Abstract
Side effects of antipsychotic drugs play a key role in nonadherence of treatment in schizophrenia spectrum disorders (SSD). While clinical observations suggest that side effect variability between patients may be considerable, statistical evidence is required to confirm this. Here, we hypothesized to find larger side effect variability under treatment compared with control. We included double-blind, placebo-controlled, randomized controlled trials (RCTs) of adults with a diagnosis of SSD treated with 1 out of 14 antipsychotics. Standard deviations of the pre-post treatment differences of weight gain, prolactin levels, and corrected QT (QTc) times were extracted. The outcome measure was the variability ratio of treatment to control for individual antipsychotic drugs and the overall variability ratio of treatment to control across RCTs. Individual variability ratios were weighted by the inverse-variance method and entered into a random-effects model. We included N = 16 578 patients for weight gain, N = 16 633 patients for prolactin levels, and N = 10 384 patients for QTc time. Variability ratios (VR) were significantly increased for weight gain (VR = 1.08; 95% CI: 1.02–1.14; P = .004) and prolactin levels (VR = 1.38; 95% CI: 1.17–1.62; P < .001) but did not reach significance for QTc time (VR = 1.05; 95% CI: 0.98–1.12; P = 0.135). We found marked differences between individual antipsychotics and increased variability in side effects in patients under treatment with antipsychotics suggesting that subgroups of patients or individual patients may benefit from treatment allocation through stratified or personalized medicine.
Keywords: antipsychotics, adverse effects, variability, weight gain, prolactin, QTc prolongation, precision medicine
Introduction
Antipsychotics are a fundamental component in the treatment of schizophrenia spectrum disorders (SSD). Yet, a major problem are side effects which play a key role in nonadherence and discontinuation.1–5 A common hypothesis among researchers and clinicians alike is that although side effects are pervasive, not all patients are equally susceptible, even when they are treated with the same drug.6 However, empirical support for this hypothesis is lacking, as randomized controlled trials (RCTs) or conventional meta-analyses by design cannot answer whether such side effect variability does exist.7,8
While it is well-established that antipsychotics are associated with sides effects for the average patient, the approach we are taking with this study moves beyond comparing group averages but instead compares group variances. By comparing variances our study can for the first time test the hypothesis that there is indeed reason to believe that subgroups or even individual patients differ in their susceptibility to side effects—something that an analysis focused on group averages cannot do.
To date, studies have established the efficacy, safety, and side effect profiles of antipsychotic medications by averaging these indices across groups of patients. Such studies can provide us with average side effects for specific drugs, but they cannot tell us anything about individual patients or subgroups.9,10 Nevertheless, before searching for potential biomarkers that might predict individual susceptibility, we should first quantify the extent to which such predictors are truly needed.
An approach to answering this question is to shift the focus from the means to the variances of side effects.11 By comparing the variances between treatment and control groups of RCTs,12 greater variability in treatment would mean that some patients are more susceptible to side effects than others.11 Note that this method13 has recently been applied for antipsychotics,7 antidepressants,8,14,15 and brain stimulation,16 but in the context of treatment effect variability. It is worth noting that these studies found little evidence for treatment effect variability.7,8,14,15 Importantly, in the case of pre-post differences used as input for a meta-analysis of variance it is crucial to think carefully about the way the variability ratio is expressed,12,15,17 as the use of the coefficient of variation ratio (CVR) that has been proposed as an alternative of the variability ratio (VR)12 may lead to unreliable results.13,17
A recently published study investigated the individual treatment response in antipsychotics and brought surprising results.7,18 By comparing the variability between treatment and control groups, no evidence was found for an increase in variability in the treatment group. What might sound counter-intuitive at first raises the question of how big the need for precision medicine really is. However, that study evaluated the evidence for treatment effect variability. It is possible that although such variability in treatment effects is not as high as sometimes assumed,19 it does exist in the susceptibility for side effects. In other words, even if there is little variability in response to treatment between patients, there may still be enough variability in side effects to justify a need for precision medicine. If true, then this would support optimization of treatment allocation with respect to side effect profiles.20
Side effects that are particularly relevant to antipsychotic treatment include weight gain,5 hyperprolactinemia, and QTc prolongation.20 Weight gain is a frequently observed side effect that can negatively impact one’s physical health and thus may also influence treatment adherence. Every additional kilogram of weight gain can contribute to an increased risk of heart failure,21 cardiovascular disease,22 and diabetes.23 In addition, treatment discontinuation is often seen in patients with increase of weight under treatment.24 High prolactin levels can lead to symptoms like decreased bone mass, gallactorhea, and fertility problems in men and women. Further possible symptoms include menstrual disturbances in female patients and decreased libido and erectile dysfunction in male patients.25 These symptoms are frequent, but often underreported by patients and unnoticed as well as untreated by clinicians.26,27 They furthermore might lead to loss in quality of life and might be a reason for treatment discontinuation1,28 and subsequent illness relapse, which together with persistent positive symptoms29–32 may severely impact recovery and therapeutic alliance.33 Prolongation of QTc was observed in 7 of 14 antipsychotics compared by placebo in the inter-group comparison by Huhn and colleagues.6 Importantly, torsade de pointes tachycardia and sudden cardiac death are possible severe consequences of QTc prolongation.34 Such cardiac events are one of the factors that lead to the loss of life expectancy observed in patients with SSD.35–37
In summary, antipsychotic side effects are highly relevant for long-term outcome and adherence in treatment of positive symptoms in SSD. The question remains whether variability in side effects is high enough to warrant efforts of treatment stratification or personalisation. If there is little or no evidence for variability in side effects there might not be a need for stratification or personalization and the already widely available data provided by intergroup comparisons might offer reasonable estimates for the individual patient. Thus, we compared the variances of side effects including weight gain, prolactin level and QTc-time between treatment and control groups to address this question and to evaluate the evidence for the presence of side effect variability. Based on the clinical impression that patients seem to vary in their susceptibility to side effects, we hypothesized that the variability in side effects would be higher in the treatment compared with the control groups across all published trials of antipsychotics in SSD.6
Methods
Search Strategy and Selection Criteria
We used the data from the recent meta-analysis by Huhn and colleagues.6 That study included placebo-controlled published and unpublished trials investigating orally administered atypical (second generation) antipsychotics and typical (first generation) antipsychotics in adults with schizophrenia spectrum disorders; and excluded patients with first episode psychosis, treatment resistance, mainly negative symptoms, comorbidity with other mental or physical illnesses and relapse-prevention studies. Long- and short-acting intramuscular injections were also excluded (as they are often used in relapse prevention or emergency treatment) and studies from mainland China were excluded because of data quality concerns.38 Data sources were MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Biosis, PsycINFO, PubMed, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform and the US Food and Drug Administration until January 8, 2019. Data quality and validity were ensured by following the PRISMA guidelines.39 For missing data, we also contacted study authors.
We decided to investigate weight gain, prolactin elevation, and QTc prolongation because these side effects are particularly relevant,5,20 and quantifiable metric data were available in most studies. Other side effects such as extrapyramidal motor symptoms (EPS), sedation and diabetes can be equally burdensome and certainly pose a health risk to patients. However, those have often not been quantified in studies but rather assessed qualitatively (e.g. through categorical variables such as EPS: yes/no; sedation: yes/no; diabetes: treated/untreated). These types of variables did not allow us to estimate variability and so we had to restrain from including them in the analysis.
For the analysis, we used the standard deviations of pre-post differences in side effects. The primary outcome was the overall variability ratio of side effects in treatment versus control groups. Standard deviations (SD) and number of patients (N) were extracted for weight gain, prolactin level and QTc time. The units used were kg for weight gain, ng/ml for prolactin levels, and ms for QTc time. Some studies provided data for all of the three side effects, whereas the majority of the studies contained less data (see Results).
Statistical Analysis
If patients or subgroups differ in their susceptibility to side effects, we would expect to observe increased variances in the treatment compared with the control group. To test this, we computed the log variability ratio (log VR) by comparing the relative variability of side effects under treatment versus control:
where was the reported sample SD for side effects under treatment, was the reported sample SD for side effects under control, was the treatment sample size, and the control sample size. The corresponding sampling variance () for each comparison can be expressed as follows:
The individual variability ratios were weighted with the inverse of this sampling variance40 and entered into a random-effects model to quantify the overall variability ratio of side effects. For better interpretability, results were back-transformed from the logarithmic scale. Here, a variability ratio greater than one would indicate a higher side effect variability in treatment compared with control, whereas a variability ratio smaller than one would indicate less side effect variability under treatment compared with control.
Data and Code Availability
The analysis was performed from September 2019 to May 2020, using the R package metafor40 (version 2.4.0). The manuscript was produced with the R packages rmarkdown (version 2.6); represearch (version 0.0.0.9000; https://github.com/phoman/represearch/); knitr (version 1.30); and papaja (version 0.1.0.9997). All data and code are freely available online to ensure reproducibility at https://github.com/homanlab/sideeffects/.
Results
Overall Reporting Details
Together, we screened N = 151 studies with 14 different antipsychotics from the original meta-analysis by Huhn and colleagues6 as these studies reported data on at least one of the three side effects that we were interested in. Of these studies, N = 94 (62%) had missing variance measures despite reported means for at least one of the three side effects. We thus included the N = 60 (40%) studies that did report variance measures for at least one of the side effects of interest.
Weight Gain
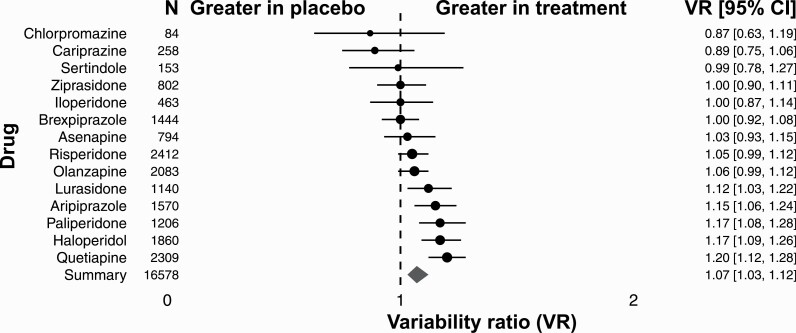
For weight gain, we included 51 RCTs, yielding 72 comparisons of antipsychotic drugs with placebo to investigate the individual occurrence of weight gain in patients. All together we included N = 16 578 patients diagnosed with schizophrenia or schizoaffective disorder. There were 11 373 (69%) patients randomly allocated to the treatment group, and 5205 (31%) to the placebo group. Patients in the treatment group received 1 out of 14 investigated antipsychotic drugs. Individual comparisons between drugs across studies indicated marked differences between individual antipsychotics. The VR for chlorpromazine, cariprazine, and sertindole was smaller than 1. The VR for ziprasidone, iloperidone, and brexpiprazole was 1. The VR for asenapine, risperidone, olanzapine, lurasidone, aripiprazol, paliperidone, haloperidol, and quetiapine was greater than 1 (VR = 1.08; 95% CI: 1.02–1.14; P = .004; figure 1). Overall, the variability for weight gain was higher under treatment than under control (VR = 1.08; 95% CI: 1.02–1.14; P = .004; supplementary figure S1).
Fig. 1.
Variability ratio for weight gain for individual antipsychotics. The forest plot shows the VR together with its 95% confidence interval (CI) for treatment versus placebo. All included studies41–96 are also listed in supplementary table S1.
Hyperprolactinemia
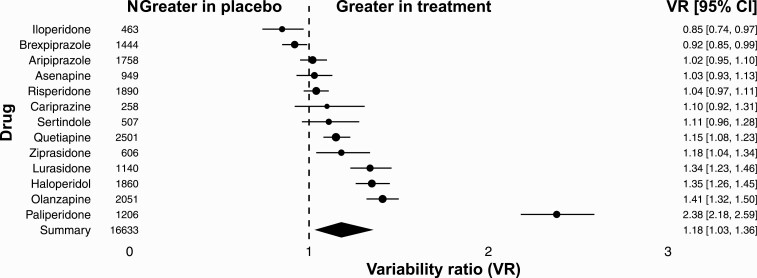
For hyperprolactinemia, we included 50 RCTs, with 71 comparisons of antipsychotic drugs with placebo. All together we included N = 16 633 patients diagnosed with schizophrenia or schizoaffective disorder. There were 11 409 (69%) patients randomly allocated to the treatment group, and 5224 (31%) to the placebo group. Patients in the treatment group received 1 out of 13 investigated antipsychotic drugs. Individual comparisons between drugs across studies indicated marked differences between individual antipsychotics. The VR for iloperidone and brexpiprazole was smaller than 1. The VR for aripiprazole, asenapine, risperidone, cariprazine, sertindole, quetiapine, ziprasidone, lurasidone, haloperidol, olanzapine, and paliperidone was greater than 1 (VR = 1.38; 95% CI: 1.17–1.62; P < .001; figure 2). Overall, the variability for hyperprolactinemia was higher under treatment than under control (VR = 1.38; 95% CI: 1.17–1.62; P < .001; figure S2).
Fig. 2.
Variability ratio for hyperprolactinemia for individual antipsychotics. The forest plot shows the VR together with its 95% confidence interval (CI) for treatment versus placebo. All included studies41,42,44–48,50–58,60,62,64,65,67–69,72,74–79,82–92,94,95,97–104 are also listed in supplementary table S1.
QTc Prolongation
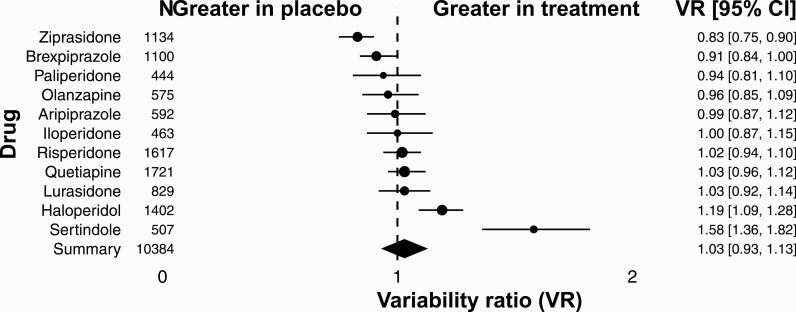
For QTc prolongation, we included 29 RCTs, with 46 comparisons of antipsychotic drugs with placebo. All together we included N = 10 384 patients diagnosed with schizophrenia or schizoaffective disorder. There were 7439 (72%) patients randomly allocated to the treatment group, and 2945 (28.00%) to the placebo group. Patients in the treatment group received 1 out of 11 investigated antipsychotic drugs. Individual comparisons between drugs across studies indicated marked differences between individual antipsychotics (VR = 1.05; 95% CI: 0.98–1.12; P = .135; figure 3). The VR for ziprasidone, brexpiprazole, paliperidone, and olanzapine was smaller than 1. The VR for iloperidone was 1. The VR for risperidone, quetiapine, lurasidone, haloperidol, and sertindole was greater than 1. Even though the variability for QTc prolongation was higher under treatment than under control, the difference did not reach statistical significance (VR = 1.05; 95% CI: 0.98–1.12; P = .135; supplementary figure S3).
Fig. 3.
Variability ratio for QTC prolongation for individual antipsychotics. The forest plot shows the VR together with its 95% confidence interval (CI) for treatment versus placebo. All included studies42,45,51,52,54–60,62,68,70,74,76–80,85,86,88,91,95,97,101,104,105 are also listed in supplementary table S1.
Discussion
Summary
This study assessed the variability in the three major side effects of antipsychotic treatment in schizophrenia spectrum disorders. We focused on side effects because their occurrence has a great impact on treatment adherence and physical health of patients, and clinical experience suggests a potential to improve treatment allocation by taking into account the variability in side effect occurrence. We also know from clinical trials and meta-analyses that some antipsychotics are more associated with specific side effects than others. For example, clozapine and olanzapine are strongly associated with weight gain,6,20,106 QTc-time prolongation is most distinct in sertindole and amisulpride,6 and prolactin level elevation in paliperidone and risperidone.6 However, these data cannot address the question whether there is variability in subgroups or individual patients. Such side effect-by-subgroup or side effect-by-patient interaction would be a prime example for the need of a more stratified or personalized medicine, respectively, which allocates treatments according to side effect profiles of subgroups or individual patients. Overall, we found that the variability for weight gain and prolactin elevation was indeed significantly increased in patients who received treatment compared with those who received placebo. For QTc prolongation, the increase was not significant. Together, our results suggest that there is indeed marked variability in the occurrence of side effects in antipsychotic treatment. Variability also differed markedly between individual drugs.
Reporting
Altogether we included 43 595 patients from 60 studies. The included studies provided data for treatment with 14 antipsychotic drugs for weight gain, 13 antipsychotic drugs for hyperprolactinemia, and 11 antipsychotic drugs for QTc prolongation compared to placebo. Only for about 40% of studies included in a previous meta-analysis6 variance data for at least one of the side effects of interest (weight gain, prolactin levels, QTc prolongation) were available. In about 62% of the studies included6 incomplete data existed such that means were reported without a measure of variance. Although we did contact authors for missing data whenever possible, we received missing data only for three studies. In summary, consistent reporting of antipsychotic side effects, specifically with respect to variability measures, is currently missing in the literature and should be improved in future studies.
Weight Gain
Weight gain, especially for second generation antipsychotics,107 is a severe side effect that can contribute to metabolic dysregulation. Importantly, every kg of weight gain leads to a linear increase in the risk of cardiovascular diseases,22 heart failure,21 and diabetes.23 Clozapine, olanzapine, zotepine, and sertindole have the most severe impact in gaining weight. Some studies showed that a lower BMI at baseline108 and sex109 can lead to more weight gain, whereas other studies found that male sex and higher BMI at baseline are related to a higher risk of metabolic disturbances.20 Our findings provide evidence that some patients are indeed more susceptible to antipsychotic weight gain than others, and that this susceptibility varies also between medications. For example, for quetiapine we found that patients differed in their susceptibility for weight gain while we did not find such evidence for olanzapine and brexpiprazole, suggesting a potential for stratified or personalized medicine for quetiapine but not olanzapine and brexpiprazole. As antipsychotics in the treatment for schizophrenia and related diseases is often recommended to be taken as a relapse prevention for a longer period,110,111 patients are likely to gain more weight during their treatment over months and years. Together, this suggests that there is a potential to improve long-term health and adherence by identifying the subgroups or individual patients that are particularly prone to weight gain. Preliminary evidence suggests that a dysregulated striatal reward circuit contributes to this weight gain susceptibility.5,112
Hyperprolactinemia
Prolactin level elevations occur in up to 70% of patients113 under the treatment with antipsychotics. By blocking dopamine D2 receptors on lacotroph cells a disinhibition of the synthesis and secretion of prolactin is observed.114,115 This can lead to both short- and long-term side effects with potentially severe impact on patients’ health. Typical short-term effects include galactorrhea, gynecomastia, menstrual irregularities, and sexual dysfunction; a typical long-term result is osteoporosis,116,117 and a potentially increased risk in developing breast cancer in association with hyperprolactinemia.118,119 Our findings suggest that these risks may be particularly relevant for some patients but not others, and more relevant for some antipsychotic drugs than others. For example, a previous study found that prolactin level elevations are more pronounced and more frequent in women than in men.115 In addition, some antipsychotics such as amisulpride, risperidone, and paliperidone are linked to a greater elevation of prolactin.6,120 The striking difference between risperidone (for which we did not find evidence for significant variability in prolactin elevation) and paliperidone (for which we did find such evidence) is puzzling as paliperidone is an active metabolite of risperidone, and previous literature suggests that paliperidone and risperidone lead to similar elevations in serum prolactin concentrations.121 A possible explanation is that the level of prolactin can be highly variable based on multiple biological and methodological factors such as stress, diurnal variation and type of assay performed. However, future studies should pay particular attention to differences in susceptibility to prolactinemia between these two antipsychotics. In summary, and in line with the weight gain findings, our findigns suggest that there is a potential to improve long-term health and antipsychotic adherence by identifying the subgroups or individual patients that are particularly likely to develop prolactin elevations under antipsychotic treatment.
QTc Prolongation
Prolongation of QTc is another important antipsychotic side effect as cardiovascular diseases remain the most common cause of natural mortality in schizophrenia spectrum disorders.121 Users of antipsychotic medication are reported to have higher rates of sudden cardiac death than nonusers.123 Prolongation of QTc (longer than 450 ms in men and longer than 470 ms in women, respectively, when corrected with Bazetts Formula124) can contribute to this.34 A prolongation of QTc can lead to torsade de pointes and subsequently to sudden death.125,126 The molecular pathway of this side effect is not completely understood.127 It is known, however, that some medications such as sertindole, amisulprid, and ziprasidone lead to more QTc prolongation than others.6 Here, we found increased variability for some antipsychotics such as haloperidol in QTc prolongation. However, altogether we did not find a statistically significant increase in variability for QTc prolongation, potentially because a smaller number of studies were available which decreased statistical power.
Limitations and Strengths
Our meta-analysis had some limitations. First, the occurrence of side effects might be a dose-dependent effect which could reflect a higher/different VR in some studies. Dose-dependent means and standard deviations are often missing but would be necessary to investigate dose-dependent effects. Second, for QTc, a reduced number of studies was available, potentially reducing statistical power to detect a significant variability increase. Third, our sample did not include predefined subgroups including patients with first-episode psychosis and treatment-resistant patients to create the most homogeneous sample possible. Finally, our method cannot determine whether the increased variability is due to variability differences in subgroups or individual patients.11 The particular strength of our study is that we included all available studies of antipsychotic treatment in SSD reporting variability measures for side effects of interest. To our knowledge, this is the first comprehensive study that provides evidence for substantial variability in side effects.
Conclusion
While we did not find convincing evidence that patients differed in their susceptibility to QTc prolongation, we did find such evidence for weight gain and prolactin elevation: for half of all antipsychotics (7 out of 14) we can assume that subgroups of patients or even individual patients would benefit from specific treatment allocation through stratified or personalized medicine, respectively. Such efforts in precision medicine might be crucial to improve adherence and long-term health under antipsychotic treatment.
Supplementary Material
Acknowledgments
The authors thank Majnu John, PhD, for advice on the analysis of the current study and Ellen Ji, PhD, for her thoughtful comments on the manuscript. These individuals received no additional compensation, outside of their usual salary, for their contributions. In the last 3 years Dr Leucht has received honoraria for service as a consultant or adviser and/or for lectures from Angelini, Böhringer Ingelheim, Geodon&Richter, Janssen, Johnson&Johnson, Lundbeck, LTS Lohmann, MSD, Otsuka, Recordati, SanofiAventis, Sandoz, Sunovion, TEVA. Dr Kane reported grants from Otsuka, Lundbeck and Janssen, as well as other from Alkermes, Allergan, Forum, Genentech, Lundbeck, Intracellular Therapies, Janssen, Johnson & Johnson, Merck, Neurocrine, Otsuka, Pierre Fabre, Reviva, Roche, Sunovion, Takeda, Teva, Vanguard Research Group, and LB Pharmaceuticals outside of the submitted work. No other disclosures were reported. M.S.N. co-analyzed and interpreted the data, wrote the first draft of the manuscript and revised the final manuscript. S.H. conceptualized the study, wrote the primary analysis code and revised the final manuscript. S.V. helped initiate the study and revised the final manuscript. E.S. helped initiate the study and revised the final manuscript. J.M.K. helped initiate the study and revised the final manuscript. M.H. collected the data and revised the final manuscript. S.L. conceptualized the study and revised the final manuscript. P.H. initiated, conceptualized and supervised the study, performed the statistical analysis, and revised the final manuscript. All authors approved the final version of the manuscript.
Funding
P.H. is supported by a NARSAD grant from the Brain & Behavior Research Foundation (28445) and by a Research Grant from the Novartis Foundation (20A058).
References
- 1. Lambert M, Conus P, Eide P, et al. Impact of present and past antipsychotic side effects on attitude toward typical antipsychotic treatment and adherence. Eur Psychiatry. 2004;19(7):415–422. [DOI] [PubMed] [Google Scholar]
- 2. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sendt KV, Tracy DK, Bhattacharyya S. A systematic review of factors influencing adherence to antipsychotic medication in schizophrenia-spectrum disorders. Psychiatry Res. 2015;225(1-2):14–30. [DOI] [PubMed] [Google Scholar]
- 4. Wade M, Tai S, Awenat Y, Haddock G. A systematic review of service-user reasons for adherence and nonadherence to neuroleptic medication in psychosis. Clin Psychol Rev. 2017;51:75–95. [DOI] [PubMed] [Google Scholar]
- 5. Homan P, Argyelan M, Fales CL, et al. Striatal volume and functional connectivity correlate with weight gain in early-phase psychosis. Neuropsychopharmacology. 2019;44(11):1948–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winkelbeiner S, Leucht S, Kane JM, Homan P. Evaluation of differences in individual treatment response in schizophrenia spectrum disorders: a meta-analysis. JAMA Psychiatry. 2019;76(10):1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munkholm K, Winkelbeiner S, Homan P. Individual response to antidepressants for depression in adults-a meta-analysis and simulation study. PLoS One. 2020;15(8):e0237950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Senn S. Mastering variation: variance components and personalised medicine. Stat Med. 2016;35(7):966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Senn S. Statistical pitfalls of personalized medicine. Nature. 2018;563(7733):619–621. [DOI] [PubMed] [Google Scholar]
- 11. Cortés J,, González JA,, Medina MN, et al. Does evidence support the high expectations placed in precision medicine? A bibliographic review. F1000Research. 2019;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakagawa S, , Poulin R,, Mengersen K, et al. Meta-analysis of variation: ecological and evolutionary applications and beyond. Methods Ecol Evol. 2015;6:143–152. [Google Scholar]
- 13. Mills HL,, Higgins JP,, Morris RW,, et al. Detecting heterogeneity of intervention effects using analysis and meta-analysis of differences in variance between arms of a trial. MedRxiv. 2020. doi: 10.1101/2020.03.07.20032516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plöderl M, Hengartner MP. What are the chances for personalised treatment with antidepressants? Detection of patient-by-treatment interaction with a variance ratio meta-analysis. BMJ Open. 2019;9(12):e034816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Volkmann CMD, Volkmann A, Mueller C. On the treatment effect heterogeneity of antidepressants in major depression. A Bayesian meta-analysis. MedRxiv. 2020. doi: 10.1101/2020.02.20.19015677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Homan S, Muscat W, Joanlanne A, et al. Treatment effect variability in brain stimulation across psychiatric disorders: a meta-analysis of variance. Neurosci Biobehav Rev. 2021;124:54–62. [DOI] [PubMed] [Google Scholar]
- 17. Volkmann A. On the relationship between treatment effect heterogeneity and the variability ratio effect size statistic. arXiv:2006.11848. 2020. [Google Scholar]
- 18. Winkelbeiner S, Homan P. Is Variance Ratio a valid indicator of heterogeneous treatment effect?-Reply. JAMA Psychiatry. 2020;77(2):217–218. [DOI] [PubMed] [Google Scholar]
- 19. Homan P, Argyelan M, DeRosse P, et al. Structural similarity networks predict clinical outcome in early-phase psychosis. Neuropsychopharmacology. 2019;44(5):915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. [DOI] [PubMed] [Google Scholar]
- 22. Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA. 1995;273(6):461–465. [DOI] [PubMed] [Google Scholar]
- 23. Cooper SJ, Reynolds GP, Barnes T, et al. ; With expert co-authors (in alphabetical order): . BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. 2016;30(8):717–748. [DOI] [PubMed] [Google Scholar]
- 24. Mustafa S, Joober R, Lepage M, Iyer S, Shah J, Malla A. Predictors of ‘all-cause discontinuation’ of initial oral antipsychotic medication in first episode psychosis. Schizophr Res. 2018;201:287–293. [DOI] [PubMed] [Google Scholar]
- 25. Thapa S, Bhusal K. Hyperprolactinemia. In: StatPearls. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 26. Bajorek T, Hafferty J. . Frequency of sexual dysfunction in patients with a psychotic disorder receiving antipsychotics. J Sex Med. 2010;7:3404–3413. [DOI] [PubMed] [Google Scholar]
- 27. Serretti A, Chiesa A. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol. 2011;26(3):130–140. [DOI] [PubMed] [Google Scholar]
- 28. Heald A. Physical health in schizophrenia: a challenge for antipsychotic therapy. Eur Psychiatry. 2010;25 Suppl 2:S6–11. [DOI] [PubMed] [Google Scholar]
- 29. Homan P, Kindler J, Hauf M, Hubl D, Dierks T. Cerebral blood flow identifies responders to transcranial magnetic stimulation in auditory verbal hallucinations. Transl Psychiatry. 2012;2:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cavelti M, Kircher T, Nagels A, Strik W, Homan P. . Neuroimaging of formal thought disorder in schizophrenia: a systematic review. Schizopr Res. 2018;2–16. [DOI] [PubMed] [Google Scholar]
- 31. Cavelti M, Winkelbeiner S, Federspiel A, et al. Formal thought disorder is related to aberrations in language-related white matter tracts in patients with schizophrenia. Psychiatry Res Neuroimaging. 2018;279:40–50. [DOI] [PubMed] [Google Scholar]
- 32. Winkelbeiner S, Cavelti M, Federspiel A, et al. Decreased blood flow in the right insula and middle temporal gyrus predicts negative formal thought disorder in schizophrenia. Schizophr Res. 2018;201:432–434. [DOI] [PubMed] [Google Scholar]
- 33. Cavelti M, Homan P, Vauth R. The impact of thought disorder on therapeutic alliance and personal recovery in schizophrenia and schizoaffective disorder: an exploratory study. Psychiatry Res. 2016;239:92–98. [DOI] [PubMed] [Google Scholar]
- 34. Funk MC, Beach SR, Bostwick JR, et al. QTc Prolongation and psychotropic medications. Am J Psychiatry. 2020;177(3):273–274. [DOI] [PubMed] [Google Scholar]
- 35. Glassman AH. Schizophrenia, antipsychotic drugs, and cardiovascular disease. J Clin Psychiatry. 2005;66 Suppl 6:5–10. [PubMed] [Google Scholar]
- 36. Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm. 2017;39(1):16–25. [DOI] [PubMed] [Google Scholar]
- 37. Koponen H, Alaräisänen A, Saari K, et al. Schizophrenia and sudden cardiac death: a review. Nord J Psychiatry. 2008;62(5):342–345. [DOI] [PubMed] [Google Scholar]
- 38. Tong Z, Li F, Ogawa Y, Watanabe N, Furukawa TA. Quality of randomized controlled trials of new generation antidepressants and antipsychotics identified in the China National Knowledge Infrastructure (CNKI): a literature and telephone interview study. BMC Med Res Methodol. 2018;18(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. [DOI] [PubMed] [Google Scholar]
- 40. Viechtbauer W. Conducting meta-analyses in R with the metafor package. Stat Software. 2010;36:1–48. [Google Scholar]
- 41. Litman RE, Smith MA, Doherty JJ, et al. AZD8529, a positive allosteric modulator at the mGluR2 receptor, does not improve symptoms in schizophrenia: a proof of principle study. Schizophr Res. 2016;172(1-3):152–157. [DOI] [PubMed] [Google Scholar]
- 42. Garcia E, Robert M, Peris F. . The efficacy and safety of blonanserin compared with haloperidol in acute-phase schizophrenia. CNS Drugs. 2009;23:615–625. [DOI] [PubMed] [Google Scholar]
- 43. Clark ML, Huber WK, Sullivan J, Wood F, Costiloe JP. Evaluation of loxapine succinate in chronic schizophrenia. Dis Nerv Syst. 1972;33(12):783–791. [PubMed] [Google Scholar]
- 44. Kahn RS, Schulz SC, Palazov VD, et al. ; Study 132 Investigators . Efficacy and tolerability of once-daily extended release quetiapine fumarate in acute schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):832–842. [DOI] [PubMed] [Google Scholar]
- 45. Ogasa M, Kimura T, Nakamura M, Guarino J. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl). 2013;225(3):519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res. 2007;93(1-3):117–130. [DOI] [PubMed] [Google Scholar]
- 47. Durgam S, Litman RE, Papadakis K, Li D, Németh G, Laszlovszky I. Cariprazine in the treatment of schizophrenia: a proof-of-concept trial. Int Clin Psychopharmacol. 2016;31(2):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coppola D, Melkote R, Lannie C, et al. Efficacy and safety of paliperidone extended release 1.5 mg/day-a double-blind, placebo- and active-controlled, study in the treatment of patients with Schizophrenia. Psychopharmacol Bull. 2011;44(2):54–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Landbloom R, Mackle M, Wu X, et al. Asenapine for the treatment of adults with an acute exacerbation of schizophrenia: results from a randomized, double-blind, fixed-dose, placebo-controlled trial with olanzapine as an active control. CNS Spectr. 2017;22(4):333–341. [DOI] [PubMed] [Google Scholar]
- 50. Kinon BJ, Zhang L, Millen BA, et al. ; HBBI Study Group . A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J Clin Psychopharmacol. 2011;31(3):349–355. [DOI] [PubMed] [Google Scholar]
- 51. van Kammen DP, McEvoy JP, Targum SD, Kardatzke D, Sebree TB. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology. 1996;124:168–175. [DOI] [PubMed] [Google Scholar]
- 52. Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42(4):233–246. [DOI] [PubMed] [Google Scholar]
- 53. Ishigooka J, Iwashita S, Tadori Y. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia in Japan: a 6-week, randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2018;72(9):692–700. [DOI] [PubMed] [Google Scholar]
- 54. Correll CU, Skuban A, Ouyang J, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870–880. [DOI] [PubMed] [Google Scholar]
- 55. Zborowski J, Schmitz P, Staser J. . Efficacy and safety of sertindole in a trial of schizophrenic patients. Biol Psychiatry. 1995;9:661–662. [Google Scholar]
- 56. Potkin SG, Litman RE, Torres R, Wolfgang CD. Efficacy of iloperidone in the treatment of schizophrenia: initial phase 3 studies. J Clin Psychopharmacol. 2008;28(2 Suppl 1):S4–11. [DOI] [PubMed] [Google Scholar]
- 57. Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(6):829–836. [DOI] [PubMed] [Google Scholar]
- 58. Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo-and risperidone-controlled trial. Journal of Clinical Psychiatry 2007;68:1492–1500. [DOI] [PubMed] [Google Scholar]
- 59. Keck P Jr, Buffenstein A, Ferguson J, et al. Ziprasidone 40 and 120 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 4-week placebo-controlled trial. Psychopharmacology (Berl). 1998;140(2):173–184. [DOI] [PubMed] [Google Scholar]
- 60. Cutler AJ, Tran-Johnson T, Kalali A, Aström M, Brecher M, Meulien D. A failed 6-week,randomized, double-blind, placebo-controlled study of once-daily extended release quetiapine fumarate in patients with acute schizophrenia: lessons learned. Psychopharmacol Bull. 2010;43(4):37–69. [PubMed] [Google Scholar]
- 61. Clark ML, Huber WK, Sakata K, Fowles DC, Serafetinides EA. Molindone in chronic schizophrenia. Clin Pharmacol Ther. 1970;11(5):680–688. [DOI] [PubMed] [Google Scholar]
- 62. Borison RL, Arvanitis LA, Miller BG. ICI 204,636, an atypical antipsychotic: efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. U.S. SEROQUEL Study Group. J Clin Psychopharmacol. 1996;16(2):158–169. [DOI] [PubMed] [Google Scholar]
- 63. Ahmed S, Casey DE, Yeung PP, et al. Lipid profile among patients with schizophrenia randomized to bifeprunox, placebo, or olanzapine: a comparison of results. Schizophrenia Bull. 2007;33:417–417. [Google Scholar]
- 64. Durgam S, Greenberg WM, Li D, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo-and active-controlled trial. J Clin Psychiatry. 2015;76:e1574–82. [DOI] [PubMed] [Google Scholar]
- 65. Meltzer H, Barbato L, Heisterberg J, Yeung P, Shapira N. A randomized, double-blind, placebo-controlled efficacy and safety study of bifeprunox as treatment for patients with acutely exacerbated schizophrenia. Schizophrenia Bull. 2007;33:446–446. [Google Scholar]
- 66. Meltzer HY, Arvanitis L, Bauer D, Rein W; Meta-Trial Study Group . Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975–984. [DOI] [PubMed] [Google Scholar]
- 67. Kane JM, Cohen M, Zhao J, Alphs L, Panagides J. Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30(2):106–115. [DOI] [PubMed] [Google Scholar]
- 68. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–771. [DOI] [PubMed] [Google Scholar]
- 69. Hirayasu Y, Tomioka M, Iizumi M, Kikuchi H. A double-blind, placebo-controlled, comparative study of paliperidone extended release (ER) tablets in patients with schizophrenia. Japanese J Psychopharmacol. 2010;13: 2077–2103. [Google Scholar]
- 70. Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20(5):491–505. [DOI] [PubMed] [Google Scholar]
- 71. Cooper SJ, Tweed J, Raniwalla J, Butler A, Welch C. A placebo-controlled comparison of zotepine versus chlorpromazine in patients with acute exacerbation of schizophrenia. Acta Psychiatr Scand. 2000;101(3):218–225. [PubMed] [Google Scholar]
- 72. Casey DE, Sands EE, Heisterberg J, Yang HM. Efficacy and safety of bifeprunox in patients with an acute exacerbation of schizophrenia: results from a randomized, double-blind, placebo-controlled, multicenter, dose-finding study. Psychopharmacology (Berl). 2008;200(3):317–331. [DOI] [PubMed] [Google Scholar]
- 73. Bugarski-Kirola D, Wang A, Abi-Saab D, Blättler T. A phase II/III trial of bitopertin monotherapy compared with placebo in patients with an acute exacerbation of schizophrenia–results from the CandleLyte study. Eur Neuropsychopharmacol. 2014;24:1024–1036. [DOI] [PubMed] [Google Scholar]
- 74. Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102–1107. [DOI] [PubMed] [Google Scholar]
- 75. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of Schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952–961. [DOI] [PubMed] [Google Scholar]
- 76. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681–690. [DOI] [PubMed] [Google Scholar]
- 77. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450–457. [DOI] [PubMed] [Google Scholar]
- 78. Nasrallah HA, Silva R, Phillips D, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47(5):670–677. [DOI] [PubMed] [Google Scholar]
- 79. Marder SR, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry. 2007;62(12):1363–1370. [DOI] [PubMed] [Google Scholar]
- 80. Borison RL, Pathiraja AP, Diamond BI, Meibach RC. Risperidone: clinical safety and efficacy in schizophrenia. Psychopharmacol Bull. 1992;28(2):213–218. [PubMed] [Google Scholar]
- 81. Shen JH, Zhao Y, Rosenzweig-Lipson S, et al. A 6-week randomized, double-blind, placebo-controlled, comparator referenced trial of vabicaserin in acute schizophrenia. J Psychiatr Res. 2014;53:14–22. [DOI] [PubMed] [Google Scholar]
- 82. Kinoshita T, Bai YM, Kim JH, Miyake M, Oshima N. Efficacy and safety of asenapine in Asian patients with an acute exacerbation of schizophrenia: a multicentre, randomized, double-blind, 6-week, placebo-controlled study. Psychopharmacology (Berl). 2016;233(14):2663–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. McEvoy JP, Daniel DG, Carson WH Jr, McQuade RD, Marcus RN. A randomized, double-blind, placebo-controlled, study of the efficacy and safety of aripiprazole 10, 15 or 20 mg/day for the treatment of patients with acute exacerbations of schizophrenia. J Psychiatr Res. 2007;41(11):895–905. [DOI] [PubMed] [Google Scholar]
- 84. Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res. 2007;90(1-3):147–161. [DOI] [PubMed] [Google Scholar]
- 85. Harvey PD., Loebel A., Cucchiaro J., et al. Is quality of life related to cognitive performance or negative symptoms in patients with schizophrenia? Results from a double-blind, active-controlled, lurasidone extension study. Neuropsychopharmacology. 2013;38:S515–S515. [Google Scholar]
- 86. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957–967. [DOI] [PubMed] [Google Scholar]
- 87. Canuso CM, Schooler N, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized, controlled study comparing a flexible dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30(5):487–495. [DOI] [PubMed] [Google Scholar]
- 88. Potkin SG, Kimura T, Guarino J. A 6-week, double-blind, placebo- and haloperidol-controlled, phase II study of lurasidone in patients with acute schizophrenia. Ther Adv Psychopharmacol. 2015;5(6):322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Beasley CM Jr, Sanger T, Satterlee W, Tollefson G, Tran P, Hamilton S. Olanzapine versus placebo: results of a double-blind, fixed-dose olanzapine trial. Psychopharmacology (Berl). 1996;124(1-2):159–167. [DOI] [PubMed] [Google Scholar]
- 90. Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo-controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71(5):587–598. [DOI] [PubMed] [Google Scholar]
- 91. Beasley CM Jr, Tollefson G, Tran P, Satterlee W, Sanger T, Hamilton S. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111–123. [DOI] [PubMed] [Google Scholar]
- 92. Loebel A, Silva R, Goldman R, et al. Lurasidone dose escalation in early nonresponding patients with Schizophrenia: a randomized, placebo-controlled study. J Clin Psychiatry. 2016;77(12):1672–1680. [DOI] [PubMed] [Google Scholar]
- 93. Litman RE, Smith MA, Desai DG, Simpson T, Sweitzer D, Kanes SJ. The selective neurokinin 3 antagonist AZD2624 does not improve symptoms or cognition in schizophrenia: a proof-of-principle study. J Clin Psychopharmacol. 2014;34(2):199–204. [DOI] [PubMed] [Google Scholar]
- 94. Kane JM, Skuban A, Hobart M, et al. Overview of short- and long-term tolerability and safety of brexpiprazole in patients with schizophrenia. Schizophr Res. 2016;174(1-3):93–98. [DOI] [PubMed] [Google Scholar]
- 95. Lindenmayer JP, Brown D, Liu S, Brecher M, Meulien D. The efficacy and tolerability of once-daily extended release quetiapine fumarate in hospitalized patients with acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled study. Psychopharmacol Bull. 2008;41(3):11–35. [PubMed] [Google Scholar]
- 96. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of Schizophrenia: results from an international, Phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367–373. [DOI] [PubMed] [Google Scholar]
- 97. Kane JM, Skuban A, Ouyang J, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164(1-3):127–135. [DOI] [PubMed] [Google Scholar]
- 98. Small JG, Hirsch SR, Arvanitis LA, Miller BG, Link CG. Quetiapine in patients with schizophrenia. A high- and low-dose double-blind comparison with placebo. Seroquel Study Group. Arch Gen Psychiatry. 1997;54(6):549–557. [DOI] [PubMed] [Google Scholar]
- 99. Schmidt ME, et al. A double-blind, randomized, placebo-controlled study with JNJ-37822681, a novel, highly selective, fast dissociating D2 receptor antagonist in the treatment of acute exacerbation of schizophrenia. Eur Neuropsychopharmacol. 2012;22:721–733. [DOI] [PubMed] [Google Scholar]
- 100. Geffen Y, Keefe R, Rabinowitz J, Anand R, Davidson M. Bl-1020, a new -aminobutyric acid-enhanced antipsychotic: Results of 6-week, randomized, double-blind, controlled, efficacy and safety study. J Clin Psychiatry. 2012;73:e1168–74. [DOI] [PubMed] [Google Scholar]
- 101. Zimbroff DL, Kane JM, Tamminga CA, et al. Controlled, dose-response study of sertindole and haloperidol in the treatment of schizophrenia. Sertindole Study Group. Am J Psychiatry. 1997;154(6):782–791. [DOI] [PubMed] [Google Scholar]
- 102. Center for Drug Evaluation and Research. A multicenter, randomized, double-blind, fixed-dose, 6-week trial of the efficacy and safety of asenapine compared with placebo using olanzapine positive control in subjects with an acute exacerbation of schizophrenia. Med Rev. 2009;22–117. [Google Scholar]
- 103. Center for Drug Evaluation and Research. A multicenter, randomized, double-blind, flexible-dose, 6-week trial of the efficacy and safety of asenapine compared with placebo using olanzapine positive control in subjects with an acute exacerbation of schizophrenia. Med Rev. 2009;23–117. [Google Scholar]
- 104. Center for Drug Evaluation and Research. Study 9420 2002. Med Rev. 2002;S9:21–436. [Google Scholar]
- 105. Center for Drug Evaluation and Research. Study 115 2000. Med Rev. 2000;S1:20–825. [Google Scholar]
- 106. Homan P, Kane JM. Clozapine as an early-stage treatment. Acta Psychiatr Scand. 2018;138(4):279–280. [DOI] [PubMed] [Google Scholar]
- 107. Osborn DP, Petersen I, Beckley N, Walters K, Nazareth I, Hayes J. Weight change over two years in people prescribed olanzapine, quetiapine and risperidone in UK primary care: Cohort study in THIN, a UK primary care database. J Psychopharmacol. 2018;32(10):1098–1103. [DOI] [PubMed] [Google Scholar]
- 108. Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, et al. Antipsychotic-induced body weight gain: predictors and a systematic categorization of the long-term weight course. J Psychiatr Res. 2009;43(6):620–626. [DOI] [PubMed] [Google Scholar]
- 109. Najar H, Joas E, Kardell M, Pålsson E, Landén M. Weight gain with add-on second-generation antipsychotics in bipolar disorder: a naturalistic study. Acta Psychiatr Scand. 2017;135(6):606–611. [DOI] [PubMed] [Google Scholar]
- 110. Leucht S, Tardy M, Komossa K., et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2012;Nr5:CD008016. [DOI] [PubMed] [Google Scholar]
- 111. Homan P, Schooler NR, Brunette MF, et al. Relapse prevention through health technology program reduces hospitalization in schizophrenia. bioRxiv. 2019.. doi: 10.1101/626663 [DOI] [PubMed] [Google Scholar]
- 112. Nielsen MØ, Rostrup E, Wulff S, Glenthøj B, Ebdrup BH. Striatal reward activity and antipsychotic-associated weight change in patients with schizophrenia undergoing initial treatment. JAMA Psychiatry. 2016;73(2):121–128. [DOI] [PubMed] [Google Scholar]
- 113. Inder WJ., Castle D. Antipsychotic-Induced Hyperpro lactinaemia. Austr N Z J Psychiatry. 2011;45(10):830-837. [DOI] [PubMed] [Google Scholar]
- 114. Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22(6):724–763. [DOI] [PubMed] [Google Scholar]
- 115. Bushe CJ, Bradley A, Pendlebury J. A review of hyperprolactinaemia and severe mental illness: are there implications for clinical biochemistry? Ann Clin Biochem. 2010;47(Pt 4):292–300. [DOI] [PubMed] [Google Scholar]
- 116. Mazziotti G, Frara S, Giustina A. Pituitary diseases and bone. Endocr Rev. 2018;39(4):440–488. [DOI] [PubMed] [Google Scholar]
- 117. Byerly M, Suppes T, Tran QV, Baker RA. Clinical implications of antipsychotic-induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorders: recent developments and current perspectives. J Clin Psychopharmacol. 2007;27(6):639–661. [DOI] [PubMed] [Google Scholar]
- 118. Johnston AN, Bu W, Hein S, et al. Hyperprolactinemia-inducing antipsychotics increase breast cancer risk by activating JAK-STAT5 in precancerous lesions. Breast Cancer Res. 2018;20(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. George A, Sturgeon SR, Hankinson SE, Shadyab AH, Wallace RB, Reeves KW. Psychotropic medication use and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2020;29(1):254–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Veselinović T, Schorn H, Vernaleken IB, Schiffl K, Klomp M, Gründer G. Impact of different antidopaminergic mechanisms on the dopaminergic control of prolactin secretion. J Clin Psychopharmacol. 2011;31(2):214–220. [DOI] [PubMed] [Google Scholar]
- 121. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010;24(7):1011–1018. [DOI] [PubMed] [Google Scholar]
- 122. Riordan HJ, Antonini P, Murphy MF. Atypical antipsychotics and metabolic syndrome in patients with schizophrenia: risk factors, monitoring, and healthcare implications. Am Health Drug Benefits. 2011;4(5):292–302. [PMC free article] [PubMed] [Google Scholar]
- 123. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bazett HC. An analysis of the time-relations of electrocardiograms. Ann Noninvasive Electrocardiol. 1997;2:177–194. [Google Scholar]
- 125. Glassman AH, Bigger JT Jr. Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001;158(11):1774–1782. [DOI] [PubMed] [Google Scholar]
- 126. Nielsen J, Graff C, Kanters JK, Toft E, Taylor D, Meyer JM. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473–490. [DOI] [PubMed] [Google Scholar]
- 127. Spellmann I, Reinhard MA, Veverka D, et al. QTc prolongation in short-term treatment of schizophrenia patients: effects of different antipsychotics and genetic factors. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):383–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The analysis was performed from September 2019 to May 2020, using the R package metafor40 (version 2.4.0). The manuscript was produced with the R packages rmarkdown (version 2.6); represearch (version 0.0.0.9000; https://github.com/phoman/represearch/); knitr (version 1.30); and papaja (version 0.1.0.9997). All data and code are freely available online to ensure reproducibility at https://github.com/homanlab/sideeffects/.