Abstract
Pomaglumetad methionil (POM), a group 2 metabotropic glutamate receptor (mGluR2/3) agonist, showed promise as a novel antipsychotic in preclinical research but failed to show efficacy in clinical trials, though it has been suggested that it may be effective in certain patient populations, including early in disease patients. We used the methyazoxymethanol acetate (MAM) rat model of schizophrenia to determine whether POM may prevent the development of dopamine (DA) system dysfunction in a model representative of the hyperdopaminergic state thought to underlie psychosis, compared to control (SAL) rats. MAM and SAL rats were administered either POM (3 mg/kg, i.p.), vehicle (1 ml/kg), or no injection during postnatal day (PD) 31–40. In either late adolescence (PD 47–56) or adulthood (PD 83–96), novel object recognition (NOR) was tested, followed by anesthetized in vivo electrophysiological recordings of VTA DA neuron activity or ventral hippocampal (vHPC) pyramidal neuron activity. MAM rats treated with POM demonstrated increased NOR in adulthood compared to no injection MAM rats, but not compared to vehicle-treated MAM rats. POM-treated MAM rats demonstrated normalized DA neuron population activity and vHPC pyramidal neuron activity compared to vehicle and no injection MAM rats in both late adolescence and adulthood. No significant differences were observed across treatment groups in SAL rats. These results suggest that peripubertal mGluR2/3 agonist administration can prevent the emergence of vHPC pyramidal neuron hyperactivity and increased DA neuron population activity in adult MAM rats.
Keywords: antipsychotic, glutamate, hippocampus, VTA, neurodevelopmental
Introduction
Schizophrenia is a chronic and debilitating disorder characterized by psychosis and a broad range of other cognitive and behavioral symptoms that typically emerge in late adolescence and early adulthood.1 The neurodevelopmental hypothesis of schizophrenia states that early disruption in brain development, due to genetic and environmental factors, interacts with later maturational processes to result in full manifestation of the disorder.2,3 It has been suggested that targeting critical periods of maturation has the potential to alter the course of schizophrenia,4,5 which has been supported by recent animal model research.6,7 Current treatments are generally effective in alleviating psychotic symptoms that are already established; however, recent research has suggested that early-intervention treatments may specifically target the development of pathophysiological processes.
The methazoxymethanol acetate (MAM) rodent model has been used to study the neurodevelopment of a number of phenotypes relevant to psychotic symptoms in schizophrenia.8 Both MAM rats9–11 and patients with schizophrenia12 demonstrate a reduction in parvalbumin (PV)+ GABA interneurons in the hippocampus. Converging evidence from animal and human studies suggest that loss of PV+ interneuron regulation of pyramidal neuron activity is associated with an imbalance between excitatory and inhibitory neurotransmission.10,13,14 Indeed, hypermetabolism of the anterior hippocampus has been observed in patients with schizophrenia and the clinical high-risk population,15–19 with a spreading increase in hippocampal metabolism following the onset of psychosis.13 Increased hippocampal glutamate levels have been reported in patients with schizophrenia20–24 and clinical high-risk individuals who later transition to psychosis.25 Work in the MAM model has further shown that the ventral hippocampus (vHPC), analogous to the anterior hippocampus in humans,26 regulates the number of spontaneously active DA neurons in the ventral tegmental area (VTA).27,28 Greater vHPC activity, as observed in MAM rats, results in higher DA neuron population activity, which allows for greater responsivity of the DA system.29 This may translate to increased measures of presynaptic DA function in patients that are thought to underlie psychotic symptoms.30,31
The prodromal phase of schizophrenia is characterized by a period of sub-clinical symptoms in the years prior to the first episode of psychosis. Attenuated psychotic signs generally begin after puberty and are progressive in severity.32 The maturational processes involved in puberty can shape brain development to support alterations in physiology and behavior across adolescence. When combined with genetic risk, these dynamic neurobiological changes are thought to contribute to the vulnerability of the developing brain to environmental factors that can lead to the emergence of psychiatric disorders.33,34 This may account for why adolescence is a period of peak onset of many psychiatric disorders, including schizophrenia.35,36 Prodromal interventions, such as protection of excitatory-inhibitory circuits within the hippocampus, may prevent or ameliorate the progression of psychosis in at-risk individuals.37
Group II metabotropic glutamate receptors (mGluR2/3) showed promise in preclinical research as a target to treat the aberrant excitatory-inhibitory balance implicated as a central component of the development and pathophysiology of schizophrenia.38,39 The mGluR2/3 pomaglumetad methionil (POM) failed during phase III clinical trials40,41; however, later analyses found that early in disease patients treated with POM demonstrated significant improvement in symptoms, which was not observed in chronic patients.42 This suggested that regulating glutamate early in the disease may be particularly useful in the treatment of schizophrenia.43,44 Our previous work demonstrated that POM can indirectly regulate DA neuron activity via the vHPC circuit.45 We therefore aimed to determine whether peripubertal administration of POM can prevent vHPC dysfunction and increased DA neuron population activity observed in adult MAM rats.
Methods
Subjects
All procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Timed pregnant Sprague-Dawley dams (Envigo) were obtained on gestational day (GD) 15 and MAM (20 mg/kg, i.p., Midwest Research Institute) or saline (SAL; 1 ml/kg, i.p.) was administered on GD 17. Male pups were weaned on postnatal day (PD) 23. All rats were housed in groups of 2–3 with littermates in a temperature (22°C) and humidity (47%)-controlled facility with ad libitum food and water in a normal 12-hour light-dark cycle.
Drug Administration
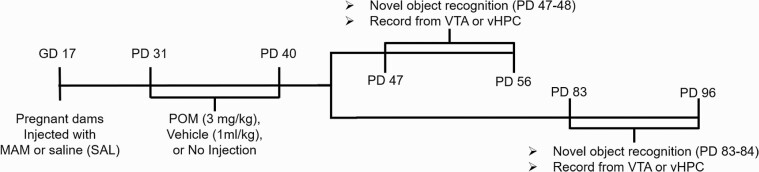
LY2140023 (pomaglumetad methionil, POM), the prodrug of mGluR2/3 agonist LY404039, was obtained from Selleck Chemicals and dissolved in 0.9% sterile saline with dropwise addition of 1M NaOH to POM (pH ~7) at a volume of 1 ml/kg. Vehicle-treated rats received 0.9% sterile saline. Peripubertal treatments occurred from PD 31–40. All rats were weighed daily and received 3 mg/kg POM (i.p.), 1 ml/kg vehicle (i.p.) or no injection (NI). The POM dose was selected based on a previous dose-response study demonstrating normalization of DA neuron population activity and to control levels in adult MAM rats.45 Rats within each cohort were divided between the 2 experimental timepoints (figure 1).
Fig. 1.
Experimental timeline. Methyazoxymethanol acetate (MAM) and SAL rats received peripubertal treatments (PD 31–40) and cohorts were randomly split into groups that were either tested during late adolescence (PD 47–56) or adulthood (PD 83–96). A random subset of each group was used for novel object recognition test during the dark cycle, which occurred either PD 47–48 or PD 83–84. Rats were then used for in vivo electrophysiological recordings to record either DA neuron activity in the ventral tegmental area (VTA) or pyramidal neuron activity in the hippocampus.
Novel Object Recognition
Novel Object Recognition (NOR) was performed on PD 47–48 or PD 83–84. Each rat was habituated to a rectangular box (L70 × W40 × H30 cm) for 10 minutes 1 day prior to the test. The test day involved two 5 min trials separated by a 1 h intertrial interval. In the first trial (T1), rats were placed in the box containing 2 identical objects. In the second trial (T2), one of the objects presented in T1 was replaced by a novel object. The familiar and novel objects were too heavy to be displaced by the animals and had different shape, color, and texture. The box and objects were cleaned between each trial. Habituation and behavioral tests were performed during the dark cycle. Behavior was recorded on video and object interaction was averaged between 2 experimenters blinded to treatment group. Interaction time of each object in each trial was recorded manually with stopwatches and defined as time when the rat interacts directly with the object, such as licking, sniffing, or touching it with its forepaws. Recognition memory was assessed using the discrimination index (discrimination index = (novel – familiar / novel + familiar)), corresponding to the difference between the time exploring the novel and the familiar object, corrected for total time exploring both objects. Two-way ANOVA was used to compare the differences between MAM and SAL groups, treatment group, and the possible interaction between MAM and treatment on behavioral measures. Tukey’s post hoc comparisons were conducted for significant interaction effects.
Electrophysiological Recordings
In vivo extracellular recordings occurred on either PD 47–56 or PD 83–96. Rats were anesthetized with chloral hydrate (400 mg/kg; i.p.) and placed on a stereotaxic frame (Kopf). Supplemental anesthesia was administered i.p. to maintain suppression of the hind limb withdrawal reflex. Body temperature was maintained at 37°C with a temperature-controlled heating pad (CWE Inc.). Single-barrel glass electrodes (WPI) were pulled vertically (PE-2, Narasige, Japan), broken under a microscope to an impedance of 6–8 MΩ, and filled with 2 M NaCl containing 2% Chicago Sky Blue dye in 2 M saline. Electrodes were lowered with a hydraulic micropositioner (Kopf) to sample neural activity in the region of interest. Single-unit activity was obtained using an amplifier (Fintronics) using a highpass filter at 30 Hz and lowpass at 16 kHz. Neural activity was recorded for at least 1 minute of stable spontaneous activity using LabChart software (AD Instruments). At the end of each recording, electrode placement was verified following each experiment via electrophoretic ejection of Chicago Sky Blue dye from the tip of the recording electrode (−20 µA constant current, 20 min). Rats were then overdosed with chloral hydrate and decapitated. The brains were removed and fixed for at least 48 hours (8% paraformaldehyde in PBS), cryoprotected (25% sucrose in PBS) until saturated, and sliced on a cryostat into 60µm sections, which were mounted onto gelatin-coated slides. Slides were stained with a mixture of cresyl violet and neutral red for verification of electrode sites with reference to a stereotaxic atlas.46
VTA Recordings and Dopamine Neuron Identification Criteria.
Recordings were performed by making 6–9 vertical electrode passes (“tracks”) in a predetermined grid pattern with each track separated by 0.2 mm (AP: −5.3 to −5.7 mm and ML: 0.6 to 1.0 mm from bregma, DV: −6.5 to −9.0 mm from the top of brain). DA neurons were identified by established criteria, including a biphasic action potential with duration >2.2 ms, 1–10 Hz firing rate, and irregular and burst firing patterns with burst initiation defined as interspike interval of ≤80 ms and termination as >160 ms.47–49
Hippocampal Recordings and Pyramidal Neuron Identification Criteria.
Recordings were performed by making 6 electrode tracks as described above, with coordinates AP: −5.5 to −5.9 mm and ML: 4.6 to 5.0 mm from bregma, DV: −5.5 to −8.5 mm from the top of brain. Neurons with a long biphasic action potential duration >2.0 ms and ≤2.0 Hz firing rate were classified as putative pyramidal neurons. Burst firing was characterized with burst firing criteria described above.
Electrophysiological Recording Analysis.
Three parameters of DA neuron activity were analyzed: (1) the average number of spontaneously active DA neurons encountered per electrode track (“population activity”), (2) average firing rate, and (3) the percentage of spikes that occurred in bursts (%SIB). Firing rate and %SIB were analyzed for pyramidal neurons. Analysis of firing rate and bursting activity was performed using NeuroExplorer (Plexon). Population activity was averaged within each animal and then across animals in each group, whereas the firing rate and burst activity of each neuron was counted as an independent replicate and averaged across animals in a group. Significance was assessed with a 2-way ANOVA (MAM × Treatment) followed by Tukey post hoc comparisons.
Results
Peripubertal Pomaglumetad Treatment Prevents Increased DA Neuron Population Activity in Adult MAM Rats
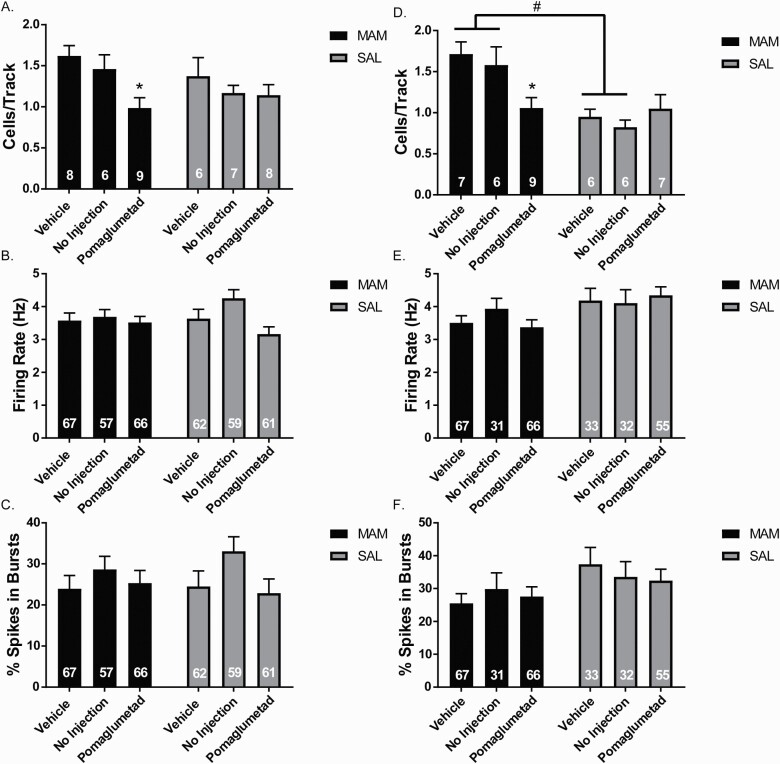
Peripubertal (PD 31–40) POM treatment significantly reduced DA neuron population activity in the VTA of rats recorded from PD 47–56 or “late adolescence” (figure 2A; 2-way ANOVA main effects: for Treatment, F(2,38) = 4.801, P = .014; post hoc vehicle MAM vs POM MAM: P = .020) and from PD 83–96 or “adulthood” (figure 2D; 2-way ANOVA main effects: for MAM: F(1,35) = 17.29, P < .001, MAM-by-POM interaction: F(2,35)=4.518, P = .018; post hoc vehicle MAM vs POM MAM: P = .022). There was no significant difference in DA neuron population activity between vehicle and NI in MAM or SAL rats in late adolescence (figure 2A) or adulthood (figure 2D). In adulthood, vehicle-treated MAM rats displayed significantly greater DA neuron population activity compared to vehicle-treated SAL rats (P = .0138) and NI MAM rats displayed significantly greater DA neuron population activity compared to NI SAL rats (P = .020). There was no significant difference of peripubertal POM treatment in SAL rats in late adolescence (figure 2A) or adulthood (figure 2D). There was no significant difference in firing rate or percentage of spikes in burst of DA neurons in late adolescence (figure 2B-C) or adulthood (figures 2E and 2F).
Fig. 2.
Peripubertal pomaglumetad treatment prevents increased DA neuron population activity in adult methyazoxymethanol acetate (MAM) rats. (A) In rats that were recorded in late adolescence, POM-treated MAM rats displayed reduced DA neuron population activity in the ventral tegmental area (VTA) compared to vehicle and no injection (NI)-treated MAM rats. There were no significant differences in the firing rate (B) or bursting activity (C) of the DA neurons in MAM or SAL rats in late adolescence. (D) In rats that were recorded in adulthood, POM-treated MAM rats displayed reduced DA neuron population activity compared to vehicle and NI-treated MAM rats. There were no significant differences in the firing rate. (E) or bursting activity (F). For population activity graphs (A, D), the numbers in bars represent the number of rats per group and for firing rate and bursting activity graphs (B–C, E–F), the numbers in bars represent the number of DA neurons per group. *P < .05 within groups #P < .05 between groups.
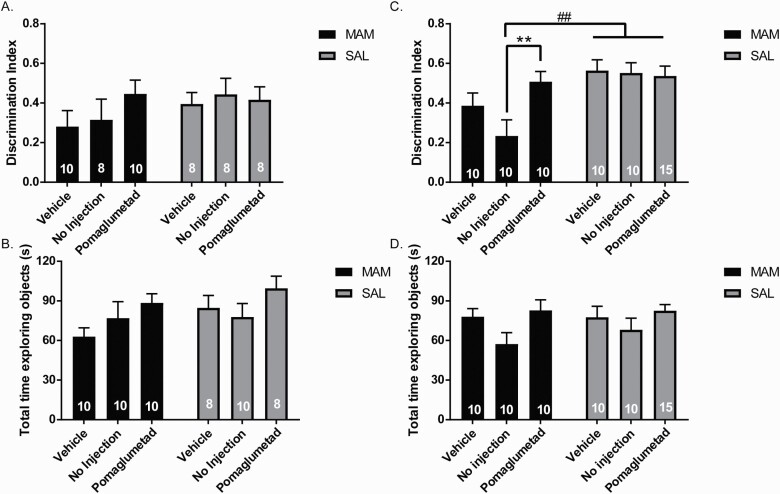
Reduced NOR Only in Adult MAM Rats That Did Not Receive Peripubertal Injections
No significant effects were observed in the NOR task in MAM or SAL rats across treatment groups in late adolescence, both in discrimination index (figure 3A) and total time exploring the objects (figure 3B). A main effect of MAM was observed in rats that were treated around puberty and tested in the NOR task during adulthood (figure 3C; F(1,59) = 12.67, P < .001). Adult MAM rats that received peripubertal POM treatment displayed significantly increased discrimination index compared to MAM rats that received NI during puberty (figure 3C; P = .031), but it was not significantly different from MAM rats that received vehicle during peripuberty (figure 3C). Adult NI-MAM rats displayed significantly lower NOR compared to NI-SAL rats (P = .007), but vehicle-MAM rats did not show a significant difference in NOR compared to vehicle-SAL rats (figure 3C). A main effect of treatment was observed in total time exploring objects in adulthood (figure 3D; 2-way ANOVA main effect for treatment: F(2,53) = 3.633, P = .0332.
Fig. 3.
Reduced novel object recognition only in adult methyazoxymethanol acetate (MAM) rats that did not receive peripubertal injections. (A) In late adolescence (PD 47–48), MAM and SAL rats displayed no differences in time interacting with a novel object compared to a familiar object as measured by a discrimination index ((novel-familiar)/(novel+familiar)) or in locomotor activity as measured by total time spent exploring objects (B). (C) In rats tested in adulthood (PD 83–84), POM-treated MAM rats displayed an increase in time spent exploring the novel object as measured by the discrimination index compared to no injection (NI)-treated MAM rats, though not significantly reduced compared to vehicle-treated MAM rats. (D) No significant differences were observed in total time spent exploring the objects. For all graphs, the numbers in bars represent the number of rats per group. **P < .01 within groups ##P < .01 between groups.
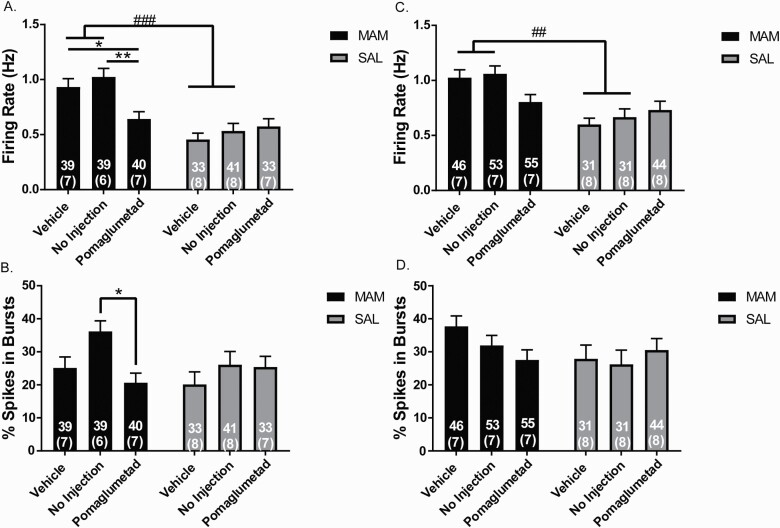
Peripubertal Pomaglumetad Treatment Prevents Increased Firing Rate of Ventral Hippocampal Pyramidal Neurons in Adult MAM Rats
MAM rats that were treated with vehicle or NI during peripuberty and recorded during late adolescence displayed significantly increased firing rate of pyramidal neurons in the vHPC compared to SAL rats that were treated with vehicle or NI (figure 4A; 2-way ANOVA main effects for: MAM: F(1,219) = 36.54, P < .001, MAM-by-POM interaction: F(2,219) = 5.782, P = .004; post hoc, vehicle-MAM-vehicle vs vehicle-SAL P < .001, NI-MAM vs NI-SAL P < .001). Peripubertal POM treatment in MAM rats significantly reduced the firing rate of pyramidal neurons in the vHPC compared to vHPC of vehicle-MAM rats (P = .035) and NI-MAM rats (P = .001) in late adolescence. There was no significant difference in the firing rate of vHPC pyramidal neurons between NI and vehicle treatment in MAM rats and no significant difference in firing rate across all treatment groups in SAL rats (figure 4A). NI-MAM rat vHPC pyramidal neurons demonstrated significantly increased bursting activity compared to POM-MAM rats (P = .015) and there were no significant differences in bursting activity in SAL rats recorded during late adolescence (figure 4B).
Fig. 4.
Peripubertal pomaglumetad treatment prevents increased firing rate of ventral hippocampal pyramidal neurons in adult methyazoxymethanol acetate (MAM) rats. (A) In late adolescence (PD 47–56), MAM rats that received vehicle or no injection (NI) during puberty displayed increased firing rate of pyramidal neurons compared to pyramidal neuron firing rate in SAL rats, which was normalized in MAM rats that received peripubertal POM. (B) MAM rats that did not receive injections showed an increase in percentage of spikes in bursts in late adolescence, compared to POM-treated MAM rats. (C) Vehicle and NI-MAM rats recorded in adulthood (PD 83–96) also displayed increased vHPC pyramidal neuron firing rate compared to vehicle and NI-SAL rats. MAM rats that received peripubertal POM had an average pyramidal neuron firing rate not significantly different from SAL rats. (D) No significant differences were observed in the percentage of spikes in burst in vHPC pyramidal neurons recorded in MAM and SAL rats at adulthood. For all graphs, the numbers outside of parenthesis represent the number of neurons per group and the number in parenthesis represents the number of rats per group. *P < .05; **P < .01 within groups ##P < .01 ###P < .001 between groups.
MAM rats that were treated with vehicle or NI during puberty and recorded during adulthood displayed significantly increased firing rate of pyramidal neurons in the vHPC compared to SAL rats that were treated with vehicle or NI (figure 4C; 2-way ANOVA main effects for: MAM: F(1,254) = 23.65, P < .001, MAM-by-POM interaction: F(2,254) = 3.678, P = .027; post hoc, MAM-vehicle vs SAL-vehicle P = .002, NI-MAM vs NI-SAL P = .004). Peripubertal POM treatment in MAM rats significantly reduced the firing rate of pyramidal neurons in the vHPC compared to vHPC of vehicle-MAM rats (P = .035) and NI-MAM rats (figure 4C; P = .001). No significant differences were observed in bursting activity in MAM or SAL rats recorded during adulthood (figure 4D).
Discussion
In this study, we examined the effects of peripubertal administration of the mGluR2/3 agonist, POM, on DA system dysregulation in the MAM model. Peripubertal administration of POM prevented several of the adult phenotypes of the MAM model, including increased DA neuron population activity in the VTA and increased vHPC pyramidal neuron activity. Previous studies have shown that the increased DA neuron population activity observed in adult MAM rats is indirectly driven by the vHPC, which shows increased pyramidal neuron firing rate in MAM rats.10,29,50 Accordingly, treatments that reduce vHPC activity have been shown to reduce DA neuron activity in MAM rats.51–54
Previous work has shown that early-intervention strategies can prevent the emergence of MAM phenotypes in adulthood. Peripubertal (PD 31–40) treatment of MAM rats with the anxiolytic drug, diazepam, prevented the hyperdopaminergic state, anxiety-like behavior and the higher neuronal firing rates within the basolateral amygdala normally present in adult MAM rats.6,55 Increasing GABA function during this period may protect PV+ interneurons that are still forming perineuronal nets,56 as it has been shown previously that severe peripubertal stress can produce a loss of PV+ interneurons in the vHPC and hyperdopaminergic state in normal rats in adulthood, similar to MAM rats.57,58 These studies emphasize the importance of disease phase as a factor in clinical trials that can play a role in heterogeneity of treatment response, whether due neurophysiological changes in the pathophysiology over time43 or other potential confounding variables, such as prior antipsychotic drug treatment.59
This leads to the question of how early-intervention targeting increased glutamatergic activity may produce long-lasting changes in preventing DA neuron hyperactivity. Puberty is a critical time in the stabilization of PV+ interneuron connections within the hippocampus through the formation of perineuronal nets, which is thought to underlie the closure of critical periods in development.56,60 Early life stress, particularly in those with a predisposition to increased stress responsivity, may lead to loss of PV+ neurons during this time and subsequent dysregulation of pyramidal neuron activity.58 MAM offspring display increased stress responsivity, prior to the manifestation of a hyperdopaminergic state,61,62 which has led to the proposal that treatments aimed at reducing anxiety and intervening in the development of glutamate dysfunction may prevent the deleterious effects of stress on the DA system during this critical time in development.7,63 Indeed, mGluR2/3 agonists have been shown to have anxiolytic potential in both animal models and humans. They demonstrate anxiolytic-like activity in rodents in tasks including stress-induced hyperthermia and the elevated plus maze,64–68 although further studies are needed to determine their anxiolytic-like effects in MAM rats. In humans, they reduce fear-potentiated startle without sedative effects69 and demonstrated therapeutic potential for patients with panic disorder70 and generalized anxiety disorder.71 It is of note that the effects observed in the present study were present even following the stress of repeated i.p. injections. Activation of mGluR2/3 in the amygdala may play a role in its regulation of fear and anxiety states. For example, infusion of mGluR2/3 agonists into the BLA disrupts fear-potentiated startle72 and blocking mGluR2/3 receptors can impair fear extinction in rats.73 These results suggest that mGluR2/3 activation may in part suppress anxiety by inhibiting projection neurons within the BLA. Future experiments are needed to determine whether mGluR2/3 agonist action in the BLA during peripubertal treatment is necessary for its long-term effects on DA neuron activity in adulthood, or whether normalizing vHPC activity alone is sufficient.
The present study was limited to male rats due to previously reported differences in DA neuron population activity across the estrous cycle between female MAM and SAL rats,74 including differences in response to mGluR2/3 agonist administration.45 Additional studies are needed to clarify the effects of early intervention in female rats that account for differences between MAM and SAL females across the estrous cycle. Additionally, female rats have shown resilience to stressors during PD 31–40 that are sufficient to result in increased DA neuron population activity in adult male rats.57,75 Therefore, a better understanding of the time course of stress responsivity and how it impacts circuit function in female rats would help guide these critical future studies.
In the NOR test, both MAM rats that received vehicle injections and POM injections displayed NOR similar to SAL rats. Only MAM rats that did not receive injections displayed impaired NOR in adulthood, as previously observed in adult MAM rats.57 All rats were handled equally and one possibility is that repeated injections during puberty, regardless of POM or vehicle, may improve NOR in adult MAM rats. Stress during puberty can produce mixed results on memory-related behavior76 and has been shown to result in greater adaptation to stressful experiences in adulthood, such as increased foraging behavior under threat,77 consistent with the concept of stress inoculation.78,79 It is therefore possible that the mild stress of i.p. injections during puberty may have improved their task performance, which did not appear to be linked to pyramidal neuron firing rate.
MAM rats treated with peripubertal vehicle or NI and tested during late adolescence displayed increased firing rate of pyramidal neurons in the vHPC, but their DA neuron activity was not significantly different from vehicle and NI-treated SAL rats recorded in late adolescence. However, MAM rats treated in the same cohorts and tested during adulthood displayed both increased vHPC pyramidal neuron firing rates and increased DA neuron population activity. The developmental period captured during PD 47–56 may represent an intermediate phenotype in which the increased vHPC pyramidal neuron activity was present prior to increased DA neuron population activity, which emerged in adulthood. It is unclear how rapid the transition to a hyperactive DA system occurs in adult MAM rats; however, these results suggest that it follows the onset of increased vHPC pyramidal neuron activity.
In sum, this study demonstrates that peripubertal administration of an mGluR2/3 agonist can prevent the emergence of vHPC pyramidal neuron hyperactivity and increased DA neuron population activity in adult MAM rats. These results support the hypothesis that targeting excitatory-inhibitory dysfunction has the potential to prevent DA system dysfunction relevant to schizophrenia.
Acknowledgments
We thank Niki MacMurdo and Christy Smolak for their technical assistance and Sara Minemyer for assistance with behavioural experiments. S.F.S. declares no conflicts of interest. A.A.G. receives consulting fees from Alkermes, Lundbeck, Takeda, Roche, Lyra, Concert, and research funding from Lundbeck.
Funding
This work is supported by the National Institutes of Health (NIH MH57440 to A.A.G.). We thank Niki MacMurdo and Christy Smolak for their technical assistance and Sara Minemyer for assistance with behavioral experiments.
References
- 1. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3–10. [DOI] [PubMed] [Google Scholar]
- 2. Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed). 1987;295(6600):681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. [DOI] [PubMed] [Google Scholar]
- 4. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485–515. [DOI] [PubMed] [Google Scholar]
- 5. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. [DOI] [PubMed] [Google Scholar]
- 6. Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38(10):1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu X, Grace AA. Prepubertal environmental enrichment prevents dopamine dysregulation and hippocampal hyperactivity in mam schizophrenia model rats. Biol Psychiatry. 2021;89(3):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Modinos G, Allen P, Grace AA, McGuire P. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38(3):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penschuck S, Flagstad P, Didriksen M, Leist M, Michael‐Titus AT. Decrease in parvalbumin‐expressing neurons in the hippocampus and increased phencyclidine‐induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci. 2006;23(1):279–284. [DOI] [PubMed] [Google Scholar]
- 10. Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29(8):2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gill KM, Grace AA. Corresponding decrease in neuronal markers signals progressive parvalbumin neuron loss in MAM schizophrenia model. Int J Neuropsychopharmacol. 2014;17(10):1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Konradi C, Yang CK, Zimmerman EI, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131(1-3):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schobel SA, Chaudhury NH, Khan UA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belforte JE, Zsiros V, Sklar ER, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talati P, Rane S, Kose S, et al. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clin. 2014;5:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Provenzano FA, Guo J, Wall MM, et al. Hippocampal pathology in clinical high-risk patients and the onset of schizophrenia. Biol Psychiatry. 2020;87(3):234–242. [DOI] [PubMed] [Google Scholar]
- 17. McHugo M, Talati P, Armstrong K, et al. Hyperactivity and reduced activation of anterior hippocampus in early psychosis. Am J Psychiatry. 2019;176(12):1030–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allen P, Azis M, Modinos G, et al. Increased resting hippocampal and basal ganglia perfusion in people at ultra high risk for psychosis: replication in a second cohort. Schizophr Bull. 2017;44(6):1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schobel SA, Lewandowski NM, Corcoran CM, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70(12):1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kraguljac NV, Morgan CJ, Reid MA, et al. A longitudinal magnetic resonance spectroscopy study investigating effects of risperidone in the anterior cingulate cortex and hippocampus in schizophrenia. Schizophr Res. 2019; 210:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kraguljac NV, White DM, Hadley J, Reid MA, Lahti AC. Hippocampal-parietal dysconnectivity and glutamate abnormalities in unmedicated patients with schizophrenia. Hippocampus. 2014;24(12):1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Elst LT, Valerius G, Büchert M, et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58(9):724–730. [DOI] [PubMed] [Google Scholar]
- 24. Kegeles LS, Shungu DC, Anjilvel S, et al. Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry Res. 2000;98(3): 163–175. [DOI] [PubMed] [Google Scholar]
- 25. Bossong MG, Antoniades M, Azis M, et al. Association of hippocampal glutamate levels with adverse outcomes in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2019;76(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. Circuit-based corticostriatal homologies between Rat and Primate. Biol Psychiatry. 2016;80(7):509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21(13):4915–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–973. [DOI] [PubMed] [Google Scholar]
- 29. Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27(42):11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358–371. [DOI] [PubMed] [Google Scholar]
- 31. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Häfner H, Maurer K, Löffler W, Riecher-Rössler A. The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry. 1993;162:80–86. [DOI] [PubMed] [Google Scholar]
- 33. Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. [DOI] [PubMed] [Google Scholar]
- 34. Casey BJ, Duhoux S, Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67(5):749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 36. Jones PB. Adult mental health disorders and their age at onset. Br J Psychiatry Suppl. 2013;54:s5–10. [DOI] [PubMed] [Google Scholar]
- 37. Lieberman JA, Girgis RR, Brucato G, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23(8):1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rorick-Kehn LM, Johnson BG, Knitowski KM, et al. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl). 2007;193(1):121–136. [DOI] [PubMed] [Google Scholar]
- 39. Mezler M, Geneste H, Gault L, Marek GJ. LY-2140023, a prodrug of the group II metabotropic glutamate receptor agonist LY-404039 for the potential treatment of schizophrenia. Curr Opin Investig Drugs. 2010;11(7):833–845. [PubMed] [Google Scholar]
- 40. Adams DH, Zhang L, Millen BA, Kinon BJ, Gomez J-C. Pomaglumetad methionil (LY2140023 monohydrate) and aripiprazole in patients with schizophrenia: a phase 3, multicenter, double-blind comparison. Schizophrenia Research and Treatment 2014;2014:758212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marek GJ. When is a Proof-of-Concept (POC) not a POC? Pomaglumetad (LY2140023) as a Case Study for Antipsychotic Efficacy. Curr Pharm Des. 2015;21(26):3788–3796. [DOI] [PubMed] [Google Scholar]
- 42. Kinon BJ, Millen BA, Zhang L, McKinzie DL. Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol Psychiatry. 2015;78(11):754–762. [DOI] [PubMed] [Google Scholar]
- 43. Krystal JH, Anticevic A. Toward illness phase-specific pharmacotherapy for schizophrenia. Biol Psychiatry. 2015;78(11):738–740. [DOI] [PubMed] [Google Scholar]
- 44. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794–810. [DOI] [PubMed] [Google Scholar]
- 45. Sonnenschein SF, Grace AA. The mGluR2/3 agonist pomaglumetad methionil normalizes aberrant dopamine neuron activity via action in the ventral hippocampus. Neuropsychopharmacology. 2020;45(12):2106–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Paxinos G, Watson C.. The Rat Brain in Stereotaxic Coordinates. Beijing, China: Qingchuan Zhuge translate People’s Medical Publishing House; 2007:32. [Google Scholar]
- 47. Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35(7):422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization. Neuroscience. 1983;10(2):301–315. [DOI] [PubMed] [Google Scholar]
- 49. Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4(11):2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31(7):1356–1361. [DOI] [PubMed] [Google Scholar]
- 51. Perez SM, Shah A, Asher A, Lodge DJ. Hippocampal deep brain stimulation reverses physiological and behavioural deficits in a rodent model of schizophrenia. Int J Neuropsychopharmacol. 2013;16(6):1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perez SM, Lodge DJ. Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Mol Psychiatry. 2013;18(11):1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36(9):1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gastambide F, Cotel MC, Gilmour G, O’Neill MJ, Robbins TW, Tricklebank MD. Selective remediation of reversal learning deficits in the neurodevelopmental MAM model of schizophrenia by a novel mGlu5 positive allosteric modulator. Neuropsychopharmacology. 2012;37(4):1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Du Y, Grace AA. Amygdala hyperactivity in mam model of schizophrenia is normalized by peripubertal diazepam administration. Neuropsychopharmacology. 2016;41(10):2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cabungcal JH, Steullet P, Morishita H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110(22):9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gomes FV, Grace AA. Prefrontal cortex dysfunction increases susceptibility to schizophrenia-like changes induced by adolescent stress exposure. Schizophr Bull. 2016;43(3):592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomes FV, Zhu X, Grace AA. The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Mol Psychiatry. 2020;25(12):3278–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gill KM, Cook JM, Poe MM, Grace AA. Prior antipsychotic drug treatment prevents response to novel antipsychotic agent in the methylazoxymethanol acetate model of schizophrenia. Schizophr Bull. 2014;40(2):341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. [DOI] [PubMed] [Google Scholar]
- 61. Zimmerman EC, Bellaire M, Ewing SG, Grace AA. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38(11):2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60(3):253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gomes FV, Rincón-Cortés M, Grace AA. Adolescence as a period of vulnerability and intervention in schizophrenia: Insights from the MAM model. Neurosci Biobehav Rev. 2016;70:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther. 1998;284(2):651–660. [PubMed] [Google Scholar]
- 65. Shekhar A, Keim SR. LY354740, a potent group II metabotropic glutamate receptor agonist prevents lactate-induced panic-like response in panic-prone rats. Neuropharmacology. 2000;39(7):1139–1146. [DOI] [PubMed] [Google Scholar]
- 66. Linden AM, Greene SJ, Bergeron M, Schoepp DD. Anxiolytic activity of the MGLU2/3 receptor agonist LY354740 on the elevated plus maze is associated with the suppression of stress-induced c-Fos in the hippocampus and increases in c-Fos induction in several other stress-sensitive brain regions. Neuropsychopharmacology. 2004;29(3):502–513. [DOI] [PubMed] [Google Scholar]
- 67. Kłodzińska A, Chojnacka-Wójcik E, Pałucha A, Brański P, Popik P, Pilc A. Potential anti-anxiety, anti-addictive effects of LY 354740, a selective group II glutamate metabotropic receptors agonist in animal models. Neuropharmacology. 1999;38(12):1831–1839. [DOI] [PubMed] [Google Scholar]
- 68. Spooren WP, Schoeffter P, Gasparini F, Kuhn R, Gentsch C. Pharmacological and endocrinological characterisation of stress-induced hyperthermia in singly housed mice using classical and candidate anxiolytics (LY314582, MPEP and NKP608). Eur J Pharmacol. 2002;435(2-3):161–170. [DOI] [PubMed] [Google Scholar]
- 69. Grillon C, Cordova J, Levine LR, Morgan CA III. Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist (LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology (Berl). 2003;168(4):446–454. [DOI] [PubMed] [Google Scholar]
- 70. Tizzano JP, Griffey KI, Schoepp DD. The anxiolytic action of mGlu2/3 receptor agonist, LY354740, in the fear-potentiated startle model in rats is mechanistically distinct from diazepam. Pharmacol Biochem Behav. 2002;73(2):367–374. [DOI] [PubMed] [Google Scholar]
- 71. Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. 2008;33(7):1603–1610. [DOI] [PubMed] [Google Scholar]
- 72. Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71(3):379–392. [DOI] [PubMed] [Google Scholar]
- 73. Kim J, An B, Kim J, et al. mGluR2/3 in the Lateral Amygdala is Required for Fear Extinction: Cortical Input Synapses onto the Lateral Amygdala as a Target Site of the mGluR2/3 Action. Neuropsychopharmacology. 2015;40(13):2916–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Perez SM, Chen L, Lodge DJ. Alterations in dopamine system function across the estrous cycle of the MAM rodent model of schizophrenia. Psychoneuroendocrinology. 2014;47:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Klinger K, Gomes FV, Rincón-Cortés M, Grace AA. Female rats are resistant to the long-lasting neurobehavioral changes induced by adolescent stress exposure. Eur Neuropsychopharmacol. 2019;29(10):1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chaby LE, Cavigelli SA, Hirrlinger AM, Lim J, Warg KM, Braithwaite VA. Chronic Stress during adolescence impairs and improves learning and memory in adulthood. Front Behav Neurosci. 2015;9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chaby LE, Sheriff MJ, Hirrlinger AM, Braithwaite VA. Does early stress prepare individuals for a stressful future? Stress during adolescence improves foraging under threat. Anim Behav. 2015;105:37–45. [Google Scholar]
- 78. Brockhurst J, Cheleuitte-Nieves C, Buckmaster CL, Schatzberg AF, Lyons DM. Stress inoculation modeled in mice. Transl Psychiatry. 2015;5:e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meichenbaum D. Stress inoculation training: A preventative and treatment approach. Principles and Practice of Stress Management. Vol. 3. New York: The Guilford Press; 2017:497–518. [Google Scholar]