Abstract
Type 2 diabetes mellitus (T2DM) is a strong risk tfactor for osteosarcopenia. The relationship between musculoskeletal index and β-cell function remains controversial. We aimed to describe the clinical characteristics of osteosarcopenia and to explore the association between osteosarcopenia and β-cell function, as well as insulin resistance in patients with T2DM. A total of 150 middle-aged and older nonobese patients with T2DM were recruited. Bone mineral density (BMD) and body composition were measured by the dual-energy X-ray absorptiometry scanner. The homeostasis model assessment of insulin resistance and Matsuda index were used to evaluate insulin resistance status. β-Cell function was estimated by the area under the curve insulin/glucose (AUC-Ins/Glu) and the area under the curve C-peptide/glucose (AUC-CP/Glu). T2DM patients with osteosarcopenia had lower body mass index, waist circumference, body fat percentage, AUC-Ins/Glu, and AUC-CP/Glu. Both AUC-Ins/Glu (OR = 0.634, P = 0.008) and AUC-CP/Glu (OR = 0.491, P = 0.009) were negatively associated with the presence of osteosarcopenia. Multivariate linear regression analysis showed that β-cell function was positively associated with the skeletal muscle mass index, whereas it showed no correlation with lumbar or hip BMD. β-Cell function is associated with osteosarcopenia in middle-aged and older nonobese patients with T2DM. These findings suggest that β-cell function might be a protective factor against osteosarcopenia.
Keywords: β-cell function, osteosarcopenia, T2DM, skeletal muscle mass index
1. Introduction
The term “osteosarcopenia” designates, specifically, the simultaneous presence of sarcopenia and osteopenia/osteoporosis [1,2]. This new concept is now known as geriatric syndrome, which associates with an increased risk of falls, fractures, and impaired mobility. It has been reported that the prevalence of osteosarcopenia ranges from 4.7 to 40% in older adults [3]. The patients with osteosarcopenia had a significantly higher risk of falls and fractures than those having either sarcopenia or osteoporosis [4]. Recently, type 2 diabetes mellitus (T2DM) was considered as a strong risk factor for osteosarcopenia [2].
It has been well known that patients with T2DM tend to have an increased risk of muscle loss and a higher rate of fractures [5,6,7]. The co-occurrence of T2DM and osteosarcopenia may aggravate musculoskeletal health and increase the risk of falls and fractures. However, few studies have focused on the clinical characteristics and risk factors of osteosarcopenia in T2DM patients.
Both insulin resistance and β-cell dysfunction are believed to play a critical role in the pathogenesis of T2DM and its complications [8,9,10]. However, the relationship between musculoskeletal index and β-cell function, as well as insulin resistance, is debated [11,12,13]. Fasting insulin and glucose were used to assess insulin resistance and β-cell function in most of the recent studies, which may not be accurate [14,15]. Euglycemic hyperinsulinemic clamp and hyperglycemic clamp are considered as “gold standard,” but they are costly and not feasible to be carried out in large trials. The concentration of stimulated glucose, insulin, and C-peptide has been recognized as a reliable measure of residual β-cell function and insulin resistance [16,17].
Therefore, this study aimed to investigate the clinical characteristics of osteosarcopenia and to explore the association between the occurrence of osteosarcopenia and β-cell function, as well as insulin resistance in patients with T2DM by using the 75 g-oral glucose test (OGTT).
2. Methods
2.1. Subjects
A total of 150 (80 men and 70 postmenopausal women) patients with T2DM aged ≥50 years at Qilu Hospital of Shandong University from January 2017 to December 2019. Diabetes was diagnosed based on the 2006 World Health Organization criteria [18]. Patients with a body mass index (BMI) ≤18.5 and ≥30 kg/m2 were excluded. Patients with severe liver disease (liver cirrhosis or apparently abnormal liver function defined as serum aspartate aminotransferase or alanine aminotransferase levels >120 U/L), severe kidney disease (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), and any malignant disease or hematologic disease were excluded. This study was approved by the ethics committee of Qilu Hospital of Shandong University.
2.2. Clinical evaluation
Age, gender, height, weight, waist circumference, blood pressure (BP), history of smoking and drinking, and medication history of metformin, sulfonylurea, and insulin treatment were assessed on admission. BMI was calculated as weight (kg) divided by height squared (m2). Fasting blood was drawn for the measurement of HbA1c, albumin, LDL-C, HDL-C, total cholesterol, triglyceride, and creatinine. At the same time, all diabetic patients underwent 75 g-OGTT. Serum insulin, C-peptide, and glucose concentrations were obtained at 0, 30, 60, 120, and 180 min after OGTT. Insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR) and Matsuda index [19,20]. β-Cell function was measured by the area under the curve insulin/glucose (AUC-Ins/Glu) and the area under the curve C-peptide/glucose (AUC-CP/Glu), which were calculated using the trapezoidal rule applied to the insulin, C-peptide, and glucose curves [20,21].
Lumbar spine and hip bone mineral density (BMD), appendicular lean mass (ALM), and body fat percentage were measured by the dual-energy X-ray absorptiometry scanner (Hologic Inc., Bedford, MA, USA). Lumbar spine or hip BMD based on T-score was classified as follows: osteoporosis (T-score less than −2.5 SD), osteopenia (T-score between −2.5 SD and <(−1) SD), and normal (T-score greater than −1 SD). Skeletal muscle mass index (SMI) was calculated as follows: SMI (kg/m2) = ALM (kg)/height2 (m2). Sarcopenia was defined as SMI below a cutoff of 7.0 kg/m2 (men) and 5.4 kg/m2 (women) [22]. Osteosarcopenia was defined as the presence of osteopenia/osteoporosis and sarcopenia [2]. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [23].
2.3. Statistical analysis
The continuous variables are expressed as the mean ± standard deviation (SD) and the mean (interquartile range). Between-group differences were detected using Student’s t-test or the Mann–Whitney U-test or chi-square test. The data were graphed using Prism software (Graphpad, California, USA). Binary logistic regression was used to evaluate the risk factors for osteosarcopenia in T2DM patients. The relationship between β-cell function and musculoskeletal indices was assessed by multiple linear regression. The statistical analysis was done using SPSS 22.0 software (SPSS Inc., Chicago, USA), and P < 0.05 was considered statistically significant.
3. Results
3.1. General characteristics of participants
The demographics and laboratory results of all T2DM patients are shown in Table 1. Age and gender distributions were not different between the two groups (nonosteosarcopenia vs osteosarcopenia). The patients with osteosarcopenia showed lower BMI, waist circumference, and body fat percentage. There was no significant difference in the history of smoking and drinking, diabetes duration, BP, lipid profile, renal function, albumin, HbA1c, or antidiabetic medication between the two groups.
Table 1.
Demographic and clinical parameters of the study population
| Nonosteosarcopenia | Osteosarcopenia | P | |
|---|---|---|---|
| n | 106 | 44 | |
| Age (years) | 60.17 ± 6.45 | 61.84 ± 6.76 | 0.156 |
| Female | 57.5% | 43.2% | 0.150 |
| Smoking | 20.8% | 27.3% | 0.398 |
| Drinking | 23.6% | 29.5% | 0.537 |
| Diabetes duration months | 150 (96–216) | 126 (81–204) | 0.544 |
| SBP (mmHg) | 136.85 ± 19.00 | 132.66 ± 22.84 | 0.249 |
| DBP (mmHg) | 78.88 ± 10.33 | 75.14 ± 12.55 | 0.060 |
| BMI | 26.03 ± 2.44 | 22.75 ± 2.45 | <0.001 |
| Waist circumference (cm) | 95.58 ± 9.51 | 89.42 ± 9.68 | <0.001 |
| Body fat percentage (%) | 32.37 ± 5.61 | 29.96 ± 6.37 | 0.023 |
| Total cholesterol (mmol/L) | 4.61 (3.90–5.36) | 4.36 (3.98–5.14) | 0.980 |
| LDL-C (mmol/L) | 2.79 (2.32–3.43) | 2.67 (2.23–3.13) | 0.212 |
| HDL-C (mmol/L) | 1.17 (1.06–1.32) | 1.23 (1.00–1.54) | 0.249 |
| Triglyceride (mmol/L) | 1.48 (1.09–2.17) | 1.29 (0.91–1.93) | 0.649 |
| Albumin (g/L) | 43.68 ± 3.92 | 43.47 ± 4.57 | 0.790 |
| eGFR (mL/min/1.73 m2) | 96.67 ± 11.91 | 98.58 ± 9.59 | 0.347 |
| HbA1c (%) | 8.55 ± 1.87 | 8.64 ± 1.83 | 0.720 |
| FBG (mmol/L) | 8.12 (5.94–9.47) | 8.63 (6.32–10.08) | 0.278 |
| 0.5 h glucose (mmol) | 10.98 (9.09–13.28) | 11.52 (9.55–13.28) | 0.261 |
| 1 h glucose (mmol) | 15.28 (13.23–17.88) | 16.14 (13.68–18.30) | 0.172 |
| 2 h glucose (mmol) | 18.06 (15.70–20.51) | 19.39 (17.30–20.98) | 0.061 |
| 3 h glucose (mmol) | 16.61 (14.04–19.07) | 18.04 (16.12–20.06) | 0.058 |
| Fasting insulin (mIU/L) | 19.32 (8.78–21.95) | 16.25 (6.43–21.00) | 0.319 |
| 0.5 h insulin (mIU/L) | 25.76 (14.27–29.97) | 19.84 (9.43–25.79) | 0.046 |
| 1 h insulin (mIU/L) | 36.55 (21.00–48.49) | 27.96 (13.90–35.64) | 0.020 |
| 2 h insulin (mIU/L) | 46.20 (23.76–62.63) | 36.18 (19.31–43.45) | 0.059 |
| 3 h insulin (mIU/L) | 39.30 (20.89–54.23) | 28.01 (15.10–36.53) | 0.010 |
| Fasting C-peptide (ng/mL) | 1.50 (0.76–1.90) | 1.20 (0.60–1.55) | 0.065 |
| 0.5 h C-peptide (ng/mL) | 2.00 (1.11–2.49) | 1.57 (0.94–2.16) | 0.034 |
| 1 h C-peptide (ng/mL) | 2.81 (1.63–3.76) | 2.14 (1.24–2.75) | 0.016 |
| 2 h C-peptide (ng/mL) | 4.17 (2.42–5.40) | 3.52 (2.13–4.33) | 0.140 |
| 3 h C-peptide (ng/mL) | 4.38 (2.71–5.50) | 3.40 (1.99–4.44) | 0.018 |
| AUC-Ins/Glu | 2.56 (1.46–3.40) | 1.84 (0.93–2.31) | 0.010 |
| AUC-CP/Glu | 0.23 (0.12–0.29) | 0.17 (0.09–0.21) | 0.013 |
| Metformin usage (%) | 80.2% | 70.5% | 0.205 |
| Sulfonylurea usage (%) | 51.9% | 45.5% | 0.591 |
| Insulin usage (%) | 52.4% | 59.1% | 0.476 |
Data are shown as the mean ± SD, median (interquartile range), or number (%).
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; and eGFR, estimated glomerular filtration rate.
Significant P values (<0.05) are indicated in bold.
3.2. β-Cell function is different between osteosarcopenia and nonosteosarcopenia groups
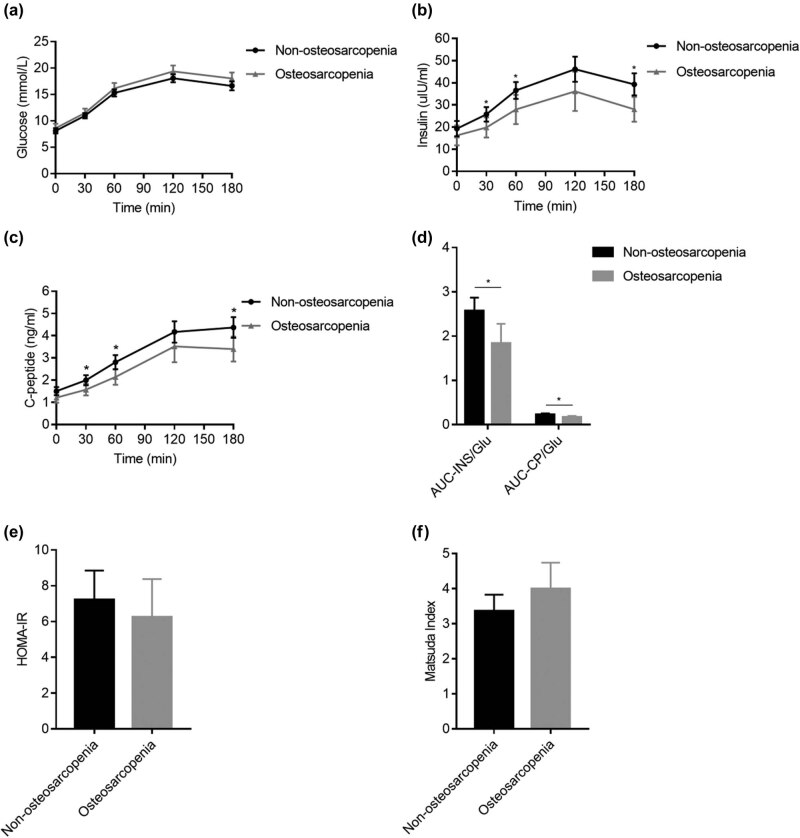
75 g-OGTT was performed to explore insulin resistance and β-cell function in T2DM patients with osteosarcopenia. As shown in Table 1 and Figure 1, fasting serum glucose, insulin, and C-peptide concentrations were not significantly different between the two groups (Figure 1a–c). Postprandial insulin and C-peptide (30, 60, and 180 min) were significantly lower in the osteosarcopenia group compared with nonosteosarcopenia group. Furthermore, patients with osteosarcopenia showed lower AUC-Ins/Glu and AUC-CP/Glu levels (Figure 1d); nevertheless, no difference was observed in HOMA-IR and Matsuda index between the two groups (Figure 1e–f).
Figure 1.
Comparison of the levels of OGTT-induced serum glucose, insulin, C peptide, β-cell function, and insulin resistance status of the two groups. (a–c) Serum glucose (a), insulin (b), and C-peptide (c) during 180 min OGTT. (d) β-Cell function measured by AUC-Ins/Glu and AUC-CP/Glu. (e–f) Insulin resistance status measured by HOMA-IR (e) and Matsuda index (f). Each error bar is constructed using a 95% confidence interval of the mean. *P < 0.05 versus nonosteosarcopenia.
3.3. β-Cell function is independently associated with the presence of osteosarcopenia in T2DM patients
To elucidate whether β-cell function is independently associated with the presence of osteosarcopenia, binary logistic regression using three models was conducted to assess the participants, as shown in Table 2. Before adjustment, AUC-Ins/Glu and AUC-CP/Glu showed a decreased odds ratio for osteosarcopenia. After adjusting for age, gender, duration of T2DM, medical history of sulfonylurea and insulin, and body fat percentage in model 2, both AUC-Ins/Glu and AUC-CP/Glu were still negatively associated with osteosarcopenia. Smoking and drinking status, renal dysfunction, malnutrition, and hyperglycemia were known causes of osteosarcopenia [2], and after an additional adjustment for these traditional risk factors, β-cell function was still significantly associated with a lower risk of osteosarcopenia. Notably, there was no correlation between the presence of osteosarcopenia and fasting insulin, as well as fasting C-peptide.
Table 2.
Binary logistic regression analysis of the relationship between β-cell function and osteosarcopenia
| AUC-Ins/Glu | (AUC-CP) ∗ 10/Glu | Fasting insulin | Fasting C-peptide | |||||
|---|---|---|---|---|---|---|---|---|
| Model | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Model 1 | 0.687 (0.511–0.924) | 0.013 | 0.672 (0.485–0.930) | 0.016 | 0.988 (0.966–1.012) | 0.321 | 0.655 (0.415–1.033) | 0.069 |
| Model 2 | 0.664 (0.477–0.923) | 0.015 | 0.596 (0.392–0.905) | 0.015 | 0.987 (0.962–1.012) | 0.303 | 0.603 (0.347–1.046) | 0.072 |
| Model 3 | 0.634 (0.453–0.887) | 0.008 | 0.491 (0.287–0.840) | 0.009 | 0.980 (0.953–1.008) | 0.163 | 0.745 (0.407–1.364) | 0.340 |
Model 1: not adjusted.
Model 2: adjusted for age, gender, duration of T2DM, insulin and sulfonylurea usage, and body fat percentage.
Model 3: adjusted for age, gender, duration of T2DM, insulin and sulfonylurea usage, body fat percentage, history of smoking and drinking, HbA1C, albumin, and eGFR.
Significant P values (<0.05) are indicated in bold.
3.4. β-Cell function was positively associated with SMI in T2DM patients
Multiple linear regression analysis was conducted to explore the potential relationship between β-cell function and musculoskeletal indices in T2DM patients. As shown in Table 3, both AUC-Ins/Glu and AUC-CP/Glu were positively associated with SMI, independent of age, gender, history of smoking and drinking, duration of T2DM, medical history of sulfonylurea and insulin, albumin, eGFR, and body fat percentage. No correlation was found between β-cell function and lumbar spine or hip BMD (Table 3).
Table 3.
Multiple linear regression analysis of β-cell function and musculoskeletal indices in T2DM patients
| AUC-Ins/Glu | AUC-CP/Glu | |||
|---|---|---|---|---|
| β coefficient (95% CI) | P | β coefficient (95% CI) | P | |
| SMI | 0.084 (0.004–0.164) | 0.040 | 0.132 (0.009–0.255) | 0.036 |
| Lumbar BMD | 0.005 (−0.012 to 0.022) | 0.591 | 0.011 (−0.016 to 0.036) | 0.446 |
| Hip BMD | 0.009 (−0.004 to 0.023) | 0.173 | 0.018 (−0.002 to 0.039) | 0.084 |
Adjusted for age, gender, duration of T2DM, insulin and sulfonylurea usage, body fat percentage, history of smoking and drinking, HbA1C, albumin, and eGFR.
Significant P values (<0.05) are indicated in bold.
4. Discussion
In this study, we first compared the clinical characteristics of osteosarcopenia and nonosteosarcopenia patients with T2DM. Patients with osteosarcopenia showed lower BMI, waist circumference, body fat percentage, and worse β-cell function. However, neither insulin resistance nor glucose control showed differences between the groups. The presence of osteosarcopenia was found to be negatively correlated with β-cell function independent of other factors, such as age, diabetes duration, history of smoking and drinking, malnutrition, body fat percentage, renal dysfunction, and glucose control.
T2DM patients have lost 50% of β-cell function at the time of diagnosis [24], and this value gradually increases with longer disease duration [25]. Residual insulin secretion capacity is considered a preventive factor against diabetic complications [26]. However, the relationship between musculoskeletal index and β-cell function is controversial. SMI was negatively associated with glucagon-stimulated C-peptide in T2DM patients <65 years, but the correlation was not found between SMI and blood glucose control [11]. In contrast, one study found that endogenous insulin secretion capacity was positively associated with muscle mass, and high HbA1c was associated with the occurrence of sarcopenia in T2DM subjects [12]. Moreover, some studies demonstrated that BMD was positively associated with insulin and C-peptide [27,28], whereas other studies obtained the opposite results [29,30].
Our results differ from the above studies, which may be due to the following several points: First, OGTT-induced glucose, insulin, and C-peptide were measured to reflect β-cell function and insulin resistance status, which are more accurate than fasting insulin and C-peptide [16,17]. Compared with the control group, in our study, no difference was identified in the fasting insulin and C-peptide concentration in the osteosarcopenia group. Neither fasting insulin nor C-peptide was associated with the presence of osteosarcopenia. Second, we studied specifically age-matched and nonobese T2DM subjects with similar disease duration, which can minimize these confounding factors. Third, both insulin secretion capacity and musculoskeletal index are highly correlated with fat mass [31,32,33]. However, few studies have considered the effect of fat mass on the association between β-cell function and musculoskeletal indices. Our study revealed that the association between the presence of osteosarcopenia and β-cell function persisted after adjusting body fat percentage, suggesting that the association is independent of body fat percentage.
Moreover, we found that SMI showed a positive correlation with β-cell function, whereas BMD did not show any correlation. The interaction among pancreas, bone, and muscle is uncertain. Bone and muscle are connected anatomically, and they interact with each other through multiple mechanisms. Several growth factors secreted by myotubes, such as fibroblast growth factor 2 and insulin-like growth factor 1 (IGF1), have an anabolic effect on bone [34], and IGF1 could also promote proliferation and differentiation in osteoblasts [35]. In addition, muscle loss is associated with the deficiency of insulin and C-peptide in type 1 diabetes [36,37]. Insulin exerts an anabolic effect on skeletal muscle either through stimulation of protein synthesis or through inhibition of proteolysis [38,39]. C-peptide has also been found to have a protective effect against cell death in myoblasts [40]. Thus, our findings support the hypothesis that β cells might have an indirect effect on bone through skeletal muscle [41].
To our knowledge, this is the first study to report the correlation of β-cell function with osteosarcopenia in T2DM patients. There were several limitations to our studies. First, this was a cross-sectional study with a small sample size. However, the statistically significant results encourage multiple-center and large-sample studies to verify our findings. Second, we did not measure IGF1 or Vitamin D, which is essential for skeletal muscle maintenance and bone remodeling [35,42]. Finally, sarcopenia was just defined by the SMI, and grip strength or physical performance was not evaluated in this study.
5. Conclusion
We found that β-cell function might be a protective factor against osteosarcopenia in middle-aged and older nonobese patients withT2DM. Moreover, this relationship was independent of other osteosarcopenia risk factors, such as age, duration of diabetes, smoking, drinking, malnutrition, and glucose control. These findings suggest that interventions to preserve β-cell function are important for the prevention of osteosarcopenia in patients with T2DM.
Footnotes
Funding information: The present study was supported by National Natural Science Foundation of China (No. 81700739, 81900756), Key Research and Development Plan of Shandong Province (2019GSF108099), and the Natural Science Foundation of Shandong province (No. ZR2019PH078).
Author contributions: J.B.L. and J.D.L. designed the study and drafted the initial manuscript. C.W. and J.D.L. collected the data. C.W., X.F.Y., Y.J.S., and C.L.W. conducted analysis. All authors approved the final manuscript as submitted.
Conflict of interest: the authors state no conflict of interest.
Data availability statement: The data used to support the findings of this study are available from the corresponding author upon request.
Contributor Information
Chuan Wang, Email: 1175533920@qq.com.
Jinbo Liu, Email: jinboliu@sdu.edu.cn.
References
- [1].Binkley N, Buehring B. Beyond FRAX: it’s time to consider “sarco-osteopenia.” J Clin Densitom J Int Soc Clin Densitom. 2009;12(4):413–6. [DOI] [PubMed]; Binkley N, Buehring B. Beyond FRAX: it’s time to consider “sarco-osteopenia.”. J Clin Densitom J Int Soc Clin Densitom. 2009;12(4):413–6. doi: 10.1016/j.jocd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [2].Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11(3):609–18. [DOI] [PMC free article] [PubMed]; Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11(3):609–18. doi: 10.1002/jcsm.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kirk B, Al Saedi A, Duque G. Osteosarcopenia: a case of geroscience. Aging Med Milton NSW. 2019;2(3):147–56. [DOI] [PMC free article] [PubMed]; Kirk B, Al Saedi A, Duque G. Osteosarcopenia: a case of geroscience. Aging Med Milton NSW. 2019;2(3):147–56. doi: 10.1002/agm2.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R. FiAT intervention group Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28(5):895–9. [DOI] [PubMed]; Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28(5):895–9. doi: 10.1007/s40520-015-0494-1. FiAT intervention group . [DOI] [PubMed] [Google Scholar]
- [5].Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–19. [DOI] [PubMed]; Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL. et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–19. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- [6].Sarodnik C, Bours SPG, Schaper NC, van den Bergh JP, van Geel TA. The risks of sarcopenia, falls and fractures in patients with type 2 diabetes mellitus. Maturitas. 2018;109:70–7. [DOI] [PubMed]; Sarodnik C, Bours SPG, Schaper NC, van den Bergh JP, van Geel TA. The risks of sarcopenia, falls and fractures in patients with type 2 diabetes mellitus. Maturitas. 2018;109:70–7. doi: 10.1016/j.maturitas.2017.12.011. [DOI] [PubMed] [Google Scholar]
- [7].Wang T, Feng X, Zhou J, Gong H, Xia S, Wei Q, et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci Rep. 2016;6(1):38937. [DOI] [PMC free article] [PubMed]; Wang T, Feng X, Zhou J, Gong H, Xia S, Wei Q. et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci Rep. 2016;6(1):38937. doi: 10.1038/srep38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96(8):2354–66. [DOI] [PubMed]; DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96(8):2354–66. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- [9].LeRoith D. Beta-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med. 2002;113(Suppl 6A):3S–11S. [DOI] [PubMed]; LeRoith D. Beta-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med. 2002;113(Suppl 6A):3S–11S. doi: 10.1016/s0002-9343(02)01276-7. [DOI] [PubMed] [Google Scholar]
- [10].Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol. 2013;4:37. [DOI] [PMC free article] [PubMed]; Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol. 2013;4:37. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shishikura K, Tanimoto K, Sakai S, Tanimoto Y, Terasaki J, Hanafusa T. Association between skeletal muscle mass and insulin secretion in patients with type 2 diabetes mellitus. Endocr J. 2014;61(3):281–7. [DOI] [PubMed]; Shishikura K, Tanimoto K, Sakai S, Tanimoto Y, Terasaki J, Hanafusa T. Association between skeletal muscle mass and insulin secretion in patients with type 2 diabetes mellitus. Endocr J. 2014;61(3):281–7. doi: 10.1507/endocrj.ej13-0375. [DOI] [PubMed] [Google Scholar]
- [12].Tanaka K, Kanazawa I, Sugimoto T. Reduction in endogenous insulin secretion is a risk factor of sarcopenia in men with type 2 diabetes mellitus. Calcif Tissue Int. 2015;97(4):385–90. [DOI] [PubMed]; Tanaka K, Kanazawa I, Sugimoto T. Reduction in endogenous insulin secretion is a risk factor of sarcopenia in men with type 2 diabetes mellitus. Calcif Tissue Int. 2015;97(4):385–90. doi: 10.1007/s00223-015-9990-8. [DOI] [PubMed] [Google Scholar]
- [13].Ruppert K, Cauley J, Lian Y, Zgibor JC, Derby C, Solomon DH. The effect of insulin on bone mineral density among women with type 2 diabetes: a SWAN Pharmacoepidemiology study. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2018;29(2):347–54. [DOI] [PMC free article] [PubMed]; Ruppert K, Cauley J, Lian Y, Zgibor JC, Derby C, Solomon DH. The effect of insulin on bone mineral density among women with type 2 diabetes: a SWAN Pharmacoepidemiology study. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2018;29(2):347–54. doi: 10.1007/s00198-017-4276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC, et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33(6):1379–81. [DOI] [PMC free article] [PubMed]; Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC. et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33(6):1379–81. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu T, Willett WC, Giovannucci E. Plasma C-peptide is inversely associated with calcium intake in women and with plasma 25-hydroxy vitamin D in men. J Nutr. 2009;139(3):547–54. [DOI] [PMC free article] [PubMed]; Wu T, Willett WC, Giovannucci E. Plasma C-peptide is inversely associated with calcium intake in women and with plasma 25-hydroxy vitamin D in men. J Nutr. 2009;139(3):547–54. doi: 10.3945/jn.108.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53(1):250–64. [DOI] [PubMed]; Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H. et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53(1):250–64. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- [17].Lachin JM, McGee P, Palmer JP. Impact of C-peptide preservation on metabolic and clinical outcomes in the diabetes control and complications trial. Diabetes. 2014;63(2):739–48. [DOI] [PMC free article] [PubMed]; Lachin JM, McGee P, Palmer JP. Impact of C-peptide preservation on metabolic and clinical outcomes in the diabetes control and complications trial. Diabetes. 2014;63(2):739–48. doi: 10.2337/db13-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].World Health Organization, International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation [Internet]; 2006. Available from: http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/; World Health Organization. International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation [Internet] 2006. http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ Available from:
- [19].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed]; Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- [20].Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. [DOI] [PubMed]; Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- [21].Wang H, Hu B, Lu W, Liu F, Feng B. The relationship of angiographically defined coronary artery disease with insulin sensitivity and secretion in subjects with different glucose tolerance. J Cardiol. 2012;60(5):367–71. [DOI] [PubMed]; Wang H, Hu B, Lu W, Liu F, Feng B. The relationship of angiographically defined coronary artery disease with insulin sensitivity and secretion in subjects with different glucose tolerance. J Cardiol. 2012;60(5):367–71. doi: 10.1016/j.jjcc.2012.06.008. [DOI] [PubMed] [Google Scholar]
- [22].Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. [DOI] [PubMed]; Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS. et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- [23].Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed]; Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96(8):2354–66. [DOI] [PubMed]; DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96(8):2354–66. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- [25].U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective diabetes study group. Diabetes. 1995;44(11):1249–58. [PubMed]; U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective diabetes study group. Diabetes. 1995;44(11):1249–58. [PubMed] [Google Scholar]
- [26].Kuo JZ, Guo X, Klein R, Klein BE, Weinreb RN, Genter P, et al. Association of fasting insulin and C peptide with diabetic retinopathy in Latinos with type 2 diabetes. BMJ Open Diabetes Res Care. 2014;2(1):e000027. [DOI] [PMC free article] [PubMed]; Kuo JZ, Guo X, Klein R, Klein BE, Weinreb RN, Genter P. et al. Association of fasting insulin and C peptide with diabetic retinopathy in Latinos with type 2 diabetes. BMJ Open Diabetes Res Care. 2014;2(1):e000027. doi: 10.1136/bmjdrc-2014-000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–32. [DOI] [PMC free article] [PubMed]; Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z. et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–32. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2007;18(4):427–44. [DOI] [PubMed]; Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2007;18(4):427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- [29].Srikanthan P, Crandall CJ, Miller-Martinez D, Seeman TE, Greendale GA, Binkley N, et al. Insulin resistance and bone strength: findings from the study of midlife in the United States. J Bone Min Res J Am Soc Bone Min Res. 2014;29(4):796–803. [DOI] [PMC free article] [PubMed]; Srikanthan P, Crandall CJ, Miller-Martinez D, Seeman TE, Greendale GA, Binkley N. et al. Insulin resistance and bone strength: findings from the study of midlife in the United States. J Bone Min Res J Am Soc Bone Min Res. 2014;29(4):796–803. doi: 10.1002/jbmr.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li Y, Liu H, Sato Y. The association between the serum C-peptide level and bone mineral density. PLoS One. 2013;8(12):e83107. [DOI] [PMC free article] [PubMed]; Li Y, Liu H, Sato Y. The association between the serum C-peptide level and bone mineral density. PLoS One. 2013;8(12):e83107. doi: 10.1371/journal.pone.0083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aguirre L, Napoli N, Waters D, Qualls C, Villareal DT, Armamento-Villareal R. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J Clin Endocrinol Metab. 2014;99(9):3290–7. [DOI] [PMC free article] [PubMed]; Aguirre L, Napoli N, Waters D, Qualls C, Villareal DT, Armamento-Villareal R. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J Clin Endocrinol Metab. 2014;99(9):3290–7. doi: 10.1210/jc.2013-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hainer V, Aldhoon-Hainerova I. Obesity paradox does exist. Diabetes Care. 2013;36(Supplement_2):S276–81. [DOI] [PMC free article] [PubMed]; Hainer V, Aldhoon-Hainerova I. Obesity paradox does exist. Diabetes Care. 2013;36(Supplement_2):S276–81. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Conte C, Epstein S, Napoli N. Insulin resistance and bone: a biological partnership. Acta Diabetol. 2018;55(4):305–14. [DOI] [PubMed]; Conte C, Epstein S, Napoli N. Insulin resistance and bone: a biological partnership. Acta Diabetol. 2018;55(4):305–14. doi: 10.1007/s00592-018-1101-7. [DOI] [PubMed] [Google Scholar]
- [34].Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10(1):64–70. [PMC free article] [PubMed]; Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- [35].Bikle DD, Tahimic C, Chang W, Wang Y, Philippou A, Barton ER. Role of IGF-I signaling in muscle bone interactions. Bone. 2015;80:79–88. [DOI] [PMC free article] [PubMed]; Bikle DD, Tahimic C, Chang W, Wang Y, Philippou A, Barton ER. Role of IGF-I signaling in muscle bone interactions. Bone. 2015;80:79–88. doi: 10.1016/j.bone.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Krause MP, Riddell MC, Hawke TJ. Effects of type 1 diabetes mellitus on skeletal muscle: clinical observations and physiological mechanisms. Pediatr Diabetes. 2011;12(4pt1):345–64. [DOI] [PubMed]; Krause MP, Riddell MC, Hawke TJ. Effects of type 1 diabetes mellitus on skeletal muscle: clinical observations and physiological mechanisms. Pediatr Diabetes. 2011;12(4pt1):345–64. doi: 10.1111/j.1399-5448.2010.00699.x. [DOI] [PubMed] [Google Scholar]
- [37].Wierzbicka E, Swiercz A, Pludowski P, Jaworski M, Szalecki M. Skeletal status, body composition, and glycaemic control in adolescents with type 1 diabetes mellitus. J Diabetes Res. 2018;2018:1–14. [DOI] [PMC free article] [PubMed]; Wierzbicka E, Swiercz A, Pludowski P, Jaworski M, Szalecki M. Skeletal status, body composition, and glycaemic control in adolescents with type 1 diabetes mellitus. J Diabetes Res. 2018;2018:1–14. doi: 10.1155/2018/8121634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95(2):811–9. [DOI] [PMC free article] [PubMed]; Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95(2):811–9. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Denne SC, Liechty EA, Liu YM, Brechtel G, Baron AD. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol. 1991;261(6 Pt 1):E809–14. [DOI] [PubMed]; Denne SC, Liechty EA, Liu YM, Brechtel G, Baron AD. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol. 1991;261(6 Pt 1):E809–14. doi: 10.1152/ajpendo.1991.261.6.E809. [DOI] [PubMed] [Google Scholar]
- [40].Essid SM, Bevington A, Brunskill NJ. Proinsulin C-peptide enhances cell survival and protects against simvastatin-induced myotoxicity in L6 rat myoblasts. Int J Mol Sci. 2019;20(7):1654. [DOI] [PMC free article] [PubMed]; Essid SM, Bevington A, Brunskill NJ. Proinsulin C-peptide enhances cell survival and protects against simvastatin-induced myotoxicity in L6 rat myoblasts. Int J Mol Sci. 2019;20(7):1654. doi: 10.3390/ijms20071654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Klein GL. Insulin and bone: recent developments. World J Diabetes. 2014;5(1):14–6. [DOI] [PMC free article] [PubMed]; Klein GL. Insulin and bone: recent developments. World J Diabetes. 2014;5(1):14–6. doi: 10.4239/wjd.v5.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gunton JE, Girgis CM, Baldock PA, Lips P. Bone muscle interactions and vitamin D. Bone. 2015;80:89–94. [DOI] [PubMed]; Gunton JE, Girgis CM, Baldock PA, Lips P. Bone muscle interactions and vitamin D. Bone. 2015;80:89–94. doi: 10.1016/j.bone.2015.02.029. [DOI] [PubMed] [Google Scholar]