Abstract
Peripheral nerve injuries commonly occur due to trauma, like a traffic accident. Peripheral nerves get severed, causing motor neuron death and potential muscle atrophy. The current golden standard to treat peripheral nerve lesions, especially lesions with large (≥ 3 cm) nerve gaps, is the use of a nerve autograft or reimplantation in cases where nerve root avulsions occur. If not tended early, degeneration of motor neurons and loss of axon regeneration can occur, leading to loss of function. Although surgical procedures exist, patients often do not fully recover, and quality of life deteriorates. Peripheral nerves have limited regeneration, and it is usually mediated by Schwann cells and neurotrophic factors, like glial cell line-derived neurotrophic factor, as seen in Wallerian degeneration. Glial cell line-derived neurotrophic factor is a neurotrophic factor known to promote motor neuron survival and neurite outgrowth. Glial cell line-derived neurotrophic factor is upregulated in different forms of nerve injuries like axotomy, sciatic nerve crush, and compression, thus creating great interest to explore this protein as a potential treatment for peripheral nerve injuries. Exogenous glial cell line-derived neurotrophic factor has shown positive effects in regeneration and functional recovery when applied in experimental models of peripheral nerve injuries. In this review, we discuss the mechanism of repair provided by Schwann cells and upregulation of glial cell line-derived neurotrophic factor, the latest findings on the effects of glial cell line-derived neurotrophic factor in different types of peripheral nerve injuries, delivery systems, and complementary treatments (electrical muscle stimulation and exercise). Understanding and overcoming the challenges of proper timing and glial cell line-derived neurotrophic factor delivery is paramount to creating novel treatments to tend to peripheral nerve injuries to improve patients’ quality of life.
Key Words: electrical muscle stimulation, exercise, glial cell line-derived neurotrophic factor, glial cell line-derived neurotrophic factor delivery, motor neuron, nerve gap, neurotrophic factor, peripheral nerve injury, Schwann cells, skeletal muscle atrophy
Introduction
The peripheral nervous system (PNS) is the nervous system branch composed of peripheral nerves branching out the central nervous system’s two main organs, the brain and the spinal cord. These peripheral nerves are in charge of sending and receiving messages from the brain and spinal cord to target tissues and vice versa. Peripheral nerves are made of axons wrapped in endoneurium, perineurium, and epineurium. These nerves are considered the most fragile structure in our body due to the ease of getting damaged by crush, compression, and trauma (Hussain et al., 2020). Peripheral nerve injuries (PNIs) can occur due to trauma, complicated childbirth, and tumor extirpation (Eggers et al., 2020; Fadia et al., 2020; Li et al., 2020). PNIs resulting from trauma are responsible for approximately 5% of patients admitted to a Level I trauma facility in the USA, costing around 150 billion US dollars in health-related costs per year (Taylor et al., 2008).
Neurotrophic factors (NFs) are a group of proteins known for their ability to promote neuronal survival, influence cell proliferation, and differentiation, regulate synaptic plasticity, and modulate both axonal and dendritic elaborations. Additionally, NFs facilitate communication between neurons and their respective target tissues (Henderson et al., 1994; Zhu et al., 2008; Morcuende et al., 2013). NFs include the neurotrophin family where nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4) belong. The glial cell line-derived neurotrophic factor (GDNF) family ligands have four members neurturin, persephin, artemin, and GDNF (Oppenheim et al., 1995; Cobianchi et al., 2017). Although the previously mentioned neurotrophic factors play a role at times of peripheral nerve injuries (Henderson et al., 1994; Glat et al., 2016; Tajdaran et al., 2016; Zheng et al., 2016), this review will be focusing on GDNF due to its formidable role as a regulator for the survival of motor neurons that innervate skeletal muscle, thus having an essential presence in the PNS and making it a target for future treatment developments for PNIs.
This review aims to discuss GDNF, Schwann cells’ role and GDNF in nerve repair mechanism, various exogenous GDNF delivery methods to treat PNIs, proper delivery challenges, and the potential role of electrical muscle stimulation and exercise as a complementary treatment following a PNI.
Search Strategy and Selection Criteria
Data retrieval is shown in Figure 1.
Figure 1.
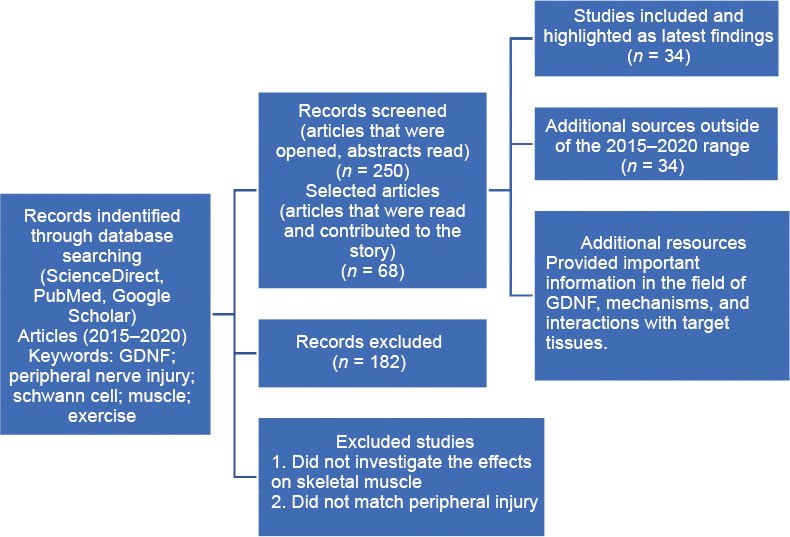
Flow chart of article retrieval.
Glial Cell Line-Derived Neurotrophic Factor
GDNF was first isolated from cultured B49 rat glial cells and came to prominence due to the capability of enhancing survival and differentiation of dopaminergic neurons in primary cultures by promoting dopamine uptake. GDNF, similar to the other GDNF family ligand members, functions as a homodimer for the activation of the tyrosine kinase rearranged during transfection (RET) receptor (Lin et al., 1993, 1994; Cintrón-Colón et al., 2020). As reviewed by Cintrón-Colón et al. (2020), in order to activate RET, GDNF first binds to a glycosylphosphatidylinositol-linked GDNF (GFRα) receptor, preferably a GFRα1 receptor or a GFRα2, with less affinity, and forms a high-affinity complex. The formed complex attracts two RET molecules causing transphosphorylation of specific tyrosine residues in their domains and initiating intracellular signaling like the mitogen-activated protein kinase, phosphoinositositide-3-kinase, Akt, or Erk pathways. The previously mentioned pathways are distinguished for promoting cell survival (Sariola and Saarma, 2003; Kim and Kim, 2018; Cintrón-Colón et al., 2020).
GDNF is a protein distributed in both peripheral nervous system and PNS, and its synthesis and secretion occur in various cells, which include a variety of glial cells like astrocytes, oligodendrocytes, and Schwann cells; neurons like motor, enteric, sympathetic, and dopaminergic neurons; and target tissues like a skeletal muscle (Henderson et al., 1994; Springer et al., 1995; Sariola and Saarma, 2003). Additional interest for studying GDNF is the ability to promote survival of motor neurons, myelination enhancement post-injury, neurite outgrowth promotion, and play a role as a synaptotrophin to promote both terminal branching and remodeling at the neuromuscular junction (NMJ) (Nguyen et al., 1998; Sariola and Saarma, 2003; Li et al., 2007).
Schwann Cell and Glial Cell Line-Derived Neurotrophic Factor Mechanism in Nerve Repair
PNIs have a limited ability of regeneration. Trupp et al. (1995) were the first to report that GDNF expression is induced following a PNI. In the years that followed, several studies have confirmed that after several modalities of nerve injuries (axotomy, sciatic nerve crush, and compression) GDNF expression was upregulated as well (Trupp et al., 1995; Chao et al., 2008). In severe denervation models, GDNF expression is upregulated in the distal stump up to 48 hours post-injury, but as denervation remains for a prolonged period, expression decreases possibly due to Schwann cell senescence and weakened ability to make GDNF (Höke et al., 2002).
As reviewed by Bolívar et al. (2020), Schwann cells are glial cells that originate from the neural crest and have high plasticity capability. These cells differentiate into two mature phenotypes, either myelinating or non-myelinating (Jessen and Mirsky, 2008, 2016; Bolívar et al., 2020). Schwann cells’ maturation starts with migrating neural crest cells forming Schwann cell precursors (multipotent embryonic progenitors). These Schwann cell precursors differentiate into immature Schwann cells, and the association of this immature form with a specific axon determines its course as a mature Schwann cell as reviewed by Jessen and Mirsky (Jessen and Mirsky, 2005).
After an injury, the distal nerve stump undergoes Wallerian degeneration, in which the nerve fiber (distal stump) degenerates. Markers related to myelin formation are downregulated, and Schwann cells change to a repair phenotype that bears a resemblance to the immature form as shown in Figure 2A (Jessen and Mirsky, 2016; Carr and Johnston, 2017; Welleford et al., 2020; Wilcox et al., 2020). Schwann cells with a repair phenotype cast off their myelin, acquire mobility and secrete neurotrophic factors like NGF, BDNF, NT-3, and GDNF to promote axon regeneration (Höke et al., 2006; Xu et al., 2013; Park and Höke, 2014; Jessen and Mirsky, 2016; Figure 2A). An outstanding study by Welleford et al. (2020) used RNA sequencing to properly analyze the human peripheral nerve’s whole transcriptome profile following injury. The researchers discovered 3641 genes that were significantly differentially expressed, the majority related to growth factor upregulation, most noticeably GDNF (Welleford et al., 2020).
Figure 2.

PNI repair mechanism.
(A) After injury, mature Schwann cell changes phenotype to a repair phenotype. Repair Schwann cells shed off their myelin, acquire mobility and secrete neurotrophic factors like NGF, BDNF, NT-3, and GDNF to promote axon regeneration. (B) Macrophages and the repair Schwann cells clear debris (represented by yellow dots). Schwann cells associate with fibroblasts to synthesize adhesion proteins and extracellular matrix, allowing for bridging between proximal and distal stumps. Repair Schwann cells align at the distal stump, forming bands of Büngner and provide trophic and physical support and direct axonal regeneration. (C) After regeneration, repair Schwann cells return to a mature myelinating phenotype and re-myelinate the axons. BDNF: Brain-derived neurotrophic factor; GDNF: glial cell line-derived neurotrophic factor; NGF: nerve growth factor; NT-3: neurotrophin-3.
Macrophages, neutrophils, and the reprogrammed Schwann cells breakdown myelin debris and clear the injured axonal membrane and cytoskeleton as seen in Figure 2B (Tomlinson et al., 2018). Additionally, Schwann cells associate with fibroblasts to synthesize adhesion proteins and extracellular matrix, allowing for successful bridging between proximal and distal stumps (review by Jessen and Mirsky, 2016; Figure 2B). Schwann cells align at the distal stump, forming bands of Büngner to provide trophic and physical support and direct axonal regeneration (Figure 2B). Once axonal regeneration has concluded, Schwann cells differentiate to their mature phenotype and re-myelinate the axons as reviewed by Boerboom et al. (2017; Figure 2C).
At times of injury, skeletal muscle serves as an additional source of GDNF. When skeletal muscles become denervated, GDNF upregulation occurs and remains elevated from weeks to months with the goal of motor neuron survival and reducing atrophy of skeletal muscle (Springer et al., 1995; Zhao et al., 2004). Zahavi et al. (2015, 2017) used an in vitro microfluidic platform that contained the cell body of a motor neuron and muscle cells connected with motor axons extending through microgrooves that allowed NMJ formation. This model’s set-up allowed the researchers to study the spatial specificity effects of GDNF. When GDNF is added to skeletal muscle cells, axonal tip growth and muscle innervation were promoted, and retrograde transport of the trophic factor was visualized (Zahavi et al., 2015, 2017). This research provides further evidence of the importance of GDNF in the innervation and synapsis of skeletal muscle.
Exogenous Glial Cell Line-Derived Neurotrophic Factor Delivery in Peripheral Nerve Injuries
Nerve allograft and drug delivery system
In cases of a transected peripheral nerve, the current surgical standard consists of using autografts from nerve tissue from the same patient in order to connect the nerve gap. In some cases, these nerve autografts may not be an option where considerable repair is needed due to the limited available length (Tajdaran et al., 2016). An alternate option to using nerve autographs are acellular nerve allografts (ANAs). These allografts maintain the nerve tissue framework and are non-immunogenic, eliminating the need for immunosuppressants, functioning as a medium for nerve regeneration. ANAs contain low amounts of neurotrophic factors compared to injured/denervated nerve stumps, where upregulation of neurotrophic factors, like GDNF, is increased (Boyd and Gordon, 2003; Tajdaran et al., 2016). Previous work from Woods et al. (2013a, b) developed a microsphere-based biodegradable drug delivery system that sustains GDNF release to the injured site in periods of days to weeks. This delivery drug system proved to increase both axon regeneration and functional recovery after a delayed nerve repair (Wood et al., 2013a, b). Tajdaran et al. (2016) combined the previously described drug delivery system with the rat analog of the ANAs and determined how this combination supported nerve repair and regeneration. The experimental groups received the drug delivery system treatment at the suture sites of the allografts for two or four weeks and compared to rats that did not receive the drug delivery treatment. The treated rats had a significantly higher number of regenerated axons from both motor and sensory neurons; and larger fiber diameters than the non-treatment group (Tajdaran et al., 2016).
Glial cell line-derived neurotrophic factor pre-treated sensory graft for motor nerve injuries
Motor nerve grafts are hardly used because of the potential damage to movement functions in the donor area. Instead, sensory nerve grafting has been used to bridge and repair extended segmental motor nerve defects. The problem of doing sensory nerve bridging is that Schwann cells residing in sensory nerves have a subpar ability to support motor neuron axon growth (Chu et al., 2008, 2009; Fang et al., 2019). In vitro experiments using microfluidic devices performed by Marquadt and Sakiyama-Elbert (2015) and in vivo experiments by Chu et al. (2009) showed that Schwann cells could overcome phenotypic mismatch-induced growth inhibition after exogenous GDNF application and as a result induce motor neuron axonal growth. Taking this previous research into consideration, Fang et al. (2019) designed a brilliant novel surgery starting with a denervated sensory nerve that was pre-treated with a sustained release of GDNF. GDNF was encapsulated into a self-assembling peptide nanofiber scaffold RADA-161 in the donor area in vivo. Following the pre-treatment, the sensory grafts were transplanted to fix motor nerve injury. Following this novel surgery approach, the exogenous GDNF pre-treatment resulted in regeneration and re-myelination of proximal motor axons. Furthermore, muscle function recovery was noted following electrophysiological analysis of the quadriceps femoris muscle (Fang et al., 2019).
Topical addition of glial cell line-derived neurotrophic factor in delayed root reimplantation
A standard model used to study brachial plexus injuries is the root avulsion model. A root avulsion injury is seen in traffic accidents and complicated childbirths. It consists of spinal roots being severed from the spinal cord, and as a consequence, motor neurons die, and peripheral nerves deteriorate (Ruven et al., 2018; Eggers et al., 2020). Ruven and colleagues (2018) used the root avulsion model combined with a 2-week delayed root reimplantation to test the effectiveness of GDNF treatment along with fetal lumbar cell transplantation on motor neuron death and muscle atrophy prevention. A peculiarity of this work is that GDNF treatment was topically added right after the injury. Two weeks after the manipulation, root reimplantation surgery was performed to determine if the motor neurons saved by the trophic factor could regenerate after delayed reimplantation. Results from this research suggest that GDNF treatment significantly prevented motor neuron death and incremented axonal sprouting and regeneration. Due to this regeneration, more neurons managed to make contact with the affected muscles, resulting in some muscle atrophy prevention compared to the delayed surgery-only group (Ruven et al., 2018).
Gene therapy as a glial cell line-derived neurotrophic factor delivery system
Patients that suffer a brachial plexus injury, even after surgical repair, could result in dysfunction and pain for the rest of the patient’s life (Eggers et al., 2020). Gene therapy has been suggested as a form of delivering GDNF to treat PNIs. As reviewed by Eggers et al. (2020), using gene therapy for GDNF can result in a continual supply of biologically active protein limited to the site of injury or where the viral vector was applied. A barrier for this approach is by-passing a potential immune response against the gene being inserted. An additional obstacle that has to be tackled is the time of expression and amount of expression (Tajdaran et al., 2016; Eggers et al., 2020). Eggers et al. (2019) completed a preclinical study testing these challenges. The researchers developed an immune-evasive, doxycycline-inducible, GDNF gene switch, with a time-restricted expression of one month. With this novel GDNF delivery system, the researchers managed to reduce the localized entrapment in avulsed reimplanted ventral spinal roots, promoted long-term motor neuron survival, and augmented long-distance regeneration motor axons. These results provide evidence that controlling the time of expression of GDNF can rectify the harmful effects of uncontrolled GDNF delivery. Also, gene therapy can improve axon regeneration post-surgical repair.
Biodegradable nerve conduit
A constant problem with PNIs is the length of the nerve gap after injury. If the nerve gap is over two to three centimeters is considered a large nerve injury, and the standard remedy is autografting (Fadia et al., 2020). PNIs that surpass three centimeters may encounter challenges with unresponsive growth cones from the proximal stump to NF signals released by the distal stump that lead axonal sprouting causing substandard nerve regrowth (Grinsell and Keating, 2014). Autografting could potentially lead to loss of sensory function and neuroma, leading Fadia et al. (2020) to explore a biodegradable poly(caprolactone) (PCL) conduit with an inserted double-walled polymeric microspheres that contain GDNF. This PCL conduit can offer a continuous release of GDNF for more than 50 days in a 5-cm nerve injury in a non-human primate (rhesus macaque). The investigators compared the PCL/GDNF conduit with a median nerve autograft and a PCL conduit with empty (no GDNF) microspheres (PCL/empty). Fadia et al. (2020) found that the groups with PCL/GDNF conduit treatment and the autograft-treatment had improved functional recuperation compared to the PCL/empty treated groups. Additionally, the groups treated with the biodegradable nerve conduit with encapsulated GDNF have better nerve conduction velocity 1-year post-surgery. Histological analysis revealed a larger average area inhabited by Schwann cells at the distal nerve when compared to the other two groups (Fadia et al., 2020). This work adds a novel approach on solving PNIs, especially when a large peripheral nerve gap is present in a non-human primate.
Challenges of Glial Cell Line-Derived Neurotrophic Factor Delivery
GDNF poses as a potential NF to treat PNIs in future human trials, but delivery challenges exist. As reviewed by Eggers et al., understanding the proper relationship between GDNF concentrations and duration of treatment to achieve adequate therapeutic GDNF levels for proper motor neuron survival and axonal outgrowth needs to be further studied. When high or low concentrations of GDNF are prolonged, there is a tendency of axonal coil formation. In low levels of GDNF, proper motor neuron survival does not occur (Eggers et al., 2008, 2013, 2020; Santos et al., 2016; Wang et al., 2018).
An additional challenge for proper GDNF delivery is maintaining exogenous GDNF in the correct location. Direct injection of the NF in a transplanted nerve tends to leak and causes unorganized axonal regeneration in the leakage site (Fang et al., 2019). Differences between distal and proximal stumps when treated with GDNF exist. When treated at the distal end, it did not improve motor neuron survival, in contrast to the peripheral end (Eggers et al., 2008, 2019).
Although a powerful tool, gene therapy can be hindered by the immune system. Thus finding ways to bypass the potential immune response is essential. Hoyng et al. and Eggers et al. have developed a successful way of doing so, as previously discussed, by developing a doxycycline-inducible GDNF system (Hoyng et al., 2014b; Eggers et al., 2019).
Electrical Muscle Stimulation as a Complementary Treatment
A common feature of PNIs is the disconnection of the active nerve from the muscle, eventually causing muscle atrophy. Previous research has demonstrated that when there is a disruption in the NMJ, in other words, a disconnection of axon and muscle, GDNF is upregulated and expressed by muscle in higher quantity to try and re-establish that connection (Springer et al., 1995; Zhao et al., 2004). Electrical stimulation is used as a form of therapy to prevent muscle atrophy and build up strength in patients with injuries. Previous work done in rat models has demonstrated that daily electrical muscle stimulation (EMS) increases reinnervation following a PNI and repair. Willand et al. (2016) provide a possible explanation of why reinnervation is increased following EMS by using a rat model with a transected tibial nerve and immediately repaired post-injury. The rat model had intramuscular electrodes implanted in the gastrocnemius muscle (calf muscle) for electrical stimulation. The researchers used two sets of rats with the same injury. One set was used to measure functional reinnervation using electromyographic recordings, and the other set had the muscle and distal nerve stump removed for molecular analysis. GDNF mRNA levels from muscles that had daily EMS were significantly upregulated compared to the no stimulation groups. However, there was no difference in trophic factor mRNA levels in the distal stump compared to the non-stimulated rats, suggesting that EMS does not regulate Schwann cell-derived GDNF transcription. This work suggests that adding electrical stimulation to a denervated muscle after a PNI upregulates intramuscular levels of GDNF mRNA. This increase in trophic factor diffuses into the distal nerve stump providing the beneficial effects of axon regeneration at the growth cone facilitating nerve regeneration (Willand et al., 2016).
Exercise as a Complementary Treatment
There is increasing evidence of exercise having beneficial regenerative, rehabilitative, and neuro-plasticity-associated effects in central and peripheral nervous systems (Park and Höke, 2014; Arbat-Plana et al., 2017; Theisen et al., 2017). Additionally, exercise has been linked with possible neurotrophic factor signaling regulation, as reviewed by Cobianchi et al. (2017) and Cintrón-Colón et al. (2020). A study from Wehrwein et al. (2002) suggests that GDNF is regulated in an activity-dependent manner by using a hindlimb unloading model and walk-training exercise. After following a 4-week walk regimen, the researchers noticed that GDNF protein content in the soleus, gastrocnemius, and pectoralis major muscles increased. In contrast, when hindlimb unloading was performed, GDNF protein content significantly decreased in the soleus and gastrocnemius but increased in the pectoralis major muscle due to recruitment of the muscle. This study indicates that mechanical activity in the form of stretch from increased physical activity might be a sufficient stimulus for neurotrophic factor production (Wehrwein et al., 2002).
Park and Höke (2014) used a peripheral nerve regeneration model to study treadmill exercise’s impact. The animals were divided into a control group, nerve repair without exercise group, and a nerve repair plus exercise group. The animals had the median nerve transected and repaired, while the ulnar nerve was prevented from regeneration after the injury. The researchers noticed that following a daily treadmill exercise regimen; there was improved regeneration after due to a higher number of axons regenerated and increased myofiber size in the target muscles. Additionally, there was an increment in serum, muscle, and nerve of various neurotrophic factors that included GDNF, BDNF, and insulin-like growth factor-1. Also, there was a faster functional recovery as demonstrated by grip power and inverted holding test. This study suggests that muscle derived neurotrophic factors, like GDNF, and an appropriate exercise regimen might offer enhanced regeneration following injury (Park and Höke, 2014).
Gyorkos et al. (2014) explored swimming and walk-training exercises. After two weeks of either swimming or walk-training exercises, the researchers noticed that it promoted changes in both GDNF protein content and NMJ structures in slow-twitch and fast-twitch muscles. Muscle-derived GDNF protein content significantly increased, and the total area of the motor end plates significantly increased in slow-twitch muscles but decreased in fast-twitch muscles. These results suggest that increased physical activity is enough to increment GDNF protein content and alter the NMJ components offering insights for potential exercise program developments as an added treatment for patients with PNIs.
Conclusion
The faulty recovery of function following PNIs is attributed to the continuous degeneration and reduction in the motor neurons’ ability to regenerate their axons that lead to chronically denervated muscles (Eggers et al., 2019). Even though surgical repair for PNIs exist, functional recovery in patients remains inefficient and could lead to long-lasting or lifelong dysfunction (Eggers et al., 2020). GDNF is a neurotrophic factor that evokes interest because of its ability for axonal outgrowth, role in neuron differentiation, and its role as a potent survival factor for motor neurons (Henderson et al., 1994). Although GDNF shows promise due to its pro-survival effects on motor neurons, it is challenging to deliver this protein. Therefore, further research is needed to understand the optimum amount of the protein and time needed to treat a PNI and better develop methods of keeping exogenous GDNF in the correct location. Previous work has found that uncontrolled GDNF distribution could cause irregular sprouting and axon and nerve entrapment (Höke et al., 2002, 2003; Eggers et al., 2008; Su et al., 2009; Hoyng et al., 2014a; Ee et al., 2017). Recently, researchers have advanced in the development of conduits that are biocompatible and possess enough mechanical strength to aid injured neurons and Schwann cells from potential apoptosis (Cebral et al., 2017; Alsmadi et al., 2018; Du et al., 2018; Sarker et al., 2018; Subbiah and Guldberg, 2018). EMS and exercise can be considered as complementary treatments following surgery. Previous research has shown that EMS increases levels of trophic factors providing positive effects for axonal regeneration (Willand et al., 2016). Exercise has been suggested to trigger the release of GDNF from skeletal muscle in an activity-dependent manner and be retrogradely shipped to the soma of motor neurons triggering pro-survival genes (Wehrwein et al., 2002; McCullough et al., 2013; Gyorkos et al., 2014; Cintrón-Colón et al., 2020). Finally, figuring out the proper dosage, time, and delivery method for GDNF therapy and combining it with a proper physical activity regimen or EMS during rehabilitation can ameliorate and enhance a patient’s quality of life post-injury.
Footnotes
P-Reviewer: Torii T, Weng J; C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was funded by the NIH Grant 1R15AG022908-01A2 and the Western Michigan University (to JMS).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Tomohiro Torii, Doshisha Daigaku, Japan; Jian Weng, Peking University People’s Hospital, China.
Funding: This work was funded by the NIH Grant 1R15AG022908-01A2 and the Western Michigan University (to JMS).
References
- 1.Alsmadi NZ, Bendale GS, Kanneganti A, Shihabeddin T, Nguyen AH, Hor E, Dash S, Johnston B, Granja-Vazquez R, Romero-Ortega MI. Glial-derived growth factor and pleiotrophin synergistically promote axonal regeneration in critical nerve injuries. Acta Biomater. 2018;78:165–177. doi: 10.1016/j.actbio.2018.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbat-Plana A, Navarro X, Udina E. Effects of forced, passive, and voluntary exercise on spinal motoneurons changes after peripheral nerve injury. Eur J Neurosci. 2017;12:2885–2892. doi: 10.1111/ejn.13739. [DOI] [PubMed] [Google Scholar]
- 3.Boerboom A, Dion V, Chariot A, Franzen R. Molecular mechanisms involved in schwann cell plasticity. Front Mol Neurosci. 2017;10:1–18. doi: 10.3389/fnmol.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolívar S, Navarro X, Udina E. Schwann cell role in selectivity of nerve regeneration. Cells. 2020;9:2131. doi: 10.3390/cells9092131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd J, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 6.Carr MJ, Johnston AP. Schwann cells as drivers of tissue repair and regeneration. Curr Opin Neurobiol. 2017;47:52–57. doi: 10.1016/j.conb.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Cebral RL, Silva-Correia J, Rui LR, Silva TH, Oliveira JM. Peripheral nerve injury: current challenges, conventional treatment approaches, and new trends in biomaterials-based regenerative strategies. ACS Biomater Sci Eng. 2017;3:3098–3122. doi: 10.1021/acsbiomaterials.7b00655. [DOI] [PubMed] [Google Scholar]
- 8.Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia. J Comp Neurol. 2008;506:180–193. doi: 10.1002/cne.21537. [DOI] [PubMed] [Google Scholar]
- 9.Chu TH, Li SY, Guo A, Wong WM, Yuan Q, Wu W. Implantation of neurotrophic factor-treated sensory nerve graft enhances survival and axonal regeneration of motoneurons after spinal root avulsion. J Neuropathol Exp Neurol. 2009;68:94–101. doi: 10.1097/NEN.0b013e31819344a9. [DOI] [PubMed] [Google Scholar]
- 10.Chu TH, Du Y, Wu W. Motor nerve graft is better than sensory nerve graft for survival and regeneration of motoneurons after spinal root avulsion in adult rats. Exp Neurol. 2008;212:562–565. doi: 10.1016/j.expneurol.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Cintrón-Colón AF, Almeida-Alves G, Boynton AM, Spitsbergen JM. GDNF synthesis, signaling, and retrograde transport in motor neurons. Cell Tissue Res. 2020;382:47–56. doi: 10.1007/s00441-020-03287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobianchi S, Arbat-Plana A, Lopez-Alvarez VM, Navarro X. Neuroprotective effects of exercise treatments after injury: the dual role of neurotrophic factors. Curr Neuropharmacol. 2017;15:495–518. doi: 10.2174/1570159X14666160330105132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du J, Chen H, Qing L, Yang X, Jia X. Biomimetic neural scaffolds: a crucial step towards optimal peripheral nerve regeneration. Biomater Sci. 2018;6:1299–1311. doi: 10.1039/c8bm00260f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ee X, Yan Y, Hunter DA, Schellhardt L, Sakiyama-Elbert SE, Mackinnon SE, Wood MD. Transgenic SCs expressing GDNF-IRES-DsRed impair nerve regeneration within acellular nerve allografts. Biotechnol Bioeng. 2017;114:2121–2130. doi: 10.1002/bit.26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggers R, De Winter F, Hoyng SA, Hoeben RC, Malessy MJA, Tannemaat MR, Verhaagen J. Timed GDNF gene therapy using an immune-evasive gene switch promotes long distance axon regeneration. Brain. 2019;142:295–311. doi: 10.1093/brain/awy340. [DOI] [PubMed] [Google Scholar]
- 16.Eggers R, de Winter F, Hoyng SA, Roet KC, Ehlert EM, Malessy MJ, Verhaagen J, Tannemaat MR. Lentiviral vector-mediated gradients of GDNF in the injured peripheral nerve: effects on nerve coil formation, Schwann cell maturation and myelination. PLoS One. 2013;8:e71076. doi: 10.1371/journal.pone.0071076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggers R, de Winter F, Tannemaat MR, Malessy MJA, Verhaagen J. GDNF gene therapy to repair the injured peripheral nerve. Front Bioeng Biotechnol. 2020;8:1–10. doi: 10.3389/fbioe.2020.583184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggers R, Hendriks WTJ, Tannemaat MR, van Heerikhuize JJ, Pool CW, Carlstedt TP, Zaldumbide A, Hoeben RC, Boer GJ, Verhaagen J. Neuroregenerative effects of lentiviral vector-mediated GDNF expression in reimplanted ventral roots. Mol Cell Neurosci. 2008;39:105–117. doi: 10.1016/j.mcn.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Fadia NB, Bliley JM, DiBernardo GA, Crammond DJ, Schilling BK, Sivak WN, Spiess AM, Washington KM, Waldner M, Liao HT, James IB, Minteer DM, Tompkins-Rhoades C, Cottrill AR, Kim DY, Schweizer R, Bourne DA, Panagis GE, Asher Schusterman M, 2nd, Egro FM, et al. Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates. Sci Transl Med. 2020;12:1–14. doi: 10.1126/scitranslmed.aav7753. [DOI] [PubMed] [Google Scholar]
- 20.Fang X, Zhang C, Yu Z, Li W, Huang Z, Zhang W. GDNF pretreatment overcomes Schwann cell phenotype mismatch to promote motor axon regeneration via sensory graft. Exp Neurol. 2019;318:258–266. doi: 10.1016/j.expneurol.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Glat MJ, Benninger F, Barhum Y, Ben-Zur T, Kogan E, Steiner I, Yaffe D, Offen D. Ectopic muscle expression of neurotrophic factors improves recovery after nerve injury. J Mol Neurosci. 2016;58:39–45. doi: 10.1007/s12031-015-0648-9. [DOI] [PubMed] [Google Scholar]
- 22.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int 2014. 2014 doi: 10.1155/2014/698256. 698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyorkos AM, McCullough MJ, Spitsbergen JM. Glial cell line-derived neurotrophic factor (GDNF) expression and NMJ plasticity in skeletal muscle following endurance exercise. Neuroscience. 2014;257:111–118. doi: 10.1016/j.neuroscience.2013.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simpson LC, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A. GDNF: A potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 25.Höke A, Gordon T, Zochodne DW, Sulaiman OAR. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- 26.Höke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon Schwann cell units and promotes myelination in unmyelinated nerve fibers. J Neurosci. 2003;23:561–567. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höke A, Redett R, Hameed H, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyng SA, De Winter F, Gnavi S, de Boer R, Boon LI, Korvers LM, Tannemaat MR, Malessy MJA, Verhaagen J. A comparative morphological, electrophysiological and functional analysis of axon regeneration through peripheral nerve auto-grafts genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or VEGF. Exp Neurol. 2014a;261:578–593. doi: 10.1016/j.expneurol.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Hoyng SA, Gnavi S, De Winter F, Eggers R, Ozawa T, Zaldumbide A, Hoeben RC, Malessy MJA, Verhaagen J. Developing a potentially immunologically inert tetracycline-regulatable viral vector for gene therapy in the peripheral nerve. Gene Ther. 2014b;21:549–557. doi: 10.1038/gt.2014.22. [DOI] [PubMed] [Google Scholar]
- 30.Hussain G, Wang J, Rasul A, Anwar H, Qasim M, Zafar S, Aziz N, Razzaq A, Hussain R, de Aguilar JLG, Sun T. Current status of therapeutic approaches against peripheral nerve injuries: a detailed story from injury to recovery. Int J Biol Sci. 2020;16:116–134. doi: 10.7150/ijbs.35653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jessen K, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 32.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 33.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim M, Kim DJ. GFRA1: a novel molecular target for the prevention of osteosarcoma chemoresistance. Int J Mol Sci. 2018;19:1–15. doi: 10.3390/ijms19041078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R, Li DH, Zhang HY, Wang J, Li XK, Xiao J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol Sin. 2020;41:1289–1300. doi: 10.1038/s41401-019-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Brakefield D, Pan Y, Hunter D, Myckatyn T, Parsadanian A. Muscle-derived but not centrally derived transgene GDNF is neuroprotective in G93A-SOD1 mouse model of ALS. Exp Neurol. 2007;203:457–471. doi: 10.1016/j.expneurol.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 37.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 38.Lin LH, Zhang TJ, Collins F, Armes LG. Purification and initial characterization of rat B49 glial cell line-derived neurotrophic factor. J Neurochem. 1994;63:758–768. doi: 10.1046/j.1471-4159.1994.63020758.x. [DOI] [PubMed] [Google Scholar]
- 39.Marquadt LM, Sakiyama-Elbert SE. GDNF preconditioning can overcome Schwann cell phenotypic memory. Exp Neurol. 2015;265:1–7. doi: 10.1016/j.expneurol.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullough MJ, Gyorkos AM, Spitsbergen JM. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience. 2013;240:258–268. doi: 10.1016/j.neuroscience.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morcuende S, Muñoz-Hernández R, Benítez-Temiño B, Pastor AM, de la Cruz RR. Neuroprotective effects of NGF, BDNF, NT-3 and GDNF on axotomized extraocular motoneurons in neonatal rats. Neuroscience. 2013;250:31–48. doi: 10.1016/j.neuroscience.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen QT, Parsadanian AS, Snider WD, Lichtmant JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- 43.Oppenheim RW, Houenou LJ, Johnson JE, Lin LFH, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by gdnf. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- 44.Park JS, Höke A. Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PLoS One. 2014;9:e90245. doi: 10.1371/journal.pone.0090245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruven C, Badea SR, Wong WM, Wu W. Combination treatment with exogenous GDNF and fetal spinal cord cells results in better motoneuron survival and functional recovery after avulsion injury with delayed root reimplantation. J Neuropathol Exp Neurol. 2018;77:325–343. doi: 10.1093/jnen/nly009. [DOI] [PubMed] [Google Scholar]
- 46.Santos D, Gonzalez-Perez F, Navarro X, del Valle J. Dose-dependent differential effect of neurotrophic factors on in vitro and in vivo regeneration of motor and sensory neurons. Neural Plast. 2016;2016:4969523. doi: 10.1155/2016/4969523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 48.Sarker MD, Naghieh S, McInnes AD, Schreyer DJ, Chen X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog Neurobiol. 2018;171:125–150. doi: 10.1016/j.pneurobio.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Springer JE, Seeburger JL, Jin HE, Gabrea A, Blankenhorn EP, Bergman LW. cDNA sequence and differential mRNA regulation of two forms of glial cell line-derived neurotrophic factor in Schwann cells and rat skeletal muscle. Exp Neurol. 1995;131:47–52. doi: 10.1016/0014-4886(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 50.Su X, Kells AP, Huang EJ, Lee HS, Hadaczek P, Beyer J, Bringas J, Pivirotto P, Penticuff J, Eberling J, Federoff HJ, Forsayeth J, Bankiewicz KS. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum Gene Ther. 2009;20:1627–1640. doi: 10.1089/hum.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subbiah R, Guldberg RE. Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv Heal Mater. 2018;8:e1801000. doi: 10.1002/adhm.201801000. [DOI] [PubMed] [Google Scholar]
- 52.Tajdaran K, Gordon T, Wood MD, Shoichet MS, Borschel GH. A glial cell line-derived neurotrophic factor delivery system enhances nerve regeneration across acellular nerve allografts. Acta Biomater. 2016;29:62–70. doi: 10.1016/j.actbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Theisen CC, Sachdeva R, Austin S, Kulich D, Kranz V, Houle JD. Exercise and peripheral nerve grafts as a strategy to promote regeneration after acute or chronic spinal cord injury. J Neurotrauma. 2017;34:1909–1914. doi: 10.1089/neu.2016.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomlinson JE, Žygelyte E, Grenier JK, Edwards MG, Cheetham J. Temporal changes in macrophage phenotype after peripheral nerve injury. J Neuroinflammation. 2018;15:17–19. doi: 10.1186/s12974-018-1219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trupp M, Rydén M, Jörnvall H, Funakoshi H, Timmusk T, Arenas E, Ibáñez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang ZZ, Wood MD, Mackinnon SE, Sakiyama-Elbert SE. A microfluidic platform to study the effects of GDNF on neuronal axon entrapment. J Neurosci Methods. 2018;308:183–191. doi: 10.1016/j.jneumeth.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wehrwein EA, Roskelley EM, Spitsbergen JM. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerve. 2002;26:206–211. doi: 10.1002/mus.10179. [DOI] [PubMed] [Google Scholar]
- 58.Welleford AS, Quintero JE, Seblani N El, Blalock E, Gunewardena S, Shapiro SM, Riordan SM, Huettl P, Guduru Z, Stanford JA, van Horne CG, Gerhardt GA. RNA sequencing of human peripheral nerve in response to injury: distinctive analysis of the nerve repair pathways. Cell Transplant. 2020;29:1–13. doi: 10.1177/0963689720926157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilcox MB, Laranjeira SG, Eriksson TM, Jessen KR, Mirsky R, Quick TJ, Phillips JB. Characterising cellular and molecular features of human peripheral nerve degeneration. Acta Neuropathol Commun. 2020;8:1–17. doi: 10.1186/s40478-020-00921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willand MP, Rosa E, Michalski B, Zhang JJ, Gordon T, Fahnestock M, Borschel GH. Electrical muscle stimulation elevates intramuscular BDNF and GDNF mRNA following peripheral nerve injury and repair in rats. Neuroscience. 2016;334:93–104. doi: 10.1016/j.neuroscience.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 61.Wood MD, Gordon T, Kemp SWP, Liu EH, Kim H, Shoichet MS, Borschel GH. Functional motor recovery is improved due to local placement of GDNF microspheres after delayed nerve repair. Biotechnol Bioeng. 2013a;110:1272–1281. doi: 10.1002/bit.24800. [DOI] [PubMed] [Google Scholar]
- 62.Wood MD, Gordon T, Kim H, Szynkaruk M, Phua P, Lafontaine C, Kemp SWP, Shoichet MS, Borschel GH. Fibrin gels containing GDNF microspheres increase axonal regeneration after delayed peripheral nerve repair. Regen Med. 2013b;8:27–37. doi: 10.2217/rme.12.105. [DOI] [PubMed] [Google Scholar]
- 63.Xu P, Rosen KM, Hedstrom K, Rey O, Guha S, Hart C, Corfas G. Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in Schwann cells through purinergic signaling and the PKC-PKD pathway. Glia. 2013;61:1029–1040. doi: 10.1002/glia.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zahavi EE, Ionescu A, Gluska S, Gradus T, Ben-Yaakov K, Perlson E. A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions. J Cell Sci. 2015;128:1241–1252. doi: 10.1242/jcs.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zahavi EE, Maimon R, Perlson E. Spatial-specific functions in retrograde neuronal signalling. Traffic. 2017;18:415–424. doi: 10.1111/tra.12487. [DOI] [PubMed] [Google Scholar]
- 66.Zhao C, Veltri K, Li S, Bain JR, Fahnestock M. NGF, BDNF, NT-3, and GDNF mRNA expression in rat skeletal muscle following denervation and sensory protection. J Neurotrauma. 2004;21:1468–1478. doi: 10.1089/neu.2004.21.1468. [DOI] [PubMed] [Google Scholar]
- 67.Zheng J, Sun J, Lu X, Zhao P, Li K, Li L. BDNF promotes the axonal regrowth after sciatic nerve crush through intrinsic neuronal capability upregulation and distal portion protection. Neurosci Lett. 2016;621:1–8. doi: 10.1016/j.neulet.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Zhu B, Pennack JA, McQuilton P, Forero MG, Mizuguchi K, Sutcliffe B, Gu CJ, Fenton JC, Hidalgo A. Drosophila neurotrophins reveal a common mechanism for nervous system formation. PLoS Biol. 2008;6:2476–2495. doi: 10.1371/journal.pbio.0060284. [DOI] [PMC free article] [PubMed] [Google Scholar]


