Abstract
Rett syndrome (RTT) is a neurodevelopmental disorder with X-linked dominant inheritance caused mainly by mutations in the methyl-CpG-binding protein 2 (MECP2) gene. The effects of various Mecp2 mutations have been extensively assessed in mouse models, but none adequately mimic the symptoms and pathological changes of RTT. In this study, we assessed the effects of Mecp2 gene deletion on female rats (Mecp2+/−) and found severe impairments in social behavior [at 8 weeks (w), 12 w, and 23 w of age], motor function [at 16 w and 26 w], and spatial cognition [at 29 w] as well as lower plasma insulin-like growth factor (but not brain-derived neurotrophic factor) and markedly reduced acetylcholine (30%–50%) in multiple brain regions compared to female Mecp2+/+ rats [at 29 w]. Alternatively, changes in brain monoamine levels were relatively small, in contrast to reports on mouse Mecp2 mutants. Female Mecp2-deficient rats express phenotypes resembling RTT and so may provide a robust model for future research on RTT pathobiology and treatment.
Introduction
Rett syndrome (RTT) (OMIM 312750) is an X-linked progressive neurodevelopmental disorder with an incidence of about 1:10,000 among newborn females, making it the second most frequent cause of female mental retardation after Down syndrome [1]. It also shortens life expectancy by ~30‒40 years in females, while males usually demonstrate early onset and die shortly after birth. Mutations in the gene encoding transcription regulator methyl-CpG-binding protein 2 (MeCP2) are identified in more than 95% RTT patients [2, 3]. Females with RTT appear to develop normally until 6 to 18 months after birth, but then begin to exhibit deficits in cognitive function, motor function, and sociality, with continued developmental regression characterized by a loss of acquired communication and purposeful hand skills [4, 5].
Over 300 MECP2 mutations and genomic abnormalities have been documented in RTT patients as well as in other mental health disorders, including intellectual disability, autism spectrum disorder, bipolar disorder, and schizophrenia [6]. Based on these genetic studies, several mouse models with various Mecp2 mutations have been developed and studied over the past two decades [7, 8]. One of the most striking findings is that many phenotypes reported in Mecp2 gene null mutants [9] are also observed in mouse mutants generated using a post-mitotic neuron-specific knockout strategy [10], consistent with postnatal neurodevelopmental regression as a predominant symptom. Moreover, some of these phenotypes can be normalized by re-expression of the Mecp2 gene after birth [11–13]. This potential postnatal reversibility of RTT phenotypes has prompted research on therapeutics to compensate for Mecp2 and associated deficiencies [14], but there has been no successful clinical translation of these findings.
While mouse models allow for convenient genetic manipulation and large-scale breeding, small body and brain sizes limit critical research methods such as region-specific in vivo electrophysiology and neurochemistry (e.g., microdialysis). Further, some complex and sophisticated behaviors are not observed in mice [15, 16], which may make it difficult to capture RTT-related regressive pathologies. Considering these shortcomings, a rat model was recently developed to investigate the effects of Mecp2 deficiency on brain development, cognition, and behavior [17–19].
Since human RTT is limited to females, it is preferable to conduct all animal model experiments on females, although few studies have examined female mutant mice exclusively, likely because of the more delayed and complex phenotypic progression associated with cellular mosaicism [8]. To further explore the pathological progression associated with Mecp2 gene deficiency, we examined differences in spatial learning and memory, histopathology, and regional neurotransmitter levels between wild-type (Mecp2+/+) and Mecp2+/− female rats. These studies revealed progressive deficits in social behavior and motor function as well as impaired spatial learning and memory. Further, regional acetylcholine levels were substantially reduced, suggesting that disrupted cholinergic transmission or metabolism may contribute to the behavioral and cognitive abnormalities of RTT.
Materials and methods
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Nihon Bioresearch Inc. (protocol number: 201601), and performed in compliance with the Guidelines for Management and Welfare of Experimental Animals of both Nihon Bioresearch and the National Institutes of Health. All efforts were made to minimize animal suffering and to reduce the number of animals used. We adopted the following protocol: if a rat showed any symptoms of suffering, such as abnormal breathing and pulse, decreased body temperature, lying down with less response to external stimuli, or showed rapid weight loss (>20% in several days), we would take euthanasia measures by exsanguination with the opening of the abdominal aorta under isoflurane anesthesia. None of the rats, however, showed any such symptoms. We therefore used all the datasets without any exclusion throughout this study.
Animals
Female Mecp2 gene mosaic heterozygotic Sprague Dawley (SD) rats (Mecp2+/−) were obtained from SAGE Labs (part of Horizon Discovery, UK). The genotype of these animals is guaranteed by SAGE Labs. The zinc-finger nuclease technology was used to establish an animal model for generating a 71-base pair deletion in exon 4 [18]. Female wild-type control SD rats (Mecp2+/+) were obtained from Charles River Laboratories (Yokohama, Japan) at 6 weeks (w) of age and housed under the same conditions as mutants (n = 6 for each genotype, respectively). Briefly, all animals were housed in an animal room with temperature maintained at 18.0°C to 28.0°C, relative humidity at 30.0%–80.0%, and a 12-hour/12-hour light/dark cycle (lighting: 6:00 a.m. to 6:00 p.m.) with filtered fresh air changes 12 times per hour. The animals were housed individually in plastic cages (W: 310 × D: 360 × H: 175 mm) lined with autoclaved paper bedding and allowed free access to food and water.
Study schedule
Behavioral test batteries are described in the order conducted. The social interaction test was performed at 8, 12, and 23 w of age, the locomotor activity test at 16 and 23W, rotarod performance test at 26W, and Morris water maze (MWM) test at 29 w (Fig 1). One week after the final MWM tests, blood was collected from the postcava into 1-mL disposable polypropylene syringe (Terumo Corporation, Tokyo, Japan) using a 23G needle (Terumo Corporation) under isoflurane anesthesia (Isoflurane Inhalation Solution [Pfizer], Mylan Inc., Osaka, Japan). The animals were then euthanized by bleeding, and the brain was collected. Half of the brain was fixed in 4% paraformaldehyde phosphate buffer solution and processed for immunohistology while the other half was used for measurement of brain neurotransmitters.
Fig 1. Experimental schedules.
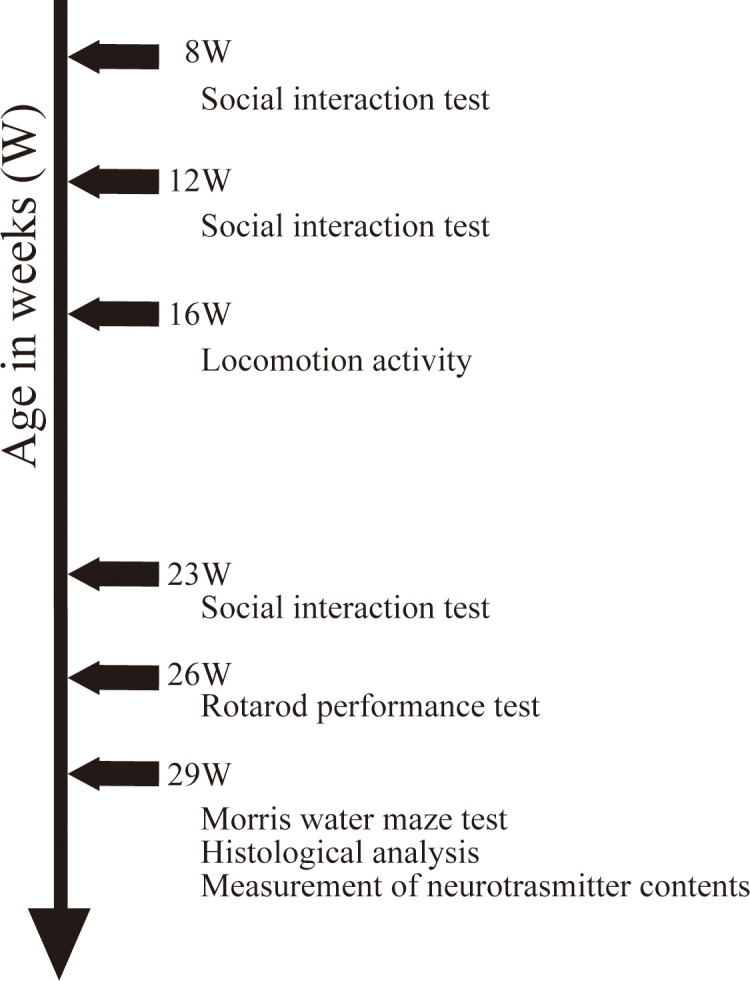
This figure describes the experimental schedules for behavioral test batteries and tissue sample preparations.
Behavioral test batteries
Social interaction test
The social interaction test was performed in a clean plastic cage of the same type used for animal housing. Two female Mecp2+/−or Mecp2+/+ rats that had never been in contact were placed together in the cage, and social behaviors were observed for 20 minutes under interior light through a transparent cage top with air vents. Frequencies and accumulated durations of contact behaviors (genital investigation, sniffing, and social grooming) and independent self-grooming were measured separately with a counter and a stopwatch.
Locomotor activity test
Locomotor activity over 2 consecutive 24-periods with 12-hour light and dark cycles (48 h) was analyzed at 30-minute intervals in the home cage using a computerized system (Multi digital 32 port count system, Neuroscience, Inc. Osaka, Japan).
Rotarod test
To evaluate motor coordination, the animal was placed on a rotarod (Rota-rod, ENV-577, Med. Associates, Inc., St. Albans, VT, U.S.A). The rotation speed was gradually accelerated from 4 to 40 rpm and the time (in seconds) and speed at which the rat fell off were measured within 5 minutes.
Morris water maze (MWM) test
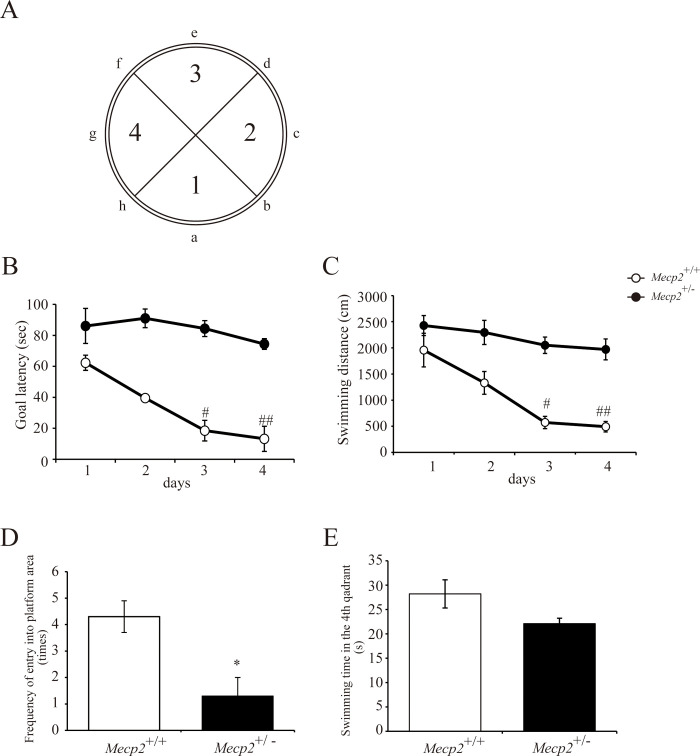
Spatial learning and memory were assessed in the MWM. The MWM apparatus consisted of a gray vinyl chloride circular pool (diameter: 148 cm, height: 44 cm) filled with water (17°C to 18°C) up to a height of about 32 cm so that a clear acrylic escape platform (12-cm in diameter) was submerged approximately 2 cm underneath the surface. The pool was divided onto four equal quadrants, with the escape platform located in the center of the fourth quadrant (see Fig 6A). In the acquisition phase, the animal was randomly placed in the water at a wall position between ‘a’ and ‘e’ (see Fig 6A) with the head facing the wall, and swimming behavior was recorded by a video camera set above the pool and displayed on a TV monitor. In acquisition trials, time to reach the platform (escape latency in seconds) and swimming distance (cm) were analyzed using a video tracking system (Etho Vision XT, Noldus Information Technology Inc., PA Wageningen, Netherlands) as indices of spatial learning. Acquisition trials were performed twice a day for 4 days (8 times in total). If the animal did not find the platform within 90 s, latency was recorded as 91 s and it was gently guided onto the platform and allowed to remain for 30 s. One day following the final acquisition trial, animals were subjected to a probe trial for spatial memory in which the escape platform was removed. The animal was released from c and allowed to swim freely for 90 s. Time in the former platform quadrant (quadrant 4) and number of platform position crossings were recorded as indices of spatial memory.
Fig 6. Impaired spatial learning and memory among female Mecp2+/− rats in the Morris water maze test.
The Morris water maze test was performed at 27 weeks of age. (A) Images of the Morris water maze indicating the release positions (a–h) and the first to fourth (target) quadrants. Acquisition trials for spatial learning with a hidden escape platform in quadrant 4 were performed twice per day for 4 days (8 times in total). Escape latency (B) and swimming distance (C) were measured. Graphs are mean ± standard error of the mean (SE) for the two daily trials (n = 6 rats per genotype). #p < 0.05 and ##p < 0.01 vs. female Mecp2+/+ rats on the same acquisition day, Friedman test). A probe trial was performed without the hidden platform for the confirmation of spatial memory on the day after completing acquisition trials. The frequency of entry into the platform area (D) and swimming time in the fourth (target) quadrant (E) were measured. Bar graphs are mean ± SE (n = 6 rats per genotype). *p < 0.05 vs. female Mecp2+/+ rats (unpaired two-tailed Mann–Whitney U-test).
High-performance liquid chromatography (HPLC) analysis of biogenic amine levels
Regional neurotransmitter levels were measured in excised brain tissue by isocratic HPLC separation and electrochemical detection. Briefly, the animals were euthanized by bleeding from the postcava under isoflurane anesthesia one week after the MWM test, and the brain tissues were collected in ice-cooled physiological saline. Using a metallic zinc brain matrix (ASI Instruments Inc. Warren, MI, U.S.A), each hemisphere was sliced into 2-mm thick coronal sections and divided into frontal cortex, amygdala, hippocampus, caudate nucleus, thalamus, hypothalamus, and medulla oblongata according to the rat brain atlas of Paxinos and Watson [20]. Each region was collected in a separate container, weighed, frozen in liquid nitrogen, and stored at −90°C to −70°C in a deep freezer (ULT-1186-3SI-A36, Thermo Electron Corporation Co., Ltd., Tokyo, Japan) until neurotransmitter measurements.
The frozen brain tissue was homogenized in 0.2 mol/L perchloric acid and centrifuged at 20227g and 4°C for 5 minutes. The following neurotransmitters and metabolites were measured from the supernatant using the HPLC-ECD system (Eicom Corporation, Kyoto, Japan): dopamine (DA) and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), serotonin (5-HT) and its metabolite 5-hydroxy indole-3-acetic acid (5-HIAA), norepinephrine (NE) and its metabolite 3-methoxyphenyl-4-hydroxy-glycol (MHPG), glutamate, γ-aminobutyric acid (GABA), and acetylcholine as follows.
The levels of monoamines (DA, 5-HT, and NE) and monoamine metabolites (DOPAC, HVA, 5-HIAA, and MHPG) in the brain tissue were estimated using an HPLC system equipped with a C18 reverse-phase column (Eicompak SC-5ODS, 150 mm × 3.0 ID, Eicom Corporation, Kyoto, Japan), guard column (PC-04, Eicom Corporation, Kyoto, Japan), and column thermostat (ATC-700) set to 25°C. Molecules were separated by a mixture of 0.1 mol/L acetic acid–citrate buffer (pH 3.5), methanol, 100 mg/mL sodium 1-octanesulfonate solution, and 5 mg/mL ethylenediaminetetraacetic acid (EDTA)-2Na solution (820:180:2.2:1) under the control of a pump system (EP-700) at a flow rate of 0.5 mL/min. For each measurement, 20 μL of the brain lysate supernatant was injected automatically using an automated sample injector (M-500). An electrochemical detector (ECD-700) with a graphite electrode (WE-3G), gasket, and Ag/AgCl reference electrode (RE-500) set to +750 mV was used for quantification.
Glutamate level was measured using an HPLC system equipped with a C18 reverse-phase column (Eicompak E-GEL, 150 mm × 4.6 ID, Eicom Corporation, Kyoto, Japan), enzyme column (E-EMZYMPAK, 4 mm × 3.0 ID, Eicom Corporation, Kyoto, Japan), guard column (PC-03, Eicom Corporation, Kyoto, Japan), and column thermostat (ATC-700) set to 33°C. A mixture of 60 mM ammonium chloride–ammonia solution (pH 7.2), hexadecyltrimethylammonium bromide, and 5 mg/mL EDTA-2Na solution (1000 mL:250 mg:0.01 mL) was used as the mobile phase under the control of the pump system (EP-700) at 0.37 mL/min. For each measurement, 20 μL of the brain lysate supernatant was injected automatically and the level measured using the electrochemical detector (ECD-700) with a platinum electrode (WE-PT), gasket, and Ag/AgCl reference electrode (RE-500) set at +450 mV.
GABA level was measured using an HPLC system equipped with a C18 reverse-phase column (Eicompak FA-3ODS, 50 mm × 3.0 ID, Eicom Corporation, Kyoto, Japan), guard column (PC-03, Eicom Corporation, Kyoto, Japan), and column thermostat (ATC-700) set to 40°C. A mixture of 0.1 mol/L phosphate buffer solution (pH 6.0), acetonitrile, and 5 mg/mL EDTA-2Na solution (500 mL:500 mL:1 mL) was used as the mobile phase under the control of the pump system (EP-700) at a flow rate of 0.5 mL/min. For each measurement, 20 μL sample was injected automatically using the automated sample injector (M-500) and the level measured using the electrochemical detector (ECD-700) with a graphite electrode (WE-GC), gasket, and Ag/AgCl reference electrode (RE-500) set at +600 mV.
Acetylcholine level was measured using an HPLC system equipped with a C18 reverse-phase column (Eicompak AC-GEL, 150 mm × 4.6 ID, Eicom Corporation, Kyoto, Japan), enzyme column (E-EMZYMPAK, 4 mm × 1.0 ID, Eicom Corporation, Kyoto, Japan), guard column (PC-03, Eicom Corporation, Kyoto, Japan), and column thermostat (ATC-700) set to 33°C. A mixture of 0.1 mol/L potassium bicarbonate solution, sodium decanesulfonate, and 5 mg/mL EDTA-2Na solution (1000 mL:400 mg:0.01 mL) was used as the mobile phase under the control of the pump system (EP-700) at a flow rate of 0.15 mL/min. For each measurement, 20 μL sample was injected automatically using the automated sample injector (M-500) and the level measured by the electrochemical detector (ECD-700) with a platinum electrode (WE-PT), gasket, and Ag/AgCl reference electrode (RE-500) set at +450 mV.
Tissue preparation and immunohistochemical analysis
The fixed brain tissue was embedded in paraffin using the routine method of our testing facility and cut into 5-μm thick sections. Every third section was processed for immunohistochemical staining with anti-glial fibrillary acidic protein (GFAP) antibody (×2000, Abcam, ab 7260). The three to five sections of choice were those closest to dorsal–ventral level interaural 3.20 mm (for frontal cortex) and -3.80 mm (for the hippocampus, hypothalamus, and amygdala) according to Paxinos and Watson [20]. Immunostained sections of the frontal cortex and hippocampus were photographed using an optical microscope with a digital camera, and GFAP-positive cells were counted using WinROOF V7.0 software.
Hematological examination
Plasma was collected by centrifugation of the collected blood (at 4°C and 2150×g for 15 minutes). Plasma insulin-like growth factor 1 (IGF-1) was estimated using the Quantikine® ELISA kit (Mouse/Rat IGF-I immunoassay, Cosmo Bio Co., Ltd., Tokyo, Japan) and plasma BDNF using the RayBio® Rat BDNF ELISA Kit (Ray Biotech. Inc., Peachtree Corners, GA, U.S.A).
Statistical analysis
Data are presented as the mean ± standard error of the mean (SE). All statistical analyses were performed using GraphPad Prism version 9.1.2 for Windows (GraphPad Software Inc., San Diego, CA, U.S.A, www.graphpad.com), according to Prism9 Statistics Guide (https://www.graphpad.com/guides/prism/latest/statistics/index.htm). All details of statistics have been indicated in figure legends. Because the sample size for each experiment was small, it was not appropriate to consider that the sample was normally distributed. Therefore, when comparing two independent groups, the unpaired two-tailed Mann–Whitney U-test was used. For the analysis of statistical significance in comparisons involving more than two groups, Kruskal–Wallis with Dunn’s post hoc multiple comparisons test was used. When comparing three or more matched groups, the Friedman test was used. In all cases, statistical significance was assessed with a 95% confidence interval; therefore, p < 0.05 was considered significant.
Results
Impaired social behaviors in Mecp2-deficient female rats
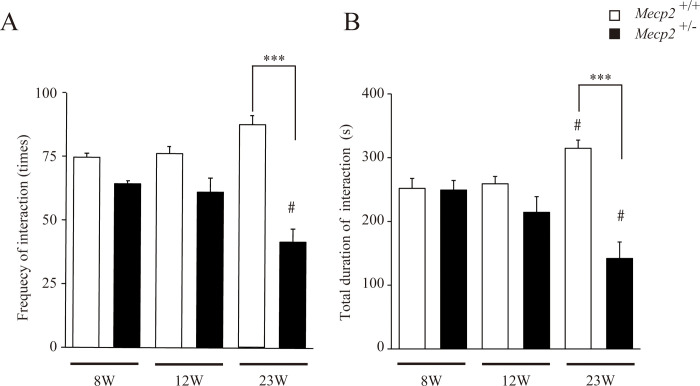
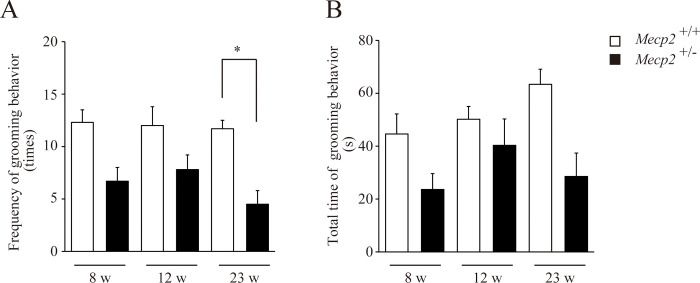
We first analyzed the effect of Mecp2 gene deficiency on rat social behaviors at 8, 12, and 23 w. The frequency and duration of contact behaviors gradually increased with age among female Mecp2+/+ rats but gradually declined with age among female Mecp2+/− rats, and both parameters were significantly lower compared to female Mecp2+/+ rats by 23 w (Fig 2A and 2B). We next analyzed self-grooming behavior during the social interaction test as higher frequency and longer duration are considered endophenotypic hyper-repetitive behaviors of autism spectrum disorder [21–23] and male Mecp2 mutant mice [24–26]. The frequencies (Fig 3A) of self-grooming were significantly reduced and the duration (Fig 3B) tended to be reduced but not significant in female Mecp2+/− rats. Thus, the deficiency in Mecp2 impaired social behaviors with reduced self-grooming.
Fig 2. Age-dependent alterations in social behaviors among female Mecp2+/− rats.
Female Mecp2+/− rats exhibited progressively less frequent social interactions with age, while social interactions increased with age among female Mecp2+/+ (wild-type) rats. Bar graphs indicate frequency of contact behaviors (A) and total duration of interactions (B) during a 20-min social interaction test (mean ± SE; n = 6 rats per genotype). #p < 0.05, and ***p < 0.001 vs. same genotyped female Mecp2+/− rats at 8 weeks (w) (Friedman test), and the age-matched female Mecp2+/+ rats at 23 w of age (Kruskal–Wallis with Dunn’s post hoc multiple comparisons test), respectively.
Fig 3. Age-dependent changes in self-grooming behavior among female Mecp2+/− rats.
Both frequency (A) and total time (B) of self-grooming tended to be lower in female Mecp2+/− rats compared to female Mecp2+/+ rats during the 20-min social interaction test at 8, 12, and 23 w of age (mean ± SE; n = 6 rats per genotype). *p < 0.05 vs. age-matched female Mecp2+/+ rats (Kruskal–Wallis with Dunn’s post hoc multiple comparisons test).
Impaired spontaneous locomotor activity and motor coordination in Mecp2-deficient female rats
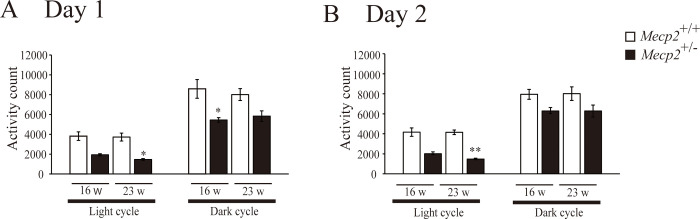
To evaluate the effects of Mecp2 deficiency on motor function, we first analyzed spontaneous locomotor activity over 2 consecutive 24-periods with 12-h/12-h light/dark cycles at 16 w and 23 w. Spontaneous locomotor activity was significantly or tended to be lower among female Mecp2+/− rats compared to female Mecp2+/+ rats (Fig 4) during both light and dark phases. In addition, judging from the locomotor activity counts every 30 minutes, Mecp2 deficiency did not seem to affect circadian rhythms because female Mecp2+/- rat shows a tendency to decrease activity over the entire measurement time, not at any specific time point (S1 Fig). To assess motor coordination, animals were tested on the accelerating rotarod at 26 w. Both rotation time and speed at which the rat fell off were lower in the Mecp2+/− group compared with the Mecp2+/+ group (Fig 5A and 5B). Thus, based on the RTT symptoms, both spontaneous and task-evoked motor activity were impaired by Mecp2+/− deficiency.
Fig 4. Impaired locomotor activity among female Mecp2+/− rats during both light and dark phases at 16 and 23 weeks of age.
Bar graphs are mean ± SE (n = 6 rats per genotype). *p < 0.05, and **p < 0.01 vs. age-matched female Mecp2+/+ rats (Kruskal–Wallis with Dunn’s post hoc multiple comparisons test).
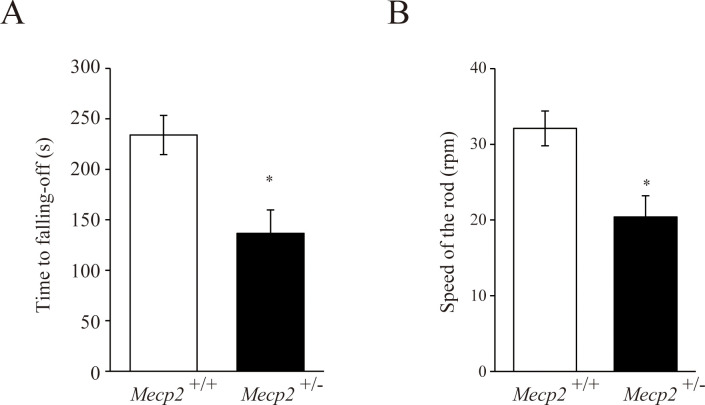
Fig 5. Impaired motor coordination among female Mecp2+/− rats at 26 weeks of age.
The animal was placed on a rotarod, which was accelerated from 4 to 40 rpm. The time at which the animal fell off (A) and the speed at which the animal fell off (B) were measured. Bar graphs are mean ± SE (n = 6 rats per genotype). *p < 0.05 vs. female Mecp2+/+ rats (unpaired two-tailed Mann–Whitney U-test).
Impaired spatial learning and memory in Mecp2-deficient female rats
To examine the effect of Mecp2 deficiency on spatial learning and memory, we compared escape latency and target memory between female Mecp2+/− and Mecp2+/+ rats in the MWM starting on 27 w (first acquisition trial). Compared to Mecp2+/+ rats, Mecp2+/− rats demonstrated significantly longer escape latencies (Fig 6B) and swimming distances (Fig 6C) over the four acquisition trial days. Further, Mecp2+/− rats made fewer crossing over the former platform location (Fig 6D) and tended to spend less time in the target (former platform) quadrant (Fig 6E). Thus, Mecp2 deficiency appears to impair spatial learning and memory.
Reduced acetylcholine but relatively normal biogenic amine levels in multiple brain regions of Mecp2-deficient rats
Several studies have reported significant downregulation of biogenic amines in the brains of Mecp2 mouse mutants [27–31]. Surprisingly, however, biogenic amine and its metabolite levels (Table 1) and the glutamate/GABA ratio (Table 2) were also near normal in most measured regions of female Mecp2+/− rats. The genotypic differences in the biogenic amine/metabolite levels were observed in such brain regions as follows: DA and DOPAC levels were comparable to those in the caudate nucleus and medulla oblongata, although HVA level tended to be lower in the caudate nucleus and was slightly but significantly lower in the medulla oblongata of female Mecp2+/− rats. 5-HT level was lower in the thalamus and hypothalamus of female Mecp2+/− rats (Table 1). By contrast, the relative reductions in acetylcholine level were greater than those of other transmitters, including biogenic amines: acetylcholine level was substantially lower in the amygdala, hippocampus, caudate nucleus, and medulla oblongata (Table 3). These results suggest that impaired cholinergic signaling could severely affect the behavioral abnormalities observed in Mecp2+/− rats. Indeed, cholinergic inputs to the hippocampus are essential for spatial learning and memory [32, 33].
Table 1. Monoamine and monoamine metabolite levels in various brain regions of wild-type (Mecp2+/+) and Mecp2-deficient (Mecp2+/−) female rats.
| Levels (ng/g wet weight) | ||||||||
|---|---|---|---|---|---|---|---|---|
| NE | MHPG | DA | DOPAC | HVA | 5-HT | 5-HIAA | ||
| Frontal cortex | Mecp2 +/+ | 521.4 ± 69.2 | 281.9 ± 38.0 | 67.0 ± 7.5 | 23.3 ± 2.0 | 6.9 ± 0.9 | 193.8 ± 27.2 | 130.9 ± 16.3 |
| Mecp2 +/− | 400.2 ± 16.5 | 321.5 ± 34.5 | 83.2 ± 4.3 | 30.6 ± 2.7 | 6.7 ± 1.6 | 215.7 ± 23.6 | 162.0 ± 10.7 | |
| Amygdala | Mecp2 +/+ | 645.7 ± 55.9 | 239.4 ± 24.3 | 132.4 ± 14.8 | 23.1 ± 5.1 | 8.9 ± 2.7 | 263.9 ± 44.3 | 170.1 ± 22.7 |
| Mecp2 +/− | 590.7 ± 79.7 | 313.2 ± 45.8 | 103.1 ± 22.8 | 19.3 ± 2.1 | 5.8 ± 1.1 | 231.6 ± 61.3 | 190.9 ± 33.4 | |
| Hippocampus | Mecp2 +/+ | 741.6 ± 59.7 | 334.4 ± 17.9 | 23.0 ± 2.1 | 1.4 ± 0.4 | 1.1 ± 0.1 | 200.1 ± 15.9 | 243.5 ± 10.9 |
| Mecp2 +/− | 606.7 ± 43.0 | 368.2 ± 30.9 | 23.8 ± 1.7 | 1.6 ± 0.3 | 0.9 ± 0.0 | 175.5 ± 8.2 | 220.8 ± 9.1 | |
| Caudate nucleus | Mecp2 +/+ | 21.2 ± 0.9 | 194.8 ± 17.9 | 4106.8 ± 267.1 | 621.4 ± 62.6 | 183.2 ± 28.0 | 92.9 ± 8.0 | 207.5 ± 16.2 |
| Mecp2 +/− | 31.0 ± 5.2 | 263.4 ± 51.5 | 4188.6 ± 472.2 | 607.6 ± 82.7 | 125.4 ± 28.8 | 89.5 ± 14.8 | 216.0 ± 38.2 | |
| Thalamus | Mecp2 +/+ | 858.0 ± 32.8 | 324.6 ± 21.8 | 131.3 ± 3.3 | 16.7 ± 1.3 | 9.4 ± 1.6 | 512.0 ± 11.0 | 447.5 ± 18.0 |
| Mecp2 +/− | 847.9 ± 56.7 | 349.0 ± 26.5 | 114.7 ± 10.4 | 15.8 ± 1.9 | 7.3 ± 1.2 | 423.4 ± 22.5* | 422.8 ± 27.9 | |
| Hypothalamus | Mecp2 +/+ | 2244.2 ± 160.9 | 286.9 ± 30.2 | 234.1 ± 44.0 | 30.4 ± 5.3 | 5.3 ± 0.9 | 439.3 ± 29.6 | 311.5 ± 27.7 |
| Mecp2 +/− | 2150.7 ± 286.9 | 328.5 ± 30.9 | 192.5 ± 20.7 | 31.4 ± 5.6 | 5.2 ± 1.1 | 322.2 ± 38.7* | 266.7 ± 19.5 | |
| Medulla oblongata | Mecp2 +/+ | 940.1 ± 37.6 | 435.0 ± 34.1 | 39.8 ± 1.4 | 7.4 ± 0.6 | 5.6 ± 0.9 | 432.5 ± 14.2 | 219.9 ± 11.3 |
| Mecp2 +/− | 1004.4 ± 35.3 | 540.3 ± 37.6 | 38.5 ± 0.9 | 6.4 ± 0.4 | 3.2 ± 0.2* | 434.3 ± 22.6 | 225.9 ± 12.7 | |
NE: norepinephrine; MHPG: 3-methoxyphenyl-4-hydroxy-glycol hemipiperazium; DA: dopamine; DOPAC: 3,4-dihydroxyphenylacetic acid; HVA: homovanillic acid; 5-HT: 5-hydroxytryptamine (serotonin); 5-HIAA: 5-hydroxy indole-3-acetic acid.
Data was expressed as mean ± SE (n = 6 samples per region and genotype).
*p < 0.05 female Mecp2+/- vs. female Mecp2+/+ rats by unpaired two-tailed Mann–Whitney U-test.
Table 2. Glutamate/GABA ratios across brain regions.
| Frontal cortex | Amygdala | Hippocampus | Caudate nucleus | Thalamus | Hypothalamus | Medulla oblongata | |
|---|---|---|---|---|---|---|---|
| Mecp2 +/+ | 7.9 ± 0.9 | 5.5 ± 0.3 | 6.5 ± 0.3 | 6.9 ± 0.4 | 2.3 ± 0.2 | 1.4 ± 0.2 | 4.7 ± 0.3 |
| Mecp2 +/− | 8.3 ± 0.8 | 6.1 ± 0.8 | 6.9 ± 0.4 | 5.0 ± 0.5 | 2.5 ± 0.1 | 1.5 ± 0.1 | 5.1 ± 0.6 |
Data expressed as mean ± SE (n = 6 for each genotype). Non significance female Mecp2+/- vs. Mecp2+/+ rats (unpaired two-tailed Mann–Whitney U-test).
Table 3. Acetylcholine levels across brain regions.
| Frontal cortex | Amygdala | Hippocampus | Caudate nucleus | Thalamus | Hypothalamus | Medulla oblongata | |
|---|---|---|---|---|---|---|---|
| Mecp2 +/+ | 480.9 ± 129.6 | 1066.1 ± 139.1 | 949.0 ± 86.3 | 742.1 ± 77.4 | 771.5 ± 49.0 | 546.7 ± 80.8 | 776.1 ± 146.0 |
| Mecp2 +/− | 333.1 ± 38.5 | 475.7 ± 85.5** | 649.4 ± 64.6* | 478.3 ± 35.6* | 675.8 ± 82.8 | 376.4 ± 81.9 | 401.8 ± 23.0** |
Data expressed as mean ± SE (n = 6 for each genotype).
*p < 0.05 and
**p < 0.01 female Mecp2+/- vs. Mecp2+/+ rats (unpaired two-tailed Mann–Whitney U-test).
Changes in GFAP-positive astrocytes and serum IGF
To further examine neurodevelopment abnormalities associated with Mecp2 deficiency, we examined the number and morphological characteristics of GFAP-positive astrocytes. Astrocytes are known to support neuronal function through production and secretion of various extrinsic factors and by the uptake of neurotransmitters, and the number and morphological features are associated with neurodevelopmental abnormalities as well as neurodegeneration and neuroinflammation [34, 35]. Further, abnormal astrocyte morphology has been observed in RTT patients and models [36, 37]. Comparing with those of female Mecp2+/+ rats (hippocampus, Fig 7A, a-c; frontal cortex, Fig 7B, a and b; amygdala, Fig 8A, a and b; hypothalamus, Fig 8B, a and b; respectively), the somata of GFAP-positive cells were smaller and possessed less complex processes in the hippocampus (Fig 7A, d-f), frontal cortex (Fig 7B, c and d) and amygdala (Fig 8A, c and d) but not in the hypothalamus (Fig 8B, c and d) of female Mecp2+/− rats. In addition, cell numbers were significantly lower in frontal cortex, hippocampus and amygdala, but not in hypothalamus (Table 4). Finally, we also examined potential neurotrophin signaling deficits by measuring serum levels of IGF and BDNF [38]. Both neurotrophic factors play important roles in survival, differentiation and functional development for brain neuronal circuits. Compared to female Mecp2+/+ rats, plasma IGF-I was significantly reduced but plasma BDNF was normal in the female Mecp2+/− rats (Table 5).
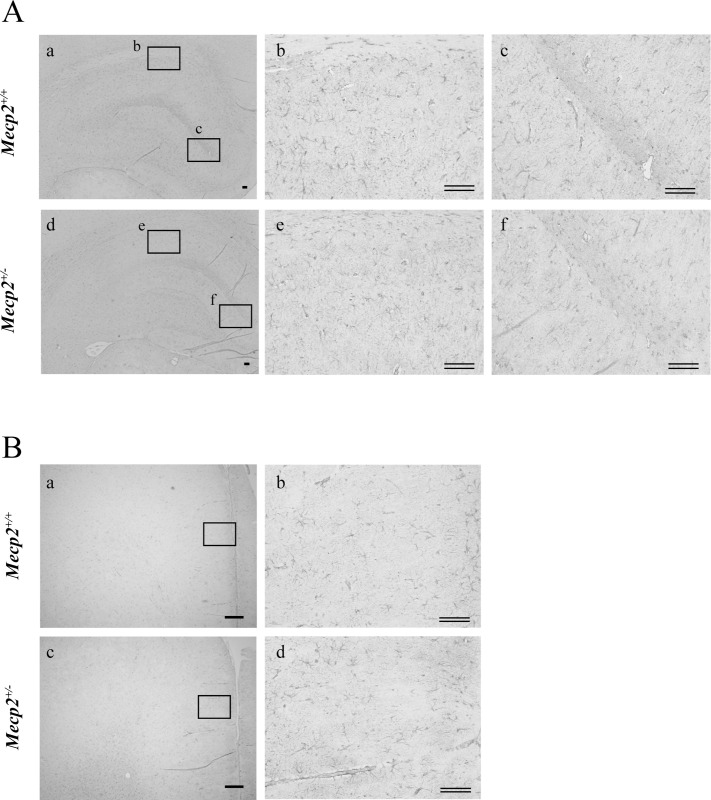
Fig 7. Changes in the number of glial fibrillary acidic protein (GFAP)-positive cells (astrocytes) in the hippocampus and frontal cortex of a female Mecp2+/− rat brain.
The brain was collected one week after the Morris water maze test and immunostained for the astrocytic marker GFAP. Shown are the morphology and the number of GFAP-positive cells in the hippocampus (A: a-c: Mecp2+/+, b and c: higher magnification of square in a; d-f, Mecp2+/−, e and f: higher magnification of square in d), the frontal cortex (B: a and b: Mecp2+/+; c, d: Mecp2+/−; b and d: higher magnification of square in a and c, respectively). Scale bar = 300 μm; double bars = 100 μm, respectively.
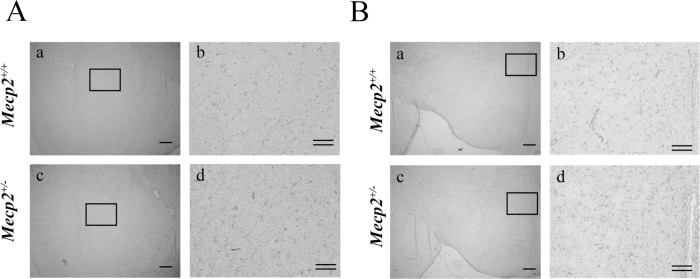
Fig 8. Number of glial fibrillary acidic protein (GFAP)-positive cells (astrocytes) in the hypothalamus and amygdala of a female Mecp2+/− rat brain.
Shown are the morphology and number of GFAP-positive cells (A. amygdala, B. hypothalamus: a and b: Mecp2+/+; c and d: Mecp2+/−; b and d: higher magnification of square in a and c, respectively). Scale bar = 300 μm; double bars = 100 μm, respectively.
Table 4. Number of GFAP-positive cells across brain regions.
| Number of GFAP-positive cells/slice | ||
|---|---|---|
| Mecp2 +/+ | Mecp2 +/− | |
| Frontal cortex | 1108.2 ± 33.5 | 609.5 ± 66.0 ** |
| Hippocampus | 476.5 ± 15.4 | 347.3 ± 11.7 ** |
| Amygdala | 51.8 ± 1.5 | 49.5 ± 2.3** |
| Hypothalamus | 39.8 ± 4.6 | 39.0 ± 2.7 |
Data expressed as mean ± SE (n = 6 for each genotype).
**p < 0.01 female Mecp2+/- vs. Mecp2+/+ rats (unpaired two-tailed Mann–Whitney U-test).
Table 5. Plasma IGF and BDNF levels.
| Mecp2 +/+ | Mecp2 +/− | |
|---|---|---|
| IGF-1 (ng/mL) | 1080.2 ± 70.3 | 769.4 ± 52.4 ** |
| BDNF (pg/mL) | 46.4 ± 5.9 | 51.9 ± 18.2 |
Data expressed as mean ± SE [IGF-1: n = 6 and BDNF: n = 2 (n = 4, under detection limit), for each genotype, respectively)].
**p < 0.01 female Mecp2+/- vs. Mecp2+/+ rats (unpaired two-tailed Mann–Whitney U-test).
Discussion
We demonstrated that Mecp2-deficient female rats (Mecp2+/−) exhibit multiple behavioral abnormalities resembling those of RTT, including progressively impaired social behavior with reduced self-grooming (at 8, 12, and 23 w, Figs 2 and 3), reduced spontaneous locomotor activity (at both 16 and 23 w, Fig 4, and S1 Fig), poor motor coordination (at 26 w, Fig 5), and deficient spatial cognition (at 27 w, Fig 6). Further, these animals exhibited substantially lower acetylcholine levels in multiple brain regions compared to wild types (Mecp2+/+), suggesting pervasive deficits in cholinergic signaling. The phenotype of this rat model basically recapitulates that of Mecp2-deficient mouse mutants [8, 17, 18] but in addition also suggests the importance of cholinergic signaling deficits to RTT pathogenesis or symptom expression. In fact, the regional reductions in acetylcholine were relatively larger than the reductions or elevations in other neurotransmitters and their metabolites, including the changes in monoamine levels. The relative stability of monoamine levels is in contrast to predominantly male Mecp2tm 1.1 Bird exon 3/4 null mutant mice [27–30] and Mecp2tm 1.1 Jae exon 3 null mutant mice [31]. We suggest that these low acetylcholine levels across multiple brain regions may contribute to the postnatal behavioral abnormalities observed in Mecp2+/− rats and possibly to RTT [4, 5].
Progressive impairments in social behavior and abnormalities in motor coordination and spatial cognition
Patients with RTT have autistic symptoms such as social behavior abnormalities. While these symptoms have been reported to improve with age [39], some persist through life [40, 41]. Consistent with findings in female Mecp2tm1.1Bird mice [42], female Mecp2+/− rats demonstrated a lower frequency of social contact in the open field, and both frequency and duration of contact decreased with age (from 8 to 23 w). Certain abnormalities in social behavior have been observed among Mecp2+/− female rats even before 4 w [17, 18], so social deficits appear to be among the earliest onset and most severely progressive. However, the influence of progressive motor dysfunction on social behaviors cannot be excluded, especially at early ages [17, 18] (Figs 2 and 3). For instance, Adcock et al. reported no significant differences in social behavior between female Mecp2+/− and Mecp2+/+ rats following auditory discrimination training or skilled forelimb motor training, suggesting that poor perceptual and (or) motor skills may contribute to social deficits in untrained rats [43]. Further, environmental enrichment [44, 45], which is known to improve motor function and cognition, can partially mitigate the developmental symptoms in female Mecp2+/− rats. Despite such limitations, the influence of training for behavioral test battery and environment and so on, should be considered on the interpretation of results; the consistency and progressive nature of these social deficits may be useful for preclinical studies on treatments to improve autistic symptoms among RTT patients.
Poor spatial memory is also a critical feature of the rat model as severe intellectual disability is a hallmark of RTT [46, 47]. Although spatial learning and memory impairments are not included in the diagnostic criteria for RTT [3, 48, 49], spatial learning and memory are critical for rodent survival and thus impairments are sensitive indicators of brain maldevelopment and dysfunction, particularly hippocampal dysfunction. Further, hippocampal dysfunction is a shared cause of cognitive deficits among numerous neurodegenerative and neurodevelopmental diseases. In this study, we used MWM [50, 51] to demonstrate that spatial learning and memory are markedly impaired in adult (29W) female Mecp2+/− rats (Fig 6). The effects of Mecp2 gene mutations on spatial learning in the MWM are known to vary by mutation type, with impairments observed among Mecp2tm 1.1 Bird mice [43, 44] but not female Mecp2 tm 1.1 Jae mice [52, 53]. Alternatively, abnormalities in recognition memory have been observed across many mutations. Since the Mecp2+/− rat is an outbred strain, it may be useful for extracting phenotypes common across species and genotypes. Although further studies using inbred strains and rats with different Mecp2 mutations are needed, the differences in hippocampus-dependent cognition between mouse and rat models may provide important clues to the underlying neuroanatomical and neurochemical changes. For instance, our current findings suggest that poor MWM performance may stem from deficient cholinergic signaling in the hippocampus of female Mecp2+/− rats.
Relationships between cholinergic and behavioral abnormalities
Many neuroanatomical and neurochemical changes have been reported in RTT patients, but the molecular mechanisms through which MECP2 gene mutations lead to pathological alterations are unknown. Acetylcholine level was significantly reduced in multiple brain regions of female Mecp2-deficient rats and associated with impaired social behavior, motor activity, and spatial cognition (Table 3). In particular, the importance of cholinergic neurons to cognitive function and mood disorders is well established [32, 33]. Clinical studies have shown decreases in the cholinergic neuron number [54], acetylcholine biosynthetic enzymes choline acetyltransferase (ChAT) and vesicular acetylcholine transporter activities, and cholinergic receptor expression [55, 56] in the brains of patients with RTT, and the changes in cholinergic neuron markers are linked to the severity of symptoms [57, 58]. Abnormalities in the cholinergic system have also been reported in Mecp2 mutant mice, including reduced ChAT expression in basal forebrain and striatum [59, 60] and locus coeruleus (LC) neurons [61]. In addition, abnormalities in memory performance and social behavior among Mecp2 null mutants were reversed by administration of acetylcholine receptor agonists and the cholinesterase inhibitor donepezil [58, 62].
While acetylcholine level was markedly reduced in female Mecp2+/− rats, relative changes in monoamines, monoamine metabolites, and the glutamic acid/GABA ratio were smaller and observed in a more limited number of brain regions. This result was unexpected because the levels of many monoamine metabolites as well as expression levels of rate-limiting biosynthetic enzymes have been shown to decline with age in male Mecp2tm1.1Bird mice [27, 30]. The result was also unexpected because the disorders of involuntary movements in RTT (progressive rigidity, dyskinesia, and dystonia) have been suggested to be associated with the maldevelopment of the dopaminergic nervous system [63–65]. It is possible that some compensatory mechanism may make it difficult to detect impairment in the monoaminergic nervous system because approximately half of the neurons in female Mecp2-mutant mouse brain maintain normal Mecp2 expression [31, 36]. Recently, Wong et al. reported that the density of dopamine D2 receptor was significantly decreased in the striatum of Mecp2tm 1.1 Bird null/heterozygous mutant mice at 7–10 w but not after 15 w. They pointed out that such developmental stage-dependent alteration could affect the measured density of D2 receptor in the RTT female brain postmortem [66]. Therefore, we could not exclude the possibility that some behavioral abnormalities observed in this study may be influenced by monoaminergic disturbances—particularly at earlier developmental stages. In fact, 5-HT levels in the thalamus and hypothalamus as well as the level of the dopamine metabolite HVA in the medulla oblongata were significantly reduced (Table 1), suggesting some abnormalities in the monoaminergic system development.
Self-grooming behavior in Mecp2+/− rats was reported to be equivalent to that of the control group (at 4 w [18]) and tended to be, or even was, significantly decreased in this study (at 8 w, 12 w, and 23 w, Fig 3). Excessive self-grooming behavior is known to be suppressed by acetylcholine antagonists [67–69]. Therefore, progressive impairment in the cholinergic nervous system is likely to explain the self-grooming behavioral abnormalities. Excessive self-grooming behavior, however, is a typical symptom of autism model mice [21–23] and has also been observed in Mecp2 null male mice [24–26]. Monoamine reductions have been found not only in postmortem brain tissue but also in the cerebrospinal fluid (CSF) of patients with RTT [28, 70]; CSF measures during development should therefore be performed in the rat model also to clarify the relationship of disturbances in the cholinergic/monoaminergic system and behavioral changes. Such efforts, in future studies, would lead to an understanding of the complex pathological changes due to mutation in the MECP2 gene, leading to the development of optimal drug treatment strategies.
Value of the Mecp2+/− rat model for RTT diagnosis and treatment
We also observed reduced numbers of astrocytes (GFAP-positive cells) in the frontal cortex and hippocampus and as well as lower plasma IGF in female Mecp2+/− rats (Fig 7 and Tables 4 and 5), consistent with previous findings of stunted morphological and functional development of astrocytes [36, 37, 71] and reduced IGF signaling [38, 72, 73] in both RTT patients and Mecp2 mutant mice. Astrocytes regulate neuronal functions through production and secretion of growth factors, nutrients, and cytokines and by the uptake of transmitters and metabolites [74, 75], while IGF is involved in the differentiation and functional maintenance of neurons as a blood–brain barrier permeable neurotrophic factor [76, 77]. The identification of blood or CSF biomarkers associated with specific neuropathological processes may aid in RTT diagnosis and provide clues to pathogenic mechanisms, and the female Mecp2+/− rat model may be a powerful tool for this purpose. Candidate biomarkers for pathogenesis may include metabolites generated by astrocytes [78] and/or molecules associated with IGF signaling [79].
Supporting information
A-D: 24-h locomotor activity in female Mecp2+/+ (open symbols) and Mecp2+/- (filled symbols) rats. Data are the average ± SE of data collected at each half an hour from 16 w (A, B) or 23 w (C, D) old rats over 2 consecutive 24-h periods (A, C: day1; B, D: day2) (n = 6, for each geneotype). Non significance female Mecp2+/- vs. Mecp2+/+ rats (Kruskal–Wallis with Dunn’s post hoc multiple comparisons test).
(TIF)
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- ChAT
Choline acetyltransferase
- CSF
Cerebrospinal fluid
- DA
Dopamine
- DBH
Dopamine β-hydroxylase
- DOPAC
3,4-dihydroxyphenylacetic acid
- EDTA
Ethylenediaminetetraacetic acid
- ELISA
Enzyme-linked immunosorbent assay
- 5-HT
Serotonin
- 5-HIAA
5-hydroxy indole-3-acetic acid
- GABA
γ-aminobutyric acid
- GFAP
Glial fibrillary acidic protein
- IGF-1
Insulin-like growth factor 1
- LC
Locus coeruleus
- MECP2
Methyl-CpG-binding protein 2
- MHPG
3-methoxyphenyl-4-hydroxy-glycol
- MWM
Morris water maze
- NE
Norepinephrine
- NMDA
N-methyl-D-aspartate
- HPLC
High-performance liquid chromatography
- HPLC-ECD
High-performance liquid chromatography with an electrochemical detector
- HVA
Homovanillic acid
- RTT
Rett syndrome
- SD
Sprague Dawley
- SE
Standard error of the mean
- TH
Tyrosine hydroxylase
- TPH
Tryptophan hydroxylase
- w
Weeks
Data Availability
All relevant data are within the manuscript.
Funding Statement
The funder, Nihon Bioresearch Inc, provided support in the form of salaries for authors: HM, HK, JI, and TN, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1.Laurvick CL, de Klerk N, Bower C, Christodoulou J, Ravine D, Ellaway C, et al. Rett syndrome in Australia: a review of the epidemiology. J Pediatr. 2006;148: 347–352. doi: 10.1016/j.jpeds.2005.10.037 [DOI] [PubMed] [Google Scholar]
- 2.Percy AK, Neul JL, Glaze DG, Motil KJ, Skinner SA, Khwaja O, et al. Rett syndrome diagnostic criteria: Lessons from the Natural History Study. Ann Neurol. 2010;68: 951–955. doi: 10.1002/ana.22154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, et al. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68: 944–950. doi: 10.1002/ana.22124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neul JL. The relationship of Rett syndrome and MECP2 disorders to autism. Dial Clin Neurosci. 2012;14: 253–262. doi: 10.31887/DCNS.2012.14.3/jneul [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weng SM, Bailey ME, Cobb SR. Rett syndrome: from bed to bench. Pediatr Neonatol. 2011;52: 309–316. doi: 10.1016/j.pedneo.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 6.Christodoulou J, Grimm A, Maher T, Bennetts B. RettBASE: The IRSA MECP2 variation database-a new mutation database in evolution. Hum Mutat. 2003;21: 466–472. doi: 10.1002/humu.10194 [DOI] [PubMed] [Google Scholar]
- 7.Jin XR, Chen XS, Xiao L. MeCP2 deficiency in neuroglia: new progress in the pathogenesis of Rett syndrome. Front Mol Neurosci. 2017;10: 316. doi: 10.3389/fnmol.2017.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro MC, MacDonald JL. Sex differences in Mecp2-mutant Rett syndrome model mice and the impact of cellular mosaicism in phenotype development. Brain Res. 2020;1729: 146644. doi: 10.1016/j.brainres.2019.146644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27: 322–326. doi: 10.1038/85899 [DOI] [PubMed] [Google Scholar]
- 10.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27: 327–331. doi: 10.1038/85906 [DOI] [PubMed] [Google Scholar]
- 11.Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci U S A. 2007;104: 1931–1936. doi: 10.1073/pnas.0610593104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315: 1143–1147. doi: 10.1126/science.1138389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luikenhuis S, Giacometti E, Beard CF, Jaenisch R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci U S A. 2004;101: 6033–6038. doi: 10.1073/pnas.0401626101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomathi M, Padmapriya S, Balachandar V. Drug studies on Rett syndrome: From bench to bedside. J Autism Dev Disord. 2020;50: 2740–2764. doi: 10.1007/s10803-020-04381-y [DOI] [PubMed] [Google Scholar]
- 15.Pellis SM, Pellis VC. Play fighting of rats in comparative perspective: a schema for neurobehavioral analyses. Neurosci Biobehav Rev. 1998;23: 87–101. doi: 10.1016/s0149-7634(97)00071-7 [DOI] [PubMed] [Google Scholar]
- 16.Einon DF, Humphreys AP, Chivers SM, Field S, Naylor V. Isolation has permanent effects upon the behavior of the rat, but not the mouse, gerbil, or guinea pig. Dev Psychobiol. 1981;14: 343–355. doi: 10.1002/dev.420140407 [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Zhong W, Cui N, Johnson CM, Xing H, Zhang S, et al. Characterization of Rett syndrome-like phenotypes in Mecp2-knockout rats. J Neurodev Disord. 2016;8: 23. doi: 10.1186/s11689-016-9156-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veeraragavan S, Wan YW, Connolly DR, Hamilton SM, Ward CS, Soriano S, et al. Loss of MeCP2 in the rat models regression, impaired sociability and transcriptional deficits of Rett syndrome. Hum Mol Genet. 2016;25: 3284–3302. doi: 10.1093/hmg/ddw178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson KC, Hawkins VE, Arps KM, Mulkey DK, Olsen ML. MeCP2 deficiency results in robust Rett-like behavioural and motor deficits in male and female rats. Hum Mol Genet. 2016;25: 3303–3320. doi: 10.1093/hmg/ddw179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paxinos G, Watoson C. The rat brain in stereotaxic coordinates. 6th ed. London: Academic Press; 2007. [Google Scholar]
- 21.Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472: 437–442. doi: 10.1038/nature09965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17: 400–406. doi: 10.1038/nn.3641 [DOI] [PubMed] [Google Scholar]
- 23.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11: 490–502. doi: 10.1038/nrn2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Z, Liu Z, Mao W, Wang X, Zheng X, Chen S, et al. Locus-specific DNA methylation of Mecp2 promoter leads to autism-like phenotypes in mice. Cell Death Dis 2020;11: 85. doi: 10.1038/s41419-020-2290-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrette LLG, Blum R, Ma W, Kelleher RJ 3rd, Lee JT. Tsix–Mecp2 female mouse model for Rett syndrome reveals that low-level MECP2 expression extends life and improves neuromotor function Proc Natl Acad Sci U S A. 2018;115: 8185–8190. doi: 10.1073/pnas.1800931115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahgoub M, Adachi M, Suzuki K, Liu X, Kavalali ET, Chahrour MH, et al. MeCP2 and Histone Deacetylases 1 and 2 in dorsal striatum collectively suppress repetitive behaviors. Nat Neurosci 2016;19: 1506–1512. doi: 10.1038/nn.4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ide S, Itoh M, Goto Y. Defect in normal developmental increase of the brain biogenic amine concentrations in the mecp2-null mouse. Neurosci Lett. 2005;386: 14–17. doi: 10.1016/j.neulet.2005.05.056 [DOI] [PubMed] [Google Scholar]
- 28.Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci U S A. 2009;106: 21966–21971. doi: 10.1073/pnas.0912257106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos M, Summavielle T, Teixeira-Castro A, Silva-Fernandes A, Duarte-Silva S, Marques F, et al. Monoamine deficits in the brain of methyl-CpG binding protein 2 null mice suggest the involvement of the cerebral cortex in early stages of Rett syndrome. Neuroscience. 2010;170: 453–467. doi: 10.1016/j.neuroscience.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 30.Panayotis N, Ghata A, Villard L, Roux JC. Biogenic amines and their metabolites are differentially affected in the Mecp2-deficient mouse brain. BMC Neurosci. 2011;12: 47. doi: 10.1186/1471-2202-12-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gantz SC, Ford CP, Neve KA, Williams JT. Loss of Mecp2 in substantia nigra dopamine neurons compromises the nigrostriatal pathway. J Neurosci. 2011;31: 12629–12637 doi: 10.1523/JNEUROSCI.0684-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6: 48–56. doi: 10.1038/nrn1588 [DOI] [PubMed] [Google Scholar]
- 33.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron. 2016;91: 1199–1218. doi: 10.1016/j.neuron.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KM, Hawi ZH, Parkington HC, Parish CL, Kumar PV, Polo JM, et al. The application of human pluripotent stem cells to model the neuronal and glial components of neurodevelopmental disorders. Mol Psychiatry. 2020;25: 368–378. doi: 10.1038/s41380-019-0495-0 [DOI] [PubMed] [Google Scholar]
- 35.Cresto N, Pillet LE, Billuart P, Rouach N. Do Astrocytes Play a Role in Intellectual Disabilities? Trends Neurosci. 2019;42: 518–527. doi: 10.1016/j.tins.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 36.Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29: 5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahanovitch U, Patterson KC, Hernandez R, Olsen ML. Glial dysfunction in MeCP2 deficiency models: implications for Rett syndrome. Int J Mol Sci. 2019;20: 3813. doi: 10.3390/ijms20153813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itoh M, Ide S, Takashima S, Kudo S, Nomura Y, Segawa M, et al. Methyl CpG-binding protein 2 (a mutation of which causes Rett syndrome) directly regulates insulin-like growth factor binding protein 3 in mouse and human brains. J Neuropathol Exp Neurol. 2007;66: 117–123. doi: 10.1097/nen.0b013e3180302078 [DOI] [PubMed] [Google Scholar]
- 39.Zappella M, Meloni I, Longo I, Canitano R, Hayek G, Rosaia L, et al. Study of MECP2 gene in Rett syndrome variants and autistic girls. Am J Med Genet B Neuropsychiatr Genet. 2003;119B: 102–107. doi: 10.1002/ajmg.b.10070 [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann WE, Tierney E, Rohde CA, Suarez-Pedraza MC, Clarke MA, Salorio CF, et al. Social impairments in Rett syndrome: characteristics and relationship with clinical severity. J Intellect Disabil Res. 2012;56: 233–247. doi: 10.1111/j.1365-2788.2011.01404.x [DOI] [PubMed] [Google Scholar]
- 41.Cosentino L, Vigli D, Franchi F, Laviola G, De Filippis B. Rett syndrome before regression: A time window of overlooked opportunities for diagnosis and intervention. Neurosci Biobehav Rev. 2019;107: 115–135. doi: 10.1016/j.neubiorev.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 42.Samaco RC, McGraw CM, Ward CS, Sun Y, Neul JL, Zoghbi HY. Female Mecp2(+/−) mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum Mol Genet. 2013;22: 96–109. doi: 10.1093/hmg/dds406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adcock KS, Blount AE, Morrison RA, Alvarez-Dieppa A, Kilgard MP, Engineer CT, et al. Deficits in skilled motor and auditory learning in a rat model of Rett syndrome. J Neurodev Disord. 2020;12: 27. doi: 10.1186/s11689-020-09330-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol Psychiatry. 2010;67: 657–665. doi: 10.1016/j.biopsych.2009.12.022 [DOI] [PubMed] [Google Scholar]
- 45.Hsu WL, Ma YL, Liu YC, Tai DJC, Lee EHY. Restoring Wnt6 signaling ameliorates behavioral deficits in MeCP2 T158A mouse model of Rett syndrome. Sci Rep. 2020;10: 1074. doi: 10.1038/s41598-020-57745-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kee SE, Mou X, Zoghbi HY, Ji D. Impaired spatial memory codes in a mouse model of Rett syndrome. eLife. 2018;7: e31451. doi: 10.7554/eLife.31451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao S, Tang B, Wu Z, Ure K, Sun Y, Tao H, et al. Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature. 2015;526: 430–434. doi: 10.1038/nature15694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rett A. Uber ein bisher nicht bekanntes Krankheitsbild einer angeborenen Stoffwechselst örung [On an until now unknown disease of a congenital metabolic disorder]. Krankenschwester. 1966;19: 121–2. German. . [PubMed] [Google Scholar]
- 49.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14: 471–479. doi: 10.1002/ana.410140412 [DOI] [PubMed] [Google Scholar]
- 50.Brandeis R, Brandys Y, Yehuda S. The use of the Morris water Maze in the study of memory and learning. Int J Neurosci. 1989;48: 29–69. doi: 10.3109/00207458909002151 [DOI] [PubMed] [Google Scholar]
- 51.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36: 60–90. doi: 10.1016/s0165-0173(01)00067-4 [DOI] [PubMed] [Google Scholar]
- 52.Stearns NA, Schaevitz LR, Bowling H, Nag N, Berger UV, Berger-Sweeney J. Behavioral and anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience. 2007;146: 907–921. doi: 10.1016/j.neuroscience.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 53.Li W, Bellot-Saez A, Phillips ML, Yang T, Longo FM, Pozzo-Miller L. A small-molecule TrkB ligand restores hippocampal synaptic plasticity and object location memory in Rett syndrome mice. Dis Model Mech. 2017;10: 837–845. doi: 10.1242/dmm.029959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wenk GL, Hauss-Wegrzyniak B. Altered cholinergic function in the basal forebrain of girls with Rett syndrome. Neuropediatrics. 1999;30: 125–129. doi: 10.1055/s-2007-973476 [DOI] [PubMed] [Google Scholar]
- 55.Wenk GL, Mobley SL. Choline acetyltransferase activity and vesamicol binding in Rett syndrome and in rats with nucleus basalis lesions. Neuroscience. 1996;73: 79–84. doi: 10.1016/0306-4522(96)00019-x [DOI] [PubMed] [Google Scholar]
- 56.Wenk GL. Rett syndrome: neurobiological changes underlying specific symptoms. Prog Neurobiol. 1997;51: 383–391. doi: 10.1016/s0301-0082(96)00059-7 [DOI] [PubMed] [Google Scholar]
- 57.Brašić JR, Bibat G, Kumar A, Zhou Y, Hilton J, Yablonski ME, et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse. 2012;66: 471–482. doi: 10.1002/syn.21515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gogliotti RG, Fisher NM, Stansley BJ, Jones CK, Lindsley CW, Conn PJ, et al. Total RNA sequencing of Rett syndrome autopsy samples identifies the M4 muscarinic receptor as a novel therapeutic target. J Pharmacol Exp Ther. 2018;365: 291–300. doi: 10.1124/jpet.117.246991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ricceri L, De Filippis B, Fuso A, Laviola G. Cholinergic hypofunction in MeCP2-308 mice: beneficial neurobehavioural effects of neonatal choline supplementation. Behav Brain Res. 2011;221: 623–629. doi: 10.1016/j.bbr.2011.03.051 [DOI] [PubMed] [Google Scholar]
- 60.Zhou H, Wu W, Zhang Y, He H, Yuan Z, Zhu Z, et al. Selective preservation of cholinergic MeCP2 rescues specific Rett-syndrome-like phenotypes in MeCP2(stop) mice. Behav Brain Res. 2017;322: 51–59. doi: 10.1016/j.bbr.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 61.Oginsky MF, Cui N, Zhong W, Johnson CM, Jiang C. Alterations in the cholinergic system of brain stem neurons in a mouse model of Rett syndrome. Am J Physiol Cell Physiol. 2014;307: C508–C520. doi: 10.1152/ajpcell.00035.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ballinger EC, Schaaf CP, Patel AJ, de Maio A, Tao H, Talmage DA, et al. Mecp2 deletion from cholinergic neurons selectively impairs recognition memory and disrupts cholinergic modulation of the perirhinal cortex. eNeuro. 2019;6: ENEURO.0134-19.2019. doi: 10.1523/ENEURO.0134-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dunn HG. Importance of Rett syndrome in child neurology. Brain Dev. 2001;23 Suppl 1: S38–S43. doi: 10.1016/s0387-7604(01)00335-7 [DOI] [PubMed] [Google Scholar]
- 64.Humphreys P, Barrowman N. The incidence and evolution of Parkinsonian rigidity in Rett syndrome: A pilot study. Can J Neurol Sci. 2016;43: 567–573. doi: 10.1017/cjn.2016.8 [DOI] [PubMed] [Google Scholar]
- 65.Segawa M. Early motor disturbances in Rett syndrome and its pathophysiological importance. Brain Dev. 2005;27 Suppl 1: S54–S58. doi: 10.1016/j.braindev.2004.11.010 [DOI] [PubMed] [Google Scholar]
- 66.Wong DF, Blue ME, Brašić JR, Nandi A, Valentine H, Stansfield KH, et al. Are dopamine receptor and transporter changes in Rett syndrome reflected in Mecp2-deficient mice? Exp Neurol. 2018;307: 74–81. doi: 10.1016/j.expneurol.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 67.Dunn AJ, Vigle G. Grooming behavior induced by ACTH involves cerebral cholinergic neurons and muscarinic receptors. Neuropharmacology. 1985;24: 329–331. doi: 10.1016/0028-3908(85)90139-x [DOI] [PubMed] [Google Scholar]
- 68.Torre E, Celis ME. Cholinergic mediation in the ventral tegmental area of alpha-melanotropin induced excessive grooming: changes of the dopamine activity in the nucleus accumbens and caudate putamen. Life Sci. 1988;42: 1651–1657. doi: 10.1016/0024-3205(88)90444-4 [DOI] [PubMed] [Google Scholar]
- 69.Berberian V, Sanchez MS, Celis ME. Participation of the cholinergic system in the excessive grooming behavior induced by neuropeptide (N) glutamic acid (E) isoleucine (I) amide (NEI). Neurochem Res. 2002;27: 1713–1717. doi: 10.1023/a:1021655631754 [DOI] [PubMed] [Google Scholar]
- 70.Roux JC, Villard L. Biogenic amines in Rett syndrome: the usual suspects. Behav Genet. 2010;40: 59–75. doi: 10.1007/s10519-009-9303-y [DOI] [PubMed] [Google Scholar]
- 71.Nguyen MVC, Du F, Felice CA, Shan X, Nigam A, Mandel G, et al. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J Neurosci. 2012;32: 10021–10034. doi: 10.1523/JNEUROSCI.1316-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riikonen R, Makkonen I, Vanhala R, Turpeinen U, Kuikka J, Kokki H. Cerebrospinal fluid insulin-like growth factors IGF-1 and IGF-2 in infantile autism. Dev Med Child Neurol. 2006;48: 751–755. doi: 10.1017/S0012162206001605 [DOI] [PubMed] [Google Scholar]
- 73.Hara M, Nishi Y, Yamashita Y, Hirata R, Takahashi S, Nagamitsu S, et al. Relation between circulating levels of GH, IGF-1, ghrelin and somatic growth in Rett syndrome. Brain Dev. 2014;36: 794–800. doi: 10.1016/j.braindev.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 74.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60: 430–440. doi: 10.1016/j.neuron.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 75.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468: 223–231. doi: 10.1038/nature09612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ip JPK, Mellios N, Sur M. Rett syndrome: insights into genetic, molecular and circuit mechanisms. Nat Rev Neurosci. 2018;19: 368–382. doi: 10.1038/s41583-018-0006-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reim D, Schmeisser MJ. Neurotrophic factors in mouse models of autism spectrum disorder: focus on BDNF and IGF-1. Adv Anat Embryol Cell Biol. 2017;224: 121–134. doi: 10.1007/978-3-319-52498-6_7 [DOI] [PubMed] [Google Scholar]
- 78.Pacheco NL, Heaven MR, Holt LM, Crossman DK, Boggio KJ, Shaffer SA, et al. RNA sequencing and proteomics approaches reveal novel deficits in the cortex of Mecp2-deficient mice, a model for Rett syndrome. Mol Autism. 2017;8: 56. doi: 10.1186/s13229-017-0174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smalley I, Law V, Wyatt C, Evernden B, Fang B, Koomen JM, et al. Proteomic analysis of CSF from patients with leptomeningeal melanoma metastases identifies signatures associated with disease progression and therapeutic resistance. Clin Cancer Res. 2020;26: 2163–2175. doi: 10.1158/1078-0432.CCR-19-2840 [DOI] [PMC free article] [PubMed] [Google Scholar]