Abstract
Treatment resistance (TR) in patients with first-episode psychosis (FEP) is a major cause of disability and functional impairment, yet mechanisms underlying this severe disorder are poorly understood. As one view is that TR has neurodevelopmental roots, we investigated whether its emergence relates to disruptions in synchronized cortical maturation quantified using gyrification-based connectomes. Seventy patients with FEP evaluated at their first presentation to psychiatric services were followed up using clinical records for 4 years; of these, 17 (24.3%) met the definition of TR and 53 (75.7%) remained non-TR at 4 years. Structural MRI images were obtained within 5 weeks from first exposure to antipsychotics. Local gyrification indices were computed for 148 contiguous cortical regions using FreeSurfer; each subject’s contribution to group-based structural covariance was quantified using a jack-knife procedure, providing a single deviation matrix for each subject. The latter was used to derive topological properties that were compared between TR and non-TR patients using a Functional Data Analysis approach. Compared to the non-TR patients, TR patients showed a significant reduction in small-worldness (Hedges’s g = 2.09, P < .001) and a reduced clustering coefficient (Hedges’s g = 1.07, P < .001) with increased length (Hedges’s g = −2.17, P < .001), indicating a disruption in the organizing principles of cortical folding. The positive symptom burden was higher in patients with more pronounced small-worldness (r = .41, P = .001) across the entire sample. The trajectory of synchronized cortical development inferred from baseline MRI-based structural covariance highlights the possibility of identifying patients at high-risk of TR prospectively, based on individualized gyrification-based connectomes.
Keywords: first-episode psychosis, treatment-resistant, clozapine, longitudinal, gyrification, MRI, schizophrenia
Introduction
Approximately 30% of patients with a diagnosis of schizophrenia spectrum disorders will experience insufficient treatment response to available treatments, a phenomenon known as treatment resistance (TR).1,2 In line with existing evidence,3 we have previously shown that around 70% of patients with first-episode psychosis, who later develop TR, already exhibit a lack of response to non-clozapine antipsychotic treatments from their first contact with mental health services, while a remaining 30% become non-responsive at later stages.4,5 This, in combination with evidence that a history of obstetric complications6,7 and a younger age of illness onset are associated with a greater risk of developing TR,4,8,9 suggests that neurodevelopmental disruption may play a crucial role in the pathophysiology of TR.
Alterations in cortical folding (gyrification), which is an excellent marker of the integrity of axonal connectivity during the prenatal period,10 as demonstrated in the classic lesional studies of Goldman-Rakic and Rakic,11 have been reported in patients with psychosis,12,13 including in association with lack of response to antipsychotic treatment.14 While age-related reductions in the degree of gyrification occur after birth, when compared to the effects on cortical thickness and volume, regional alterations in gyrification index with age are minimal.15 Regional or mass univariate whole brain analysis of morphological measures such as gyrification, reveals brain regions that are most susceptible to focal disease-related change.16–19 Nevertheless, in the presence of significant pathophysiological heterogeneity, as that suspected in TR, the presence of these changes may be subtle and differ across patients, leading towards the identification of null results and to inconsistency on repeated case-control sampling.20 Focussing on regional alterations also fails to quantify the relationship between concomitant changes across different brain areas, which is crucial to uncovering the presence of defects in synchronized maturation. Graph-based measures of structural covariance provide a more powerful mode of capturing defects in synchronized maturation.21 When applied to the gyrification index, this approach likely captures the state of axonal connectivity occurring during prenatal development,22 making it one of the most promising methods for tracing the basis of TR back to factors that predate the onset of symptoms in psychosis. Additionally, deriving network metrics for the individual patient, which is at the core of the perturbation-based network analyses that compute an individual’s distance from a group norm,23 further allows for quantification of the heterogeneity inherent to many clinical subgroups.
Therefore, this study investigated if the emergence of TR in the 4 years following the first episode of psychosis is already associated with alterations in individual structural covariance of cortical gyrification at illness onset. While there are multiple metrics available to study cortical structure,24,25 the utility of structural covariance approaches in the study of psychoses, including schizophrenia, has been established only for thickness26 and gyrification,22 and among these, gyrification appears to be less malleable to environmental influences.22 In this context, and in continuity with our prior work,14,27,28 we chose the gyrification index as the metric of interest for this study. As treatment-resistant psychosis appears to have a unique neurobiological basis, different from that of partially treatment-responsive psychosis, we used a categorical definition of TR. We hypothesized that patients with first-episode psychosis who later developed TR would already show evidence of alterations in synchronized cortical maturation at the time of their first contact with mental health services.
Methods
Sample Ascertainment
Participants were recruited as part of the National Institute of Health Research (NIHR) Biomedical Research Centre (BRC) Genetics and Psychosis (GAP) study conducted in South London, United Kingdom.29 In line with previous research,3,30 we included all individuals aged 16–65 years with a first episode of schizophrenia spectrum disorders [codes F20–F29], affective psychoses [F30–F33] or other psychoses as defined in the International Classification of Diseases, 10th Revision (ICD-10) manual.31 These diagnoses were further validated by administration of the Schedules for Clinical Assessment in Neuropsychiatry (SCAN).32 The study exclusion criteria were evidence of (1) psychotic symptoms precipitated by an organic cause; (2) transient psychotic symptoms resulting from acute intoxication as defined in ICD-10; (3) head injury causing clinically significant loss of consciousness; and (4) intellectual disability (IQ < 70).
Ethics
The GAP study was granted ethical approval by the South London and Maudsley and Institute of Psychiatry Local Research Ethics Committee (reference number: 05/Q0706/158). All cases gave informed written consent after reading a detailed information sheet.
Data at Baseline
Sociodemographic Characteristics.
Sociodemographic details were collated using the Medical Research Council (MRC) Sociodemographic Schedule modified version.33 Age at first contact with services was defined as age at which a patient was first in contact with mental health services due to their psychotic symptoms.34 Ethnicity was self-ascribed using the 16 categories of the UK Census in 2001 (www.statistics.gov.uk/census 2001).
Clinical Assessments.
Duration of untreated psychosis (DUP) was defined (in weeks) as the difference between the date of onset of psychotic symptoms and the date of treatment with antipsychotic medications.35,36 The Global Assessment of Functioning (GAF) scale was used to measure both overall symptoms severity and functional disability at study entry.37 The GAF was completed from face-to-face interviews with good inter-rater reliability (κ = 0.90). The degree of psychopathology over the preceding week was evaluated with the 30-item Positive and Negative Syndrome Scale (PANSS),38,39 in face-to-face interviews. Each PANSS item was rated on a 7-point scale (1 = absent, 7 = extreme), across all 3 subscales: positive symptoms (7 items), negative symptoms (7 items), and general psychopathology (16 items).
MR Image Acquisition and Processing
To ensure minimal exposure to antipsychotic medications in patients, MRI scans were obtained within a 3-month period following the first contact with psychiatric services. A 3 Tesla GE (General Electric, Milwaukee) Signa HDx scanner at the Centre for Neuroimaging Sciences (CNS), Institute of Psychiatry, Psychology and Neuroscience (London, UK) was used to acquire 3-dimensional MPRAGE volumetric scans (matrix size of 256 × 256 × 166 voxels, with in-plane voxel size of 1.02 × 1.02 mm and a slice thickness of 1.2 mm (echo time/repetition time/inversion time = 2.848/6.988/650 ms, excitation flip angle 20°, one data average). Full brain and skull coverage was required for the MRI datasets and detailed quality control was carried out on all MR images according to previously published criteria.14,27,40 A summary of the quality control criteria used is presented in the supplementary material.
Cortical Gyrification Analysis.
We computed local gyrification indexes (LGIs) for various anatomically defined sulcal and gyral regions of the cortical mantle using Freesurfer (FreeSurfer, version 4.5.0; http://surfer.nmr.mgh.harvard.edu/), based on the method by Schaer et al.41 The steps for constructing an individual-specific LGI network are presented in figure 1; more details on surface extraction are provided in the supplementary material. LGIs were computed for 148 parcellated brain regions (74 in each hemisphere; no subcortical structures included) according to the atlas of Destrieux et al42 and in line with our prior work on structural covariance.14,27 All pre-processing steps followed the standard description given by Fischl et al.43 This included skull-stripping, intensity correction, determination of the gray-white matter boundary followed by tessellation to generate multiple vertices across the whole brain and the expansion of this boundary to re-create the pial surface, followed by spherical morphing and registration using sulcogyral landmarks. Schaer’s automated vertex-wise method computes Zilles’ gyrification index,44 a ratio of the inner folded contour to the outer perimeter of the cortex, using a 25 mm hull surface around each vertex of the reconstructed pial surface.41 Averaging the gyrification values assigned to the vertices within each anatomical boundary of Destrieux parcellations provided the regional LGI measure for each of the 148 parcellated brain regions. An LGI value closer to 1 suggests that the region has an approximately flat pial surface with almost no “buried cortex” in sulcal ridges.
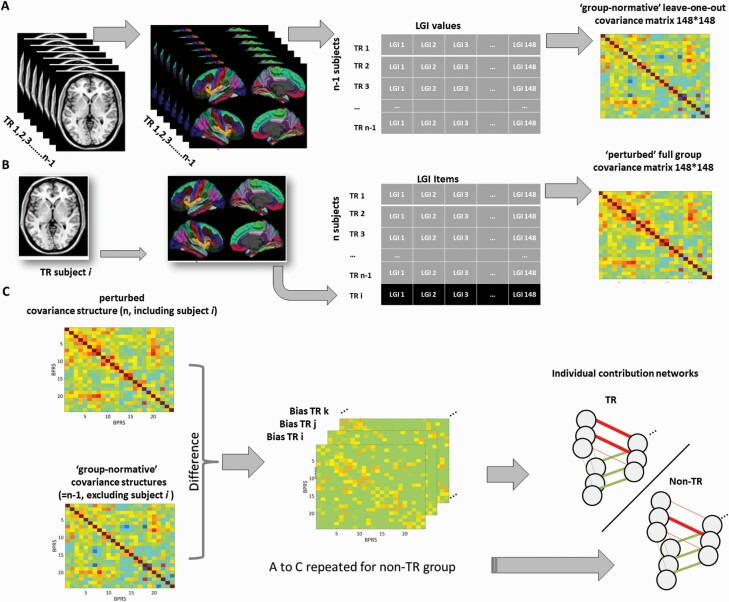
Fig. 1.
Depicts 3 steps (referred to as A, B, and C) followed to construct an individual-specific LGI network in the present study. (A) For a single group of (N-1) subjects (treatment resistance [TR] or non-TR), a specific group-based network is constructed by the correlations between LGI values based on regional LGI measures from 148 parcellations in this group, with the exclusion of a single subject i. This group network (based on N-1 matrix) has the “normative” covariance structure of that group’s gyrification pattern. (B) A new subject i belonging to that patient group is added to the group, and the perturbed network with this additional individual is constructed in the same way as the (N-1) matrix. The difference between the (N) and the (N-1) network is due to the individual i (or j or k…). (C) An individual contribution-based network is constructed using the difference of the corresponding edge between the (N) and (N-1) matrix. For illustrative purposes, only one group (TR) and only 3 nodes are shown. LGI-based networks in this study were made of 148 nodes, each node representing a single region of the parcellation scheme.
Constructing Gyrification-Based Individual Covariance Networks.
We first generated a 148 × 148 correlation matrix based on LGI values, separately for the 2 study groups. We estimated the contribution of each individual to their group matrix using a jack-knife bias estimation procedure, in line with our prior study.45 Bias values for each cell in the matrix of an individual subject quantified the contribution of that subject to the structural covariance of their group. Higher values signify greater relationship between the 2 given nodes in that subject, relative to the group norm. As a result, everyone’s matrix is a representation of the absolute contribution of that individual to their group’s overall covariance structure. This approach is mathematically similar to individual-specific gene expression networks used in bioinformatics23,46 and cancer studies.47 The bias values that constitute the edges in a group perturbation-based deviance matrix show a smooth, symmetric distribution around the absolute group correlation value, enabling parametric inferences, as shown by Liu et al.46 We then used the Graph Analysis Toolbox,48 to generate binary undirected graphs with a range of network thresholds based on connection density (ie, 0.05–0.25, with interval steps of 0.01). The interval steps were chosen to generate sufficient data points (20) for the functional data analysis (FDA) described below. This proportional thresholding and binarization based on edge density ensured that differences in absolute bias values between the 2 groups (that relate to the degree of freedom in jack-knife estimates) did not influence the estimates of topological parameters. The proportional thresholding range was chosen to enable between-group comparisons without inducing disconnection (percolation threshold) or losing small-worldness (randomness threshold) in any of the groups.49 Topological properties become unstable when sparsity levels are too high as some nodes are fully disconnected, while weak pairwise relationships that are likely to introduce randomness are included at lower sparsity values.49 See figure 1 for further details.
Tracing Patients at Follow-up
Approximately 4 years (mean = 3.8, SD = 1.4; range = 1–9, 283 person years) after the first contact with psychiatric services, we sought to trace all patients who had given consent for their clinical records to be accessed for research purposes. Follow-up data on illness course were extracted retrospectively from electronic clinical records, the primary clinical record keeping system within the Trust,50 using the WHO Life Chart Schedule (LCS) extended version,51,52 with has good reliability.53 All deaths and migrations up to, and including those that occurred during, the final year of follow-up were identified by a case-tracing procedure with the Office for National Statistics (ONS) for England and Wales and the General Register Office (GRO) for Scotland.
Definitions of TR.
Patients were defined as having TR if they had been treated with clozapine and/or they showed little or no symptomatic improvement to 2 consecutive treatments with antipsychotic medications of adequate dose and duration (at least 6 weeks) during the follow-up period.4,54 The presence of no symptomatic improvement following antipsychotic treatment was established if (1) having been treated with an antipsychotic medication of adequate dose and for an adequate duration, patients did not show improvement in their clinical presentation as recorded by treating clinicians, and/or (2) the documented reason for switching antipsychotic medication was a lack of therapeutic response, and not intolerance to antipsychotic medications or self-discontinuation of medications.4 An adequate daily dose of antipsychotic medication was defined as a daily dose of at least 400mg of chlorpromazine equivalents.55
Data Analysis
Confirmatory Factor Analysis.
As the Wallwork/Fortgang’s model56 offered the most robust model for exploring symptom profiles, we conducted a confirmatory factor analysis (CFA) to identify and evaluate the statistical fit57 of these symptom dimensions in our sample. The Goodness-of-Fit Index (GFI) statistics included the comparative fit index (CFI; values greater than 0.90 indicate good model fit), the root mean square error of approximation (RMSEA; values less than 0.06 indicate good model fit), and the standardized root mean square residual (SRMR; values less than 0.08 indicate good model fit).57 To improve the model fit, we further included correlated measurement errors into the model, based on significantly correlated residuals as indicated by modification indices.58 With this approach, 5 core symptom dimensions (positive, negative, excited, disorganized/concrete, and depressed59) were estimated for each patient.
Topological Analyses.
Global network properties were quantified using small-worldness (σ), characteristic path length (λ) and clustering coefficient (ϒ). While there are multiple measures of network properties available, we chose a minimal set with an index reflecting the “segregation” that may result from modular (communal) development of gyrification within a cluster of regions that increases clustering (ϒ); and an index of “integration” indicating a coordinated maturational process in gyrification across the entire brain, that reduces path length (λ). The presence of high segregation in the context of optimum integration gives rise to small-worldness, measured using the small-world index (σ). Network measures were normalized to equivalent values derived from 20 random (“null”) networks with the same degree of distribution, in line with previous studies.45,60 To perform statistical group comparisons across the range of chosen densities, we constructed curves showing the change in measures of interest as a function of the density. Functional data analysis (FDA) was performed with network measures treated as a function of y = f(x), allowing summation across densities and obviating the need for multiple testing (inline with61) using the GAT software.48 One-way ANOVA was used to compare the density function obtained using FDA between the 2 groups, followed by an estimation of the unbiased effect sizes using Hedges g.
Results
Core Analytic Cohort
The sample comprised 84 patients at first contact with services for an episode of psychosis, with an average age of 29.6 (SD = 9.8) years. After an average of 4 years, 10 (11.9%) were lost to follow-up and 74 (88.1%) were successfully followed up (mean years = 3.7, SD = 1.4). At onset, patients lost to follow-up were more likely to live with others (x2(1) = 3.88, P =.049) and to have a higher GAF symptom score (t(53) = 3.08, P =.003). There were no other significant differences between those patients who were followed up and those who were lost to follow-up (supplementary table 1). Among patients who were followed up, 4 (5.4%) did not have sufficient information to establish whether they had developed TR. Therefore, the core analytic sample included in subsequent analyses comprised 70 patients with an average age of 28.2 years (SD = 7.3). Of these, 17 (24.3%) met criteria for TR at the end of the follow-up period, while 53 (74.7%) were defined as non-TR.
Comparisons Between TR and Non-TR Groups
Baseline sociodemographic and clinical characteristics of the TR and non-TR groups are presented in table 1. There were no significant differences between the 2 groups in baseline sociodemographic and clinical characteristics. There were also no differences in age at first contact with services between the TR (meanyears = 25.8, SD = 5.4) and non-TR groups (meanyear = 28.8, SD = 7.8; t(68) = 1.47, P =.146).
Table 1.
Baseline Sample Characteristics for the Non-TR and TR Groups
| Baseline Characteristic | Non-TR, N = 53 (75.7%) | TR, N = 17 (24.3%) | Test Statistics | ||
|---|---|---|---|---|---|
| Mean (SD)/N(%) | Mean (SD)/N(%) | t/U/x 2 | df | P | |
| Ageyears | 28.8 (7.8) | 25.8 (5.4) | 1.47 | 68 | .146 |
| Follow-up lengthyears | 3.5 (1.5) Range = 1–9 |
4.6 (0.78) Range = 3–6 |
−3.00 | 69 | .004 |
| Time from starting AP to MRIdays | 40.5 (31.7) | 48.3 (37.0) | −0.688 | .491 | |
| DUP weeks | 64.0 (207.8) | 50.7 (114.2) | −1.32 | .187 | |
| Gender | 0.002 | 1 | .969 | ||
| Female | 12 (23.1) | 4 (23.5) | |||
| Male | 40 (76.9) | 13 (76.5) | |||
| Ethnicity | 0.44 | 2 | .803 | ||
| White ethnic groups | 19 (36.5) | 8 (44.4) | |||
| Black ethnic groups | 18 (34.6) | 6 (33.3) | |||
| Other | 15 (28.9) | 4 (22.2) | |||
| Living arrangements | 1.18 | 1 | .277 | ||
| Alone | 21 (46.7) | 10 (62.5) | |||
| Not alone | 24 (53.3) | 6 (37.5) | |||
| Relationship status | 3.42 | 1 | .064 | ||
| Single/separated | 32 (71.1) | 15 (93.8) | |||
| Stable relationship | 13 (28.9) | 1 (6.2) | |||
| Clinical presentation | |||||
| GAF symptoms | 48.3 (20.0) | 37.1 (12.7) | 1.51 | 63 | .138 |
| GAF disability | 49.5 (20.0) | 55.9 (18.3) | −0.83 | 63 | .412 |
| Positive symptom dimension | −0.28 (1.21) | 0.01 (1.37) | −0.78 | 63 | .441 |
| Negative symptom dimension | −0.17 (0.96) | 0.36 (0.30) | −1.77 | 62 | .082 |
| Disorganisation dimension | −0.13 (0.61) | −0.01 (0.52) | −0.65 | 62 | .519 |
| Excited symptom dimension | −0.13 (0.42) | −0.17 (0.51) | 0.29 | 63 | .770 |
| Depressed symptom dimension | −0.13 (0.61) | −0.05 (0.51) | −0.49 | 63 | .628 |
| Baseline diagnosis | 1.31 | 1 | .520 | ||
| Schizophrenia spectrum disorders | 38 (71.7) | 15 (82.3) | |||
| Affective psychoses | 13 (24.5) | 2 (11.8) | |||
| Other | 2 (3.8) | 1 (5.9) |
Note: TR, treatment resistant; AP antipsychotic medications; MRI, Magnetic resonance imaging; df, degrees of freedom; DUP, Duration of untreated psychosis; GAF, Global Assessment of Functioning Scale.
Confirmatory Factor Analysis
The CFA of our core analytic sample produced an excellent fit of the model: CFI = 0.959, RMSEA = 0.052 (90% CI 0.037–0.067), and SRMR = 0.071. There were no significant differences between TR and non-TR groups in these symptom dimensions. Scores for PANSS items the 2 are presented in supplementary table 2.
Gyrification-Based Connectome and TR
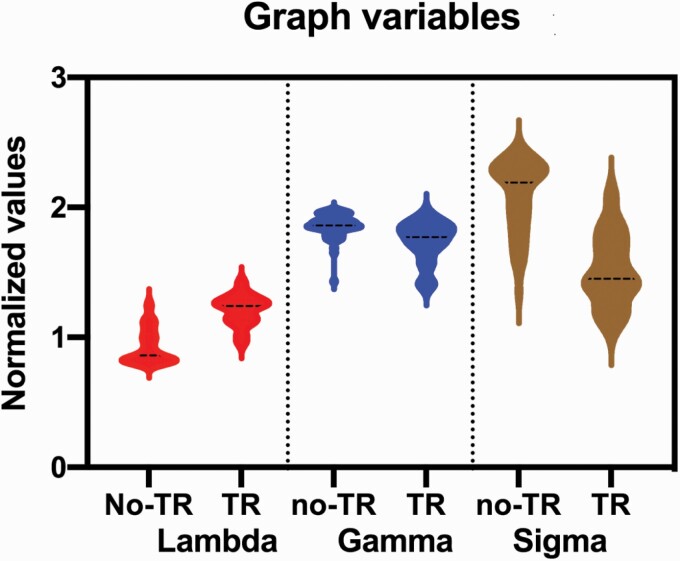
Results from the graph analyses are presented in table 2. Compared to patients in the non-TR group, patients in the TR group had a significant reduction in small-worldness (Hedges’s g = 2.09, P < .001) and reduced clustering coefficient (Hedges’s g = 1.07, P < .001) with increased path length (Hedges’s g = −2.17, P < .001). As shown in figure 2, having adjusted the analyses for age, gender and TR status, the positive symptom dimension was positively correlated with higher small-worldness (r = .41, P =.001) across the entire sample. This relationship was in the same direction, although not statistically significant, when the correlation was performed in the TR and non-TR groups separately (r = .26 to 0.35, P = .07–.2; supplementary figure 2). There were no similar correlations with the other symptom dimensions (table 3).
Table 2.
Graph Variables and Their Effect Sizes in the TR and non-TR Groups
| Graph Variables | FDA Mean (SD) | F value (P-value) | Hedges’ g | |
|---|---|---|---|---|
| Non-TR(N = 53) | TR (N = 17) | |||
| Path length (λ) | 0.93 (0.13) | 1.21 (0.12) | 60.39 (<.001) | −2.17 |
| Clustering coefficient (ϒ) | 1.86 (0.10) | 1.74 (0.16) | 14.66 (<.001) | 1.07 |
| Small-worldness (σ) | 2.09 (0.28) | 1.51 (0.28) | 56.02 (<.001) | 2.09 |
Note: TR, treatment resistance; FDA, Functional Data Analysis; all 3 comparisons survived Bonferroni correction for multiple testing (ie, P < .016). Also see supplementary figure 1.
Fig. 2.
Plot depicting the relationship, adjusted for TR status, age and sex and the positive symptom dimension, between the residuals of small-worldness index (σ) and positive symptom dimension in the entire sample. Also, see supplementary figure 2.
Table 3.
Correlation Between Graph Variables and Symptom Dimensions
| Small-Worldness (σ) | Path Length (λ) | Clustering Coefficient (ϒ) | |
|---|---|---|---|
| r (P-value) | r (P-value) | r (P-value) | |
| Positive dimension | .41 (.001)a | −.34 (.006) | .33 (.008) |
| Negative dimension | −.14 (.30) | .08 (.52) | −.15 (.24) |
| Disorganized dimension | .20 (.12) | −.18 (.18) | .12 (.34) |
| Excited symptom dimension | −.19 (.14) | .16 (.21) | .21 (.09) |
| Depressed symptom dimension | −.038 (.76) | −.23 (.06) | −.07 (.59) |
Note: Unstandardized residuals adjusted for binary TR status, age and sex. r = Pearson correlation coefficient; degree of freedom df = 64 for each correlation.
aSurvives Bonferroni correction for multiple testing (ie, P < .005).
We also undertook a direct comparison of the LGI values of the 148 regions between the TR and non-TR groups, with False Discovery Rate (FDR) corrected at P < .05 as threshold for significance. This comparison did not identify any between-group difference, indicating that disruptions in the covariance pattern of gyrification (based on (σ, ϒ, and λ) are more pronounced than any subtle difference in absolute regional cortical folding. The uncorrected comparisons of LGI values for the 148 regions are presented in supplementary table 3.
Discussion
To our knowledge, this is the first study to have examined the value of gyrification at illness onset as a predictor of TR occurring over the subsequent 4 years. Our results point to an association between a reduced coordination of processes driving cortical folding at the whole brain level (σ), stemming from both a neighborhood-level disruption (λ) and a reduced distributed relationship (ϒ), and the onset of TR. Thus, patients with first-episode psychosis who later developed TR, already displayed, at first presentation, a significant aberration in cortex-wide covariance of folding patterns when compared to patients who do not go on to develop TR. This indicates that the development of TR is likely related to the maturational coordination of the entire cortex, rather than to the degree of folding of any single region.
While structural covariance has not been examined in relation to prospectively determined TR to date, a recent study reported that thickness-based group-level structural covariance was not altered at whole brain level in patients with chronic schizophrenia resistant to treatment; instead, covariance was only higher among those regions that showed reduced thickness in TR.62 In contrast, here we report an overall reduction in gyrification-based, perturbation-derived patient-level structural covariance in relation to TR. While thickness-based covariance has been interpreted as an index of intra-cortical connectivity,62 gyrification-based covariance is best interpreted as an index of synchronized early maturation, given the in-utero synchronization of sulcal development.63 Thus, the reduction of small-worldness in perturbation-based LGI networks probably indicates a loss of coordination in cortical folding at the whole brain level, likely occurring during early development.
In contrast to our previous observations when contrasting short-term (12-week) responders with non-responders,27 we did not observe localized differences in gyrification between patients who later developed TR and those who did not. This supports the prevailing notion that TR is unlikely to be related to specific neuroanatomical defects.64,65 Instead, it may rather relate to a diffuse but subtle neurodevelopmental aberration that occurs either in utero or in early life. Consequently, it is likely that we observe no localizable effects, and that instead disrupted maturational relationships (covariance) among brain regions result in abnormal network-level topology. Here, we observe reduced regional segregation (clustering) and reduced overall integration of the morphological connectome, resulting in reduced small-worldness in covariance. Given the major role played by axonal tension in the formation of cortical folds,66,67 we surmise that TR results from a weakening of axonal tensions that arise from reduced inter-regional connectivity in the neonatal brain. As a result, the folding of spatially proximal, physically connected brain regions may not be synchronized and result in reduced clustering. Although the large effect size changes we find indicates >90% chance68 that a randomly selected patient with first-episode psychosis could be correctly identified to come from the TR group based on the reduced small-worldness of their cortical morphology, it remains unclear how well this prediction would perform at a single patient level.
Intriguingly, we found higher small-worldness was correlated with higher levels of positive symptoms at illness onset, and that small-worldness was reduced among patients with TR. Some evidence on the neurobiology of TR suggests that patients with TR may have a normodopaminergic/hyperglutamatergic status, while a predominantly hyperdopaminergic/normoglutamatergic status (ie, increased presynaptic dopamine) could relate to a higher degree of treatment responsiveness.3,69–71 Consistent with these suggestions, we speculate that a substantial number of individuals with a predominantly hyperdopaminergic pattern and higher positive symptoms at presentation may not have a notable cortical maturational deficit resulting in increased small-worldness of gyrification networks. However, those with less maturational coordination of cortical folding may go on to exhibit TR. The case for a neurodevelopmental characterization of TR is further strengthened by the observation that a younger age of onset,72 low IQ and family history of schizophrenia are all associated with TR.73 Prospectively designed hybrid PET/MR and glutamate MRS studies would help test the notion that a neurodevelopmental subtype with predominant glutamatergic deficits is specifically associated with TR.
It is noteworthy that network methods used in previous cross-sectional structural covariance studies also relied on group-based correlation,74 but the edges of those networks represented population-level (ie, between-subjects) relationships among regional gyrification indices. In contrast, the edges derived from our approach using deviance matrices represent the estimated relationship at an individual level (within-subject). Thus, the topological metrics from our network approach, based on second-order statistics (distance vs. correlation coefficient), are likely idiographic. The edges between 2 regions (nodes) in the individual gyrification networks do not imply direct connectivity but represent the absolute contribution each individual makes to the observed group-specific coordination in gyrification.
Methodological Considerations
The strengths of this study include the evaluation of brain morphology at the time of the first presentation to services, and the prospective evaluation of illness course over the first 4 years of illness. We have examined the onset of TR from the time of first contact with mental health services. The factor model of psychosis symptoms we used was derived from previous studies56 and shown to be optimal for the evaluation of patients with first-episode psychosis.75–77 The symptom dimensions were based on the PANSS, which has been shown to be resilient to the effects of age, chronicity of illness78 and short-term medication withdrawal.79 As the MRI data were obtained at the first-episode, before the evolution to TR status, the findings are not likely to be confounded by chronicity of illness or prolonged exposure to medications.
Although our length of DUP was consistent with that of other cohorts of first-episode psychoses,80–82 it was still shorter than that reported in some other studies.83–85 Given the variability in the definitions of DUP,86 we urge caution when generalizing our observations to all studies of first-episode psychosis. It is also possible that some patients in the non-TR group could have been TR but were unable did not accept or tolerate clozapine; similarly, it is also possible that some individuals in the non-TR group could have developed TR if they were followed up over a longer time period of time. However, we believe this latter possibility is unlikely, as we have previously shown that in most cases, TR becomes apparent early in the course of illness. Although evidence suggests there may be an early and a late treatment-resistant subtype,4,5 we did not have a sufficient number of patients to investigate if gyrification can discriminate between them. Similarly, it is feasible that a relatively small and unbalanced number of patients could have had an impact on our results. Nonetheless, our sample size is consistent with that of previous reports,3,16,22,25 and the definition of TR we used took into account instances when antipsychotics were stopped due to side effects rather than lack of improvement, thus ensuring it reflected true TR. Finally, the lack of a healthy control group limits our ability to comment on whether the patterns seen in non-TR individuals are “deviant” from those of individuals without a diagnosis of psychosis. As we now know what are the demographic and socioeconomic profiles of patients that are likely to develop TR over 4 years, we anticipate undertaking future matched data-collection with cohorts of healthy subjects that match the predicted non-TR and TR groups.
Conclusion
Several putative mechanistic pathways that ultimately result in treatment resistance in psychotic disorders may converge on disruption of coordinated cortical maturation. Our observations raise the possibility of defining a therapeutically meaningful “neurotype” of psychosis based on an easily accessible structural imaging assay of cortical folding patterns.
Supplementary Material
Acknowledgments
L.P. reports personal fees from Otsuka Canada, SPMM Course Limited, UK, Canadian Psychiatric Association; book royalties from Oxford University Press; investigator-initiated educational grants from Janssen Canada, Sunovion and Otsuka Canada outside the submitted work. R.M.M. has received honoraria from Janssen, Astra-Zeneca, Lilly, BMS, and is an editor of Psychological Medicine journal. PD has received speaker’s fees from Janssen and Lundbeck.
Funding
This study was funded in part by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. This study was further funded by CIHR Foundation Grant (375104/2017); the Bucke Family Fund, the Chrysalis Foundation and the Arcangelo Rea Family Foundation (London, Ontario) to L.P. L.P. acknowledges salary support from the Tanna Schulich Chair of Neuroscience and Mental Health and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario (AMOSO). O.A. is funded by the National Institute for Health Research (NIHR Post-Doctoral Fellowship - PDF-2018-11-ST2-020). R.M.M. receives salary support from the NIHR Maudsley BRC. M.D.F. is funded by Clinician Scientist Medical Research Council fellowship (project reference MR/M008436/1). A.S.D. is funded by the NIHR University College London Hospitals BRC. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1. Elkis H, Buckley PF. Treatment-resistant schizophrenia. Psychiatr Clin North Am. 2016;39(2):239–265. [DOI] [PubMed] [Google Scholar]
- 2. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. [DOI] [PubMed] [Google Scholar]
- 3. Demjaha A, Egerton A, Murray RM, et al. . Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75(5):e11–e13. [DOI] [PubMed] [Google Scholar]
- 4. Lally J, Ajnakina O, Di Forti M, et al. . Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016;8:1–10. [DOI] [PubMed] [Google Scholar]
- 5. Ajnakina O, Agbedjro D, Lally J, et al. . Predicting onset of early- and late-treatment resistance in first-episode schizophrenia patients using advanced shrinkage statistical methods in a small sample. Psychiatry Res. 2020;294:113527. [DOI] [PubMed] [Google Scholar]
- 6. Alvir JM, Woerner MG, Gunduz H, Degreef G, Lieberman JA. Obstetric complications predict treatment response in first-episode schizophrenia. Psychol Med. 1999;29(3):621–627. [DOI] [PubMed] [Google Scholar]
- 7. Froudist-Walsh S, Bloomfield MA, Veronese M, et al. . The effect of perinatal brain injury on dopaminergic function and hippocampal volume in adult life. Elife 2017:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vanelle JM. Treatment refractory schizophrenia. Encephale. 1995:13–21. [PubMed] [Google Scholar]
- 9. Meltzer HY, Rabinowitz J, Lee MA, et al. . Age at onset and gender of schizophrenic patients in relation to neuroleptic resistance. Am J Psychiatry. 1997;154(4):475–482. [DOI] [PubMed] [Google Scholar]
- 10. Dubois J, Benders M, Cachia A, et al. . Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2008;18(6):1444–1454. [DOI] [PubMed] [Google Scholar]
- 11. Goldman-Rakic PSRP. Experimental modification of gyral patterns. In: Geschwind N, Galaburda A, eds. Cerebral Dominance. Cambridge, MA: Harvard University Press; 1984:179–195. [Google Scholar]
- 12. Cachia A, Paillère-Martinot ML, Galinowski A, et al. . Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage. 2008;39(3):927–935. [DOI] [PubMed] [Google Scholar]
- 13. Friedman L, Knutson L, Shurell M, Meltzer HY. Prefrontal sulcal prominence is inversely related to response to clozapine in schizophrenia. Biol Psychiatry. 1991;29(9):865–877. [DOI] [PubMed] [Google Scholar]
- 14. Palaniyappan L, Marques TR, Taylor H, et al. . Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70(10):1031–1040. [DOI] [PubMed] [Google Scholar]
- 15. Raznahan A, Shaw P, Lalonde F, et al. . How does your cortex grow? J Neurosci. 2011;31(19):7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palaniyappan L, Liddle PF. Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatry Neurosci. 2012;37(6):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nanda P, Tandon N, Mathew IT, et al. . Local gyrification index in probands with psychotic disorders and their first-degree relatives. Biol Psychiatry. 2014;76(6):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris JM, Yates S, Miller P, Best JJ, Johnstone EC, Lawrie SM. Gyrification in first-episode schizophrenia: a morphometric study. Biol Psychiatry. 2004;55(2):141–147. [DOI] [PubMed] [Google Scholar]
- 19. Falkai P, Honer WG, Kamer T, et al. . Disturbed frontal gyrification within families affected with schizophrenia. J Psychiatr Res. 2007;41(10):805–813. [DOI] [PubMed] [Google Scholar]
- 20. Lawrie SM. Parsing heterogeneity. JAMA Psychiatry. 2017;74(11):1089–1090. [DOI] [PubMed] [Google Scholar]
- 21. Evans AC. Networks of anatomical covariance. Neuroimage. 2013;80:489–504. [DOI] [PubMed] [Google Scholar]
- 22. Palaniyappan L, Park B, Balain V, Dangi R, Liddle P. Abnormalities in structural covariance of cortical gyrification in schizophrenia. Brain Struct Funct. 2015;220(4):2059–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuijjer ML, Tung MG, Yuan G, Quackenbush J, Glass K. Estimating sample-specific regulatory networks. iScience. 2019;14:226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacKinley ML, Sabesan P, Palaniyappan L. Deviant cortical sulcation related to schizophrenia and cognitive deficits in the second trimester. Transl Neurosci. 2020;11(1):236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palaniyappan L, Al-Radaideh A, Gowland PA, Liddle PF. Cortical thickness and formal thought disorder in schizophrenia: An ultra high-field network-based morphometry study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;101:109911. [DOI] [PubMed] [Google Scholar]
- 26. Guo S, Palaniyappan L, Liddle PF, Feng J. Dynamic cerebral reorganization in the pathophysiology of schizophrenia: a MRI-derived cortical thickness study. Psychol Med. 2016;46(10):2201–2214. [DOI] [PubMed] [Google Scholar]
- 27. Palaniyappan L, Marques TR, Taylor H, et al. . Globally efficient brain organization and treatment response in psychosis: a connectomic study of gyrification. Schizophr Bull. 2016;42(6):1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dazzan P, Lawrence AJ, Reinders A, et al. . Symptom remission and brain cortical networks at first clinical presentation of psychosis: The OPTiMiSE study. Schizophr Bull. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Forti M, Sallis H, Allegri F, et al. . Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kravariti E, Demjaha A, Zanelli J, et al. . Neuropsychological function at first episode in treatment-resistant psychosis: findings from the ÆSOP-10 study. Psychol Med. 2019;49(12):2100–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 32. WHO. Schedules for Clinical Assessment in Neuropsychiatry: Version 2: Manual. Geneva, Switzerland: World Health Organization, Division of Mental Health; 1994. [Google Scholar]
- 33. Mallett R, Leff J, Bhugra D, Pang D, Zhao JH. Social environment, ethnicity and schizophrenia. A case-control study. Soc Psychiatry Psychiatr Epidemiol. 2002;37(7):329–335. [DOI] [PubMed] [Google Scholar]
- 34. McKenzie K, Samele C, Van Horn E, Tattan T, Van Os J, Murray R. Comparison of the outcome and treatment of psychosis in people of Caribbean origin living in the UK and British Whites. Report from the UK700 trial. Br J Psychiatry. 2001;178:160–165. [DOI] [PubMed] [Google Scholar]
- 35. Singh SP, Cooper JE, Fisher HL, et al. . Determining the chronology and components of psychosis onset: The Nottingham Onset Schedule (NOS). Schizophr Res. 2005;80(1):117–130. [DOI] [PubMed] [Google Scholar]
- 36. Malla A, Norman R, Schmitz N, et al. . Predictors of rate and time to remission in first-episode psychosis: a two-year outcome study. Psychol Med. 2006;36(5):649–658. [DOI] [PubMed] [Google Scholar]
- 37. Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. [DOI] [PubMed] [Google Scholar]
- 38. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 39. Kay SR, Opler LA, Lindenmayer JP. The positive and negative syndrome scale (PANSS): rationale and standardisation. British Journal of Psychiatry Supplement 1989;7:59–67. [PubMed] [Google Scholar]
- 40. Simmons A, Westman E, Muehlboeck S, et al. . The AddNeuroMed framework for multi-centre MRI assessment of Alzheimer’s disease: experience from the first 24 months. Int J Geriatr Psychiatry. 2011;26(1):75–82. [DOI] [PubMed] [Google Scholar]
- 41. Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27(2): 161–170. [DOI] [PubMed] [Google Scholar]
- 42. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 44. Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl). 1988;179(2):173–179. [DOI] [PubMed] [Google Scholar]
- 45. Das T, Borgwardt S, Hauke DJ, et al. . Disorganized gyrification network properties during the transition to psychosis. JAMA Psychiatry. 2018;75(6):613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu X, Wang Y, Ji H, Aihara K, Chen L. Personalized characterization of diseases using sample-specific networks. Nucleic Acids Res. 2016;44(22):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manem VSK, Salgado R, Aftimos P, Sotiriou C, Haibe-Kains B. Network science in clinical trials: a patient-centered approach. Semin Cancer Biol. 2018;52(Pt 2):135–150. [DOI] [PubMed] [Google Scholar]
- 48. Hosseini SM, Hoeft F, Kesler SR. GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One. 2012;7(7):e40709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–140. [DOI] [PubMed] [Google Scholar]
- 50. Stewart R, Soremekun M, Perera G, et al. . The south london and maudsley NHS foundation trust biomedical research centre (SLAM BRC) case register: development and descriptive data. BMC Psychiatry 2009;9(51):9–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jablensky A, Sartorius N, Ernberg G, et al. . Schizophrenia: manifestations, incidence and course in different cultures. A world health organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. [DOI] [PubMed] [Google Scholar]
- 52. Sartorius N, Gulbinat W, Harrison G, Laska E, Siegel C. Long-term follow-up of schizophrenia in 16 countries. A description of the international study of SChizophrenia conducted by the world health organization. Soc Psychiatry Psychiatr Epidemiol. 1996;31(5):249–258. [DOI] [PubMed] [Google Scholar]
- 53. Susser E, Finnerty M, Mojtabai R, et al. . Reliability of the life chart schedule for assessment of the long-term course of schizophrenia. Schizophr Res. 2000;42(1):67–77. [DOI] [PubMed] [Google Scholar]
- 54. Ajnakina O, Horsdal HT, Lally J, et al. . Validation of an algorithm-based definition of treatment resistance in patients with schizophrenia. Schizophr Res. 2018;( 19):294–297. [DOI] [PubMed] [Google Scholar]
- 55. Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40(2):314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophr Res. 2012;137(1-3):246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stefanovics EA, Elkis H, Zhening L, Zhang XY, Rosenheck RA. A cross-national factor analytic comparison of three models of PANSS symptoms in schizophrenia. Psychiatry Res. 2014;219(2):283–289. [DOI] [PubMed] [Google Scholar]
- 58. Liemburg E, Castelein S, Stewart R, van der Gaag M, Aleman A, Knegtering H; Genetic Risk and Outcome of Psychosis (GROUP) Investigators . Two subdomains of negative symptoms in psychotic disorders: established and confirmed in two large cohorts. J Psychiatr Res. 2013;47(6):718–725. [DOI] [PubMed] [Google Scholar]
- 59. Liddle PF. The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. Br J Psychiatry. 1987;151:145–151. [DOI] [PubMed] [Google Scholar]
- 60. Hosseini SM, Kesler SR. Influence of choice of null network on small-world parameters of structural correlation networks. PLoS One. 2013;8(6):e67354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO. Altered resting state complexity in schizophrenia. Neuroimage. 2012;59(3):2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wannan CMJ, Cropley VL, Chakravarty MM, et al. . Evidence for Network-Based Cortical Thickness Reductions in Schizophrenia. Am J Psychiatry. 2019;176(7):552–563. [DOI] [PubMed] [Google Scholar]
- 63. Rajagopalan V, Scott J, Habas PA, et al. . Local tissue growth patterns underlying normal fetal human brain gyrification quantified in utero. J Neurosci. 2011;31(8):2878–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Molent C, Olivo D, Wolf RC, Balestrieri M, Sambataro F. Functional neuroimaging in treatment resistant schizophrenia: a systematic review. Neurosci Biobehav Rev. 2019;104:178–190. [DOI] [PubMed] [Google Scholar]
- 65. Gillespie AL, Samanaite R, Mill J, Egerton A, MacCabe JH. Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? a systematic review. BMC Psychiatry. 2017;17(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997;385(6614):313–318. [DOI] [PubMed] [Google Scholar]
- 67. Wang Y, Necus J, Kaiser M, Mota B. Universality in human cortical folding in health and disease. Proc Natl Acad Sci U S A. 2016;113(45):12820–12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Coe R. It’s the effect size, stupid what effect size is and why it is important. Paper presented at the Annual Conference of the British Educational Research Association, University of Exeter, England, 2002.
- 69. Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169(11):1203–1210. [DOI] [PubMed] [Google Scholar]
- 70. Jauhar S, Veronese M, Nour MM, et al. . Determinants of treatment response in first-episode psychosis: an 18F-DOPA PET study. Mol Psychiatry. 2019;24(10):1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mouchlianitis E, Bloomfield MA, Law V, et al. . Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr Bull. 2016;42(3):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smart SE, Kepinska AP, Murray RM, MacCabe JH. Predictors of treatment resistant schizophrenia: a systematic review of prospective observational studies. Psychol Med. 2019;51(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kowalec K, Lu Y, Sariaslan A, et al. . Increased schizophrenia family history burden and reduced premorbid IQ in treatment-resistant schizophrenia: a Swedish national register and genomic study. Mol Psychiatry. 2019. doi: 10.1038/s41380-019-0575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fried EI, van Borkulo CD, Cramer AO, Boschloo L, Schoevers RA, Borsboom D. Mental disorders as networks of problems: a review of recent insights. Soc Psychiatry Psychiatr Epidemiol. 2017;52(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Langeveld J, Andreassen OA, Auestad B, et al. . Is there an optimal factor structure of the positive and negative syndrome scale in patients with first-episode psychosis? Scand J Psychol. 2013;54(2):160–165. [DOI] [PubMed] [Google Scholar]
- 76. Ajnakina O, Lally J, Di Forti M, et al. . Utilising symptom dimensions with diagnostic categories improves prediction of time to first remission in first-episode psychosis. Schizophr Res. 2018;193:391–398. [DOI] [PubMed] [Google Scholar]
- 77. Ajnakina O, Trotta A, Oakley-Hannibal E, et al. . Impact of childhood adversities on specific symptom dimensions in first-episode psychosis. Psychol Med. 2016;46(2):317–326. [DOI] [PubMed] [Google Scholar]
- 78. White L, Harvey PD, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the positive and negative syndrome scale. The PANSS study group. Psychopathology. 1997;30(5):263–274. [DOI] [PubMed] [Google Scholar]
- 79. Lindenmayer JP, Bernstein-Hyman R, Grochowski S. Five-factor model of schizophrenia. Initial validation. J Nerv Ment Dis. 1994;182(11):631–638. [DOI] [PubMed] [Google Scholar]
- 80. Morgan C, Lappin J, Heslin M, et al. . Reappraising the long-term course and outcome of psychotic disorders: the AESOP-10 study. Psychol Med. 2014;44(13):2713–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Salem MO, Moselhy HF, Attia H, Yousef S. Psychogenic psychosis revisited: a follow up study. Int J Health Sci (Qassim). 2009;3(1):45–49. [PMC free article] [PubMed] [Google Scholar]
- 82. Fraguas D, Del Rey-Mejías A, Moreno C, et al. . Duration of untreated psychosis predicts functional and clinical outcome in children and adolescents with first-episode psychosis: a 2-year longitudinal study. Schizophr Res. 2014;152(1):130–138. [DOI] [PubMed] [Google Scholar]
- 83. Barnes TR, Hutton SB, Chapman MJ, Mutsatsa S, Puri BK, Joyce EM. West London first-episode study of schizophrenia. Clinical correlates of duration of untreated psychosis. Br J Psychiatry. 2000;177(3):207–211. [DOI] [PubMed] [Google Scholar]
- 84. Loebel AD, Lieberman JA, Alvir JM, Mayerhoff DI, Geisler SH, Szymanski SR. Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry. 1992;149(9):1183–1188. [DOI] [PubMed] [Google Scholar]
- 85. Hoff AL, Sakuma M, Razi K, Heydebrand G, Csernansky JG, DeLisi LE. Lack of association between duration of untreated illness and severity of cognitive and structural brain deficits at the first episode of schizophrenia. Am J Psychiatry. 2000;157(11):1824–1828. [DOI] [PubMed] [Google Scholar]
- 86. Howes OD, Whitehurst T, Shatalina E, et al. . The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.