It is well known that diabetic retinopathy is a neurovascular disease that is accompanied by dysfunction of neurovascular units composed of neurons, glial cells, and vascular cells (Antonetti et al., 2012; Figure 1). Many studies have reported that the neuronal abnormalities, such as neuronal cell death, frequently precedes vascular abnormalities including neovascularization (Sohn et al., 2016). Neuronal cell death and axonal degeneration are irreversible changes under normal physiological conditions, and they are directly linked to the vision decrease. In fact, there is a reduction of the thickness of retinal nerve fiber layer in patients without diabetic retinopathy (Sohn et al., 2016). The reduction in the thickness of the ganglion cell complex, which is made up of the retinal nerve fiber layer, ganglion cell layer, and inner plexiform layer, is 0.54 μm/year which is similar to the reduction observed in advanced glaucoma (Sohn et al., 2016). These clinical findings strongly indicated that the axonal degeneration is associated with the pathogenesis of neuronal abnormalities in diabetic retinas. Thus, not only neuroprotection but also regenerative therapies are required for the protection and maintenance of visual function of eyes with diabetic retinopathy.
Figure 1.
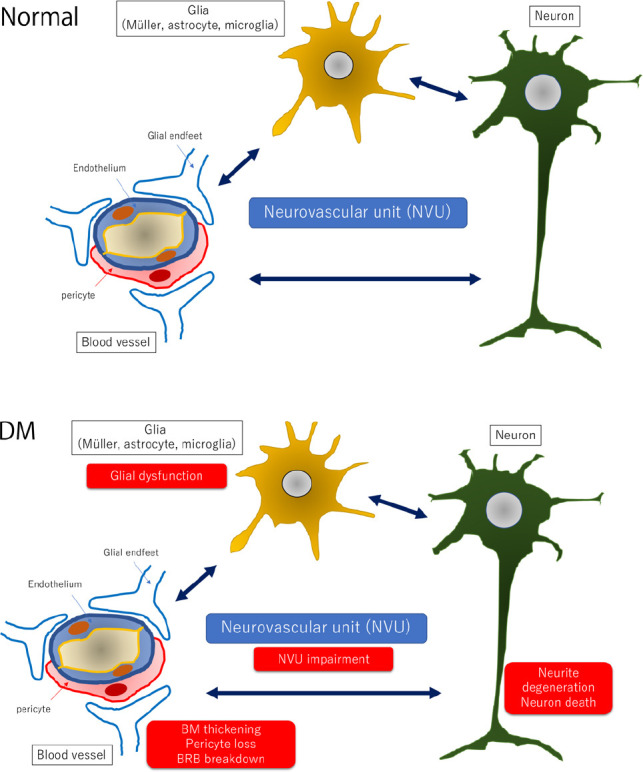
Hypothetic schemes of neurovascular units and pathological changes of the diabetic retina.
In the normal retina (upper panel), the neurovascular units are composed of vascular cells (pericyte and endothelial cell), glial cells (Müller cell, microglia and astrocyte), and neurons. Vascular cells and glial processes form the BRB, and pericytes are considered to maintain the function of retinal cells by maintaining the appropriate environment because the loss of platelet-derived growth factor signaling in pericytes causes a breakdown of the BRB and neuronal cell death. Glial cells including microglia may monitor the changes in the vessels and the condition of the retina and transfer the changes into neuronal cells. In the diabetic retina (lower panel), glial dysfunction may occur first by an increase expression of glial fibrillary acidic protein in Müller cells and reduction of glial fibrillary acidic protein expression in astrocytes. Activated glial cells are partly associated with an increase of cytokines such as tumor necrosis factor-α, interleukin-1β, interleukin-6, interleukin-8, monocyte chemoattractant protein-1, and vascular endothelial growth factor and a decrease of growth factors such as nerve growth factor, brain-derived growth factor, and pigment epithelium-derived growth factor. These glial changes contribute to neuronal abnormalities including neuronal cell death and neurite degeneration. Pericytes are affected faster than endothelial cells and increase the expression of extracellular matrix components, partly contributing to BRB breakdown. BM: Basement membrane; BRB: blood-retinal barrier; DM: diabetes mellitus.
Diabetic retinopathy is a chronic disease, and the methods used for neuroprotective therapies must be simple and easy to perform. For these reasons, several clinical trials using topical instillation of neuroprotective drugs, e.g., brimonidine and somatostatin, have been performed in Europe (the EUROCONDOR Clinical Trials) (Simo et al., 2019). Unfortunately, these large clinical trials could not reach the primary end points even though the neuroprotective effects of these drugs had been demonstrated in many animal studies (Saylor et al., 2009; Hernandez et al., 2013). One of the reasons for the absence of protection of visual function in diabetic patients was that multiple pathways of neuronal cell death are activated during the process of neuronal cell death in diabetic eyes, and only one drug cannot inhibit all pathways involved in the neuronal cell death. Another reason is that even if the neuronal cell bodies are protected, once axonal degeneration occurs, neuronal function cannot be recovered because degenerated axons cannot be regenerated under normal physiological conditions. Therefore, axonal regeneration as well as neuroprotection are required. Unfortunately, most clinicians, especially vitreous surgeons, see only retinas and vascular abnormalities. They do not realize that the optic nerve axons are degenerated during the progression of diabetic retinopathy. In fact, few studies have focused on retinal neuronal regeneration in diabetic retinopathy worldwide.
To resolve these issues, we have tried to determine a combination therapy for neuroprotection and regeneration for diabetic retinopathy (Bikbova et al., 2017; Kitamura et al., 2019). We have examined citicoline which is an intermediate molecule in the metabolism of phosphatidyl choline because phosphatidyl choline is the major phospholipids of neuronal cells in the retina and the brain. Exogenous citicoline has been shown to have neuroprotective and regenerative effects on retinal ganglion cells (Oshitari et al., 2002). This is probably because citicoline indirectly inhibits phospholipase A2 and stabilizes the mitochondrial membrane as a mitochondrial stabilizer (Oshitari et al., 2002). In patients with diabetic retinopathy, the mitochondria- and caspase-dependent cell death pathways are associated with neuronal degeneration (Oshitari et al., 2008). Thus, a mitochondrial stabilizer, citicoline, is one of the candidates for a neuroprotective drug.
Taurourusodeoxycholic acid (TUDCA) is known to be an anti-endoplasmic reticulum stress agents (Oshitari et al., 2014). It is a major component of bear bile and has been used in traditional Chinese medicine for a long time. Because endoplasmic reticulum stress is also associated with neuronal cell death under diabetic conditions, we have selected TUDCA as one of the combination drugs (Oshitari et al., 2014).
For axonal regeneration, the endogenous regenerative pathways, mammalian target of rapamycin (mTOR) pathways (Lim et al., 2016) and/or Jak-STAT pathways (Mak et al., 2020) must be activated. The mTOR pathway is known to be activated by trk receptors binding with neurotrophic factors (Pernet and Schwab, 2014). Nerve growth factor family members including brain-derived neurotrophic factor and neurottophin-4 (NT-4) bind to the trkB receptor and stimulate neuronal cell survival and regeneration by the activation of the mTOR pathways (Pernet and Schwab, 2014). In a series of retinal culture studies, we selected NT-4 for facilitating regeneration because NT-4 has been shown to have the most neuroprotective and regenerative effects compared to the other neurotrophic factors. In cultured retinas, a combination of citicoline, TUDCA, and NT-4 was the most effective for neuroprotection and regeneration compared to a single agent (Bikbova et al., 2017). Similarly, in the optic nerve crush model, an acute injury model, topical instillation of a combination of the three agents was the most effective for the regeneration of the optic nerve axons (Kitamura et al., 2019).
These combination therapies may be useful for neuroprotection and regeneration of chronic retinal disease such as diabetic retinopathy. However, there is no guarantee that the same strategies which are effective for acute injuries are effective for chronic retinal diseases. Because the cell death pathways of chronic retinal diseases may be more complicated than that of acute injuries and the same neuroprotectants which are effective in acute injuries may not be able to rescue neuronal cell death in chronic retinal diseases completely. In addition, regenerative medicines must be applied for a long period, and thus topical application of neuroprotective and regenerative agents has been used in the clinical trials (Simo et al., 2019). Blood supplied methods are one of the options but general side effects must be considered for a long period of supplementation. That is why researchers have selected topical instillation methods for neuroprotective therapies for diabetic retinopathy (Simo et al., 2019).
We are planning to perform topical combination therapies for diabetic animal models in the near future. Briefly, Spontaneously Diabetic Torii rats will be used for the study and the drugs of topical instillation will be 100 mM TUDCA, 100 mM citicoline, 10 ng/mL NT-4, combined TUDCA and NT-4 (doublet), and combined TUDCA, citicoline and NT-4 (triplet) and PBS. These drugs will be applied twice per day for 3 months. After three months, the effect of these drugs will be examined by retinal ganglion cell counts, GAP-43 immunostaining, optical coherence tomography findings and electroretinograms.
For the complete management of preventing the progression of diabetic retinopathy, clinicians should not ignore the progression of neuronal abnormalities following the progression of vascular abnormalities in diabetic retinopathy. At the same time, not only neuroprotection but also axonal protection and regeneration for protecting visual function should be required because axonal degeneration is definitely associated with vision loss of patients with diabetic retinopathy. The pathological mechanisms of diabetic retinopathy are not simple which means neuroprotective and regenerative therapies are not so easy to establish. Furthermore, therapeutic strategies for neuroprotection and regeneration of diabetic retinopathy must be reconsidered before performing clinical trials. Otherwise, we may lose much time and efforts as well as patients with diabetic retinopathy.
We thank Professor Emeritus Duco Hamasaki of the Bascom Palmer Eye Institute of the University of Miami for editing the manuscript.
The present work is supported by a Grant-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japanese Government.
Footnotes
P-Reviewers: Yin Q, Hu Y; C-Editors: Zhao M, Zhao LJ, Qiu Y; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 2.Bikbova G, Oshitari T, Baba T, Yamamoto S. Combination of neuroprotective and regenerative agents for AGE-induced retinal degeneration (in vitro study) BioMed Res Int. 2017;2017:8604723. doi: 10.1155/2017/8604723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernández C, García-Ramírez M, Corraliza L, Fernández-Carneado J, Farrera-Sinfreu J, Ponsati B, González-Rodríguez A, Valverde AM, Simó R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2013;62:2569–2578. doi: 10.2337/db12-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitamura Y, Bikbova G, Baba T, Yamamoto S, Oshitari T. In vivo effects of single or combined topical neuroprotective and regenerative agents on degeneration of retinal ganglion cells in rat optic nerve crush model. Sci Rep. 2019;9:101. doi: 10.1038/s41598-018-36473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci. 2016;19:1073–1084. doi: 10.1038/nn.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mak HK, Ng SH, Ren T, Ye C, Leung CK. Impact of PTEN/SOCS3 deletion on amelioration of dendritic shrinkage of retinal ganglion cells after optic nerve injury. Exp Eye Res. 2020;192:107938. doi: 10.1016/j.exer.2020.107938. [DOI] [PubMed] [Google Scholar]
- 7.Oshitari T, Fujimoto N, Adachi-Usami E. Citicoline has a protective effect on damaged retinal ganglion cells in mouse culture retina. Neuroreport. 2002;13:2109–2111. doi: 10.1097/00001756-200211150-00023. [DOI] [PubMed] [Google Scholar]
- 8.Oshitari T, Yamamoto S, Hata N, Roy S. Mitochondria- and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy. Br J Ophthalmol. 2008;92:552–556. doi: 10.1136/bjo.2007.132308. [DOI] [PubMed] [Google Scholar]
- 9.Oshitari T, Bikbova G, Yamamoto S. Increased expression of phosphorylated c-Jun and phosphorylated c-Jun N-terminal kinase associated with neuronal cell death in diabetic and high glucose exposed rat retinas. Brain Res Bull. 2014;101:18–25. doi: 10.1016/j.brainresbull.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Pernet V, Schwab ME. Lost in the jungle: new hurdles for optic nerve axon regeneration. Trends Neurosci. 2014;37:381–387. doi: 10.1016/j.tins.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Saylor M, McLoon LK, Harrison AR, Lee MS. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: an evidence-based review. Arch Ophthalmol. 2009;127:402–406. doi: 10.1001/archophthalmol.2009.9. [DOI] [PubMed] [Google Scholar]
- 12.Simó R, Hernández C, Porta M, Bandello F, Grauslund J, Harding SP, Aldington SJ, Egan C, Frydkjaer-Olsen U, García-Arumí J, Gibson J, Lang GE, Lattanzio R, Massin P, Midena E, Ponsati B, Ribeiro L, Scanlon P, Lobo C, Costa MÂ, et al. Effects of topically administered neuroprotective drugs in early stages of diabetic retinopathy: results of the EUROCONDOR clinical trial. Diabetes. 2019;68:457–463. doi: 10.2337/db18-0682. [DOI] [PubMed] [Google Scholar]
- 13.Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N, Garmager A, Wit F, Kucukevcilioglu M, van Velthoven ME, DeVries JH, Mullins RF, Kuehn MH, Schlingemann RO, Sonka M, Verbraak FD, Abràmoff MD. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113:E2655–2664. doi: 10.1073/pnas.1522014113. [DOI] [PMC free article] [PubMed] [Google Scholar]


