Introduction
Leptospirosis is a ubiquitous zoonotic infection caused by bacterial spirochetes that are equally adapted to life in the aqueous environment as they are to infection of their eucaryotic hosts. Leptospires owe their ubiquity to having evolved from free-living saprophytes to become nonpathogenic commensals of a wide range of mammals and, although not as well documented, birds, amphibians, and reptiles [1,2]. They colonize the proximal renal tubules of the host, in which they proliferate in the nutrient-rich glomerular filtrate, and from which they are shed into the environment by host urination. Most infections are mild or asymptomatic, but others result in organ failure and death (Fig 1). Significant impacts on human well-being have been documented, with an estimated 1 million cases and approximately 59,000 deaths per year, many of which occur in tropical, medically underserved regions of the world [3]. Leptospirosis affects not only human health but also livestock farming, causing great economic or subsistence resources losses. Despite the fact that leptospirosis has been much less investigated than other illnesses with comparable or even lower burden [4], a number of remarkable discoveries have recently emerged about these organisms and the infections they cause.
Fig 1. Overview of leptospirosis.
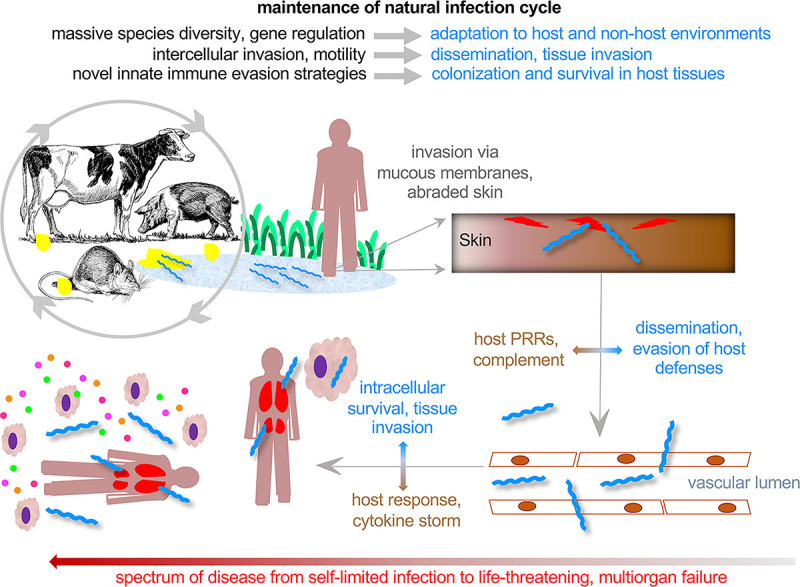
Potentially human pathogenic leptospires are maintained in zoonotic infection cycles in wildlife and domestic animals. Leptospires colonize the renal proximal tubule of reservoir hosts and are shed in the urine. Urine contamination of water and mud are common sources of human exposure. In humans and disease susceptible animals, leptospires disseminate and cause symptomatic disease ranging from mild to severe and, in some cases, death.
Massive species diversity
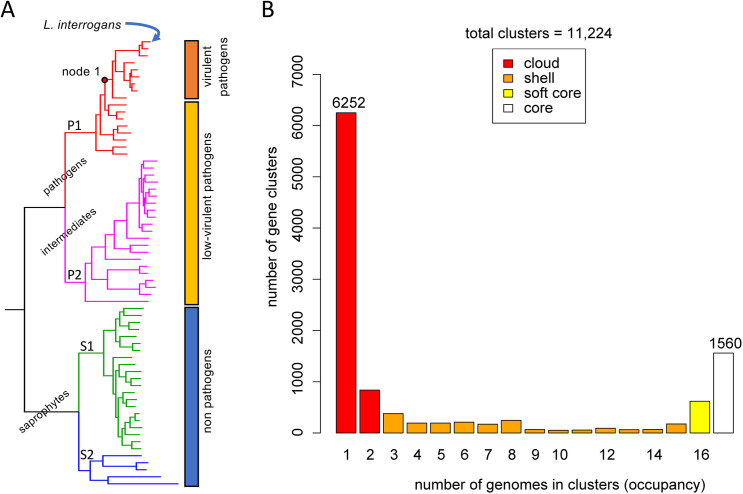
With the description of many new leptospiral genomes, a striking pattern of massive species diversity has emerged: Leptospira species belonging to the P1 clade, which includes human pathogens, have an open pan-genome with a relatively high number of genes found only in a single species (Fig 2) [5]. The open pan-genome reflects the leptospiral life cycle, which includes the ability of leptospiral pathogens to form biofilms to withstand environmental stress and survive for prolonged periods in milieux such as soil and aqueous habitats [6]. Such settings contain complex microbial and chemical compositions, facilitating a high rate of horizontal gene transfer that enables reworking of cellular functions to allow rapid adaptation to new environmental conditions and hosts. Leptospiral genes and gene fragments are derived both from unrelated bacteria and other leptospires, resulting in mosaic genes with diverse phylogenetic ancestry [7,8]. Clues to which pathogen-specific genes are required for virulence have been obtained from RNAseq studies examining differential expression during adaptation to the mammalian host [9]. Determining the impact of specific genes on in vivo fitness has also been determined by high-throughput sequencing of tissues from animals challenged with transposon mutant pools [10]. Targeted mutagenesis in leptospiral pathogens has been challenging and has only recently become more reliable through the development of the CRISPR dCas9 and transcription activator-like effectors (TALEs) as gene silencing approaches [11,12]. These novel tools made it possible to show that the pathogen-specific, multifunctional leptospiral immunoglobulin-like domain (Lig) proteins were required for virulence [11,12].
Fig 2. Massive species diversity.
(A) Phylogenetic tree showing the relatedness of the 64 Leptospira species. Leptospira species are clustered as non-pathogens, low-virulent pathogens, and virulent pathogens according to their virulence status in animal models, prevalence in severe infections, and presence of virulence factors. Node 1 indicates the node from which descend pathogenic species are most frequently involved in human disease. (B) Distribution of gene clusters in the P1 clade revealing an open pan-genome with a relatively high number of gene clusters found only in a single species. Adapted from Vincent and colleagues [5].
Rapid dissemination
Rapid and widespread dissemination to all organs, including the eye and brain, is a hallmark of leptospirosis. Remarkably, Leptospira interrogans is detectable in all organs of perfused animals within 1 hour after intraperitoneal inoculation of hamsters [13]. Consistently, flagellar mutants with decreased motility are attenuated for virulence in animal models [14,15]. Leptospires are uniquely equipped for dissemination through their corkscrew morphology, 200 nM diameter (in Greek, leptos means “thin”), and powerful propulsion by their endoflagella. As in other spirochetes, the organs of motility are called “endoflagella” because they are not surface exposed; instead, they are entirely within the periplasm. Leptospires are unique in that they have a single supercoiled endoflagellum extending axially toward the middle of the cell from a flagellar motor embedded in the inner membrane at each pole, without overlap. Endoflagellar rotation imparts a swimming motility that enables leptospires to be particularly invasive at liquid–gel borders, such as the interface between the vascular lumen and the endothelium, with 30 times the swimming force of Escherichia coli [16]. Recent elegant high-resolution cryo-electronic tomography studies have shown that the function of leptospiral endoflagellar filaments relies on an asymmetric sheath that imparts their supercoiled structure [17].
Host tissue barrier invasion
Another key to efficient transmigration across host tissue barriers appears to be targeting of E- and VE-cadherins, which are integral to the intercellular adherens junctions of epithelium and endothelium, respectively [18,19]. Virulent L. interrogans, but not the saprophytic Leptospira biflexa, disrupt adherens junctions of endothelial and epithelial cells in vitro, resulting in loss of VE- and E-cadherins and disruption of the associated catenins, which link cadherins to the intracellular actin cytoskeleton. A clue to the molecular mechanisms for endothelial barrier disruption emerged from a study examining the crystal structures of 4 L. interrogans leucine-rich repeat (LRR) proteins [20]. As has been observed with other LRR proteins, the 23-residue repeating leucine-rich motifs formed a characteristic curved solenoid structure. One of these LRR proteins, LIC10831, has a structure and binding pocket similar to that of the Listeria monocytogenes internalin InlA. InlA mediates the first step of listerial invasion of the intestinal epithelium by binding to unpaired E-cadherin proteins as they become accessible through the normal sloughing of intestinal epithelial cells at the tip of the brush border. LIC10831 is actively secreted by L. interrogans and specifically binds E- and VE-cadherins at a binding coefficient 10-fold lower than InlA [21]. Importantly, LIC10831, but not an LRR protein (LIC12234) that bound other host factors, bound specifically to the cell–cell junctions of endothelial cells. Two other VE-cadherin adhesins were identified using phage display [22], suggesting that targeting of endothelial barrier integrity may be a key feature of pathogenic Leptospira species.
Evasion of innate immune recognition
Reflecting their ancient history of coevolution with eukaryotes, pathogenic leptospires have evolved an array of novel strategies to evade and/or alter the innate immune response. These strategies may well be important in extending the time of persistence in the renal tubules of reservoir hosts for shedding in the urine. Leptospires are stealth pathogens that evade recognition with altered microbial-associated molecular patterns (MAMPs) structures [23]. For example, Leptospira spp. escape murine TLR5 recognition through the peculiar subsurface localization and stability of the FlaB flagellar subunits, combined with specific down-regulation of FlaB transcription during mammalian infection [23]. An additional immune evasion strategy involves leptospiral lipoproteins that bind MAMPs and block their recognition by host pattern recognition receptors (PRRs) of the Toll- and NOD-like families. In some cases, these PRR-blocking lipoproteins are some of the most abundant proteins in pathogenic leptospires. This strategy applies to leptospiral lipid A, which is similar to gram-negative endotoxin but differs structurally in key ways such that, although it is recognized by mouse TLR4, it is not recognized by human TLR4 [24]. In fact, C3H/HeJ mice lacking TLR4 are more susceptible to leptospiral infection have a higher leptospiral burden than C3H mice with an intact TLR4 [25]. However, even in mice with intact TLR4, leptospiral lipopolysaccharide (LPS) O-antigen and multiple LPS-binding lipoproteins reduce TLR4-mediated uptake by macrophages and their TRIF-dependent activation [26]. Similarly, LipL21, a major leptospiral lipoprotein has now been recognized as a peptidoglycan-binding lipoprotein that enables escape from NOD1 and NOD2 recognition [27].
Evasion of innate killing mechanisms
Leptospires have evolved a variety of strategies for evasion of host innate killing mechanisms. These include escape from complement by surface presentation of LigA, LigB, and other proteins that bind host complement regulators [28,29]. Pathogenic leptospires also express catalase, encoded by katE, which is required for resistance to reactive oxygen species (ROS) and for virulence in the hamster model [30]. Expression of katE is transcriptionally controlled by the peroxide stress regulator PerR, a novel H2O2 sensor [31–33]. Recently, a second potential PerR was identified, called PerRB, which is present only in pathogenic Leptospira species. Inactivating perRA or perRB led to an increased tolerance to 2 different components of the phagocytic oxidative burst, H2O2 and superoxide, respectively, indicating that the 2 regulators do not have a redundant functions [34]. While single perRA and perRB mutants were virulent for hamsters, the double mutant was avirulent for hamsters [34]. These results indicate that, although the double mutant has the metabolic pathways required for infection, it lacks expression of specific virulence-related gene products. Interestingly, RNAseq and protein expression studies involving perRA and perRB single and double mutants revealed complex regulons, including many coding and noncoding RNAs, some of which are likely unrelated to resistance to oxidative stress [34].
Rapid diagnostics
Leptospirosis is a common cause of acute febrile illness in areas where dengue, malaria, and rickettsial infections are also common. Though the majority of cases are mild, life-threatening leptospirosis is common in many tropical countries with a case fatality rate exceeding that of other common febrile illnesses. Our ability to identify the etiology of febrile illness and provide targeted therapy is limited by the nonspecific clinical presentations, low sensitivity of molecular methods, and low specificity and delayed utility of serological methods. Standard biomarkers such as C-reactive protein (CRP) and procalcitonin have relatively low specificity for leptospirosis [35]. In contrast, the transcriptional response to leptospirosis and scrub typhus is distinguishable from that of patients with viral etiologies [35]. Likewise, proteomic approaches utilizing multiple inflammatory biomarkers such as serum amyloid A and leucine-rich alpha-2 glycoprotein provide diagnostic utility greatly superior to CRP or PCT alone and are more readily translated to rapid diagnostic platforms suitable for use in low resource healthcare settings. Host response signatures and biomarkers such as decreased cathelicidin may also enable prediction of disease severity among patients with early infection [36] and identify patients who would benefit from antibiotics and other interventions.
Conclusions
While much more work is needed to validate and expand these discoveries, leptospirosis research is providing unprecedented insights regarding these ancient pathogens and their host interactions. Further elucidation of leptospiral genetic diversity and its global health impacts are needed, both in humans and in both domestic and wild animals using a One Health perspective. The coevolution of leptospires with their eukaryotic hosts is reflected in their novel mechanisms for immune evasion and escape from innate killing strategies. A better understanding of the remarkably rapid and efficient dissemination process will provide opportunities for improved prevention strategies including vaccine development. Finally, host response and biomarker approaches to diagnosis are needed to rapidly diagnose suspected cases and expedite their management to improve patient outcomes. A better disease burden estimate of causes of fever, including leptospirosis, in developing countries would also be most useful for policy and decision makers.
Funding Statement
This work was supported by National Institute of Health grants AI128560 (to D.A.H.), AI147573 (to J.C.), and AI112920 (to J.C.) and by the Department of Veterans Affairs Research award BX002003 (to D.A.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Picardeau M, Bulach DM, Bouchier C, Zuerner RL, Zidane N, Wilson PJ, et al. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS ONE. 2008;3(2):e1607. doi: 10.1371/journal.pone.0001607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Y, Zhu Y, Wang Y, Chang YF, Zhang Y, Jiang X, et al. Whole genome sequencing revealed host adaptation-focused genomic plasticity of pathogenic Leptospira. Sci Rep. 2016;6:20020. Epub 2016/02/03. doi: 10.1038/srep20020 ; PubMed Central PMCID: PMC4735792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015;9(9):e0003898. Epub 2015/09/18. doi: 10.1371/journal.pntd.0003898 ; PubMed Central PMCID: PMC4574773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goarant C, Picardeau M, Morand S, McIntyre KM. Leptospirosis under the bibliometrics radar: evidence for a vicious circle of neglect. J Glob Health. 2019;9(1):010302. Epub 2019/01/04. doi: 10.7189/jogh.09.010302 ; PubMed Central PMCID: PMC6304173 form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no conflict of interest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent AT, Schiettekatte O, Goarant C, Neela VK, Bernet E, Thibeaux R, et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl Trop Dis. 2019;13(5):e0007270. Epub 2019/05/24. doi: 10.1371/journal.pntd.0007270 ; PubMed Central PMCID: PMC6532842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thibeaux R, Soupe-Gilbert ME, Kainiu M, Girault D, Bierque E, Fernandes J, et al. The zoonotic pathogen Leptospira interrogans mitigates environmental stress through cyclic-di-GMP-controlled biofilm production. NPJ Biofilms Microbiomes. 2020;6(1):24. Epub 2020/06/14. doi: 10.1038/s41522-020-0134-1 ; PubMed Central PMCID: PMC7293261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haake DA, Suchard MA, Kelley MM, Dundoo M, Alt DP, Zuerner RL. Molecular evolution and mosaicism of leptospiral outer membrane proteins involves horizontal DNA transfer. J Bacteriol. 2004;186(9):2818–28. Epub 2004/04/20. doi: 10.1128/JB.186.9.2818-2828.2004 ; PubMed Central PMCID: PMC387810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoke DE, Egan S, Cullen P, Adler B. LipL32 is an extracellular matrix-interacting protein of Leptospira spp. and Pseudoalteromonas tunicata. Infect Immun. 2008;76:2063–9. doi: 10.1128/IAI.01643-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caimano MJ, Sivasankaran SK, Allard A, Hurley D, Hokamp K, Grassmann AA, et al. A model system for studying the transcriptomic and physiological changes associated with mammalian host-adaptation by Leptospira interrogans serovar Copenhageni. PLoS Pathog. 2014;10(3):e1004004. Epub 2014/03/15. doi: 10.1371/journal.ppat.1004004 ; PubMed Central PMCID: PMC3953431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lourdault K, Matsunaga J, Haake DA. High-Throughput Parallel Sequencing to Measure Fitness of Leptospira interrogans Transposon Insertion Mutants during Acute Infection. PLoS Negl Trop Dis. 2016;10(11):e0005117. doi: 10.1371/journal.pntd.0005117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes LGV, Hornsby RL, Nascimento A, Nally JE. Genetic manipulation of pathogenic Leptospira: CRISPR interference (CRISPRi)-mediated gene silencing and rapid mutant recovery at 37 degrees C. Sci Rep. 2021;11(1):1768. Epub 2021/01/21. doi: 10.1038/s41598-021-81400-7 ; PubMed Central PMCID: PMC7815788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappas CJ, Picardeau M. Control of Gene Expression in Leptospira spp. by Transcription Activator-Like Effectors Demonstrates a Potential Role for LigA and LigB in Leptospira interrogans Virulence. Appl Environ Microbiol. 2015;81(22):7888–92. Epub 2015/09/06. doi: 10.1128/AEM.02202-15 ; PubMed Central PMCID: PMC4616954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wunder EA Jr., Figueira CP, Santos GR, Lourdault K, Matthias MA, Vinetz JM, et al. Real-Time PCR Reveals Rapid Dissemination of Leptospira interrogans after Intraperitoneal and Conjunctival Inoculation of Hamsters. Infect Immun. 2016;84(7):2105–15. Epub 2016/05/04. doi: 10.1128/IAI.00094-16 ; PubMed Central PMCID: PMC4936353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wunder EA Jr., Figueira CP, Benaroudj N, Hu B, Tong BA, Trajtenberg F, et al. A novel flagellar sheath protein, FcpA, determines filament coiling, translational motility and virulence for the Leptospira spirochete. Mol Microbiol. 2016;101(3):457–70. Epub 2016/04/27. doi: 10.1111/mmi.13403 ; PubMed Central PMCID: PMC4979076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert A, Picardeau M, Haake DA, Sermswan RW, Srikram A, Adler B, et al. FlaA proteins in Leptospira interrogans are essential for motility and virulence but are not required for formation of the flagellum sheath. Infect Immun. 2012;80(6):2019–25. Epub 2012/03/28. doi: 10.1128/IAI.00131-12 ; PubMed Central PMCID: PMC3370569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe K, Kuribayashi T, Takabe K, Nakamura S. Implications of back-and-forth motion and powerful propulsion for spirochetal invasion. Sci Rep. 2020;10(1):13937. Epub 2020/08/20. doi: 10.1038/s41598-020-70897-z ; PubMed Central PMCID: PMC7434897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson KH, Trajtenberg F, Wunder EA, Brady MR, San Martin F, Mechaly A, et al. An asymmetric sheath controls flagellar supercoiling and motility in the leptospira spirochete. Elife. 2020;9. Epub 2020/03/12. doi: 10.7554/eLife.53672 ; PubMed Central PMCID: PMC7065911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato H, Coburn J. Leptospira interrogans causes quantitative and morphological disturbances in adherens junctions and other biological groups of proteins in human endothelial cells. PLoS Negl Trop Dis. 2017;11(7):e0005830. Epub 2017/07/28. doi: 10.1371/journal.pntd.0005830 ; PubMed Central PMCID: PMC5549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sebastian I, Okura N, Humbel BM, Xu J, Hermawan I, Matsuura C, et al. Disassembly of the apical junctional complex during the transmigration of Leptospira interrogans across polarized renal proximal tubule epithelial cells. Cell Microbiol. 2021. Epub 2021/04/18. doi: 10.1111/cmi.13343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miras I, Saul F, Nowakowski M, Weber P, Haouz A, Shepard W, et al. Structural characterization of a novel subfamily of leucine-rich repeat proteins from the human pathogen Leptospira interrogans. Acta Crystallogr D Biol Crystallogr. 2015;71(Pt 6):1351–9. Epub 2015/06/10. doi: 10.1107/S139900471500704X . [DOI] [PubMed] [Google Scholar]
- 21.Eshghi A, Gaultney RA, England P, Brule S, Miras I, Sato H, et al. An extracellular Leptospira interrogans leucine-rich repeat protein binds human E- and VE-cadherins. Cell Microbiol. 2019;21(2):e12949. Epub 2018/09/02. doi: 10.1111/cmi.12949 ; PubMed Central PMCID: PMC7560960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evangelista KV, Hahn B, Wunder EA Jr., Ko AI, Haake DA, Coburn J. Identification of cell-binding adhesins of Leptospira interrogans. PLoS Negl Trop Dis. 2014;8(10):e3215. Epub 2014/10/03. doi: 10.1371/journal.pntd.0003215 ; PubMed Central PMCID: PMC4183468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holzapfel M, Bonhomme D, Cagliero J, Vernel-Pauillac F, Fanton d’Andon M, Bortolussi S, et al. Escape of TLR5 Recognition by Leptospira spp.: A Rationale for Atypical Endoflagella. Front Immunol. 2020;11:2007. Epub 2020/08/28. doi: 10.3389/fimmu.2020.02007 ; PubMed Central PMCID: PMC7431986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint Girons I, et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2(4):346–52. Epub 2001/03/29. doi: 10.1038/86354 . [DOI] [PubMed] [Google Scholar]
- 25.Viriyakosol S, Matthias MA, Swancutt MA, Kirkland TN, Vinetz JM. Toll-like receptor 4 protects against lethal Leptospira interrogans serovar icterohaemorrhagiae infection and contributes to in vivo control of leptospiral burden. Infect Immun. 2006;74(2):887–95. Epub 2006/01/24. doi: 10.1128/IAI.74.2.887-895.2006 ; PubMed Central PMCID: PMC1360355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonhomme D, Santecchia I, Vernel-Pauillac F, Caroff M, Germon P, Murray G, et al. Leptospiral LPS escapes mouse TLR4 internalization and TRIFassociated antimicrobial responses through O antigen and associated lipoproteins. PLoS Pathog. 2020;16(8):e1008639. Epub 2020/08/14. doi: 10.1371/journal.ppat.1008639 ; PubMed Central PMCID: PMC7447051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratet G, Santecchia I, Fanton d’Andon M, Vernel-Pauillac F, Wheeler R, Lenormand P, et al. LipL21 lipoprotein binding to peptidoglycan enables Leptospira interrogans to escape NOD1 and NOD2 recognition. PLoS Pathog. 2017;13(12):e1006725. Epub 2017/12/07. doi: 10.1371/journal.ppat.1006725 ; PubMed Central PMCID: PMC5764436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breda LC, Hsieh CL, Castiblanco Valencia MM, da Silva LB, Barbosa AS, Blom AM, et al. Fine Mapping of the Interaction between C4b-Binding Protein and Outer Membrane Proteins LigA and LigB of Pathogenic Leptospira interrogans. PLoS Negl Trop Dis. 2015;9(10):e0004192. Epub 2015/10/31. doi: 10.1371/journal.pntd.0004192 ; PubMed Central PMCID: PMC4627802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbosa AS, Isaac L. Strategies used by Leptospira spirochetes to evade the host complement system. FEBS Lett. 2020;594(16):2633–44. Epub 2020/03/11. doi: 10.1002/1873-3468.13768 . [DOI] [PubMed] [Google Scholar]
- 30.Eshghi A, Lourdault K, Murray GL, Bartpho T, Sermswan RW, Picardeau M, et al. Leptospira interrogans catalase is required for resistance to H2O2 and for virulence. Infect Immun. 2012;80:3892–9. doi: 10.1128/IAI.00466-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zavala-Alvarado C, Sismeiro O, Legendre R, Varet H, Bussotti G, Bayram J, et al. The transcriptional response of pathogenic Leptospira to peroxide reveals new defenses against infection-related oxidative stress. PLoS Pathog. 2020;16(10):e1008904. Epub 2020/10/07. doi: 10.1371/journal.ppat.1008904 ; PubMed Central PMCID: PMC7567364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kebouchi M, Saul F, Taher R, Landier A, Beaudeau B, Dubrac S, et al. Structure and function of the Leptospira interrogans peroxide stress regulator (PerR), an atypical PerR devoid of a structural metal-binding site. J Biol Chem. 2018;293(2):497–509. Epub 2017/11/18. doi: 10.1074/jbc.M117.804443 ; PubMed Central PMCID: PMC5767856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo M, Murray GL, Khoo CA, Haake DA, Zuerner RL, Adler B. Transcriptional response of Leptospira interrogans to iron limitation and characterization of a putative fur mutant. [Submitted for Publication]. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zavala-Alvarado C, Samuel Garcia Huete S, Vincent AT, Sismeiro S, Legendre R, Varet H, et al. The oxidative stress response of pathogenic Leptospira is controlled by two peroxide stress regulators which putatively cooperate in controlling virulence. bioRxiv. 2021; 2020.11.06.371039v2. doi: 10.1101/2020.11.06.371039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tillekeratne LG, Suchindran S, Ko ER, Petzold EA, Bodinayake CK, Nagahawatte A, et al. Previously Derived Host Gene Expression Classifiers Identify Bacterial and Viral Etiologies of Acute Febrile Respiratory Illness in a South Asian Population. Open Forum Infect Dis. 2020;7(6):ofaa194. Epub 2020/07/04. doi: 10.1093/ofid/ofaa194 ; PubMed Central PMCID: PMC7314590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindow JC, Wunder EA Jr., Popper SJ, Min JN, Mannam P, Srivastava A, et al. Cathelicidin Insufficiency in Patients with Fatal Leptospirosis. PLoS Pathog. 2016;12(11):e1005943. Epub 2016/11/05. doi: 10.1371/journal.ppat.1005943 ; PubMed Central PMCID: PMC5094754. [DOI] [PMC free article] [PubMed] [Google Scholar]