Abstract
Stress CMR is a cost-effective, non-invasive test that accurately assesses myocardial ischemia, myocardial viability, and cardiac function without the need for ionizing radiation. There is a large body of literature, including randomized-controlled trials, validating its diagnostic performance, risk stratification capabilities, and ability to guide appropriate use of coronary intervention. Specifically, stress CMR has demonstrated higher diagnostic sensitivity than SPECT in detecting angiographically significant coronary artery disease. Stress CMR is particularly valuable for the evaluation of patients with moderate to high pre-test probability of having stable ischemic heart disease and for patients known to have challenging imaging characteristics such as women, individuals with prior revascularization, and left ventricular dysfunction. In this article, we review the basics principles of stress CMR, the data supporting its clinical use, the added-value of myocardial blood flow quantification, and the assessment of myocardial function and viability routinely obtained during a stress CMR study.
Keywords: stable ischemic heart disease, coronary artery disease, magnetic resonance imaging, myocardial perfusion
Condensed Abstract:
Stress CMR is a non-invasive test that accurately assesses myocardial ischemia, myocardial viability, and cardiac function without the need for ionizing radiation. There is a growing body of literature, which includes randomized-controlled trials, validating its diagnostic performance, risk stratification capabilities, and ability to guide treatment decisions. In this article, we review the basics principles of stress CMR, the data supporting its clinical use, the added-value of myocardial blood flow quantification, and the assessment of myocardial function and viability routinely obtained during a stress CMR study.
Introduction
A number of recent studies have demonstrated the clinical utility of stress cardiac magnetic resonance imaging (CMR) for the evaluation of patients with known or suspected coronary artery disease (CAD) . Given the availability of highly effective medical therapy (OMT) for CAD, noninvasive stress testing must not only accurately diagnose the cause of symptoms but also provide risk stratification and guide treatment strategies. Following the FAME (Fractional flow reserve (FFR) versus Angiography for Multivessel coronary artery disease Evaluation) trials, coronary revascularization is now preferentially performed to treat a coronary stenosis on the basis of whether it is hemodynamically significant rather than the degree of luminal stenosis (1). Amongst the various cardiac imaging modalities, stress testing using CMR and positron emission tomography (PET) had demonstrated high sensitivities (89% and 84%) and specificities (87% each) in their correlations with invasive FFR (2). Stress CMR also has a number of technical strengths, including high spatial and temporal resolution in detecting subendocardial ischemia or infarction, freedom from soft tissue attenuation or the requirement of an acoustic window, sensitive tissue characterization for silent myocardial infarction and myocardial viability, excellent safety profile, and lack of ionizing radiation or iodinated contrast exposure.(4,5) On the other hand, various logistical issues partially limit widespread use of stress CMR. In this article, we review the basic principles of stress CMR, the diagnostic accuracy of stress CMR, the prognostic utility of stress CMR, quantification of myocardial blood flow, and the assessment of function and myocardial viability (See Central Illustration).
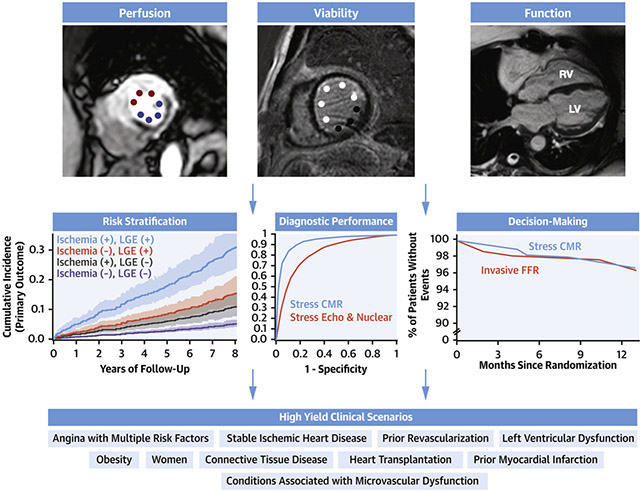
Central Illustration. Overview of Stress CMR.
Stress CMR includes assessment of ischemia, viability, and function. It is accurate, can be used for risk stratification capabilities, impacts decision making, and can be used for several clinical scenarios. Figure includes adoptions from (2,14,20)
Basic Principles of Stress CMR
Stress CMR has several technical advantages that likely result in a higher diagnostic performance when compared to more commonly used imaging modalities such as SPECT. Because of its higher spatial resolution, larger field of view and better ability to differentiate between tissues, CMR is not limited by attenuation artifacts or from contamination of the myocardium by signal sources not related to myocardial perfusion that can mimic or disguise perfusion defects (Figure 1). Additionally, its high spatial resolution allows for the assessment of perfusion within the different layers of the myocardium. Since myocardial perfusion abnormalities preferentially involve the subendocardial layers, the presence of a perfusion defect can be identified within a single myocardial segment without consideration of the perfusion in the other myocardial segments. This is in contrast to SPECT where the spatial resolution is poor enough that ischemic segments are best identified in the context of a normally perfused remote myocardial segment. This difference makes stress CMR less susceptible to challenging situations such as balanced ischemia (Figure 2).
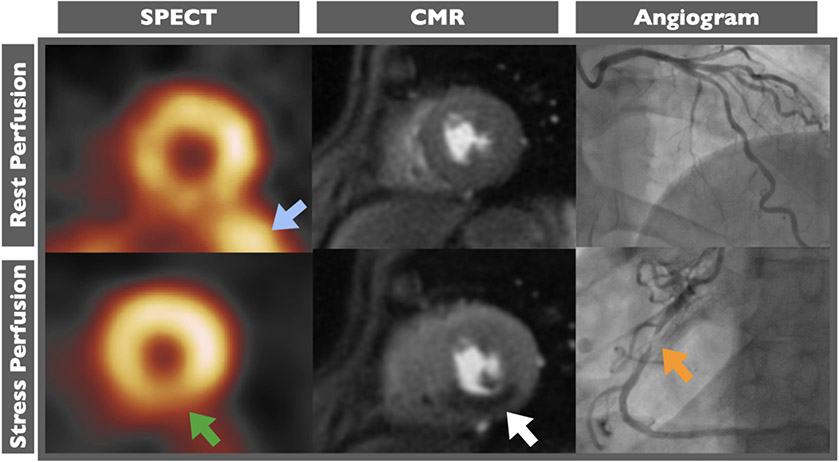
Figure 1. Improved visualization of inferior wall using stress cardiac magnetic resonance.
In patient with severe right coronary artery stenosis (orange arrow), perfusion defect in inferior wall (white arrow) clearly seen on stress cardiac magnetic resonance (CMR) images but obscured by excessive signal being emitted from the abdomen (blue arrow) and diaphragmatic attenuation (green arrow) on single photon emission tomography (SPECT) images.
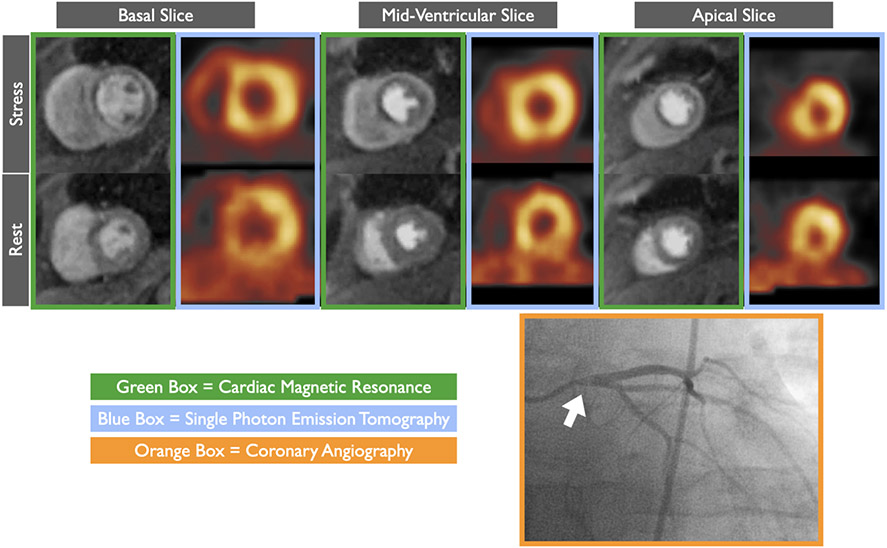
Figure 2. Stress cardiac magnetic resonance (CMR) is less susceptible to balanced ischemia.
In patient with severe left main stenosis (white arrow), circumferential perfusion defect noted throughout all stress CMR (green boxes) images but as evident on single photon emission tomography (SPECT, blue boxes) images due to balanced ischemia.
Currently, the most common approach for performing stress CMR is to induce hyperemia with the use of vasodilators such as adenosine, regadenoson, or dipyridamole. Once hyperemia is achieved, a gadolinium-based contrast agent (GBCA) functioning as a blood flow tracer (3) is injected into a peripheral vein. Serial T1-weighted CMR images are acquired during every heart beat to visualize the GBCA as it transits through the chambers of the heart and perfuses the myocardium. The greater the concentration of the GBCA in a region of interest, the greater the signal generated on a T1-weighted CMR image. The GBCA will enter into normally perfused myocardial segments more quickly and at higher concentrations, resulting in a more rapid and greater increase in T1-signal when compared to abnormally perfused myocardial segments (Figure 3).
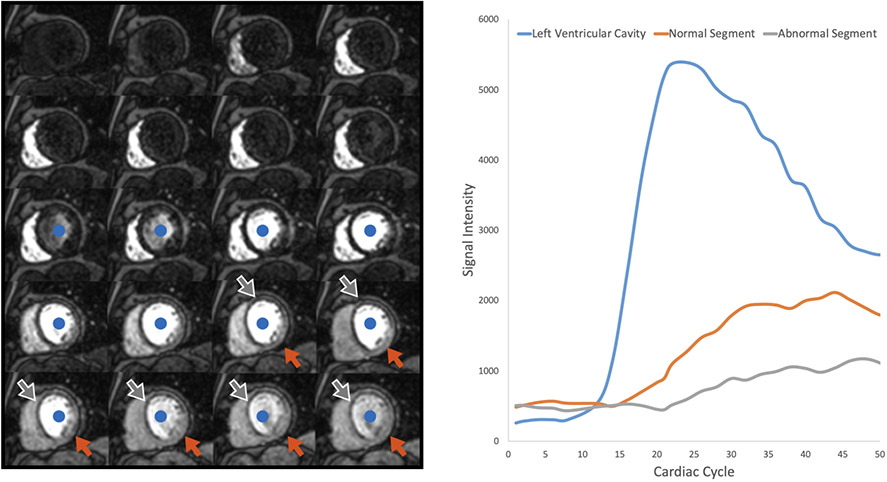
Figure 3. First pass perfusion.
Left Panel: A single mid-ventricular short axis slice acquired repetitively as contrast first enters the right ventricle, then the left ventricle (blue dot), and finally perfuses the myocardium. More contrast enters the non-ischemic segments of the myocardium (orange arrow) when compared to the ischemic segments (grey arrow). Time intensity curves representing the left ventricular cavity, an ischemic segment, and a normal segment are shown.
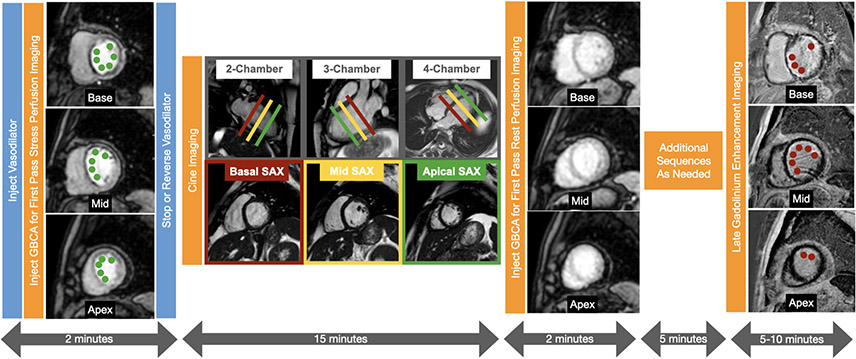
A typical stress perfusion CMR protocol (4) is shown in Figure 4. After the acquisition of a series of scout images, a vasodilator is administered and a series of dynamic stress perfusion images covering the basal, mid, and apical levels of the left ventricular short axis are acquired during the infusion of a GBCA. These images allow for visualization of each of the 16 myocardial segments as standardized by the American Heart Association and represent typical coronary artery territories. Once the stress perfusion images are acquired, the vasodilator is stopped or a pharmacologic agent such as aminophylline may be administered to reverse hyperemia. Next, a series of cine CMR images are acquired in 3 distinct long axis planes and in a stack of short axis planes covering the entirety of the ventricles from the apex through the base. Approximately 10-15 minutes after the stress perfusion images are acquired, resting perfusion images using the same technique and slice position of the stress perfusion images are acquired during injection of additional GBCA. After a 5-minute delay to allow for washout of the GBCA from healthy myocardium, late gadolinium enhancement (LGE) images are acquired to assess for myocardial viability. This protocol typically takes approximately 30 minutes.
Figure 4. Typical vasodilator stress cardiac magnetic resonance protocol.
First pass perfusion images using a gadolinium-based contrast agent (GBCA) are acquired in 3 short axis slices of the left ventricle during hyperemic conditions to assess for ischemia. During the next 15 minutes, cine images are acquired in the multiple short axis and long axis views to assess cardiac anatomy and function. Next first pass perfusion images are acquired in 3 short axis slices during resting conditions. After a 5 minute delay, late gadolinium enhancement images are acquired in multiple short axis and long axis views to assess for viability. Large perfusion defects involving most of the basal slice and the mid to apical septal, anterior, and anterolateral segments are present (adjacent to the green dots) on the stress perfusion images but not on the rest perfusion images which are normal. The perfusion defect extends beyond the thin rim of late gadolinium enhancement (red dots) consistent with subendocardial myocardial infarction with significant peri-infarction ischemia.
Stress CMR perfusion images are typically displayed as a dynamic series of images acquired as contrast perfuses into the myocardium (Figure 3). A perfusion defect will appear as a hypointensity along the subendocardium in a coronary distribution. The abnormality is most pronounced 2-3 heart beats after the left ventricular cavity is maximally enhanced with contrast and persists for several more seconds as the contrast washes out of the myocardium (5). The perfusion images are interpreted in context of the LGE images (described in more detail below) to classify any perfusion defects as either being due to myocardial ischemia or due to myocardial infarction. See Figure 4 and Figure 5 for examples of peri-infarct ischemia and reversible ischemia, respectively. In addition to determining the presence or absence of ischemia, the overall ischemic burden can be evaluated by counting the number of segments with perfusion defects due to ischemia. A perfusion defect must be differentiated from imaging artifacts such as dark rim artifacts which can also occur along the subendocardial layer of the myocardium which is more pronounced in the setting of reduced spatial resolution and temporal resolution. Dark rim artifacts are typically identified when thin subendocardial perfusion defects are present during both resting and stress conditions in the absence underlying late gadolinium enhancement.
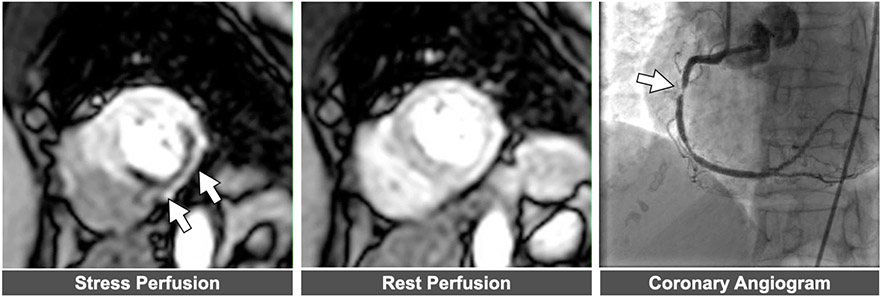
Figure 5. Example of abnormal stress CMR perfusion.
Left panel: Perfusion defect in mid-ventricular short axis slice denoted by arrows. . Middle panel Normal perfusion noted in same slice position during resting conditions. Right panel: Severe right coronary artery stenosis corresponding to perfusion defect.
Diagnostic Accuracy of Stress CMR.
More than 35 single-center observational studies and their meta-analyses (6)over the past 2 decades have consistently demonstrated excellent accuracy of stress CMR in diagnosing stable ischemic heart disease (SIHD). The prospective CE-MARC trial (Clinical Evaluation of Magnetic Resonance imaging in Coronary heart disease) enrolled 752 patients with suspected angina to undergo stress CMR and single photon emission computed tomography (SPECT) imaging and compared them to invasive coronary angiography (ICA) as a reference standard. With an angiographic prevalence of 39% for CAD, stress CMR demonstrated a sensitivity of 87% that was superior to SPECT (67%), whereas specificities of both modalities were similar at 83%. CE-MARC concluded that stress CMR was more sensitive for ruling out significant CAD than SPECT based on its superior negative predictive value. (7) Since then, several multicenter studies have firmly established the diagnostic accuracy of stress CMR (8,9). The most recent of these studies, GadaCad (Gadobutrol-enhanced CMR to detect Coronary Artery Disease), consisted of two combined phase III multivendor clinical trials evaluating the diagnostic accuracy of stress CMR for the detection of angiographically-significant CAD amongst 764 patients with suspected SIHD from 47 international centers (10). To minimize verification bias, all patients underwent ICA or cardiac computed tomography angiography (CCTA) within 4 weeks. With an angiographic stenosis prevalence of 28% (defined as ≥70% stenosis), stress CMR had sensitivities of 79% and 87%, and specificities of 87% and 73%, for detecting single vessel and multivessel CAD, respectively. Six blinded expert readers reported excellent interobserver reproducibility, lending credence to the robustness of the image quality. Results of the GadaCad study were the impetus for the United States Food and Drug Administration approval of gadobutrol (Bayer AG) as the first GBCA to be used for stress CMR in patients with suspected SIHD. Multiple studies have demonstrated a high correlation between stress CMR perfusion interpreted qualitatively and invasive FFR. Watkins et al demonstrated that stress CMR has excellent positive and negative predictive values of 91% and 94% for detecting hemodynamically significant CAD as defined by invasive FFR (11). Moreover, several meta-analyses have been conducted using FFR as the gold standard and have similarly demonstrated excellent diagnostic accuracy for stress CMR. Danad and colleagues compared a number of different imaging modalities and found that stress CMR had the highest diagnostic performance against FFR as the reference standard, both for per-patient (sensitivity 90%, specificity 94%) and per-vessel (sensitivity 91%, specificity 85%) analyses (6). Finally, compared to other modalities, CMR is the most reliable method in diagnosing and risk stratifying non-coronary cardiac causes of chest pain such as pericarditis or myocarditis (12,13).
Prognostic Utility of Stress CMR
In the multicenter SPINS registry (Stress CMR Perfusion Imaging in the United States study) (14) of 2,349 patients from 13 centers, stress CMR demonstrated highly effective risk reclassification for cardiac death or nonfatal myocardial infarction (MI) and was cost-effective in the triage of chest pain patients. Sixty-seven percent of the SPINS cohort had no evidence of ischemia or infarction, with an associated event-free rate of 99.3% per year over an intermediate follow-up duration of 5.5 years. Subgroup analysis of the SPINS study also showed strong prognostic utility of stress CMR in those with reduced left ventricular function (15). In a multicenter study of over 9,000 patients with greater than 48,000 patient-years of follow-up, stress CMR was an independent predictor of mortality, as well as in subgroups with known SIHD, LGE, typical chest pain, and left ventricular systolic dysfunction (16). Importantly, an abnormal stress CMR classified patients at higher risk than would have been predicted by their Framingham risk group; whereas, a normal stress CMR identified patients at lower risk than predicted by the Framingham risk group (16). A separate meta-analysis of over 12,000 patients reported a negative predictive value for nonfatal MI and cardiac death for a normal stress CMR of 98.1% (95% CI, 97.3-98.8%) during a mean follow-up of 25 months (17). Stress CMR has shown significant improvement in risk reclassification amongst suspected SIHD. Indeed, the addition of ischemia reclassified over 90% of patients at intermediate risk of SIHD with 4.9% annual risk of cardiac death and MI in those re-classified to high risk versus 0.3% for those reclassified as low risk (18). Taken together, the currently available data suggest that individuals with a negative stress CMR examination have a <1% annualized even rate.
Following the ISCHEMIA trial (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) (19), which highlighted the remarkable success of OMT if adhered to for patients SIHD, the role of stress imaging in effective risk assessment, guiding the use of OMT, and avoiding unnecessary invasive coronary angiography should continue to be supported. In the MR-INFORM trial (The Myocardial Perfusion CMR versus Angiography and FFR to Guide the Management of Patients with Stable Coronary Artery Disease) (20) randomizing 910 patients moderate-high risk chest pain syndrome to ICA plus FFR versus stress CMR guided care, patients in the stress CMR arm required less coronary revascularization versus invasive ICA plus FFR (36% vs. 45%; P=0.005) without an increase in adverse cardiac events (3.6% vs 3.7%, risk difference −0.2) or reduction in freedom from angina (49.2% vs 43.8%, P=0.21) at 1-year. The CE-MARC 2 trial randomized over 1,200 patients with suspected SIHD to stress CMR, SPECT, and coronary computed tomography angiography (CCTA) with primary endpoint of unnecessary ICA. At 12 months, stress CMR resulted in lower probability of unnecessary ICA than CCTA (7.5% vs 28.8%, OR 0.21, 95% CI 0.12-0.34, P <0.001) (21). In the STRATEGY trial (Stress Cardiac Magnetic Resonance Versus Computed Tomography Coronary Angiography for the Management of Symptomatic Revascularized Patients), 600 patients with stable angina and previous revascularization were randomized to stress CMR versus CCTA for evaluation. After approximately 2 years of follow-up, they reported that a stress CMR strategy was associated with a lower rate of downstream noninvasive tests (28% versus 17%; P=0.0009), ICA (31% versus 20%; P=0.0009), and revascularization procedures (24% versus 16%; P=0.007); additionally, radiation exposure and cumulative costs (120±251 versus 218±298 Euro/yr; P<0.001) were lower. Importantly, adverse cardiac events were significantly reduced (5% versus 10%; P<0.010) with the stress CMR strategy (22). Table 1 and Table 2 include a select list of stress CMR studies which are comprised of more than 100 patients and include a comparison against invasive coronary angiography, invasive fractional flow reserve assessment, or patient-related outcomes. Numerous additional studies have demonstrated the potential cost effectiveness of an upfront stress CMR strategy within the United States (23), Australia (24), and the United Kingdom (25).
Table 1.
Stress CMR studies with ≥100 patients and comparison to invasive catheterization or patient-related outcomes.
| Review of Stress CMR Publications | |||||
|---|---|---|---|---|---|
| Comparison of Stress CMR Against Invasive ICA | |||||
| Study/Ye ar |
Study size (n) |
Inclusion criteria |
Ref CAD definition |
Sensitivity/ Specificity/AUC |
Results |
| (8)MR-IMPACT (2008) | 241 | Clinical indication for ischemic evaluation | ≥50% diameter stenosis in >1 coronary artery | 85% / 67% / 0.86 | CMR performance similar to SPECT in matched patients (n=42); better performance when comparing CMR with 0.1mmol/kg dose to whole SPECT population (n=212) |
| (9,57)MR - IMPACT II (2012, 2013) | 533 | Suspected CAD/clinical indication for ICA or SPECT | ≥50% diameter stenosis in >1 coronary artery | 75% /59% / 0.75 | CMR diagnostic performance superior to SPECT |
| (7)CE-MARC (2012) | 752 | Suspected angina + 1 cardiovasc ular risk factor | ≥70% area stenosis in ≥1 coronary artery or ≥50% Left main | 87% /83% / AUC 0.89 | CMR has high diagnostic accuracy over SPECT, with greater sensitivity and NPV compared to SPECT |
| Comparison of Stress CMR Against Invasive Fractional Flow Reserve (FFR) | |||||
| (11)Watkins et al (2009) | 103 | Suspected angina with indication for CA | FFR<0.75, or subtotal/total coronary occlusionm | 95%/91% | CMR has high diagnostic accuracy in the detection of functionally significant CAD when compared against FFR reference standard |
| (58)Manka et al (2012) | 120 | Known or suspected CAD | FFR <0.75 in vessels >2mm and >40% stenosis | 90% /82% | 3D stress CMR has high diagnostic accuracy for detection of functionally significant CAD and can represent myocardial ischemic burden accurately |
| (59)Bettencourt et al (2013) | 103 | Symptoma tic patients with suspected CAD or intermedia te-high probability of CAD | FFR <0.80 for any stenosis >40%, or subtotal/occluded vessel | 89% /88% | CMR has high accuracy in detection of functionally significant CAD in intermediate-high pretest probability patients |
| (60)Ebers berger et al (2013) | 116 | Known or Suspected CAD | FFR ≤0.8 OR stenosis >75% | 85% /87%/ 0.93 | 3T stress CMR has excellent diagnostic performance with high image quality |
Table 2:
Selected stress CMR studies with >100 subjects which include patient outcomes.
| Prognostic Value | |||||
|---|---|---|---|---|---|
| Study/Year | Study size (n) |
Inclusion Criteria |
Study Design/Treatment & Comparison |
Primary Outcome |
Results |
| (21)CE-MARC 2 (2016) | 1201 | Symptomatic with suspected angina | Random assignment to UK NICE guidelines, CMR or SPECT guided management | Primary Endpoint: Unncessary ICA Secondary Endpoint: MACE |
Primary Endpoint CMR vs. NICE OR 0.21 (95% CI 0.12-0.34;p<0.01) CMR vs SPECT OR 1.27 (95% CI 0.79-2.03; p=0.32) Secondary Endpoints: CMR vs. NICE HR 1.37 (95%CI 0.52-3.57;p=0.52) CMR vs. SPECT HR 0.95 (95% CI 0.46-1.95; p=0.88) |
| (22) STRATEGY (2016) | 600 | Patients with chest pain and prior revascularization | Prospective registry comparing CTCA and stress CMR | MACE (Cardiac death, nonfatal MI) | Stress CMR patients had lower MACE rate (5%) compared to CTCA arm (10%), p<0.01 |
| (61) MAGnet (2018) | 200 | Symptomatic stable CAD (intermediate or high risk) | 1:1 randomization to ICA vs. adenosine stress CMR | Composite CV death and nonfatal MI | 3.1% for ICA vs. 4.2% for CMR (p=ns) with lower revascularization rate in CMR arm |
| (20)MR-INFORM (2019) | 918 | Symptomatic patients with 2+ cardiovascular risk factors/+ETT | Unblinded assignment to upfront stress CMR with FFR-guided revascularization | Primary endpoint: death, nonfatal MI, TVR at 1 year | 3.7% for FFR vs. 3.6% for CMR (meeting criteria for noninferiority) with no difference in freedom from angina at 12 months |
| (16)Heitner et al (2019) | 9151 | Known or suspected CAD | Multicenter study of patients undergoing stress CMR | All -cause mortality | Abnormal stress associated with increased mortality (HR 1.89, 95% CI 1.68-2.1, p<0.001), including in patients with/without CAD, and for normal/reduced EF |
| (14)SPINS Registry (2019) | 2349 | Patients presenting with chest pain syndrome | Retrospective multicenter cohort study of patients undergoing stress CMR | Composite cardiovascular death and nonfatal MI | + ischemia associated with HR 1.96 (95% CI 1.35-2.86, p<.0004) No ischemia or LGE associated with low event rate up to 5 years after index exam |
CAD = Coronary Artery Disease; MI = Myocardial infarction; TVR = target vessel revascularization; ICA = invasive coronary angiography; FFR = fractional flow reserve; EF = ejection fraction
Studies >100 included
per patient level analyses
There are several patient populations where stress CMR may be a particularly appropriate stress testing modality for the evaluation of coronary artery disease. Indeed, stress CMR has been demonstrated to be effective in patients with a high pretest probability such as those with known SIHD (16), prior myocardial infarction (16), prior revascularization (22,26), and angina associated with multiple risk factors (20). Stress CMR may also be particularly beneficial in patient populations that are more challenging to image such as women (27) and those with obesity (28) where attenuation artifacts and challenging echocardiographic windows are more frequently encountered. Another group of patients that might have an added benefit from stress CMR imaging include those with left ventricular dysfunction (15,29) where balanced ischemia can be problematic with other imaging modalities. Patients with conditions such as connective tissue disease (30) and heart transplant (31) where microvascular dysfunction (32-34) is more prevalent may also be good candidates for stress CMR.
Quantification of Myocardial Perfusion.
Absolute Quantification of Myocardial Blood Flow
The basic premise of quantifying myocardial blood flow is in the measurement of an arterial input function which describes the contrast delivery into the coronary tree and measurement of the tissue function which describes the contrast concentration in the myocardium as a function of time. When a specialized pulse sequence (35) that allows for both of these parameters to be measured is used, the myocardial blood flow (MBF) can be determined using a mathematical operation called deconvolution (3) and can be used to supplement the qualitative interpretation of the images (36) (Figure 6). See appendix 1 for more details regarding quantitative MBF assessment using CMR.
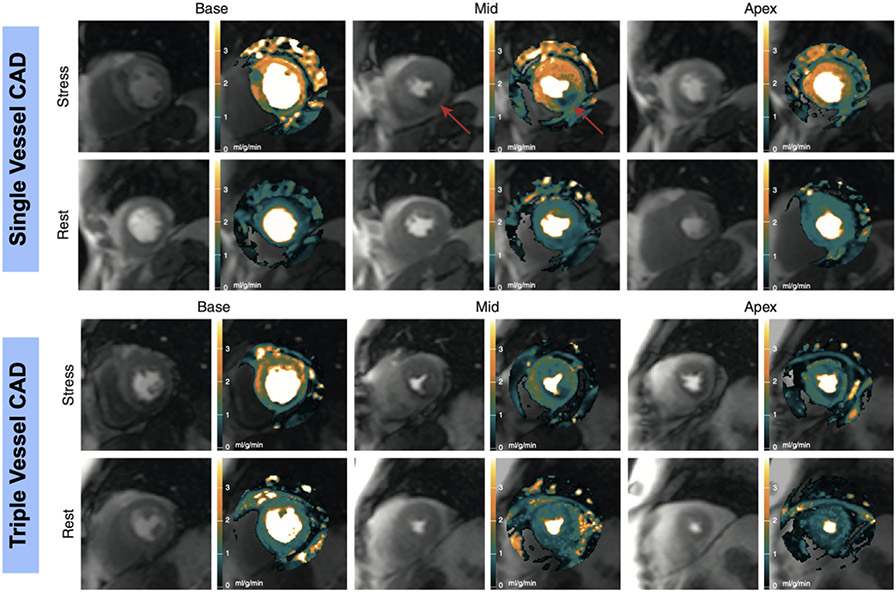
Figure 6. Quantitative Assessment of Myocardial Perfusion.
Top two rows: Quantitative myocardial blood flow maps and perfusion images from patient with single vessel coronary artery disease. Arrow denotes region ischemic myocardium. Bottom two rows: Myocardial blood flow maps and perfusion images from patient with multi-vessel coronary artery disease. Resting myocardial blood flow is approximately 1ml/g/min and remains low throughout the myocardium during stress. Adapted from Hsu LY, Jacobs M, Benovoy M et al. Diagnostic Performance of Fully Automated Pixel-Wise Quantitative Myocardial Perfusion Imaging by Cardiovascular Magnetic Resonance. JACC Cardiovasc Imaging 2018;11:697-707.
Clinical Applications of Quantitative Perfusion Imaging.
Absolute quantification of myocardial perfusion has been applied in ischemic heart disease to assess both epicardial coronary artery disease and microvascular dysfunction. The SPECT literature has elucidated how the relative assessment of perfusion has reduced sensitivity in situations associated with balanced ischemia. In contrast, CMR studies have demonstrated improved sensitivity for detecting left main and multi-vessel disease as compared to SPECT using either visual or quantitative analysis (37). However, quantitative CMR can better differentiate between single vessel disease and multi-vessel disease as compared to visual analysis (38). More recent studies using pixel-wise quantification of MBF have shown improved diagnostic utility as compared to visual analysis alone (39,40). Indeed, quantitative analysis of myocardial perfusion suggests that the ischemic burden in a given coronary territory may in fact be greater than what is initially appreciated with visual inspection of perfusion images (40). Recently, the prognostic utility of quantitative perfusion analysis was validated in patients being evaluated for CAD. One study demonstrated incremental prognostic utility of quantitative analysis over visual analysis (41). In a recent study of over 1000 patients without obstructive CAD, abnormal myocardial perfusion reserve (MPR) was independently associated with major adverse cardiac events (MACE) and cardiovascular death (42).
While beyond the scope of this review article, quantitative analysis of myocardial perfusion also has the potential to detect cardiac microvascular disease (33).
Cine and Late Gadolinium Enhancement/Viability Imaging
The assessment of left and right ventricular systolic function by cine imaging is a fundamental component of stress CMR exams, with implications for viability assessment, clinical management and overall prognosis. The presence of regional wall motion abnormalities at rest may act as a clue to the existence of myocardial infarction, with cine images also serving as a point of reference for the determination of myocardial viability. In addition to global and regional systolic function, CMR measures of ventricular volumes and left ventricular mass are highly reproducible and may offer indicators of ventricular remodeling with relevant prognostic implications for patients undergoing a stress CMR examination (43). See appendix 2 for more information regarding cine CMR imaging.
LGE imaging is an integral part of any stress CMR protocol (4). LGE is the best technique presently available for locating and sizing myocardial infarction (MI). It had been observed as far back as the 1980’s that GBCAs accumulate in areas of infarct-related scar due to increased volume of distribution within fibrosis as well as delayed washout of the contrast agent (44). The ideal CMR technique for LGE with the use of inversion recovery to null normal myocardium was subsequently developed at the turn of the millennium (45). This approach to LGE was carefully validated in a canine model of acute MI (46). In humans, it was shown to correlate well with enzymatic markers of acute MI, with the additional and consequential observation that functional recovery of infarcted myocardium relates to its transmural extent as demonstrated by LGE (47). The transmural extent of LGE relates to recovery of function both in acute and chronic MI as shown in a landmark study of patients before and several months after revascularization (48). Although the STICH trial (49) suggested that there is no interaction between myocardial viability and treatment strategy, the trial relied on SPECT and dobutamine stress echo for the assessment of viability and LGE imaging was not utilized. Indeed, a subsequent publication by Shah et al (50) revealed that the absence of transmural LGE in patients with severe myocardial thinning and LV dysfunction was associated with recovery of LV function following revascularization. Figures 7 and 8 are examples of non-viable and viable myocardium.
Figure 7. Example of non-viable myocardium.
Baseline cine images (blue box) from a patient with a large myocardial infarction in left anterior descending artery (LAD) territory are shown. Late gadolinium enhancement (LGE) images (green box) showing a transmural myocardial infarction consistent with non-viable myocardium in the LAD territory.
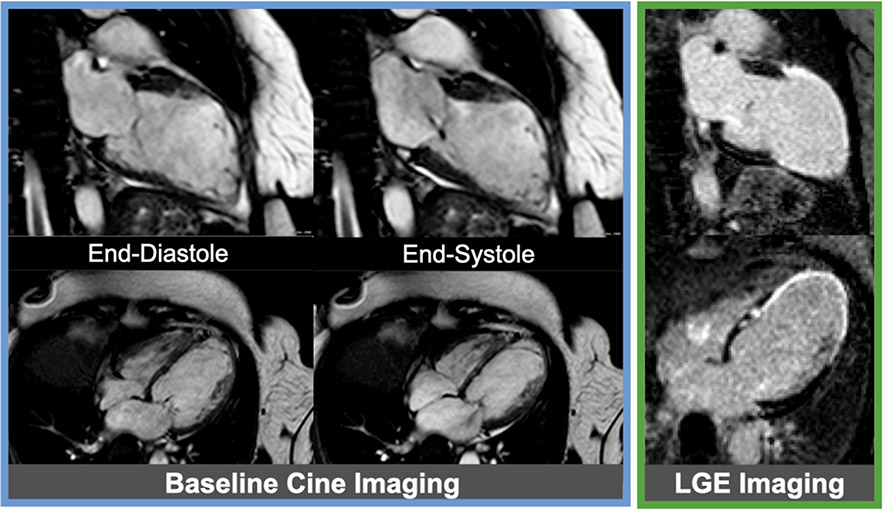
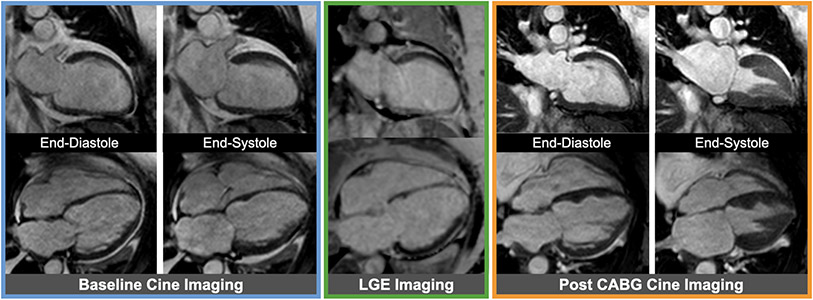
Figure 8. Example of viable myocardium.
Baseline cine images (blue box) from a patient with severe multi-vessel coronary artery disease showing severely reduced ejection fraction. Late gadolinium enhancement (LGE) images (green lox) without any significant LGE representing fully viable myocardium are shown in the same cardiac views; of note, the mid to apical septum in the 4-chamber view has mildly increased signal intensity not sufficient enough to be classified as myocardial infarction. Corresponding cine images (orange box) showing improved ejection fraction following coronary artery bypass grafting (CABG).
The reproducibility and reliability of LGE was demonstrated in a multi-center study (51) and it was also shown to be more sensitive for smaller, non Q-wave, and inferior infarcts when compared to SPECT (52). Identifying regions of LGE in an infarct pattern in elderly patients without a clinical history of MI as unrecognized MI has prognostic import as shown in the ICELAND-MI study (53). In 2349 patients with a mean age of 63 years in the SPINS study, identification of an unrecognized MI by LGE was equivalent to the prognostic value of a clinically recognized MI (54). A meta-analysis of 19 studies including 11,636 patients, of whom 29% had LGE, demonstrated that combining first pass perfusion stress CMR with LGE has significant prognostic value for predicting cardiovascular death and nonfatal MI, as individual and composite events (55). The SPINS study also showed that LGE was additive to the presence of ischemia by stress CMR in prognostication of cardiovascular death or nonfatal MI (14). Either finding alone had intermediate prognostic value, whereas the combination conferred the greatest future cardiovascular risk.
Challenges and Limitations
Although stress CMR is an excellent tool for the assessment of SIHD, it has some limitations. Notably, stress CMR cannot readily be combined with exercise at present; however, prototype treadmills that can be used in the MRI environment have been developed (56). There are also several potential patient-related factors that might limit its widespread use. Claustrophobic and obese patients may not be able to tolerate the study; however, modern wide-bore scanners combined with the use of low dose oral sedation have reduced this challenge to exist in only 3-4% of all patients. Additionally, patients with implantable devices comprised of ferromagnetic materials may not be ideal candidates for stress CMR due to device-related imaging artifacts that limit interpretability of the images. Previous concerns related to the risk of nephrogenic systemic fibrosis in patients with advanced renal disease have been greatly diminished with the use of macrocyclic GBCAs and increased screening for severe renal insufficiency. The utilization of stress CMR is also hampered by common misperceptions that consider stress CMR as an expensive procedure. Indeed, the payment for a stress CMR examination ($680.74) by the Hospital Outpatient Prospective Payment System in the year 2020 was considerably less than that for SPECT ($1,272.05) and PET ($1,443.00 or $2,250.50 if combined with computed tomography transmission scan), paving the way for it to be a highly cost-effective strategy for assessing SIHD.
Conclusions
Stress CMR has been extensively validated for the evaluation of patients with known and suspected CAD. Through its ability to assess myocardial ischemia, presence of myocardial scar, and global and regional function, it can accurately detect hemodynamically significant CAD, assess microvascular function, predict myocardial viability, guide the need for revascularization, and provide clinical risk stratification safely and effectively. It has shown superiority to SPECT and equivalence to an FFR-guided invasive angiographic approach to patient evaluation. Given its clinical advantages, lack of ionizing radiation, and cost-effectiveness, it should be expected that stress CMR will be increasingly utilized for the evaluation of ischemic heart disease.
Highlights:
Stress cardiac magnetic resonance imaging (CMR) accurately assesses myocardial ischemia, myocardial viability, and cardiac function without exposure to ionizing radiation.
A revascularization strategy guided by stress CMR has effectiveness comparable to that guided by invasive measurement of fractional flow reserve, and can be particularly helpful in clinically stable patients with a moderate to high pretest probability of ischemic heart disease.
Quantification of myocardial blood flow from stress CMR images is an emerging application for assessment of ischemic burden and coronary microvascular function.
Disclosures:
Dr. Patel has received research grant and/ or support from Philips, General Electric, Arterys, CircleCVI, and Neosoft. Dr. Salerno has received grant support from the National Institutes of Health (R01 HL131919); and research support from Siemens Healthcare. Dr.Kwong has received grant support from the American Heart Association, MyoKardia Inc., and Alnylam Pharmaceuticals. Dr. Kramer is a consultant for BMS and Lilly and receives grant support from the NIH (5R01 HL075792 and U01HL117006-01A1). Dr. Singh and Dr. Heydari have no disclosures.
Abbreviations:
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- CE-MARC
Clinical Evaluation of Magnetic Resonance imaging in Coronary heart disease
- CMR
cardiovascular magnetic resonance
- COURAGE
Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation
- FAME
Fractional flow reserve versus Angiography for Multivessel coronary artery disease Evaluation
- FFR
fractional flow reserve
- GadaCad
Gadobutrol-enhanced CMR to detect Coronary Artery Disease
- GBCA
gadolinium-based contrast agent
- ICA
invasive coronary angiography
- ISCHEMIA
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches
- LGE
late gadolinium enhancement
- MACE
major adverse cardiac event
- MBF
myocardial blood flow
- MI
myocardial infarction
- MPR
myocardial perfusion reserve
- MR-INFORM trial
The Myocardial Perfusion CMR versus Angiography and FFR to Guide the Management of Patients with Stable Coronary Artery Disease
- OMT
optimal medical therapies
- PET
positron emission tomography
- SIHD
stable ischemic heart disease
- SPECT
single photon emission computed tomography
- SPINS
Stress CMR Perfusion Imaging in the United States study
- STRATEGY
Stress Cardiac Magnetic Resonance Versus Computed Tomography Coronary Angiography for the Management of Symptomatic Revascularized Patients trial
Appendix 1
Semi-Quantitative Parameters of Myocardial Perfusion
In order to perform semi-quantitative assessment of myocardial perfusion the signal intensity in the left ventricular cavity and myocardial segments at stress and rest must be quantified. Commonly used semi-quantitative metrics include area under the myocardial signal intensity curve during first pass, and upslope methods.[1] For upslope methods, the upslope of the myocardial signal intensity curve is normalized to the upslope of the signal within the LV cavity that is used as the arterial input. By performing upslope analysis at rest and stress a Myocardial Perfusion Reserve Index (MPRI) can be defined.[2] While this technique requires minimal processing of the signal intensity curves, the signal intensity in the myocardium and blood pool have a non-linear relationship to the gadolinium concentration, which is more closely related to the myocardial perfusion. Further this method of quantifying signal intensity is dependent on the pulse sequence parameters. For these reasons specific values for upslope parameters are only valid in the context of the same measurement technique resulting in difficulty in defining universal thresholds for abnormal perfusion and perfusion reserve.
Despite these limitations, a number of studies have demonstrated good performance of semi-quantitative upslope parameters for assessing obstructive CAD.[2] Furthermore, MPRI has been used in studies for assessing microvascular disease.[3] Due to the aforementioned issues the intra-reader reproducibility of MPRI assessment is only modest.
Absolute Quantification of Myocardial Blood Flow by First Pass Perfusion
Quantification of myocardial perfusion by first-pass perfusion by first-pass CMR was proposed by Jerosch-Herold et al in 1998 using a technique proposed earlier by Leon Axel for first-pass CT perfusion.[4] Conceptually this approach is based on measuring an arterial input function (AIF) which describes the contrast delivery, and the tissue function (TF) which describes the contrast concentration in the myocardium as a function of time. The myocardial blood flow can be determined using a mathematical operation called deconvolution to derive a system response function (sometimes called the residue function) which describes a kinetic model for the passage of contrast through the myocardium. The most common approach in the literature, likely due to its simplicity and robustness, has been to constrain the system function to have the empirical shape of a fermi-function.[4] Using this approach, the myocardial blood flow is given as the initial amplitude the residue function. Other mathematical approaches include two-compartment kinetic modeling[5], distributed parameter models[6], model-independent[7], and differential equation models such as the blood-tissue exchange model (BTEX) [8]. These techniques differ in the number of parameters that are modeled, the complexity of the models, and their robustness to violation of underlying assumptions.
One very important point is that for accurate estimation of myocardial blood flow using this approach the AIF and TF need to be directly proportional to the gadolinium contrast agent. While at very low contrast doses the signal intensity of the perfusion images is roughly proportional to the contrast does, for typical doses of contrast used clinically (0.05-0.1 mmol/kg) this assumption does not hold due to the nonlinearity of signal intensity due to saturation effects from T1 recovery. Thus the signal intensity should first be converted to gadolinium concentration units using the Bloch equations.[9] This typically requires acquiring proton-density weighted images at the beginning of the dynamic acquisition. Another significant issue is that the arterial input function, which is measured typically from the left ventricular blood pool has very high gadolinium contrast and cannot be accurately measured from the signal in the blood pool from clinically available pulse sequences. Two approaches have been used: In the dual bolus approach, a mini-bolus of contrast typically diluted at a 1:10 ratio is given first to quantify the AIF and then followed by a second high contrast dose to quantify the tissue function.[10] The more commonly used approach is the dual-sequence approach where a separate pulse sequence with a short saturation pulse is used to quantify the high-concentration arterial signal, followed by traditional perfusion pulse sequences to quantify the tissue function.[10] The dual-sequence approach is easier to perform clinically, but requires care to make sure the AIF and TF are accurately converted to gadolinium contrast concentration units. Both approaches have been well validated in animal and human studies. By performing quantitative analysis at rest and under adenosine stress, myocardial flow reserve can be quantified.
Automatic Pipelines for Quantification
While traditionally quantification of perfusion required significant human effort for post-processing, improvements in image registration and segmentation and improvement in reconstruction hardware has made automatic processing feasible. First images for the AIF and TF are registered using non-rigid registration. The LV blood pool is segmented in the AIF images to derive an AIF time intensity curve. Following normalization by the PD images, the AIF signal intensity is converted to T1, and using the difference in T1 from pre-contrast images is converted to gadolinium contrast agent concentration units. The same approach is then applied to the TF images to derive time signal intensity curves for each pixel in the image which are then converted to concentration units. Finally the TF is fit to the convolution of the AIF and the RF on a pixel-by pixel basis. Maps can then be segmented to derive segmental perfusion values if desired. Recently there has been growing interest in using AI and deep learning techniques to automate different parts of this pipeline. Automatic quantification has been compared to standard techniques, validated with invasive angiography, and validated as compared to PET. Following robust vendor implementation, rapid automatic quantification of myocardial perfusion will become feasible for routine clinical practice.
References:
- 1.Jerosch-Herold M, Quantification of myocardial perfusion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson, 2010. 12: p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel E, et al. , Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation, 2003. 108(4): p. 432–7. [DOI] [PubMed] [Google Scholar]
- 3.Thomson LE, et al. , Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging, 2015. 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerosch-Herold M, Wilke N, and Stillman AE, Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys, 1998. 25(1): p. 73–84. [DOI] [PubMed] [Google Scholar]
- 5.Tofts PS, et al. , Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging, 1999. 10(3): p. 223–32. [DOI] [PubMed] [Google Scholar]
- 6.Broadbent DA, et al. , Myocardial blood flow at rest and stress measured with dynamic contrast-enhanced MRI: comparison of a distributed parameter model with a Fermi function model. Magn Reson Med, 2013. 70(6): p. 1591–7. [DOI] [PubMed] [Google Scholar]
- 7.Jerosch-Herold M, Swingen C, and Seethamraju RT, Myocardial blood flow quantification with MRI by model-independent deconvolution. Med Phys, 2002. 29(5): p. 886–97. [DOI] [PubMed] [Google Scholar]
- 8.Xue H, et al. , Automatic in-line quantitative myocardial perfusion mapping: Processing algorithm and implementation. Magn Reson Med, 2020. 83(2): p. 712–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cernicanu A and Axel L, Theory-based signal calibration with single-point T1 measurements for first-pass quantitative perfusion MRI studies. Acad Radiol, 2006. 13(6): p. 686–93. [DOI] [PubMed] [Google Scholar]
- 10.Christian TF, Aletras AH, and Arai AE, Estimation of absolute myocardial blood flow during first-pass MR perfusion imaging using a dual-bolus injection technique: comparison to single-bolus injection method. J Magn Reson Imaging, 2008. 27(6): p. 1271–7. [DOI] [PubMed] [Google Scholar]
Appendix 2
Cine CMR Imaging.
The assessment of left and right ventricular systolic function by cine imaging is a fundamental component of stress CMR exams, with implications for viability assessment, clinical management and overall prognosis. Most commonly, balanced steady-state free precession (bSSFP) sequences are used to ensure optimal visualization of the myocardial-blood pool border and adequate temporal resolution. Long axis views of the heart in the 2-, 3-, and 4-chamber views are routine acquired along with a stack of contiguous short axis slices spanning the full extent of the LV and RV acquired throughout the cardiac cycle allowing for all myocardial segments to be visualized in two different imaging planes and also in multiple contiguous slices (1). Widely acknowledged as the gold standard methodology, quantification of ejection fraction by CMR can be accurately measured and interpreted in the context of contemporary normal value references, and may also offer prognostic information in patients with suspected or known CAD (2-5). The presence of regional wall motion abnormalities at rest may act as a clue to the existence of myocardial infarction, with cine images also serving as a point of reference for the determination of myocardial viability, and myocardial contractile reserve. In addition to global and regional systolic function, CMR measures of ventricular volumes and LV mass are highly reproducible and may offer indicators of ventricular remodeling with relevant prognostic implications for patients undergoing a stress CMR examination (6).
Recognition of subclinical and regional myocardial abnormalities is aided by the use of CMR strain techniques. While beyond the scope of this review, multiple methods for CMR strain quantification exist with the most widely accepted methods utilizing myocardial tagging, which apply magnetized tag lines in a 3D grid (spatial modulation of magnetization, SPAMM) (7,8), Strain-Encoded (SENC) imaging which measures changes in K-space that occur in response to through-plane motion to measure strain (9,10), and Displacement Encoding with Stimulated Echos (DENSE) which quantifies myocardial motion in pixelwise fashion through extracting data regarding tissue displacement from phase-contrast images (11). However, these techniques each require the acquisition of additional images which limits their clinical adoption. More recently, CMR feature tracking (FT) is a technique that is analogous to speckle tracking echocardiography used to measure myocardial strain. It can be applied retrospectively to routine bSSFP cine images and may help bridge the gap which deters CMR strain from widespread clinical use (12) and appears to have several advantages to echocardiography-derived strain measurements (13).
When performed as part of a dobutamine stress CMR examination, both tagging and FT strain techniques have been associated with improvements in sensitivity for detection of resting and stress induced wall motion abnormalities and myocardial contractile reserve when compared to standard cine imaging (14-16). More recently, baseline abnormalities in FT-derived LV strain identified patients at higher risk for adverse events following vasodilator stress CMR, highlighting the potential prognostic impact of routine strain assessment in patients with suspected CAD(17). Pertinent limitations which confine CMR strain to an adjunctive rather than primary role in stress CMR include the need for specific and/or additional sequences, low temporal resolution, variable prolongation of scan time, time-consuming post-processing needs, and variability in normal values according to technique(12).
CMR offers the ability to evaluate for non-ischemic symptom etiology in selected patients, including concomitant diastolic dysfunction and valvular disease. The available CMR-derived indices for diastolic function assessment are varied, and extend well beyond the prototypical structural changes (increased LV mass/hypertrophy, left atrial dilation) to include time-volume LV filling curves, generated from cine-based volumetric data(18,19), phase-contrast imaging to define transmitral and pulmonary venous flow (akin to Doppler echocardiography), and strain techniques including tagging and FT for quantification of strain and strain rate of the LV and left atrium, though the latter is still predominantly limited to research endeavors(20).
References.
- 1.Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020; 22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellenger NG, Burgess MI, Ray SG et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 2000;21:1387–96. [DOI] [PubMed] [Google Scholar]
- 3.Grothues F, Smith GC, Moon JC et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29–34. [DOI] [PubMed] [Google Scholar]
- 4.Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B et al. Reference ranges ("normal values") for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson 2020; 22:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Aidi H, Adams A, Moons KG et al. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol 2014;63:1031–45. [DOI] [PubMed] [Google Scholar]
- 6.Abdi-Ali A, Miller RJH, Southern D et al. LV Mass Independently Predicts Mortality and Need for Future Revascularization in Patients Undergoing Diagnostic Coronary Angiography. JACC Cardiovasc Imaging 2018;11:423–433. [DOI] [PubMed] [Google Scholar]
- 7.Young AA, Imai H, Chang CN, Axel L. Two-dimensional left ventricular deformation during systole using magnetic resonance imaging with spatial modulation of magnetization. Circulation 1994;89:740–52. [DOI] [PubMed] [Google Scholar]
- 8.Fischer SE, McKinnon GC, Maier SE, Boesiger P. Improved myocardial tagging contrast. Magn Reson Med 1993;30:191–200. [DOI] [PubMed] [Google Scholar]
- 9.Korosoglou G, Lossnitzer D, Schellberg D et al. Strain-encoded cardiac MRI as an adjunct for dobutamine stress testing: incremental value to conventional wall motion analysis. Circ Cardiovasc Imaging 2009;2:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korosoglou G, Gitsioudis G, Voss A et al. Strain-encoded cardiac magnetic resonance during high-dose dobutamine stress testing for the estimation of cardiac outcomes: comparison to clinical parameters and conventional wall motion readings. J Am Coll Cardiol 2011;58:1140–9. [DOI] [PubMed] [Google Scholar]
- 11.Kim D, Epstein FH, Gilson WD, Axel L. Increasing the signal-to-noise ratio in DENSE MRI by combining displacement-encoded echoes. Magn Reson Med 2004;52:188–92. [DOI] [PubMed] [Google Scholar]
- 12.Vo HQ, Marwick TH, Negishi K. MRI-Derived Myocardial Strain Measures in Normal Subjects. JACC Cardiovasc Imaging 2018;11:196–205. [DOI] [PubMed] [Google Scholar]
- 13.Erley J, Genovese D, Tapaskar N et al. Echocardiography and cardiovascular magnetic resonance based evaluation of myocardial strain and relationship with late gadolinium enhancement. J Cardiovasc Magn Reson 2019;21:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuijpers D, Ho KY, van Dijkman PR, Vliegenthart R, Oudkerk M. Dobutamine cardiovascular magnetic resonance for the detection of myocardial ischemia with the use of myocardial tagging. Circulation 2003;107:1592–7. [DOI] [PubMed] [Google Scholar]
- 15.Schneeweis C, Qiu J, Schnackenburg B et al. Value of additional strain analysis with feature tracking in dobutamine stress cardiovascular magnetic resonance for detecting coronary artery disease. J Cardiovasc Magn Reson 2014;16:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster A, Kutty S, Padiyath A et al. Cardiovascular magnetic resonance myocardial feature tracking detects quantitative wall motion during dobutamine stress. J Cardiovasc Magn Reson 2011;13:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano S, Romer B, Evans K et al. Prognostic Implications of Blunted Feature-Tracking Global Longitudinal Strain During Vasodilator Cardiovascular Magnetic Resonance Stress Imaging. JACC Cardiovasc Imaging 2020;13:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal N, Mor-Avi V, Volpato V et al. Machine learning based quantification of ejection and filling parameters by fully automated dynamic measurement of left ventricular volumes from cardiac magnetic resonance images. Magn Reson Imaging 2020;67:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendoza DD, Codella NC, Wang Y et al. Impact of diastolic dysfunction severity on global left ventricular volumetric filling - assessment by automated segmentation of routine cine cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamsi-Pasha MA, Zhan Y, Debs D, Shah DJ. CMR in the Evaluation of Diastolic Dysfunction and Phenotyping of HFpEF: Current Role and Future Perspectives. JACC Cardiovasc Imaging 2020;13:283–296. [DOI] [PubMed] [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Tonino PA, De Bruyne B, Pijls NH et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. [DOI] [PubMed] [Google Scholar]
- 2.Takx RA, Blomberg BA, El Aidi H et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015;8. [DOI] [PubMed] [Google Scholar]
- 3.Jerosch-Herold M, Wilke N, Stillman AE. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys 1998;25:73–84. [DOI] [PubMed] [Google Scholar]
- 4.Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020;22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz-Menger J, Bluemke DA, Bremerich J et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update : Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson 2020;22:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danad I, Szymonifka J, Twisk JWR et al. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: a meta-analysis. Eur Heart J 2017;38:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenwood JP, Maredia N, Younger JF et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwitter J, Wacker CM, van Rossum AC et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J 2008;29:480–9. [DOI] [PubMed] [Google Scholar]
- 9.Schwitter J, Wacker CM, Wilke N et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J 2013;34:775–81. [DOI] [PubMed] [Google Scholar]
- 10.Arai AE S-M J, Berman D, et al. Gadobutrol-Enhanced Cardiac Magnetic Resonance Imaging for Detection of Coronary Artery Disease. J Am Coll Cardiol 2020.;76:1536–1547. [DOI] [PubMed] [Google Scholar]
- 11.Watkins S, McGeoch R, Lyne J et al. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation 2009;120:2207–13. [DOI] [PubMed] [Google Scholar]
- 12.Zurick AO, Bolen MA, Kwon DH et al. Pericardial delayed hyperenhancement with CMR imaging in patients with constrictive pericarditis undergoing surgical pericardiectomy: a case series with histopathological correlation. JACC Cardiovasc Imaging 2011;4:1180–91. [DOI] [PubMed] [Google Scholar]
- 13.Hausvater A, Smilowitz NR, Li B et al. Myocarditis in Relation to Angiographic Findings in Patients With Provisional Diagnoses of MINOCA. JACC Cardiovasc Imaging 2020;13:1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwong RY, Ge Y, Steel K et al. Cardiac Magnetic Resonance Stress Perfusion Imaging for Evaluation of Patients With Chest Pain. J Am Coll Cardiol 2019;74:1741–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge Y, Antiochos P, Steel K et al. Prognostic Value of Stress CMR Perfusion Imaging in Patients With Reduced Left Ventricular Function. JACC Cardiovasc Imaging 2020;13:2132–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heitner JF, Kim RJ, Kim HW et al. Prognostic Value of Vasodilator Stress Cardiac Magnetic Resonance Imaging: A Multicenter Study With 48000 Patient-Years of Follow-up. JAMA Cardiol 2019;4:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gargiulo P, Dellegrottaglie S, Bruzzese D et al. The prognostic value of normal stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: a meta-analysis. Circ Cardiovasc Imaging 2013;6:574–82. [DOI] [PubMed] [Google Scholar]
- 18.Shah R, Heydari B, Coelho-Filho O et al. Stress cardiac magnetic resonance imaging provides effective cardiac risk reclassification in patients with known or suspected stable coronary artery disease. Circulation 2013;128:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maron DJ, Hochman JS, Reynolds HR et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagel E, Greenwood JP, McCann GP et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N Engl J Med 2019;380:2418–2428. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood JP, Ripley DP, Berry C et al. Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scintigraphy, or NICE Guidelines on Subsequent Unnecessary Angiography Rates: The CE-MARC 2 Randomized Clinical Trial. JAMA 2016;316:1051–60. [DOI] [PubMed] [Google Scholar]
- 22.Pontone G, Andreini D, Guaricci AI et al. The STRATEGY Study (Stress Cardiac Magnetic Resonance Versus Computed Tomography Coronary Angiography for the Management of Symptomatic Revascularized Patients): Resources and Outcomes Impact. Circ Cardiovasc Imaging 2016;9. [DOI] [PubMed] [Google Scholar]
- 23.Everaars H, van Diemen PA, Bom MJ et al. Comparison between quantitative cardiac magnetic resonance perfusion imaging and [(15)O]H2O positron emission tomography. Eur J Nucl Med Mol Imaging 2020;47:1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozor R, Walker S, Parkinson B et al. Cost-Effectiveness of Cardiovascular Magnetic Resonance in Diagnosing Coronary Artery Disease in the Australian Health Care System. Heart Lung Circ 2020;30:380–387. [DOI] [PubMed] [Google Scholar]
- 25.Moschetti K, Favre D, Pinget C et al. Comparative cost-effectiveness analyses of cardiovascular magnetic resonance and coronary angiography combined with fractional flow reserve for the diagnosis of coronary artery disease. J Cardiovasc Magn Reson 2014;16:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinnel M, Sanguineti F, Pezel T et al. Prognostic value of vasodilator stress perfusion CMR in patients with previous coronary artery bypass graft. Eur Heart J Cardiovasc Imaging 2020;00:1–9. [DOI] [PubMed] [Google Scholar]
- 27.Greenwood JP, Motwani M, Maredia N et al. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circulation 2014;129:1129–38. [DOI] [PubMed] [Google Scholar]
- 28.Ge Y, Steel K, Antiochos P et al. Stress CMR in patients with obesity: insights from the Stress CMR Perfusion Imaging in the United States (SPINS) registry. Eur Heart J Cardiovasc Imaging 2020;22:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pezel T, Sanguineti F, Kinnel M et al. Safety and Prognostic Value of Vasodilator Stress Cardiovascular Magnetic Resonance in Patients With Heart Failure and Reduced Ejection Fraction. Circ Cardiovasc Imaging 2020;13:e010599. [DOI] [PubMed] [Google Scholar]
- 30.Ishimori ML, Martin R, Berman DS et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging 2011;4:27–33. [DOI] [PubMed] [Google Scholar]
- 31.Kazmirczak F, Nijjar PS, Zhang L et al. Safety and prognostic value of regadenoson stress cardiovascular magnetic resonance imaging in heart transplant recipients. J Cardiovasc Magn Reson 2019;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panting JR, Gatehouse PD, Yang GZ et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002;346:1948–53. [DOI] [PubMed] [Google Scholar]
- 33.Kotecha T, Martinez-Naharro A, Boldrini M et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction: Validation Against Invasive Coronary Physiology. JACC Cardiovasc Imaging 2019;12:1958–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doyle M, Weinberg N, Pohost GM et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging 2010;3:1030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gatehouse PD, Elkington AG, Ablitt NA, Yang GZ, Pennell DJ, Firmin DN. Accurate assessment of the arterial input function during high-dose myocardial perfusion cardiovascular magnetic resonance. J Magn Reson Imaging 2004;20:39–45. [DOI] [PubMed] [Google Scholar]
- 36.Villa ADM, Corsinovi L, Ntalas I et al. Importance of operator training and rest perfusion on the diagnostic accuracy of stress perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2018;20:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berman DS, Kang X, Slomka PJ et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol 2007;14:521–8. [DOI] [PubMed] [Google Scholar]
- 38.Patel AR, Antkowiak PF, Nandalur KR et al. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol 2010;56:561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu LY, Jacobs M, Benovoy M et al. Diagnostic Performance of Fully Automated Pixel-Wise Quantitative Myocardial Perfusion Imaging by Cardiovascular Magnetic Resonance. JACC Cardiovasc Imaging 2018;11:697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotecha T, Chacko L, Chehab O et al. Assessment of Multivessel Coronary Artery Disease Using Cardiovascular Magnetic Resonance Pixelwise Quantitative Perfusion Mapping. JACC Cardiovasc Imaging 2020;13:2546–2557. [DOI] [PubMed] [Google Scholar]
- 41.Sammut EC, Villa ADM, Di Giovine G et al. Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging 2018;11:686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knott KD, Seraphim A, Augusto JB et al. The Prognostic Significance of Quantitative Myocardial Perfusion: An Artificial Intelligence-Based Approach Using Perfusion Mapping. Circulation 2020;141:1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdi-Ali A, Miller RJH, Southern D et al. LV Mass Independently Predicts Mortality and Need for Future Revascularization in Patients Undergoing Diagnostic Coronary Angiography. JACC Cardiovasc Imaging 2018;11:423–433. [DOI] [PubMed] [Google Scholar]
- 44.Higgins CB, Herfkens R, Lipton MJ et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol 1983;52:184–8. [DOI] [PubMed] [Google Scholar]
- 45.Simonetti OP, Kim RJ, Fieno DS et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology 2001;218:215–23. [DOI] [PubMed] [Google Scholar]
- 46.Kim RJ, Fieno DS, Parrish TB et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002. [DOI] [PubMed] [Google Scholar]
- 47.Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation 2001;104:1101–7. [DOI] [PubMed] [Google Scholar]
- 48.Kim RJ, Wu E, Rafael A et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53. [DOI] [PubMed] [Google Scholar]
- 49.Bonow RO, Maurer G, Lee KL et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med 2011;364:1617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah DJ, Kim HW, James O et al. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA 2013;309:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner A, Mahrholdt H, Thomson L et al. Effects of time, dose, and inversion time for acute myocardial infarct size measurements based on magnetic resonance imaging-delayed contrast enhancement. J Am Coll Cardiol 2006;47:2027–33. [DOI] [PubMed] [Google Scholar]
- 52.Ibrahim T, Bulow HP, Hackl T et al. Diagnostic value of contrast-enhanced magnetic resonance imaging and single-photon emission computed tomography for detection of myocardial necrosis early after acute myocardial infarction. J Am Coll Cardiol 2007;49:208–16. [DOI] [PubMed] [Google Scholar]
- 53.Schelbert EB, Cao JJ, Sigurdsson S et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 2012;308:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antiochos P, Ge Y, Steel K et al. Imaging of Clinically Unrecognized Myocardial Fibrosis in Patients With Suspected Coronary Artery Disease. J Am Coll Cardiol 2020;76:945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol 2013;62:826–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raman SV, Dickerson JA, Mazur W et al. Diagnostic Performance of Treadmill Exercise Cardiac Magnetic Resonance: The Prospective, Multicenter Exercise CMR's Accuracy for Cardiovascular Stress Testing (EXACT) Trial. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwitter J, Wacker CM, Wilke N et al. Superior diagnostic performance of perfusion-cardiovascular magnetic resonance versus SPECT to detect coronary artery disease: The secondary endpoints of the multicenter multivendor MR-IMPACT II (Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial). J Cardiovasc Magn Reson 2012;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manka R, Paetsch I, Kozerke S et al. Whole-heart dynamic three-dimensional magnetic resonance perfusion imaging for the detection of coronary artery disease defined by fractional flow reserve: determination of volumetric myocardial ischaemic burden and coronary lesion location. Eur Heart J 2012;33:2016–24. [DOI] [PubMed] [Google Scholar]
- 59.Bettencourt N, Chiribiri A, Schuster A et al. Cardiac magnetic resonance myocardial perfusion imaging for detection of functionally significant obstructive coronary artery disease: a prospective study. Int J Cardiol 2013;168:765–73. [DOI] [PubMed] [Google Scholar]
- 60.Ebersberger U, Makowski MR, Schoepf UJ et al. Magnetic resonance myocardial perfusion imaging at 3.0 Tesla for the identification of myocardial ischaemia: comparison with coronary catheter angiography and fractional flow reserve measurements. Eur Heart J Cardiovasc Imaging 2013;14:1174–80. [DOI] [PubMed] [Google Scholar]
- 61.Buckert D, Witzel S, Steinacker JM, Rottbauer W, Bernhardt P. Comparing Cardiac Magnetic Resonance-Guided Versus Angiography-Guided Treatment of Patients With Stable Coronary Artery Disease: Results From a Prospective Randomized Controlled Trial. JACC Cardiovasc Imaging 2018;11:987–996. [DOI] [PubMed] [Google Scholar]