ABSTRACT
Small-molecule drugs inhibiting BK polyomavirus (BKPyV) represent a significant unmet clinical need in view of polyomavirus-associated nephropathy or hemorrhagic cystitis, which complicate 5% to 25% of kidney and hematopoietic cell transplantations. We characterized the inhibitory activity of acitretin on BKPyV replication in primary human renal proximal tubular epithelial cells (RPTECs). Effective inhibitory concentrations of 50% (EC50) and 90% (EC90) were determined in dilution series measuring BKPyV loads, transcripts, and protein expression, using cell proliferation, metabolic activity, and viability to estimate cytotoxic concentrations and selectivity indices (SI). The acitretin EC50 and EC90 in RPTECs were 0.64 (SI50, 250) and 3.25 μM (SI90, 49.2), respectively. Acitretin effectively inhibited BKPyV replication until 72 h postinfection when added 24 h before infection until 12 h after infection, but decreased to <50% at later time points. Acitretin did not interfere with nuclear delivery of BKPyV genomes, but it decreased large T-antigen transcription and protein expression. Acitretin did not inhibit the initial round of BKPyV replication following transfection of full-length viral genomes, but it affected subsequent rounds of reinfection. Acitretin also inhibited BKPyV replication in human urothelial cells and in Vero cells, but not in COS-7 cells constitutively expressing Simian virus 40 (SV40) large T antigen. Retinoic acid agonists (all-trans retinoic acid, 9-cis retinoic acid [9-cis-RA], 13-cis-RA, bexarotene, and tamibarotene) and the RAR/RXR antagonist RO41-5253 also inhibited BKPyV replication, pointing to an as-yet-undefined mechanism.
IMPORTANCE Acitretin selectively inhibits BKPyV replication in primary human cell culture models of nephropathy and hemorrhagic cystitis. Since acitretin is an approved drug in clinical use reaching BKPyV-inhibiting concentrations in systemically treated patients, further studies are warranted to provide data for clinical repurposing of retinoids for treatment and prevention of replicative BKPyV-diseases.
KEYWORDS: BKPyV, BK virus, antiviral, acitretin, retinoic acid, large T antigen, antiviral agents, hemorrhagic cystitis, nephropathy
INTRODUCTION
BK polyomavirus (BKPyV) is the main etiologic agent underlying polyomavirus-associated hemorrhagic cystitis (PyVHC) after allogeneic hematopoietic cell transplantation (HCT) as well as polyomavirus-associated nephropathy (PyVAN) and polyomavirus-associated urothelial cancer (PyVUC) after kidney transplantation (1). These diseases share the triad of failing antiviral immune control, transplant-specific procedures in an allogeneic setting, and the exquisite tropism of BKPyV for renourinary cells (2), where the virus persists lifelong after primary infection during early childhood (3, 4). Despite the high BKPyV seroprevalence of >90% in the general population (5–7) and virological and epidemiological evidence for innate and adaptive immune escape in healthy BKPyV-seropositive blood donors shedding the virus in the urine (8–10), immunocompetent individuals are hardly ever affected by BKPyV diseases. However, transplant centers around the world report that PyVAN and PyVHC complicate their kidney transplant and allogeneic HCT programs at rates of 5% to 25% (11, 12). Importantly, antivirals with proven efficacy are lacking, leaving reconstitution of BKPyV-specific adaptive immune control as the only option of current therapy (11–13). For PyVAN, screening of kidney transplant patients for BKPyV-DNAemia is recommended to guide prompt reduction of maintenance immunosuppression in order to avoid prolonged BKPyV replication, extensive allograft damage, progressive graft loss, as well as accidental chromosomal integration of viral genomes en route to cancer (1). However, reducing immunosuppression is not always successful and may be hesitantly applied because of the increased risk of rejection. In fact, reducing immunosuppression is highly controversial in allogeneic HCT patients with PyVHC due to relevant concerns of precipitating or exacerbating graft-versus-host disease. Thus, antivirals with prophylactic, preemptive, or therapeutic efficacy represent a significant unmet clinical need for managing replicative BKPyV diseases such as PyVAN and PyVHC (14). Although a number of small-molecule drugs have been reported to inhibit BKPyV replication in cell culture, the search for BKPyV-specific antivirals has been disappointing for reasons of poor efficacy, unacceptable toxicity, and missing clinical benefit (13). This sobering status quo partly results from the fact that polyomavirus replication relies heavily on host cell functions. Thus, the small circular double-stranded DNA (dsDNA) BKPyV genome of 5,100 bp is known to encode only 6 major proteins, the regulatory large T antigen (LTag) and small T antigen (sTag) in the early viral gene region (EVGR), the structural Vp1, Vp2, and Vp3 capsid proteins, and the regulatory agnoprotein (13) in the late viral gene region (LVGR). Akin to other human polyomaviruses (15), the EVGR and LVGR expression is governed by the approximately 400-bp-long noncoding control region (NCCR) carrying bidirectional promoter and enhancer elements as well as the origin of viral DNA replication (16, 17). In search for small-molecule drug candidates, we noted an earlier report of retinoic acid (RA) inhibiting polyomavirus replication in mouse embryo cells (18). The inhibitory effects were associated with stalling cellular DNA synthesis, but the key mechanism of action was not identified. RAs are administered to treat acute promyelocytic leukemia in combination with other drugs. Acitretin is an RA approved by the U.S. FDA to treat psoriasis and other skin disorders (19). Recently, acitretin was successfully used together with reducing immunosuppression to treat human polyomavirus 7 (HPyV7)-associated pruritic hyperproliferative keratinopathy in a heart transplant patient (4, 13, 20). Here, we report that acitretin inhibits BKPyV replication in primary human cells derived from renal proximal tubules and the urothelium, the main targets of PyVAN and PyVHC after kidney transplantation and allogeneic HCT, respectively.
RESULTS
Acitretin inhibits BKPyV replication in RPTECs.
To characterize the potential effects of acitretin (Fig. 1A) on BKPyV replication, primary human renal proximal tubular epithelial cells (RPTECs), the key BKPyV target in PyVAN (21), were exposed to BKPyV Dunlop (multiplicity of infection [MOI] of 1) for 2 h before supernatant removal and addition of increasing concentrations of acitretin at 2 h postinfection (hpi). Supernatants and cell lysates were harvested at 72 hpi to quantify extracellular BKPyV loads and intracellular viral protein levels, respectively. The extracellular viral loads were quantified after DNase I digestion prior to nucleic acid extraction and quantitative nucleic acid amplification testing (QNAT) using a validated diagnostic protocol (22) to determine encapsidated BKPyV genomes. The results revealed a dose-dependent reduction in extracellular BKPyV loads compared to the solvent control (Fig. 1B). Acitretin inhibition showed a 50% effective concentration (EC50) of 0.64 and an EC90 of 3.25 μM (Fig. 1C; Table 1). Viral proteins, including LTag, Vp1, and agnoprotein. were decreased in a dose-dependent fashion (Fig. 1D). Similarly, LTag transcripts were reduced (Fig. 1E), as were the intracellular BKPyV genome loads (Fig. 1F and G) and the number of infected cells (Fig. 1H).
FIG 1.
Acitretin inhibits BKPyV replication in RPTECs. (A) Chemical structure of acitretin (ACRT). (B) Extracellular BKPyV loads at 72 hpi in RPTECs measured by QNAT in response to indicated concentrations of acitretin added at 2 hpi (see Materials and Methods). Data from three independent experiments are shown unless indicated otherwise (mean ± SEM). (C) Determination of inhibitory acitretin EC50 and EC90 by curve fitting (see Materials and Methods). (D) BKPyV protein levels in RPTECs at 72 hpi analyzed by immunoblotting for LTag, Vp1, and agnoprotein, using tubulin as control in response to increasing acitretin concentrations added at 2 hpi. (E) BKPyV LTag transcript levels at 24 hpi normalized to human HPRT1 (see Materials and Methods). (F) Intracellular viral load of acitretin (0 to 40 μM)-treated RPTECs determined by QNAT and normalized to human asparto-acylase gene (see Materials and Methods). (G) Intracellular viral loads plotted as percentage of the solvent control. (H) BKPyV replication in RPTECs at 72 hpi in response to increasing acitretin concentrations added at 2 hpi using immunofluorescence for LTag, Vp1, and agnoprotein and Hoechst staining for cell nuclei (representative experiment; see Materials and Methods).
TABLE 1.
Effective and cytotoxic concentrations of retinoic acids against BKPyV replicationa
| Compound | Cell type | EC50 (95% CI) (μM) | CC50 (μM) | SI50 | EC90 (95% CI) (μM) | CC90 (μM) | SI90 |
|---|---|---|---|---|---|---|---|
| Acitretin | RPTEC | 0.64 (0.47–0.81) | >160 | >250 | 3.25 (2.17–5.89) | >160 | >49.2 |
| Acitretin | HUC | 0.62 | ND | 3.12 | ND | ||
| ATRA | RPTEC | 1.42 | ND | 5.22 | ND | ||
| 13-cis-RA | RPTEC | 1.71 | ND | 10.6 | ND | ||
| 9-cis-RA | RPTEC | 0.63 | ND | 12.9 | ND | ||
| Bexarotene | RPTEC | <1 | ND | ND | ND | ||
| Tamibarotene | RPTEC | 0.19 | ND | 16.4 | ND |
Results were obtained as described in Materials and Methods using the indicated curve-fitting procedures. EC50, 50% effective inhibitory concentration; CC50, 50% cytotoxic concentration; SI50, 50% selectivity index; EC90, 90% effective inhibitory concentration; CC90, 90% cytotoxic concentration; SI90, 90% selectivity index; ATRA, all-trans retinoic acid; 9-cis-RA, 9-cis retinoic acid; 13-cis-RA, 13-cis retinoic acid; ND, not done.
To assess the potential detrimental effects of acitretin on the host cells, we measured real-time cell proliferation of RPTECs using the xCELLigence system (Fig. 2A). The results revealed no significant change of the cell index at concentrations up to 10 μM and only transiently reduced cell proliferation at rather high acitretin concentrations at 40 μM. Consistent with the report on mouse PyV (18), acitretin reduced BrdU incorporation (serving as an independent measure of cellular DNA replication at 20 μM and higher concentrations), whereby low cell numbers were more affected (Fig. 2B). Although the 50% cytotoxic concentration (CC50) was not formally reached at the highest concentration of 160 μM (Fig. 2B), we applied this value to cautiously estimate the selectivity indices SI50 and SI90 as being 250 and 49.2, respectively (Table 1). In two additional assays (Tox8 and CellTox Green), only little and mostly transient effects above the background appeared when low cell numbers were exposed to high acitretin concentrations (Fig. 2C to E). We concluded that acitretin effectively inhibits BKPyV replication in RPTECs in the low micromolar range with low cytotoxicity, hence demonstrating a promising selectivity index of approximately 100-fold. As our previous in silico and in vivo modeling studies indicated the need for a more than 80% curtailing of BKPyV replication for clearance of BKPyV DNAemia and viruria (23), acitretin inhibition of BKPyV replication appeared, to be warranting our further characterization.
FIG 2.
Acitretin exhibits little detrimental effects on RPTECs. (A) RPTEC proliferation over time by real-time impedance in response to acitretin (see Materials and Methods). (B) Cellular DNA synthesis of RPTECs measured by BrdU incorporation assay in response to acitretin at 24 h posttreatment (see Materials and Methods). (C) Effects of acitretin on membrane integrity using CellTox Green at 24, 48, and 72 h posttreatment (see Materials and Methods). (D) Fluorescence intensity of CellTox Green dye was quantified and plotted as percentage of membrane integrity (see Materials and Methods). (E) Mitochondrial reductase activity measured by as change in fluorescence at 24 h posttreatment using Tox8 (see Materials and Methods).
Acitretin inhibition is effective before and early after BKPyV infection.
To explore whether or not acitretin inhibition of BKPyV replication is equally effective when added at different times before or after infection, we administered 5 μM acitretin, a concentration reducing the 72-hpi extracellular viral loads by >90% when added at 2 hpi, at the indicated time points (Fig. 3A and B). The results showed that acitretin inhibition was effective at 72 hpi when added from 48 h before until 24 h after infection and then progressively faded to around 50% inhibition at 36 hpi and 48 hpi (Fig. 3A and B). To assess whether or not continuous presence until 72 hpi was needed for inhibition, acitretin was added in different concentrations at 2 hpi followed by a washout at 12 hpi. The results indicated that acitretin inhibition was only slightly reduced by the washout and remained >75% effective at concentrations >2.5 μM (Fig. 3C and D). Because BKPyV Dunlop is controlled by a rearranged NCCR altering binding sites for host cell transcription factors and DNA-binding proteins, we examined the BKPyV-ww, which carries an archetype NCCR (17). Although BKPyV-ww replicates much more slowly in cell culture than the BKPyV Dunlop strain as reported (17), 1 μM and 5 μM acitretin reduced archetype BKPyV replication at 6 and 9 days postinfection (Fig. 3E and F). We concluded that acitretin inhibition did not require the continuous presence of the drug in the cell culture and elicited maximal effects when added from 24 h before until 12 h after BKPyV infection of RPTECs before gradually disappearing as the viral life cycle progressed to viral genome replication and late gene expression.
FIG 3.
Acitretin inhibition is most effective shortly before or early after BKPyV infection. (A) Extracellular BKPyV loads at 72 hpi in RPTECs measured by QNAT in response to 5 μM acitretin added at the indicated times before and after infection and expressed as percentage of mock. †, solvent added at 2 hpi (see Materials and Methods). (B) BKPyV protein levels in RPTECs at 72 hpi analyzed by immunoblotting for LTag, Vp1, and agnoprotein, using tubulin as control in response to 5 μM acitretin added at the indicated times before and after infection. †, solvent added at 2 hpi. (C) Extracellular BKPyV loads in RPTECs at 72 hpi in response to the indicated acitretin concentrations added at 2 hpi followed by washout at 12 hpi. *, no acitretin washout for comparison. (D) BKPyV protein levels in RPTECs at 72 hpi analyzed by immunoblotting for LTag, Vp1, agnoprotein, and tubulin as control in response to the indicated acitretin concentrations added at 2 hpi followed by washout at 12 hpi. *, no acitretin washout for comparison. (E) Archetype BKPyV-ww at 3, 6, and 9 dpi in RPTECs treated with 1 μM and 5 μM acitretin at 2 hpi, 3 dpi, and 6 dpi and measured by QNAT. White bar, untreated BKPyV Dunlop as control. (F) Archetype BKPyV-ww protein levels at day 9 in response to acitretin added at days 1, 3, and 6 by immunoblotting for LTag, Vp1, and agnoprotein, using tubulin as control.
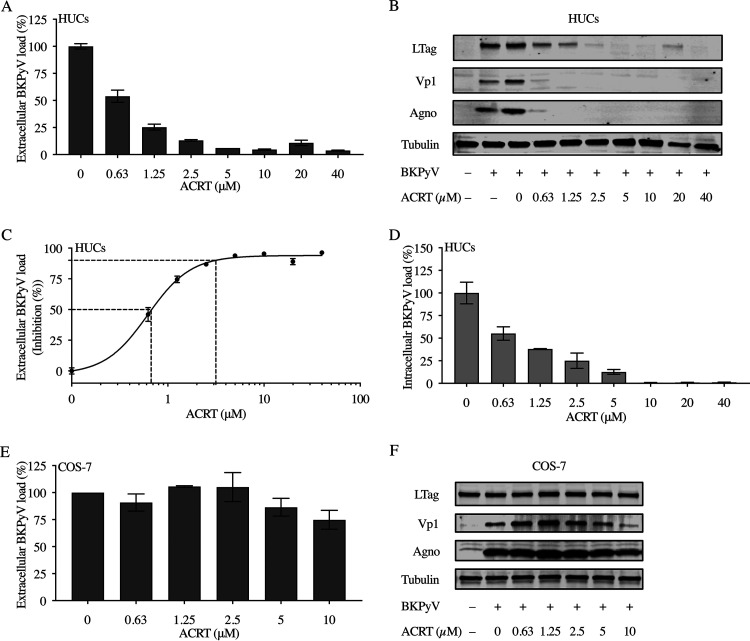
Acitretin inhibits BKPyV replication in primary HUCs but not in COS-7 cells.
To investigate whether acitretin was capable of inhibiting BKPyV replication in permissive cells other than RPTECs, we examined primary human urothelial cells (HUCs), the key viral target of PyVHC in allogeneic HCT. The results indicated that acitretin inhibited BKPyV replication with an EC50 of 0.62 μM and EC90 of 3.12 μM in HUCs (Fig. 4A and C; Table 1) and also reduced the expression of early and late viral proteins (Fig. 4B) as well as intracellular BKPyV loads (Fig. 4D). We also examined the effect of acitretin on BKPyV replication in COS-7 cells constitutively expressing SV40 LTag (17, 24). The results indicated that acitretin inhibition of BKPyV replication in COS-7 cells amounted to less than 25% and required higher concentrations (Fig. 4E). By immunoblotting, LTag protein levels were not changed, in line with the fact that it resulted mostly from constitutive SV40 LTag expression already detectable before BKPyV infection (Fig. 4F). However, expression of the BKPyV-Vp1 and agnoprotein was reduced at 5 or 10 μM acitretin, suggesting partial inhibition of BKPyV replication in SV40 LTag-expressing COS-7 cells at higher concentrations.
FIG 4.
Acitretin inhibits BKPyV replication in primary human urothelial cells (HUCs) but not in COS-7 cells. (A) Extracellular BKPyV loads in HUCs at 72 hpi measured by QNAT in response to acitretin added at 2 hpi in increasing concentrations (for details, see Materials and Methods). (B) BKPyV protein levels in HUCs at 72 hpi analyzed in response to increasing acitretin concentrations added at 2 hpi by immunoblotting for LTag, Vp1, and agnoprotein, using tubulin as control. (C) Inhibitory acitretin EC50 and EC90 in HUCs by curve fitting. (D) Intracellular viral loads of HUCs determined by QNAT at 72 hpi and expressed as percentage of the solvent control. (E) Extracellular BKPyV loads in COS-7 cells at 6 dpi measured by QNAT in response to increasing concentrations of acitretin added at 2 hpi and followed every 3 days. (F) BKPyV-protein levels in COS-7 cells at 6 dpi in response to increasing acitretin as described above by immunoblotting for LTag, Vp1, and agnoprotein, using tubulin as control.
Other RA agonists inhibit BKPyV replication in RPTECs.
We next investigated whether or not other related RA compounds inhibited BKPyV replication in RPTECs, and we therefore selected several drugs with a clinical evaluation record (Fig. 5) such as all-trans-RA (pan-RAR/RXR agonist), 9-cis-RA (pan-RAR/RXR agonist), 13-cis-RA (pan-RAR agonist), bexarotene (pan-RXR agonist), and tamibarotene (RAR-α/β agonist). The results indicated that all compounds inhibited BKPyV replication and viral protein expression in a concentration range similar to the one observed for acitretin (Fig. 5A to E). In the absence of a clear-cut advantage of these RAs regarding BKPyV inhibition or selectivity, we deferred their further characterization at this point.
FIG 5.
Retinoic acids inhibit BKPyV replication in RPTECs. RPTECs were infected with BKPyV (MOI = 1) and treated with the indicated concentrations of the indicated RA derivatives at 2 hpi and analyzed at 72 hpi. *, concentration of acitretin for comparison. For each of the tested drugs, the chemical structure, extracellular viral loads quantified by QNAT and plotted as percentage of the solvent control, and protein levels analyzed by immunoblotting LTag, Vp1 and agnoprotein are indicated (for details, see Materials and Methods). (A) All-trans retinoic acid (ATRA). (B) 13-cis retinoic acid (13-cis-RA). (C) 9-cis retinoic acid (9-cis-RA). (D) Bexarotene (bexa). (E) Tamibarotene (tami).
RAR antagonist RO41-5253 inhibits BKPyV replication in RPTECs.
As the tested RA agonists also inhibited BKPyV replication, we examined RO41-5253 (Fig. 6A), a selective antagonist which has been reported to block RAR-α and downstream transcriptional activation without affecting RAR/RXR-heterodimerization (25). We found that RO41-5253 inhibited BKPyV replication similarly to acitretin and also reduced early and late viral protein expression (Fig. 6B and C). Since BKPyV inhibition by RO41-5253 could be mediated by another mechanism, including one that still might antagonize or interfere with acitretin inhibition, we pretreated RPTECs with different concentrations of RO41-5253 for 2 h before BKPyV infection followed by acitretin treatment at 2 hpi. We observed no significant antagonistic but, rather, an additive effect being well detectable at higher RO41-5253 concentrations (Fig. 6D and E). We concluded that acitretin inhibition of BKPyV replication could not be offset by RO41-5253, suggesting that another mechanism than RAR-α blockade of transcription must be involved.
FIG 6.
RAR-α antagonist RO41-5253 inhibits BKPyV replication in RPTECs. (A) Chemical structure of RO41-5253 (RO41). (B) Extracellular BKPyV loads at 72 hpi in RPTECs measured by QNAT in response to RO41 added at 2 hpi in increasing concentrations (using 5 μM acitretin for comparison). (C) BKPyV protein levels in RPTECs at 72 hpi in response to increasing RO41 concentrations added at 2 hpi by immunoblotting for LTag, Vp1, and agnoprotein, using tubulin as control (using 5 μM acitretin for comparison). (D) Extracellular BKPyV loads were measured at 72 hpi by QNAT after RO41 pretreatment with indicated concentrations at 2 h before infection followed by acitretin treatment with indicated concentrations at 2 hpi (plotted as percentage of the solvent control). (E) BKPyV protein levels in RPTECs at 72 hpi treated, as described above, by immunoblotting for LTag, Vp1, and agnoprotein, using tubulin as control.
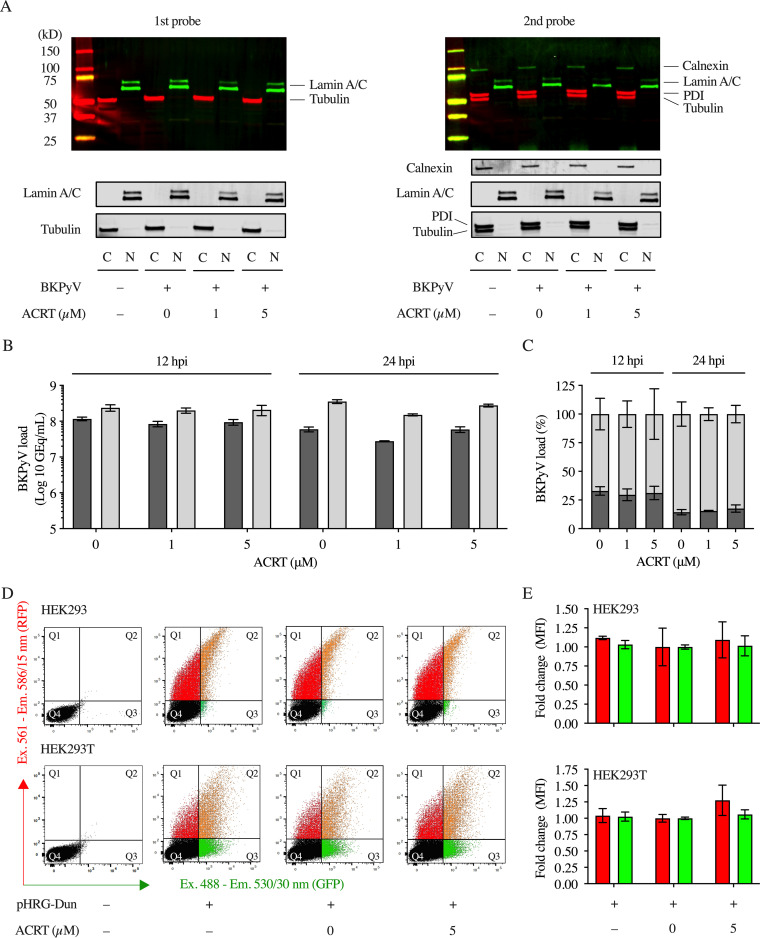
Acitretin does not prevent BKPyV genome delivery to the host cell nucleus.
Given the efficacy of acitretin being highest in the early phase of the BKPyV replication cycle, we investigated whether or not acitretin inhibited viral genome delivery to the host cell nucleus. To this end, we compared the BKPyV genome loads by QNAT in cytoplasmic and nuclear cell fractions of BKPyV-infected RPTECs without or with 1 or 5 μM acitretin added at 2 hpi. Cells were harvested at 12 hpi and 24 hpi, and their nuclear and cytoplasmic fractions were analyzed by immunoblotting using lamin A/C and tubulin as respective enrichment markers (Fig. 7A). To estimate the amount of endoplasmic reticulum (ER) materials in the respective preparations, the immunoblots were also probed with the ER markers calnexin and protein disulfide isomerase. The results indicated that both ER proteins were highly enriched in the cytoplasmic fractions but hardly detectable in the nuclear fractions (Fig. 7A). Quantification of the BKPyV genome by QNAT in the respective fractions at 12 hpi indicated higher loads in the nuclear preparations than the cytoplasmic ones (unpaired t test; P = 0.002) in the mock-treated as well as in the acitretin-treated cells (Fig. 7B). This was similarly observed at 24 hpi, although the BKPyV genome load in the cytoplasmic fraction was lower, in line with other studies reporting nuclear genome delivery at 12 hpi to 24 hpi (26). However, no significant difference was seen in response to 1 or 5 μM acitretin (Fig. 7C). As viral genome replication has been described to occur after LTag expression at 24 hpi, typically seen at 36 hpi in several studies of BKPyV infecting RPTECs (21, 26, 27), relevant differences are unlikely to be leveled off by viral genome replication. We concluded that the major inhibitory mechanism of acitretin reducing acitretin inhibition of BKPyV replication does not occur at the step of nuclear genome delivery but affected early steps downstream.
FIG 7.
Acitretin does not inhibit BKPyV genome delivery to the host cell nucleus or BKPyV NCCR reporter expression following transient transfection. (A) RPTECs lysates were prepared at 24 h with or without BKPyV infection and the indicated acitretin treatment at 2 hpi. Cytosolic (C) and nuclear (N) fractions were prepared (see Materials and Methods) and analyzed by immunoblotting using tubulin and lamin A/C as cytosolic and nuclear markers, respectively (left, 1st probe) and after reprobing for ER markers calnexin and protein disulfide isomerase (PDI) (right, 2nd probe). (B) BKPyV genome copies were quantified by QNAT in the cytosolic fraction (dark gray) and in the nuclear fraction (light gray). (C) BKPyV genome load expressed as percentage detected in the respective cytosolic (dark gray) and nuclear (light gray) fractions prepared at 12 h or at 24 h with or without acitretin treatment as indicated. (D) Flow cytometry of HEK293 and HEK293T cells at 48 h after transfection of the bidirectional pHRG-BKPyV Dunlop NCCR reporter plasmid and 5 μM acitretin addition at 6 h posttransfection (hpt). EVGR expression of dsRed2 in red (Q1), LVGR expression of EGFP in green (Q3), or both (Q2) (see Materials and Methods). (E) Mean fluorescence intensity (MFI) of EVGR (red) and LVGR (green) in HEK293 or in HEK293T cells at 48 hpt expressed as fold change after normalization to the solvent control.
Since acitretin inhibited the expression of the early viral LTag at the transcript and the protein level, we investigated whether or not acitretin decreased EVGR expression using a well-characterized bidirectional reporter assay reflecting the basal BKPyV NCCR activity (17, 28). HEK293 cells were transfected with the pHRG1-BKPyV Dunlop NCCR reporter and exposed to 5 μM acitretin at 6 h posttransfection (hpt). Quantification of the EVGR reporter protein DsRed at 48 hpt by flow cytometry revealed no significant decrease in the mean fluorescence intensity upon acitretin treatment (Fig. 7D and E, top panels). Since the NCCR-controlled reporter protein expression has been described to be higher in response to LTag expression (15), HEK293T cells were transfected and treated with 5 μM acitretin at 6 hpt (Fig. 7D and E, bottom panels). Although the mean fluorescence intensity increased compared to HEK293, no significant inhibition of EVGR reporter DsRed levels was observed. We concluded that acitretin did not confer significant inhibition of EVGR or LVGR expression from the transfected NCCR reporter constructs. Thus, the results are in line with the fact that the BKPyV NCCR does not contain classic retinoic acid response elements (RAREs) (25).
Acitretin does not inhibit BKPyV LTag expression following DNA genome transfection.
To rule out a relevant contribution of the HEK293 and 293T cell background to the failure of acitretin to inhibit NCCR reporter gene expression and to investigate whether or not other BKPyV genome sequences are necessary for mediating acitretin inhibition, we transfected full-length BKPyV Dunlop DNA genomes, released from plasmids by restriction enzyme digestion and recircularized, into RPTECs as described (8, 29). Increasing acitretin concentrations were added 12 h before and at 6 h after transfection before analyzing LTag transcript levels at 24 hpt (Fig. 8A). The results indicated that acitretin did not reduce LTag transcript levels following transfection of BKPyV genomes into RPTECs. After BKPyV infection of RPTECs, however, acitretin caused a dose-dependent inhibition of LTag transcript levels at 24 hpi (Fig. 8B). The failure of acitretin to significantly inhibit LTag transcription after transfection, but not after infection, was also observed in Vero cells, which are known to have impaired type 1 interferon (IFN) sensing (Fig. 8C and D).
FIG 8.
Acitretin inhibition is effective early after infection of BKPyV virions but not after transfection of BKPyV genomes. (A) LTag transcript levels in RPTECs at 24 hpt after transfection of recircularized full-length BKPyV Dunlop genomes and acitretin treatment at 12 h before and 6 hpt (see Materials and Methods for details). (B) LTag transcript levels in RPTECs at 24 hpi after infection with BKPyV Dunlop and acitretin treatment at 12 h before and 6 hpi. (C) LTag transcript levels in Vero cells at 24 hpt after transfection of recircularized full-length BKPyV Dunlop genomes and acitretin treatment at 12 h before and 6 hpt (see Materials and Methods for details). (D) LTag transcript levels in Vero cells at 24 hpi after infection with BKPyV Dunlop and acitretin treatment at 12 h before and 6 hpi. (E) Extracellular BKPyV loads in RPTEC after transfection or after infection and acitretin treatment at day 1 and 3 measured by QNAT at day 3 and day 6 with follow-up on day 9. (F) Extracellular BKPyV loads in RPTECs transfected or infected as described above, expressed as percentage of mock control.
To investigate whether or not secondary rounds of BKPyV replication could be inhibited by acitretin, we added acitretin on day 1 or day 3 following transfection and infection of RPTECs and compared the supernatant BKPyV loads on day 3 and day 6, with extension of the latter to day 9 (Fig. 8E and F). The results showed that acitretin addition on day 1 failed to cause a significant reduction of supernatant BKPyV loads on day 3 after transfection of RPTECS. However, addition of acitretin on day 3 after transfection caused significant reductions of supernatant BKPyV loads on day 6 and day 9. The data indicated that secondary rounds of BKPyV infection following genome delivery by transfection became susceptible to acitretin inhibition. Conversely, acitretin addition on day 1 reduced supernatant BKPyV loads after infection at day 3, as well as after secondary rounds at day 6, and the inhibitory effect was lost without further addition until day 9 (Fig. 8E and F). We concluded that acitretin inhibited BKPyV replication at an early step after nuclear delivery of the viral genome and targeted early viral gene expression after primary or secondary rounds of infection but not after viral genome transfection.
Acitretin does not inhibit BKPyV replication by activating a type I interferon response.
To investigate whether or not acitretin inhibition involved significant activation of type I IFN pathways, we examined the inhibition of the TANK-binding kinase 1 (TBK1) using the inhibitor BX795. The results showed that BX795 treatment did not antagonize acitretin inhibition of BKPyV replication (Fig. 9A and B). To directly address type I IFN induction by acitretin, mock-infected or BKPyV-infected RPTECs were treated with increasing drug concentrations at 2 hpi. Six hours later (i.e., at 8 hpi), the cells were analyzed for IFN-β transcript levels using poly(dA-dT) induction as control (8) (Fig. 9C and D). The data indicated that acitretin treatment was not associated with a significant increase in IFN-β transcript expression that would account for a major acitretin mechanism inhibiting BKPyV replication. Consistent with this notion, acitretin was able to inhibit BKPyV replication also in Vero cells (see above), which are known to be defective in the type I IFN pathway.
FIG 9.
Acitretin does not inhibit BKPyV replication by activating type I interferons. (A) Extracellular BKPyV loads at 72 hpi in RPTECs treated with indicated concentrations of acitretin at 2 hpi and TBK-1 inhibitor BX795 at 6 hpi and expressed as percentage of mock (see Materials and Methods). (B) BKPyV protein levels in RPTECs at 72 hpi as described above, analyzed by immunoblotting for LTag, Vp1, and agnoprotein, using tubulin as control. (C) IFN-β transcript levels in RPTECs were measured after mock infection and acitretin treatment at 2 hpi or after poly(dA-dT) transfection as positive control (see Materials and Methods for details). (D) IFN-β transcript levels in RPTECs were measured after BKPyV infection and acitretin treatment at 2 hpi or after poly(dA-dT) transfection as positive control (see Materials and Methods for details).
DISCUSSION
The lack of antivirals effectively inhibiting BKPyV replication in kidney transplant and allogeneic HCT patients represents a significant unmet clinical need (13). Here, we characterized acitretin and related RA compounds regarding BKPyV replication in primary human RPTECs, the key viral target in PyVAN after kidney transplantation. Our results show that acitretin inhibits BKPyV replication at EC50 and EC90 values of 0.64 (SI50, 250) and 3.25 μM (SI90, 49.2), respectively. Using different assays assessing host cell toxicity, we can estimate the respective selectivity indices as being approximately 100-fold higher. Importantly, plasma concentrations close to the EC90 value can be reached in systemically treated patients (30). Acitretin also inhibits BKPyV replication in primary HUCs in a similar concentration range. These results are encouraging since earlier in silico modeling and clinical validation of BKPyV replication kinetics in kidney transplant patients predicted that clearance of BKPyV-DNAemia can be expected within 3 to 6 weeks upon 90% effective curtailing of BKPyV replication (13, 23). Given the long-standing clinical experience of systemic acitretin therapy for acute promyelocytic leukemia as well as for several dermatologic indications, our results suggest that further clinical evaluation of acitretin for treating BKPyV replication is warranted.
The detailed characterization in RPTECs indicates that one-time acitretin administration from 24 h before to 12 h after infection is sufficient for the 80% to 90% reduction in supernatant BKPyV loads at 72 hpi, hence providing a relatively long overall treatment window of more than 48 h. Notably, acitretin inhibition decreased when added after 24 hpi, amounting to less than 50% after 36 hpi, indicating that steps after EVGR expression, namely, viral genome replication, LVGR expression, and progeny release, ceased to be susceptible. Our results also demonstrate that new rounds of infection are effectively inhibited if appropriate dosing intervals of at least 48 h are chosen. Given the asynchronous replication situation in patients with replicative BKPyV disease and the metabolic half-life of acitretin, at least daily dosing may be considered when developing the clinical evaluation of this drug.
Time-of-addition and cell fractionation studies indicate that acitretin does not mediate its major inhibitory effect by reducing the nuclear delivery of the viral genomes, but acts at a subsequent early step in reducing LTag expression at the transcript and protein levels. From 12 h to 24 h postinfection, the BKPyV load slightly decreases in the cytoplasmic fraction while increasing in the nuclear fraction of mock-treated or acitretin-treated cells alike. Thus, acitretin inhibition appears to be operative at an early stage when the vast majority of virions have passed through the ER and delivered their genomes to the nucleus (31, 32). However, we did not observe the direct inhibition of the basal EVGR gene expression from transfected NCCR reporter plasmids, which, in our previous studies, allowed for a discrete functional dissection of NCCR-embedded transcription factor-binding sites (15–17, 28). Notably, the NCCR-controlled reporter gene expression is increased in SV40 LTag-expressing cells, as reported previously (15), indicating that some regulatory responses are reliably captured by this experimental approach (16), although not permitting acitretin inhibition, in line with the COS-7 results. At first glance, these data are consistent with the fact that the BKPyV NCCR is lacking RA response elements (RAREs) in the promoter regions, for which RAR/RXR is known to regulate gene transcription. Moreover, the RAR-α inhibitor RO41-5253 did not antagonize acitretin inhibition but also strongly inhibited BKPyV replication in RPTECs, hence arguing against the role of classic RAREs in BKPyV replication. Although we observed a BKPyV inhibitory activity of a number of other RA derivatives such as all-trans retinoic acid (ATRA), 13-cis-RA, 9-cis-RA, bexarotene, and tamibarotene, our data suggest a different mechanism possibly related to their shared alkene and/or cyclic carbon chemical structure.
Acitretin inhibition of BKPyV replication was not detectable following transfection of full-length BKPyV DNA genomes in different cell types that were susceptible to acitretin inhibition following infection. This observation excluded that the host cell background of HEK293 or missing viral genome sequences represented the key reasons for the failure of the BKPyV NCCR reporter to respond to acitretin inhibition. Although the mechanisms are currently unclear and might involve undefined processes or routes of nuclear delivery being perturbed by transfection (25), we speculate that a key difference between transfected naked viral genomes and infected viral genomes resides in the organization of delivered genomes. Whereas the transfected NCCR reporter or full-length genomes may allow direct interpretation of their basal activity by the transcription machinery in the nucleus according to the respective host cell state, the nucleosomal organization of infected genomes may confer a different level of organization with differences in the initial accessibility, efficiency, and regulation of viral early gene expression, including even epigenic modification and acitretin responses. This notion is indirectly supported by our experiments, demonstrating that secondary rounds of BKPyV replication after initial transfection became increasingly susceptible to acitretin. Possibly, as progeny virions with nucleosomal genome organization are generated from the initially transfected viral genomes, the susceptibility to acitretin inhibition is restored and effective during the secondary rounds of infection. For SV40, chromatin remodeling occurs early following infection and involves AP1 and SP1 (33). RAs have been reported to interfere with AP1 activity (34, 35) as well as increasing SP1 interactions in the polycystic kidney disease promoter (36). Also, RAR was reported to bind p300/CBP, which is subject to LTag regulation (37, 38), and cells expressing LTag may have a higher steady-state level of p300/CBP (39). This could contribute to the failure of acitretin inhibition in COS-7 cells constitutively expressing SV40 LTag and to the fading acitretin inhibition after 24 hpi when LTag is increasingly active. Clearly, further work is needed to resolve these issues while keeping in mind that acitretin and related RAs are effectively inhibiting replication of BKPyV with archetype or rearranged NCCRs in RPTECs in the clinically relevant setting of BKPyV infection (40–43).
Acitretin similarly inhibited BKPyV replication in other cells that are permissive for the virus, such as primary HUCs or Vero cells. The former is notable since these are the viral target cells in hemorrhagic cystitis, one of the major clinical complications of BKPyV in allogeneic HCT, pointing to potential utility of the drug, which may include topical intravesical administration. The latter is notable because of the lack of type I IFN induction, arguing that acitretin inhibition is not primarily mediated by this pathway. Although we cannot exclude mechanisms through other interferon pathways, acitretin was not antagonized by the TBK-1 inhibitor BX795 and did not elicit significant IFN-β expression in RPTECs.
How does acitretin compare to other antiviral compounds? Data from our own group and others (26, 44) suggest that efficacy and selectivity of acitretin in cell culture may be superior to cidofovir and comparable to brincidofovir (21), although the EC90 of brincidofovir is approximately 10-fold lower. Unlike acitretin, brincidofovir acts after LTag expression at the level of viral genome replication occurring at 36 hpi as expected from its being an acyclic nucleotide phosphonate analogue of deoxycytidine causing chain termination after incorporation into the growing DNA strands synthesized by the host cell DNA polymerase complex (21). However, brincidofovir has not been associated with suppression of BKPyV events as a secondary endpoint in a large phase 3 randomized trial aiming at preventing clinically significant cytomegalovirus (CMV) events (45). Despite prominent gastrointestinal toxicity, oral brincidofovir appeared to not be reaching the renourinary compartment in sufficient concentrations. Although efficacy and serious adverse effects of acitretin treatment for BKPyV replication are difficult to predict at this time, we believe that our data warrant further clinical exploration given the well-documented experience of systemic acitretin in vulnerable patients with hematological and dermatological disorders.
To conclude, we identified acitretin and five other retinoids as potential drug candidates inhibiting BKPyV replication in relevant primary human cell culture models. Both potency and selectivity are promising. Since acitretin is already an approved drug in clinical use, it appears to be an attractive candidate to evaluate repurposing for the clinical treatment of replicative diseases such as PyVHC and PyVAN.
MATERIALS AND METHODS
Cells, culture media, and reagents.
Primary human renal proximal tubule epithelial cells (RPTECs) were cultured in epithelial cell medium (EpiCM; catalog no. 4101; ScienCell, Carlsbad, USA) supplemented with epithelial cell growth supplement (EpiCGS; catalog no. 4152; ScienCell) and 2% fetal bovine serum (FBS; catalog no. 0010; ScienCell). Human urothelial cells (catalog no. 4320; ScienCell) were cultured in urothelial cell medium (UCM; catalog no. 4321; ScienCell) supplemented with urothelial cell growth supplement (UCGS; catalog no. 4352; ScienCell). HEK293 cells (ATCC CRL-1573), HEK293T cells (ATCC CRL-3216), COS-7 cells (ATCC CRL-1651), and Vero cells (ATCC CCL-81) were cultured in Dulbecco modified Eagle medium (DMEM) high-glucose medium (catalog no. D0819; Sigma-Aldrich, Buchs, Switzerland) containing 10% FBS (catalog no. F7524; Sigma-Aldrich). Cells were passaged as follows: cells were trypsinized when reaching confluence using trypsin-EDTA solution (catalog no. T3924; Sigma-Aldrich) and inactivated with defined trypsin inhibitor (1:3 volume to trypsin) (catalog no. R-007-100; Gibco, Reinach, Switzerland). The suspension was centrifuged at 350 × g for 5 min and the supernatant discarded. The cell pellets were resuspended in fresh medium and seeded at indicated density in cell culture plates. Acitretin (catalog no. 44707), all-trans retinoic acid (catalog no. R2625), 9-cis retinoic acid (catalog no. R4643), 13-cis retinoic acid (catalog no. R3255), bexarotene (catalog no. SML0282), tamibarotene (catalog no. T3205), and RO41-5253 (catalog no. SML0573) were purchased from Sigma-Aldrich. BX795 was purchased from Selleck Chemicals (catalog no. S1274; Lucerne, Switzerland). All compounds were dissolved in dimethyl sulfoxide (DMSO) (catalog no. D2650; Sigma-Aldrich).
Virus infection and transfection.
Infectious BKPyV Dunlop and BKPyV archetype were obtained after transfection of recircularized plasmid carrying the full-length genome. pGEM-BKPyV Dunlop plasmid (46) was digested with BamHI and recircularized with T4 DNA ligase (New England Biolabs, Allschwil, Switzerland). BKPyV genomes were transfected into Vero cells, and infectious viral particles were prepared as described previously (8, 26, 29). RPTECs at passage 3 were seeded at 32,500 cells/cm2 (123,500 cells per well in 12-well plates) in 1 ml medium per 12-well plate at 24 h before infection (hbi) unless stated otherwise. BKPyV Dunlop was mixed with EpiCM (serum free) at a multiplicity of infection (MOI) of 1 and incubated with RPTECs in the volume of 500 μl per 12-well plate for 2 h. Then, supernatants were removed, and cells were washed once with culture medium (500 μl per 12-well plate) and filled with fresh EpiCM (0.5% FBS, 1 ml per 12-well plate) containing acitretin or other compounds at the indicated final concentrations until 72 h postinfection (hpi) unless mentioned otherwise. At the indicated times postinfection, the cell culture supernatants were collected and centrifuged at 350 × g for 5 min to remove cells and debris and then submitted to DNase I digestion followed by total nucleic acid (TNA) extraction as described below. Cells were washed once with phosphate-buffered saline (PBS) and harvested in triple buffer (50 mM Tris-HCl, 150 mM NaCl, 1% sodium deoxycholate [NaDOC], 1% Triton X-100, and 0.1% SDS) with protease inhibitor (complete mini-EDTA-free; Roche, Basel, Switzerland) for intracellular DNA extraction or immunoblot analysis as described below. RPTECs were infected with archetype BKPyV-ww (6 × 107 copies/12-well plate) and harvested and quantified at the 3 days postinfection (dpi), 6 dpi, and 9 dpi, as described above for BKPyV Dunlop.
Transfections of recirularized BKPyV genomes were performed using the ViaFect transfection reagent (catalog no. E4982; Promega) in RPTECs and Vero cells as described. LTag transcripts were quantified at 24 hpt (8). RPTECs (passage 3) and Vero cells were seeded at 32,500 and 50,000 cells/cm2 in 24-well plates, and acitretin at the indicated concentration was added 12 h before transfection and 6 h posttransfection after replacing the transfection reagents with fresh medium. Samples were harvested at indicated time points for reverse transcription-quantitative nucleic acid amplification testing (RT-QNAT) as shown below.
To quantify IFN-β transcripts, RPTECs were infected following the standard infection protocol or incubated in serum-free medium for 2 h (mock infection), and acitretin was added at 2 hpi. RNA was extracted at 8 hpi as described below. Transfection of poly(dA-dT) (1.5 μg per 12-well plate) at 2 hpi served as a positive control for inducing IFN-β expression as described (8).
BKPyV genome extraction and quantitative nucleic acid amplification testing.
Cell culture supernatants were first digested with DNase I (0.45 μl per 200-μl reaction) (catalog no. 18047019; Invitrogen, Reinach, Switzerland) at 37°C for 30 min to remove unprotected (not encapsidated) BKPyV genomes. DNA was extracted using QIAamp DNA blood minikit (catalog no. 51104; Qiagen, Hombrechtikon, Switzerland) following the manufacturer’s protocol. BKPyV genomes were quantified by QNAT in triplicates (Table 2). QNAT was performed as described (22) in 25 μl reaction mixture (2× quantitative PCR [qPCR] mastermix plus low ROX [Eurogentec, Liege, Belgium], forward and reverse primers [300 nM], TaqMan probe [200 nM], and template [5 μl]) on the ABI 7500 HT cycler (Applied Biosystems, Allschwil, Switzerland), following the program of 50°C for 2 min, 95°C for 10 min, 95°C for 15 s (45 cycles), and 60°C for 1 min (45 cycles). Intracellular DNA was extracted from cell lysates following the same protocol but without DNase I digestion. Intracellular BKPyV loads were quantified by QNAT and normalized to the human asparto-acylase (ACY) gene (22) (Table 2).
TABLE 2.
Primers and probes used for QNAT detection of BKPyV genomea
| Genome (reference no.) | Forward primer(s) | Reverse primer(s) | TaqMan probe(s) |
|---|---|---|---|
| BKPyV genome (22) | BKPyV-LTAG-(3.2)-4209-f | BKPyV-LTAG-(3.2)-4296-r | BKPyV-LTAG-(3.2)-4238-p |
| 5′-ARC AGG CRA GDG TTC TAT TAC TAA AT-3′ | 5′-AGA RAG GTA GAA GAC CCT AAA GAC-3′ | 5′-FAM-TCC YTS TGA TCT ACA CCA GTT TCT TAG YCA AGC-TARMRA-3′ | |
| BKPyV-LTAG-(3.3)-4209-f | BKPyV-LTAG-(3.2)-4341-r | ||
| 5′-ARC AGG CRA GDG TTC TAT TAC TRA AY-3′ | 5′-GAR RCA ACA GSA GAT TCYCAA CA-3′ | ||
| BKPyV-LTAG-(3.4)-4209-f | BKPyV-LTAG-(3.2)-4447-r | ||
| 5′-ARC AGG CRA GDG TTC TAT TAC TRA AY-3′ | 5′-GGT RCC AAC MTA TGG AAC AGA A-3′ | ||
| Asparto-acylase (ACY) | 5′-CCC TGC TAC GTT TAT CTG ATT GAG-3′ | 5′-CCC ACA GGA TAC TTG GCT ATG G-3′ | 5′ FAM-CCT TCC CTC AAA TAT GCG ACC ACT CG-TAMRA 3′ |
| BKPyV LTag (cDNA) (26) | 5′-ACT CCC ACT CTT CTG TTC CAT AGG-3′ | 5′-TCA TCA GCC TGA TTT TGG AAC CT-3′ | 5′ FAM-TTG GCA CCT CTG AGC TAC-TAMRA 3′ |
Degenerate bases are indicated as R, A, or G; D, A, or G or T; Y, T, or C; S, G, or C; and M, A, or C.
Gene transcript quantification.
Total RNA was extracted using the RNeasy minikit (catalog no. 74106; Qiagen) following the manufacturer’s protocol of animal cells, including an on-column DNase digestion step to remove residual DNA (catalog no. 79254; Qiagen). Reverse transcription-QNAT (RT-QNAT) was used to quantify gene transcripts as described previously (8) using primers targeting BKPyV LTag cDNA in a 25-μl reaction mixture (2× qPCR mastermix plus low ROX [Eurogentec] and 10× primers and probes mix and template [5 μl; catalog no. 4331182; Applied Biosystems]) using forward and reverse primers (300 nM) and TaqMan probe (200 nM) (Table 2) and targeting IFN-β1. Results were normalized to the human hypoxanthine phosphoribosyl transferase (huHPRT1) housekeeping gene (catalog no. 4333768F; Applied Biosystems) in a 25-μl reaction mixture containing 2× qPCR mastermix plus low ROX (Eurogentec) and 10× huHPRT1 primers and probe sets and template (5 μl) and expressed as fold change of gene transcripts.
Immunoblotting.
Total protein concentration was measured using bicinchoninic acid (BCA) protein assay (catalog no. 23225; Pierce, Lucerne, Switzerland). Cell lysates were diluted to the desired concentration in triple buffer and mixed with 4× Laemmli sample buffer (catalog no. 1610747; Bio-Rad, Cressier, Switzerland). We loaded 10 μg per well into a gradient minigel (4 to 20%; Bio-Rad). Gels were run at 25 mA per gel for 50 min using the Mini-Protean Tetra vertical electrophoresis cell (Bio-Rad). Proteins were transferred onto an Immobilon polyvinylidene difluoride (PVDF-FL) membrane (catalog no. IPFL00010; Millipore, Buchs, Switzerland) and then blocked with LI-COR blocking buffer (LI-COR Odyssey; LI-COR, Bad Homburg, Germany) for 30 min and incubated with primary antibody at 4°C overnight, which includes anti-SV40 LTag antibody (1:50) (catalog no. DP02; Calbiochem, Buchs, Switzerland), anti-Vp1 (1:5,000) (catalog no. MAB3204; Abnova, Lucerne, Switzerland), anti-agnoprotein (1:1,000) (Hans H. Hirsch custom made no. 1163, Eurogentec) (8), calnexin (1: 1,000) (catalog no. ADI-SPA-860; Enzo Life Sciences, Lausen, Switzerland), lamin A/C (1:1,000) (catalog no. ab108595; Abcam, Lucerne, Switzerland), PDI (protein disulfide isomerase; 1:1,000) (catalog no. ADI-SPA-891; Enzo Life Sciences), and anti-alpha tubulin (catalog no. 1:1,000) (A-11126; Invitrogen). The secondary antibodies, donkey anti-mouse Alexa 680 (catalog no. A-10038; Invitrogen) and goat anti-rabbit Alexa 800 (1:10,000) (catalog no. 926-32211; LI-COR), were incubated for 1 h at room temperature and membranes scanned with Odyssey CLx system (LI-COR) and analyzed with Image Studio Lite software (LI-COR).
Immunofluorescence staining and microscopy.
RPTECs were seeded onto glass coverslips (129 × 1; Thermo Fisher Scientific, Reinach, Switzerland) in 24-well plates at 32,500 cells/cm2. Cells were infected as described above and harvested by removing the coverslips at 72 hpi for immunofluorescence staining. Briefly, cells were washed in PBS and then fixed with 4% paraformaldehyde for 20 min at room temperature, permeabilized in 0.2% Triton X-100 solution for 10 min at room temperature, blocked with 3% bovine serum albumin (BSA) in PBS at 37°C for 15 min, and then incubated with primary antibodies using anti-SV40 LTag antibody (1:50) (catalog no. DP02; Calbiochem), anti-Vp1 (1:400) (catalog no. MAB3204; Abnova), anti-agnoprotein (1:750) (8), and secondary antibodies using anti-mouse IgG2a Alexa 568 (1:300) (catalog no. A-21134; Invitrogen), anti-mouse IgG1 Alexa 647 (1:800) (catalog no. A-21240, Invitrogen), or anti-rabbit Alexa 488 (1:1,000) (catalog no. A-21441; Invitrogen). Cell nuclei were stained with Hoechst 33342 (1 μg/ml) (catalog no. B2261; Sigma-Aldrich) for 1 h to determine the total cell number. The coverslips were then mounted on glass slides with ProLong gold antifade mountant (catalog no. P36941; Thermo Fisher Scientific). Images were acquired with a Nikon E800 microscope with the 10× objective and processed with Fiji software (version 2.0.0-rc-46) on at least 5 fields per sample.
Toxicity and viability assays.
Cell proliferation in the presence and absence of acitretin and other drugs in BKPyV-infected or mock-infected cells was measured using the real-time xCELLigence system (ACEA Biosciences) following the protocol described previously (21). Cellular DNA synthesis was quantified by a colorimetric bromodeoxyuridine (BrdU) incorporation assay (catalog no. 11647229001; Roche) using endpoint protocol according to the manufacturer’s instructions. CellTox Green assay was performed to monitor the cell membrane integrity following previously described protocols (47) (catalog no. G8741; Promega, Dübendorf, Switzerland). Mitochondrial metabolism was measured by resazurin-based Tox8 assay following the manufacturer’s instructions (catalog no. R-6892; Sigma-Aldrich). For viability assays, RPTECs were seeded at indicated density. After 24 h, cells were serum free for 2 h to mimic the virus infection conditions and thereafter treated with different concentrations of acitretin.
Cell fractionation.
Cytoplasmic and nuclear fractions of BKPyV- and mock-infected RPTECs were prepared at the indicated times postinfection from a 12-well plate. In brief, RPTECs were washed twice with ice-cold PBS, followed by trypsinization with 0.2 ml trypsin-EDTA solution and inactivated with 0.6 ml of defined trypsin inhibitor (DTI) (1:3 volume to trypsin). The mixture was transferred to a 1.5-ml Eppendorf tube and centrifuged at 2,000 rpm at 4°C for 2 min, and the supernatant was discarded. The pellets were resuspended in 50 μl hypotonic homogenization buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 2.5 mM dithiothreitol [DTT], and protease inhibitor) and incubated on ice for 30 min. We added 3 μl of 10% NP-40. Then, the mixture was vortexed and centrifuged at 13,000 rpm at 4°C for 5 min. Supernatants were collected (cytoplasmic fraction), and the pellets were dissolved in 50 μl of nuclear extraction buffer (20 mM HEPES, pH 7.9, 25% glycerol, 400 mM NaCl, 1 mM EDTA, 2.5 mM DTT, and protease inhibitor) (nuclear fraction). BKPyV genomes were quantified by QNAT and fractions assessed by immunoblotting as described above.
NCCR reporter assay.
The bidirectional NCCR reporter plasmids pHRG-BKPyV-Dunlop NCCR (28) and pRG13D12-BKPyV-Dunlop NCCR (15) (data not shown) were used to assess the expression of DsRed2 for EVGR and enhanced green fluorescent protein (EGFP) for LVGR by flow cytometry as described previously (15, 28). Reporter plasmids were transfected into HEK293 and HEK293T cells using Lipofectamine 2000 following the manufacturer’s instructions. After 6 h, transfection reagents were removed and replaced by culture medium containing acitretin (5 μM). The number of fluorescent cells and mean fluorescence intensity of EVGR (DsRed) and LVGR (EGFP) expression were determined by flow cytometry (BD LSRFortessa; BD Biosciences, Allschwil, Switzerland) at 48 hpt.
Statistics.
All statistical analyses were performed with GraphPad Prism 9 software (version 9.1.0; GraphPad Software, La Jolla, CA). Data sets are presented as the mean ± standard error of the mean (SEM). The effective and cytotoxicity concentrations were determined using a sigmoidal 4P model, , in Prism 8 software (GraphPad).
ACKNOWLEDGMENTS
We thank Maud Wilhelm, Amandeep Kaur, and Karoline Leuzinger for helpful discussion, Fabian Weissbach and Marion Wernli for technical assistance, and Erika Hofmann and Corinne Salvisberg for timely updating the reference library.
This research was funded by a personal appointment grant of the University of Basel to H.H.H. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Hans H. Hirsch, Email: hans.hirsch@unibas.ch.
Lawrence Banks, International Centre for Genetic Engineering and Biotechnology.
REFERENCES
- 1.Graf FE, Hirsch HH. 2020. BK polyomavirus after solid organ and hematopoietic cell transplantation: one virus – three diseases, p 1–26. In Morris MI, Kotton CN, Wolfe C (ed), Emerging transplant infections. Springer Nature, Cham, Switzerland. 10.1007/978-3-030-01751-4_29-1. [DOI] [Google Scholar]
- 2.Hirsch HH. 2016. Human polyomavirus and papillomavirus infection and disease posttransplant, p 631–652. In Ljungman P, Snydman D (ed), Transplant infections, 4th ed. Springer, Cham, Switzerland. 10.1007/978-3-319-28797-3_35. [DOI] [Google Scholar]
- 3.DeCaprio JA, Imperiale MJ, Hirsch HH. 2021. Fields virology: emerging viruses. In Knipe DM, Howley PM (ed), Fields virology, 7th ed., vol 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 4.Greenlee JE, Hirsch HH. 2017. Polyomaviruses, p 599–623. In Richman D, Whitley R, Hayden F (ed), Clinical virology, 4th ed. ASM Press, Washington, DC. 10.1128/9781555819439. [DOI] [Google Scholar]
- 5.Kardas P, Leboeuf C, Hirsch HH. 2015. Optimizing JC and BK polyomavirus IgG testing for seroepidemiology and patient counseling. J Clin Virol 71:28–33. 10.1016/j.jcv.2015.07.305. [DOI] [PubMed] [Google Scholar]
- 6.Kean JM, Rao S, Wang M, Garcea RL. 2009. Seroepidemiology of human polyomaviruses. PLoS Pathog 5:e1000363. 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randhawa PS, Gupta G, Vats A, Shapiro R, Viscidi RP. 2006. Immunoglobulin G, A, and M responses to BK virus in renal transplantation. CVI 13:1057–1063. 10.1128/CVI.00114-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzetti J, Weissbach FH, Graf FE, Unterstab G, Wernli M, Hopfer H, Drachenberg CB, Rinaldo CH, Hirsch HH. 2020. BK polyomavirus evades innate immune sensing by disrupting the mitochondrial network and promotes mitophagy. iScience 23:101257. 10.1016/j.isci.2020.101257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. 2009. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 199:837–846. 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 10.Kaur A, Wilhelm M, Wilk S, Hirsch HH. 2019. BK polyomavirus-specific antibody and T-cell responses in kidney transplantation: update. Curr Opin Infect Dis 32:575–583. 10.1097/QCO.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch HH, Randhawa PS, AST Infectious Diseases Community of Practice. 2019. BK polyomavirus in solid organ transplantation-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 33:e13528. 10.1111/ctr.13528. [DOI] [PubMed] [Google Scholar]
- 12.Cesaro S, Dalianis T, Hanssen Rinaldo C, Koskenvuo M, Pegoraro A, Einsele H, Cordonnier C, Hirsch HH, ECIL-6 Group. 2018. ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother 73:12–21. 10.1093/jac/dkx324. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Graf FE, Hirsch HH. 2021. Antivirals against human polyomaviruses: leaving no stone unturned. Rev Med Virol 10.1002/rmv.2220:e2220. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch HH. 2005. BK virus: opportunity makes a pathogen. Clin Infect Dis 41:354–360. 10.1086/431488. [DOI] [PubMed] [Google Scholar]
- 15.Ajuh E, Wu Z, Kraus E, Weissbach FH, Bethge T, Gosert R, Fischer N, Hirsch HH. 2018. Novel human polyomavirus noncoding control regions differ in bidirectional gene expression according to host cell, large T-antigen expression, and clinically occurring rearrangements. J Virol 92:e02231-17. 10.1128/JVI.02231-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bethge T, Ajuh E, Hirsch HH. 2016. Imperfect symmetry of Sp1 and core promoter sequences regulates early and late virus gene expression of the bidirectional BK polyomavirus noncoding control region. J Virol 90:10083–10101. 10.1128/JVI.01008-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bethge T, Hachemi HA, Manzetti J, Gosert R, Schaffner W, Hirsch HH. 2015. Sp1 sites in the noncoding control region of BK polyomavirus are key regulators of bidirectional viral early and late gene expression. J Virol 89:3396–3411. 10.1128/JVI.03625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell JK, Blalock JE. 1984. Vitamin A inhibition of polyoma virus replication. Biochem Biophys Res Commun 122:851–858. 10.1016/s0006-291x(84)80112-6. [DOI] [PubMed] [Google Scholar]
- 19.Pang ML, Murase JE, Koo J. 2008. An updated review of acitretin–a systemic retinoid for the treatment of psoriasis. Expert Opin Drug Metab Toxicol 4:953–964. 10.1517/17425255.4.7.953. [DOI] [PubMed] [Google Scholar]
- 20.Canavan TN, Baddley JW, Pavlidakey P, Tallaj JA, Elewski BE. 2018. Human polyomavirus-7-associated eruption successfully treated with acitretin. Am J Transplant 18:1278–1284. 10.1111/ajt.14634. [DOI] [PubMed] [Google Scholar]
- 21.Rinaldo CH, Gosert R, Bernhoff E, Finstad S, Hirsch HH. 2010. 1-O-hexadecyloxypropyl cidofovir (CMX001) effectively inhibits polyomavirus BK replication in primary human renal tubular epithelial cells. Antimicrob Agents Chemother 54:4714–4722. 10.1128/AAC.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leuzinger K, Naegele K, Schaub S, Hirsch HH. 2019. Quantification of plasma BK polyomavirus loads is affected by sequence variability, amplicon length, and non-encapsidated viral DNA genome fragments. J Clin Virol 121:104210. 10.1016/j.jcv.2019.104210. [DOI] [PubMed] [Google Scholar]
- 23.Funk GA, Gosert R, Comoli P, Ginevri F, Hirsch HH. 2008. Polyomavirus BK replication dynamics in vivo and in silico to predict cytopathology and viral clearance in kidney transplants. Am J Transplant 8:2368–2377. 10.1111/j.1600-6143.2008.02402.x. [DOI] [PubMed] [Google Scholar]
- 24.Hara K, Sugimoto C, Kitamura T, Aoki N, Taguchi F, Yogo Y. 1998. Archetype JC virus efficiently replicates in COS-7 cells, simian cells constitutively expressing simian virus 40 T antigen. J Virol 72:5335–5342. 10.1128/JVI.72.7.5335-5342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Tanoury Z, Piskunov A, Rochette-Egly C. 2013. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res 54:1761–1775. 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernhoff E, Gutteberg TJ, Sandvik K, Hirsch HH, Rinaldo CH. 2008. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am J Transplant 8:1413–1422. 10.1111/j.1600-6143.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 27.Low J, Humes HD, Szczypka M, Imperiale M. 2004. BKV and SV40 infection of human kidney tubular epithelial cells in vitro. Virology 323:182–188. 10.1016/j.virol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Gosert R, Rinaldo CH, Funk GA, Egli A, Ramos E, Drachenberg CB, Hirsch HH. 2008. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J Exp Med 205:841–852. 10.1084/jem.20072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myhre MR, Olsen GH, Gosert R, Hirsch HH, Rinaldo CH. 2010. Clinical polyomavirus BK variants with agnogene deletion are non-functional but rescued by trans-complementation. Virology 398:12–20. 10.1016/j.virol.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Wiegand UW, Chou RC. 1998. Pharmacokinetics of acitretin and etretinate. J Am Acad Dermatol 39:S25–S33. 10.1016/s0190-9622(98)70441-4. [DOI] [PubMed] [Google Scholar]
- 31.Moriyama T, Sorokin A. 2008. Intracellular trafficking pathway of BK virus in human renal proximal tubular epithelial cells. Virology 371:336–349. 10.1016/j.virol.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang M, Abend JR, Tsai B, Imperiale MJ. 2009. Early events during BK virus entry and disassembly. J Virol 83:1350–1358. 10.1128/JVI.02169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milavetz BI. 2002. SP1 and AP-1 elements direct chromatin remodeling in SV40 chromosomes during the first 6 hours of infection. Virology 294:170–179. 10.1006/viro.2001.1308. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson RC, Mader S, Nagpal S, Leid M, Rochette-Egly C, Chambon P. 1990. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J 9:4443–4454. 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schüle R, Rangarajan P, Yang N, Kliewer S, Ransone LJ, Bolado J, Verma IM, Evans RM. 1991. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A 88:6092–6096. 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Islam MR, Puri S, Rodova M, Magenheimer BS, Maser RL, Calvet JP. 2008. Retinoic acid-dependent activation of the polycystic kidney disease-1 (PKD1) promoter. Am J Physiol Renal Physiol 295:F1845–F1854. 10.1152/ajprenal.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckner R, Ludlow JW, Lill NL, Oldread E, Arany Z, Modjtahedi N, DeCaprio JA, Livingston DM, Morgan JA. 1996. Association of p300 and CBP with simian virus 40 large T antigen. Mol Cell Biol 16:3454–3464. 10.1128/mcb.16.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho S, Tian Y, Benjamin TL. 2001. Binding of p300/CBP co-activators by polyoma large T antigen. J Biol Chem 276:33533–33539. 10.1074/jbc.M102906200. [DOI] [PubMed] [Google Scholar]
- 39.Saenz Robles MT, Shivalila C, Wano J, Sorrells S, Roos A, Pipas JM. 2013. Two independent regions of simian virus 40 T antigen increase CBP/p300 levels, alter patterns of cellular histone acetylation, and immortalize primary cells. J Virol 87:13499–13509. 10.1128/JVI.02658-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binet I, Nickeleit V, Hirsch HH, Prince O, Dalquen P, Gudat F, Mihatsch MJ, Thiel G. 1999. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation 67:918–922. 10.1097/00007890-199903270-00022. [DOI] [PubMed] [Google Scholar]
- 41.Randhawa PS, Finkelstein S, Scantlebury V, Shapiro R, Vivas C, Jordan M, Picken MM, Demetris AJ. 1999. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation 67:103–109. 10.1097/00007890-199901150-00018. [DOI] [PubMed] [Google Scholar]
- 42.Drachenberg CB, Beskow CO, Cangro CB, Bourquin PM, Simsir A, Fink J, Weir MR, Klassen DK, Bartlett ST, Papadimitriou JC. 1999. Human polyoma virus in renal allograft biopsies: morphological findings and correlation with urine cytology. Hum Pathol 30:970–977. 10.1016/S0046-8177(99)90252-6. [DOI] [PubMed] [Google Scholar]
- 43.Drachenberg CB, Papadimitriou JC, Wali R, Cubitt CL, Ramos E. 2003. BK polyoma virus allograft nephropathy: ultrastructural features from viral cell entry to lysis. Am J Transplant 3:1383–1392. 10.1046/j.1600-6135.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 44.Bernhoff E, Tylden GD, Kjerpeseth LJ, Gutteberg TJ, Hirsch HH, Rinaldo CH. 2010. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol 84:2150–2156. 10.1128/JVI.01737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marty FM, Winston DJ, Chemaly RF, Mullane KM, Shore TB, Papanicolaou GA, Chittick G, Brundage TM, Wilson C, Morrison ME, Foster SA, Nichols WG, Boeckh MJ. 2019. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 25:369–381. 10.1016/j.bbmt.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henriksen S, Mittelholzer C, Gosert R, Hirsch HH, Rinaldo CH. 2015. Human BK polyomavirus plasmid pBKV (34–2) (Dunlop) contains mutations not found in the originally published sequences. Genome Announc 3:e00046-15. 10.1128/genomeA.00046-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tylden GD, Hirsch HH, Rinaldo CH. 2015. Brincidofovir (CMX001) inhibits BK polyomavirus replication in primary human urothelial cells. Antimicrob Agents Chemother 59:3306–3316. 10.1128/AAC.00238-15. [DOI] [PMC free article] [PubMed] [Google Scholar]