Abstract
Background:
Psoriasis is a chronic, inflammatory, immune-mediated, debilitating skin disease affecting approximately 2%–3% of the global population. Various treatment modalities are available for extensive psoriasis which include methotrexate, cyclosporine, retinoids, oral PUVA therapy, and biologic agents.
Aims and Objectives:
The aim of this study was to compare the effectiveness and safety of methotrexate vs. PUVA in severe chronic plaque psoriasis with BSA >20%.
Materials and Methods:
A randomized open-label clinical study was performed. Sixty patients with extensive stable plaque psoriasis were recruited in the study. Thirty patients received methotrexate at a dose of 0.4 mg/kg up to a maximum of 15 mg/week and the rest 30 were treated by PUVA, 8-methoxy psoralen tablet (20 mg) followed by UVA started at the dose of 1 j/cm2 thrice weekly with an increment of 20% dose every third sitting until 2.5 j/cm2 is reached. Both forms of treatment were continued for 10 weeks or until PASI 90 achieved, which-ever was earlier. Clinical examination, blood investigation, PASI scoring, and photograph were repeated in serial intervals during the study. At the end of study, the data were compiled, tabulated, and analyzed.
Result:
In the PUVA group, 90% achieved PASI-50 and 63.33% achieved PASI-90, in the methotrexate group all patients achieved both PASI-50 and PASI-90. Methotrexate acted significantly faster than PUVA in disease clearance. In the methotrexate group decreased platelet count in 13.33% patients, decreased hemoglobin (<10 gm/dl), elevated liver enzyme, each of these developed in 10% of patients. In the PUVA group, no serious side effects were observed.
Conclusions:
Methotrexate is more efficacious with lesser incidence of subjective complications and more incidence of laboratory complications compared to PUVA in extensive plaque psoriasis.
Key Words: Comparative effectiveness, extensive plaque psoriasis, safety of methotrexate, PUVA, PASI
Introduction
Psoriasis is a chronic, inflammatory, immune-mediated, debilitating skin disease affecting approximately 2%–3% of the global population.[1] Nowadays, it is considered as a systemic disease. According to National Psoriasis Foundation, patients with less than 3% of BSA involvement were considered to have mild psoriasis, between 3% and 10% of the BSA affected as moderate case and more than 10% BSA involvement is considered severe. (The surface area of the hand equals about 1% of the skin.).[2] PASI (Psoriasis Area and Severity Index) score can also be used to assess severity of psoriasis. PASI score ranges from 0 to 72, mild 0–3, moderate 3–10, and severe >10.[3] As per Roenigk et al., methotrexate is commonly indicated in case of extensive, severe plaque psoriasis: not responsive to conventional therapy (usually >20% surface involvement).[4] We have included patients with >20% BSA involvement in our study.
Methotrexate inhibits DNA synthesis by competitive inhibition of dihydrofolate reductase, inhibiting the proliferation of lymphoid cell lines in addition to keratinocytes. PUVA acts through immuno-modulatory and anti-proliferative actions. It also generates reactive oxygen species which damage DNA, cell membranes and cytoplasmic constituents.[5] In a resource-poor country such as ours, methotrexate remains the mainline therapy for moderate to severe psoriasis because of its cost-effectiveness and proven benefit. Simultaneously, photochemotherapy with psoralen-UVA (PUVA) has been mastering the field of phototherapy of extensive plaque psoriasis for years. So we intended to compare the effectiveness and safety of PUVA in comparison to methotrexate in chronic stable plaque psoriasis patients with BSA >20% with normal hepatic and renal function.
Aims and objectives
The study was performed for the following purposes:
To compare the effectiveness and safety of Methotrexate versus PUVA in severe chronic stable plaque psoriasis with BSA >20%.
To assess cumulative dose required to control psoriasis in case of PUVA and Methotrexate in Indian patients.
Materials and Methods
A randomized open-label clinical study was performed comparing the effectiveness and safety of PUVA with that of methotrexate in severe chronic stable plaque psoriasis. Sixty patients with chronic stable plaque psoriasis involving >20% BSA who were not on any systemic treatment or phototherapy at that time attending the Dermatology Out Patient Department of a tertiary care medical college of eastern India, from March 2015 to February 2016 were recruited in the study. The sample size was determined by following the formula of comparing two proportions, n =[[(Zα/2 + Zβ)2 × {(p1 (1–p1) + (p2 (1–p2))}]/(p1 – p2)2][6]
Where n = sample size required in each group,
p1 = Proportion of subject who achieve therapeutic endpoint by methotrexate = 0.40 (in accordance to reference no).[7]
p2 = proportion of subject who achieve therapeutic endpoint by PUVA = 0.84, (in accordance to reference no.[5])
p1-p2 = clinically significant difference = 0.44
Zα/2: this depends on level of significance, for 5% this is 1.96
Zβ: this depends on power, for 95% this is 1.64
Based on above formula the sample size was [(1.96 + 1.64)2× {(0.40 (1-0.40) + (0.84 (1-0.84))}]/(0.44)2
= 26 (approx) in each arm. i.e. total 52. Keeping in mind 10% dropout rate the final sample size was 60 (approx).
Patients were incorporated in the study by Restricted (Block) Randomization. Two Blocks of 30 Patients were taken and randomly allotted to either PUVA or Methotrexate by computer-generated randomization schedule.
A detailed clinical examination, PASI scoring along with photographic evidence of the most affected part of the body was taken at the beginning of the study. Baseline blood investigation was done which included Routine Hemogram, Fasting blood sugar, Electrolytes like Sodium, Potassium, Renal function test, Liver function test. Apart from this, Serum lipid profile and Serum uric acid estimation were also done.
Thirty patients received Methotrexate at a dose of 0.4 mg/kg up to a maximum 15 mg/week and rest were treated by PUVA following 8-methoxypsoralen tablet (20 mg) which was given 2 h prior to PUVA treatment. PUVA started at the dose of 1 j/cm2 thrice weekly with an increment of 20% dose every third sitting until 2.5 j/cm2 is reached. Both forms of treatment were continued for 10 weeks or until the PASI score was reduced by 90%, which-ever was earlier. Clinical examination, Blood investigation, PASI scoring, and photographs were repeated in serial intervals during the study.
At the end of the study, the data were compiled, tabulated, and analyzed with appropriate statistical tests using medical statistical software (MedCal C statistical software version 12.2.1.0).
Results
We have analyzed 60 patients of chronic stable plaque psoriasis.
Table 1 denotes the demographic profile of both groups.
Table 1.
Demographic profile
| Methotrexate group | PUVA group | P | |
|---|---|---|---|
| Average Age (years) | 43.2667+13.1698 | 40.3333+14.9720 | P=0.4237 |
| Sex ratio (M: F) | 1.5:1 | 2:1 | |
| BMI | <25-22 cases | <25 25 cases | P=0.3603 |
| >25-8 cases | >25-5 cases | ||
| Mean- 21.8830±3.5441 | Mean- 22.6520±4.0038 | ||
| Duration in years, mean±SD | 7.8±5.2 | 8.0±4.9 |
In PUVA group [Figure 1] 27 patients (90%) achieved PASI-50 (50% reduction in PASI score) and 19 patients (63.33%) achieved PASI-90 (90% reduction in PASI score), in methotrexate group [Figure 2] all patients achieved both PASI-50 and PASI-90. The difference was statistically significant (P < 0.05).
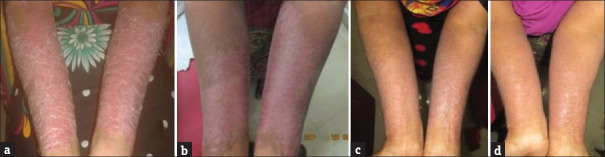
Figure 1.
Serial Picture of Patient treated with PUVA. (a) 0 week. (b) 4 weeks. (c) 8 weeks. (d) 10 weeks
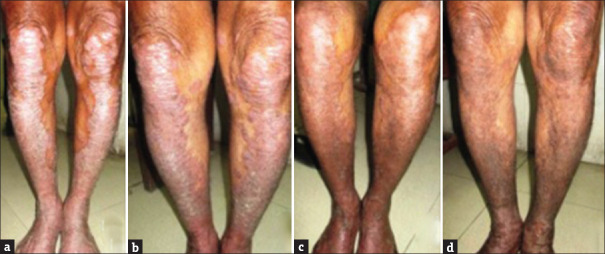
Figure 2.
Serial Picture of a Patient treated with methotrexate at (a) 0 week. (b) 4 weeks. (c) 8 weeks. (d) 10 weeks
Weeks required to achieve PASI-50 in methotrexate Group was 2.70 ± 0.79 weeks with PASI- 90 achieved in 6.17 ± 1.42 weeks. In PUVA group it took 4.96 ± 1.51 weeks (PASI-50) and 9.11 ± 0.81 weeks (PASI-90) respectively. The difference between the two groups was statistically significant (P < 0.05) in both instances.
The mean number of exposure required to achieve PASI-50 with PUVA was 14.70 ± 4.45 (median-15) and for PASI-90 it was 27.11 ± 2.42 (median-27). The mean dose required to achieve PASI-50 was 59.20 ± 11.25 j/cm2 and the mean dose required to achieve PASI-90 was 90.11 ± 6.06 j/cm2 in case of the PUVA group. The mean cumulative dose required to achieve PASI-50 was 48.00± 11.92mg and the mean dose required to achieve PASI-90 was 100.00 ± 21.24 mg in the case of the methotrexate group.
Table 2 shows comparative laboratory changes in both groups. The only change of alanine aminotransferase (ALT) in the methotrexate group (27.8033 + 11.2232 IU/L vs. 35.3800 + 15.1918 IU/L) was significant (P = 0.0320). Finally, when evaluating side effect profiles in each group it was noted as such: in methotrexate group observed side effects were (in decreasing frequency).
Table 2.
Comparison of Laboratory Parameters In Both Groups
| Laboratory tests | Groups | At 0 weeks | After 10 weeks | P |
|---|---|---|---|---|
| Haemoglobin | Methotrexate group | 12.7507+1.5254 | 12.3757+1.6229 | 0.3603(>0.05) |
| PUVA group | 12.5100+1.5988 | 12.4810+1.5115 | 0.9427(>0.05) | |
| P | 0.5531(>0.05) | 0.7957(>0.05) | ||
| TLC | Methotrexate group | 8313.3333+2228.9217 | 7883.3333+1532.8772 | 0.3880(>0.05) |
| PUVA group | 7803.3333+1862.9756 | 7840.0000+1541.2937 | 0.9341(>0.05) | |
| P | 0.3402(>0.05) | 0.9134(>0.05) | ||
| Platelet (in lakh) | Methotrexate group | 2.0533+0.4906 | 1.9310+0.6070 | 0.3942(>0.05) |
| PUVA group | 2.4113+0.5315 | 2.3897+0.4831 | 0.8693(>0.05) | |
| P | 0.0088 | 0.0020 | ||
| Bilirubin (mg/dl) | Methotrexate group | 0.6393+0.2602 | 0.7120+0.3101 | 0.3295(>0.05) |
| PUVA group | 0.7097+0.2554 | 0.7860+0.4189 | 0.3984(>0.05) | |
| P | 0.2950 | 0.4399 | ||
| ALT (IU/L) | Methotrexate group | 27.8033+11.2232 | 35.3800+15.1918 | 0.0320 |
| PUVA group | 30.5633+11.3952 | 35.3033+13.8870 | 0.1538(>0.05) | |
| P | (>0.05) | (>0.05) | ||
| AST (IU/L) | Methotrexate group | 28.2400+11.3865 | 33.9500+15.5623 | 0.1103(>0.05) |
| PUVA group | 29.0333+14.0117 | 32.8200+15.6123 | 0.3269(>0.05) | |
| P | (>0.05) | (>0.05) |
Decreased platelet count (<150000 per microliter) and nausea, vomiting. (in both cases 13.33% patients).
Decreased hemoglobin (<10 gm/dL), elevated liver enzymes (>2 times of upper normal limit), altered lipid profile (elevated LDL and Triglyceride level) (each of these developed in 10% of patients).
Decreased total leukocyte count (<4500/microliter) (developed in 3.33% patients).
No side effect was noted in 50% of cases.
In the PUVA group side effects observed were (in decreasing frequency)-
Dryness and itching (43.33% each)
Grade 1 erythema (40%)
Nausea and vomiting (33.33%)
Pigmentation (16.67%)
Exacerbation of lesion (13.33%)
Elevated bilirubin (Total bilirubin >2.0 mg/dL) (3.33%).
No side effect was observed in 33.33% of patients.
Discussion
In our study, both groups were well matched in terms of age distribution, sex, and BMI. The average age in the methotrexate group was 43.2667 ± 13.1698 years and in the PUVA population being 40.3333 ± 14.9720 years, which were not statistically different (P = 0.4237). It matches with the study from middle east Asia.[8] Male:female ratio in the methotrexate group was 1.5:1, whereas in the PUVA group it was 2:1. Indian studies like Okhlandier et al.,[9] Bedi et al.,[10] Kaur et al.[11] also had a similar sex ratio (male>female) in their studies.
In our study, PASI-90 (90% reduction in PASI score) was achieved in 100% patients after 6.17 ± 1.42 weeks in patients of methotrexate group with mean dose required to achieve PASI-50 was 48.00 ± 11.92 mg and mean dose required to achieve PASI-90 was 100.00 ± 21.24 mg. In a study,[12] response to methotrexate was showed as PASI-90 achieved in 40% of patients and the rest of the patients (60%) achieved PASI 75% after 6 ± 0.89 weeks.[12] Few other studies showed achievement of PASI 75 in 35.5% after 16 weeks,[13] PASI90 in 26.7%, PASI75 in 73.3%, PASI50 in 100% patients,[8] PASI 90 in 40% and PASI 75 in 60% patients after 16 weeks,[14] and PASI 75 only 29% after 12 weeks.[15] In another Indian study, though in below 18 years age group, all patients achieved clearance.[16] PASI 75 was achieved in 7.6 ± 3.36 weeks and PASI50 was achieved in 4.6 ± 2.46 weeks. Achievement in our study was near this. A table [Table 3] of comparison of adverse effects in various studies showed that side effects with Methotrexate observed in our study were comparable with them. Also, a study[17] showed 6.9% of patients having adverse events severe enough to limit their Methotrexate dosage, though no patient needed treatment discontinuation in our study and their adverse events were adequately controlled by simple measures. In addition, no patient in our Methotrexate study group developed any visible jaundice or any coagulation disorder during the study period.
Table 3.
Adverse effects of Methotrexate
| Study | Side effects | Pts/% | Drug continuation |
|---|---|---|---|
| Haider [12] | Raised liver enzymes | 13/86 pts | Discontinued |
| Saurat [13] | Total adverse events | 90 (81.Æ8%) | |
| Serious adverse events | 1 (0Æ9%) | ||
| Adverse events leading to discontinuation | 6 (5Æ5%) | ||
| Nausea | 8 (7Æ3%) | ||
| c-Glutamyltransferase elevation | 0 | ||
| Alanine aminotransferase >2Æ5 times the ULN | 4 (3Æ6%) | ||
| Aspartate aminotransferase >2Æ5 times the ULN | 2 (1.8%) | ||
| Total bilirubin >1Æ5 times the ULN | 4 (3.6%) | ||
| Vera M.R. Heydendael[14] | Total number of pts had adverse effect | 29/44 | 0 |
| Nausea | 19 of 43 | ||
| Serious or irreversible side effects | 0 | ||
| Shabeer [15] | Total AEs | 3 (17.65%) | 0 |
| Weakness/fatigue | 0 (0%) | ||
| Nausea/vomiting | 2 (11.74%) | ||
| Elevated liver enzymes (less than 1.5 times the normal level) | (0%) | ||
| Goyal et al.[16] | Serious or Irreversible Side Effects, Lab Derrangement | 0 | 0 |
| Nausea/Vomiting | 2/9 pts | ||
| West [17] | All adverse effects | 28.3% | 6.9% |
| Nausea/vomiting | 18.2% | ||
| Mouth ulcer | 11.1% | ||
| Abnormal LFT | 10% | ||
| Leucopenia | 3.4% | ||
| Our study | Adverse Events | 50% | Continued |
| Nausea And Vomiting | 13.33% | ||
| Decreased Platelet Count | 13.33% | ||
| Decreased Haemoglobin | 10%, | ||
| Elevated Liver Enzyme | 10% | ||
| Altered Lipid Profile | 10% | ||
| Leukopenia | 3.33% |
PASI-50 was achieved in 27 patients (90%) and near-complete remission (PASI-90) was achieved in 19 patients (63.33%) of PUVA group in our study. Table 4 shows a comparison of result of PUVA in different studies. In our study clearance rate of lesions with PUVA was lower compared to these studies. Also, our study took a longer time to achieve clearance, [Table 4]. This may be due to the difference in Fitzpatrick skin type because international studies were performed in patients of relatively lower Fitzpatrick skin types which usually respond well to phototherapy compared to Indian skin. This view is supported by another Indian Study of Kaur et al.[21]
Table 4.
Effects of PUVA in Psoriasis
| Study | Skin Type | Frequency/Week | Therapeutic Terget | Clearance Rate | Treatment Session | Mean Time Weeks | Mean Cumulative Dose j/cm2 |
|---|---|---|---|---|---|---|---|
| TAHIR AND MUJTABA[5] | NA | 3 | 85% | 17 | |||
| Yones[18] | I-IV | 2 | 84% | 17 | |||
| V-VI | 27% | ||||||
| GORDON[19] | I - IV | 2 | 84% | 16.7 | 70.1 | ||
| Archier et. Al[20] | PASI 75 | 80% | |||||
| KAUR [21] | IV- V | 3 | 75% | 22.66 | |||
| CHAUHAN[22] | IV- V | 3 | 82% | 29.8 | |||
| MELSKI[23] | 90% | ||||||
| DAYAL [24] | V-VI | 2 | PASI 50 | All | 12.7+4.9 | 7.4 | |
| PASI75 | all | ||||||
| PRESENT STUDY | V- VI | 3 | PASI 50 | 90% | 14.70+4.45 | 4.96+1.51 | 59.20+11.25 |
| PASI90 | 63.33% | 27.11+2.42 | 9.11+0.81 | 90.11+6.06 |
In our study, the most common side effects of PUVA therapy observed are dryness and itching (43.33% in both) followed by grade 1 erythema (40%). But one study[24] observed grade 1 erythema in 100% patients, grade 2 erythema in 70% of PUVA patients, whereas in our study grade 2 erythema developed in none. In another study[18] with skin type I-VI, erythema developed in 49% in total, 65% in type I-II to 17% in type V–VI patients. Other side effects like Pruritus in 80%, nausea in 75%, vertigo 75%, diffuse hair fall (70%) and headache (45%) in patients receiving PUVA patients[24] which is much higher than our patients. Though one of our patients in PUVA group developed elevated bilirubin (>2.0 mg/dL), it was an isolated finding and his pretreatment bilirubin was also high (total bilirubin – 1.5 mg/dL).
There is no study comparing Methotrexate and PUVA in Psoriasis as the modality of treatments are different. In our study as we observed Methotrexate reduces psoriasis lesion comparatively faster than PUVA (time required to achieve PASI-90 was 6.17 + 1.42 weeks for Methotrexate which is lesser in comparison to time required to achieve PASI-90 with PUVA which was 9.11 + 0.81 weeks (P < 0.0001) [Figure 1]. Also PASI reduction is much more greater with Methotrexate compared to PUVA. (In methotrexate group mean PASI reduces from 21.83 + 7.23 to 1.35 + 0.21 after 10 weeks of treatment, whereas in PUVA group it reduces from 19.74 + 5.93 to 7.22 + 8.88, initially the mean PASI score were comparable in both groups (P > 0.05), but later it became significantly different in two groups [P < 0.05]). This difference in mean PASI score became statistically significant from second week onwards, so in terms of comparative efficacy and also in terms of rapid onset of action, Methotrexate is much better in comparison to PUVA.
Now comparison of the side effect profile shows the incidence of laboratory abnormality mainly in few patients of Methotrexate Group, though on the comparative study there was no significant difference in laboratory parameters in methotrexate and PUVA group altogether except in the case of ALT which shows a significant increase in Methotrexate Group patients at the end of the study period (P < 0.05). Also, few isolated patients in methotrexate group showed altered blood parameters [decreased platelet count (13.33%), decreased hemoglobin (10%), elevated liver enzyme (10%), altered lipid profile (10%), and leukopenia (3.33%)], whereas in PUVA group it was found in only one patient [elevated total bilirubin (3.33%)]. Although none of these complications were severe and all patients with altered blood parameters were managed adequately without much problem. Now when it comes to subjective complaints, PUVA patients had a much higher incidence of such complications compared to methotrexate. In the methotrexate group, only four patients complained of nausea and vomiting, but in the PUVA group apart from 10 patients who complained of vomiting, 13 patients complained of dryness and itching which were predominant complaints among PUVA patients. Apart from this, 12 patients complained of mild erythema, and pigmentation developed in 5 patients and 4 patients had increased lesions following PUVA treatment. In methotrexate group none of these side effects were observed. Side effects were not observed in 50% of Methotrexate Group patients whereas only 33.33% of PUVA patients did not develop any side effects.
Conclusions
Methotrexate reduces psoriasis lesion much more faster than PUVA, also it is much more efficacious than PUVA in terms of reduction in lesion severity. And although Methotrexate is associated with alteration in blood parameters (namely elevated liver enzymes, reduced platelet count) in few patients, with the dose of this drug used in psoriasis it is mild enough to be controlled easily and did not need discontinuation of drug. Methotrexate is also associated with a much lesser incidence of subjective complications than PUVA. Compliance issue is also better with Methotrexate due to less frequent hospital visit.
So methotrexate is more efficacious with lesser incidence of subjective complications and more incidence of laboratory complications compared to PUVA in chronic stable plaque psoriasis involving >20% of BSA.
Limitations of the study
Duration of remission and subsequent recurrence following treatment was not studied.
DLQI was not measured to assess efficacy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gelfand JM, Stern RS, Nijsten T, Feldman SR, Thomas J, Kist J, et al. The prevalence of psoriasis in African Americans: Results from a population-based study. J Am Acad Dermatol. 2005;52:23–6. doi: 10.1016/j.jaad.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 2.National Psoriasis Foundation. About psoriasis. [[Last accessed on 2014 Oct 21]]. Available from: http://www.psoriasis.org/about-psoriasis .
- 3.Feldman SR. The design of clinical trials in psoriasis: Lessons for clinical practice. J Am Acad Dermatol. 2003;49(2 Suppl):S62–5. doi: 10.1016/s0190-9622(03)01137-x. [DOI] [PubMed] [Google Scholar]
- 4.Callen JP, Kulp-Shorten CL. Methoterxate. In: Wolverton SE, editor. Comprehensive Dermatologic Drug Therapy. 3rd ed. Edinburgh, London, New York, Oxford, Philadelphia: Elsevier Saunders; 2012. pp. 169–81. [Google Scholar]
- 5.Tahir R, Mujtaba G. Comparative efficacy of psoralen UVA photochemotherapy versus narrow band UVB phototherapy in the treatment of psoriasis. J Coll Physicians Surg Pak. 2004;14:593–5. doi: 10.2004/JCPSP.593595. [DOI] [PubMed] [Google Scholar]
- 6.Sakpal TV. Sample size estimation in clinical trial. Perspect Clin Res. 2010;1:67–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: A population-based study. Arch Dermatol. 2005;141:1537–41. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 8.Akhyani M, Chams-Davatchi C, Hemami MR, Fateh S. Efficacy and safety of mycophenolate mofetil vs.methotrexate for the treatment of chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2010;24:1447–51. doi: 10.1111/j.1468-3083.2010.03667.x. [DOI] [PubMed] [Google Scholar]
- 9.Okhandiar RP, Banerjee BN. Psoriasis in the tropics: An epidemiological survey. J Indian Med Assoc. 1963;41:550–6. [PubMed] [Google Scholar]
- 10.Bedi TR. Clinical profile of psoriasis in North India. Indian J Dermatol Venereol Leprol. 1995;61:202–5. [PubMed] [Google Scholar]
- 11.Kaur I, Kumar B, Sharma VK, Kaur S. Epidemiology of psoriasis in a clinic from north India. Indian J Dermatol Venereol Leprol. 1986;52:208–12. [PubMed] [Google Scholar]
- 12.Haider S, Wahid Z, Najam-us-Saher, Riaz F. Efficacy of methotrexate in patients with plaque type psoriasis. Pak J Med Sci. 2014;30:1050–3. doi: 10.12669/pjms.305.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558–66. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 14.Heydendael VM, Spuls PI, Opmeer BC, de Borgie CA, Reitsma JB, Goldschmidt WF, et al. Methotrexate versus cyclosporine in moderate –to -severe chronic plaque psoriasis. N Engl J Med. 2003;349:658–65. doi: 10.1056/NEJMoa021359. [DOI] [PubMed] [Google Scholar]
- 15.Shabeer D, Bhandare B, Satyanarayana V, Krishnan P. A study to compare the efficacy of methotrexate alone vs.methotrexate plus pioglitazone in the management of plaque-type psoriasis. Int J Basic Clin Pharmacol. 2017;6:859–63. [Google Scholar]
- 16.Goyal T, Pradhan S, Varshney A. The study of clinical outcome of systemic methotrexate uses in moderate to severe childhood psoriasis. Indian J Paediatr Dermatol. 2017;18:31–5. [Google Scholar]
- 17.West J, Ogston S, Foerster J. Safety and efficacy of methotrexate in psoriasis: A meta-analysis of published trials. PLoS One. 2016;11:e0153740. doi: 10.1371/journal.pone.0153740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yones SS, Palmer RA, Garibaldinos TT, Hawk JL. Randomized double- blind trial of the treatment of chronic- plaque psoriasis: Efficacy of psoralen- UV-A therapy vs narrowband UV- B therapy. Arch Dermatol. 2006;142:836–42. doi: 10.1001/archderm.142.7.836. [DOI] [PubMed] [Google Scholar]
- 19.Gordon PM, Diffey BL, Matthews JN, Farr PM. A randomized comparison of narrow-band TL-O1 phototherapy and PUVA photochemotherapy for psoriasis. J Arn Acad Dermatol. 1999;41:728–32. doi: 10.1016/s0190-9622(99)70008-3. [DOI] [PubMed] [Google Scholar]
- 20.Archier E, Devaux S, Castela E, Gallini A, Aubin F, Le Maître M, et al. Efficacy of psoralen UV-A therapy vs.narrowband UV-B therapy in chronic plaque psoriasis: A systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(Suppl 3):11–21. doi: 10.1111/j.1468-3083.2012.04519.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaur J, Sharma VK, Sethuraman G, Tejasvi T. Comparison of the efficacy of psoralen ultraviolet A with narrowband ultraviolet B phototherapy for the treatment of chronic plaque psoriasis in patients with skin types IV and V. Clin Exp Dermatol. 2008;33:513–5. doi: 10.1111/j.1365-2230.2008.02718.x. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan PS, Kaur I, Dogra S, De D, Kanwar AJ. Narrowband ultraviolet B versus psoralen plus ultraviolet A therapy for severe plaque psoriasis: An Indian perspective. Clin Exp Dermatol. 2011;36:169–73. doi: 10.1111/j.1365-2230.2010.03874.x. [DOI] [PubMed] [Google Scholar]
- 23.Melski JW, Tanenbaum L, Parrish JA, Fitzpatrick TB, Bleich HL. Oral methoxsalen photochemotherapy for the treatment of psoriasis: A co-operative clinical trial. J Invest Dermatol. 1977;68:328–35. doi: 10.1111/1523-1747.ep12496022. [DOI] [PubMed] [Google Scholar]
- 24.Dayal S, Mayanka, Jain VK. Comparative evaluation of NBUVB phototherapy and PUVA photochemotherapy in chronic plaque psoriasis. Indian J Dermatol Venereol Leprol. 2010;76:533–7. doi: 10.4103/0378-6323.69081. [DOI] [PubMed] [Google Scholar]