Abstract
Psychosis, characterized by hallucinations and delusions, is a common feature of psychiatric disease, especially schizophrenia. One prominent theory posits that psychosis is driven by abnormal sensorimotor predictions leading to the misattribution of self-related events. This misattribution has been linked to passivity experiences (PE), such as loss of agency and, more recently, to presence hallucinations (PH), defined as the conscious experience of the presence of an alien agent while no person is actually present. PH has been observed in schizophrenia, Parkinson’s disease, and neurological patients with brain lesions and, recently, the brain mechanisms of PH (PH-network) have been determined comprising bilateral posterior middle temporal gyrus (pMTG), inferior frontal gyrus (IFG), and ventral premotor cortex (vPMC). Given that the experience of an alien agent is a common feature of PE, we here analyzed the functional connectivity within the PH-network in psychotic patients with (N = 39) vs without PE (N = 26). We observed reduced fronto-temporal functional connectivity in patients with PE compared to patients without PE between the right pMTG and the right and left IFG of the PH-network. Moreover, when seeding from these altered regions, we observed specific alterations with brain regions commonly linked to auditory-verbal hallucinations (such as Heschl’s gyrus). The present connectivity findings within the PH-network extend the disconnection hypothesis for hallucinations to the specific case of PH and associates the PH-network with key brain regions for frequent psychotic symptoms such as auditory-verbal hallucinations, showing that PH are relevant to the study of the brain mechanisms of psychosis and PE.
Keywords: psychosis, hallucinations, functional connectivity, disconnection, presence hallucination network, resting-state fMRI
Introduction
Psychosis is an abnormal mental state including hallucinations and delusions that characterize psychiatric conditions such as schizophrenia.1 It is broadly defined as a loss of contact with reality and may include abnormal sensations such as somatic passivity, thought withdrawal, thought insertion, delusions of being control, or auditory-verbal hallucinations (AVH), when in the form of voices commenting, conversing and audible thoughts. These specific psychotic symptoms, formerly known as Schneiderian first-rank symptoms,2,3 have been termed passivity experiences (PE),4 arguably reflecting a failure to self-attribute one’s perceptions, thoughts, actions, or emotions.5,6 Related clinical research further suggested that PE and psychosis may be associated with impairments in self-monitoring,5–8 an essential aspect of sensorimotor functioning, based on sensorimotor and predictive mechanisms (ie, prediction of sensory consequences related to self-generated actions).9–11 Disturbances in self-monitoring have been argued to reflect a diminished demarcation of the self-other boundary and often consist of at least 2 co-occurring, but phenomenally distinct aspects of positive symptoms. A reductive aspect is characterized by a loss in subjective experience, such as a loss of self-attribution (ie, sense of agency or ownership); an additive aspect is characterized by the appearance of foreign or alien elements in subjective experience (ie, alien voices in AVH or alien thoughts in thought insertion). This classification, albeit being a phenomenological oversimplification, has recently been useful for the experimental investigation of thought insertion.12 Thought insertion, is characterized by the experience that certain thoughts, occurring in one’s mind, are not one’s own thoughts (reductive aspect; loss of thought agency; or thought ownership).13,14 However, the lack of thought agency is not sufficient to account for thought insertion, because patients with thought insertion not only experience that their thoughts are not their own, but those of another alien person (additive aspect).15–18 The additive positive element is therefore also important, because lack of self-attribution (in thought awareness) also occurs in healthy subjects (as is the case of unbidden thoughts14,19), whereas the additive aspect of thought insertion does not. A similar phenomenological dichotomy applies to AVH and other PE.
The large majority of previous neuroscience research of psychosis has targeted the first, reductive element of PE, characterized by lack or decreases of self-attribution,20–23 with very few studies on the second additive element of PE.4,24 Here we sought to target the second, additive, aspect of PE. Based on recent findings including behavioral, clinical, and imaging work4,25–27 it has been suggested that a particular complex symptom, presence hallucination (PH), and the associated neural system may be of relevance for psychosis, and especially the additive element of PE. PH is frequently observed in patients with schizophrenia28 and is defined as the vivid sensation that another alien person is nearby when no one is actually present and can neither be seen or heard. PH has recently been linked to the disturbances in self-monitoring and sensorimotor processing4,25,26 related to bodily self-consciousness.25,29,30 Although PH occurs in around 50% of patients with schizophrenia, it is often overlooked in psychiatric patients28 and it is accordingly not known how PH relates to PE in psychotic patients. However, PH is very frequent in patients with neurodegenerative disease suffering from hallucinations such as Parkinson’s disease26,31 and dementia with Lewy bodies.32,33 PH has also been studied in neurological patients suffering from focal brain damage.31,34,35 Importantly, it has also been demonstrated that PH and somatic passivity, mental states that phenomenologically resemble PE, can be experimentally induced in healthy participants.25 This controlled induction of a mild PE-like mental state was induced by robotically controlled sensorimotor stimulation, showing that PH are caused by the misperception of the source and the identity of sensorimotor signals of one’s own body. The cortical network of neurological and robot-induced PH have been investigated recently by Bernasconi, Blondiaux et al26 using lesion network mapping36 and MRI-compatible robotics.37 This led to the definition of a PH-network, consisting of bilateral inferior frontal gyrus, posterior middle temporal gyrus, and ventral premotor cortex that was impaired in Parkinson’s disease patients with symptomatic PH.26 However, it is currently unknown whether the PH-network is impaired in psychotic patients with PE.
In the present study, we studied whether functional connectivity in the PH-network differed between a group of psychotic patients with symptomatic PE vs a group of psychotic patients without PE, allowing us to distinguish possible symptom-specific neural activities from disease-related effects. Based on the disconnection hypothesis in schizophrenia38–41 we hypothesized, first, to observe decreased functional connectivity within the PH-network, especially of fronto-temporal connections, based on previous findings linking impaired sensorimotor integration in schizophrenia.42–44 Second, using the whole-brain analysis we investigated whether the affected PH-network areas would be characterized by abnormal functional connectivity, especially with areas previously shown to be involved in PE.
Methods
Participants
Sixty-five psychotic patients were included in this study. Part of the sample of patients (N = 23) was recruited from the outpatient clinic of the department of psychiatry, Lausanne University Hospitals, Switzerland, and met DSM-IV criteria for schizophrenia or schizoaffective disorder.45 Another part of the patients (N = 42), who met threshold criteria for psychosis, as defined by the “Psychosis threshold” subscale of the Comprehensive Assessment of At-Risk Mental States, were recruited from the TIPP Program (Treatment and Early Intervention in Psychosis Program, University Hospital, Lausanne, Switzerland).46 Neurological disorders and severe head trauma were exclusion criteria for all patients. Informed written consent in accordance with institutional guidelines (protocol approved by the Cantonal Ethics Commission of Vaud, Switzerland) was obtained for all subjects.
Patients underwent an in-depth clinical assessment by a trained psychiatrist where the frequency and severity of symptoms were evaluated. Symptom severity was assessed in the patient groups using the Positive and Negative Syndrome Scale (PANSS).47 PE were assessed based on the specific items of the Scale for the Assessment of Positive Symptoms (SAPS) (item 2: voices commenting; item 3: voices conversing; item 15: delusions of being controlled; item 17: thought broadcasting; item 18: thought insertion; item 19: thought withdrawal),4,48–51 as well as somatic passivity, audible thought and delusional perception (see supplementary material for a description of each symptoms). Patients were considered PE+ if they had presented at least one of these experiences (N = 39, 60% of tested patients; supplementary table S1) during the psychotic episodes. Twenty-six patients (40% of tested patients) did never show these symptoms and were thus included in the PE− group. The patient groups did not differ significantly on any demographic trait (see table 1 for the details).
Table 1.
Demographic and Clinical Data of the Patients
| Characteristic/Test | PE− | PE+ | P(t/χ 2) |
|---|---|---|---|
| Group size | 26 | 39 | |
| Gender, M/F | 20/6 | 23/16 | 0.1 (2.2) |
| Handedness, R/L | 23/3 | 34/3 | 0.4 (1.5) |
| Age, y | 29.9 ± 10 | 30.5 ± 9.7 | 0.9 (−0.08) |
| Education, y | 12.9 ± 2.5 | 12.3 ± 3.5 | 0.6 (0.55) |
| Illness duration, y | 6.2 ± 7.7 | 5.2 ± 5.1 | 0.6 (0.6) |
| Chlorpromazine, mg/d | 275 ± 247 | 365 ± 262 | 0.2 (−1.4) |
| PANSS total | 65.5 ± 17.3 | 59.8 ± 16 | 0.2 (1.3) |
| PANSS positive | 14 ± 4.9 | 13.5 ± 4.3 | 0.6 (0.4) |
| PANSS negative | 17.5 ± 6 | 14.6 ± 5.8 | 0.06 (1.9) |
| Time difference between fMRI acquisition and symptom assessment, y | 2.6 ± 2.1 | 2.7 ± 2.3 | 0.8 (−0.26) |
| Recruitment cohort (TIPP/Outpatient) | 17/9 | 25/14 | 0.9 (0.01) |
Note: Data are presented in mean ± SD. Two-tailed t-tests and χ 2 tests performed when appropriate. M, male; F, female; R, right-handed; L, left-handed; PANSS, positive and negative syndrome scale; PE, passivity experiences.
MRI was performed at the time of the patient’s inclusion in the clinical program at Lausanne University Hospital. Therefore, the duration of the interval between the fMRI recording and the clinical assessment could vary between subjects. The mean time of this interval was 2.7 ± 2.2 years for all the participants (2.7 ± 2.3 year for the PE+ group and 2.6 ± 2.1 year for the PE− group) and did not differ between the groups (t = −0.26, P = .8).
MR Image Acquisition
MRI data were acquired using a 3 Tesla scanner (Magnetom TrioTim, Siemens Medical Solutions), equipped with a 32-channel head coil. Each MRI session included a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence and a 9-minute gradient echo-planar imaging (EPI) sequence sensitive to BOLD (blood-oxygen-level-dependent) contrast. The MPRAGE acquisition had a 1mm in-plane resolution and 1.2 mm slice thickness, covering 240 × 257 × 160 voxels (TR = 2.30 ms, TE = 2.98 ms, and TI = 900 ms). The functional MRI (EPI) acquisition had isotropic 3.3mm voxel size, with a 0.3mm inter-slice gap and covering a total of 64 × 58 × 32 voxels (TR = 1920 ms and TE = 30 ms). Resting-state fMRI (rs-fMRI) was recorded; patients were instructed to lay quietly in the scanner with eyes open without focusing on any specific thought. The rs-fMRI sequence was performed at the beginning of the session, immediately after the structural scan acquisition and an experienced psychologist accompanied all patients during scanning. The acquisition process resulted in a sequence of 280 BOLD images for each participant.
Image Preprocessing
Standard preprocessing and data analyses were performed using SPM12 (fil.ion.ucl.ac.uk/spm/) and the functional connectivity toolbox CONN (conn-toolbox.org/) for MATLAB (mathworks.com). Functional images were corrected for slice time and motion, co-registered with a high-resolution anatomical scan, normalized into MNI space, resampled to 1.5 × 1.5 × 1.5 mm3 and smoothed with a 6 mm3 full-width at half maximum (FWHM) Gaussian kernel for each subject. To estimate the excessive movement, mean frame-wise displacement (FD)52 during the scanning was estimated with the exclusion threshold of 0.5 mm. The groups did not differ in terms of the movements over the scanning period (t = −0.35, P = .7 with the mean FD of 0.18 ± 0.09 mm and 0.19 ± 0.1 mm for PE− and PE+ groups, respectively). The standard pipeline for confound removal of the CONN toolbox implementing a component-based noise correction procedure (CompCor) was followed.53 Confounding effects of the individual time courses of the segmented white matter and cerebrospinal fluid, session effect of constant and linear BOLD signal trends, the 6 motion parameters with rigid body transformations and their first-order derivatives were extracted and regressed out of the data. Regressions were performed for the entire time-series. The BOLD signal data were passed through a band filter (0.009–0.08 Hz). A whole-brain mask in MNI space was used to restrict number of voxels tested during data analysis.
Networks
Presence Hallucination Network
The presence hallucination network (PH-network; figure 1) was established in an extensive previous investigation by Bernasconi, Blondiaux et al26, by combining data from neurological patients with symptomatic PH and data from healthy subjects, in whom PH was experimentally induced during fMRI acquisition. The PH-network was defined as the overlap of the brain regions associated with experimentally induced PH (healthy subjects) and symptomatic PH (neurological patients).26 The PH-network consisted of the bilateral posterior middle temporal gyri (pMTG; x = ±54, y = −54, z = 0), inferior frontal gyri (IFG; x = ±51, y = 18, z = 29) and ventral premotor cortices (vPMC; x = ±26, y = −18, z = 57).
Fig. 1.
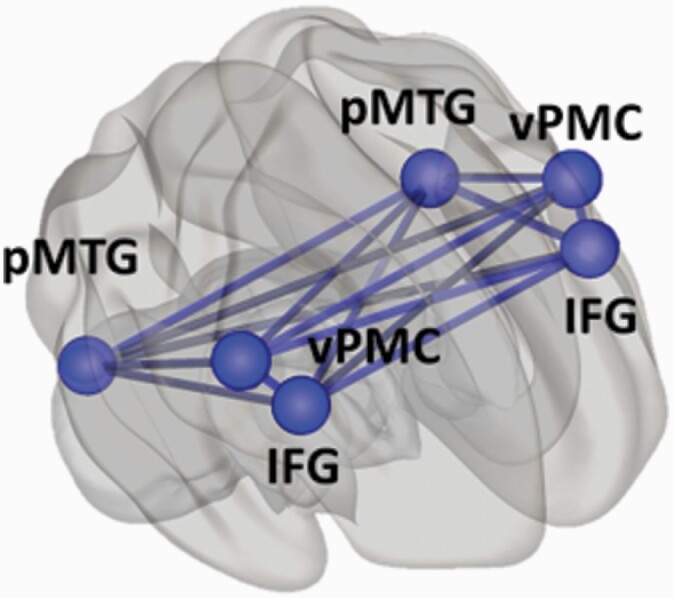
PH-network. Projection on the brain surface of 6 regions forming PH-network: bilaterally inferior frontal gyrus (IFG), posterior middle temporal gyrus (pMTG), ventral premotor cortex (vPMC). The network forms 15 connections (lines). Based on Bernasconi, Blondiaux et al26.
Control Networks
Control regions were derived by shifting each region of the PH-network but keeping the same shape and the same number of voxels as the original network26 (supplementary figure S1A). The areas were shifted to fit in the brain mask and do not comprise of white matter. The areas were shifted by the following coordinates: IFG x ± 20 y + 30 z − 15; vPMC x ± 10 y + 30 z − 15; pMTG x y + 30 z − 15. A visual network from rs-fMRI network atlas54 (supplementary figure S1B) was analyzed as an additional control network. It was comprised of 4 regions of interest (ROIs): calcarine sulcus, left thalamus, left and right middle/ superior occipital gyri.
Statistical Analyses
Demographic and Clinical Variables
Possible differences in demographic and clinical variables data between the 2 groups of patients were assessed by unpaired t-tests and Pearson’s χ 2 tests, when applicable. Correlation analyses between the PANSS scores and functional connectivity of the PH-network connections were performed (supplementary material).
fMRI. Functional connectivity ROI-to-ROI analyses were conducted by extracting bivariate correlation values (z-transformed) for all possible connections within PH-network for each subject. Statistical analyses were performed in R (R-project.org/), v3.6. To investigate possible alteration in functional connectivity between groups of patients, we used linear mixed-effects models with Group (PE+, PE−) and Connection (15 connections) as fixed effects and Patients as a random effect. Post-hoc analyses for the between-group differences were performed with FDR (P = .05) correction for multiple comparisons. Data outliers (6% of all data points) were removed based on 1.5IQR from the functional connectivity mean value for each connection. Patient’s age and dose of medication were included in the analysis as covariates due to their considerable variance between the patients, which can influence functional coupling.55,56 In addition, PANSS positive and negative sub-scores, recruitment cohort (TIPP, outpatient), and the time difference between fMRI image acquisition and symptom evaluation were included in the model as covariates. Additional control analyses were performed by splitting the PE+ group according to the presence (PE+AVH+) or absence (PE+AVH-) of AVH to investigate whether the PH-network alterations were driven by AVH or by PE in general (supplementary material).
To further study functional connectivity associated with PH-network, we performed seed-to-whole-brain analysis. The ROIs from the PH-network, which connections showed significant functional connectivity differences between groups, were used as seeds. Individual correlation maps were created by extracting the mean resting-state BOLD time course from the seed region and correlating it with the time course of each voxel in the whole brain. Subsequently, correlation coefficients were normalized using Fisher-z-transformation to create individual single-subject maps of voxel-wise functional connectivity. The resulting maps were then entered in a second-level analysis. T-contrasts for group comparisons with P < .001 peak voxel-level uncorrected and cluster level FDR P < .05 corrected thresholds were analyzed. Age and medication dose of the patients were included as the covariates to control for possible confounds in functional brain coupling.
Spatial overlap. Spatial overlap analysis between the PH-network and language, auditory and default mode (DMN) networks (defined from rs-fMRI network atlas54) was performed. See more details in supplementary material.
Results
Functional Disconnection Within the PH-Network
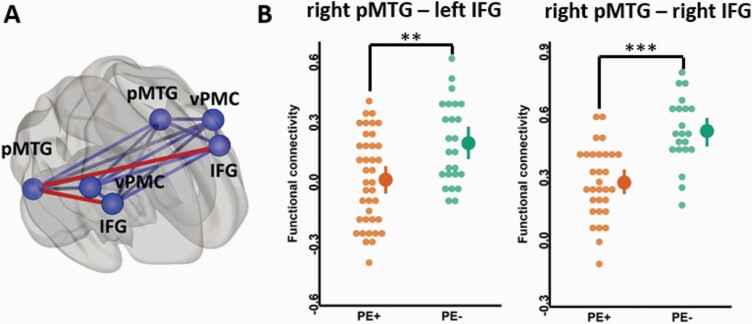
We observed a significant interaction between patient group and connection (F(14,786) = 3.4, P < .0001), suggesting that specific connections of the PH-network are associated with PE. Yet, there was no significant main effect of patient group, meaning that there was no global difference of PH-network functional connectivity between groups (PE+ patients rPH_total = .21 ± 0.26, CI(95%) = [0.19, 0.23], PE− patients rPH_total = .24 ± .28, CI(95%) = [.22, .27], F(1,56) = 2.4, P = .12). Post-hoc analysis showed that fronto-temporal connections, between the right pMTG and the right IFG (rPE+ = .235 ± .172, CI(95%) = [0.17, 0.29]; rPE− = .481 ± .165, CI(95%) = [0.40, 0.56]; t = −4.1, PFDR = .0007) and between the right pMTG and the left IFG (rPE+ = −.029 ± .223, CI(95%) = [−0.10, 0.04]; rPE− = .152 ± .206, CI(95%) = [0.07, 0.24]; t = −3.49, PFDR = .004) (figure 2B) were reduced in PE+ compared to PE− patients. None of the other connections differed between groups. There also was an expected significant main effect of connection (F(14,786) = 52.4, P < .0001), meaning that the strength of functional connectivity varied between the different connections of the PH-network independent of group.57 The covariates (age, medication dose, PANSS positive, PANSS negative, recruitment cohort and time difference between scan acquisition and symptom evaluation) did not show significant effects (all P > .25, see supplementary material for the details). No significant correlations were observed between the PH-network right pMTG - right IFG, right pMTG - left IFG functional connectivity and PANSS positive, and negative scores (all P ≥ .1, supplementary table S4).
Fig. 2.
Functional disconnectivity within the PH- network comparing patients with (PE+) and without (PE−) passivity experiences. (A) Abnormal fronto-temporal connections are marked in red and were decreased in PE+ patients vs PE− patients. (B) Functional connectivity between the right posterior middle temporal gyrus (pMTG) and the left inferior frontal gyrus (IFG; left plot), and the pMTG and the right IFG (right plot). Post-hoc FDR corrected at the threshold of P = .05. **P < .01, ***P < .001. Dots represent the individual connectivity values of each patient.
The same analyses in control regions (main effect of group F(1,52) = 0.12, P = .73; group by connection interaction F(14,766) = 1.4 P = .14; supplementary figure S3) and in a visual control network (main effect of group F(1,51) = 0.33, P = .56; group by connection interaction F(5,267) = 1.16, P = .32; supplementary figure S4) revealed no significant differences between the 2 groups.
We further show that the fronto-temporal disconnection of the PH-network is driven by PE in general and not only by the presence of AVH (all P ≥ .1) in PE + group (supplementary table S3, supplementary figure S5; more details in supplementary material).
Overlap analysis revealed that auditory and DMN networks do not overlap with the PH-network, whereas the language network overlapped in the left IFG and bilateral pMTG of the PH-network (supplementary figure S6).
Extended PH-Network Functional Connectivity Changes
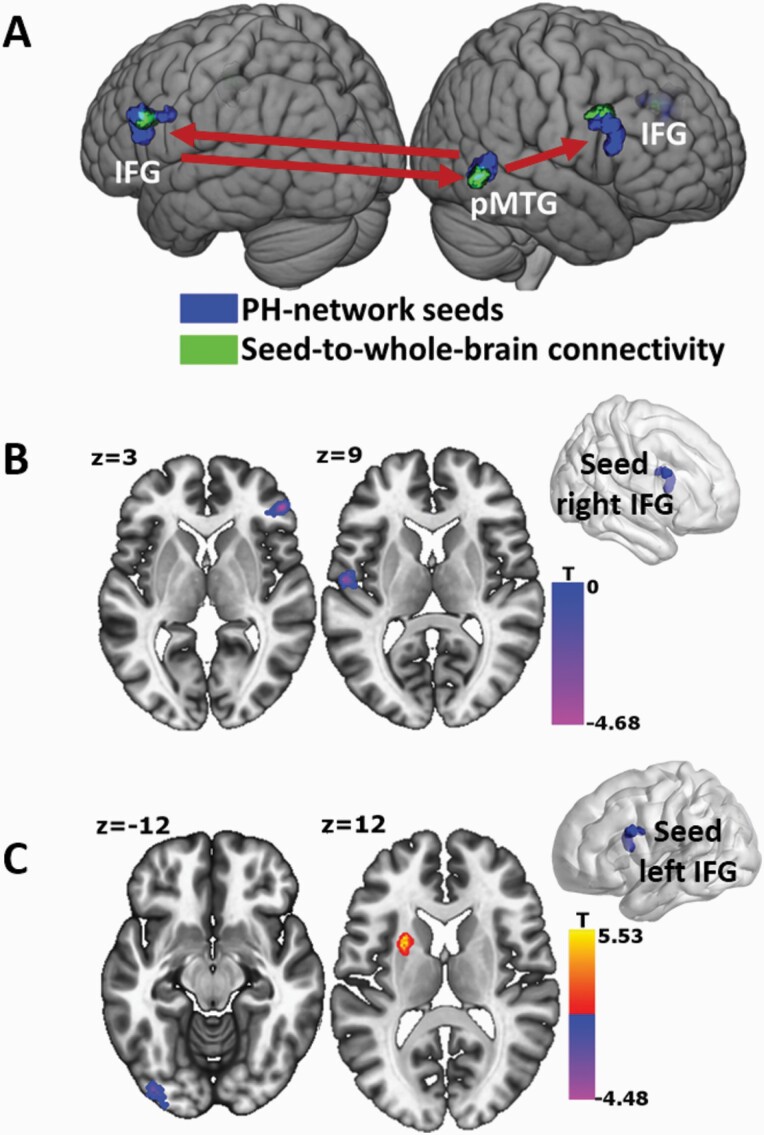
To investigate whether there are any global changes in connectivity associated with the PH-network that differed between groups, we conducted seed-based analyses from the 3 nodes forming the 2 altered connections in the PH-network indicated by the previous connectivity analysis. These 3 regions used as seeds were right pMTG, left IFG, and right IFG. With the right pMTG as seed, we observed a statistically significant decrease in functional connectivity between the right middle frontal gyrus and the left inferior frontal gyrus in the PE+ group compared to the PE− group. Both areas partly overlapped with the ROIs of the PH-network (108 voxels with the left IFG, 2 voxels with the right IFG; figure 3A). Using the right IFG as a seed, we observed a statistically significant decrease in functional coupling with the right medial superior frontal gyrus and the left Heschl’s gyrus (superior temporal gyrus) in the PE+ group vs PE− group (figure 3B). Seeding from left IFG revealed a statistically significant increase in functional coupling with the left putamen and decrease in functional coupling with the left lateral occipital cortex (inferior occipital gyrus), and the right middle temporal gyrus in the PE+ vs PE− group (figure 3C). We note that the cluster in the right middle temporal gyrus was part of the PH-network area (59 voxels overlap with the right pMTG; figure 3A). The details of all clusters are described in table 2.
Fig. 3.
Functional connectivity changes in PE+ vs PE− patients seeding from the PH-network areas to the whole brain. (A) PH-network seeds (in blue) were traced back during whole-brain functional connectivity analysis (areas in green). The red arrows represent from which seed (blue) the decreased functional connectivity in PE+ patients were observed. (B) Decreased functional connectivity in PE+ patients seeding from the right IFG with the right medial superior frontal gyrus and the left Heschl’s gyrus. (C) Decreased functional connectivity in PE+ patients seeding from the left IFG with the left inferior occipital gyrus and increased functional connectivity with the left putamen. Voxel level P < .001 uncorrected, cluster threshold at P < .05 FDR corrected.
Table 2.
Details of the Clusters From Seed-to-Whole-Brain Functional Connectivity Analysis
| Seed-to-Whole-Brain PE+ vs PE− | MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|---|
| BA | Anatomic Label | k (vox) | x | y | z | β | T | |
| right pMTG | ||||||||
| 9 | Middle frontal gyrus | R | 189 | 35 | 06 | 32 | −0.22 | −4.58 |
| 9 | Inferior frontal gyrus | L | 161 | −53 | 17 | 27 | −0.22 | −4.47 |
| right IFG | ||||||||
| 8 | Medial superior frontal gyrus (frontal pole) | R | 137 | 48 | 39 | 3 | −0.22 | −4.64 |
| 43, 22 | Heschl’s gyrus (part of superior temporal gyrus) | L | 149 | −57 | −11 | 9 | −0.17 | −4.06 |
| left IFG | ||||||||
| Putamen | L | 178 | −24 | 08 | 14 | 0.14 | 5.34 | |
| 18 | Lateral occipital cortex, inferior division (inferior occipital gyrus) | L | 211 | −39 | −87 | −12 | −0.16 | −4.18 |
| 21, 37 | Middle temporal gyrus, temporooccipital part | R | 147 | 63 | −53 | −3 | −0.19 | −4.13 |
Note: BA, Broadman area; k, cluster size in voxels; PE, passivity experiences. Voxel level P < .001 uncorrected, cluster threshold at P < .05 FDR-corrected.
Discussion
Investigating functional connectivity changes within the PH-network in psychotic patients with and without PE, we observed an alteration of fronto-temporal functional connectivity within the PH-network that was characterized by decreased connectivity in PE+ compared to PE− patients. Additional results showed that this connectivity pattern is region-specific and limited to the PH-network as analysis of 2 control networks did not reveal any significant group differences. These data were corroborated and extended by seed-to-the-whole-brain connectivity, allowing us to trace back the fronto-temporal connectivity decreases in the PH-network without any a priori regional restriction of the connections.
Altered resting-state functional connectivity in psychotic patients has been observed in numerous studies (for review see41,58–61) and it has been proposed that psychotic symptoms are related to decreases in functional connectivity formulated, eg, in the disconnection hypothesis.40,41,62,63 Our findings are compatible with and extend this proposal, as we observed reduced connectivity in PE+ patients that was restricted to the PH-network and concerned fronto-temporal connectivity within this network. Interestingly, fronto-temporal disconnectivity has previously been associated with impaired sensorimotor integration in patients with schizophrenia.42–44 As PE4–6 and PH4,25 have also been associated with sensorimotor and prediction mechanisms, our finding of fronto-temporal disconnectivity in the PH-network in PE patients associates the brain mechanism of PH with those of PE and psychosis. Fronto-temporal disconnection of the PH-network in psychotic patients extends recent work investigating symptomatic PH in other patient populations, such as those suffering from Parkinson’s disease26 and dementia with Lewy bodies,32 2 neurological neurodegenerative diseases that are frequently associated with hallucinations and psychosis. Thus, Parkinson’s disease patients with symptomatic PH had decreased functional fronto-temporal connectivity (between the left pMTG and left IFG)26 compared to Parkinson’s disease patients without PH. Moreover, PH-network functional connectivity could be used to predict the occurrence of PH as a symptom during daily life and these patients were found to have abnormal elevated sensitivity to sensorimotor stimulation.26 A PET study in dementia with Lewy bodies patients32 (who frequently experience PH) reported reduced glucose metabolism in fronto-temporal cortical areas (in patients with vs without PH), partly overlapping with the altered fronto-temporal connections in the present study. Taken together, the present findings suggest that fronto-temporal connectivity changes are not only of relevance to study PH, their neural mechanisms and disruption in neuropsychiatric diseases, but are also disrupted in psychiatric patients with psychosis suffering from a larger range of PE, extending the disconnection hypothesis to PH.42–44,64
Prominent neurological work classified PH (following focal brain damage) consistently among disorders of the body schema and the bodily self (such as autoscopic phenomena).65–71 The felt presence is generally experienced as another person, but some patients have also reported to feel the presence of a second own body next to them.70,72,73 Moreover, some patients with PH may also report autoscopic phenomena such as heautoscopy or out-of-body experiences and other alterations of the body schema (for a detailed discussion of PH and its link to disorders of the bodily self see).70,74 More recent studies corroborated these findings, inducing PH repeatedly by electrical stimulation of a cortical region involved in sensorimotor processing34 and linking PH to the misperception of the source and identity of sensorimotor signals in healthy participants12,25,27 and patients with symptomatic PH.4,26 The present findings associate the common symptom cluster of PE to neural mechanisms of PH, thereby providing further evidence for the prominent and long-standing proposal that a disturbed sense of self is of paramount importance in psychosis and schizophrenia75–77; for recent discussion see.78–80 More recent work in schizophrenia reported behavioral alterations in bodily perception and experience, thereby providing empirical evidence that disturbances of body schema and self are impaired in psychotic patients,78,81 also suggested by the present findings.
One of the most frequent PE in psychosis is AVH,28 often in the form of voices arguing and commenting about the patient. Among the many accounts for AVHs,61,82,83 a prominent one, that is of relevance for the present work on psychosis and PH, has argued that AVH are caused by altered self-monitoring associating loss of self-attribution (inner speech is not perceived as self-generated; negative aspect) with misattribution to an external agent (inner speech perceived as that of another person; additive aspect).84,85 At the neural level AVH have been associated with altered resting-state functional connectivity of the auditory, language and default mode (DMN) networks.86–88 Thus, how are AVH related to PH and to the neural mechanisms of PH? It could be argued that PH and the related PE are caused by brain mechanisms reflecting altered social processes, because PH has been linked not only to sensorimotor mechanisms and alterations of body schema,4,25,26 but also to social brain mechanisms.31 Concerning psychosis, such social mechanisms have also been underlined as an important pathomechanism, based on reports that AVH can be accompanied by co-perceived felt presences or social agents.89,90 Future work should carefully investigate the shared and distinct mechanisms between social and sensorimotor accounts of PH and their importance in psychosis. Concerning the overlap with other functional accounts of PH and psychosis, our analysis revealed no spatial overlap between the PH-network and the auditory network and the DMN, which both have been linked to psychosis.88 We found minimal overlap with a language network previously linked to psychosis, suggesting possible links between PH and language networks commonly linked to AVH.88,91 However, the observed PH-network fronto-temporal connectivity differences were driven by the PE in general, rather than by the occurrence of AVH specifically as further analysis has revealed (supplementary figure S5, supplementary table S3, see more in supplementary material). Further work is needed to directly investigate potential behavioral and neural overlaps between language and PH-related networks in psychosis.
Recent studies were able to link PH and mild psychotic states to altered sensorimotor processing and changes in voice perception. This was found in first-episode psychosis patients4 as well as healthy subjects.27 Thus, when exposed to specific sensorimotor conflicts giving rise to PH, first-episode psychosis patients (with PE) misattributed their voice to an external agent. These misattribution errors were absent in patients without PE, showing that sensorimotor stimulations leading to a mental state mimicking PH is associated with alterations in auditory-verbal self-monitoring4 and, potentially, with the PH-network.
Our findings, through applying seed-to-whole-brain analysis, provide further evidence that PH, related sensorimotor mechanisms, and the PH-network are of importance in some forms of AVH (voices conversing, commenting, audible thoughts). When the affected PH-network areas were used as seeds to the whole-brain, we observed altered functional connectivity in key auditory regions. More specifically, we link the PH to auditory processing in psychosis patients by revealing altered functional connectivity between the PH-network and Heschl’s gyrus. We found decreased functional connectivity (PE+ vs PE− group) between the right IFG and left Heschl’s gyrus that is part of the primary auditory cortex and has frequently been reported to have altered functional connectivity in psychotic patients with AVH.63,92,93 Moreover, we observed increased functional connectivity between the left IFG and the left putamen in PE+ patients, a subcortical structure that has also been linked to AVH.94–96 AVH is one of the most common symptoms in psychotic patients28 and indeed, in this study, one of the inclusion criteria for the PE+ group was the presence of AVH (characterized by voices conversing, commenting, or audible thoughts), where 56% of the patients reported to suffer from such AVHs (supplementary table S1). These findings, therefore, associate the PH-network with some forms of AVH and extend proposals that the PH and related network is important for the additive aspect of AVH and PE.4,25,27 The seed-to-whole-brain data also corroborate behavioral evidence from specific sensorimotor stimulations that have been shown to not only induce experimentally-controlled PH, but also changes in auditory perception.4,27
The current study had several limitations, which may restrict the interpretation of the results. First, the evaluation of the symptoms was considered as a lifetime occurrence. Although detailed interviews were carried out about all PE symptoms, we only had access to MRI data ranging over a large period of time. The time interval between the imaging and the PE assessment could vary between patients and we cannot state whether the participants were experiencing any of the PE at the time of the brain imaging session. Therefore, this study does not allow to answer the question whether brain activation is altered during an ongoing PE. However, it does show that the resting-state functional connectivity between brain regions previously associated with PH is impaired only in patients who have already presented PE. Future work should perform the clinical interview and scanning with a shorter and fixed delay. Second, future studies of the PH-network in psychosis should directly evaluate the occurrence of symptomatic PH, allowing comparisons of PE± patients with and without symptomatic PH and also investigating the neural correlates of experimentally induced PH and its comparison with symptomatic PH. We further note the lack of significant correlations between the PANSS and functional connectivity alterations (see supplementary material for more details) which would enable us to more directly link PH and the psychopathological profile of PE. Possibly, more refined measures specifically related to PE rather than psychotic symptoms in general are in need. However, in this study, we did not have access to any more refined measures related to the PE. Further research, beyond the present investigation, should be dedicated to study dynamic functional connectivity in relation to symptoms, such as PE and PH, and to the PH-network, potentially revealing temporal alterations of the networks we have defined here.
Conclusions
To summarize, we show that the brain regions and functional connectivity within the PH-network that was determined independently from the present study (experimentally induced PHs in healthy participants and symptomatic PHs in neurological patients)25,26 are of relevance in psychiatric patients suffering from psychosis characterized by PE. We found evidence for a specific decrease in fronto-temporal functional connectivity in psychotic patients with PE and that this decrease was driven by decreased connectivity between the right pMTG and bilateral IFG. This PH-network connectivity decrease is of relevance to the PE in general, extending fronto-temporal alterations beyond AVH research.87,88,97,98 Besides, we show that the PH-network’s altered connectivity is associated with changes in connectivity with Heschl’s gyrus and putamen, 2 areas previously associated with AVH and altered auditory-verbal processing, further supporting the importance of the neural mechanisms of PH and PH-network in psychosis and PE. Based on the present data, future neuroimaging studies evaluating the sensitivity of psychotic patients to robot-induced PH25,26 and how this affects auditory-verbal processing4,27 will be necessary. This may allow for more detailed behavioral evaluations based on robot-induced mental states and their respective brain networks, providing improved biomarkers for diagnosis and therapy.
Supplementary Material
Acknowledgments
G.S., N.F., G.R., O.B. developed the study concept and contributed to the study design. Patients’ recruitment, testing, and data collection were performed by J.P., P.P., K.D., P.C., P.H., and G.S. performed the data analysis. G.S., J.P., and O.B. drafted the paper; all authors provided critical revisions and approved the final version of the article for submission. The authors are grateful for all patients for their participation and clinical staff for data collection and patients recruitment. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This study was supported by the National Center of Competence in Research (NCCR) “Synapsy—The Synaptic Bases of Mental Diseases” grant number 51NF40-185897, 2 generous donors advised by CARIGEST SA, the first one wishing to remain anonymous and the second one being Fondazione Teofilo Rossi di Montelera e di Premuda, the Swiss National Science Foundation (SNF), the Bertarelli Foundation, Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards, and Jeantet Foundations.
References
- 1. Lysaker PH, Lysaker JT. Narrative structure in psychosis. Theory Psychol. 2002;12(2):207–220. [Google Scholar]
- 2. Schneider K. [Primary & secondary symptoms in schizophrenia]. Fortschr Neurol Psychiatr Grenzgeb. 1957;25(9):487–490. [PubMed] [Google Scholar]
- 3. Kendler KS, Mishara A. The Prehistory of Schneider’s First-Rank Symptoms: texts from 1810 to 1932. Schizophr Bull. 2019;45(5):971–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salomon R, Progin P, Griffa A, et al. Sensorimotor induction of auditory misattribution in early psychosis. Schizophr Bull. 2020;46(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham-Schmidt KT, Martin-Iverson MT, Waters FAV. Self- and other-agency in people with passivity (first rank) symptoms in schizophrenia. Schizophr Res. 2018;192:75–81. [DOI] [PubMed] [Google Scholar]
- 6. Waters FA, Badcock JC, Dragović M, Jablensky A. Neuropsychological functioning in schizophrenia patients with first-rank (passivity) symptoms. Psychopathology. 2009;42(1):47–58. [DOI] [PubMed] [Google Scholar]
- 7. Blakemore SJ, Smith J, Steel R, Johnstone CE, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000;30(5):1131–1139. [DOI] [PubMed] [Google Scholar]
- 8. Ford JM, Mathalon DH. Efference copy, corollary discharge, predictive coding, and psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(9):764–767. [DOI] [PubMed] [Google Scholar]
- 9. Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci. 1998;18(18):7511–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ford JM, Palzes VA, Roach BJ, Mathalon DH. Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophr Bull. 2014;40(4):804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frith CD. Can a problem with corollary discharge explain the symptoms of schizophrenia? Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(9):768–769. [DOI] [PubMed] [Google Scholar]
- 12. Serino A, Pozeg P, Bernasconi F, et al. Thought consciousness and source monitoring depend on robotically controlled sensorimotor conflicts and illusory states. iScience. 2021;24(1):101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sousa P, Swiney L. Thought insertion: abnormal sense of thought agency or thought endorsement? Phenomenol Cogn Sci. 2013;12(4):637–654. [Google Scholar]
- 14. Martin JR, Pacherie E. Out of nowhere: Thought insertion, ownership and context-integration. Conscious Cogn. 2013;22(1):111–122. [DOI] [PubMed] [Google Scholar]
- 15. Taylor MA. Schneiderian first-rank symptoms and clinical prognostic features in schizophrenia. Arch Gen Psychiatry. 1972;26(1):64–67. [DOI] [PubMed] [Google Scholar]
- 16. Koehler K. First rank symptoms of schizophrenia: questions concerning clinical boundaries. Br J Psychiatry. 1979;134:236–248. [DOI] [PubMed] [Google Scholar]
- 17. Schneider K. Clinical Psychopathology. New York, NY:Grune & Stratton; 1959. [Google Scholar]
- 18. Mullins S, Spence S a. Re-examining thought insertion : Semi-structured literature review and conceptual analysis. Br J Psychiatry. 2014;182:293–298. [DOI] [PubMed] [Google Scholar]
- 19. Gallagher S. Neurocognitive models of schizophrenia: a neurophenomenological critique. Psychopathology. 2004;37(1):8–19. [DOI] [PubMed] [Google Scholar]
- 20. Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10(1):48–58. [DOI] [PubMed] [Google Scholar]
- 21. Frith C. The self in action: lessons from delusions of control. Conscious Cogn. 2005;14(4):752–770. [DOI] [PubMed] [Google Scholar]
- 22. Frith CD, Done DJ. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol Med. 1989;19(2):359–363. [DOI] [PubMed] [Google Scholar]
- 23. Bansal S, Ford JM, Spering M. The function and failure of sensory predictions. Ann N Y Acad Sci. 2018;1426(1):199–220. [DOI] [PubMed] [Google Scholar]
- 24. Sommer IE, Selten JP, Diederen KM, Blom JD. Dissecting auditory verbal hallucinations into two components: audibility (Gedankenlautwerden) and alienation (thought insertion). Psychopathology. 2010;43(2):137–140. [DOI] [PubMed] [Google Scholar]
- 25. Blanke O, Pozeg P, Hara M, et al. Neurological and robot-controlled induction of an apparition. Curr Biol. 2014;24(22):2681–2686. [DOI] [PubMed] [Google Scholar]
- 26. Bernasconi F, Blondiaux E, Potheegadoo J, et al. Sensorimotor hallucinations in Parkinson’ s disease. bioRxiv. 2020. doi:10.1101/2020.05.11.054619. [Google Scholar]
- 27. Orepic P, Rognini G, Kannape OA, Faivre N. Sensorimotor conflicts induce somatic passivity and louden quiet voices in healthy listeners. bioRxiv. 2020. doi:10.1101/2020.03.26.005843. [DOI] [PubMed]
- 28. Llorca PM, Pereira B, Jardri R, et al. Hallucinations in schizophrenia and Parkinson’s disease: an analysis of sensory modalities involved and the repercussion on patients. Sci Rep. 2016;6:38152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci. 2012;13(8):556–571. [DOI] [PubMed] [Google Scholar]
- 30. Blanke O, Slater M, Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88(1):145–166. [DOI] [PubMed] [Google Scholar]
- 31. Fénelon G, Soulas T, Cleret de Langavant L, Trinkler I, Bachoud-Lévi AC. Feeling of presence in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011;82(11):1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicastro N, Eger AF, Assal F, Garibotto V. Feeling of presence in dementia with Lewy bodies is related to reduced left frontoparietal metabolism. Brain Imaging Behav. 2018;14(4):1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nagahama Y, Okina T, Suzuki N, Matsuda M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain. 2010;133(Pt 2):557–567. [DOI] [PubMed] [Google Scholar]
- 34. Arzy S, Seeck M, Ortigue S, Spinelli L, Blanke O. Induction of an illusory shadow person. Nature. 2006;443(7109):287. [DOI] [PubMed] [Google Scholar]
- 35. Reckner E, Cipolotti L, Foley JA. Presence phenomena in Parkinsonian disorders: phenomenology and neuropsychological correlates. Int J Geriatr Psychiatry. 2020;35(7):785–793. [DOI] [PubMed] [Google Scholar]
- 36. Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138(Pt 10):3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hara M, Salomon R, van der Zwaag W, et al. A novel manipulation method of human body ownership using an fMRI-compatible master-slave system. J Neurosci Methods. 2014;235:25–34. [DOI] [PubMed] [Google Scholar]
- 38. Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30(2):115–125. [DOI] [PubMed] [Google Scholar]
- 39. Hahamy A, Calhoun V, Pearlson G, et al. Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect. 2014;4(6):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skudlarski P, Jagannathan K, Anderson K, et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karbasforoushan H, Woodward ND. Resting-State Networks in Schizophrenia. Curr Top Med Chem. 2013;12(21):2404–2414. [DOI] [PubMed] [Google Scholar]
- 42. Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51(12):1008–1011. [DOI] [PubMed] [Google Scholar]
- 43. Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frith CD, Blakemore SJ, Wolpert DM. Explaining the symptoms of schizophrenia: Abnormalities in the awareness of action. Brain Res Rev. 2000;31(2–3):357–363. [DOI] [PubMed] [Google Scholar]
- 45. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., Text Revision. Washington, DC:AAmerican Psychiatric Association;2000. [Google Scholar]
- 46. Baumann PS, Crespi S, Marion-Veyron R, et al. Treatment and early intervention in psychosis program (TIPP-Lausanne): Implementation of an early intervention programme for psychosis in Switzerland. Early Interv Psychiatry. 2013;7(3):322–328. [DOI] [PubMed] [Google Scholar]
- 47. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 48. Schnell K, Heekeren K, Daumann J, et al. Correlation of passivity symptoms and dysfunctional visuomotor action monitoring in psychosis. Brain. 2008;131(Pt 10):2783–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Franck N, O’Leary DS, Flaum M, Hichwa RD, Andreasen NC. Cerebral blood flow changes associated with Schneiderian first-rank symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14(3):277–282. [DOI] [PubMed] [Google Scholar]
- 50. Farrer C, Franck N, Frith CD, et al. Neural correlates of action attribution in schizophrenia. Psychiatry Res. 2004;131(1):31–44. [DOI] [PubMed] [Google Scholar]
- 51. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Br J Psychiatry Suppl. 1984;(7):49–58. http://www.ncbi.nlm.nih.gov/pubmed/2695141. [PubMed]
- 52. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22(1):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ferreira LK, Regina AC, Kovacevic N, et al. Aging effects on whole-brain functional connectivity in adults free of cognitive and psychiatric disorders. Cereb Cortex. 2016;26(9):3851–3865. [DOI] [PubMed] [Google Scholar]
- 56. H. Roder C, Marie Hoogendam JM, van der Veen F. FMRI, antipsychotics and schizophrenia. Influence of different antipsychotics on BOLD-signal. Curr Pharm Des. 2010;16(18):2012–2025. [DOI] [PubMed] [Google Scholar]
- 57. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. [DOI] [PubMed] [Google Scholar]
- 58. González-Vivas C, Soldevila-Matías P, Sparano O, et al. Longitudinal studies of functional magnetic resonance imaging in first-episode psychosis: a systematic review. Eur Psychiatry. 2019;59:60–69. [DOI] [PubMed] [Google Scholar]
- 59. Satterthwaite TD, Baker JT. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr Opin Neurobiol. 2015;30:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mwansisya TE, Hu A, Li Y, et al. Task and resting-state fMRI studies in first-episode schizophrenia: A systematic review. Schizophr Res. 2017;189:9–18. [DOI] [PubMed] [Google Scholar]
- 61. Northoff G, Duncan NW. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Prog Neurobiol. 2016;145–146:26–45. [DOI] [PubMed] [Google Scholar]
- 62. Crossley NA, Mechelli A, Fusar-Poli P, et al. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum Brain Mapp. 2009;30(12):4129–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oertel-Knöchel V, Knöchel C, Matura S, et al. Association between symptoms of psychosis and reduced functional connectivity of auditory cortex. Schizophr Res. 2014;160(1–3):35–42. [DOI] [PubMed] [Google Scholar]
- 64. Frith C. The neural basis of hallucinations and delusions. C R Biol. 2005;328(2):169–175. [DOI] [PubMed] [Google Scholar]
- 65. Menninger-Lerchenthal E. Das Truggebilde Der Eigenen Gestalt (Heautoscopy, Doppelganger). Berlin, Germany: Karger; 1935. [Google Scholar]
- 66. Lhermitte J. L’image de Notre Corps. Paris, France: Edi.; 1939. [Google Scholar]
- 67. Hécaen H, Ajuriaguerra J.. Meconnaissances et Hallucinations Corporelles: Intégration et Désintégration de La Somatognosie (in French). Paris: Masson; 1952. [PubMed] [Google Scholar]
- 68. Lukianowicz. Autoscopic phenomena. Arch Neurol Psychiatry. 1958;80(2):199. [DOI] [PubMed] [Google Scholar]
- 69. Critchley M. The Divine Banquet of the Brain and Other Essays. New York, NY:Raven Press; 1979. [Google Scholar]
- 70. Brugger P, Regard M, Landis T. Unilaterally Felt “Presences”: The Neuropsychiatry of One’s Invisible Doppelgänger. Neuropsychiatry Neuropsychol Behav Neurol. 1996;9(2):114–122. [Google Scholar]
- 71. Brugger, Regard M, Landis T. Illusory reduplication of one’s own body: phenomenology and classification of autoscopic phenomena. Cogn Neuropsychiatry. 1997;2(1):19–38. [DOI] [PubMed] [Google Scholar]
- 72. Critchley M. The body-image in neurology. Lancet. 1950;1:335–340. [Google Scholar]
- 73. Critchley M. The idea of a presence. Acta Psychiatr Neurol Scand. 1955;30(1-2):155–168. [DOI] [PubMed] [Google Scholar]
- 74. Blanke O, Arzy S, Landis T. Illusory perceptions of the human body and self. Neuropsychology. 2008;88(3):429–458. [DOI] [PubMed] [Google Scholar]
- 75. Kraepelin E. Psychiatrie; Ein Lehrbuch Für Studierende Und Ärzte. Leipzig, Germany: Barth; 1913. [Google Scholar]
- 76. Bleuler E. Dementia praecox oder Gruppe der Schizophrenien. Deuticke. 1911;12. [Google Scholar]
- 77. Schneider K. Die Psychopathischen Persönlichkeiten. Wien: Deuticke;1950. [Google Scholar]
- 78. Costantini M, Salone A, Martinotti G, et al. Body representations and basic symptoms in schizophrenia. Schizophr Res. 2020;222:267–273. [DOI] [PubMed] [Google Scholar]
- 79. Hur JW, Kwon JS, Lee TY, Park S. The crisis of minimal self-awareness in schizophrenia: a meta-analytic review. Schizophr Res. 2014;152(1):58–64. [DOI] [PubMed] [Google Scholar]
- 80. Northoff G, Sandsten KE, Nordgaard J, Kjaer TW, Parnas J. The self and its prolonged intrinsic neural timescale in schizophrenia. Schizophr Bull. 2020;47(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Benson TL, Brugger P, Park S. Bodily self-disturbance in schizophrenia-spectrum populations: Introducing the Benson et al. Body Disturbances Inventory (B-BODI). Psych J. 2019;8(1):110–121. [DOI] [PubMed] [Google Scholar]
- 82. Humpston CS, Adams RA, Benrimoh D, et al. From Computation to the First-Person: Auditory-Verbal Hallucinations and Delusions of Thought Interference in Schizophrenia-Spectrum Psychoses. Schizophr Bull. 2019;45(45 Suppl 1):S56–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cho R, Wu W. Mechanisms of auditory verbal hallucination in schizophrenia. Front Psychiatry. 2013;4:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jones SR, Fernyhough C. Neural correlates of inner speech and auditory verbal hallucinations: a critical review and theoretical integration. Clin Psychol Rev. 2007;27(2):140–154. [DOI] [PubMed] [Google Scholar]
- 85. Tracy DK, Shergill SS. Mechanisms underlying auditory hallucinations-understanding perception without stimulus. Brain Sci. 2013;3(2):642–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Guo Q, Goff D, Wang J, Northoff G, Wang J, Yang Z. Parietal memory network and default mode network in first-episode drug-naïve schizophrenia : Associations with auditory hallucination. Hum Brain Mapp. 2020;41(8);1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bauer CCC, Okano K, Gosh SS, et al. Real-time fMRI neurofeedback reduces auditory hallucinations and modulates resting state connectivity of involved brain regions: Part 2: Default mode network -preliminary evidence. Psychiatry Res. 2020;284:112770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Alderson-Day B, Diederen K, Fernyhough C, et al. Auditory hallucinations and the brain’s resting-state networks: findings and methodological observations. Schizophr Bull. 2016;42(5):1110–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Alderson-Day B, Woods A, Moseley P, et al. Voice-hearing and personification: characterizing social qualities of auditory verbal hallucinations in early psychosis. Schizophr Bull. 2020;47(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wilkinson S, Bell V. The representation of agents in auditory verbal hallucinations. Mind Lang. 2016;31(1):104–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Backasch B, Sommer J, Klöhn-Saghatolislam F, Müller MJ, Kircher TT, Leube DT. Dysconnectivity of the inferior frontal gyrus: implications for an impaired self-other distinction in patients with schizophrenia. Psychiatry Res. 2014;223(3):202–209. [DOI] [PubMed] [Google Scholar]
- 92. Shinn AK, Baker JT, Cohen BM, Öngür D. Functional connectivity of left Heschl’s gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr Res. 2013;143(2–3):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dierks T, J Linden DE, Jandl M, Formisano E, Goebel R. Activation of Heschl’s Gyrus during auditory hallucinations of these studies could directly differentiate between the hallucinatory and nonhallucinatory states within one scanning session. Because of this restriction, they did. Neuron. 1999;22:615–621. [DOI] [PubMed] [Google Scholar]
- 94. Cui LB, Liu K, Li C, et al. Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Res. 2016;173(1-2):13–22. [DOI] [PubMed] [Google Scholar]
- 95. Hoffman RE, Fernandez T, Pittman B, Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69(5):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hoffman RE, Hampson M. Functional connectivity studies of patients with auditory verbal hallucinations. Front Hum Neurosci. 2011;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gao J, Zhang D, Wang L, et al. Altered effective connectivity in schizophrenic patients with auditory verbal hallucinations: a resting-state fMRI study with Granger causality analysis. Front Psychiatry. 2020;11(June):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bohlken MM, Hugdahl K, Sommer IEC. Auditory verbal hallucinations: neuroimaging and treatment. Psychol Med. 2016;47(2);1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.