Key Words: chronic pain, electroacupuncture, glutamic acid decarboxylase 65, glutamic acid decarboxylase 67, hyperalgesia, nicotine dependence, pain sensation, smoking, β-endorphin, μ-opioid receptor
Abstract
Tobacco smoking is considered to be one of the main risk factors in the development of chronic pain. Long-term chronic exposure to nicotine and other forms of tobacco have been shown to be associated with an increased incidence of pain. Studies have shown that acupuncture can help smokers to reduce their desire to smoke, reduce their withdrawal symptoms, and avoid a relapse after treatment. However, little has been reported about the effects of acupuncture on pain sensitivity caused by long-term smoking. Models of hyperalgesia were established in rats exposed to nicotine for 6 weeks. After 6 weeks of continuous nicotine exposure, electroacupuncture at bilateral acupoints Zusanli (ST36) and Taichong (LR3) was performed 20 minutes per day for 6 days at a continuous wave with a frequency of 2 Hz and a stimulus intensity of 1 mA. The results revealed that electroacupuncture treatment increased the mechanical response threshold of hind paw of nicotine-dependent rats with hyperalgesia and up-regulated the protein expression of pain-related factors μ-opioid receptor, β-endorphin and glutamic acid decarboxylase 65 in the spinal cord and midbrain periaqueductal gray and the protein expression of glutamic acid decarboxylase 67 in the spinal cord. These findings suggest that electroacupuncture treatment has positive analgesic effects on pain sensitivity caused by long-term chronic nicotine exposure. One possible mechanism for the improved analgesia is that electroacupuncture increases the expression of pain-related factors in the spinal cord and midbrain periaqueductal gray. This study was approved by Institutional Animal Care and Use Committee (IACUC) of the University of Miami (#18-167) on December 12, 2018.
Chinese Library Classification No. R454.1; R749; S884.9+1
Introduction
Nicotine dependence and chronic pain are highly prevalent in many societies. Both entities have a profound impact on public health and lead to significant morbidity. Long-term chronic exposure to nicotine and other forms of tobacco have been shown to be associated with an increased incidence of pain (Shi et al., 2010; D’ Silva et al., 2014). Tobacco smoking is considered to be one of the main risk factors in the development of chronic pain, and smokers are at a higher risk of developing back pain, musculoskeletal pain, fibromyalgia, and other pain disorders (Shiri et al., 2010; Goesling et al., 2015). Furthermore, clinical evidences have repeatedly demonstrated that smokers have more pain locations, more severe pain, greater physical and mental functional impairment, and require more intraoperative and postoperative narcotic analgesics than nonsmokers (Hooten et al., 2009; Orhurhu et al., 2015). The complex interaction between smoking addiction and chronic pain has been a problem of increasing interest to clinicians and researchers in the field of health care.
Acupuncture is a non-pharmacological pain relief therapy derived from traditional Chinese medicine and is gaining popularity in many countries (Deng and Mao, 2019). Clinical reports have shown that acupuncture is effective in treating nicotine dependence and different types of chronic pain (Lin et al., 2012; Deng and Mao, 2019). In some instances, acupuncture can be considered as a first-line treatment for pain before opioids are prescribed and may reduce the use of opioid-like medications overall (Fan et al., 2017).
Many studies have focused on the effects of acupuncture on smoking cessation, indicating that acupuncture can help smokers to reduce their desire to smoke, reduce their withdrawal symptoms, help smokers to quit smoking overall, and avoid a relapse after treatment (Wang et al., 2016a, 2019). However, little has been reported about the effects of acupuncture on pain sensitivity caused by long-term smoking. The purpose of this study was to investigate the effects of electroacupuncture at bilateral Zusanli (ST36) and Taichong (LR3) acupoints on the pain sensation in hyperalgesia rats subjected to chronic nicotine exposure. Furthermore, this study was to investigate the effects of electroacupuncture on pain-related factors μ-opioid receptor (MOR), β-endorphin (β-EP), glutamic acid decarboxylase 67 (GAD67), and glutamic acid decarboxylase 65 (GAD65) in an animal model.
Materials and Methods
Animal preparation
The animal experiment protocols (#18-167) were approved by Institutional Animal Care and Use Committee (IACUC) of the University of Miami on December 12, 2018. To avoid the cycle of estrogen from affecting the results, 5-week-old male Sprague-Dawley rats weighing 130–150 g were purchased from Charles River Laboratories International, Inc. (Wilmington, MA, USA). The rats were kept in pairs in padded plastic cages with free access to water and food, maintained in a 12-hour light/dark cycle at 22 ± 2°C with 50–60% relative humidity, and were acclimated in the cages for 1 week before the experiments were performed. The rats were randomly divided into two groups: age-matched control group (CTR, n = 8), and nicotine-exposed group (n = 16). The models of hyperalgesia were established in nicotine-exposed rats. The nicotine-exposed rats were then randomly divided into nicotine-exposed group (NIC, n = 8) and nicotine-exposed/electroacupuncture-treated group (NIC + EA, n = 8).
Nicotine exposure
A nicotine solution was added to the rats’ drinking water for 6 weeks plus 6 days. Water consumption daily was measured to adjust the nicotine concentration in drinking water to a standard level. Nicotine hemisulfate salt (Cat# MKCG2560, Sigma, St. Loius, MO, USA) was dosed at 1.0 mg/kg per day during the first week, 1.2 mg/kg per day during the second week, 1.5 mg/kg per day during the third week, then 2 mg/kg per day for the remaining weeks. Urine cotinine levels (a long-term metabolite of nicotine (Gaalema et al., 2011)) were measured by immunochromatography (NicAlert, Knoxville, TN, USA; urine samples with NicAlert®) to monitor nicotine levels. The dipstick was inserted into urine samples to wait for the results to appear. Urinary cotinine concentrations above 1000 ng/mL in rats are considered to be similar to those found in chronically addicted subjects (Zhang et al., 2021). In this study, urine cotinine levels (at 6 weeks) in all nicotine-exposed rats were > 1000 ng/mL. Rats in the CTR group were provided drinking water with no nicotine.
Mechanical sensory behavioral testing
Behavioral testing was performed twice a week during the 6 weeks of nicotine exposure, and once a day during the 6-day period of electroacupuncture treatment. Behavioral baseline values were measured twice in the week before nicotine exposure. We used a von Frey filaments sensory evaluator (North Coast Medical, Inc., Wood Dale, IL, USA) to measure hind paw mechanical withdrawal thresholds so as to analyze the effects of nicotine and electroacupuncture on pain sensation. For each behavioral testing, the rat was placed on a wire-mesh platform with a clear plastic cover, and allowed 20 minutes for habituation. In the von Frey filament testing, the up-down method was used for titration (Bonin et al., 2014). Testing was done randomly beginning with either the left or right hind paw. When a positive response (rapid flinching or paw shrinking or licking) was performed within 5 seconds, the next measurement was performed with a thinner filament (or a thicker filament if the response negative). Rat hind paw responses were measured five times for each behavioral test, and the mean value was calculated. The mechanical withdrawal thresholds were expressed in grams as the tolerance level.
Electroacupuncture treatment
According to the mechanical sensory behavioral testing, after 6 weeks of continuous nicotine exposure, all the nicotine-induced hyperalgesic rats in the NIC + EA group received electroacupuncture at bilateral Zusanli and Taichong acupoints. The comparative anatomy was used to locate the rat acupoints (Guo and Fang, 2008). As shown in Figure 1.
Figure 1.
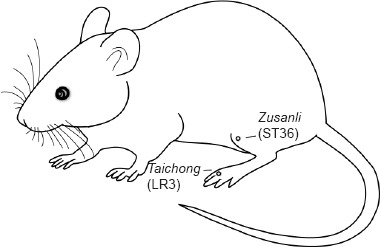
The location of the acupoints Zusanli (ST36) and Taichong (LR3) in rats.
Zusanli acupoint is posterolateral to the knee joint and approximately 5 mm below the fibular head. Taichong acupoint is in the depression between the first and second metatarsals of the dorsal surface of the foot. A 0.25 mm × 13.0 mm stainless steel filiform needle (Huatuo Medical Equipment Co., Ltd., Suzhou, China) was inserted into the acupoint Zusanli at an angle of 90° and the insertion depth was 7 mm. The Taichong acupoint was needled at an angle of 45° and the insertion depth was 4 mm. A G6805-2 Electroacupuncture Therapy Instrument (Shanghai Medical Equipment Co., Ltd., Shanghai, China) was used as a continuous wave with a frequency of 2 Hz and a stimulus intensity of 1 mA. Electroacupuncture treatment was administered daily, 20 minutes once, for 6 days. Electroacupuncture was applied to the rats in the NIC + EA group at the same time in the morning, every day by the same researcher. Rats in the CTR and NIC groups received grip fixation similar to those in the NIC + EA group but without electroacupuncture treatment. At 24 hours after each electroacupuncture treatment, mechanical sensory behavioral testing was performed in all three groups. During the 6-day period of electroacupuncture treatment, nicotine was continuously provided to the rats in the NIC + EA group.
Tissue preparation
To analyze the possible mechanisms underlying the effect of electroacupuncture on pain sensitivity caused by long-term nicotine exposure, spinal cord and the midbrain periaqueductal gray (PAG) samples were taken from the rats of the three groups on the same day of the final behavioral testing, 24 hours after the sixth electroacupuncture treatment. Rats were given an overdose of isoflurane (Piramal Enterprises Limited, Andhra Pradesh, India), sacrificed, and transcardially perfused with normal saline. The rat’s L4–5 spinal cord and the brain tissue were removed, and the PAG was quickly dissected. Then the spinal cord and the PAG were rapidly frozen and stored at –80°C. Six rats from each group were randomly selected for western blot assay.
Western blot assay
The tissues of the spinal cord (L4–5) and PAG were homogenized in radioimmunoprecipitation assay lysis buffer containing phosphatase inhibitors and protease inhibitors (Sigma-Aldrich). The homogenate was centrifuged at 12,000 × g for 15 minutes. Protein concentrations from the supernatant were assayed using the PierceTM Bicinchoninic acid assay Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). Protein of each tissue was denatured with sodium dodecyl-sulfate polyacrylamide gel electrophoresis loading buffer at 95°C for 5 minutes. Proteins were separated by 10% tris-glycine sodium dodecyl-sulfate polyacrylamide gel electrophoresis, transferred onto a polyvinylidene difluoride membrane, and blocked with RapidBlockTM (Amresco, Solon, OH, USA) for 1 hour. The membranes were incubated with primary antibodies at 4°C overnight. The internal controls were goat anti-β-actin (1:1000, Cat# SC-1616, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse anti-tubulin (1:2000, Cat# T8023, Sigma). Target antibodies were rabbit anti-MOR (1:300, Cat# 44-308G, Invitrogen, Camarillo, CA, USA), goat anti-β-EP (1:300, Cat# SC-18264, Santa Cruz Biotechnology), mouse anti-GAD67 (1:300, Cat# SC-58531, Santa Cruz Biotechnology) and rabbit anti-GAD65 (1:500, Cat# PA5-21297, Thermo Fisher, Rockford, IL, USA). After three washes with phosphate-buffered saline with 0.5% Tween-20 wash buffer, the membranes were then incubated with horseradish peroxidase conjugated secondary antibodies from Santa Cruz Biotechnology: goat anti-mouse secondary antibody (1:5000, Cat# SC-2005), donkey anti-goat secondary antibody (1:5000, Cat# SC-2020) or goat anti-rabbit secondary antibody (1:5000, Cat# SC-2004) for 1 hour at room temperature. After three washes with phosphate-buffered saline with 0.5% Tween-20 wash buffer, the membranes were incubated with an enhanced chemiluminescence reagent (Thermo Scientific). The obtained chemiluminescence values were used for quantitative western blotting. Targeted protein band was standardized by measuring the level of the internal control.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6.0 software (GraphPad Software, San Diego, CA, USA). Behavioral testing data were presented as mean ± standard error of mean (SEM) and used by two-way analysis of variance followed by Bonferroni post hoc tests to compare the differences over the same period of time. Western blot data were presented as relative values to control levels. One-way analysis of variance followed by Newman-Keuls multiple comparison test was performed. P values < 0.05 were considered statistically different.
Results
Pain sensation of nicotine-exposed rats at 6 weeks
Behavioral testing results in the NIC (n = 16) and CTR (n = 8) groups of rats were evaluated during the 6 weeks of nicotine exposure. Figure 2 shows the differences of pain sensation in the NIC and CTR groups at 6 weeks. Mechanical thresholds in the NIC group gradually increased during the nicotine exposure period of weeks 1–3 and were significantly higher in weeks 2–3 compared to the CTR group (P < 0.001), indicating an expected initial analgesic effect. In the fourth week of nicotine exposure, the mechanical thresholds in the NIC group decreased but were still higher compared to the CTR group (P > 0.05). In the nicotine exposure period of weeks 5–6, the mechanical thresholds in the NIC group continued to decrease and were significantly lower compared to the CTR group (P < 0.001), indicating hyperalgesia from long-term nicotine exposure. The results indicate that short-term acute nicotine exposure produced an analgesic effect, and long-term chronic nicotine exposure led to an increase in pain sensitivity.
Figure 2.
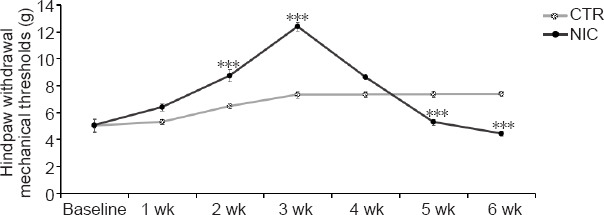
Pain sensation of nicotine-exposed rats at 6 weeks.
Data are presented as mean ± SEM (CTR: n = 8, NIC: n = 16). ***P < 0.001, vs. CTR group (two-way analysis of variance followed by Bonferroni post-hoc tests). CTR: Age-matched control group; NIC: nicotine-exposed group.
Mechanical sensory effects of electroacupuncture in nicotine-induced hyperalgesic rats
Behavioral testing results in the CTR (n = 8), NIC (n = 8) and NIC + EA (n = 8) groups of rats were evaluated during the 6-day period of electroacupuncture treatment. Twenty-four hours after each electroacupuncture treatment, mechanical sensory behavioral testing was performed in all three groups of rats. Figure 3 shows the differences of pain sensation in the three groups during the 6-day period of electroacupuncture treatment. The NIC group consistently demonstrated lower mechanical thresholds compared to the CTR group indicating hyperalgesia (P < 0.001). After electroacupuncture treatments, the mechanical thresholds increased in the nicotine exposed rats and were significantly higher than those in the NIC group without electroacupuncture (P < 0.001). After the fifth and the sixth electroacupuncture treatment, the mechanical thresholds were significantly higher in the nicotine exposed rats than even those in the CTR group (P < 0.05 or 0.01). The results indicate that electroacupuncture treatment had positive analgesic effects on pain sensitivity in the nicotine exposed rats. This analgesic effect was maintained for at least 24 hours after each electroacupuncture treatment.
Figure 3.
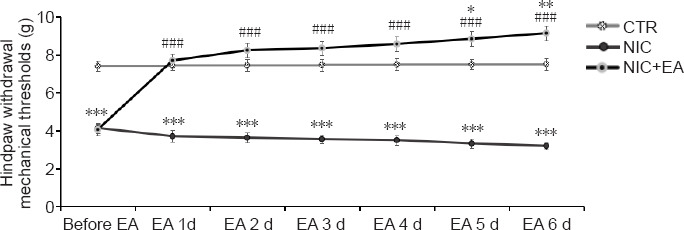
Mechanical sensory effects of electroacupuncture in nicotine-induced hyperalgesic rats.
Data are presented as mean ± SEM (CTR: n = 8, NIC: n = 8, NIC + EA: n = 8). *P < 0.05, **P < 0.01, ***P < 0.001, vs. CTR group; ###P < 0.001, vs. NIC group (two-way analysis of variance followed by Bonferroni post-hoc tests). CTR: Age-matched control group; NIC: nicotine-exposed group; NIC + EA: nicotine-exposed/electroacupuncture-treated group.
Electroacupuncture effects on pain-related factor protein expression in the spinal cord and PAG of nicotine-induced hyperalgesic rats
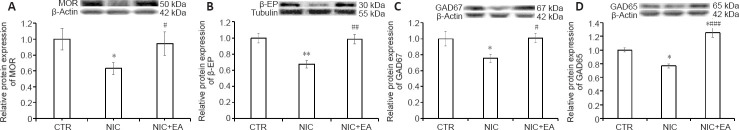
MOR, β-EP, GAD67, and GAD65 protein expression in the spinal cord and PAG of the CTR (n = 6), NIC (n = 6) and NIC + EA (n = 6) groups of rats were analyzed by western blotting assay after the 6-day period of electroacupuncture treatment. Figure 4 shows the differences in MOR, β-EP, GAD67, and GAD65 protein expression in the spinal cord of rats in the three groups after the 6-day period of electroacupuncture treatment. In the NIC group, MOR, β-EP, GAD67, and GAD65 protein expression in the spinal cord were significantly lower compared to those in the CTR group (P < 0.05 or 0.01). After electroacupuncture treatments, MOR, β-EP, GAD67, and GAD65 protein expression in the spinal cord increased in the nicotine exposed rats and were significantly higher than those in the NIC group without electroacupuncture (P < 0.05 or 0.001). The protein expression of GAD65 in the spinal cord in the NIC + EA group was significantly higher than that in the CTR group (P < 0.05). The results indicate that MOR, β-EP, GAD67, and GAD65 protein expression in the spinal cord were significantly increased by electroacupuncture treatment in the nicotine-induced hyperalgesic rats.
Figure 4.
Electroacupuncture effects on MOR, β-EP, GAD67, and GAD65 protein expression in the spinal cord of nicotine-induced hyperalgesic rats.
(A–D) Western blot analysis of MOR (A), β-EP (B), GAD67 (C), and GAD65 (D) in the spinal cord of rats in the three groups. Data (mean ± SEM) are presented as relative values to control levels (CTR: n = 6, NIC: n = 6, NIC + EA: n = 6). *P < 0.05, **P < 0.01, vs. CTR group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. NIC group (one-way analysis of variance followed by Newman-Keuls multiple comparison test). CTR: Age-matched control group; NIC: nicotine-exposed group; NIC + EA: nicotine-exposed/electroacupuncture-treated group. GAD65: glutamic acid decarboxylase 65; GAD67: glutamic acid decarboxylase 67; MOR: μ-opioid receptor; β-EP: β-endorphin.
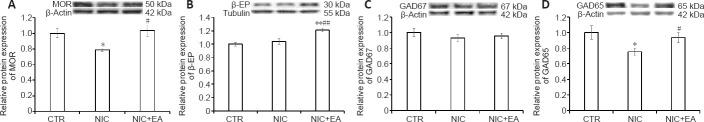
Figure 5 shows the differences of MOR, β-EP, GAD67, and GAD65 protein expression in the PAG of the three groups of rats. In the NIC group, MOR and GAD65 protein expression in the PAG were significantly lower than those in the CTR group (P < 0.05). There were no significant differences in the protein expression of β-EP or GAD67 in the PAG in the NIC group compared to the CTR group (P > 0.05). After electroacupuncture treatment, MOR, β-EP, and GAD65 protein expression in the PAG increased in the nicotine exposed rats and were significantly higher than those in the NIC group without electroacupuncture (P < 0.05 or 0.01). There was no significant difference in the protein expression of GAD67 in the PAG between NIC + EA and NIC groups (P > 0.05), and the protein expression of β-EP in the PAG in the NIC + EA group was significantly higher than that in the CTR group (P < 0.01). The results indicate that MOR, β-EP, and GAD65 protein expression in the PAG could be significantly increased by electroacupuncture treatment in nicotine-induced hyperalgesic rats. The electroacupuncture effect on the protein expression of GAD67 in the PAG in nicotine-induced hyperalgesic rats was not significantly changed.
Figure 5.
Electroacupuncture effects on MOR, β-EP, GAD67, and GAD65 protein expression in the PAG of nicotine-induced hyperalgesic rats.
(A–D) Western blot analysis of MOR (A), β-EP (B), GAD67 (C), and GAD65 (D) in the PAG of rats in the three groups. Data (mean ± SEM) are presented as relative values to control levels (CTR: n = 6, NIC: n = 6, NIC + EA: n = 6). *P < 0.05, **P < 0.01, vs. CTR group; #P < 0.05, ##P < 0.01, vs. NIC group (one-way analysis of variance followed by Newman-Keuls multiple comparison test). CTR: Age-matched control group; NIC: nicotine-exposed group; NIC + EA: nicotine-exposed/electroacupuncture-treated group. GAD65: glutamic acid decarboxylase 65; GAD67: glutamic acid decarboxylase 67; MOR: μ-opioid receptor; PAG: periaqueductal gray; β-EP: β-endorphin.
Discussion
Cigarette smoking introduces nicotine into the human body and causes notable changes in physiology, leading to respiratory problems, cardiovascular disease, and chronic pain conditions. Repeated exposure to nicotine can lead to neuroadaptation and produce nicotine dependence (D’ Silva et al., 2014; Zanaty, 2014). One of the main factors making tobacco use particularly addictive is the large amounts of nicotine, which is repeatedly introduced into the bloodstream. Nicotine dependence and chronic pain often occur together. Smoking can enhance chronic pain, and pain can stimulate the desire to use tobacco, interfering with attempts at smoking cessation (Orhurhu et al., 2015; LaRowe and Ditre, 2020). The interrelationship between pain and tobacco smoking is of increasing interest because of its high prevalence and profound impact on public health (LaRowe and Ditre, 2020). A previous survey indicated that people who smoke every day have higher levels of bodily pain than those who never smoked, across all ages (Perski et al., 2020). Smokers with pain are more likely to use e-cigarettes and alternative nicotine products to self-medicate pain (Powers et al., 2020). Studies also reported that smokers who were motivated to use tobacco to alleviate pain may unintentionally aggravate their pain by increasing the frequency of smoking, thus forming a vicious circle and leading to more severe nicotine dependence (Ditre and Brandon, 2008; Zale et al., 2016).
Acupuncture is a promising adjuvant treatment for chronic pain. Furthermore, acupuncture analgesia is more effective, faster, and tolerated better compared with morphine. Studies have suggested that the one way to avoid chronic opioid dependence is the use of acupuncture as a non-pharmacological intervention, effectively reducing the need to prescribe opioids (Grissa et al., 2016; Deng and Mao, 2019). Electroacupuncture therapy is a method of combining acupuncture and electrical stimulation via the filiform needles which have been inserted into the body points, using a stable frequency pulse current through the electroacupuncture therapeutic apparatus. Electroacupuncture may be a better treatment for chronic pain compared to manual manipulation of the needles because acupuncturists do not have to constantly stimulate the needles by hand (Deare et al., 2013). The stimulus frequency, intensity, and duration of electroacupuncture are kept stable throughout the treatment. In this study, we used electroacupuncture at bilateral Zusanli and Taichong acupoints to treat pain sensitivity caused by long-term exposure to nicotine. Zusanli is the “Lower He-sea” point and the “He-sea” point of the Stomach Meridian of the Foot-Yangming. Taichong is the “Yuan-source” point and the “Shu-stream” point of the Liver Meridian of the Foot-Jueyin. Zusanli and Taichong are the commonly used points in treating nicotine dependence and smoking cessation (Ma et al., 2015; Wang et al., 2016b). The two points can work together to regulate the qi movement of the spleen, stomach, and liver so as to relieve the pain and discomfort caused by smoking addiction.
This study demonstrated that short-term nicotine exposure had an analgesic effect, and long-term nicotine exposure increased pain sensitivity. These findings are consistent with our previous study as well as others (Ditre et al., 2011; Zhang et al., 2020). In this trial, the mechanical sensory behavioral testing results indicate that electroacupuncture treatment had significant analgesic effects on nicotine-induced hyperalgesia in rats. A western blot assay was used to detect the changes of MOR, β-EP, GAD67, and GAD65 in the spinal cord and midbrain PAG of the central nervous system.
The spinal cord is the lower part of the central nervous system, and the midbrain PAG is the higher part of the central nervous system that regulates a variety of complex behaviors, including pain. β-EP is an endogenous opioid neuropeptide and peptide hormone, which is similar to opioid drugs in its analgesic properties. It is an opioid receptor agonist and is the main endogenous ligand of MOR. β-EP has the highest binding affinity to the MOR compared to any of the endogenous opioids. It can combine with MOR to produce the same analgesic effect as morphine and other opiates, and is often referred to as the human body’s natural analgesic (Smyth, 2016). γ-Aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the mammalian central nervous system and plays an important role in analgesic mechanism (Jasmin et al., 2004). Glutamic acid decarboxylase (GAD) is a key enzyme in GABA biosynthesis. GAD has two major isoforms, GAD67 and GAD65, which are encoded by the GAD1 and GAD2 genes respectively (Kanao-Kanda et al., 2020).
The results of our study demonstrated that MOR, β-EP, GAD67, and GAD65 protein expression in the spinal cord significantly decreased in nicotine-induced hyperalgesic rats. After electroacupuncture treatment, MOR, β-EP, GAD67, and GAD65 protein expression in the spinal cord increased in the same rat population. The protein expression of GAD65 in the spinal cord in the NIC + EA group was also significantly higher than that in the CTR group. Electroacupuncture treatment appears to promote the release of β-EP and increase the expression of MOR in the spinal cord. The increased β-EP combined with the increased MOR appears to produce improved analgesic effects. Electroacupuncture treatment also increased the expression of GAD67 and GAD65 in the spinal cord. The increased levels of GAD67 and GAD65 lead to an increase in GABA synthesis, promoting the release of GABA and apparently producing increased analgesic effects.
Changes in the levels of pain-related factors MOR, β-EP, GAD67, and GAD65 in the PAG were different from those in the spinal cord. MOR and GAD65 protein expression in the PAG decreased significantly in the nicotine-induced hyperalgesic rats. But there was no significant difference in the protein expression of β-EP in the PAG in the NIC group compared to the CTR group. The results may indicate that β-EP in the midbrain PAG contributes to the rewarding and emotional effects of nicotine, which is consistent with some other studies (Berrendero et al., 2010; Gudehithlu et al., 2012). After electroacupuncture treatment, MOR, β-EP, and GAD65 protein expression in the PAG increased in the nicotine-exposed rats, and the protein expression of β-EP in the PAG in the NIC + EA group was significantly higher than that in the CTR group. However, there was no significant difference in the protein expression of GAD67 in the PAG between the NIC + EA and NIC groups. There was also no significant difference in the protein expression of GAD67 in the PAG in the NIC group compared to the CTR group. The results suggest that presynaptic GAD65 plays a role in the pain mechanism, which is consistent with prior studies (Patel et al., 2006; Zhang et al., 2011). GAD65 preferentially acts on the presynaptic terminals of central neurons for GABA synthesis in the synaptic vesicles, and is necessary for active GABA synaptic release. GAD67 is a GABA-synthesizing enzyme producing cytoplasmic GABA. Electroacupuncture treatment significantly increased the expression of GAD65 in the spinal cord and midbrain PAG in nicotine-induced hyperalgesic rats, producing analgesic effects. Simultaneously, electroacupuncture treatment increased the release of β-EP and improved the expression of MOR in the midbrain PAG and appeared to increase analgesic effects.
Based on this trial, electroacupuncture treatment appears to improve pain sensitivity in the nicotine-induced hyperalgesic rats. The possible mechanism for the positive effects of the electroacupuncture treatment appears to be related to the release of β-EP and the increased expression of MOR in the spinal cord and the midbrain PAG of the central nervous system. The increased β-EP combines with the increased MOR, producing enhanced analgesic effects. Electroacupuncture treatment can also improve the expression of GAD67 and GAD65 in the spinal cord, and improve the expression of GAD65 in the midbrain PAG, promoting the release of GABA and again, produced analgesic effects. If electroacupuncture will have a similar effect in improving the effects of nicotine withdrawal in humans remains unknown at this time.
In condition, short-term acute nicotine exposure can induce analgesic effects, and long-term chronic nicotine exposure can cause pain hypersensitivity. Electroacupuncture treatment has positive analgesic effects on the increased pain sensitivity noted in the nicotine-exposed rats. The enhancing analgesic effects of electroacupuncture treatment appear to be related to the increased expression of β-EP, MOR, GAD67, and GAD65 in the spinal cord, and the increased expression of β-EP, MOR, and GAD65 in the midbrain PAG. Electroacupuncture treatment may be an effective adjuvant analgesic for chronic pain caused by long-term chronic nicotine exposure.
Additional file: Open peer review reports 1 (86.5KB, pdf) and 2 (88.1KB, pdf) .
Acknowledgments:
We sincerely thank the Department of Anesthesiology, Perioperative Medicine and Pain Management, University of Miami, Miller School of Medicine for offering experimental areas and instruments.
Footnotes
P-Reviewers: Gomez-Pinedo U, Herrera-Valdez MA; C-Editor: Zhao M; S-Editors: Yu J, Song LP; L-Editor: Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare no competing interests.
Financial support: This study was supported by the Department of Anesthesiology, Perioperative Medicine and Pain Management, University of Miami Miller School of Medicine, USA. The funding source had no role in study conception and design, data analysis or interpretation, paper writing or decision to submit this paper for publication.
Institutional review board statement: The study was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Miami (#18-167) on December 12, 2018.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Ulises Gomez-Pinedo, Hospital Clinico Universitario San Carlos, Spain; Marco Arieli Herrera-Valdez, Universidad Nacional Autónoma de México, Mexico.
Funding: This study was supported by a grant from Department of Anesthesiology, Perioperative Medicine and Pain Management, University of Miami Miller School of Medicine, USA.
References
- 1.Berrendero F, Robledo P, Trigo JM, Martín-García E, Maldonado R. Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav Rev. 2010;35:220–231. doi: 10.1016/j.neubiorev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014;10:26. doi: 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’ Silva J, Devadiga S, Dengody P, Chalathadka M, Bhoj M, Jain V. Smoking and chronic pain. J Health Res Rev. 2014;1:34–39. [Google Scholar]
- 4.Deare JC, Zheng Z, Xue CC, Liu JP, Shang J, Scott SW, Littlejohn G. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev 2013. 2013 doi: 10.1002/14651858.CD007070.pub2. CD007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng G, Mao JJ. Acupuncture to reduce Opioid Consumption in Patients with Pain: Getting to the Right Points. Pain Med. 2019;20:207–208. doi: 10.1093/pm/pny258. [DOI] [PubMed] [Google Scholar]
- 6.Ditre JW, Brandon TH. Pain as a motivator of smoking: effects of pain induction on smoking urge and behavior. J Abnorm Psychol. 2008;117:467–472. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. 2011;137:1065–1093. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan AY, Miller DW, Bolash B, Bauer M, McDonald J, Faggert S, He H, Li YM, Matecki A, Camardella L, Koppelman MH, Stone JAM, Meade L, Pang J. Acupuncture’s role in solving the opioid epidemic: evidence, cost-effectiveness, and care availability for acupuncture as a primary, non-pharmacologic method for pain relief and management-white paper 2017. J Integr Med. 2017;15:411–425. doi: 10.1016/S2095-4964(17)60378-9. [DOI] [PubMed] [Google Scholar]
- 9.Gaalema DE, Higgins ST, Bradstreet MP, Heil SH, Bernstein IM. Using NicAlert strips to verify smoking status among pregnant cigarette smokers. Drug Alcohol Depend. 2011;119:130–133. doi: 10.1016/j.drugalcdep.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goesling J, Brummett CM, Meraj TS, Moser SE, Hassett AL, Ditre JW. Associations between pain, current tobacco smoking, depression, and fibromyalgia status among treatment-seeking chronic pain patients. Pain Med. 2015;16:1433–1442. doi: 10.1111/pme.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grissa MH, Baccouche H, Boubaker H, Beltaief K, Bzeouich N, Fredj N, Msolli MA, Boukef R, Bouida W, Nouira S. Acupuncture vs intravenous morphine in the management of acute pain in the ED. Am J Emerg Med. 2016;34:2112–2116. doi: 10.1016/j.ajem.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Gudehithlu KP, Duchemin AM, Tejwani GA, Neff NH, Hadjiconstantinou M. Nicotine-induced changes of brain β-endorphin. Neuropeptides. 2012;46:125–131. doi: 10.1016/j.npep.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Fang J. Experimental acupuncture science. Beijing: China Press of Traditional Chinese Medicine; 2008. [Google Scholar]
- 14.Hooten WM, Townsend CO, Bruce BK, Schmidt JE, Kerkvliet JL, Patten CA, Warner DO. Effects of smoking status on immediate treatment outcomes of multidisciplinary pain rehabilitation. Pain Med. 2009;10:347–355. doi: 10.1111/j.1526-4637.2008.00494.x. [DOI] [PubMed] [Google Scholar]
- 15.Kanao-Kanda M, Kanda H, Liu S, Roy S, Toborek M, Hao S. Viral vector-mediated gene transfer of glutamic acid decarboxylase for chronic pain treatment: a literature review. Hum Gene Ther. 2020;31:405–414. doi: 10.1089/hum.2019.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaRowe LR, Ditre JW. Pain, nicotine, and tobacco smoking: current state of the science. Pain. 2020;161:1688–1693. doi: 10.1097/j.pain.0000000000001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin JG, Chan YY, Chen YH. Acupuncture for the treatment of opiate addiction. Evid Based Complement Alternat Med. 2012;2012:739045. doi: 10.1155/2012/739045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma E, Chan T, Zhang O, Yang JS, Wang YY, Li YC, Ho R, Lai C, Lam PY. Effectiveness of acupuncture for smoking cessation in a Chinese population. Asia Pac J Public Health. 2015;27:NP2610–2622. doi: 10.1177/1010539513503867. [DOI] [PubMed] [Google Scholar]
- 19.Orhurhu VJ, Pittelkow TP, Hooten WM. Prevalence of smoking in adults with chronic pain. Tob Induc Dis. 2015;13:17. doi: 10.1186/s12971-015-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J Neurochem. 2006;97:385–396. doi: 10.1111/j.1471-4159.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- 21.Perski O, Garnett C, Shahab L, Brown J, West R. Associations between smoking status and bodily pain in a cross-sectional survey of UK respondents. Addict Behav. 2020;102:106229. doi: 10.1016/j.addbeh.2019.106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powers JM, Heckman BW, LaRowe LR, Ditre JW. Smokers with pain are more likely to report use of e-cigarettes and other nicotine products. Exp Clin Psychopharmacol. 2020;28:601–608. doi: 10.1037/pha0000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Weingarten TN, Mantilla CB, Hooten WM, Warner DO. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977–992. doi: 10.1097/ALN.0b013e3181ebdaf9. [DOI] [PubMed] [Google Scholar]
- 24.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. Am J Med. 2010;123:87.e7–35. doi: 10.1016/j.amjmed.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Smyth DG. 60 YEARS OF POMC: Lipotropin and beta-endorphin: a perspective. J Mol Endocrinol. 2016;56:T13–25. doi: 10.1530/JME-16-0033. [DOI] [PubMed] [Google Scholar]
- 26.Wang JH, van Haselen R, Wang M, Yang GL, Zhang Z, Friedrich ME, Wang LQ, Zhou YQ, Yin M, Xiao CY, Duan AL, Liu SC, Chen B, Liu JP. Acupuncture for smoking cessation: a systematic review and meta-analysis of 24 randomized controlled trials. Tob Induc Dis. 2019;17:48. doi: 10.18332/tid/109195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Liu Z, Zhang O, Chen M, Huang L, Wu Y, Zhang F, Wu G, He X, Yang J. Effect of acupuncture treatment on smoking cessation in smokers from Hong Kong. J Tradit Chin Med. 2016a;36:634–639. doi: 10.1016/s0254-6272(16)30083-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang YY, Liu Z, Wu Y, Zhang O, Chen M, Huang LL, He XQ, Wu GY, Yang JS. Acupuncture for smoking cessation in Hong Kong: a prospective multicenter observational study. Evid Based Complement Alternat Med. 2016b;2016:2865831. doi: 10.1155/2016/2865831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zale EL, Maisto SA, Ditre JW. Anxiety and depression in bidirectional relations between pain and smoking: implications for smoking cessation. Behav Modif. 2016;40:7–28. doi: 10.1177/0145445515610744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanaty OM. Nicotine smoking: influences on perioperative pain management. Egypt J Anaesth. 2014;30:373–376. [Google Scholar]
- 31.Zhang Y, Sevilla A, Weller R, Wang S, Gitlin MC, Candiotti KA. The role of α7-nicotinic acetylcholine receptor in a rat model of chronic nicotine-induced mechanical hypersensitivity. Neurosci Lett. 2021;743:135566. doi: 10.1016/j.neulet.2020.135566. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Yang J, Sevilla A, Weller R, Wu J, Su C, Zheng C, Rodriguez-Blanco YF, Gitlin M, Candiotti KA. The mechanism of chronic nicotine exposure and nicotine withdrawal on pain perception in an animal model. Neurosci Lett. 2020;715:134627. doi: 10.1016/j.neulet.2019.134627. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med. 2011;17:1448–1455. doi: 10.1038/nm.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.