Abstract
Objective
Brain-based Biotypes for psychotic disorders have been developed as part of the B-SNIP consortium to create neurobiologically distinct subgroups within idiopathic psychosis, independent from traditional phenomenological diagnostic methods. In the current study, we aimed to validate the Biotype model by assessing differences in volume and shape of the amygdala and hippocampus contrasting traditional clinical diagnoses with Biotype classification.
Methods
A total of 811 participants from 6 sites were included: probands with schizophrenia (n = 199), schizoaffective disorder (n = 122), psychotic bipolar disorder with psychosis (n = 160), and healthy controls (n = 330). Biotype classification, previously developed using cognitive and electrophysiological data and K-means clustering, was used to categorize psychosis probands into 3 Biotypes, with Biotype-1 (B-1) showing reduced neural salience and severe cognitive impairment. MAGeT-Brain segmentation was used to determine amygdala and hippocampal volumetric data and shape deformations.
Results
When using Biotype classification, B-1 showed the strongest reductions in amygdala-hippocampal volume and the most widespread shape abnormalities. Using clinical diagnosis, probands with schizophrenia and schizoaffective disorder showed the most significant reductions of amygdala and hippocampal volumes and the most abnormal hippocampal shape compared with healthy controls. Biotype classification provided the strongest neuroanatomical differences compared with conventional DSM diagnoses, with the best discrimination seen using bilateral amygdala and right hippocampal volumes in B-1.
Conclusion
These findings characterize amygdala and hippocampal volumetric and shape abnormalities across the psychosis spectrum. Grouping individuals by Biotype showed greater between-group discrimination, suggesting a promising approach and a favorable target for characterizing biological heterogeneity across the psychosis spectrum.
Keywords: psychosis, Biotype, schizophrenia, bipolar, amygdala, hippocampus
Introduction
Traditional diagnostic criteria among schizophrenia, schizoaffective, and bipolar disorders rely on clinical manifestations, but do not take into consideration the underlying pathologic mechanisms.1 Considering the increasing evidence of a biological overlap between these diagnoses,2,3 the concept of psychosis dimension representing a continuum based on phenomenological description has been proposed.4 The psychosis dimension concept is further supported by the lack of success in identifying brain-based biomarkers differentiating people with schizophrenia, schizoaffective, and bipolar disorders.5,6 Furthermore, treatment modalities of psychosis spectrum disorders oftentimes overlap, targeting specific symptoms rather than diagnosis.7 This highlights the increased need for distinction based on underlying pathophysiology to improve identification of more similar subgroups of probands across the psychosis spectrum, and in turn facilitate the selection of treatments promoting optimal responses.8
Two specific limbic structures involved in emotional and cognitive processing, the amygdala and the hippocampus, have been consistently found to be deviant in psychotic disorders.9–11 More specifically, compared with the general population, reductions in amygdala and hippocampal volumes have been observed in people with schizophrenia and schizoaffective disorders,12,13 as well as in people with bipolar disorder14 and bipolar disorder with psychosis.15 Two recent meta-analyses identified these structures as being among the most affected in these disorders.13,14 Although these disorders share common neuroanatomical abnormalities, some studies have shown more extensive reduction of amygdala and hippocampal volumes in people with schizophrenia compared with bipolar disorder.16–18
Aside from volume measures, subtle but meaningful changes in brain structures can be captured by their morphology. If a structure shows more subtle morphological properties along its surface, that may not be captured by its volume average, but these surface-based measures are meaningful19 and can provide more sensitive distinctions between subgroups of probands.20 For instance, amygdala shape abnormalities differ between bipolar disorder with psychosis and schizophrenia, representing a distinguishing morphologic feature.17 The shapes of the hippocampus can also demonstrate regional deformations despite the similar hippocampal volumes that can be seen across psychotic disorders.21 Hence, considering the shape of these structures can provide additional and novel perspectives on the nature of brain structural alterations in psychotic illnesses.19
In an attempt to improve psychotic disorder classification, our team has developed psychosis Biotypes that are biologically distinctive subgroups of individuals across the psychosis spectrum based on shared neurobiological phenotypes, which are independent of clinical diagnosis.8,22 These Biotypes were created using an unsupervised machine-learning approach and various dimensions of cognition and electrophysiological brain responses. Three distinct Biotypes of probands were then identified: Biotype-1 (B-1), defined by reduced neural response to salient stimuli, and significant cognitive impairment; Biotype-2 (B-2) with accentuated intrinsic electrophysiological neural activity, comparable cognitive impairment to B-1; and Biotype-3 (B-3) with minimal deviation from normal function with the best cognitive and electrophysiological responses of all Biotypes. Initial findings showed that B-1 has the most prominent gray matter reduction and impaired social function, suggesting that Biotypes meaningfully discriminate probands based on both brain function outcomes and structural brain markers.22–24 Nonetheless, further evidence of validation of Biotypes is critically needed.
As the amygdala and hippocampus were not used in the development of these Biotypes and are showing large volume reduction when subgroups of probands with psychosis are compared with healthy individuals, they are great candidates to validate the Biotype classification. Hence, we aimed to determine if these brain structures can validate whether the Biotypes are more neurobiologically distinct compared with traditional clinical diagnoses. Based on their respective profiles, we hypothesized that (a) B-1 probands will show the most extensive amygdala and hippocampal volume reductions and shape abnormality, (b) B-2 probands will have moderate amygdala and hippocampal volume reductions and shape abnormality, and (c) B-3 probands will show “near normal” amygdala and hippocampus compared with healthy controls. We also predicted that these limbic structural measures would provide better discrimination between the Biotypes subgroups of probands than the traditional clinical diagnoses.
Methods
A total of 984 participants with magnetic resonance scans were recruited from 6 sites in the United States (Baltimore, Boston, Chicago, Dallas, Detroit, and Hartford). Psychosis cases were recruited through advertisements and hospital clinics. Controls were also recruited from these sites in the community via advertisements. Diagnostic, clinical, and scanning procedures were standardized across all sites, and each individual biomarker was processed at the same site, as reported in prior studies.9,22,23 After visual scan inspection, quality control, and removing probands who did not receive Biotype classification (B-1, B-2, and B-3), a total of 811 participants were included in the current study, including 199 individuals with schizophrenia, 122 with schizoaffective disorder, 160 with bipolar disorder with psychosis, and 330 healthy controls (supplementary figure S1).
A Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)25 was done to confirm diagnoses of schizophrenia, schizoaffective, or bipolar disorder with a history of psychosis, in addition with available medical and psychiatric history from health records and relatives. The Positive and Negative Symptom Scale (PANSS),26 Montgomery Asberg Depression Rating Scale (MADRS),27 and Young Mania Rating Scale (YMRS)28 were all used to rate clinical symptoms in participants with an Axis 1 clinical psychosis diagnosis. For cognition assessment, the Brief Assessment of Cognition in Schizophrenia (BACS) was administered to all participants and the composite z-scores of all 6 subtests were used.29 Chlorpromazine equivalent antipsychotic daily dose was also calculated using the method described in Andreasen et al.30
Healthy controls were not part of the Biotype classification. To determine the Biotype classification of each proband, recording and testing conditions were standardized across all sites. The approach to Biotype classification is detailed in Clementz et al.22 Traditional intermediate phenotypes were assessed via brain function and phenotypic data derived from B-SNIP assessments and used in Biotype development. The BACS, stop-signal tasks, pro- and anti-saccade tasks, and auditory-paired stimuli and oddball evoked brain responses were used to create the cognitive control and sensorimotor reactivity measures used for the Biotype classification.22 Further details on Biotype stratification are provided in supplementary methods. A total of 73 participants did not have enough data to be included in the Biotype classification and were therefore removed from this study (supplementary figure S1). All clinical diagnoses were represented in all Biotypes (see table 2). The distribution of diagnoses in each Biotype was significantly different (χ 2 = 26.01; df = 4; P < .001). Specifically, B-1 had fewer probands with bipolar disorder than B-2 and B-3. B-1 also had fewer probands with schizoaffective disorder than B-3.
Table 2.
Descriptive Statistics of Clinical and Demographic Information of All Participants Based on Biotype (N = 811)
| Healthy Controls | B-1 | B-2 | B-3 | Pairwise Comparisons | |
|---|---|---|---|---|---|
| n | 330 | 121 | 161 | 199 | |
| Age, mean (SD) | 37.19 (12.42) | 36.27 (13.09) | 34.59 (11.77) | 35.13 (12.49) | |
| Sex (%) | |||||
| Female | 179 (54.2) | 61 (50.4) | 83 (51.6) | 100 (50.3) | |
| Male | 150 (45.5) | 60 (49.6) | 78 (48.4) | 99 (49.7) | |
| Race (%) | |||||
| Caucasian | 211 (63.9) | 45 (37.2) | 101 (62.7) | 132 (66.3) | B-1 vs B-2*** B-1 vs B-3*** B-1 vs HC*** |
| Other | 119 (36.1) | 76 (62.8) | 60 (37.3) | 67 (33.7) | |
| American Indian/Alaska Native | 3 (0.9) | 0 (0) | 0 (0) | 0 (0) | |
| Asian | 16 (4.8) | 0 (0) | 6 (3.7) | 4 (2.0) | |
| Black or African American | 86 (26.1) | 69 (57) | 48 (29.8) | 52 (26.1) | |
| Hawaiian or Pacific Islander | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | |
| More than one race | 6 (1.8) | 5 (4.1) | 2 (1.2) | 8 (4.0) | |
| Other | 6 (1.8) | 2 (1.7) | 4 (2.5) | 3 (1.5) | |
| Site (%) | |||||
| Baltimore | 50 (15.2) | 51 (42.1) | 24 (14.9) | 43 (21.6) | B-1 vs B-2*** B-1 vs B-3** B-1 vs HC*** B-2 vs B-3* B-2 vs HC** B-3 vs HC*** |
| Boston | 34 (10.3) | 4 (3.3) | 2 (1.2) | 6 (3.0) | |
| Chicago | 71 (21.5) | 23 (19.0) | 34 (21.1) | 64 (32.2) | |
| Dallas | 69 (20.9) | 14 (11.6) | 35 (21.7) | 26 (13.1) | |
| Detroit | 37 (11.2) | 14 (11.6) | 16 (9.9) | 17 (8.5) | |
| Hartford | 69 (20.9) | 15 (12.4) | 50 (31.1) | 43 (21.6) | |
| Handedness (%) | |||||
| Ambidextrous | 4 (1.2) | 3 (2.5) | 2 (1.2) | 4 (2.0) | |
| Left handed | 33 (10.0) | 11 (9.1) | 16 (9.9) | 26 (13.1) | |
| Right handed | 281 (85.2) | 106 (87.6) | 141 (87.6) | 168 (84.4) | |
| PANSS Total Score, mean (SD) | N/A | 64.20 (17.37) | 63.82 (17.00) | 60.59 (16.56) | |
| MADRS Total Score, mean (SD) | N/A | 9.61 (9.33) | 11.02 (8.87) | 10.40 (9.31) | |
| YMRS Total Score, mean (SD) | N/A | 5.46 (5.50) | 6.16 (6.32) | 6.35 (6.36) | |
| CPZ Equivalency, mean (SD) | N/A | 527.55 (438.26) | 519.99 (496.86) | 379.52 (298.41) | B-1 vs B-3* B-2 vs B-3* |
| BACS Cognition Score, mean (SD) | 0.02 (1.14) | −2.62 (0.90) | −1.93 (0.93) | −0.19 (0.84) | HC vs B-1*** HC vs B-2*** HC vs B-3* B-1 vs B-2*** B-1 vs B-3*** B-2 vs B-3*** |
| Diagnosis (%) | |||||
| Schizophrenia | N/A | 69 (57.0) | 67 (41.6) | 63 (31.7) | B-1 vs B-2* B-1 vs B-3*** |
| Schizoaffective disorder | N/A | 30 (24.8) | 42 (26.1) | 50 (25.1) | |
| Bipolar disorder with psychosis | N/A | 22 (18.2) | 52 (32.3) | 86 (43.2) | |
| Healthy controls | N/A | N/A | N/A | N/A |
Note: HC, healthy controls; B-1, Biotype-1; B-2, Biotype-2; B-3, Biotype-3; PANSS, The Positive and Negative Syndrome Scale; MADRS, The Montgomery–Åsberg Depression Rating Scale; YMRS, Young Mania Rating Scale; CPZ, chlorpromazine. Participants with missing data were removed from the analyses of the fields of their missing data (1 healthy control did not have data for age and sex, 16 participants did not have data for handedness, 11 probands did not have data for PANSS, 12 probands did not have data for MADRS, 12 probands did not have data for YMRS, and 172 probands did not have data for antipsychotic intake).
*P < .05, **P < .01, ***P < .001 false discovery rate corrected.
Inclusion and Exclusion Criteria
Healthy controls were to have no lifetime history of psychotic or mood disorder confirmed by completing the Structured Clinical Interview for DSM-IV Axis I Disorders and no family history of psychotic or bipolar disorders in their first‐ or second‐degree relatives. Exclusion criteria for all participants included the presence of a serious medical or neurological illness (eg, cancer, seizure disorders, and encephalopathy), mental retardation (ie, IQ < 65 on the Wide Range Achievement Test, 4th Edition31), current substance abuse (within 3 months), dependence within 6 months or extensive history of drug dependence (DSM‐IV), and any magnetic resonance imaging contraindications.
Magnetic Resonance Imaging Acquisition and Preprocessing
Sequence parameters were standardized across all sites and established from the Alzheimer’s Disease Neuroimaging Initiative protocol.22 We acquired T1-weighted scans using 3T scanners from different manufacturers such as Achieva, Philips, GE Signa, Siemens Allegra, and Siemens Trio. There were slight variations in MPRAGE or IR-SPGR parameters at each site, as appropriate for each scanner brand or model. Overall, all sites adopted the following parameters: 3D acquisitions, sagittal slab, shot interval 2300 or 3000 ms, inversion time 650–900 ms, TR 6.8–7.2 ms, TE 2.74–3.1 ms, flip angle 8 or 9º, FOV 176–270 (foot-to-head) × 240–260 (anterior-to-posterior) mm2, matrix approximately 256 × 256, in-pane resolution 1 × 1 mm2, 160–170 slices, slice thickness 1.2 mm, voxel size 1 × 1 × 1.2 mm3, total scan duration 9 minutes 4 seconds to 10 minutes 28 seconds. Site-specific imaging parameters are provided in supplementary methods and materials.
Preprocessing of T1-weighted images was carried out using the minc bpipe library (https://github.com/CobraLab/minc-bpipe-library). Quality control on all scans were performed by visual inspection. Motion artifacts were first graded (0 = no/very subtle motion, 1 = moderate motion, and 2 = severe motion), and scans with moderate-to-severe motion artifacts were excluded from the study (supplementary figure S1). Freesurfer 6.0 was used to estimate the total intracranial volume to control for its variability among participants in our analyses.
MAGeT-Brain Amygdala-Hippocampus Segmentation and Morphometric Modeling
The amygdala, hippocampus, and hippocampal subfields were segmented automatically on the preprocessed T1-weighted images. This was performed using the Multiple Automatically Generated Templates (MAGeT) Brain Segmentation algorithm,32 to improve segmentation accuracy by using neuroanatomical variability from the participant population, as previously described.33,34 This method provides accurate and reliable segmentations, resulting in a closer estimation to manual segmentation in clinical populations.34 The selected template library from unlabeled participants was representative in terms of diagnosis/Biotypes category, site, age, sex, and race. Each atlas was matched to each template via the template layer using linear and nonlinear transformation estimates (carried out using the Advanced Normalization Tools [ANTS] registration suite for MINC formatted images).35 Then, surface-based models of the amygdala and hippocampal structures were separately defined and morphologically smoothed.36 A single averaged transformation was estimated to estimate surface-based deformations, by concatenating individual nonlinear deformations from each of the subjects to a generated model from the atlases.37,38 To increase accuracy and precision and reduce noise, transformations were averaged for each model-to-subject pathway into a single nonlinear transformation. Each participant was matched to surface-based representations, warped to fit the corresponding template. Surface vertices were redefined using a Vornoi diagram to ensure homology prior to MAGeT-Brain segmentations.39 A surface-based diffusion smoothing kernel of 5 mm was used to blur all surface area values for both structures, after representing surface area as a sum for each polygon in the surface. A total of 41 scans were removed due to low segmentation quality through quantitative outlier detection (more than 3 SD from the mean) and qualitative visual inspection of all MAGeT segmentations using a similar grading as for the quality control of motion artifacts (supplementary figure S1).
Statistical Analysis
All statistical analyses were performed in R (version 3.6), except the shape analysis that was performed using the SurfStat toolbox (http://www.math.mcgill.ca/keith/surfstat/) in Matlab (R2018b). Multiple comparison correction for Type 1 error was applied when necessary, using the false discovery rate (FDR) Benjamini–Hochberg procedure.
Clinical and demographic analyses presented in tables 1 and 2 were conducted using Chi-square for categorical variables and ANOVA for continuous variables.
Table 1.
Descriptive Statistics of Clinical and Demographic Information of All Participants Based on Clinical Diagnosis (N = 811)
| Healthy Controls | Schizophrenia | Schizoaffective Disorder | Bipolar Disorder With Psychosis | Pairwise Comparisons | |
|---|---|---|---|---|---|
| n | 330 | 199 | 122 | 160 | |
| Age, mean (SD) | 37.19 (12.42) | 34.69 (12.24) | 35.31 (11.98) | 35.86 (12.94) | |
| Sex (%) | |||||
| Female | 179 (54.2) | 66 (33.2) | 69 (56.6) | 109 (68.1) | SZ vs SZA*** SZA vs BDP*** SZ vs HC*** BDP vs HC** |
| Male | 150 (45.5) | 133 (66.8) | 53 (43.4) | 51 (31.9) | |
| Race (%) | |||||
| Caucasian | 211 (63.9) | 93 (46.7) | 67 (54.9) | 118 (73.8) | SZ vs BDP*** SZ vs HC*** SZA vs BDP** |
| Other | 119 (36.1) | 106 (53.3) | 55 (45.1) | 42 (26.2) | |
| American Indian/Alaska Native | 3 (0.9) | 0 (0) | 0 (0) | 0 (0) | |
| Asian | 16 (4.8) | 4 (2.0) | 2 (1.6) | 4 (2.5) | |
| Black or African American | 86 (26.1) | 89 (44.7) | 47 (38.5) | 33 (20.6) | |
| Hawaiian or Pacific Islander | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | |
| More than one race | 6 (1.8) | 8 (4.0) | 5 (4.1) | 2 (1.2) | |
| Other | 6 (1.8) | 5 (2.5) | 1 (0.8) | 3 (1.9) | |
| Site (%) | |||||
| Baltimore | 50 (15.2) | 65 (32.7) | 26 (21.3) | 27 (16.9) | SZ vs SZA*** SZ vs BDP** SZ vs HC*** SZA vs BDP*** SZA vs HC*** BDP vs HC** |
| Boston | 34 (10.3) | 5 (2.5) | 2 (1.6) | 5 (3.1) | |
| Chicago | 71 (21.5) | 40 (20.1) | 25 (20.5) | 56 (35.0) | |
| Dallas | 69 (20.9) | 18 (9.0) | 33 (27.0) | 24 (15.0) | |
| Detroit | 37 (11.2) | 26 (13.1) | 1 (0.8) | 20 (12.5) | |
| Hartford | 69 (20.9) | 45 (22.6) | 35 (28.7) | 28 (17.5) | |
| Handedness (%) | |||||
| Ambidextrous | 4 (1.2) | 4 (2.0) | 4 (3.3) | 1 (0.6) | |
| Left handed | 33 (10.0) | 20 (10.1) | 9 (7.4) | 24 (15.0) | |
| Right handed | 281 (85.2) | 171 (85.9) | 109 (89.3) | 135 (84.4) | |
| PANSS Total Score, mean (SD) | N/A | 65.77 (17.06) | 68.58 (15.98) | 54.14 (14.31) | SZ vs BDP*** SZA vs BDP*** |
| MADRS Total Score, mean (SD) | N/A | 8.57 (8.14) | 14.02 (10.06) | 9.83 (8.93) | SZA vs BDP*** SZA vs SZ*** |
| YMRS Total Score, mean (SD) | N/A | 5.43 (5.50) | 7.22 (6.11) | 5.96 (6.81) | SZA vs SZ* |
| CPZ Equivalency, mean (SD) | N/A | 528.40 (425.44) | 525.35 (462.31) | 332.52 (335.20) | SZA vs BDP** SZ vs BDP** |
| BACS Cognition Score, mean (SD) | 0.02 (1.14) | −1.72 (1.30) | −1.44 (1.36) | −0.90 (1.30) | HC vs BDP*** HC vs SZ *** HC vs SZA*** SZA vs BDP*** SZ vs BDP*** SZ vs SZA* |
Note: HC, healthy controls; SZ, schizophrenia; SZA, schizoaffective disorder; BDP, bipolar disorder with psychosis; PANSS, The Positive and Negative Syndrome Scale; MADRS, The Montgomery–Åsberg Depression Rating Scale; YMRS, Young Mania Rating Scale; CPZ, chlorpromazine. Participants with missing data were removed from the analyses of the fields of their missing data (1 healthy control did not have data for age and sex, 16 participants did not have data for handedness, 11 probands did not have data for PANSS, 12 probands did not have data for MADRS, 12 probands did not have data for YMRS, and 172 probands did not have data for antipsychotic intake).
*P < .05, **P < .01, ***P < .001 false discovery rate corrected.
First, we performed a series of general linear models to investigate group differences in amygdala-hippocampal volumes and hippocampal subfields volumes between both healthy control group and psychosis cases group. Then, post hoc contrasts were used to compare all psychosis subgroups (based on either diagnoses or Biotypes) to healthy controls in one general linear model. Separate general linear models were also used to investigate possible interaction between diagnoses and Biotypes. Age, sex, race, intracranial volume, site, and handedness of participants were entered as covariates in all models.
To investigate the shape differences, we specified a general linear model with between-group contrasts at each vertex with surface area as a dependent variable to compare all psychosis subgroups (based either on diagnoses or Biotypes) to healthy controls in one model. FDR corrections were then performed across all vertices of each structure separately. Separate general linear models were also used to investigate the possible interaction between diagnoses and Biotypes. Age, sex, race, intracranial volume, site, and handedness of participants were entered as covariates in all models.
A series of logistic regressions using adjusted mean for age, sex, race, intracranial volume, site, and handedness of participants were then performed to determine whether amygdala-hippocampal volumes could discriminate the various subgroups of probands from each other. In addition, receiver operating characteristic (ROC) curves were used to compare subgroup discrimination sensitivity and specificity. ROC curves analyses allowed us to directly compare the sensitivity of clinical diagnosis and Biotypes at discriminating subgroups of probands.
Finally, we explored the effect of cognitive performance and antipsychotic intake on these brain structures (see supplementary methods and materials).
Results
Clinical and Demographic Data
Descriptive statistics of clinical and demographic information of all participants are shown in tables 1 and 2.
Amygdala and Hippocampal Volumes
After FDR correction, significant group differences were observed while using traditional clinical diagnosis classification for the left amygdala (F(3,781) = 10.60, corrected P < .001), right amygdala (F(3,781) = 6.39, corrected P < .001), left hippocampus (F(3,781) = 6.37, corrected P < .001), and right hippocampus (F(3,781) = 5.29, corrected P = .001). We also observed significant differences between subgroups of probands across different Biotypes for the left amygdala (F(3,781) = 7.70, corrected P < .001), right amygdala (F(3,781) = 8.85, corrected P < .001), left hippocampus (F(3,781) = 7.53, corrected P < .001), and the right hippocampus (F(3,781) = 10.97, corrected P < .001).
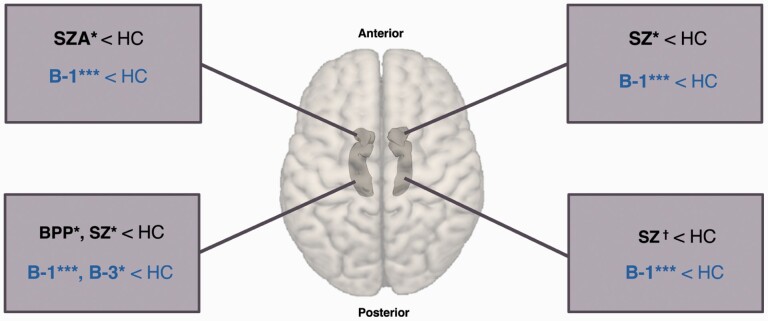
Figure 1 and table 3 summarize the post hoc between-group contrasts results for the left and right amygdala and hippocampus. Individuals with schizophrenia showed significantly smaller right amygdala volumes and left hippocampal volume and trending significant smaller right hippocampus when compared with controls. People with schizoaffective disorder showed significantly smaller left amygdala, whereas people with bipolar disorder with psychosis showed significantly smaller volume of the left hippocampus compared with healthy controls.
Fig. 1.
Results of volume analyses for left and right amygdala and hippocampus. †P < .10, *P < .05, ***P < .001 from post hoc contrasts comparing all psychosis subgroups to healthy controls in one general linear model. HC, healthy controls (n = 330); SZ, schizophrenia (n = 199); SZA, schizoaffective disorder (n = 122); BDP, bipolar disorder with psychosis (n = 160); B-1, Biotype-1 (n = 121); B-2, Biotype-2 (n = 161); B-3, Biotype-3 (n = 199).
Table 3.
Results of Between-Group Contrasts for the Left and Right Amygdala and Hippocampus
| Left Amygdala | Right Amygdala | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Volume, mean (SD), mm3 | Comparisons | Volume, mean (SD), mm3 | Comparisons | |||||||
| P | d | P | d | |||||||
| Diagnosis | HC | 1149.61 (104.70) | HC | 1068.64 (97.51) | ||||||
| SZ | 1134.24 (111.53) | SZ vs HC | .17 | 0.14 | SZ | 1049.44 (99.99) | SZ vs HC | .04 | 0.2 | |
| SZA | 1126.65 (106.01) | SZA vs HC | .05 | 0.22 | SZA | 1052.97 (94.25) | SZA vs HC | .15 | 0.16 | |
| BDP | 1134.69 (106.19) | BDP vs HC | .17 | 0.14 | BDP | 1065.29 (95.72) | BDP vs HC | .88 | 0.03 | |
| Biotype | B-1 | 1108.36 (105.45) | B-1 vs HC | <.001 | 0.39 | B-1 | 1028.60 (91.73) | B-1 vs HC | <.001 | 0.42 |
| B-2 | 1139.27 (114.55) | B-2 vs HC | .39 | 0.10 | B-2 | 1068.00 (101.74) | B-2 vs HC | .91 | 0.01 | |
| B-3 | 1141.62 (102.81) | B-3 vs HC | .45 | 0.08 | B-3 | 1062.01 (93.95) | B-3 vs HC | .55 | 0.07 | |
| Left Hippocampus | Right Hippocampus | |||||||||
| Diagnosis | HC | 1946.69 (199.34) | HC | 1816.16 (190.48) | ||||||
| SZ | 1901.38 (212.92) | SZ vs HC | .03 | 0.22 | SZ | 1778.94 (200.74) | SZ vs HC | .07 | 0.2 | |
| SZA | 1910.92 (220.97) | SZA vs HC | .13 | 0.17 | SZA | 1780.93 (196.85) | SZA vs HC | .11 | 0.18 | |
| BDP | 1894.24 (223.70) | BDP vs HC | .01 | 0.25 | BDP | 1788.11 (188.57) | BDP vs HC | .18 | 0.15 | |
| Biotype | B-1 | 1863.20 (204.78) | B-1 vs HC | <.001 | 0.42 | B-1 | 1723.20 (208.97) | B-1 vs HC | <.001 | 0.48 |
| B-2 | 1922.33 (205.31) | B-2 vs HC | .30 | 0.12 | B-2 | 1809.27 (196.64) | B-2 vs HC | .96 | 0.04 | |
| B-3 | 1907.75 (233.86) | B-3 vs HC | .05 | 0.18 | B-3 | 1796.88 (178.67) | B-3 vs HC | .32 | 0.1 |
Note: HC, healthy controls (n = 330); SZ, schizophrenia (n = 199); SZA, schizoaffective disorder (n = 122); BDP, bipolar disorder with psychosis (n = 160); B-1, Biotype-1 (n = 121); B-2, Biotype-2 (n = 161); B-3, Biotype-3 (n = 199); d, Cohen’s d estimate. P values reported here are from post hoc contrasts comparing all psychosis subgroups to healthy controls in one general linear model. Means and SD are adjusted for age, sex, race, intracranial volume, site, and handedness. Bold values indicate d > 0.30.
For probands across different Biotypes, probands of B-1 had significantly smaller amygdala-hippocampal volumes, compared with healthy controls. In addition, probands of B-3 showed significantly smaller volume for the left hippocampus, when compared with healthy controls.
The effect sizes observed for significant differences when Biotypes were compared with controls were generally higher than when clinical diagnoses were used (see table 3). There was no significant interaction between Biotype and diagnosis for any of the structures (P > .18). Means and standard deviations for each Biotype group within each diagnosis are illustrated in supplementary figure S4.
Hippocampal Subfields
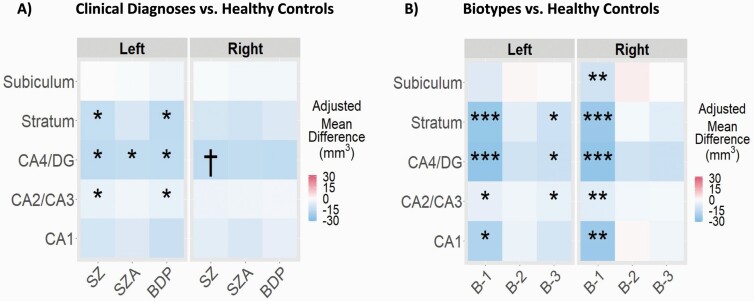
Figure 2 illustrates the differences in hippocampal subfield volumes between healthy controls and probands across different clinical diagnoses and Biotypes. After FDR correction, the left cornu ammonis (CA) 4 and dentate gyrus (DG) was significantly smaller in probands across all clinical diagnoses compared with controls. In addition, the left stratum and CA2/CA3 were significantly smaller in individuals with schizophrenia and bipolar disorder with psychosis compared with controls. The right CA4/DG was marginally significantly smaller for people with schizophrenia.
Fig. 2.
Hippocampal subfield volume differences for individuals across diagnoses and Biotypes when compared with healthy controls. †P < .10, *P < .05, **P < .01, ***P < .001 false discovery rate corrected. SZ, schizophrenia (n = 199); SZA, schizoaffective disorder (n = 122); BDP, bipolar disorder with psychosis (n = 160); B-1, Biotype-1 (n = 121); B-2, Biotype-2 (n = 161); B-3, Biotype-3 (n = 199); CA, cornu ammonis; DG, dentate gyrus. The mean differences are adjusted for age, sex, race, intracranial volume, site, and handedness. All regions showing a significant difference represent smaller subfield volumes in psychosis cases compared with controls.
Significantly smaller volumes were also observed in all hippocampal subfields in B-1 compared with healthy controls. The left stratum, CA4/DG, and CA2/CA3 were significantly smaller in B-3 when compared with healthy controls.
Finally, there was no significant group interaction between diagnoses and Biotypes for any hippocampal subfield (P > .23).
Amygdala and Hippocampal Shape Analysis
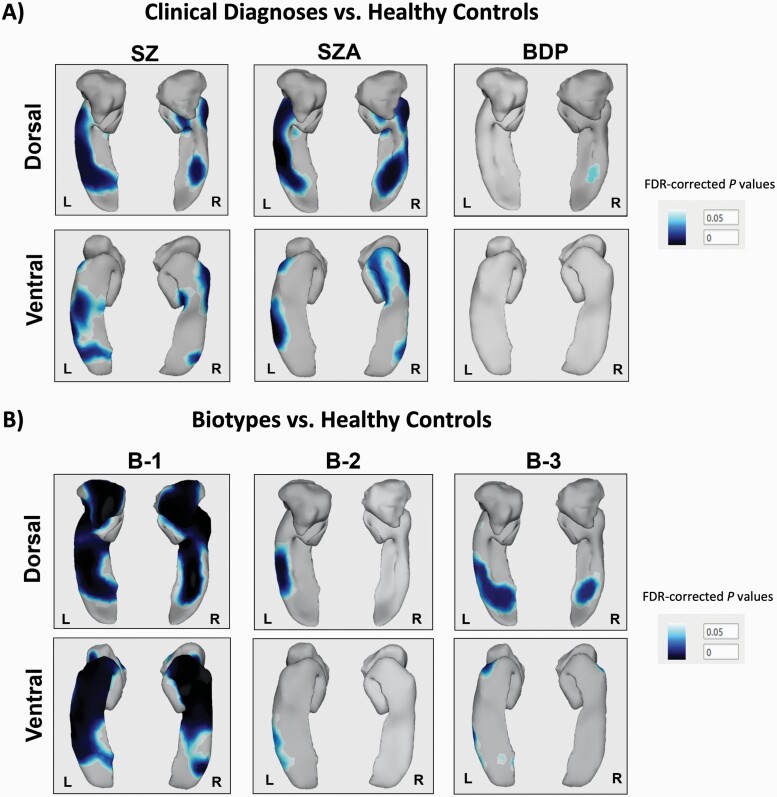
Figure 3 depicts shape differences in amygdala and hippocampus between probands and healthy controls. As illustrated, the significant differences in the shape of the left and right amygdala were widespread and particularly notable in the comparison between B-1 and healthy controls (corrected P < .05). Among diagnoses, the comparison between people with schizophrenia and healthy controls showed a small significant abnormal shape on the dorsomedial posterior region on the right amygdala (corrected P < .05).
Fig. 3.
Significant surface shape deformation for the left and right amygdala and hippocampus for each group of probands compared with healthy controls. Darker regions represent significant surface-based deformations (ie, smaller surfaces, false discovery rate corrected) observed in psychosis cases when compared with healthy controls. HC, healthy controls (n = 330); SZ, schizophrenia (n = 199); SZA, schizoaffective disorder (n = 122); BDP, bipolar disorder with psychosis (n = 160); B-1, Biotype-1 (n = 121); B-2, Biotype-2 (n = 161); B-3, Biotype-3 (n = 199).
In contrast to amygdala, significant shape differences of the left and right hippocampus were present in almost all subgroups of probands (corrected P < .05). B-1, B-3, schizophrenia, and schizoaffective groups demonstrated more significant differences in both anterior and posterior hippocampus compared with healthy controls. Overall, B-1 was the subgroup of probands that showed the most widespread abnormal shape for both left and right hippocampus (corrected P < .05). Subtle shape differences were also observed in the left posterior hippocampus in B-2 and in the right posterior hippocampus in individuals with bipolar disorder with psychosis. There was no significant diagnosis by Biotype interaction for the shape of either of these structures (P > .78).
Discrimination Between Different Subgroups of Probands
When using the amygdala volumes, we observed significant discrimination between B-1 and the other Biotypes (left amygdala [B-1 vs B-3]: B = 0.003, SE = 0.001, z-ratio = 2.72, x2 = 7.39, corrected P = .03; right amygdala [B-1 vs B-2]: B = 0.004, SE = 0.001, z-ratio = 3.23, x2 = 10.41, corrected P = .01; right amygdala [B-1 vs B-3]: B = 0.004, SE = 0.001, z-ratio = 3.03, x2 = 9.17, corrected P = .01), whereas no significant discrimination was observed between B-2 and B-3 or between any clinical diagnoses (corrected P > .05).
When using the hippocampal volumes, we observed significant discrimination between B-1 and the other Biotypes for the right hippocampus ([B-1 vs B-2]: B = 0.002, SE = 0.001, z-ratio = 3.40, x2 = 11.50, corrected P = .01; [B-1 vs B-3]: B = 0.002, SE = 0.001, z-ratio = 3.23, x2 = 10.43, corrected P = .01). No other significant discrimination was observed between any Biotypes nor clinical diagnoses using hippocampal volumes (corrected P > .05).
ROC curves analyses showed that amygdala and hippocampal volumes are more sensitive for separating Biotypes than clinical diagnosis (supplementary figure S5). The greatest discriminations were observed between B-1 and other Biotypes (AUC range = 0.573–0.617).
Discussion
In the current study, we examined amygdala and hippocampal structures across the psychosis spectrum, using both volumetric and shape data in traditional DSM-IV diagnoses and psychosis Biotypes. Various patterns of differences across psychosis subgroups were observed, but overall, our results demonstrated that using Biotype classification was more effective at identifying a specific subgroup of cases with greater abnormalities (ie, B-1) than using traditional clinical diagnosis. Hence, using volume and shape data of the amygdala and hippocampus provide additional validation for the Biotype model as a more efficient way of detecting neurobiologically meaningful subgroups of probands.
B-1 showed the most significant and widespread volume and shape abnormalities on various amygdala and hippocampal measures from all subgroups of probands. These results are in line with the cognitive profile of individuals in B-1 showing impaired cognition and profoundly deficient neural responses to sensory stimuli.22 Other B-SNIP studies have also shown that probands in B-1 have reduced gray matter density, reduced hippocampal volume, abnormal low intrinsic neural activity, and functional connectivity.23,40–42 Together, these findings suggest that there are extensive impairments in brain structure and function seen in B-1 psychosis cases.
Our results showed that differences in bilateral amygdala and hippocampal volumes provided the best discrimination between psychosis subgroups when B-1 was compared with other Biotypes. B-1 was also the only Biotype that had significant volume reductions in bilateral amygdala and hippocampus, with the largest effect size of all subgroups of probands. Amygdala and hippocampal alterations are commonly seen in psychosis, which have also been associated with cognitive deficits.11,43,44 In addition, shape results showed widespread abnormalities for B-1. As B-1 has severe cognitive impairments combined with profound electrophysiological responses, it could reflect why they also show the largest amygdala and hippocampal abnormalities. It is also in line with our current findings showing that lower cognitive performance was associated with lower volumes of the amygdala and hippocampus in our participants (see supplementary results for more details).
In the initial Biotype construction, individuals in B-2 also showed cognitive impairments, but a normal ability to respond to sensory stimuli combined with accentuated background brain activity; B-3 cases had the most similar profiles to healthy controls.22 Additionally, when measuring overall gray matter density, the most extensive gray matter loss was in B-1, intermediate loss in B-2, and near-normal gray matter density in B-3.23 The current sample used in this study showed a similar pattern of cognitive profile, with more severe cognitive deficits in B-1, intermediate cognitive deficits in B-2, and near-normal cognition in B-3. However, contrary to our initial hypothesis based on previous gray matter findings, we only observed subtle significant abnormalities in the shape of the left posterior hippocampus for B-2, similarly as for B-3, who also showed significant smaller volumes of the left CA2–CA3, CA4, and stratum hippocampal subfields. Interestingly, the left CA2–CA3 was not significantly associated with cognitive scores on the BACS in psychosis cases (see supplementary results for more details). Therefore, it is possible that subfields and shape analysis can identify more subtle abnormalities on the posterior hippocampus, especially on the left hemisphere, that could be related more to psychosis pathology, than general cognitive profiles.45
When using traditional clinical diagnoses, individuals with schizophrenia showed significant reductions for the right amygdala and the left hippocampus, as well as trending smaller right hippocampus when compared with healthy controls. Significant volume reductions compared with healthy controls were also observed in the left amygdala in schizoaffective probands and in the left hippocampus in bipolar disorder with psychosis probands. Our findings are consistent with previous research reporting reduced amygdala and hippocampal volume in schizophrenia and schizoaffective disorders13,46 and bipolar disorder with psychosis14,47 compared with healthy controls. Significant shape abnormalities of the left and right hippocampus were also seen mostly in schizophrenia and schizoaffective subgroups compared with healthy controls. These findings are in line with previous studies showing that individuals with schizophrenia and schizoaffective disorder showed hippocampal shape deformations that differ from individuals with bipolar disorder with psychosis.17,19
The current study is the first to utilize shape along with volume data of the amygdala and hippocampus, comparing both conventional clinical diagnoses and the psychosis Biotype model in a large sample of people on the psychosis spectrum. Biotype categories were based on cognitive and electrophysiological biomarkers, but not imaging biomarkers. Importantly, the Biotype defining parameters were not markers related to amygdala nor hippocampal structures. Furthermore, no significant interaction was observed between the traditional diagnoses and Biotypes, suggesting that regardless of the clinical diagnosis of the probands, Biotypes presented consistent patterns of results. Biotype classification provided the greatest neuroanatomical differences for the amygdala and hippocampus compared with controls, but the specificity and sensitivity to discriminate within subgroups of probands remains modest. Hence, more work is needed in the field to improve the identification of distinct subgroups of probands across the psychosis spectrum.
Nonetheless, the Biotype organization can be seen as a first step toward more effective classification methods in psychosis. This first step could lead to a more effective way to identify subgroups of probands, which are biologically meaningful across the psychosis spectrum.8 Hence, our findings support Biotype classification compared with clinical diagnoses and highlight the need for more effective objective methods of classification that could benefit early intervention and personalized treatment.48 Our findings also support the value of shape abnormalities as potentially valuable biomarkers to characterize biologically distinct subgroups of psychotic disorders for future pathophysiological research. For instance, our shape analysis was more sensitive in detecting subgroup differences between probands and controls than our volume analysis. Finally, amygdala and hippocampal shape alterations may be potential endophenotypic markers, suggesting that investigating these biomarkers in relatives at risk for psychotic disorders are likely to be fruitful.
Our findings should be appreciated in the context of some limitations. First, most individuals with psychosis were medicated, creating a potential confound of medication effects and state of illness of the probands. Although antipsychotic treatment may affect brain measures, our post hoc analysis did not show a significant effect of medication dose on volume and shape of the amygdala or hippocampus, suggesting that dose differences between groups unlikely account for neuroanatomic differences (see supplementary results for more details). Nonetheless, it would be interesting to further investigate Biotype classification in unmedicated cases and high-risk populations. In addition, our subgroups of probands were not perfectly matched. Further cross-diagnostic investigation with more homogeneous subgroups of probands on sex, race, and antipsychotic uptake is warranted. The current study was an a priori hypothesis-driven investigation of 2 important limbic regions involved in psychosis (ie, the amygdala in the hippocampus) and further investigation of subcortical regions also involved in these disorders (eg, the thalamus) could be of interest. Importantly, trained individuals who performed motion and segmentation quality control were blind to the diagnosis and Biotype conditions. Therefore, although, like in most structural imaging studies, it is possible that some motion-related noise may have remained in our data, it is unlikely that it would explain the between-group findings. Nonetheless, future studies should aim to include continuous measures of image quality as covariates in statistical analyses. Finally, the cross-sectional design is a limitation, and the longitudinal trajectory of these observations across the whole course of illness needs to be characterized in future studies.
Conclusion
The results from the current study provide further insight into psychiatric disease classification. B-1 showed greater amygdala and hippocampal volume and shape abnormalities and better discrimination from other psychosis case subtypes compared with differences observed using traditional clinical diagnoses. Using more objective methods to identify neuroanatomically distinct subgroups of probands on the psychosis spectrum, such as the Biotype classification, could have important contributions in progressing toward more personalized treatment in psychosis.
Supplementary Material
Acknowledgments
We acknowledge all of the individuals who have volunteered for the studies reported in this article and their contributions to progress in psychiatric research. The authors thank Dr. Gunvant Thaker for his contribution to the B-SNIP study, all the participants who took part in the study, the staff who supported the study, and the National Institute of Mental Health for funding the study.
Funding
This research was supported by the National Institute of Mental Health (MH078113 to M.S.K, MH077945 to G.D.P., MH077851 to C.A.T., and MH077862 to J.A.S.). S.G. was supported by the Fonds de recherche du Québec – Santé (FRQS) and the Emerging Research Innovators in Mental Health (eRIMh) award.
Conflict of Interest
J.A.S. is a scientific advisor for VeraSci, C.T. is on the Advisory Board of Karuna and Kynexis and a DSB Member for Merck, as well as an ad hoc consultant for Sunovion and Astellas. All other authors reported no conflict of interest.
References
- 1. Keshavan MS, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA. Reimagining psychoses: an agnostic approach to diagnosis. Schizophr Res. 2013;146(1–3):10–16. [DOI] [PubMed] [Google Scholar]
- 2. Hill SK, Reilly JL, Keefe RS, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170(11):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howes O, Juahar S, Brugger S, Pepper F. Brain structural and neurochemical heterogeneity and homogeneity in psychotic disorders: transdiagnostic PET and MRI imaging findings in schizophrenia and bipolar affective disorder. Schizophr Bull. 2018;44(suppl 1):S13. [Google Scholar]
- 4. Tamminga CA, Pearlson GD, Stan AD, et al. Strategies for advancing disease definition using biomarkers and genetics: the bipolar and schizophrenia network for intermediate phenotypes. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(1):20–27. [DOI] [PubMed] [Google Scholar]
- 5. Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17(12):1174–1179. [DOI] [PubMed] [Google Scholar]
- 6. Hager BM, Keshavan MS. Neuroimaging biomarkers for psychosis. Curr Behav Neurosci Reports. 2015;2(2):102–111. [PMC free article] [PubMed] [Google Scholar]
- 7. Keshavan MS, Kelly S, Hall MH. The core deficit of “Classical” schizophrenia cuts across the psychosis spectrum. Can J Psychiatry. 2020;65(4):231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clementz BA, Trotti RL, Pearlson GD, et al. Testing psychosis phenotypes from bipolar–schizophrenia network for intermediate phenotypes for clinical application: biotype characteristics and targets. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(8):808–818. [DOI] [PubMed] [Google Scholar]
- 9. Mathew I, Gardin TM, Tandon N, et al. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71(7):769–777. [DOI] [PubMed] [Google Scholar]
- 10. van Winkel R, van Nierop M, Myin-Germeys I, van Os J. Childhood trauma as a cause of psychosis: linking genes, psychology, and biology. Can J Psychiatry. 2013;58(1):44–51. [DOI] [PubMed] [Google Scholar]
- 11. Makowski C, Bodnar M, Shenker JJ, et al. Linking persistent negative symptoms to amygdala-hippocampus structure in first-episode psychosis. Transl Psychiatry. 2017;7(8):e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zierhut KC, Graßmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain. 2013;136(pt 3):804–814. [DOI] [PubMed] [Google Scholar]
- 13. Van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hibar DP, Westlye LT, Van Erp TGM, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016;21(12):1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strasser HC, Lilyestrom J, Ashby ER, et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry. 2005;57(6):633–639. [DOI] [PubMed] [Google Scholar]
- 16. Watson DR, Bai F, Barrett SL, et al. Structural changes in the hippocampus and amygdala at first episode of psychosis. Brain Imaging Behav. 2012;6(1):49–60. [DOI] [PubMed] [Google Scholar]
- 17. Mahon PB, Lee DS, Trinh H, et al. Morphometry of the amygdala in schizophrenia and psychotic bipolar disorder. Schizophr Res. 2015;164(1–3):199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maggioni E, Bellani M, Altamura AC, Brambilla P. Neuroanatomical voxel-based profile of schizophrenia and bipolar disorder. Epidemiol Psychiatr Sci. 2016;25(4):312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mamah D, Alpert KI, Barch DM, Csernansky JG, Wang L. Subcortical neuromorphometry in schizophrenia spectrum and bipolar disorders. Neuroimage Clin. 2016;11:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith MJ, Wang L, Cronenwett W, Mamah D, Barch DM, Csernansky JG. Thalamic morphology in schizophrenia and schizoaffective disorder. J Psychiatr Res. 2011;45(3):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiu A, Wang L, Younes L, et al. Neuroanatomical asymmetry patterns in individuals with schizophrenia and their non-psychotic siblings. Neuroimage. 2009;47(4):1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ivleva EI, Clementz BA, Dutcher AM, et al. Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biol Psychiatry. 2017;82(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamminga CA, Ivleva EI, Keshavan MS, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170(11):1263–1274. [DOI] [PubMed] [Google Scholar]
- 25. First MD, Spitzer RL, Gibbon M, Williams JB.. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition. New York, NY: Biometrics Research Department; 1996. [Google Scholar]
- 26. Kay SR, Flszbeln A, Qpjer LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 27. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 28. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 29. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283–297. [DOI] [PubMed] [Google Scholar]
- 30. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilkinson GS, Robertson GJ.. Wide Range Achievement Test. 4th ed. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 32. Chakravarty MM, Steadman P, van Eede MC, et al. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp. 2013;34(10):2635–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pipitone J, Park MT, Winterburn J, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage. 2014;101:494–512. [DOI] [PubMed] [Google Scholar]
- 34. Treadway MT, Waskom ML, Dillon DG, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77(3):285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Avants B, Tustison N, Song G. Advanced normalization tools (ANTS). Insight J. 2008;1–35. [Google Scholar]
- 36. Lorensen WE, Cline HE. Marching cubes: a high resolution 3D surface construction algorithm. In: Proceedings of the 14th Annual Conference on Computer Graphics and Interactive Techniques, SIGGRAPH 1987. New York, NY: Association for Computing Machinery, Inc; 1987:163–169. [Google Scholar]
- 37. Borghammer P, Østergaard K, Cumming P, et al. A deformation-based morphometry study of patients with early-stage Parkinson’s disease. Eur J Neurol. 2010;17(2):314–320. [DOI] [PubMed] [Google Scholar]
- 38. Voineskos AN, Winterburn JL, Felsky D, et al. Hippocampal (subfield) volume and shape in relation to cognitive performance across the adult lifespan. Hum Brain Mapp. 2015;36(8):3020–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lyttelton OC, Karama S, Ad-Dab’bagh Y, et al. Positional and surface area asymmetry of the human cerebral cortex. Neuroimage. 2009;46(4):895–903. [DOI] [PubMed] [Google Scholar]
- 40. Arnold SJM, Ivleva EI, Gopal TA, et al. Hippocampal volume is reduced in schizophrenia and schizoaffective disorder but not in psychotic bipolar I disorder demonstrated by both manual tracing and automated parcellation (FreeSurfer). Schizophr Bull. 2015;41(1):233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hudgens-Haney ME, Ethridge LE, McDowell JE, et al. Psychosis subgroups differ in intrinsic neural activity but not task-specific processing. Schizophr Res. 2018;195:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ji L, Meda SA, Tamminga CA, et al. Characterizing functional regional homogeneity (ReHo) as a B-SNIP psychosis biomarker using traditional and machine learning approaches. Schizophr Res. 2020;215:430–438. [DOI] [PubMed] [Google Scholar]
- 43. Fan F, Xiang H, Tan S, et al. Subcortical structures and cognitive dysfunction in first episode schizophrenia. Psychiatry Res Neuroimaging. 2019;286:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kelly S, Guimond S, Lyall A, et al. Neural correlates of cognitive deficits across developmental phases of schizophrenia. Neurobiol Dis. 2019;131:104353. [DOI] [PubMed] [Google Scholar]
- 45. Li W, Ghose S, Gleason K, et al. Synaptic proteins in the hippocampus indicative of increased neuronal activity in CA3 in schizophrenia. Am J Psychiatry. 2015;172(4):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Okada N, Fukunaga M, Yamashita F, et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 2016;21(10):1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bearden CE, Thompson PM, Dutton RA, et al. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33(6):1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pearlson GD, Clementz BA, Sweeney JA, Keshavan MS, Tamminga CA. Does biology transcend the symptom-based boundaries of psychosis? Psychiatr Clin North Am. 2016;39(2):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.