intense inflammation due to vasculitis-induced epidermal/dermal damage in the early stages of HZ).[18]
Comparative analysis between PV and BP also showed a statistically significant difference (P < 0.001) in terms of (i) a distorted pigment network which was prominent in BP due to subepidermal vesiculation causing elevation of superficial structures but absent in PV due to intraepidermal vesiculation pushing the basal layer down as well as (ii) perifollicular and perieccrine pigmentation notable in BP but not in PV. According to Nayak et al.,[13] the yellow translucent areas without follicular/eccrine openings in IBR correlated to intraepidermal blisters and inflammatory reactions, while craters corresponded to erosions. In a case of blister beetle dermatitis, dermoscopy showed brown/black dots on a gray background surrounded by a white halo (due to dermal edema) and erythema.
In EM cases, Kaliyadan reported red/blue/purple/black clods in the central dusky zone and a pale edematous zone and peripherally along with a few short linear vessels, characteristic of the typical target or iris lesions.[19,20] In BFDE, color variations depend on the location of melanin in the skin—jet black in stratum corneum and upper epidermis; brown in the basal layer and dermoepidermal junction; blue-gray in papillary dermis; and steel blue in reticular dermis. These variations are due to the Tyndall effect caused by short-wavelength visible light (blue) dispersed and reflected more than long-wavelength light (red). These resonate with the findings of Nayak et al.[13] and Valdebran et al.[21] Pearly white Wickham striae are the diagnostic feature for LP, histopathologically related to compact orthokeratosis over wedge-shaped hypergranulosis, acanthosis, and dermal fibrosis.[22] Blue-gray globules (pigment-laden melanophages in the dermis) are also specific for LP diagnosis.[23] EB lesions are characterized by shiny white rosettes (resembling a four-leaf clover, 0.2–0.5 mm in size).[24,25] They are postulated to result from the optical effect of polarized light, narrowing of infundibula, blockage by keratin, or alternating areas of focal hyperkeratosis, normal corneal layer, and keratin-filled acrosyringeal openings.[25,26] The findings seen in HHD are in line with those of Kelati et al.[27 and Chauhan et al.[28] The crumpled fabric appearance represents grouped flaccid vesiculo-pustules.
Our study establishes dermoscopic features for various VBD, correlating them histologically. Thorough knowledge of the physics of the device and dermoscopic patterns is imperative to use this technique efficiently. This in-office handy procedure may obviate the need for skin biopsy for diagnosis and follow-up in the future. However, currently, dermoscopy is not yet an alternative but a complementary tool to histopathological examination. In our study, various dermoscopic findings are consistent in vesiculobullous diseases. Knowledge of these findings can help in the early diagnosis and differentiation of similar diseases. It gives an added benefit to the accuracy of clinical diagnosis.
This study has its limitations in being a single-centered, cross-sectional study with limited sample size. Also, very few studies are available for review and comparison. Multicentric, prospective studies with larger sample size and longer follow-up periods are encouraged to validate the results.
Conclusion
Some dermoscopic patterns are observed consistently with certain diseases, which can be used for their diagnosis. These dermoscopic findings can aid the diagnosis and may obviate the need for biopsy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Magro CM, Roberts-Barnes J, Crowson AN. Direct immunofluorescence testing in the diagnosis of immunobullous disease, collagen vascular disease, and vascular injury syndromes. Dermatol Clin. 2012;30:763–98. doi: 10.1016/j.det.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi V, Sharma R, Misra SR, Yadav L. Diagnostic procedures for autoimmune vesiculobullous diseases: A review. J Oral Maxillofac Pathol. 2014;18:390–7. doi: 10.4103/0973-029X.151324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boĭtinskiĭ E, Gendel's BS, Leonov VM, Ryzhova TP, Tsinzerling VF. Clinico-electrophysiologic studies of experimental herpetic encephalitis. Zh Nevropatol Psikhiatr Im S S Korsakova. 1977;77:171–8. [PubMed] [Google Scholar]
- 4.Zalaudek I, Argenziano G, Di Stefani A, Ferrara G, Marghoob AA, Hofmann-Wellenhof R, et al. Dermoscopy in general dermatology. Dermatology. 2006;212:7–18. doi: 10.1159/000089015. [DOI] [PubMed] [Google Scholar]
- 5.Grimaldi L, Silvestri A, Brandi C, Nisi G, Brafa A, Calabrò M, et al. Digital epiluminescence dermoscopy for pigmented cutaneous lesions, primary care physicians, and telediagnosis: A useful tool? J Plast Reconstr Aesthet Surg. 2009;62:1054–8. doi: 10.1016/j.bjps.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Braun RP, Rabinovitz H, Tzu JE, Marghoob AA. Dermoscopy research-An update. Semin Cutan Med Surg. 2009;28:165–71. doi: 10.1016/j.sder.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Lallas A, Argenziano G. Dermatoscope-The dermatologist's stethoscope. Indian J Dermatol Venereol Leprol. 2014;80:493–4. doi: 10.4103/0378-6323.144141. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Marchetti MA, Marghoob AA. Dermoscopy: Not just for dermatologists. Melanoma Manag. 2015;2:63–73. doi: 10.2217/mmt.14.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: An update. J Am Acad Dermatol. 2018;79:1117–32. doi: 10.1016/j.jaad.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Kreusch JF. Vascular patterns in skin tumors. Clin Dermatol. 2002;20:248–54. doi: 10.1016/s0738-081x(02)00227-4. [DOI] [PubMed] [Google Scholar]
- 11.Zalaudek I, Giacomel J, Cabo H, Di Stefani A, Ferrara G, Hofmann-Wellenhof R, et al. Entodermoscopy: A new tool for diagnosing skin infections and infestations. Dermatology. 2008;216:14–23. doi: 10.1159/000109353. [DOI] [PubMed] [Google Scholar]
- 12.Errichetti E, Zalaudek I, Kittler H, Apalla Z, Argenziano G, Bakos R, et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): An expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol. 2020;182:454–67. doi: 10.1111/bjd.18125. [DOI] [PubMed] [Google Scholar]
- 13.Nayak SS, Mehta HH, Gajjar PC, Nimbark VN. Dermoscopy of general dermatological conditions in Indian population: A descriptive study. Clin Dermatol Rev. 2017;1:41–51. [Google Scholar]
- 14.Bajaj S, Marchetti MA, Navarrete-Dechent C, Dusza SW, Kose K, Marghoob AA. The role of color and morphologic characteristics in dermoscopic diagnosis. JAMA Dermatol. 2016;152:676–82. doi: 10.1001/jamadermatol.2016.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celebi ME, Kingravi HA, Uddin B, Iyatomi H, Aslandogan YA, Stoecker WV, et al. A methodological approach to the classification of dermoscopy images. Comput Med Imaging Graph. 2007;31:362–73. doi: 10.1016/j.compmedimag.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johr RH, Stolz W. Johr and Stolz's Dermoscopy-An Illustrated Self-Assessment Guide. 1st ed. New York: McGraw-Hill; 2010. Dermoscopy from A to Z; pp. 1–26. [Google Scholar]
- 17.Weismann K, Lorentzen HF. Dermoscopic color perspective. Arch Dermatol. 2006;142:1250. doi: 10.1001/archderm.142.9.1250. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Yun SJ, Erickson L, Chen L. Disease caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, Murphy GF, Rubin AI, Xu X, editors. Lever's Histopathology of the Skin. 11th ed. Philadelphia: Wolters Kluwer; 2015. pp. 781–815. [Google Scholar]
- 19.Kaliyadan F. Dermoscopy of erythema multiforme. Indian Dermatol Online J. 2017;8:75. doi: 10.4103/2229-5178.198771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vázquez-López F, Kreusch J, Marghoob AA. Dermoscopic semiology: Further insights into vascular features by screening a large spectrum of nontumoral skin lesions. Br J Dermatol. 2004;150:226–31. doi: 10.1111/j.1365-2133.2004.05753.x. [DOI] [PubMed] [Google Scholar]
- 21.Valdebran M, Salinas RI, Ramirez N, Rodriguez A, Guzman L, Marte S, et al. Fixed drug eruption of the eyelids: A dermoscopic evaluation. Our Dermatol Online. 2013;4:344–6. [Google Scholar]
- 22.Ankad BS, Beergouder SL. Hypertrophic lichen planus versus prurigonodularis: A dermoscopic perspective. Dermatol Pract Concept. 2016;6:9–15. doi: 10.5826/dpc.0602a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanumaiah B, Joseph JM. Role of dermoscopy in the diagnosis of hypertrophic lichen planus and prurigo nodularis. Indian J Dermatol. 2019;64:341–5. doi: 10.4103/ijd.IJD_123_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuellar F, Vilalta A, Puig S, Palou J, Salerni G, Malvehy J. New dermoscopic pattern in actinic keratosis and related conditions. Arch Dermatol. 2009;145:732. doi: 10.1001/archdermatol.2009.86. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Alvarez T, Armengot-Carbo M, Barriero A, Alarcón I, Carrera C, García A, et al. Dermatoscopic rosettes as a clue for pigmented incipient melanoma. Dermatology. 2014;228:31–3. doi: 10.1159/000356822. [DOI] [PubMed] [Google Scholar]
- 26.Haspeslagh M, Noe M, De Wispelaere I, Degryse N, Vossaert K, Lanssens S, et al. Rosettes and other white shiny structures in polarized dermoscopy: Histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311–3. doi: 10.1111/jdv.13080. [DOI] [PubMed] [Google Scholar]
- 27.Kelati A, Argenziano G, Mernissi FZ. Dermoscopic presentation of Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:S31–3. doi: 10.1016/j.jaad.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan P, Meena D, Hazarika N. Dermoscopy of Hailey Hailey disease. Indian Dermatol Online J. 2018;9:139–40. doi: 10.4103/idoj.IDOJ_202_17. [DOI] [PMC free article] [PubMed] [Google Scholar]