Abstract
Research in schizophrenia (SZ) emphasizes the need for new therapeutic approaches based on antioxidant/anti-inflammatory compounds and psycho-social therapy. A hallmark of SZ is a dysfunction of parvalbumin-expressing fast-spiking interneurons (PVI), which are essential for neuronal synchrony during sensory/cognitive processing. Oxidative stress and inflammation during early brain development, as observed in SZ, affect PVI maturation. We compared the efficacy of N-acetyl-cysteine (NAC) and/or environmental enrichment (EE) provided during juvenile and/or adolescent periods in rescuing PVI impairments induced by an additional oxidative insult during childhood in a transgenic mouse model with gluthation deficit (Gclm KO), relevant for SZ. We tested whether this rescue was promoted by the inhibition of MMP9/RAGE mechanism, both in the mouse model and in early psychosis (EP) patients, enrolled in a double-blind, randomized, placebo-controlled clinical trial of NAC supplementation for 6 months. We show that a sequential combination of NAC+EE applied after an early-life oxidative insult recovers integrity and function of PVI network in adult Gclm KO, via the inhibition of MMP9/RAGE. Six-month NAC treatment in EP patients reduces plasma sRAGE in association with increased prefrontal GABA, improvement of cognition and clinical symptoms, suggesting similar neuroprotective mechanisms. The sequential combination of NAC+EE reverses long-lasting effects of an early oxidative insult on PVI/perineuronal net (PNN) through the inhibition of MMP9/RAGE mechanism. In analogy, patients vulnerable to early-life insults could benefit from a combined pharmacological and psycho-social therapy.
Keywords: antioxidant, physical exercice, mechanism, brain development, early psychosis, cognition
Introduction
The search for effective treatments in schizophrenia (SZ) is challenging due to the complexity of the disorder and the high heterogeneity of patients at genetic, pathophysiological, and clinical levels. Following a shift in clinical approach towards early intervention and preventive strategies for a better patient prognosis, there is also a need for preclinical studies to uncover pathophysiology and translational mechanism-based therapeutic approach linked to impaired brain function during adolescent development. SZ pathophysiology implicates both genetic and environmental factors interacting especially at early stage of brain development, triggering, among others, oxidative stress and neuroinflammation.1–4 Environmental stressors such as obstetrical complication, maternal infection during pregnancy, but also adverse childhood experiences (ACE), that are well-known risk factors for SZ, induce oxidative stress and inflammation.1,5–15
Based on these evidence, several clinical studies focusing on the use of antioxidants and anti-inflammatory compounds as add-on to antipsychotics show promising results. Specifically, the antioxidant and glutathione (GSH) precursor N-Acetyl Cysteine (NAC) has been shown to improve symptoms,16–19 white matter integrity in fornix,20 and EEG mismatch negativity21 in SZ or early psychosis (EP) patients.
Although 2 recent meta-analyses22,23 concluded that, in general, NAC demonstrates some efficacy for the treatment of SZ symptoms, some studies found limited benefits24 or improvement only in specific subgroups of patients.17,19
In addition to pharmacological treatments, physical exercise, which modulates the immune system,25–27 showed beneficial effects in SZ.28–34 In SZ rodent models, physical exercise and animal housing in an enriched environment (EE) reduces oxidative stress,35,36 neuroinflammation,37,38 and SZ-like behaviors.39–42 Thus, NAC and EE may have similar or overlapping effects on pathological mechanisms mediated by oxidative stress and neuroinflammation, such as impairments of parvalbumin interneurons (PVI). Anomaly of PVI and their enwrapping perineuronal nets (PNN) is a hallmark of SZ43–46 and contributes to abnormal high-frequency neuronal synchronization,47,48 impacting multiple information processing critical for sensory perception and cognition, and possibly promoting hyperdopaminergia related to positive symptoms.49–53 PVI are vulnerable to oxidative stress and inflammation, especially during their development.54–58 A deleterious feedforward interaction between oxidative stress and neuroinflammation through MMP9/RAGE pathway during specific developmental periods appears to be central to PVI impairments.59
In order to intervene early on precise pathophysiological mechanisms mediated by oxidative stress/neuroinflammation, it is crucial to better define both the optimal timing and type of such interventions that would lead to long-term beneficial effects.
The aim of this study was to (1) compare the efficacy of NAC and/or EE provided during juvenile and/or adolescent periods for PVI/PNN impairments rescue in an SZ model, (2) understand the underlying mechanisms associated with the timely beneficial effects, and (3) explore in EP patients the effect of NAC on the described mechanisms.
Here, we studied Gclm KO mice which have a 70% decrease in brain GSH due to the lack of the GCL (glutamate-cysteine ligase) modulatory subunit (Gclm).60,61 These mice display increased oxidative stress in some brain regions, such as the anterior cingulate cortex (ACC) and show SZ-related phenotypes.54,60,62–67 Most importantly, an additional oxidative stress challenge applied during childhood, to mimic stressful events, induces perduring PVI impairments into adulthood, in line with the developmental aspect of SZ.54
We evaluated the short and long-term benefits of NAC and/or EE provided at different timing on PVI, following the early-life oxidative challenge. We found that NAC administration during the juvenile/peripubertal period, when followed by EE during the adolescence, prevents the long-term impairment of PVIs through inhibition of the MMP9/RAGE cascade.59 NAC add-on treatment in EP patients also mitigates the MMP9/RAGE mechanism in association with increased prefrontal GABA levels and improvements of clinical symptoms and cognitive function.
Materials and Methods
Animals
Gclm KO mice68 were backcrossed with C57BL/6J mice as described previously.54 To induce an additional oxidative stress, GBR-12909dihydrochloride (GBR, BioTrend, 5mg/kg), a dopamine reuptake inhibitor, was subcutaneously injected to Gclm-KO and WT mice, postnatal days (PND10)-PND20. NAC (900mg/L, PharmaNAC, BioAdvantexPharma) was delivered in drinking water, PND21-PND35. EE was provided either PND21-PND40 or PND36-PND57 (supplementary information).
Immunohistochemistry
Animals were sacrificed at PND40 or PND90 and coronal slices (40 µm) were used for the IH quantification. The antibodies list is presented in the supplementary information.
Confocal and Image Analysis
Immunohistological images were obtained with a Zeiss LSM780 Quasar confocal microscope. More details in the supplementary information, and elsewhere.54,59
Electrophysiology
Paracoronal slices (400-μm, Bregma~1.4–0.6) containing the ACC were prepared as described in Steullet et al.69 The specific details in the supplementary information.
Subjects Recruitment
Data from EP patients included in the present study comes from the original sample of the NAC randomised controlled trial that was already published.17,70 More details in the supplementary information.
Magnetic Resonance Spectroscopy Acquisition and Analysis
All MR measurements were carried out on a 3T-MR scanner (Magnetom TimTrio, Siemens Healthcare) with a transverse electromagnetic (TEM3000) head coil (MRInstruments, Inc).71 More details elsewhere59,72,73 and in the supplementary information.
Results
Short-Term Effect at Peripuberty: Juvenile/Peripubertal NAC Treatment but not EE Rescues PVI/PNN Through MMP9/RAGE-Dependent Mechanism
In Gclm KO mice, an early-life GBR (PND10-PND20) induces an additional oxidative stress which affects maturation of PVI in the ACC and subsequently their long-term impairments.54,59 These effects are mediated by a mechanism involving the shedding of RAGE by MMP9 during juvenile/peripubertal stage that maintains a perduring oxidative stress and neuroinflammation through a feedforward loop.59
First, we examined the short-term effect (at PND40) of either a juvenile/peripubertal NAC (PND21-PND35) or EE (PND21-PND40), on the maturation of PVI in the ACC of GBR-treated KO mice (figure 1A). PVI maturation was evaluated by quantifying the density of parvalbumin-immoreactive (PV-IR) cells and the number of PV-IR cells surrounded by PNN (stained with WFA).74–76 The decrease in PV-IR cell density and PNN in GBR-treated KO mice was completely abolished by NAC, but not EE (figure 1B).
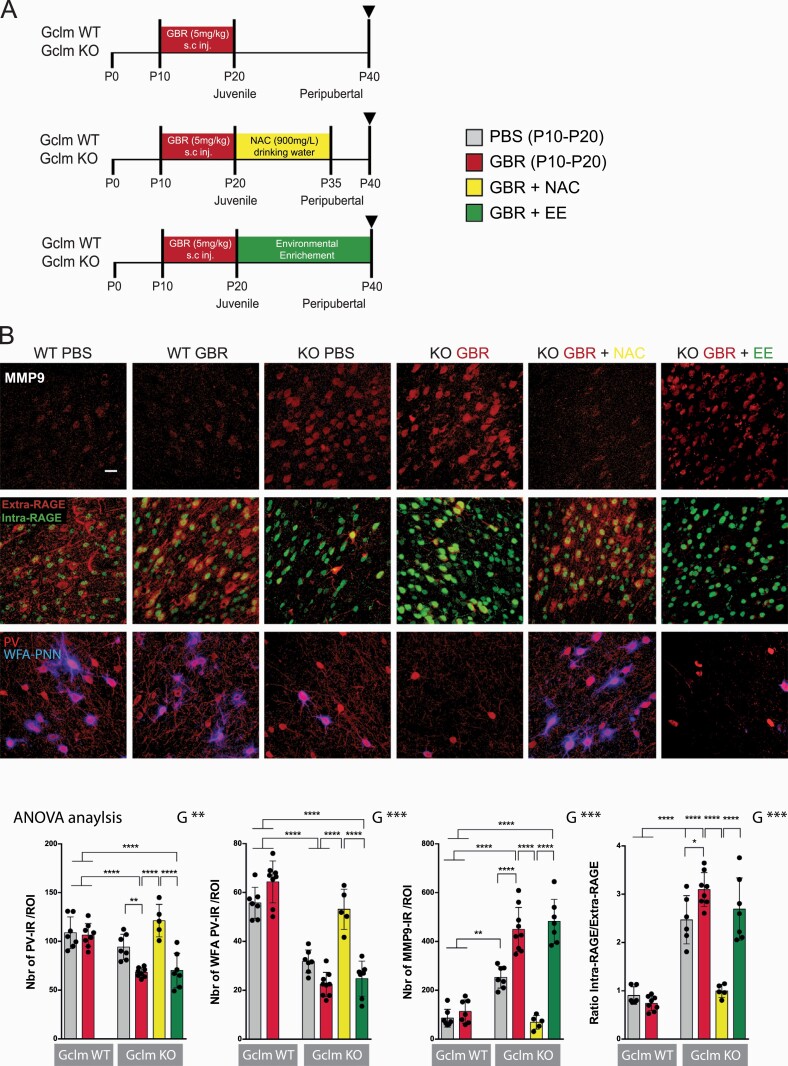
Fig. 1.
NAC, but not EE, after an additional early-life oxidative challenge, prevents MMP9/RAGE activation and PV/PNN decrease in Gclm KO at PND40. (A) Protocol scheme. (B) MMP9, intraRAGE/extraRAGE ratio, PV and WFA in Gclm KO and WT mice: confocal images (scale bar:30 μm) and quantification graphs. Data expressed as mean±s.e.d. (mice N = 5–7). ANOVA analysis: G = genotype, T = treatment, R = recovery, x = interaction; *P < .05; **P < .01; ***P < .001, analyzed by Tukey post-hoc test.
Then, we investigated MMP9/RAGE pathway activation, by measuring MMP9 protein expression and RAGE shedding. The shedding of RAGE is assessed with 2 antibodies targeting its extra and intra-cellular domains, respectively. The extracellular-domain antibody binds to the uncleaved membrane-bound RAGE (extra-RAGE). The intracellular antibody recognizes the intracellular domain of RAGE (intra-RAGE) that translocates to the nucleus after shedding.59 As previously shown,59 MMP9 expression and RAGE shedding (ratio of intra- over extra-RAGE) were elevated in PND40 KO mice, and further increased by early-life GBR (figure 1B). Juvenile/peripubertal NAC, but not EE, decreased both MMP9 expression and RAGE shedding (figure 1B).
Finally, we observed that oxidative stress (8-oxoDG) and microglia activation (Iba1 and CD68) were high in PND40 KO mice and further increased by GBR (supplementary figure 1B). Juvenile/peripubertal NAC, but not EE, decreased these markers of oxidative stress and neuroinflammation to levels found in WT mice (supplementary figure 1B).
Collectively, these show that NAC treatment at PND21-35 blocks the oxidative stress/MMP9/RAGE pathway and rescues PVI alterations at peripuberty. This was linked to the antioxidant and/or anti-inflammatory property of NAC, as EE applied at the same time period had no effect on MMP9/RAGE and PVI recovery.
Long-Term Effect at Adulthood: Neither Juvenile/Peripubertal NAC Nor EE Rescue PVI/PNN
As juvenile/peripubertal NAC blocked the MMP9/RAGE pathway during the PVI maturation period allowing PVI recovery at PND40, we examined the long-term effect of such NAC treatment on PVI/PNN at adulthood (PND90). As previously shown,54 early-life GBR decreased PV-IR cell density and PNN in ACC of adult KO but not WT mice (figure 2B). This was accompanied with increased MMP9 expression, microglial activation, and oxidative stress (figure 2B, supplementary figure 2B).
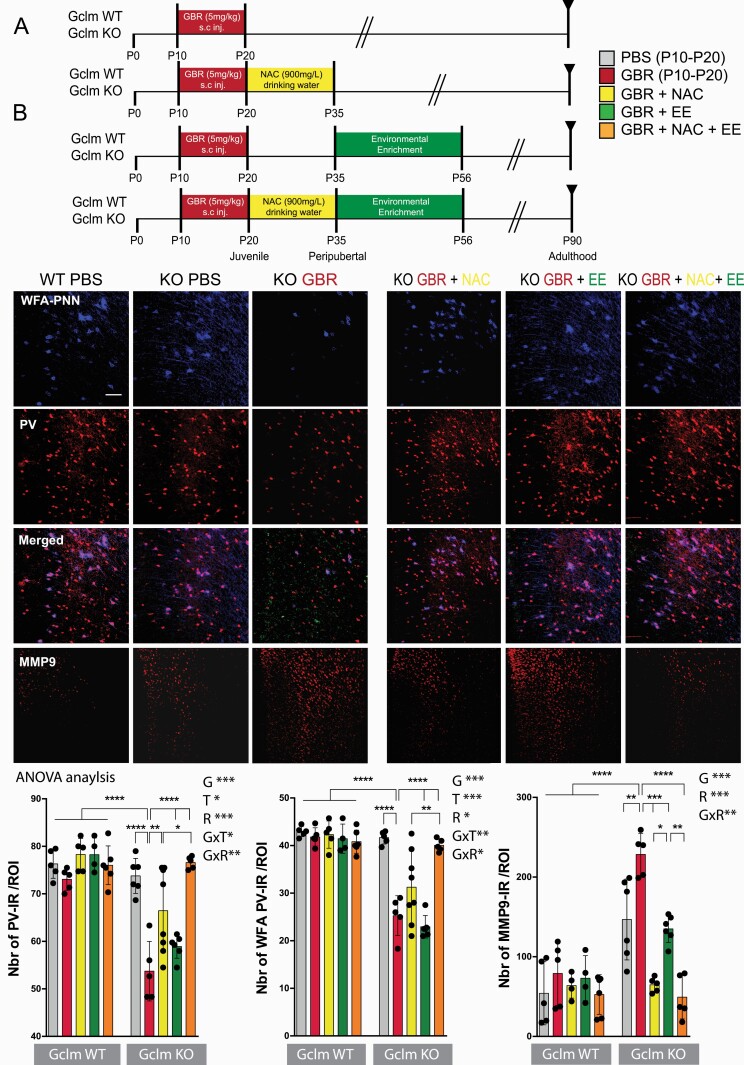
Fig. 2.
NAC+EE after an additional early-life oxidative challenge recover PV/PNN and MMP9 in Gclm KO at adulthood. (A) Protocol scheme. (B) PV, WFA and MMP9 in Gclm KO and WT mice: confocal images (scale bar: 50 μm) and quantification graphs. Data expressed as mean±s.e.d. (mice N = 5–8). ANOVA analysis: G = genotype, T = treatment, R = recovery, x = interaction; *P < .05; **P < .01; ***P < .001, analyzed by Tukey post-hoc test.
Although juvenile/peripubertal NAC restored PVI/PNN integrity at PND40 (figure 1B) and maintained low levels of oxidative stress, microglia activation, and MMP9 (figure 2B, supplementary figure 2B) until adulthood, it did not fully succeed in preserving long-term PVI/PNN integrity at PND90 (figure 2B).
As juvenile/peripubertal EE did not show any short-term protection of PVI/PNN in ACC of PND40 GBR-treated KO mice, we assessed the impact of EE when applied during the adolescent period (PND36-PND57). We found that adolescent EE also had no long-term effect on PVI/PNN (figure 2B). However, adolescent EE fully prevented oxidative stress and microglia activation at adulthood (supplementary figure 2B), while only partially decreasing MMP9 at the levels of PBS-treated KO, without reaching the levels observed in WT (figure 2B).
Long-Term Effect at Adulthood: Combined Juvenile/Peripubertal NAC and Adolescent EE Rescue PVI/PNN Through MMP9/RAGE-Dependent Mechanism
As both juvenile/peripubertal NAC and EE, individually failed to fully rescue PVI in adulthood, we tested the long-term effect of a combined treatment consisting of juvenile/peripubertal NAC (PND21-35) followed by EE during adolescence (PND36-57). We hypothesized that juvenile/peripubertal NAC would prevent oxidative stress-induced impairment of PVI/PNN by blocking MMP9/RAGE pathway, and the following EE during adolescence would further enhance their final maturation, function, and integrity, as well as keeping oxidative stress and neuroinflammation to low levels.36,56
The combination of NAC+EE completely prevented the perduring GBR-induced PVI/PNN impairments in adult KO mice (figure 2B). NAC+EE also fully normalized MMP9 (figure 2B), microglia activation, and oxidative stress levels in adult KO mice (supplementary figure 2B). These results suggest that timely combination of NAC+EE following an early-life stress allows the full recovery of PVI/PNN integrity at adulthood.
Combined Juvenile/Peripubertal NAC and Adolescent EE Rescue High-Frequency Oscillations
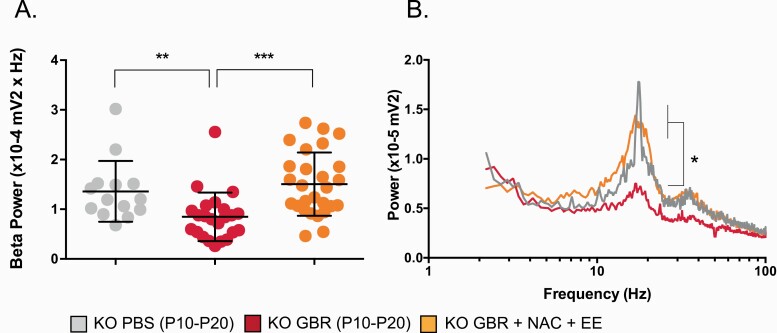
We then examined whether an early-life GBR treatment also affected the fast-oscillatory neuronal activity in the ACC of adult KO mice, and if so, whether NAC combined with EE could restore it. High-frequency (β) oscillations were induced pharmacologically in ACC slices via the perfusion of carbachol, kainate, and quinpirole. In previous studies conducted in the ACC of adults mice (ie, 3 mo), we reported that these oscillations, generated within local networks of excitatory and inhibitory neurons,67 were not significantly different between PBS-treated KO and WT mice,54 and that WT mice were not affected by a GBR administration PND10-20.77 Here, we observed that an early-life GBR treatment in KO mice significantly decreased β-power at PND90, concomitant with the impaired PVI/PNN network (figures 3A and 3B). More importantly, the sequential treatment of juvenile/peripubertal NAC followed by adolescent EE not only restored normal integrity of PVI/PNN (figure 2B) but also fully restored the ability of the ACC to generate and sustain high-frequency oscillations at PND90.
Fig. 3.
Pharmacologically-induced high-frequency(β) oscillations in ACC slices are affected by an early-life oxidative challenge in Gclm KO mice, but are fully normalized by NAC+EE. (A–B) Effect of GBR and NAC+EE in KO mice. (D) Illustration of the average power spectrum for each experimental group. Box plots depict medians and quartiles; bars show values in the 1.5 box length. KO (mice N = 5, cells N = 14), KO+GBR(mice N = 7, cells N = 23), KO+GBR +NAC+EE (mice N = 8, cells N = 27). **P = .004; ***P < .001.
Reverse Translation to Early Psychosis Patients: NAC Add-on Treatment Reduces MMP9/RAGE Pathway Overactivation in Association With Increased Prefrontal GABA Levels and Improvement in Processing Speed and Positive Symptoms
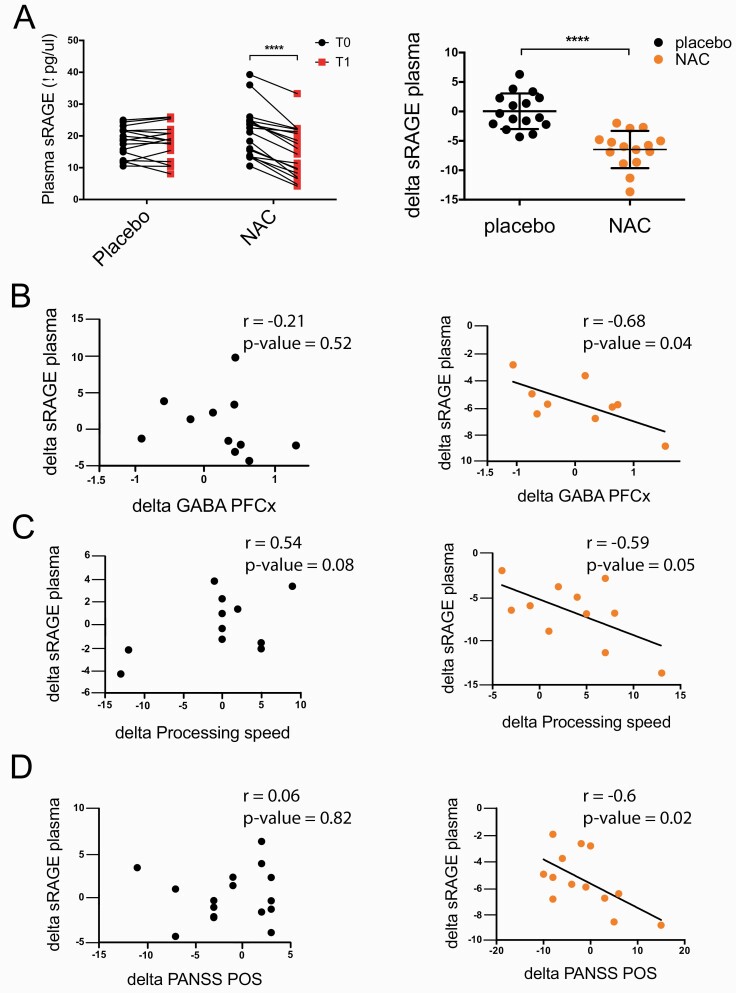
Previously, we reported that the soluble form of RAGE (sRAGE) was increased in the plasma of EP patients, reflecting an enhanced RAGE shedding via oxidative stress-induced MMP9 activation.59 Moreover, higher sRAGE levels were associated with lower GABA levels in patients’ prefrontal cortex as assessed by magnetic resonance spectroscopy (MRS),59 suggesting a potential functional link between RAGE shedding and interneuron integrity. Here, we investigated whether NAC inhibits RAGE shedding in EP patients, as observed in brains of Gclm KO. We measured sRAGE in the plasma of EP patients enrolled in a double-blind, randomized, placebo-controlled trial of NAC supplementation for 6 months.17 Six-month NAC increased prefrontal GSH levels, improved processing speed, as well as positive symptoms in a sub-group of patients with high peripheral oxidative status.17 Here, we showed that NAC also decreased sRAGE levels, with no change in the placebo group (figure 4A). Moreover, in the NAC- but not placebo-treated group, we found a negative correlation between the changes in peripheral sRAGE and in prefrontal GABA (figure 4B), indicating that NAC leads to a normalization of sRAGE levels in association with an increase in prefrontal GABA levels. Interestingly, NAC-induced sRAGE decrease was significantly correlated with improvement in processing speed (figure 4C), a domain that was already shown to be improved by NAC.17 A similar trend was found for working memory after NAC treatment (supplementary figure 3A). These suggest that decreased level of peripheral sRAGE following NAC is associated with amelioration of some cognitive functions in particular processing speed.17,78,79 Finally, in the NAC-treated group, we also found an association between decreased sRAGE and an improvement of clinical symptoms scores (PANSS positive and PANSS total) (figure 4D, supplementary figure 3B). Overall, our results indicate that the normalization of sRAGE levels by NAC treatment in EP patients correlates with an increase in prefrontal GABA levels and an improvement of cognition and psychotic symptoms.
Fig. 4.
sRAGE level is decreased after 6-mo NAC add-on treatment in EP patients and is associated with increased GABA level in the PFCx, as well as improvements in cognition and positive symptoms. (A) sRAGE decrease in the NAC-treated group (N = 18; P < .0001) compared to the placebo group (N = 18). (B) Negative correlation between delta GABA and delta sRAGE in NAC-treated group (N = 9; r = −0.68, P = .04). (C) Negative correlation between delta processing speed and delta sRAGE in NAC-treated group (N = 11; r = −0.59, P = .05). (D) Negative correlation between delta PANSS positive symptoms and delta sRAGE in NAC-treated group (N = 13; r = −0.6, P = .02).
Discussion
We show that the sequential combination of an antioxidant treatment and EE applied during the juvenile and adolescent periods respectively normalizes the integrity and function of PVI/PNN networks in ACC of adult Gclm KO after an early-life oxidative insult. This recovery is mediated by NAC, possibly via the inhibition of oxidative stress-induced MMP9/RAGE pathway. NAC interrupts this deleterious feedforward mechanism that maintains perduring high levels of oxidative stress and neuroinflammation, allowing PVI/PNN maturation. A subsequent EE during adolescence promotes the final maturation of PVI, providing a long-term neuroprotection to PVI/PNN networks. Our clinical study suggests that NAC engages similar neuroprotective mechanisms in EP patients. Indeed, a 6-month NAC add-on treatment reduces plasma sRAGE in association with an increase in prefrontal GABA level, and with improvements of working memory, processing speed, and positive symptoms.
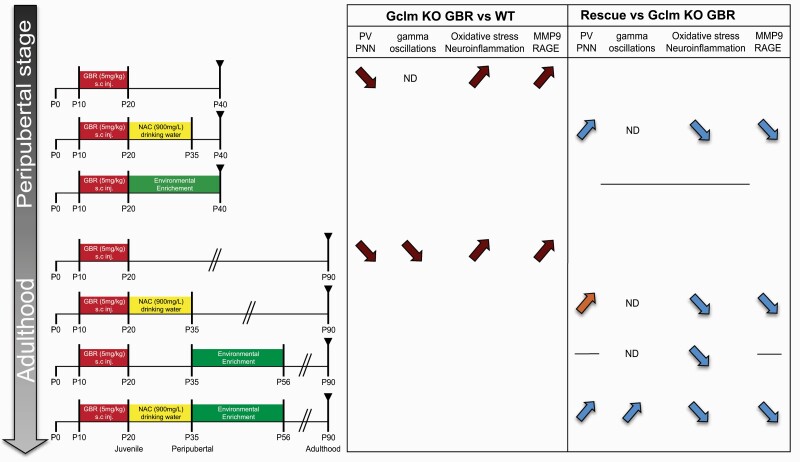
Although converging evidence indicates that both NAC and EE can confer neuroprotection via overlapping mechanisms, our results show a different effect of NAC and EE on neuroinflammation, oxidative stress, and PVI/PNN recovery in an animal model, with a genetic risk for impaired antioxidant defense (figure 5).
Fig. 5.
Summary of NAC and/or EE effects at different developmental periods. Comparison of the Gclm KO+GBR to WT PBS/GBR, and to Gclm KO+GBR. Red-arrows = pathological changes; blue-arrows = rescue; orange-arrow = not significant rescue; line = no changes; ND = not determined (more details in the supplementary information).
Effect of NAC Treatment
Juvenile/peripubertal NAC, but not EE, decreases microglia activation and oxidative stress, and fully recovers PVI/PNN maturation at PND40 (figure 5). Indeed, NAC during PVIs maturation blocks the overactivation of the MMP9/RAGE pathway initiated by the early-life oxidative challenge that is responsible for the long-lasting PVI/PNN impairments in Gclm KO.59 This may stop the perduring feedforward process between oxidative stress and microglia activation, allowing normal maturation of PNN, and subsequently PVIs at PND40. However, an early NAC treatment alone is not sufficient to fully maintain a long-term protection of PVI/PNN networks in adult Gclm KO (figure 2). This may be due to the genetically compromised antioxidant GSH system which may impinge the long-term maintenance of PVI/PNN integrity despite minimal oxidative stress and microglia activation. Previously, NAC has been shown to decrease oxidative stress, rescue PVI deficit and SZ-like behaviors in several animal models, including Gclm KO mice.54,77–81 However, in these studies, NAC was provided either before or during the early-life oxidative stress insult and maintained until the end of the experiments. The demonstration that a short NAC administration after the early-life oxidative challenge has also an effect is promising in view of clinical settings.
Effect of EE
EE alone during adolescence, but not during the juvenile/peripubertal period, reduces oxidative stress and microglial activation. The EE-effect during adolescence may be due to the combined reduction of ROS produced by microglia,37 increase of neurotrophic factors such as BDNF, and activation of NMDAR,82 which enhances the antioxidant defenses.3 The lack of impact of EE during the juvenile/peripubertal period may be due to the impaired GSH system in Gclm KO and its lack of effect on MMP9.
Actually, in animals with intact antioxidant defenses, EE during juvenile/peripuberty boosts PVI-associated network maturation83–85 and prevents PVI impairment induced by either early postnatal hypoxia or anesthesia.36,56,86 This strongly suggests that the protective effect of EE on PVI/PNN during juvenile/peripubertal requires an intact GSH system. Juvenile/peripubertal EE favors PVI maturation by enhancing PV expression,83 glutamatergic input onto PVI as well as inhibitory synapses on pyramidal neurons, in a NDMAR-dependent manner.84,87 This activity-dependent maturation of PVI induces more ROS production by the mitochondria, due to the fast-spiking interneurons high energy demand. Therefore, the beneficial effect of EE on PVI maturation may be limited in Gclm KO as they lack intact antioxidant system. Although EE at adolescence strongly diminishes oxidative stress and microglia activation, suggesting a less critical role for the GSH system, this effect is not sufficient to recover PVI/PNN in adulthood, but still maintain PVI/PNN recovery provided by NAC treatment during juvenile/peripubertal (figure 5).
Effect of the Combination of NAC and EE
Collectively, juvenile/peripubertal NAC combined to EE during adolescence induces a long-term recovery of the PVI in the Gclm KO mice (figure 5). Our results suggest that NAC administration during the sensitive juvenile/peripubertal period of maturation, may not only decrease oxidative stress but may also block the activation of the MMP9/RAGE pathway. Addition of EE during adolescence may stimulate the final maturation of PVI in an activity-dependent manner, maintaining the integrity of these neurons. Interestingly, spontaneous excitatory postsynaptic currents (EPSC) measured on PVI in the PFCx increase drastically between the juvenile/peripubertal (PND25-35) and adolescent (PND45-55) periods,88 suggesting that increased glutamatergic stimulation by EE (from PND36-57) could be beneficial to PVI maturation. This is in line with our findings regarding the functional recovery of the gamma oscillation power in the adult Gclm KO after NAC and EE (figure 3). Altogether, our results reveal a pathological mechanism involving MMP9/RAGE, that can be blocked by NAC at a precise timing, followed by a beneficial effect of EE during adolescence, contributing to the final recovery of the inhibitory network.
Translation to Patients
We investigated a novel combination of 2 therapeutic approaches in a mouse model carrying a genetic vulnerability to redox dysregulation and exposed to an environmental insult during brain development. Indeed, the GBR injections during early postnatal days mimic the dopamine increase in the PFCx, possibly induced by a psychosocial stress89 and leading to ROS increase via the catabolism of dopamine.90,91 This study paves the way for a novel therapeutic approach that could be proposed to patients after an early psychotraumatic event. This consists of: (1) reestablishing the redox/antioxidant balance and neutralizing the deleterious feedforward interaction between oxidative stress and neuroinflammation with NAC during the juvenile/peripubertal period, and (2) stimulating the activity-dependent PVI/PNN microcircuitry with EE during adolescence, eventually leading to the timely maturation of functional local network, critical for gamma oscillations and cognition. Noteworthy, NAC decreases plasmatic sRAGE in EP patients in association with increased prefrontal GABA levels, suggesting that NAC in EP may act on similar oxidative stress-mediated pathological mechanisms than those underlying PVI/PNN impairment in the animal model. This is further supported by the observation that elevated plasmatic sRAGE levels correlate with low prefrontal GABA in EP who carry a genetic susceptibility to redox dysregulation.59 Together, these results highlight that peripheral sRAGE may be a valid proxy marker of central I/E imbalance in EP. Moreover, the NAC-induced decrease in sRAGE is associated with improvement of positive symptoms and cognitive functions, including working memory and processing speed. NAC has been previously reported to reduce symptoms and improve cognition in EP,22,23 including processing speed in EP.17 Interestingly, the beneficial effect of NAC on symptoms was observed mostly in EP displaying high peripheral oxidative status.17 This suggests the need of mechanism based biomarker allowing patients stratification and targeting precise underlying mechanism.
In a translational train of thought, it is tempting to hypothesize that individuals who carry a genetic vulnerability have a risk to develop psychosis if they are exposed to environmental insults during brain development. Those insults, which can be infectious, traumatic or psychosocial, generate additional oxidative stress. Indeed, ACE during childhood are more prevalent in at-risk-mental-state (ARMS) for psychosis as compared to control individuals,15,92 increase the risk for developing psychosis93,94 and are associated with a redox dysregulation along with reduced hippocampal volume and more severe symptoms and cognitive deficits in EP.11 Moreover, in various animal models,46,80,95–97 known genetic and environmental risks converge to a pathophysiological hub of oxidative stress and consequently long-term decreased PVI, affecting cognitive functions.98
Altogether, the present findings highlight the need to (1) identify individuals who are most likely to profit from antioxidant treatment based on mechanism-based biomarker and (2) timely involve the equivalent of “EE” in the treatment, which could include physical training, nutrition, social activities and psychotherapy. Physical exercise and cognitive remediation were shown to improve cognitive performance, psychosocial functioning, and overall symptoms in SZ.33,99–104 Besides, a decrease in schizotypal personality was observed in adolescents enrolled when they were children in an enrichment program, consisting of nutrition, education, and physical exercise, compared with those that were not,105 stressing the importance of childhood environment. These data provide a framework for the implementation of early therapeutic strategies based on antioxidants and psychosocial/cognitive therapy/physical exercise/nutrition, oriented mostly towards individuals exposed to ACE presenting high oxidative status. A limitation of this paradigm is to directly translate these findings into real-world clinic, in particular regarding the optimal timing and duration of NAC treatment followed by physical/psychosocial intervention. Moreover, the characterization of precise cognitive/dimension-related brain circuitry to be stimulated still need to be clarified.
To conclude, our results highlight an innovative mechanism-based therapeutic strategy, aiming to block MMP9/RAGE pathway inducing oxidative stress and neuroinflammation during childhood, to improve long-lasting integrity and function of PVI/PNN networks. This may pave the way to novel interventions that may improve symptoms and cognition EP patients.
Supplementary Material
Acknowledgments
We are grateful to Adeline Cottier, Audrey Goupil, Morgane Baumgartner and Gloria Reuteler for technical assistance and to Section Minkowski collaborators for their precious help. We thank all patients for their enduring participation. MRS was performed in the Centre d’Imagerie BioMédicale (CIBM/UNIL-CHUV-HUG-EPFL). Thanks to Fulvio Magara and his team from the Centre d’Etudes du Comportement(CEC-CNP/CHUV) and Jean-Yves Chatton and his team, at the Cellular Imaging Facility(CIF/UNIL). The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by the Swiss-National-Science-Foundation (320030_122419 to P.C.andKQ.D.), National-Center-of-Competence-in-Research (NCCR)“SYNAPSY-The-Synaptic-Bases-of-Mental-Diseases (n°51AU40_125759), and Damm-Etienne and Alamaya Foundations.
References
- 1. Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19(2):220–230. [DOI] [PubMed] [Google Scholar]
- 2. Steullet P, Cabungcal JH, Monin A, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr Res. 2016;176(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. 2016;17(2):125–134. [DOI] [PubMed] [Google Scholar]
- 4. Perkins DO, Jeffries CD, Do KQ. Potential roles of redox dysregulation in the development of Schizophrenia. Biol Psychiatry. 2020;88(4):326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P. Signs of asphyxia at birth and risk of schizophrenia. Population-based case-control study. Br J Psychiatry. 2001;179:403–408. [DOI] [PubMed] [Google Scholar]
- 6. Laurens KR, Luo L, Matheson SL, et al. Common or distinct pathways to psychosis? A systematic review of evidence from prospective studies for developmental risk factors and antecedents of the schizophrenia spectrum disorders and affective psychoses. BMC Psychiatry. 2015;15(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suvisaari JM, Taxell-Lassas V, Pankakoski M, Haukka JK, Lönnqvist JK, Häkkinen LT. Obstetric complications as risk factors for schizophrenia spectrum psychoses in offspring of mothers with psychotic disorder. Schizophr Bull. 2013;39(5):1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HW, Rutten BP. An environmental analysis of genes associated with schizophrenia: hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiatry. 2012;17(12):1194–1205. [DOI] [PubMed] [Google Scholar]
- 9. Geoffroy PA, Etain B, Houenou J. Gene x environment interactions in schizophrenia and bipolar disorder: evidence from neuroimaging. Front Psychiatry. 2013;4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiavone S, Jaquet V, Trabace L, Krause KH. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal. 2013;18(12):1475–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alameda L, Fournier M, Khadimallah I, et al. Redox dysregulation as a link between childhood trauma and psychopathological and neurocognitive profile in patients with early psychosis. Proc Natl Acad Sci U S A. 2018;115(49):12495–12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of Schizophrenia. Obstet Gynecol Surv. 2005;60(2):77–78. [Google Scholar]
- 13. Piotrowski P, Frydecka D, Kotowicz K, et al. A history of childhood trauma and allostatic load in patients with psychotic disorders with respect to stress coping strategies. Psychoneuroendocrinology. 2020;115:104645. [DOI] [PubMed] [Google Scholar]
- 14. Hepgul N, Pariante CM, Psychological SD. Childhood trauma is associated with increased Body Mass Index and increased C-reactive protein levels in first-episode psychosis patients. Psychol Med. 2012;42(9):1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stowkowy J, Goldstein BI, MacQueen G, et al. Trauma in youth at-risk for serious mental illness. J Nerv Ment Dis. 2020;208(1):70–76. [DOI] [PubMed] [Google Scholar]
- 16. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64(5):361–368. [DOI] [PubMed] [Google Scholar]
- 17. Conus P, Seidman LJ, Fournier M, et al. N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. Schizophr Bull. 2018;44(2):317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farokhnia M, Azarkolah A, Adinehfar F, et al. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2013;36(6):185–192. [DOI] [PubMed] [Google Scholar]
- 19. Rapado-Castro M, Berk M, Venugopal K, Bush AI, Dodd S, Dean OM. Towards stage specific treatments: effects of duration of illness on therapeutic response to adjunctive treatment with N-acetyl cysteine in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:69–75. [DOI] [PubMed] [Google Scholar]
- 20. Klauser P, Xin L, Fournier M, et al. N-acetylcysteine add-on treatment leads to an improvement of fornix white matter integrity in early psychosis: a double-blind randomized placebo-controlled trial. Transl Psychiatry. 2018;8(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lavoie S, Murray MM, Deppen P, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33(9):2187–2199. [DOI] [PubMed] [Google Scholar]
- 22. Zheng W, Zhang QE, Cai DB, et al. N-acetylcysteine for major mental disorders: a systematic review and meta-analysis of randomized controlled trials. Acta Psychiatr Scand. 2018;137(5):391–400. [DOI] [PubMed] [Google Scholar]
- 23. Yolland CO, Hanratty D, Neill E, et al. Meta-analysis of randomised controlled trials with N-acetylcysteine in the treatment of schizophrenia. Aust N Z J Psychiatry. 2020;54(5):453–466. [DOI] [PubMed] [Google Scholar]
- 24. Willborn RJ, Hall CP, Fuller MA. Recycling N-acetylcysteine: a review of evidence for adjunctive therapy in schizophrenia. Ment Health Clin. 2019;9(3):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu D, Wang R, Grant AR, Zhang J, Gordon PM. Immune adaptation to chronic intense exercise training: new microarray evidence. BMC Genomics. 2017;18(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gjevestad GO, Holven KB, Ulven SM. Effects of exercise on gene expression of inflammatory markers in human peripheral blood cells: a systematic review. Curr Cardiovasc Risk Rep. 2015;9(7):115–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gómez-Rubio P, Trapero I. The effects of exercise on IL-6 levels and cognitive performance in patients with schizophrenia. Diseases (Basel, Switzerland). 2019;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wood L, Byrne R, Varese F, Morrison AP. Psychosocial interventions for internalised stigma in people with a schizophrenia-spectrum diagnosis: a systematic narrative synthesis and meta-analysis. Schizophr Res. 2016;176(2–3):291–303. [DOI] [PubMed] [Google Scholar]
- 29. Fernandez-Gonzalo S, Turon M, Jodar M, et al. A new computerized cognitive and social cognition training specifically designed for patients with schizophrenia/schizoaffective disorder in early stages of illness: a pilot study. Psychiatry Res. 2015;228(3):501–509. [DOI] [PubMed] [Google Scholar]
- 30. Bechi M, Bosia M, Spangaro M, et al. Combined social cognitive and neurocognitive rehabilitation strategies in schizophrenia: neuropsychological and psychopathological influences on theory of mind improvement. Psychol Med. 2015;45(15):3147–3157. [DOI] [PubMed] [Google Scholar]
- 31. Scheewe TW, Backx FJ, Takken T, et al. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatr Scand. 2013;127(6):464–473. [DOI] [PubMed] [Google Scholar]
- 32. Vera-Garcia E, Mayoral-Cleries F, Vancampfort D, Stubbs B, Cuesta-Vargas AI. A systematic review of the benefits of physical therapy within a multidisciplinary care approach for people with schizophrenia: an update. Psychiatry Res. 2015;229(3):828–839. [DOI] [PubMed] [Google Scholar]
- 33. Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med. 2015;45(7):1343–1361. [DOI] [PubMed] [Google Scholar]
- 34. Yoon S, Ryu JK, Kim CH, et al. Preliminary effectiveness and sustainability of group aerobic exercise program in patients with schizophrenia. J Nerv Ment Dis. 2016;204(9):644–650. [DOI] [PubMed] [Google Scholar]
- 35. Sun XR, Zhang H, Zhao HT, et al. Amelioration of oxidative stress-induced phenotype loss of parvalbumin interneurons might contribute to the beneficial effects of environmental enrichment in a rat model of post-traumatic stress disorder. Behav Brain Res. 2016;312:84–92. [DOI] [PubMed] [Google Scholar]
- 36. Zhang M, Wu J, Huo L, et al. Environmental enrichment prevent the juvenile hypoxia-induced developmental loss of parvalbumin-immunoreactive cells in the prefrontal cortex and neurobehavioral alterations through inhibition of NADPH Oxidase-2-Derived Oxidative Stress. Mol Neurobiol. 2016;53(10):7341–7350. [DOI] [PubMed] [Google Scholar]
- 37. Singhal G, Jaehne EJ, Corrigan F, Baune BT. Cellular and molecular mechanisms of immunomodulation in the brain through environmental enrichment. Front Cell Neurosci. 2014;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. do Prado CH, Narahari T, Holland FH, Lee H-N, Murthy SK, Brenhouse HC. Effects of early adolescent environmental enrichment on cognitive dysfunction, prefrontal cortex development, and inflammatory cytokines after early life stress. Dev Psychobiol. 2016;58(4):482–491. [DOI] [PubMed] [Google Scholar]
- 39. Murueta-Goyena A, Ortuzar N, Gargiulo PÁ, Lafuente JV, Bengoetxea H. Short-Term exposure to enriched environment in adult rats restores mk-801-induced cognitive deficits and gabaergic interneuron immunoreactivity loss. Mol Neurobiol. 2018;55(1):26–41. [DOI] [PubMed] [Google Scholar]
- 40. Murueta-Goyena A, Morera-Herreras T, Miguelez C, et al. Effects of adult enriched environment on cognition, hippocampal-prefrontal plasticity and NMDAR subunit expression in MK-801-induced schizophrenia model. Eur Neuropsychopharmacol. 2019;29(5):590–600. [DOI] [PubMed] [Google Scholar]
- 41. Faatehi M, Basiri M, Nezhadi A, et al. Early enriched environment prevents cognitive impairment in an animal model of schizophrenia induced by MK-801: Role of hippocampal BDNF. Brain Res. 2019;1711:115–119. [DOI] [PubMed] [Google Scholar]
- 42. Bator E, Latusz J, Wędzony K, Maćkowiak M. Adolescent environmental enrichment prevents the emergence of schizophrenia-like abnormalities in a neurodevelopmental model of schizophrenia. Eur Neuropsychopharmacol. 2018;28(1):97–108. [DOI] [PubMed] [Google Scholar]
- 43. Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67(2):155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mauney SA, Athanas KM, Pantazopoulos H, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74(6):427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steullet P, Cabungcal JH, Coyle J, et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry. 2017;22(7):936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75(6):963–980. [DOI] [PubMed] [Google Scholar]
- 48. Grent-’t-Jong T, Gross J, Goense J, et al. Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. eLife. 2018;7:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin⁺ GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345(6196):1255263. [DOI] [PubMed] [Google Scholar]
- 50. Kim H, Ährlund-Richter S, Wang X, Deisseroth K, Carlén M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164(1-2):208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bicks LK, Yamamuro K, Flanigan ME, et al. Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior. Nat Commun. 2020;11(1):1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32(9):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Perez SM, Boley A, Lodge DJ. Region specific knockdown of Parvalbumin or Somatostatin produces neuronal and behavioral deficits consistent with those observed in schizophrenia. Transl Psychiatry. 2019;9(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry. 2013;73(6):574–582. [DOI] [PubMed] [Google Scholar]
- 55. Cabungcal JH, Steullet P, Morishita H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110(22):9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Komitova M, Xenos D, Salmaso N, et al. Hypoxia-induced developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain. J Neurosci. 2013;33(33):13375–13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Soares AR, Gildawie KR, Honeycutt JA, Brenhouse HC. Region-specific effects of maternal separation on oxidative stress accumulation in parvalbumin neurons of male and female rats. Behav Brain Res. 2020;388:112658. [DOI] [PubMed] [Google Scholar]
- 58. Sullivan EM, O’Donnell P. Inhibitory interneurons, oxidative stress, and schizophrenia. Schizophr Bull. 2012;38(3):373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dwir D, Giangreco B, Xin L, et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: a reverse translation study in schizophrenia patients. Mol Psychiatry. 2020;25(11):2889–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Steullet P, Cabungcal JH, Kulak A, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30(7):2547–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. das Neves Duarte JM, Kulak A, Gholam-Razaee MM, Cuenod M, Gruetter R, Do KQ. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry. 2012;71(11):1006–1014. [DOI] [PubMed] [Google Scholar]
- 62. Kulak A, Cuenod M, Do KQ. Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behav Brain Res. 2012;226(2):563–570. [DOI] [PubMed] [Google Scholar]
- 63. Monin A, Baumann PS, Griffa A, et al. Glutathione deficit impairs myelin maturation: relevance for white matter integrity in schizophrenia patients. Mol Psychiatry. 2015;20(7):827–838. [DOI] [PubMed] [Google Scholar]
- 64. Corcoba A, Steullet P, Duarte JM, et al. Glutathione deficit affects the integrity and function of the Fimbria/Fornix and Anterior Commissure in Mice: relevance for Schizophrenia. Int J Neuropsychopharmacol. 2015;19(3):pyv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen Y, Curran CP, Nebert DW, Patel KV, Williams MT, Vorhees CV. Effect of chronic glutathione deficiency on the behavioral phenotype of Gclm-/- knockout mice. Neurotoxicol Teratol. 2012;34(4):450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Castagné V, Cuénod M, Do KQ. An animal model with relevance to schizophrenia: sex-dependent cognitive deficits in osteogenic disorder-Shionogi rats induced by glutathione synthesis and dopamine uptake inhibition during development. Neuroscience. 2004;123(4):821–834. [DOI] [PubMed] [Google Scholar]
- 67. Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. A developmental redox dysregulation leads to spatio-temporal deficit of parvalbumin neuron circuitry in a schizophrenia mouse model. Schizophr Res. 2019;213:96–106. [DOI] [PubMed] [Google Scholar]
- 68. Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(-/-) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem. 2002;277(51):49446–49452. [DOI] [PubMed] [Google Scholar]
- 69. Steullet P, Cabungcal JH, Cuénod M, Do KQ. Fast oscillatory activity in the anterior cingulate cortex: dopaminergic modulation and effect of perineuronal net loss. Front Cell Neurosci. 2014;8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baumann PS, Crespi S, Marion-Veyron R, et al. Treatment and early intervention in psychosis program (TIPP-Lausanne): Implementation of an early intervention programme for psychosis in Switzerland. Early Interv Psychiatry. 2013;7(3):322–328. [DOI] [PubMed] [Google Scholar]
- 71. Xin L, Mekle R, Fournier M, et al. Genetic polymorphism associated prefrontal glutathione and its coupling with brain glutamate and peripheral redox status in early psychosis. Schizophr Bull. 2016;42(5):1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61(6):1279–1285. [DOI] [PubMed] [Google Scholar]
- 73. Mlynárik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med. 2006;56(5):965–970. [DOI] [PubMed] [Google Scholar]
- 74. Caballero A, Granberg R, Tseng KY. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci Biobehav Rev. 2016;70:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Reh RK, Dias BG, Nelson CA III, et al. Critical period regulation across multiple timescales. Proc Natl Acad Sci U S A. 2020;117(38):23242–23251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sorg BA, Berretta S, Blacktop JM, et al. Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci. 2016;36(45):11459–11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cardis R, Cabungcal JH, Dwir D, Do KQ, Steullet P. A lack of GluN2A-containing NMDA receptors confers a vulnerability to redox dysregulation: consequences on parvalbumin interneurons, and their perineuronal nets. Neurobiol Dis. 2018;109(Pt A):64–75. [DOI] [PubMed] [Google Scholar]
- 78. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. [DOI] [PubMed] [Google Scholar]
- 79. Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165(2):214–220. [DOI] [PubMed] [Google Scholar]
- 80. Cabungcal JH, Counotte DS, Lewis E, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83(5):1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Phensy A, Duzdabanian HE, Brewer S, et al. Antioxidant treatment with n-acetyl cysteine prevents the development of cognitive and social behavioral deficits that result from perinatal ketamine treatment. Front Behav Neurosci. 2017;11:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Burrows EL, McOmish CE, Buret LS, Van den Buuse M, Hannan AJ. Environmental enrichment ameliorates behavioral impairments modeling schizophrenia in Mice lacking Metabotropic Glutamate Receptor 5. Neuropsychopharmacology. 2015;40(8):1947–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Urakawa S, Takamoto K, Hori E, Sakai N, Ono T, Nishijo H. Rearing in enriched environment increases parvalbumin-positive small neurons in the amygdala and decreases anxiety-like behavior of male rats. BMC Neurosci. 2013;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shojaei A, Anaraki AK, Mirnajafi-Zadeh J, Atapour N. Modifications of inhibitory transmission onto pyramidal neurons by postnatal exposure to MK-801: Effects of enriched environment. Int J Dev Neurosci. 2017;57:56–61. [DOI] [PubMed] [Google Scholar]
- 85. Slaker M, Barnes J, Sorg BA, Grimm JW. Impact of environmental enrichment on perineuronal nets in the prefrontal cortex following early and late abstinence from Sucrose self-administration in Rats. PLoS One. 2016;11(12):e0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ji MH, Wang ZY, Sun XR, et al. Repeated neonatal sevoflurane exposure-induced developmental delays of parvalbumin interneurons and cognitive impairments are reversed by environmental enrichment. Mol Neurobiol. 2017;54(5):3759–3770. [DOI] [PubMed] [Google Scholar]
- 87. Caballero A, Tseng KY. GABAergic function as a limiting factor for prefrontal maturation during adolescence. Trends Neurosci. 2016;39(7):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct. 2014;219(1):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lataster J, Collip D, Ceccarini J, et al. Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: a positron emission tomography study using [¹⁸F]fallypride. Neuroimage. 2011;58(4):1081–1089. [DOI] [PubMed] [Google Scholar]
- 90. Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32(2):117–131. [DOI] [PubMed] [Google Scholar]
- 91. Rabinovic AD, Hastings TG. Role of endogenous glutathione in the oxidation of dopamine. J Neurochem. 1998;71(5):2071–2078. [DOI] [PubMed] [Google Scholar]
- 92. Rosenthal A, Meyer MS, Mayo D, et al. Contributions of childhood trauma and atypical development to increased clinical symptoms and poor functioning in recent onset psychosis. Early Intervention in Psychiatry. 2020;8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Turner S, Harvey C, Hayes L, et al. Childhood adversity and clinical and psychosocial outcomes in psychosis. Epidemiol Psychiatr Sci. 2019;29:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cullen AE, Addington J, Bearden CE, et al. Stressor-Cortisol concordance among individuals at clinical high-risk for psychosis: novel findings from the NAPLS Cohort. Psychoneuroendocrinology. 2020;115:104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sawa A, Seidman LJ. Is prophylactic psychiatry around the corner? combating adolescent oxidative stress for adult psychosis and schizophrenia. Neuron. 2014;83(5):991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gomes FV, Zhu X, Grace AA. The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Nature Publishing Group. 2019;1(1):15067. [Google Scholar]
- 97. Gomes FV, Rincón-Cortés M, Grace AA. Adolescence as a period of vulnerability and intervention in schizophrenia: insights from the MAM model. Neurosci Biobehav Rev. 2016;70:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Goodwill HL, Manzano-Nieves G, LaChance P, et al. Early life stress drives sex-selective impairment in reversal learning by affecting parvalbumin interneurons in orbitofrontal cortex of mice. Cell Rep. 2018;25(9):2299–2307.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. [DOI] [PubMed] [Google Scholar]
- 101. Malchow B, Keller K, Hasan A, et al. Effects of endurance training combined with cognitive remediation on everyday functioning, symptoms, and cognition in multiepisode Schizophrenia Patients. Schizophr Bull. 2015;41(4):847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Noordsy DL, Burgess JD, Hardy KV, Yudofsky LM, Ballon JS. Therapeutic potential of physical exercise in early psychosis. Am J Psychiatry. 2018;175(3):209–214. [DOI] [PubMed] [Google Scholar]
- 103. Soundy A, Roskell C, Stubbs B, Probst M, Vancampfort D. Investigating the benefits of sport participation for individuals with schizophrenia: a systematic review. Psychiatr Danub. 2015;27(1):2–13. [PubMed] [Google Scholar]
- 104. Falkai P, Malchow B, Schmitt A. Aerobic exercise and its effects on cognition in schizophrenia. Curr Opin Psychiatry. 2017;30(3):171–175. [DOI] [PubMed] [Google Scholar]
- 105. Raine A, Mellingen K, Liu J, Venables P, Mednick SA. Effects of environmental enrichment at ages 3–5 years on schizotypal personality and antisocial behavior at ages 17 and 23 years. Am J Psychiatry. 2003;160(9):1627–1635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.