Abstract
Schizophrenia often requires long-term treatment with antipsychotic medication. This study aims to measure the continuity of antipsychotic treatment over the course of illness in schizophrenia, as well as factors involved in the interruption of treatment. For this, we followed up a national cohort of first-episode psychosis patients in Finland for up to 18 years. Stratified Cox proportional hazards regressions were conducted for “within-participant” risk of discontinuation of subsequent treatments compared to the first, and by specific antipsychotic compared to oral olanzapine, the most prescribed antipsychotic in this cohort. Adjusted hazard ratios (aHRs) and 95% confidence intervals (95% CIs) were calculated. Among 3343 participants followed up for a mean of 8 years (SD = 4.93), the median number of continuous treatment episodes was 6 (interquartile range [IQR] = 3–11) with a median duration of 11.4 months (IQR = 5.3–25.6). In the first year after diagnosis, the incidence rate of treatment discontinuation was 30.12 (95% CI = 29.89–30.35) events per 100 participant-years, decreasing to 8.90 (95% CI = 8.75–9.05) in the 10th year. The risk of discontinuation progressively decreased over successive treatment episodes (aHR = 0.30; 95% CI = 0.20–0.46 for episodes after the 15th compared to the first). Individuals were 67% less likely to interrupt treatment with long-acting injectable than oral antipsychotics (aHR = 0.33; 95% CI = 0.27–0.41). Treatment for schizophrenia over the long term is often characterized by recurrent cycles of interruptions and reintroductions of antipsychotic medication, which is typically not recommended by management guidelines. Greater utilization of long-acting injectable formulations earlier in the course of illness may facilitate the continuity of antipsychotic treatment in schizophrenia.
Keywords: pharmacoepidemiology, treatment discontinuation, course of treatment
Introduction
Schizophrenia is most often a chronic disorder, requiring long-term treatment.1 Despite the demonstrated efficacy of antipsychotics in preventing relapse,2 most guidelines do not make recommendations for treatment beyond 2 years, since this is the longest period of time for which there are randomized data.3 However, there is no evidence that the risk of relapse associated with interrupting antipsychotic treatment decreases after 2 years of antipsychotic treatment. Instead, long-term national registry data indicates that this risk may indeed increase.4 Interruption of antipsychotic treatment has been consistently associated with worse psychiatric and medical outcomes. In a systematic review and meta-analysis of risk factors for relapse following the initial treatment of schizophrenia, interrupting antipsychotic treatment was the predictor with the largest effect, with 4-fold risk compared to treatment continuation, surpassing other risk factors such as co-occurring drug use.5 Lack of antipsychotic treatment in individuals with schizophrenia has also been associated with premature mortality due to medical comorbidities and suicide.6
The general consensus favoring long-term antipsychotic maintenance treatment for relapse prevention in schizophrenia has been questioned, however, by some authors. The Dutch MESIFOS study found that individuals randomized to treatment discontinuation did have greater rates of long-term functional recovery,7 which has prompted some authors to caution against the universal long-term antipsychotic maintenance treatment for schizophrenia.8 However, this finding was not replicated in a study from Hong Kong with a similar design and larger sample.9 Additionally, given their naturalistic designs, other studies cited in support of better prognosis associated with treatment discontinuation10 may have been exposed to selection and attrition bias (ie, including and retaining disproportionately individuals with better prognosis) and confounder by indication (ie, treatment discontinuation is consequence but not cause of better prognosis). Thus, some degree of controversy remains on this important area, although we would argue that most evidence supports the recommendation of continuous long-term antipsychotic treatment in most individuals with schizophrenia.1 In a recent systematic review of treatment guidelines, 5 out of 6 advised against discontinuation of treatment in multiepisode schizophrenia, and 9 out of 9 converged recommending against intermittent/targeted use of antipsychotic drugs.3 Given this important discussion about the long-term treatment of schizophrenia at the level of supporting evidence and recommendations, it is relevant to study the actual utilization of antipsychotic medication over the long-term. Understanding such patterns of treatment utilization is essential to inform policies aimed at closing the gap between actual and recommended use.
It is well known that that there is a high rate of interruption in long-term antipsychotic treatment. For example, a study from the veteran affairs (VA) system in the United States (n = 2138) found that 84% of patients discontinued antipsychotic treatment during a follow-up of up to 33 months.11 Also in a VA cohort, another group found that 61% of individuals had difficulty with consistent adherence over a 4-year period, with younger age, non-white race, co-occurring substance use, and previous hospitalizations contributing to greater risk for poor adherence over this period of time.12 Similarly in Europe, the SOHO study (n = 7728) reported treatment discontinuation rates between 34% and 66% over 36 months of follow-up.13 Thus, it is well established that most individuals will interrupt antipsychotic treatment during their course of illness. What is less understood, and what this study intends to address, is how such a high rate of treatment interruption evolves over the long term and what factors might contribute. To our knowledge, there are no studies that measure treatment utilization in a representative cohort starting from the early treatment and continuing to the chronic phase of illness.
In this study, we aim to measure the continuity of antipsychotic treatment over a significant portion of the course of illness in a national cohort of individuals with early phase schizophrenia followed for up to 18 years. In particular, we use a within-participant analysis to examine the risk factors associated with the interruption of each treatment period for any given participant, which mitigates residual confounding and reverse causation by comparing treatment episodes for each individual participant, instead of comparing between groups of participants.
Methods
Study Population
The nationwide source population included all individuals residing in Finland between January 1, 2000 and December 31, 2014 who were first diagnosed with schizophrenia or schizoaffective disorder (ICD-10 F20, F25) during this time, were younger than 40 years when diagnosed, and had no antipsychotic exposure within 1 year before diagnosis.
Antipsychotic Exposure
Medication use from the Prescription Register included reimbursed drug expenditures during the period from January 1, 1995 to December 31, 2017 in outpatient care. Measurement of medication use prior to January 1, 2000, when the patient population started being registered, was conducted to exclude antipsychotic utilization prior to schizophrenia diagnosis (relationship between measurement periods for study populations, medication use, and outcomes in supplementary figure 1). Medication use information in the register is categorized according to the Anatomical Therapeutic Chemical (ATC) classification, and the purchased amount is recorded in defined daily dose together with information on drug package and formulation. Antipsychotics are defined as ATC code N05A excluding lithium (ATC code N05AN01). Antipsychotics used in monotherapy by the study cohort were divided by antipsychotic type and formulation. Polypharmacy periods were categorized as such, but not specifically assessed since treatment interruption of one drug would still mean that there is ongoing treatment. By using only monotherapy episodes, we also mitigated the confounder of drugs being used for indications other than antipsychotic, as it would be expected that use of antipsychotics for non-antipsychotic indications would be done as add on to another antipsychotic in individuals with schizophrenia (eg, quetiapine for sleep). When antipsychotics were aggregated by formulation (ie, long-acting injectable [LAI] vs oral antipsychotic [OAP]), concomitant use of both formulations was categorized as LAI. Treatment discontinuation was defined as interruption (ie, no antipsychotic at all) in use >30 days for reasons other than hospitalization, treatment switch, death, or end of follow-up. Treatment episodes therefore consisted in the periods between discontinuations, and were modeled with the PRE2DUP method, which takes into consideration the amount of antipsychotic purchased, personal purchasing regularity, hospital days, and stockpiling, to determine the date when antipsychotic use is ended.14 For example, for LAIs, this meant that the end of the period was calculated according to a window that included the time when the next injection was due plus 30 days (supplementary figure 2).
Outcomes and Covariates
Primary outcome was time to treatment discontinuation between January 1, 2000 and December 31, 2017, defined as end of treatment period followed by >30 days without antipsychotic exposure (ie, death, hospitalization, end of study, or switch to another antipsychotic were censoring points, meaning that they were not included as treatment discontinuation events). The second outcome was the proportion of days of outpatient time covered (PDC) by antipsychotic treatment between time of diagnosis and end of follow-up. Covariates are defined in supplementary table 1.
Statistical Analyses
We measured the incidence rate of treatment discontinuation in events per 100 participant-years with 95% confidence intervals (95% CIs) for the entire duration of follow-up, and by year after diagnosis for the first decade of follow-up, which we also stratified by duration of the first hospitalization for schizophrenia. In addition, we measured the PDC by ≥1 antipsychotic between diagnosis and end of follow-up (except for periods of hospitalization), using a threshold of >80% as reflective of meaningful adherence.15
Next, we conducted a “within-participant” comparison of the risk of discontinuation by order of treatment episode for any given individual. This approach is preferable to a group comparison, since as a group, only individuals with greater number of interruptions (and with shorter duration of treatment episodes) would contribute to the treatment episodes with a later order. Furthermore, by comparing between treatment episodes for each individual, we mitigate residual confounding and reverse causation. For this approach, we conducted a stratified Cox proportional hazards regression model in which each individual formed his or her own stratum. This method has been used previously in large cohorts with recurring events.16 Since the exposure periods are compared for the same individual, the only factors that need to be adjusted for are those that change as a function of time: time since cohort entry, and antipsychotic medication polypharmacy. By doing this, we mitigate the confounding of drugs being typically used later in treatment (ie, LAIs and clozapine). Follow-up time was reset to zero after each outcome event to allow comparison of treatment periods within each individual (supplementary figure 1).
Next, we also used a “within-participant” comparison of time to discontinuation for each antipsychotic drug monotherapy compared to oral olanzapine. The reason to use this drug as comparison is that it was the most commonly prescribed antipsychotic in this cohort. In this case, we conducted a stratified Cox proportional hazards regression model adjusted for order of treatments, time since cohort entry, and antipsychotic polypharmacy. Only individuals with variation in exposure and outcome contributed to the model. This “within-participant” approach was also applied to compare the risk of discontinuation for any LAI vs any OAP, and for each LAI vs their OAP counterpart among individuals in the cohort who were prescribed both formulations. In the case of paliperidone LAI, since paliperidone OAP is not marketed in Finland, it was compared to risperidone OAP, as risperidone is predominantly metabolized to paliperidone.
Finally, we conducted a traditional Cox proportional hazards regression analysis for the whole cohort to measure the predictive effect of a set of covariates on treatment discontinuation. All the regression analyses were adjusted for all the covariates in the model, yielding adjusted hazard ratios (aHRs) and 95% CIs.
Ethics of Research
Permissions were granted by pertinent institutional authorities at the Finnish National Institute for Health and Welfare (permission THL/847/5.05.00/2015), the Social Insurance Institution of Finland (65/522/2015), and Statistics Finland (TK53-1042-15).
Results
Cohort Characteristics and Incidence of Treatment Discontinuation
The total cohort consisted of 3343 participants, of which 512 (15.3%) had schizoaffective disorder. The mean follow-up time was 8 years (SD = 4.93) (table 1). The incidence of treatment discontinuation was 14.82 events per 100 participant-years of use (95% CI = 14.77–14.88). Each subject had a median of 6 (interquartile range [IQR] = 3–11) distinct treatment periods during follow-up, with a median duration of 11.4 (IQR = 5.3–25.6) months each. Over the whole follow-up period, the proportion of the cohort for which there were >80% of days covered by antipsychotic treatment was 62.7%, whereas 9.2% had 60%–80%, 6.3% had 40%–60%, 5.7% had 20%–40%, and 16.1% of the cohort had <20% days covered, respectively.
Table 1.
Characteristics of the Cohort of Individuals With Their First Diagnosis With Schizophrenia in Finland Between 2000 and 2014
| Covariate | Total Exposed Cohort (n = 3343) | |
|---|---|---|
| n | % | |
| Sociodemographic | ||
| Male gender | 2117 | 63.32 |
| Age | ||
| <25 | 1218 | 36.4 |
| 25–34 | 1471 | 44.0 |
| ≥35 | 654 | 19.6 |
| Duration of first hospital stay due to schizophrenia | ||
| <1 mo | 693 | 20.7 |
| 1–2 mo | 718 | 21.5 |
| 2–4 mo | 907 | 27.1 |
| ≥4 mo | 1025 | 30.7 |
| History of comorbid psychiatric conditions | ||
| Substance use disorder | 564 | 16.87 |
| Suicidality | 11 | 0.33 |
| Intellectual disability | 49 | 1.46 |
| History of comorbid medical conditions | ||
| Cancer | 18 | 0.54 |
| Cardiovascular disease | 108 | 3.23 |
| Diabetes | 19 | 0.57 |
| Asthma/COPD | 106 | 3.23 |
| Co-treatment upon treatment initiation | ||
| Antidepressant | 788 | 23.57 |
| Antiparkinsonian | 56 | 1.67 |
| Benzodiazepine | 367 | 10.98 |
| Z-drugs | 103 | 3.08 |
| Lithium | 93 | 2.78 |
| Mood stabilizer | 349 | 10.44 |
| Number of treatment episodes during follow-up | ||
| 1 | 161 | 4.8 |
| 2 | 551 | 16.5 |
| 3 | 336 | 10.1 |
| 4–7 | 1280 | 38.3 |
| 8–11 | 609 | 18.2 |
| 12–15 | 253 | 7.6 |
| >15 | 153 | 4.6 |
Note: COPD, chronic obstructive pulmonary disease.
Treatment Discontinuation Over the Illness Course
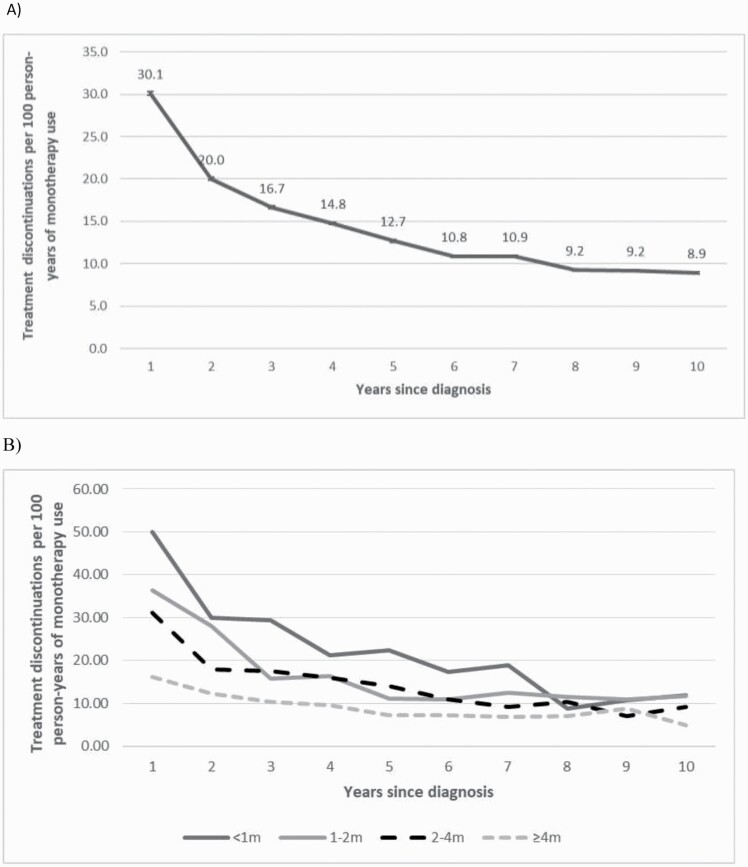
The incidence of treatment discontinuation decreased over the course of illness, beginning at 30.12 (95% CI = 29.89–30.35) events per 100 participant-years of antipsychotic use for individuals in their first year after diagnosis, and decreasing to 8.90 (95% CI = 8.75–9.05) 10 years later. Individuals with a shorter duration of their first hospitalization for schizophrenia started with a much greater risk of discontinuation that decreased over time, whereas those with longer initial hospitalizations tended to have relatively low risk of discontinuation from the beginning throughout the next decade (figure 1). For each individual, compared to their first treatment episode, the risk of discontinuation was aHR = 0.59, 95% CI = 0.44–0.79 for the second episode; aHR = 0.59, 95% CI = 0.44–0.80 for the third episode; aHR = 0.40, 95% CI = 0.29–0.56 for the fourth to seventh episode; aHR = 0.32; 95% CI = 0.22–0.46 for the eighth to 11th episode; aHR = 0.31, 95% CI = 0.20–0.46 for the 12th to 15th episode; and aHR = 0.30, 95% CI = 0.20–0.46 for episodes after the 15th episode.
Fig. 1.
Risk of treatment discontinuation per year within the first decade since diagnosis, (A) among all, (B) stratified by duration of the first hospital stay (in months) due to schizophrenia.
Risk of Discontinuation for Specific Antipsychotics
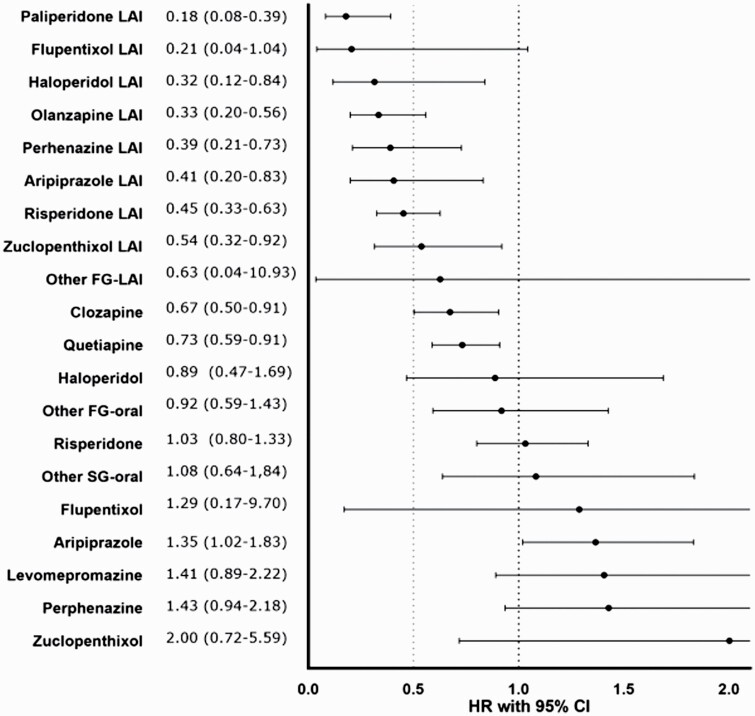
Compared to oral olanzapine, treatment periods on oral aripiprazole (aHR = 1.35; 95% CI = 1.02–1.83) were more likely to be discontinued. Alternatively, periods on paliperidone LAI (aHR = 0.18; 95% CI = 0.08–0.39), haloperidol LAI (aHR = 0.21; 95% CI = 0.12–0.84), olanzapine LAI (aHR = 0.33; 95% CI = 0.20–0.56), perphenazine LAI (aHR = 0.39; 95% CI = 0.21–0.73), aripiprazole LAI (aHR = 0.41; 95% CI = 0.20–0.83), risperidone LAI (aHR = 0.45; 95% CI = 0.33–0.63), zuclopenthixol LAI (aHR = 0.54; 95% CI = 0.32–0.92), clozapine (aHR = 0.67; 95% CI = 0.50–0.91), and quetiapine (aHR = 0.73; 95% CI = 0.59–0.91) were less likely to be discontinued (figure 2).
Fig. 2.
Risk of treatment discontinuation during monotherapy compared with oral olanzapine in a within-individual analysis. 95% CI, 95% confidence interval;AHR, adjusted hazard ratio; FG, first generation; LAI, long-acting injectable; SG, second generation.
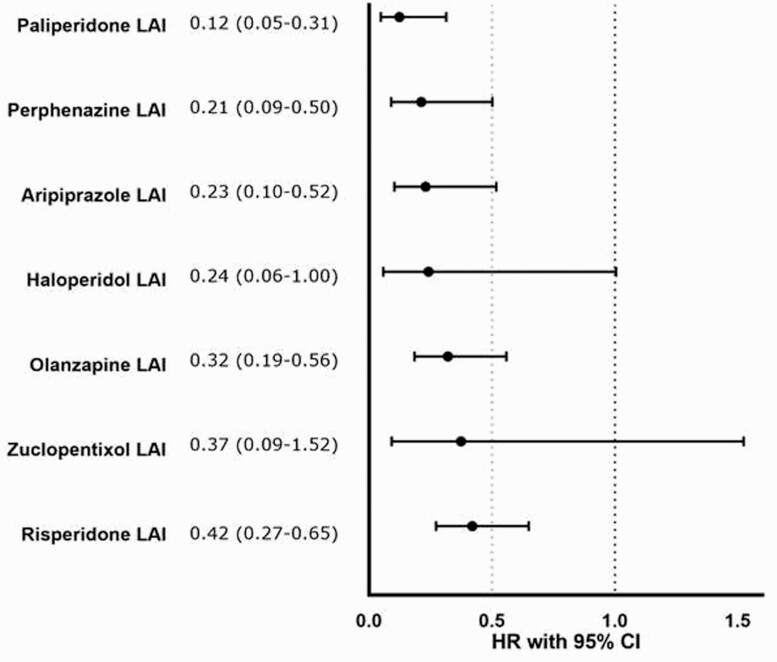
Among the cohort that used both LAIs and OAPs (n = 833), individuals were 67% less likely to discontinue LAI than OAP formulations (aHR = 0.33; 95% CI = 0.27–0.41). Compared to their oral counterparts, paliperidone LAI (aHR = 0.12; 95% CI = 0.05–0.31), perphenazine LAI (aHR = 0.21; 95% CI = 0.09–0.50), aripiprazole LAI (aHR = 0.23; 95% CI = 0.10–0.52), olanzapine LAI (aHR = 0.32; 95% CI = 0.19–0.56), and risperidone LAI (aHR = 0.42; 95% CI = 0.27–0.65) were less likely to be discontinued (figure 3).
Fig. 3.
Risk of treatment discontinuation in head-to-head comparison of LAIs vs their oral counterparts in a within-individual analysis. 95% CI, 95% confidence interval; AHR, adjusted hazard ratio; LAI, long-acting injectable.
Predictors of Antipsychotic Discontinuation
The risk of treatment discontinuation was lower for men than women (aHR = 0.83; 95% CI = 0.77–0.88), for individuals for whom the duration of their first hospitalization for schizophrenia was 1–2 months (aHR = 0.76; 95% CI = 0.69–0.84), 2–4 months (aHR = 0.68; 95% CI = 0.62–0.75), and >4 months (aHR = 0.44; 95% CI = 0.40–0.48), compared to <1 month, and for individuals who had comorbid cardiovascular disease (aHR = 0.80; 95% CI = 0.65–0.98). Alternatively, individuals aged in the ranges of <25 (aHR = 1.24; 95% CI = 1.13–1.37) and 25–34 (aHR = 1.14; 95% CI = 1.04–1.25) had greater risk of antipsychotic treatment discontinuation than individuals aged >35 years, and also individuals with comorbid substance use disorders were more likely to discontinue treatment than those without (aHR = 1.30; 95% CI = 1.18–1.42) (table 2).
Table 2.
Predictors of Antipsychotic Treatment Discontinuation in a Between-Individual Analysis
| Covariate | P Value | AHR | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Sociodemographic | ||||
| Male gender | <.0001 | 0.83 | 0.77 | 0.88 |
| Age | ||||
| <25 | <.0001 | 1.24 | 1.13 | 1.37 |
| 25–34 | .0067 | 1.14 | 1.04 | 1.25 |
| ≥35a | Reference | |||
| Duration of first hospital stay due to schizophrenia | ||||
| <1 moa | Reference | |||
| 1–2 mo | <.0001 | 0.76 | 0.69 | 0.84 |
| 2–4 mo | <.0001 | 0.68 | 0.62 | 0.75 |
| ≥4 mo | <.0001 | 0.44 | 0.40 | 0.48 |
| History of comorbid psychiatric conditions | ||||
| Substance abuse disorder | <.0001 | 1.30 | 1.18 | 1.42 |
| Suicidality | .0949 | 1.57 | 0.93 | 2.67 |
| Intellectual disability | .0596 | 0.75 | 0.55 | 1.01 |
| History of comorbid medical conditions | ||||
| Cancer | .2669 | 0.76 | 0.46 | 1.24 |
| Cardiovascular disease | .0302 | 0.80 | 0.65 | 0.98 |
| Diabetes | .0731 | 1.48 | 0.96 | 2.28 |
| Asthma/COPD | .101 | 0.84 | 0.68 | 1.03 |
| Co-treatment upon treatment initiation | ||||
| Antidepressant | .4587 | 0.97 | 0.90 | 1.05 |
| Antiparkinsonian | .1063 | 0.78 | 0.58 | 1.05 |
| Benzodiazepine | .4302 | 0.96 | 0.86 | 1.07 |
| Z-drugs | .2869 | 1.11 | 0.92 | 1.34 |
| Lithium | .3852 | 1.09 | 0.90 | 1.32 |
| Mood stabilizer | .7338 | 1.02 | 0.92 | 1.14 |
Note: 95% CI, 95% confidence interval; AHR, adjusted hazard ratio; COPD, chronic obstructive pulmonary disease.
aReference.
Discussion
We present data on the long-term continuity of antipsychotic medication in a national cohort of patients with schizophrenia. The results suggest a common pattern of repetitive treatment discontinuations and reintroductions, which tends to slow down over the course of illness, with a consistently decreasing risk of treatment discontinuation after each reintroduction. Such decrement in the risk of discontinuation in chronically ill patients reached a relatively low risk over time. However, most often this occurs after having had multiple interruptions which may have led to potentially avoidable relapses.
These results highlight the challenges to the continuity of long-term antipsychotic treatment in schizophrenia.1 In this national cohort, participants had a median of 6 treatment interruptions over a median of 8 years of follow-up. In approximately one-third of participants, those interruptions resulted in less than a meaningful proportion of their course of illness (ie, <80%) covered by antipsychotic treatment. This group of patients may include individuals in recovery without antipsychotic treatment, but this is unfortunately an uncommon outcome in schizophrenia,17 and even less so without antipsychotic treatment.18 Thus, very possibly many of the individuals with only limited portions of their course of illness covered by antipsychotic treatment, despite residual symptoms, may not perceive that the benefits of long-term antipsychotic treatment outweigh its disadvantages. Several challenges may account for the frequent treatment interruptions over the long term. The burden of antipsychotics is usually perceived shortly after initiating treatment.19 This may include stigma or side effects such as weight gain.20 On the other hand, the benefits of treatment are often experienced later on, especially in the maintenance phase of treatment, since the goals are mostly preventing relapses and in the long run possibly reducing premature mortality.2,21 This discrepancy between the timing of the benefits and disadvantages of treatment may result in earlier discontinuations. Additionally, it is important to recognize the challenges faced in adhering to the recommendations for continuous long-term treatment in any chronic illness. Overall, these data might reflect a natural human tendency to discontinue medications regardless of the recommendations.
We identified illness characteristics that make individuals more likely to discontinue treatment. We found quite consistently, in between-patient and within-patient analyses, that those in the early phase of illness are most prone to treatment interruptions. Each subsequent cycle of interrupting and reinitiating treatment was longer than the previous one. This could reflect withdrawal of antipsychotic treatment in the early phase of illness in the hopes that there would not be a subsequent clinical worsening, with more cautious attempts to discontinue over time. This was particularly true for individuals for whom the initial hospitalization was shorter, which may reflect patient-driven requests of early discharge reflecting lack of appreciation of need for treatment of their illness, and/or the perception of a milder form of illness with quick stabilization which may have indicated to the patient and/or prescriber that long-term continuation of antipsychotic would not be necessary. Regardless of the potential explanations, our data show though that most patients went through many of those cycles over their course of illness. Most guidelines recommend against intermittent treatment,3 since it may be associated with potentially avoidable risk of relapse,21 lesser effect of antipsychotic treatment upon reintroduction,22 and increased risk for tardive dyskinesia.23 Thus, this common pattern of intermittent treatment should be minimized, given the low likelihood of successfully interrupting antipsychotic treatment if doing so previously required to reinitiate treatment.24
Another illness characteristic that was associated with greater likelihood of treatment discontinuation is comorbid substance use disorder. This finding should raise concern, since individuals with co-occurring substance use tend to have worse overall prognosis.25 Given the potential of drugs of abuse to exacerbate psychosis, antipsychotic drugs may have a protective effect. Future research should prioritize this population in developing antipsychotic intervention strategies that facilitate their engagement into treatment.
Our results suggest recommendations that might facilitate the continuity of antipsychotic treatment. First, the early phase of the illness seems to be a critical period for psychoeducation. Discussions about treatment needs with individuals recently diagnosed with schizophrenia should emphasize that most evidence indicates that long-term treatment may be beneficial for the majority of individuals with schizophrenia, especially in regards to prevention of psychiatric and medical morbidity and mortality.1 To date, some experts still advocate for guided dose reduction and discontinuation of antipsychotics over the long term in schizophrenia.8 When discussing this approach in the shared decision process during the initial phase of treatment, it should be noted that if antipsychotic withdrawal fails (ie, if dose reduction or discontinuation results in clinical deterioration), it may be a sign for the need for continuous treatment over the long run, given the low likelihood of future interruptions to succeed.24
Second, we consistently found that the time to discontinuation of LAIs was substantially longer than that of their oral counterparts, indicating that these formulations may be advantageous, not only by assuring continuous drug delivery, but also by facilitating longer treatment periods for the same individual, compared to oral drugs. The potential benefits of increased use of this formulation aligns with previous findings of greater effectiveness of LAIs compared to oral antipsychotics in preventing rehospitalization.16 Our data suggest that LAIs should be prioritized in general, and in particular for individuals at the greatest risk for treatment interruptions, including those early in the illness course and those with comorbid substance use disorders. LAIs may have benefits early in the illness, clinically as well as neurobiologically,26 arguably due to the assured continuity of treatment. Recent data demonstrated that the vast majority of individuals with recent-onset psychosis will accept LAIs with simple staff training, and that greater utilization of LAIs in individuals at the early phase of illness can reduce hospitalization risk.27 Therefore, the recommendation of initiating LAIs earlier in the course of illness is scalable and may result in improved tangible outcomes. The acceptability and effectiveness of LAIs among individuals with co-occurring substance use has been less studied. To our knowledge, only one randomized controlled trial has addressed this issue, in individuals with schizophrenia and history of incarceration, many of whom also had concurrent substance use. This study demonstrated superiority of the LAI formulation in preventing treatment failure compared to oral antipsychotics.28 In exploratory analyses comparing individuals with and without comorbid substance use disorders, the LAI was superior to the oral form in both subcohorts, although the effect size was lower among those with dual diagnosis.29 Thus, future research should address methods to facilitate the engagement into treatment, including with LAIs, in individuals with dual diagnosis (ie, schizophrenia and comorbid substance use disorder).
In addition to most LAIs, clozapine was also less likely to be discontinued than oral olanzapine. By finding this in within-participant analyses adjusted for time since cohort entry mitigates the confounder of clozapine being used in patients prone to be most adherent with any medication in general, instead reflecting that individuals with treatment resistant schizophrenia who start treatment with clozapine may tend to stay longer on this drug than on previous medications. Clinicians may be concerned about starting clozapine on individuals with a history of multiple treatment interruptions; however, these data suggest that this should not be a major deterrent to initiate clozapine.
Several limitations need to be considered when interpreting these results. First, these results have absolute external validity in Finland during the observed period of time, yet there might be less generalizability of the results across different health systems. However, to date, there are very few other datasets that would allow one to address a window of observation long enough to cover a significant portion of the course of illness without cohort attrition. It is necessary to develop pharmacoepidemiological capabilities comparable to those in Scandinavia in other world regions, in order to examine those potential differences. Second, our data are based on pharmacy purchases, and may not correspond exactly with taken treatment. This gap though was virtually nonexistent for LAIs. Also, it is possible that interruptions <30 days, the minimum reflected in the study, were already clinically significant. This is a conservative potential bias since it may overestimate actually taken treatment. Third, national registry datasets do not have information on additional factors that might influence treatment continuity, such as family support, therapeutic alliance, insight, symptom severity, or patient’s beliefs on treatment, which could provide additional context to the reasons for treatment discontinuation. Fourth, the main aim of our study was to describe the actual pattern of utilization of long-term antipsychotic treatment in schizophrenia, not including clinical outcomes as a result of discontinuation, as we would consider this a separate clinical question deserving its own study.
In conclusion, in a national cohort, most patients received antipsychotic treatment for most of their course of illness, yet most often in recurrent cycles of interruption and reintroduction. Individuals earlier in the course of illness and with comorbidities such as substance use disorder are at greater risk of interrupting treatment. The use of LAIs in this population may facilitate the continuity of antipsychotic maintenance treatment.
Supplementary Material
Acknowledgments
J.M.R. has been a consultant or has received speaker/consulting honoraria from Lundbeck, Teva, and Medscape. He has also received royalties from UpToDate and grant support from Alkermes. H.T. reports personal fees from Janssen-Cilag. C.U.C. has been a consultant and/or advisor to or has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He received royalties from UpToDate and grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma. J.M.K. has been a consultant and/or advisor for or has received honoraria from Alkermes, Allergan, LB Pharmaceuticals, H. Lundbeck, Intracellular Therapies, Janssen Pharmaceuticals, Johnson and Johnson, Merck, Minerva, Neurocrine, Newron, Otsuka, Pierre Fabre, Reviva, Roche, Sumitomo Dainippon, Sunovion, Takeda, Teva, and UpToDate and is a shareholder in LB Pharmaceuticals and Vanguard Research Group. J.T. reports personal fees from the Finnish Medicines Agency (Fimea), European Medicines Agency (EMA), Eli Lilly, Janssen-Cilag, Lundbeck, and Otsuka; is a member of advisory board for Lundbeck; and has received grants from the Stanley Foundation and Sigrid Jusélius Foundation. J.T., A.T., and H.T. have participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to their employing institution.
Funding
This study was funded with departmental support of the Zucker Hillside Hospital (Glen Oaks, NY), the Department of Clinical Neuroscience, Karolinska Institutet (Stockholm, Sweden), and the Academy of Finland (grants: 315969 and 320107 to H.T.). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
References
- 1. Rubio J, Correll CU, Kane JM. What is the risk–benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry Off J World Psychiatr Assoc WPA. 2018;17(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. [DOI] [PubMed] [Google Scholar]
- 3. Shimomura Y, Kikuchi Y, Suzuki T, Uchida H, Mimura M, Takeuchi H. Antipsychotic treatment in the maintenance phase of schizophrenia: an updated systematic review of the guidelines and algorithms. Schizophr Res. 2020;215:8–16. [DOI] [PubMed] [Google Scholar]
- 4. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765–773. [DOI] [PubMed] [Google Scholar]
- 5. Alvarez-Jimenez M, Priede A, Hetrick SE, et al. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res. 2012;139(1–3):116–128. [DOI] [PubMed] [Google Scholar]
- 6. Vermeulen J, van Rooijen G, Doedens P, Numminen E, van Tricht M, de Haan L. Antipsychotic medication and long-term mortality risk in patients with schizophrenia; a systematic review and meta-analysis. Psychol Med. 2017;47(13):2217–2228. [DOI] [PubMed] [Google Scholar]
- 7. Wunderink L, Nieboer RM, Wiersma D, Sytema S, Nienhuis FJ. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry. 2013;70(9):913–920. [DOI] [PubMed] [Google Scholar]
- 8. Murray RM, Quattrone D, Natesan S, et al. Should psychiatrists be more cautious about the long-term prophylactic use of antipsychotics? Br J Psychiatry. 2016;209(5):361–365. [DOI] [PubMed] [Google Scholar]
- 9. Hui CLM, Honer WG, Lee EHM, et al. Long-term effects of discontinuation from antipsychotic maintenance following first-episode schizophrenia and related disorders: a 10 year follow-up of a randomised, double-blind trial. Lancet Psychiatry. 2018;5(5):432–442. [DOI] [PubMed] [Google Scholar]
- 10. Harrow M, Jobe TH, Faull RN, Yang J. A 20-year multi-follow-up longitudinal study assessing whether antipsychotic medications contribute to work functioning in schizophrenia. Psychiatry Res. 2017;256:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kreyenbuhl J, Slade EP, Medoff DR, et al. Time to discontinuation of first- and second-generation antipsychotic medications in the treatment of schizophrenia. Schizophr Res. 2011;131(1–3):127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valenstein M, Ganoczy D, McCarthy JF, Myra Kim H, Lee TA, Blow FC. Antipsychotic adherence over time among patients receiving treatment for schizophrenia: a retrospective review. J Clin Psychiatry. 2006;67(10):1542–1550. [DOI] [PubMed] [Google Scholar]
- 13. Haro JM, Suarez D, Novick D, Brown J, Usall J, Naber D; SOHO Study Group . Three-year antipsychotic effectiveness in the outpatient care of schizophrenia: observational versus randomized studies results. Eur Neuropsychopharmacol. 2007;17(4):235–244. [DOI] [PubMed] [Google Scholar]
- 14. Tanskanen A, Taipale H, Koponen M, et al. From prescription drug purchases to drug use periods—a second generation method (PRE2DUP). BMC Med Inform Decis Mak. 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nau D. Proportion of Days Covered (PDC) as a Preferred Method of Measuring Medication Adherence [Internet].: http://www.pqaalliance.org/files/PDCvsMPRfinal.pdf. Accessed June 6, 2020.
- 16. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jääskeläinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gitlin M, Nuechterlein K, Subotnik KL, et al. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry. 2001;158(11):1835–1842. [DOI] [PubMed] [Google Scholar]
- 19. Llorca PM, Lançon C, Hartry A, et al. Assessing the burden of treatment-emergent adverse events associated with atypical antipsychotic medications. BMC Psychiatry. 2017;17(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–126. [DOI] [PubMed] [Google Scholar]
- 21. Taipale H, Tanskanen A, Mehtälä J, Vattulainen P, Correll CU, Tiihonen J. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry. 2020;19(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solmi M, Pigato G, Kane JM, Correll CU. Clinical risk factors for the development of tardive dyskinesia. J Neurol Sci. 2018;389:21–27. [DOI] [PubMed] [Google Scholar]
- 24. Hui CLM, Honer WG, Lee EHM, Chang WC, Chan SKW, Chen EYH. Factors associated with successful medication discontinuation after a randomized clinical trial of relapse prevention in first-episode psychosis: a 10-year follow-up. JAMA Psychiatry. 2019;76(2):217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bartzokis G, Lu PH, Raven EP, et al. Impact on intracortical myelination trajectory of long acting injection versus oral risperidone in first-episode schizophrenia. Schizophr Res. 2012;140(1–3):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76(5):554–561. [DOI] [PubMed] [Google Scholar]
- 29. Lynn Starr H, Bermak J, Mao L, Rodriguez S, Alphs L. Comparison of long-acting and oral antipsychotic treatment effects in patients with schizophrenia, comorbid substance abuse, and a history of recent incarceration: an exploratory analysis of the PRIDE study. Schizophr Res. 2018;194:39–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.