Abstract
(a) Background.
Morningness-eveningness refers to interindividual differences in preferred timing of behavior (i.e., bed- and waketimes). Older people have earlier waketimes and rate themselves as more morning-like than young adults. It has been reported that the phase of circadian rhythms is earlier in morning-types than in evening types, and that older people have earlier phases than young adults. These changes in phase have been have been considered to be the chronobiological basis of differences in preferred bed- and waketimes and age-related changes therein. Whether such differences in phase are associated with changes in the phase relationship between endogenous circadian rhythms and the sleep-wake cycle has not been investigated previously.
(b). Methods.
We investigated the association between circadian phase, the phase relationship between the sleep-wake cycle and circadian rhythms, and morningness-eveningness, and their interaction with aging in 68 young and 40 older subjects who participated in a circadian rhythm study and completed a morningness-eveningness questionnaire.
(c). Results.
Among the young subjects, the phase of the melatonin and core temperature rhythms occurred earlier in morning than in evening types and the interval between circadian phase and usual waketime was longer in morning types. Thus, while evening types woke at a later clock hour than morning types, morning types actually woke at a later circadian phase.
Comparing young and older morning types we found that older morning types had an earlier circadian phase and a shorter phase-waketime interval. The shorter phase-waketime interval in older “morning types” is opposite to the change associated with morningness in young people, and is more similar to young evening types.
(d) Conclusions.
These findings demonstrate an association between circadian phase, the relationship between the sleep-wake cycle and circadian phase, and morningness-eveningness in young adults. Furthermore, they demonstrate that age-related changes in phase angle cannot be attributed fully to an age-related shift toward morningness. These findings have important implications for understanding individual preferences in sleep-wake timing and age-related changes in the timing of sleep.
Keywords: morningness, eveningness, constant routine, aging, circadian rhythms
INTRODUCTION
The endogenous circadian pacemaker has been shown to contribute to daily variations in a number of physiologic and behavioral/psychological rhythms1–5. Several investigations of individual differences in circadian rhythms have focused on the morningness/eveningness dimension. So-called “morning types” show a marked preference for waking at an early hour and find it difficult to remain awake beyond their usual bedtime, while those at the other end of the morningness/eveningness dimension, “evening types”, show a preference for sleeping at later hours and often find it difficult to get up in the morning. Furthermore, there have been reports that older people are more likely to rate themselves as more morning-like6–10, a finding thought to be related to the earlier preferred waketimes among older people7;10.
Morning and evening type individuals were investigated experimentally as early as the 1930’s by Nathaniel Kleitman, who compared performance on a number of tasks between self-described morning or evening type subjects5; [see11;12 for review]. A number of more recent attempts to classify individuals according to morningness/eveningness11;13–15 were focused on determining individual suitability for night or shift work, after it was reported that evening type individuals were better suited for night work due to their greater ability to sleep during the day16. Daily rhythms of many physiologic variables have been reported to occur at an earlier time of day in morning types than in evening types, including those of oral temperature17–23, heart rate15;23, adrenaline excretion24;25, and salivary cortisol26 and melatonin27 levels. In addition to preferring different sleep and wake times, morning and evening types have been reported to show differences in sleep, including sleep latency, REM latency, EEG power density, and the ability to sleep at other than their habitual times28–33. The timing and daily pattern of behavioral and performance rhythms have also been shown to differ between morning and evening types9;15–17;19;23;25.
Despite reports that many daily rhythms of performance and behavior show differences between morning and evening types, many studies collected data infrequently or did not collect data throughout a complete circadian cycle. Thus, accurate estimation of rhythm acrophase or nadir was difficult. Furthermore, a number of studies collected data under ambulatory conditions, in which activity, posture, sleep or food intake can obscure the endogenous component of underlying endogenous rhythms. Under such experimental conditions, it is difficult to ascertain whether the earlier timing of rhythms in morning-type subjects is due to a direct effect of sleeping and waking at earlier times of day, or whether an underlying biological difference leads morning types to sleep and wake at an earlier time of day.
In the present analysis we used the constant routine technique34–36 to unmask the endogenous component of the core temperature rhythm of young and older subjects in order to investigate the relationship between this established marker of the circadian system, and habitual sleep-wake behavior and morningness/eveningness. Morningness-Eveningness was determined using the English language Morningness-Eveningness Questionnaire developed by Horne and stberg21. In a subset of young subjects who were identified as morning or evening types, a second marker of the endogenous circadian pacemaker, plasma melatonin, was analyzed.
MATERIALS AND METHODS
Subjects.
68 healthy men between the ages of 18 and 30 (mean age ⩝ SD = 23.5 ⩝ 3.4 years) and 40 healthy men and women between the ages of 64 and 81 (68.2 ⩝ 3.8 years) who participated in studies in our laboratory between 1990 and 1996 were included in the present analysis. Subjects had no medical, psychiatric, or sleep disorders as determined from their medical history, physical examination, electrocardiogram, biochemical screening tests on their blood and urine, and psychological screening questionnaires (the Minnesota Multiphasic Personality Inventory and the Beck Inventory). All were free from medications as assessed by urine toxicologic analysis for alcohol, nicotine, caffeine, and common drugs of abuse carried out upon admission to the laboratory.
To minimize potential effects of irregular schedules on entrained circadian phase, only subjects who denied performing regular night work during the three years prior to study and transmeridien travel in the three months prior to study were included. Furthermore, each subject was required to choose a target bedtime and waketime that would result in an 8-hour sleep period, and to adhere to this self-selected schedule for at least three weeks prior to admission to the laboratory. The subjects were asked to record their actual sleep and wake times, and all reported regular bedtimes and waketimes (± 1 hour) for at least one week immediately prior to their study. Those self-reported sleep-wake times were verified by wrist activity monitoring (PMS-8 Recorder, Vitalog Monitoring, Inc., Redwood City, CA). Subjects whose self-reported sleep-wake times differed significantly from the times recorded by the activity monitor or who did not report maintaining a regular schedule were disempaneled from study prior to admission to the laboratory.
As part of their pre-study screening, each subject completed a modified version of Horne and ⤡stberg’s Morningness-Eveningness Questionnaire21.
Study protocol.
Each study began with three baseline days and nights in the laboratory, under normal room light conditions (~150 lux ambient light during the day, and <0.02 lux during scheduled bedrest episodes). Each subject was scheduled to sleep for eight hours per night according to his or her sleep-wake diary from the week prior to entering the laboratory. Upon awakening after the third baseline night of sleep, subjects began a constant routine (CR) which lasted from 26 to 45.5 hours. The CR used was a modification35;36 of the method first proposed by Mills et al.34, and was designed to unmask the endogenous component of circadian rhythms. During the constant routine, subjects were kept awake in a semi-recumbent posture in low level room light (10–15 lux), and were fed a diet of equivalent hourly snacks. A trained technician attended the subjects during the constant routine to ensure adherence to the protocol. All studies continued after the CR, but only data from the baseline days until the end of the CR were included in the present analysis.
The protocols were approved by the Human Research Committee of the Brigham and Women’s Hospital, and each subject gave signed informed consent prior to study.
Data collection.
During the baseline period and the CR, core body temperature was collected each minute from a rectal thermistor (Yellow Springs Instrument Company, Yellow Springs, OH), and from many subjects plasma samples were collected hourly via an indwelling catheter inserted into a forearm vein. The samples were frozen at −20EC and were later assayed for melatonin using radioimmunoassay kits (Elias USA, Osceola, WI) or by the method of Arendt et al.37.
Data Analysis.
After each study was complete, the subject’s Morningness-Eveningness Questionnaire was scored according to the criteria of Horne and ⤡stberg21.
The average habitual bedtime and waketime for each subject was calculated from the self-recorded sleep log using the seven nights immediately prior to entering the laboratory, and the average habitual sleep length was calculated as the elapsed time from average habitual bedtime to average habitual waketime.
Phase of the core body temperature rhythm minimum (CBTmin) was estimated by the maximum likelihood fit of a 2-harmonic regression model with first-order autoregressive noise fit to the CR temperature data38. Phase of the melatonin rhythm for the subset of young subjects identified as morning or evening types was estimated as the midpoint between the upper and lower mean crossing of the data from the CR. Phase angle was defined as the difference between the phase of the temperature or melatonin rhythm and the habitual waketime.
Average waveforms of temperature and melatonin were compiled with respect to actual clock hour. To do this, all data for each subject were averaged per hour beginning on the morning of the CR, and these hourly average values per subject were then averaged across all subjects in each group (morning and evening types). In order to account for interindividual differences in mean temperature and melatonin, and differences in usual time of awakening, average waveforms of temperature and melatonin z-scores were compiled with respect to the habitual waketime of each subject. To do this, all data for each subject were first expressed relative to their own mean (= 0) and standard deviation (= 1). Then the data for each individual were averaged hourly beginning at waketime on the morning of the CR, and these hourly average values per subject were averaged across all subjects in each group.
Comparisons between young and older subjects, and between morning and evening types, were performed using unpaired Student’s t-test, after verification of variance homogeneity. All statistical analyses were two-tailed. Correlation analysis was performed using Pearson’s correlation coefficient. The SAS System was used for data analysis (SAS Institute, Inc., Cary, NC). Results are presented as mean ⩝ SD unless otherwise indicated.
RESULTS
Overall Results.
Average Morningness-Eveningness score for the 108 subjects was 54.1 ⩝ 9.9. A comparison between the young and older groups of subjects revealed a significant difference in Morningness-Eveningness score (young: 49.6 ⩝ 8.3; old: 61.8 ⩝ 7.6; p < 0.00001). Among the young subjects, the distribution of Morningness-Eveningness types centered around the intermediate type (see Figure 1), while among the older subjects the distribution of Morningness-Eveningness centered around the moderate morning type with only one evening type among the 40 older subjects (see Table 1). Although there were moderate morning and evening types in the young population, there were no definite morning or evening types. In contrast to the young subjects, none of who were definite types of either category, 12.5% of the older subjects were definite morning types.
Figure 1.
Distribution of scores on the Morningness-Eveningness Questionnaire among the 68 young men (upper panel) and the 40 older men and women (lower panel). As indicated on the lower axis, individuals scoring 41 or below are classified as evening types and those scoring above 59 are classified as morning types 21.
TABLE 1.
Distribution of Types on the Morningness-Eveningness Questionnaire by Age.
| Young | Old | |||
| Definite | ||||
| Morning | 0 | 5 | (12.5%) | |
| Moderate | ||||
| Morning | 10 | (15%) | 24 | (60%) |
| Intermediate | 45 | (66%) | 10 | (25%) |
| Moderate | ||||
| Evening | 13 | (19%) | 1 | (2.5%) |
| Definite | ||||
| Evening | 0 | 0 | ||
For the subjects as a whole, the Morningness-Eveningness score was negatively correlated with habitual waketime (r = −0.69, p < 0.0001), habitual bedtime (r = −0.64, p < 0.0001), habitual sleep length (r = −0.21, p < 0.03), and endogenous circadian phase of the core body temperature rhythm (CBTmin; r = −0.42, p < 0.0001; see Figure 2). Separate correlation analyses on the young and older groups revealed a significant correlation between score on the Morningness-Eveningness questionnaire and habitual waketime and bedtime in both groups (p < 0.01). However, there was no longer a significant correlation between Morningness-Eveningness score and habitual sleep length or age within either group. Furthermore, while the correlation between Morningness-Eveningness score and CBTmin remained significant in the young group (r = −0.47, p < 0.0001), it did not reach significance for the older group (r = −0.27, p = 0.087).
Figure 2.
Score on the Morningness-Eveningness Questionnaire vs. circadian phase of the core body temperature minimum (CBTmin) for the 108 subjects. Filled squares: young subjects; open squares: older subjects. A significant correlation was found between morningness-eveningness score and CBTmin when all subjects were considered together. This correlation remained significant when young subjects were analyzed separately, but did not reach statistical significance when the older subjects were analyzed separately (see text for details).
Comparison of Morning and Evening Types.
Comparisions between morning and evening types could only be done using young subjects because there was only one evening type identified in the older group. Among the 68 young subjects, 10 were moderate morning types and 13 were moderate evening types. Habitual waketime and bedtime were earlier for the morning types than for the evening types (waketime: 07:52 ⩝ 0:44 vs. 09:08 ⩝ 1:03, p < 0.004; bedtime: 23:42 ⩝ 1:09 vs. 24:52 ⩝ 0:48, p < 0.01), although habitual sleep length was not significantly different (8.16 ⩝ 0.54 hours vs. 8.27 ⩝ 0.42 hours, p = 0.6). The time of both the CBTmin and the endogenous melatonin maximum was significantly earlier in the morning types than in the evening types (CBTmin: 04:34 ⩝ 1:30 vs. 06:52 ⩝ 1:19, p < 0.001; melatonin maximum: 03:33 ⩝ 0:37 vs. 05:16 ⩝ 1:08, p < 0.001; see Figure 3).
Figure 3.
Clock hour of endogenous circadian phase in young morning and evening types. The time of the endogenous temperature minimum (circles) and the melatonin maximum (triangles) was significantly later in the 13 evening types than in the 10 morning types.
Analysis of the average constant routine temperature curves for the young morning and evening types revealed that the average core temperature of the morning types was approximately 0.2EC higher than that of the evening types throughout the morning hours, reached a plateau by the early afternoon, began to decline by 8 pm, reached a nadir between 3 and 5 am, and then began to rise sharply by 6 am on the second morning of the CR (see Figure 4). In contrast, the average temperature curve of the evening types did not reach a plateau until approximately 8 pm, had a narrower crest, and did not begin to decline until approximately 11 pm. After reaching a nadir at approximately 8 am on the second morning of the CR, the temperature of the evening types began to rise, although not as steeply as did that of the morning types.
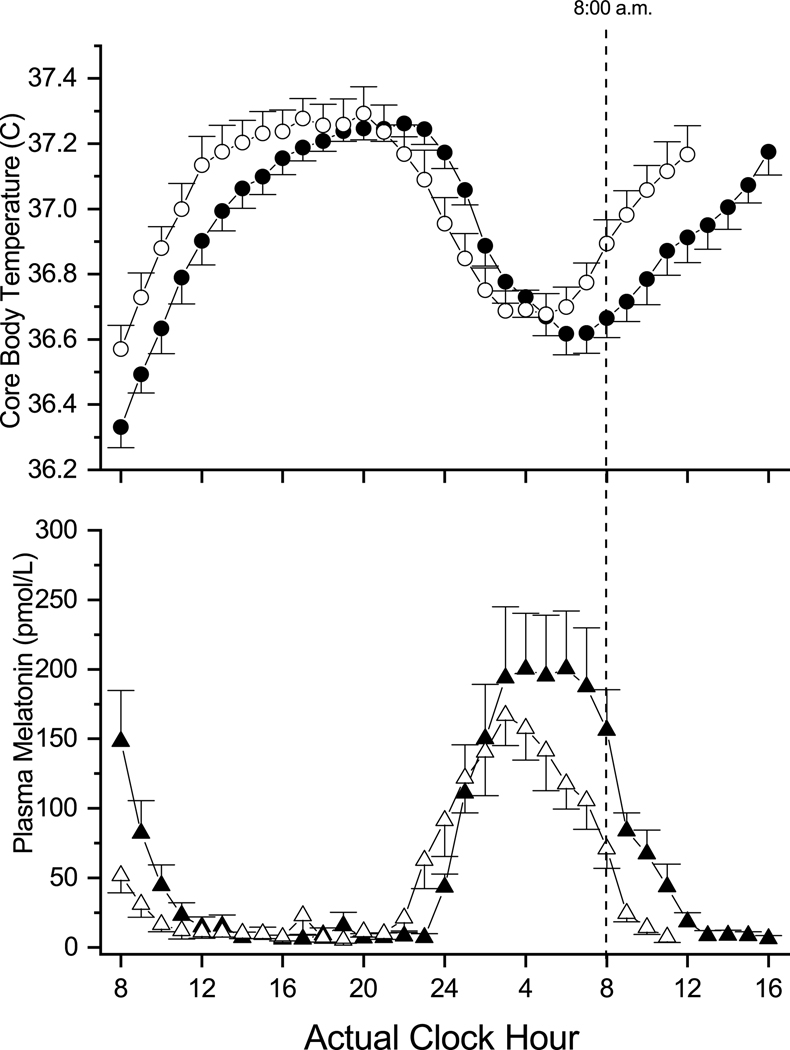
Figure 4.
Temperature and melatonin curves of morning and evening types from a constant routine (CR) averaged with respect to time of day. Upper panel: average endogenous temperature curves of morning (open circles) and evening (filled circles) types during the CR. Lower panel: average plasma melatonin curves of morning (open triangles) and evening (filled triangles) types during the CR. Average curves were complied with respect to actual time of day. The average hourly value for each individual was first calculated, and those hourly values were then averaged across subjects for each of the two groups.
The average melatonin curves were also different between the morning and evening types. The average plasma melatonin level of the morning types began to rise between 10 and 11 pm, peaking between 1 and 3 am. At that point, average plasma melatonin levels began to decline, reaching basal levels by 9 am on the second morning of the CR. In contrast, average melatonin levels of the evening types began to rise at approximately midnight and reached peak levels from 3 to 7 am. The average peak in the evening types was broader than in the morning types. The average plasma melatonin levels of the evening types began to decline at 7 am on the second morning of the CR, and did not reach basal levels until approximately noon, in contrast to that of the morning types which reached basal levels by 9 am.
When the average temperature and melatonin curves of the morning and evening types were expressed as z-scores and aligned with respect to each subject’s habitual waketime, the curves were quite similar throughout the first 12–14 hours of the CR, corresponding to the habitual waking day (see Figure 5). Just prior to the usual bedtime hours, differences in average temperature and melatonin curves between the two groups became apparent. The average temperature curves of the two groups began to diverge 2–3 hours before habitual bedtime, with the temperature of the morning types declining earlier and rising again earlier with respect to habitual bed- and waketime, and remaining higher throughout the second morning of the CR. The average melatonin curve of the morning types was also slightly phase-advanced with respect to the evening types, despite that the data were aligned with respect to each subject’s habitual waketime. Like the temperature data, the average plasma melatonin rhythms of both types followed a similar pattern throughout the habitual daytime hours on the first day of the CR. However, just prior to habitual bedtime the average plasma melatonin curve of the morning types began to rise sooner than did that of the evening types, and began to decline earlier relative to waketime than did that of the evening types (see Figure 5).
Figure 5.
Temperature and melatonin z-score curves of morning and evening types averaged with respect to habitual waketime. Symbols as in Figure 4. Data for each subject were first expressed relative to the subject’s mean (= 0) and standard deviation (=1). Average curves were complied with respect to each subject’s habitual waketime, with habitual waketime set to a reference value of 08:00 for all subjects. The average hourly z-score for each individual was first calculated, and those hourly values were then averaged across subjects for each of the two groups.
Finally, the phase angle between the CBTmin and habitual waketime was significantly longer in the morning types than in the evening types (3.3 ⩝ 1.06 hours vs. 2.26 ⩝ 0.97 hours, p < 0.03; see Figure 6) and the phase angle between the endogenous melatonin maximum and habitual waketime followed the same trend, although it did not reach significance (interval between melatonin maximum and habitual waketime: 4.47 ⩝ 0.45 hours vs. 3.88 ⩝ 0.86 hours, p = 0.07). Thus, while the morning types wake up at an earlier clock hour than do the evening types, the morning types actually wake at a later circadian phase than do the evening types.
Figure 6.
Average interval between endogenous circadian temperature phase and habitual waketime in young evening (upper bar) and morning (middle bar) types, and older morning (lower bar) types.
Comparison of Young and Older Morning Types.
Overall, there were 29 older morning types (24 moderate and 5 definite) and 10 young morning types (all moderate). When only moderate morning types of the two age groups were compared, habitual waketime of the older morning types was significantly earlier than that of the young morning types (older: 06:50 ⩝ 0:49; young: 07:52 ⩝ 0:44; p < 0.002), with the older subjects tending to also have earlier bedtimes (23:07 ⩝ 0:45 vs. 23:43 ⩝ 1:09, p = 0.08). This resulted in the habitual sleep length of the older morning types being significantly shorter than that in the young morning types (7.72 ⩝ 0.57 h vs. 8.16 ⩝ 0.54 h, p < 0.05). The clock hour of the endogenous circadian temperature phase in the older morning types was slightly later than that of the young morning types (05:18 ⩝ 2:15 vs. 04:34 ⩝ 1:30, p = 0.3). This, together with the earlier waketimes in the older morning types, resulted in a significantly shorter phase angle between the temperature minimum and habitual waketime in the older morning types (older: 1.53 ⩝ 1.89 h vs. 3.3 ⩝ 1.06 h, p < 0.01) (see Figure 6).
DISCUSSION
In a number of studies, morningness-eveningness preference has been shown to be related to sleep architecture, sleep timing, and daytime alerteness and performance levels6;19;24;25;28;29;31–33;39–44. There has been the presumption that underlying biological differences, perhaps in the circadian timing system18;28, contribute to such morning or evening preferences. However, most previous studies failed to collect data throughout a full circadian cycle45 or collected ambulatory data confounded by the very differences in behavior (i.e., sleep-wake timing) used to differentiate morning and evening types17;18;42;46. The present study is one of the first to examine the underlying endogenous component of circadian rhythms in morning types and in evening types with respect to both clock hour and the subjects’ habitual waketimes. Given their earlier bedtimes and wake times, we expected to find that the endogenous circadian temperature and melatonin phases would occur at a earlier clock hour in morning types than in evening types, as had been reported previously27;47. In fact, we found that the average endogenous circadian temperature and melatonin phases occurred much earlier in the morning types as compared to the evening types than could be accounted for by the average difference in their usual bedtimes and wake times. This is the first such report of its kind and offers new insight in understanding the physiological basis of morningness-eveningness preferences.
Our study used endogenous circadian temperature data obtained during a constant routine, rather than temperature data masked by changes in posture, activity or sleep. This allowed us to demonstrate that the observed difference in clock hour of circadian temperature phase between morning and evening types is not simply a direct effect of their different sleep-wake schedules, but reflects a difference in the timing of the underlying circadian component of the core body temperature rhythm. The significant correlation between the clock hour of the endogenous circadian temperature phase and Morningness-Eveningness score is consistent with previous reports27;47, some of which used masked ambulatory temperature data14;17;18;29;42;46.
That there is a difference in the timing of the underlying circadian rhythm is supported by our examination of the rhythm of plasma melatonin secretion in the subset of young subjects we identified as morning and evening types. Similar to our findings with the endogenous core body temperature rhythm, we found that the phase of the plasma melatonin rhythm occurred at a later clock hour in the evening types than it did in the morning types.
This finding, which is consistent with prior reports, does not in itself demonstrate that there is a fundamental biological difference between morning and evening types. One could postulate, for example, that differences in light exposure patterns between the two groups secondary to their different self-selected sleep-wake schedules might provide a simple explanation for the difference in the clock hour of the temperature and melatonin phases. Such differences in phases would be expected between any two groups of subjects living on such different sleep-wake and light-dark schedules, even if there were no underlying biological differences between them.
However, in the present study we have demonstrated for the first time that there are internal differences in entrained circadian phase even when the habitual sleep-wake cycle is taken into account. We found that while evening types do have later waketimes, their waketimes occur earlier with respect to their endogenous temperature and melatonin phases than do the waketimes of morning types. This should expose more of the phase-advance portion of the PRC in evening types and less in morning types. Thus, a simple difference in morning light exposure cannot explain the differences we observed in the timing of circadian phase between morning and evening types.
There are a number of alternative possibilities as to why morning and evening types not only have different sleep-wake times and endogenous circadian phases, but also have a difference in the phase relationship between the sleep-wake cycle and underlying circadian rhythms. On any given day it is possible that societal factors, such as school, work, etc. could lead an individual or group of individuals to awaken earlier than they would have if they were free to follow their biological urges, thus causing an artificially shorter or longer interval between their circadian phase and self-reported waketime. However, all subjects included in this study maintained a regular sleep-wake (and associated light-dark) schedule for three weeks prior to entering the study. If there were no difference in underlying circadian biology, at least 20 days should have been more than sufficient48 to entrain all subjects to the same phase relationship (such that the interval between their temperature and melatonin phase and usual waketime would be the same). Furthermore, a previous study found a high degree of test-retest reliability when a morningness-eveningness questionnaire was administered to students first during the school term and again three months later during vacation or nine months later after working on rotating shifts, suggesting that a preference for morningness or eveningness is independent of social requirements49.
A second possibility is that there is a difference in the daily light exposure pattern between the two groups. If evening types receive brighter light in the evening hours and/or morning types receive brighter light in the morning hours, it might result in the phase angle differences we observed in the present study50. It is also possible that there might be an underlying difference in the circadian timing system that leads individuals to become morning or evening types. For example, if the shape of the PRC to light were different between the two groups, this could lead to differences in the phase angle between circadian phase and usual waketime.
The third possibility is that there are differences in intrinsic period between morning and evening types. In 1971, Konopka and Benzer found that by selective breeding of Drosophila that eclosed early or late relative to the light-dark cycle, they could identify genetically mutant strains with abnormally short (19 hours) or long (28 hours) circadian periods51. Genetically-determined differences in circadian period have since been identified in other species, including mammals52;53, and it has been demonstrated that these genetic differences are related to fundamental clock mechanisms at the molecular level54;55. That there might be a difference in intrinsic period between morning and evening types is supported by very preliminary data from a forced desynchrony protocol conducted in our laboratory. When the intrinsic period of the core body temperature rhythm of 17 young men was compared with their Morningness-Eveningness score, a significant negative correlation was observed, such that subjects with shorter intrinsic periods rated themselves as more morning-like56 (Pearson correlation coefficient: r = −0.59, p < 0.02). While this observation is preliminary, it is known that interindividual differences in circadian period are associated with differences in phase angle of entrainment57–60. Furthermore, a recent study found an association between a polymorphism in the Clock gene and diurnal preference in humans61. Taken together, these provocative findings suggest the possibility of a genetic basis to morningness-eveningness preferences in humans.
The results of the present study also provide important information for understanding age-related changes in habitual sleep times, and the possible causes of those changes. We found that older people systematically rate themselves as more morning-like than do young adults, confirming previous reports6–10. We also found that the time of the endogenous core body temperature phase of the older morning types occurred at an earlier clock hour, as we might expect based on previous reports from older persons in general62–69 and on our current findings from young morning types. However, we found a profound difference in the phase angle between the core body temperature nadir and habitual waketime between young and older morning types, with the older morning types waking at a significantly later circadian phase than the young morning types. In fact, the phase angle of older morning types was more like that of young evening types. Thus, the chronobiological features associated with self-reported morningness are quite different in young and older subjects.
These findings, together with other recent findings from our laboratory69;70, suggest that the primary reason older people awaken at an earlier hour is due to an inability to sustain sleep at or just after the minimum of the core body temperature rhythm, rather than simply being the consequence of a circadian phase advance. This may lead older people to awaken at a relatively early circadian phase, at which time they are sensitive to the phase advancing effects of morning light exposure, which in turn will further advance their circadian phase and their ability to maintain sleep to an even earlier hour. In contrast, we hypothesize that the primary mechanism underlying morningness-eveningness in young adult men is interindividual differences in circadian period. The results of this study have important implications for understanding circadian rhythm sleep disorders such as delayed and advanced sleep phase syndrome, which may in fact represent extreme forms of the morningness-eveningness continuum described here.
ACKNOWLEDGMENTS
We wish to thank the subject-volunteers for their participation in the studies; the subject recruitment staff of our laboratory for administering the morningness/eveningness questionnaires; the supervisory staff of the GCRC Environmental Scheduling Facility (T.L. Shanahan, E.B. Martin, Jr., A.E. Ward, D.W. Rimmer, G. Jayne, S. Driscoll); the laboratory technicians for subject monitoring and temperature and plasma data collection; J. Emens, D. Chen and J. Whittemore for organization of the plasma melatonin data; E.J. Silva for help with data analysis; J.M. Ronda and A. McCollom for assistance with computer software and hardware; and J.M. Zeitzer, D.B. Boivin, D.W. Rimmer and E.B. Klerman who supervised some of the studies included in the present analysis.
Supported in part by awards AG09975, AG06072, and MH45130 from the National Institutes of Health; and by grants NAG9-524 and NAGW-4033 from the National Aeronautics and Space Administration. These studies were carried out in a General Clinical Research Center supported by grant RR02635.
Preliminary results were presented at the Association of Professional Sleep Societies Meeting, San Francisco, CA, June 1997.
REFERENCES
- 1.Moore RY: Circadian rhythms: Basic neurobiology and clinical applications. Annu.Rev.Med 1997;48:253–266. [DOI] [PubMed] [Google Scholar]
- 2.Van Cauter E: Diurnal and ultradian rhythms in human endocrine function: A minireview. Horm.Res 1990;34:45–53. [DOI] [PubMed] [Google Scholar]
- 3.Folkard S, Monk TH: Circadian performance rhythms, in Folkard S, Monk TH (eds): Hours of Work: Temporal Factors in Work-Scheduling. New York, John Wiley & Sons; 1985:37–52. [Google Scholar]
- 4.Monk TH: Circadian rhythms in subjective activation, mood, and performance efficiency, in Kryger MH, Roth T, Dement WC (eds): Principles and Prcatice of Sleep Medicine. Philadelphia, W.B.Saunders Company; 1994:321–330. [Google Scholar]
- 5.Kleitman N: Sleep and Wakefulness, Chicago, University of Chicago Press; 1939. [Google Scholar]
- 6.Ishihara K, Miyake S, Miyasita A, Miyata Y: Morningness-eveningness preference and sleep habits in Japanese office workers of different ages. Chronobiologia 1991;18:9–16. [PubMed] [Google Scholar]
- 7.Carrier J, Monk TH, Buysse DJ, Kupfer DJ: Sleep and morningness-eveningness in the ‘middle’ years of life (20–59y). J.Sleep Res 1997;6:230–237. [DOI] [PubMed] [Google Scholar]
- 8.Drennan MD, Klauber MR, Kripke DF, Goyette LM: The effects of depression and age on the Horne-Ostberg morningness-eveningness score. J.Affect.Disord 1991;23:93–98. [DOI] [PubMed] [Google Scholar]
- 9.Costa G, Lievore F, Ferrari P, Gaffuri E: Usual meal times in relation to age, sex, work activity and morningness-eveningness. Chronobiologia 1987;14:383–391. [PubMed] [Google Scholar]
- 10.Monk TH, Reynolds III CF, Buysse DJ, Hoch CC, Jarrett DB, Jennings JR, Kupfer DJ: Circadian characteristics of healthy 80-year-olds and their relationship to objectively recorded sleep. J.Gerontol 1991;46:M171–M175 [DOI] [PubMed] [Google Scholar]
- 11.Kerkhof GA: Inter-individual differences in the human circadian system: A review. Biol.Psychol 1985;20:83–112. [DOI] [PubMed] [Google Scholar]
- 12.Lacoste V, Wetterberg L: Individual variations of rhythms in morning and evening types with special emphasis on seasonal differences, in Wetterberg L (ed): Light and Biological Rhythms in Man. Oxford, Pergamon Press; 1993:287–304. [Google Scholar]
- 13.Moog R: Morning-evening types and shift work. A questionaire study, in Reinberg A, Vieux N, Andlauer P (eds): Night and Shift Work: Biological and Social Aspects. Oxford, Pergamon Press; 1981:481–488. [Google Scholar]
- 14.Hildebrandt G, Stratmann I: Circadian system response to night work in relation to the individual circadian phase position. Int.Arch.Occup.Environ.Health 1979;43:73–83. [DOI] [PubMed] [Google Scholar]
- 15.Breithaupt H, Hildebrandt G, Döhre D, Josch R, Sieber U, Werner M: Tolerance to shift of sleep, as related to the individual’s circadian phase position. Ergonomics 1978;21:767–774. [DOI] [PubMed] [Google Scholar]
- 16.Östberg O: Interindividual differences in circadian fatigue patterns of shift workers. Br.J.Indust.Med 1973;30:341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Östberg O: Circadian rhythms of food intake and oral temperature in ‘morning’ and ‘evening’ groups of individuals. Ergonomics 1973;16:203–209. [DOI] [PubMed] [Google Scholar]
- 18.Foret J, Benoit O, Royant-Parola S: Sleep schedules and peak times of oral temperature and alertness in morning and evening ‘types’. Ergonomics 1982;25:821–827. [DOI] [PubMed] [Google Scholar]
- 19.Horne JA, Brass CG, Pettitt AN: Circadian performance differences between morning and evening `types’. Ergonomics 1980;23:29–36. [DOI] [PubMed] [Google Scholar]
- 20.Horne JA, Östberg O: Individual differences in human circadian rhythms. Biol.Psychol 1977;5:179–190. [DOI] [PubMed] [Google Scholar]
- 21.Horne JA, Östberg O: A self-assessment questionnaire to determine morningness- eveningness in human circadian rhythms. Int.J.Chronobiol 1976;4:97–110. [PubMed] [Google Scholar]
- 22.Vidaek S, Kaliterna L, Radoevi-Vidaek B, Folkard S: Personality differences in the phase of circadian rhythms: A comparison of morningness and extraversion. Ergonomics 1988;31:873–888. [DOI] [PubMed] [Google Scholar]
- 23.Breithaupt H, Hildebrandt G, Werner M: Circadian type questionnaire and objective circadian characteristics, in Reinberg A, Vieux N, Andlauer P (eds): Night and shift work: Biological and social aspects. Oxford, Pergamon Press; 1981:435–440. [Google Scholar]
- 24.Pátkai P: Interindividual differences in diurnal variations in alertness, performance and adrenaline excretion. Acta Physiol.Scand 1971;81:35–46. [DOI] [PubMed] [Google Scholar]
- 25.Fröberg JE: Twenty-four-hour patterns in human performance, subjective and physiological variables and differences between morning and evening active subjects. Biol.Psychol 1977;5:119–134. [DOI] [PubMed] [Google Scholar]
- 26.Bailey SL, Heitkemper MM: Morningness-eveningness and early-morning salivary cortisol levels. Biol.Psychol 1991;32:181–192. [DOI] [PubMed] [Google Scholar]
- 27.Lack LC, Bailey M: Endogenous circadian rhythms of evening and morning types. Sleep Res. 1994;23:501 [Google Scholar]
- 28.Kerkhof GA: Differences between morning-types and evening-types in the dynamics of EEG slow wave activity during night sleep. Electroenceph.clin.Neurophysiol 1991;78:197–202. [DOI] [PubMed] [Google Scholar]
- 29.Ishihara K, Miyasita A, Inugami M, Fukuda K, Miyata Y: Differences in sleep-wake habits and EEG sleep variables between active morning and evening subjects. Sleep 1987;10:330–342. [DOI] [PubMed] [Google Scholar]
- 30.Kerkhof GA, van Dongen HPA, Treep JA: Sleep at habitual and shifted bed-times in morning-types and evening-types. J.Sleep Res 1996;5:107 [Google Scholar]
- 31.Lavie P, Segal S: Twenty-four-hour structure of sleepiness in morning and evening persons investigated by ultrashort sleep-wake cycle. Sleep 1989;12:522–528. [PubMed] [Google Scholar]
- 32.Lancel M, Kerkhof GA: Sleep structure and EEG power density in morning types and evening types during a simulated day and night shift. Physiol.Behav 1991;49:1195–1201. [DOI] [PubMed] [Google Scholar]
- 33.Kerkhof GA, Lancel M: EEG slow wave activity, REM sleep, and rectal temperature during night and day sleep in morning-type and evening-type subjects. Psychophysiol. 1991;28:678–688. [DOI] [PubMed] [Google Scholar]
- 34.Mills JN, Minors DS, Waterhouse JM: Adaptation to abrupt time shifts of the oscillator[s] controlling human circadian rhythms. J.Physiol.(Lond.) 1978;285:455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE: Exposure to bright light and darkness to treat physiologic maladaptation to night work. N.Engl.J.Med 1990;322:1253–1259. [DOI] [PubMed] [Google Scholar]
- 36.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA: Sensitivity of the human circadian pacemaker to moderately bright light. J.Biol.Rhythms 1994;9:315–331. [DOI] [PubMed] [Google Scholar]
- 37.Arendt J, Paunier L, Sizonenko PC: Melatonin radioimmunoassay J.Clin.Endocrinol.Metab 1975;40:347–350. [DOI] [PubMed] [Google Scholar]
- 38.Brown EN, Czeisler CA: The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J.Biol.Rhythms 1992;7:177–202. [DOI] [PubMed] [Google Scholar]
- 39.Mecacci L, Zani A: Morningness-eveningness preferences and sleep-waking diary data of morning and evening types in student and worker samples. Ergonomics 1983;26:1147–1153. [DOI] [PubMed] [Google Scholar]
- 40.Sexton-Radek K, Harris D: Morningness versus eveningness arousal patterns in young adults. Percept.Mot.Skills 1992;74:115–119. [DOI] [PubMed] [Google Scholar]
- 41.Ishihara K, Miyake S, Miyasita A, Miyata Y: Comparisons of sleep-wake habits of morning and evening types in Japanese worker sample. J.Human Ergol 1988;17:111–118. [PubMed] [Google Scholar]
- 42.Foret J, Touron N, Benoit O, Bouard G: Sleep and body temperature in “morning” and “evening” people. Sleep 1985;8:311–318. [DOI] [PubMed] [Google Scholar]
- 43.Weir CJ, Ogilvie RD, Simons IA: Morningness and eveningness as related to sleepiness and performance. Sleep Res. 1992;21:393 [Google Scholar]
- 44.Webb WB, Bonnet MH: The sleep of ‘morning’ and ‘evening’ types. Biol.Psychol 1978;7:29–35. [DOI] [PubMed] [Google Scholar]
- 45.Watts C, Cox T, Robson J: Morningness-eveningness and diurnal variations in self-reported mood. J.Psychol 1983;113:251–256. [DOI] [PubMed] [Google Scholar]
- 46.Andrade MMM, Benedito-Silva AA, Menna-Barreto L: Correlations between morningness-eveningness character, sleep habits and temperature rhythm in adolescents. Brazilian J.Med.Biol.Res 1992;25:835–839. [PubMed] [Google Scholar]
- 47.Kerkhof GA, van Dongen HPA: Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci.Lett 1996;218:153–156. [DOI] [PubMed] [Google Scholar]
- 48.Kronauer RE, Czeisler CA: Understanding the use of light to control the circadian pacemaker in humans, in Wetterberg L (ed): Light and Biological Rhythms in Man. Oxford, Pergamon Press; 1993:217–236. [Google Scholar]
- 49.Greenwood KM: Long-term stability and psychometric properties of the composite scale of morningness. Ergonomics 1994;37(2):377–383. [DOI] [PubMed] [Google Scholar]
- 50.Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM: Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science 1989;244:1328–1333. [DOI] [PubMed] [Google Scholar]
- 51.Konopka RJ, Benzer S: Clock mutants of Drosophila melanogaster. Proc.Natl.Acad.Sci.USA 1971;68:2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ralph MR, Menaker M: A mutation of the circadian system in golden hamsters. Science 1988;241:1225–1227. [DOI] [PubMed] [Google Scholar]
- 53.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH: Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994;264:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TDL, Weitz CJ: Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 1998;280:1599–1603. [DOI] [PubMed] [Google Scholar]
- 55.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ: Role of CLOCK protein in the mammalian circadian mechanism. Science 1998;280:1564–1569. [DOI] [PubMed] [Google Scholar]
- 56.Duffy JF, Rimmer DW, Silva EJ, Czeisler CA: Correlation of intrinsic circadian period with morningness-eveningness in young men. Sleep 1999;in press (Abstract). [DOI] [PubMed] [Google Scholar]
- 57.Sharma VK, Chandrashekaran MK, Singaravel M: Relationship between period and phase angle differences in Mus booduga under abrupt versus gradual light-dark transitions. Naturwissenschaften 1998;85:183–186. [DOI] [PubMed] [Google Scholar]
- 58.Pittendrigh CS: On the mechanism of the entrainment of a circadian rhythm by light cycles, in Aschoff J (ed): Circadian Clocks. Amsterdam, North-Holland Publishing Company; 1965:277–297. [Google Scholar]
- 59.Pittendrigh CS, Daan S: A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J.Comp.Physiol.[A] 1976;106:291–331. [Google Scholar]
- 60.Hoffmann K: Zur beziehung zwischen phasenlage und spontanfrequenz bei der endogenen tagesperiodik. Z.Naturforsch 1963;18:154–157. [Google Scholar]
- 61.Katzenberg D, Young T, Finn L, Lin L, King DP, Takahashi JS, Mignot E: A clock polymorphism associated with human diurnal preference. Sleep 1998;21:569–576. [DOI] [PubMed] [Google Scholar]
- 62.Lieberman HR, Wurtman JJ, Teicher MH: Circadian rhythms of activity in healthy young and elderly humans. Neurobiol.Aging 1989;10:259–265. [DOI] [PubMed] [Google Scholar]
- 63.Webb WB: The measurement and characteristics of sleep in older persons. Neurobiol.Aging 1982;3:311–319. [DOI] [PubMed] [Google Scholar]
- 64.Touitou Y, Sulon J, Bogdan A, Touitou C, Reinberg A, Beck H, Sodoyez J-C, Demey-Ponsart E, Van Cauwenberge H: Adrenal circadian system in young and elderly human subjects: A comparative study. J.Endocrinol 1982;93:201–210. [DOI] [PubMed] [Google Scholar]
- 65.Sherman B, Wysham C, Pfohl B: Age-related changes in the circadian rhythm of plasma cortisol in man. J.Clin.Endocrinol.Metab 1985;61:439–443. [DOI] [PubMed] [Google Scholar]
- 66.Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, Nair NPV: Circadian rhythms of melatonin and cortisol in aging. Biol.Psychiatry 1989;25:305–319. [DOI] [PubMed] [Google Scholar]
- 67.Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC: Chronobiology of aging: Temperature, sleep-wake rhythms and entrainment. Neurobiol.Aging 1982;3:299–309. [DOI] [PubMed] [Google Scholar]
- 68.Monk TH, Buysse DJ, Reynolds III CF, Kupfer DJ, Houck PR: Circadian temperature rhythms of older people. Exp.Gerontol 1995;30:455–474. [DOI] [PubMed] [Google Scholar]
- 69.Duffy JF, Dijk D-J, Klerman EB, Czeisler CA: Later endogenous circadian temperature nadir relative to an earlier waketime in older people. Am.J.Physiol 1998;275:R1478–R1487. [DOI] [PubMed] [Google Scholar]
- 70.Dijk D-J, Duffy JF, Riel E, Czeisler CA: Altered interaction of circadian and homeostatic aspects of sleep propensity results in awakening at an earlier circadian phase in older people. Sleep Res. 1997;26:710 [Google Scholar]