Abstract
Introduction
Sex differences in dementia with Lewy bodies (DLB) have been reported in clinically defined cohorts; however, clinical diagnostic accuracy in DLB is suboptimal and phenotypic differences have not been assessed in pathologically confirmed participants.
Methods
Core DLB features were compared across 55 women and 156 men with pathologically defined DLB in the National Alzheimer’s Coordinating Center. These analyses were repeated for 55 women and 55 men matched for age, education and tau burden.
Results
In the total sample, women died older, had fewer years of education, had higher tau burden but were less likely to be diagnosed with dementia and clinical DLB. In the matched sample, visual hallucinations continued to be less common in women, and fewer women met clinical DLB criteria.
Discussion
Sex impacts clinical manifestations of underlying pathologies in DLB. Despite similar underlying Lewy body pathology, women are less likely to manifest core DLB features and may be clinically underdiagnosed.
INTRODUCTION
Dementia with Lewy bodies (DLB) is the second most common neurodegenerative dementia accounting for approximately 5% of dementia cases in lder people.1 Although clinical criteria are available for DLB,2 diagnostic accuracy is still suboptimal in part due to high clinical heterogeneity.3 An important factor influencing that clinical heterogeneity is the co-occurring Alzheimer’s disease (AD) pathology that is often observed alongside the primary Lewy body (LB) pathology. The presence of this copathology is associated with an AD-like cognitive impairment and a lower likelihood of manifesting core DLB features like visual hallucinations.4 Consequently, current DLB diagnostic criteria require neuropathologic assessment of both LB and AD pathology when determining the likelihood of pathologic findings being associated with a typical DLB phenotype.2
Previous studies have reported that sex is associated with different neuropathological changes in the older people; men are more likely to have pure neocortical LB, whereas women have more AD pathology and cerebrovascular disease.5,6 Studies of clinical DLB prevalence in men and women are inconsistent, although the majority report a higher prevalence in men.7–11 Inconsistent findings may be attributed to differences in methodology, but also possibly a greater likelihood of a more typical DLB phenotype manifesting in men due to more common pure LB pathology. Sex differences can also affect clinicopathologic correlations. In AD, it has been reported that women bear more tau burden before the onset of cognitive decline.12,13 There is no similar analysis in DLB to date. Thus, to investigate whether pure LB pathology2 is associated with different clinical phenotypes in women and men, we studied neuropathologically confirmed cases from the National Alzheimer’s Coordinating Center (NACC) database with a high likelihood of exhibiting a DLB phenotype.14–17 Given that the neuropathology criteria for a high likelihood of a DLB phenotype exclude those with a high likelihood of AD neuropathologic change,2 those with LB and AD copathology were not included in our analysis and the analysis was limited to those with pure LB pathology. We hypothesised that the women will be less likely to have DLB core features despite underlying pure LB pathology.
METHODS
Participants
Data were obtained from the NACC Neuropathology Data Set, Genetic Data and Uniform Data Set (UDS)14–17 for visits conducted between September 2005 and August 2019 at 39 past and present AD research centres. The included data are collected by trained clinicians and clinic personnel from participants and their coparticipants enrolled in AD research centres using a standardised evaluation. Cognitive status of participants includes those with normal cognition, mild cognitive impairment and dementia. All contributing centres of NACC were approved from their local Institutional Review Boards and obtained informed consents from their participants prior to participation. We selected participants with neuropathological assessments showing a high likelihood of exhibiting a DLB phenotype,2 namely, those with either (a) diffuse neocortical LB pathology with no, low or intermediate likelihood of AD neuropathologic change18 (Braak tau stage <V19) or (b) limbic LB pathology with no, or low likelihood of AD neuropathologic change (Braak tau stage <III).2 Participants with any other pathologic diagnoses defined in the NACC dataset were excluded (ie, AD, multiple system atrophy, frontotemporal degeneration, other tauopathies, trinucleotide repeat diseases, traumatic brain injury, infections). Participants clinically diagnosed with Parkinson’s disease (PD) were excluded to avoid results being driven by well-described sex differences in PD.20 The inclusion and exclusion criteria provided a sample of 55 women and 156 men from 29 past and present AD research centres in the NACC. Given the more common pure LB pathology in men than women,5,6 men made up the majority of the initial sample. We performed the first set of analyses in this initial sample to avoid selection bias. Subsequently, we performed analysis in a subsample matched for sex and other unequal variables in the initial samples including age, education and Braak tau stage (n=55 for men and women).
CDR Dementia Staging Instrument-Sum of Boxes (CDR-SOB), Neuropsychiatric Inventory-Questionnaire (NPI-Q) and Unified Parkinson’s Disease Rating Scale (UPDRS)-Part III scores at last visit before death were analysed. Clinician report of the DLB core clinical features (dementia, cognitive fluctuations, visual hallucinations, rapid eye movement sleep behaviour disorder (RBD), parkinsonism)2 at any visit during longitudinal data collection was analysed. Although the newer NACC UDS version includes validated forms for DLB core features, such as Mayo Fluctuations Scale for cognitive fluctuations, Noise Pareidolia Test for visual hallucinations, participant and coparticipant forms of Mayo and SCOPA Sleep Scales for sleep problems,15 this UDS version was implemented in 2015, and data collected before this date lack this more systematic evaluation. For the older data, the presence of core features was determined by the clinician based on participant and informant interview and examination without using these scales.
Cognitive status and the related clinical syndrome may be made by a single clinician, a group of clinicians or an ad hoc consensus group (two or more clinicians or other informal group) in the NACC assessments. Normal cognition was defined as (1) no diagnosis of mild cognitive impairment or dementia and (2) global CDR=0 or neuropsychological testing within normal range (or both). Dementia was defined based on the all-cause dementia criteria described by McKhann and colleagues in 2011.21 If the participant did not have normal cognition or behaviour and was not clinically demented, they were deemed as having mild cognitive impairment.22 The predominant cognitive domain that was first recognised to decline is determined by the clinician based on any available information, coparticipant report or the clinician’s best clinical judgement. Clinical diagnoses at the last visit before death were included in our analysis. Clinical diagnoses were made by the clinicians examining the participant based on the available clinical diagnostic criteria for AD (probable and possible), DLB and other cognitive/behavioural syndromes at the date of examination.
LB pathology staging2 and Thal phase (amyloid-β plaque score), Braak tau stage (neurofibrillary tangle stage), Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) score (neuritic plaque score), which were subsequently used to determine levels of AD neuropathological change,18 were available for analysis. Level of substantia nigra neuron loss in the NACC Neuropathology Data Set was also recorded with values ranging from 0 (none) to 3 (severe). Postmortem interval data (time between death and brain removal) was available for 72 participants and ranged from 2 hours to 90 hours, without any sex differences (women mean (SD)=10.3 (8.9) vs men mean (SD)=14.1 (10.6) hours; p>0.17).
Statistics
IBM SPSS V.27.0 (Armonk, New York, USA), R V.4.0.2,23 mice package24 and MatchIt package25 were used for statistical analysis. Multiple imputation by fully conditional specification (Markov chain Monte Carlo) was used for handling missing data (11% of the values were missing for the initial sample, 12.3% missing for the matched sample).26 This method was adopted to overcome loss of precision and power with complete case analysis, to avoid potentially biased estimates with single imputation and to account for missing data in both categorical and continuous variables.26 Because multiple imputation approaches may be problematic in small samples, both raw and imputed data for core DLB features and neuropathology variables are presented. Demographics at last visit and clinical features were compared with χ2 and t-tests as appropriate. Pathological severity scales were compared with Mann-Whitney U tests. Additionally, 55 women were matched 1:1 with 55 men for the covariates consisting of age, education and Braak tau stage using the propensity score matching with the R MatchIt package25 to exclude skewness caused by a male-dominant sample and confounding effects of these variables (demographics and tau burden level), which differed for men and women in the overall sample. Clinical features were compared between these men and women in this matched sample. False discovery rate correction was used for multiple comparisons and q (pFDR-adjusted) <0.05 was considered statistically significant.
RESULTS
Comparison of men and women from the overall sample
Demographics, clinical features and neuropathological features of the overall and matched samples are shown in table 1, figures 1 and 2. There was no difference in McKeith LB stage2 between men and women. Women were significantly older at last visit and time of death (q=0.008, q=0.009) and had a tendency for fewer years of education (q=0.05). Sex of informant was different for women and men (q<0.001); the majority of informants for male participants was women (96.4%), and for female participants, male and female informants were equally represented (men: 47.6%, women: 52.4%).
Table 1.
Demographics, clinical features and pathology findings in the overall (n=211) and matched subsamples (n=110) including the same 55 women
| Women (n=55) | Men overall sample (n=156) | Men-matched sample (n=55) | |
|---|---|---|---|
| Age at last visit | 80.0 (8.7) | 75.9 (8.4) ** | 80.4 (7.9) |
| Age at death | 81.6 (8.5) | 77.4 (8.2) ** | 81.8 (7.9) |
| Length of follow-up, years | 2.9 (3.1) | 2.6 (2.5) | 2.7 (2.7) |
| Interval between last visit and death, months | 19.5 (17.5) | 17.0 (17.3) | 16.8 (18.4) |
| Education, years | 14.6 (3.5) | 15.8 (3.1) | 14.8 (3.5) |
| Cognition at last visit, % | |||
| Cognitively normal | 14.50% | 2.5% * | 7.30% |
| Mild cognitive impairment | 7.30% | 5.8% * | 7.30% |
| Dementia | 78.20% | 91.7% * | 85.50% |
| Core DLB features, pooled % | |||
| Cognitive fluctuations | 48.70% | 67.20% | 56.40% |
| Visual hallucinations | 40 | 64.1% ** | 63.6% * |
| REM sleep behaviour disorder | 29.10% | 56% ** | 51.30% |
| Parkinsonism | 76.40% | 92.2% ** | 86.20% |
| Clinical diagnosis, % | |||
| Dementia with Lewy bodies | 50.90% | 79.7% *** | 74.5% * |
| Alzheimer’s disease | 62.20% | 46.30% | 55.60% |
| Other | 21.80% | 8.30% | 10.90% |
| Age at onset of cognitive decline | 72.6 (8.8) | 68.5 (8.1) ** | 71.9 (8.0) |
| First cognitive change, pooled % | |||
| Memory | 57.10% | 64% | 63.60% |
| Executive function | 7.60% | 14.20% | 11.30% |
| Attention | 8.70% | 6.20% | 7.30% |
| Visuospatial | 6.50% | 5.40% | 5.50% |
| Language | 4% | 4.50% | 7.60% |
| Cognitive fluctuations | 8.40% | 2.80% | 1.80% |
| Orientation | 7.60% | 2.90% | 2.90% |
| CDR—Sum of Boxes | 9.0 (6.5) | 10.5 (5.3) | 10.8 (5.8) |
| NPI-Q | 6.2 (8.8) | 8.8 (8.1) * | 8.1 (6.1) |
| UPDRS-Part III | 21.7 (20.6) | 23.3 (16.9) | 22.6 (18.4) |
| APOE-e4 carrier, pooled % | 45.10% | 49.10% | 50.20% |
| Lewy body pathology, % | |||
| Diffuse (neocortical) | 51 (92.7%) | 146 (93.6%) | 51 (92.7%) |
| Limbic (transitional) | 4 (7.3%) | 10 (6.4%) | 4 (7.3%) |
| Thal phase (amyloid-β plaque score) | 3.2 (1.8) | 3.1 (2.0) | 3.2 (2.4) |
| Braak tau stage (neurofibrillary tangle stage) | 3.2 (1.0) | 2.8 (1.1) * | 3.1 (1.0) |
| CERAD score (neuritic plaque score) | 1.7 (1.0) | 1.6 (1.1) | 1.7 (1.1) |
| Substantia nigra neuronal loss | 2.0 (1.1) | 2.1 (1.0) | 1.8 (1.5) |
Values are reported as mean (standard deviation) orpercentage as appropriate. For variables with missing data, imputed means arereported. Pooled percentages represent the percentages in imputed data. Statisticalsignificance for comparisons to women are bolded and marked with
for q<0.05,
for q<0.01,
for q<0.001.
APOE, Apolipoprotein E; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; DLB, dementia with Lewy bodies; NPI-Q, Neuropsychiatric Inventory-Questionnaire; REM, rapid eye movement; UPDRS, Unified Parkinson’s Disease Rating Scale.
Figure 1.
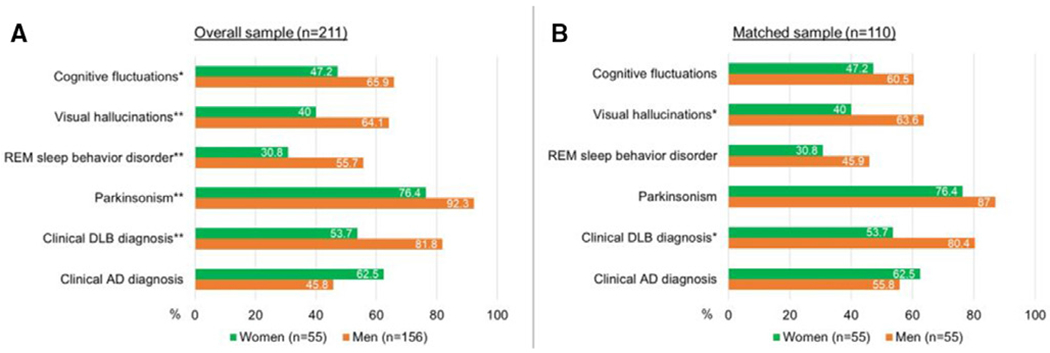
(A) Ratios of participants with core DLB features and clinical diagnoses in the overall sample using raw data (n=211), (B) ratios of participants with core DLB features and clinical diagnoses in the matched subsample using raw data (n=110). Statistical significance for comparisons between women and men are marked with * for q<0.05, ** for q<0.01. Sex difference for clinical AD diagnosis in the overall sample did not reach significance (q=0.05). Analysis with raw data yielded similar findings to the analysis with imputed data except for cognitive fluctuations. AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; REM, rapid eye movement.
Figure 2.
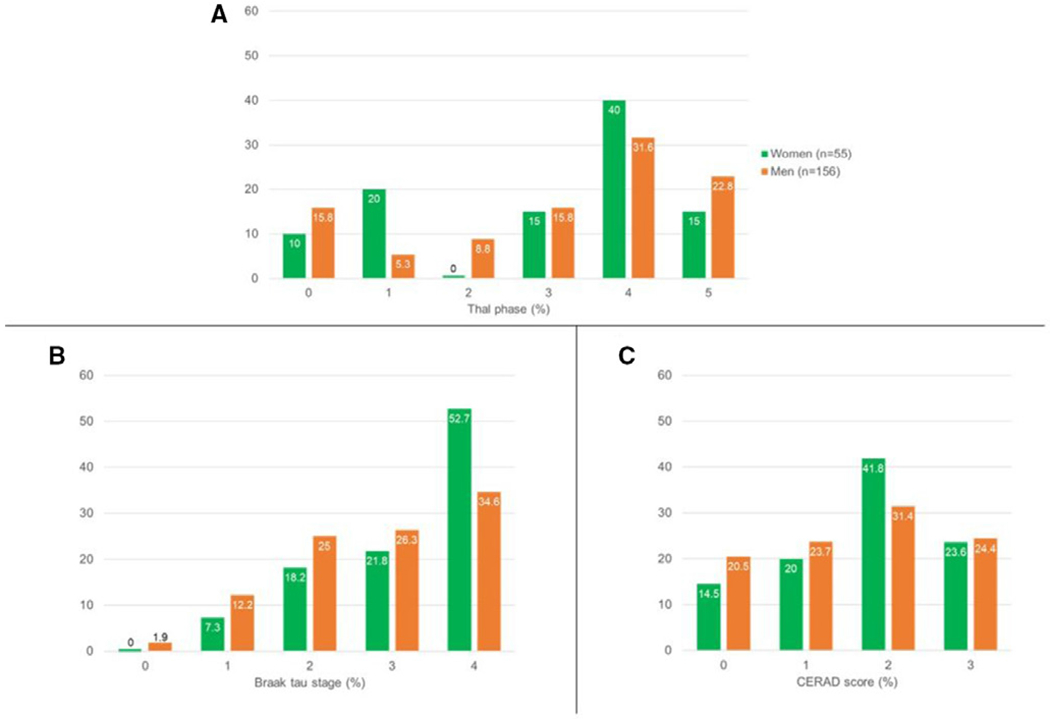
Ratios of participants in each (A) Thal phase (amyloid-β plaque score), (B) Braak tau stage (neurofibrillary tangle stage) and (C) Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) score (neuritic plaque score) in the overall sample (n=211) using raw data.
The majority of participants met clinical diagnostic criteria of probable DLB or AD (n=186, 88.2%), and of these, 56 (30.1%) were assessed as having both DLB and AD by their NACC clinicians at time of death. Of the 25 participants who had neither a diagnosis of DLB nor AD, 7 (28%) had been diagnosed with frontotemporal dementia, 4 (16%) with depression, 1 (4%) with normal pressure hydrocephalus and 1 (4%) with age-related psychomotor slowing. Aetiology could not be clinically determined in 2 (8%) participants, 10 (40%) did not have a clinical aetiological diagnosis.
A greater proportion of men than women met the criteria for dementia (q=0.02). Men were younger at cognitive decline onset (q=0.009). Men and women had similar rates of apolipoprotein E (APOE)-e4 alleles and reported similar types of initial cognitive changes with memory being the most commonly reported domain in clinician-filled forms. Men and women had similar degrees of motor impairment (UPDRS-Part III) and CDR-SOB scores at last visit (table 1). Compared with men, women had less severe behavioural disturbances as assessed by NPI-Q scores at their last visit (q=0.02). Compared with men, fewer women had visual hallucinations (q=0.009), RBD (q=0.007), or parkinsonism (q=0.007); and accordingly, fewer women met the clinical criteria for probable DLB (q<0.001). There was a trend towards fewer women having cognitive fluctuations (q=0.07) and a trend for women to be more likely to meet clinical criteria for AD during life (q=0.09). Braak tau stage was higher in women (q=0.03), while Thal phase, CERAD score and degree of substantia nigra neuronal loss were similar for men and women (q>0.49 for all).
Comparison of men and women from the matched sample
Because the original cohort showed differences between men and women in education, age at last visit and death, and Braak tau stage, we undertook subsequent analysis using a subset of subjects from the cohort above matched on these features (women n=55, men n=55). In this matched set, women and men had similar cognitive status at last visit, age at onset of cognitive decline, first cognitive domain to change, CDR-SOB, NPI-Q and UPDRS-Part III scores at last visit (table 1, q>0.60 for all). Sex of informant remained different for female and male participants (q<0.001) (female informant 94.5% for men, 52.7% for women). Sex of informant was not significantly associated with cognitive status, core features, clinical diagnosis, CDR-SOB or NPI-Q scores that require informant input (q>0.34 for all).
In the matched sample, men and women had similar rates of cognitive fluctuations, RBD and parkinsonism (q>0.29 for all) (table 1, figure 1B). Women still had a lower likelihood of having visual hallucinations than men (q=0.046) and remained less likely to meet clinical criteria for probable DLB (q=0.049). Clinical AD diagnosis rates were similar for men and women (q=0.76).
DISCUSSION
Using a large autopsy series of well-characterised participants with pathological findings indicating a high likelihood of manifesting a DLB phenotype, this study showed that despite older age, lower education and more severe tau, women were less likely to have a diagnosis of dementia. Women were also less likely to exhibit core DLB features, were older at onset of cognitive decline and had less severe behavioural disturbances. Accordingly, women were less likely to meet clinical diagnostic criteria for probable DLB and greater proportions of women were clinically diagnosed with AD despite underlying LB pathology. Despite women being less likely to have dementia or parkinsonism, when present, severity of dementia and motor impairment (determined by CDR-SOB and UPDRS-Part III scores) did not differ from men. In PD, women may have greater disease severity than men despite similar disease duration.27 Our findings may suggest that severity of decline is similar or even worse in women who present with these symptoms compared with men with DLB, and it warrants future investigation with a specific focus on the severity of symptoms. Nevertheless, the wide variability for the scores in our sample limits the reliability of our interpretation. In AD, memory performance is better in women up until reaching a Braak tau stage of 5, and after this stage, memory performance is similar for men and women.13 A similar instance can occur in those with LB pathology, while women bear a higher pathological burden before developing symptoms, severity can become equal or surpass the level in men after a certain level of pathological burden. We also performed analyses in a matched sample to specifically investigate the sex differences potentially associated with LB pathology. After matching for age, education and Braak tau stage, we observed similar ratios of dementia, clinical AD misdiagnosis, cognitive fluctuations, RBD and parkinsonism for men and women. Age at cognitive decline and level of behavioural disturbance became similar for men and women as well, suggesting age-related factors and tau pathology association with phenotype in the initial sample despite inclusion of individuals with lower tau burden. Age can impact the presence and severity of pathological burden28 and clinicopathological correlations can differ by age.29 In fact, in some studies, older adults with and without dementia can have similar neuropathological burden.29 Thus, higher ratio of older men in the matched sample compared with the overall sample with younger men may have led to the disappearance of several significant sex differences due to age-related factors. The number of participants meeting clinical criteria for probable DLB (dementia with at least two core features)2 continued to be significantly lower for women, which can partially explain the male predominance for DLB prevalence reported in some DLB studies7–9 and raise questions about the accuracy of clinical diagnostic criteria in predicting underlying pathology especially in women.
Previous studies have shown women to be less likely to have pure LB pathology than men in a cohort of over 1500 community-dwelling older people5 and 3830 participants in the University of Kentucky AD Center and NACC database.6 In our study, we aimed to instead determine whether pure LB pathology on its own is associated with phenotypic differences between men and women. Our findings suggest that LB pathology may not have the same clinical impact in women as men. Despite high likelihood of typical DLB phenotype based on underlying LB pathology with low AD pathology staging, only half of the women met the clinical diagnostic criteria for DLB. Core features of DLB in the NACC UDS are based on participant, informant and clinician report, which can be less reliable than more objective measures (eg, noise pareidolia test for visual hallucinations, polysomnography for RBD). Validated forms for DLB core features, such as the Mayo Fluctuations Scale, Noise Pareidolia Test, Mayo and SCOPA Sleep Scales and neuroimaging findings (including DaTscans) that are included in the NACC UDS version implemented in 2015,15 can help with the clinical differentiation. However, we were unable to investigate the sex differences in these forms in our study given that only 7.6% (n=16) of our total sample had a clinical visit after 2015. Sex differences found in our sample may also change with the inclusion of supporting features and indicative biomarkers from the current diagnostic criteria2 as well as novel biomarkers. Informant’s sex was different for men and women, which can impact the findings while relying on informant report. Nonetheless, our analysis showed that informant’s sex was not associated with the presence or absence of core features, and, thus, our findings on sex differences for the core features are likely not driven from the difference for the informants’ sex.
Both women and men having a clinical diagnosis of AD despite underlying LB-dominant pathology emphasise the low clinical diagnostic accuracy in DLB3 and the importance of developing reliable biomarkers and predictors to differentiate the underlying LB and AD pathologies in a clinical setting. Cognitive profiles were not investigated in detail in our study and future investigations with detailed neuropsychological assessments can determine differences between those with accurate AD diagnosis and AD misdiagnosis. Neuroimaging (eg, 123I-FP-CIT SPECT, 123I-MIBG myocardial scintigraphy, positron emission tomography with novel amyloid and tau ligands) as well as biofluid biomarkers (eg, α-synuclein RT-QuIC in the cerebrospinal fluid, proteome of the plasma-derived extracellular vesicles) can help differentiate LB and AD pathology during life. Women being more likely to have a clinical AD diagnosis than a clinical DLB diagnosis despite underlying pathology in our study further emphasises the sex differences for clinicopathologic correlations and the need for sex-specific biomarkers for differentiation. Additionally, LB and AD pathologies frequently co-occur4 and the value of these biomarkers to determine the primary and secondary pathologies for dementia is yet to be clarified.
Sex differences for DLB symptoms have been reported by studies, which included clinical samples of over 150 probable DLB participants without pathological confirmation.30–32 These previous reports showed higher proportions of men with RBD and parkinsonism32 similar to our study, and higher proportions of women with visual hallucinations.30,31 However, in our study, visual hallucinations were the only core feature remaining to have a lower frequency in women than men after matching for age, education and Braak tau stage. This discrepancy is likely due to our inclusion of participants based on pathology rather than clinical diagnosis alone. Complex, well-formed and recurrent visual hallucinations occurring in early stages of dementia can be used to differentiate DLB from AD, as visual hallucinations are less common in mild early AD.33–35 These observations suggest that the accurate clinical diagnosis of DLB in women will continue to be challenging in the potential absence of core features. Visual hallucinations in DLB have been shown to be associated with more LB pathology in the temporal lobe36; however, the NACC database provides limited information regarding regional pathologic burden and future detailed neuropathologic studies will be needed to further clarify the relationship of regional pathologic burden, clinical features and sex. On the other hand, we excluded those with a clinical diagnosis of PD to avoid the results being driven by sex differences in PD20 and to focus on the sex differences in DLB that have not been studied as much. However, the differentiation between PD and DLB is based on the onset of dementia in comparison to parkinsonism, and this differentiation is done clinically, not pathologically.2 Our exclusion criteria may have led to the exclusion of those with pure LB pathology that had a high likelihood for a DLB phenotype. A future direction is to assess sex differences in LB dementias, including both PD dementia and DLB, by defining the sample solely by neuropathology. Our findings underscore the relevance of sex for phenotypic differences across those with LB pathology, and future studies are needed to expand on these sex differences.
Copathologies are common in DLB and impact symptoms.4 In AD, tau is thought to more strongly associate with cognitive dysfunction than amyloid β.37 Similarly, tau has been shown to be associated with cognitive profile and behavioural symptoms for participants with LB pathology.38–40 Despite having higher level of tau burden, older age and less education compared to men, women had lower rates of dementia. This finding suggests that women may be more resilient to tau pathology than men, which is similar to reports in AD where women are also more resilient to tau pathology.13 This is perhaps due to inclusion of participants with only low or intermediate Braak tau stages, as women were shown to withstand tau burden within lower Braak tau stages better than men in AD.13 Assessment of cohorts including participants with higher degrees of tau pathology may be needed to more fully understand the relationship between tau and cognition in women in DLB; however, we chose to study those subjects harbouring neuropathologic changes that were highly likely to manifest as typical DLB to better understand the presentation of those clinical features in particular. Genetic risk factors for DLB, such as frequency of GBA mutations,41 gene expressions in the substantia nigra42 and environmental factors,43 differ for men and women and may explain the different effects of pathology for women and men with DLB. Future studies should assess the combined effect of genetic and environmental factors on the clinicopathologic correlations in men and women. Determining such sex-specific effects can guide future efforts for precision medicine.
There are some limitations to this study. There was a small number of participants with limbic or neocortical stage LB pathology without cognitive impairment. These cases could be indicative of incidental LB disease, which has been reported in 30%–40% in certain autopsy series44,45 and could represent prodromal PD or DLB. It is possible that subtle motor or cognitive changes were missed or not reported by participants or informants or emerged in the interval between last assessment and death. Given our inclusion criteria based on neuropathology, but not clinical status, these participants were included. We do not believe that the inclusion of these asymptomatic participants significantly change the findings here as they constitute a small minority of the cohort (n=12, 5.7%). To allow assessment of phenotype associated with pure LB pathology, we excluded participants with higher AD neuropathological staging, limiting the range of tau burden. Thus, the higher tau staging in women should be interpreted cautiously and future studies are needed. There is an inevitable time interval between last clinical assessment and neuropathological assessment. This interval was relatively short, however, approximately 18 months for the cohort, and did not differ for men and women. Variable length of follow-up may also limit the interpretation of our findings, although this also did not differ for men and women. For our analysis, we did not take into account history of the drugs being taken for motor, behavioural and cognitive symptoms. Symptom presence and severity can be affected by various drugs and this was not assessed in our study, which could be a focus of future studies. Symptom presence based on participant and informant report, lack of data on clinical DLB supporting features, biomarkers and imaging also limited our analysis but represent standard procedure for the NACC database at that time. Current efforts, including by the DLB Consortium in the US and Europe, to develop DLB focused data sets will provide more insight into sex differences and their underlying aetiology. Pathological assessment recorded in NACC is limited by use of more traditional approaches that largely rely on topographic spread of pathology rather than regional severity, which precludes more finely grained comparisons of regional pathology between sexes and its impact on clinical features. Future studies, of the regional pathologic burden, will be needed to further explore pathological differences across men and women in DLB. The NACC is a rich resource, but there are inherent limitations to retrospective research with multisite datasets. Participants are best regarded as a referral-based or volunteer case series and are not representative of the general population (see naccdata.org for more information). Currently, the NACC database contains clinical data of 43 517 participants (42.8% men, 57.2% women) and neuropathological data of 6416 participants (53.9% men, 46.1% women) (see naccdata.org for up-to-date numbers). We are unable to determine whether this is a sex difference for signing up for a research study such as NACC and signing up for autopsy among the dementia population. Such sex differences may impact the outcomes in research studies and limit the generalisation of the findings. In addition, over 73% of the participants in the NACC database have more than 12 years of education and over 79% are White, which further limit the generalisation of the findings. Efforts to recruit participants across different races and with different levels of education will allow making more reliable assumptions about the dementia population.
In conclusion, these results suggest that women with autopsy-confirmed significant LB pathology are clinically underdiagnosed with DLB. These women are often instead clinically diagnosed with AD because of a lower likelihood of manifesting core DLB features, which may explain the lower prevalence of clinical DLB in women. There may be a need for sex-specific diagnostic criteria given these significant phenotypic differences across the sexes. Our findings emphasise the importance of neuropathological confirmation of clinical diagnosis to improve our understanding of DLB and increase diagnostic accuracy during life and to promote appropriate enrolment of women participants into disease-modifying clinical trials. Determining sex differences in DLB can help elucidate pathophysiology, consequently leading to development of agents for prevention and treatment for DLB, a dementia type with a substantial burden on the affected individuals, caregivers and healthcare.
Acknowledgements
Data used in this study were obtained from the National Alzheimer’s Coordinating Center (NACC) database. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG062428-01 (PI James Leverenz, MD) P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P30 AG062421-01 (PI Bradley Hyman, MD, PhD), P30 AG062422-01 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P30 AG062429-01(PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P30 AG062715-01 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests
DGC: supported by the American Academy of Neurology, American Brain Foundation, and the Parkinson’s Foundation Clinical Research Training Scholarship in Parkinson’s disease (2059) and by NIA P30AG 062429. SJB: consulting relationship with Boston University on the DIAGNOSE CTE project (U01NS093334); and research funding from the NIA/NIH (R01AG066088), Alzheimer’s Association and California Department of Public Health.IL: supported by the National Institutes of Health grants: 2R01AG038791-06A, U01NS090259, U01NS100610, U01NS80818, R25NS098999, P20GM109025; U19 AG063911-1; 1R21NS114764-01A1; Michael J Fox Foundation, Lewy Body Association, Abbvie, Biogen, Centogene, Roche, EIP-Pharma and Biohaven Pharmaceuticals. She was member of a Lundbeck Advisory Board. She receives her salary from the University of California San Diego and is the Chief Editor of Frontiers in Neurology.
Footnotes
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data were made available to the authors by the NACC database and may be obtained following the approval of a data request to the NACC database.
REFERENCES
- 1.Hogan DB, Fiest KM, Roberts JI, et al. The prevalence and incidence of dementia with Lewy bodies: a systematic review. Can J Neurol Sci 2016;43 Suppl 1:S83–95. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzo G, Arcuti S, Copetti M, et al. Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2018;89:358–66. [DOI] [PubMed] [Google Scholar]
- 4.Coughlin DG, Hurtig HI, Irwin DJ. Pathological influences on clinical heterogeneity in Lewy body diseases. Mov Disord 2020;35:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes LL, Lamar M, Schneider JA. Sex differences in mixed neuropathologies in community-dwelling older adults. Brain Res 2019;1719:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson PT, Schmitt FA, Jicha GA, et al. Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol 2010;257:1875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kane JPM, Surendranathan A, Bentley A, et al. Clinical prevalence of Lewy body dementia. Alzheimers Res Ther 2018;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savica R, Grossardt BR, Bower JH, et al. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol 2013;70:1396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fereshtehnejad S-M, Religa D, Westman E, et al. Demography, diagnostics, and medication in dementia with Lewy bodies and Parkinson’s disease with dementia: data from the Swedish dementia quality registry (SveDem). Neuropsychiatr Dis Treat 2013;9:927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouton A, Blanc F, Gros A, et al. Sex ratio in dementia with Lewy bodies balanced between Alzheimer’s disease and Parkinson’s disease dementia: a cross-sectional study. Alzheimers Res Ther 2018;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price A, Farooq R, Yuan J-M, et al. Mortality in dementia with Lewy bodies compared with Alzheimer’s dementia: a retrospective naturalistic cohort study. BMJ Open 2017;7:e017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes LL, Wilson RS, Bienias JL, et al. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 2005;62:685–91. [DOI] [PubMed] [Google Scholar]
- 13.Digma LA, Madsen JR, Rissman RA, et al. Women can bear a bigger burden: ante- and post-mortem evidence for reserve in the face of tau. Brain Commun 2020;2:fcaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besser LM, Kukull WA, Teylan MA, et al. The revised national Alzheimer’s coordinating center’s neuropathology Form-Available data and new analyses. J Neuropathol Exp Neurol 2018;77:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord 2018;32:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s coordinating center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord 2007;21:249–58. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC, Weintraub S, Chui HC, et al. The uniform data set (UDS): clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis Assoc Disord 2006;20:210–6. [DOI] [PubMed] [Google Scholar]
- 18.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerri S, Mus L, Blandini F. Parkinson’s disease in women and men: what’s the difference? J Parkinsons Dis 2019;9:501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 2005;62:1160–3. [DOI] [PubMed] [Google Scholar]
- 23.R Core Team. R: a language and environment for statistical computing, 2020. Available: https://www.r-project.org/
- 24.Buuren Svan, Groothuis-Oudshoorn K. mice : Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 25.DE H, Imai K, King G. MatchIt : Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw 2011;42. [Google Scholar]
- 26.Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res 2015;4:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahodwala N, Pei Q, Schmidt P Sex differences in the clinical progression of Parkinson’s disease. J Obstet Gynecol Neonatal Nurs 2016;45:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018;141:2181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: the 90+ study. Curr Alzheimer Res 2012;9:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu P-Y, Teng P-R, Wei C-Y, et al. Gender difference in the association and presentation of visual hallucinations in dementia with Lewy bodies: a cross-sectional study. Int J Geriatr Psychiatry 2018;33:193–9. [DOI] [PubMed] [Google Scholar]
- 31.van de Beek M, Babapour Mofrad R, van Steenoven I, et al. Sex-Specific associations with cerebrospinal fluid biomarkers in dementia with Lewy bodies. Alzheimers Res Ther 2020;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Utsumi K, Fukatsu R, Yamada R, et al. Characteristics of initial symptoms and symptoms at diagnosis in probable dementia with Lewy body disease: incidence of symptoms and gender differences. Psychogeriatrics 2020;20:737–45. [DOI] [PubMed] [Google Scholar]
- 33.Apostolova LG, Di LJ, Duffy EL, et al. Risk factors for behavioral abnormalities in mild cognitive impairment and mild Alzheimer’s disease. Dement Geriatr Cogn Disord 2014;37:315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry 2014;171:572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment. JAMA 2002;288:1475. [DOI] [PubMed] [Google Scholar]
- 36.Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain 2002;125:391–403. [DOI] [PubMed] [Google Scholar]
- 37.Giannakopoulos P, Herrmann FR, Bussière T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003;60:1495–500. [DOI] [PubMed] [Google Scholar]
- 38.Coughlin D, Xie SX, Liang M, et al. Cognitive and pathological influences of tau pathology in Lewy body disorders. Ann Neurol 2019;85:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 2003;60:1586–90. [DOI] [PubMed] [Google Scholar]
- 40.Peavy GM, Edland SD, Toole BM, et al. Phenotypic differences based on staging of Alzheimer’s neuropathology in autopsy-confirmed dementia with Lewy bodies. Parkinsonism Relat Disord 2016;31:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gámez-Valero A, Prada-Dacasa P, Santos C, et al. Gba mutations are associated with earlier onset and male sex in dementia with Lewy bodies. Mov Disord 2016;31:1066–70. [DOI] [PubMed] [Google Scholar]
- 42.Mariani E, Lombardini L, Facchin F, et al. Sex-specific transcriptome differences in substantia Nigra tissue: A meta-analysis of parkinson’s disease data. Genes 2018;9:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement 2018;14:1171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaccai J, Brayne C, McKeith I, et al. Patterns and stages of alpha-synucleinopathy: relevance in a population-based cohort. Neurology 2008;70:1042–8. [DOI] [PubMed] [Google Scholar]
- 45.Wakisaka Y, Furuta A, Tanizaki Y, et al. Age-Associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol 2003;106:374–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data were made available to the authors by the NACC database and may be obtained following the approval of a data request to the NACC database.


