Abstract
Despite the established benefits of regular physical activity in cardiovascular disease prevention, coronary events in the context of atherosclerotic coronary artery disease are the most common cause of exercise-related sudden death. A paradoxical development of an increased coronary calcification burden is likely associated with endurance training even in the absence of any of the traditional cardiovascular risk factors. In this case report, we present a 50-year-old male long-distance runner with excessive subclinical myocardial ischemia.
Keywords: marathon running, endurance exercise, subclinical myocardial ischemia, coronary calcification, cardiovascular risk stratification
Key Points
Endurance training is associated with increased calcification of the coronary arteries of unclarified clinical significance.
Routine screening tests may be ineffective for detecting coronary artery disease in athletes with low cardiovascular risk profiles, as predicted by conventional cardiovascular risk scores.
Regular physical activity is considered a basic component of a healthy lifestyle with well-established benefits for preventing atherosclerotic cardiovascular disease (CVD).1 In particular, even at lower doses, running has been associated with reduced all-cause and CVD mortality.2 These benefits are mainly attributed to the positive effect of regular exercise on endothelial function.
Paradoxically, coronary events in the context of previously clinically silent atherosclerotic coronary artery disease were the most common cause of exercise-related sudden death in long-distance athletes with an otherwise low atherosclerotic risk profile.3 Although it is recognized that vigorous physical activity may increase the short-term risk of coronary events, especially in the absence of any previous habitual exercise, the relationship of endurance training with coronary artery disease seems controversial.4 Researchers5 have addressed an association between long-term marathon training and the formation of coronary artery atherosclerotic plaques. The underlying pathogenetic background as well as the clinical relevance of this association remain to a great extent unclear. Given that endurance athletes are usually asymptomatic, their very low cardiovascular risk profile (as assessed by conventional risk scores), and the poor diagnostic value of standard clinical exercise tests, an optimal cardiovascular screening strategy for this population is yet to be determined.
In this case report, we highlight the importance of the medical history and detailed imaging evaluation for the prompt detection of coronary artery disease in asymptomatic healthy athletes.
CASE PRESENTATION
Patient Section
A 50-year-old male marathon runner was referred to our department for a routine cardiologic evaluation. The athlete was apparently healthy with the exception of a history of deep vein thrombosis of the left lower limb treated with rivaroxaban for 6 months. The laboratory investigation of that episode revealed heterozygosity for a 677T mutation in the gene for methylenetetrahydrofolate reductase (MTHFR), the enzyme responsible for homocysteine recycling in the human body, and the thrombosis was finally attributed to exhaustive exercise.
Traditional cardiovascular risk factors, namely arterial hypertension, diabetes mellitus, family history of CVD, and smoking, were absent; therefore, he was considered to have a very low cardiovascular risk. He had a strict training program consisting of approximately 15 km of continuous and interval training running per day, with participation in multiple marathon races over the past 10 years. Furthermore, he had never experienced chest pain, arrhythmia, or any other symptom that could indicate underlying cardiovascular dysfunction.
Evaluation
At his first evaluation in our department, physical examination revealed no pathologic heart sounds. Arterial blood pressure was within normal limits. Electrocardiography showed sinus rhythm and no repolarization abnormalities (Figure 1). The athlete underwent a treadmill test, performed according to the standard Bruce protocol. He exercised for 14 minutes, achieving a peak heart rate of 145 beats/min and a total workload of 12.1 metabolic equivalents. No chest pain or other discomfort was reported during the test, and the exercise electrocardiogram revealed no significant ST-segment depression. Routine laboratory tests revealed no pathologic values. More specifically, a complete blood count showed normal values, and a comprehensive metabolic panel demonstrated no signs of abnormal liver or renal function. Cholesterol values were also within normal ranges (total cholesterol = 187 mg/dL, high-density lipoprotein cholesterol = 56 mg/dL, low-density lipoprotein cholesterol = 95 mg/dL, triglyceride = 87 mg/dL).
Figure 1.
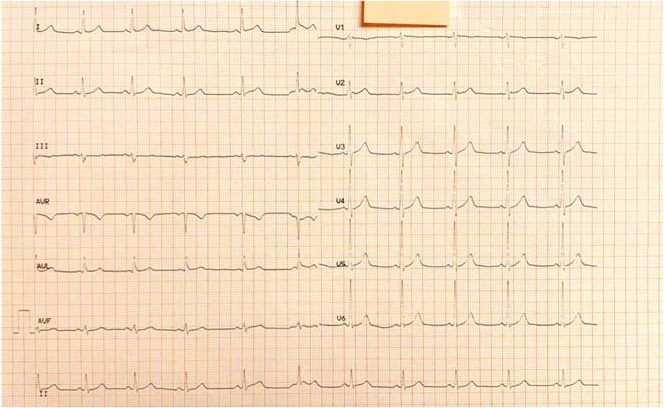
Electrocardiogram at first evaluation showing no significant abnormalities.
An echocardiography study was also performed, demonstrating mildly increased left ventricular dimensions, with preserved contractility and an ejection fraction of 50%, without any specific wall-motion abnormalities, along with left atrial enlargement. Although these findings were considered consistent with “athlete's heart,” further evaluation with stress echocardiography and a cardiac magnetic resonance (CMR) scan was ordered, mainly due to the observed left ventricular enlargement and borderline ejection fraction.
The stress echocardiography study was to follow several weeks later and be performed before the CMR. The patient came to his scheduled appointment in the outpatient clinic directly after running 12 km during his morning training. Surprisingly, the repeat electrocardiogram showed T-wave inversions in precordial leads V1–V5 and in limb lead augmented vector left (Figure 2), which were absent in the previous electrocardiogram. In addition to these findings, striking dyskinesia of the apical parts of the left ventricular wall along with a reduced systolic function and an estimated fraction of 40% were detected echocardiographically, with the patient remaining free of any symptoms.
Figure 2.
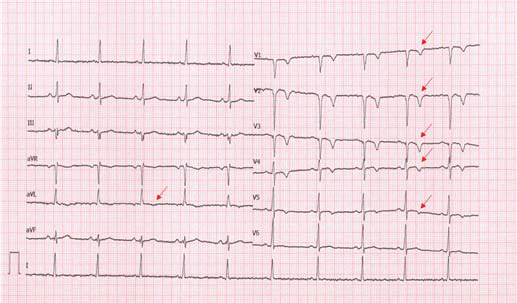
Electrocardiogram at second evaluation demonstrating T-wave inversion in the precordial leads V1–V5 and in limb lead augmented vector left (arrows).
Outcomes
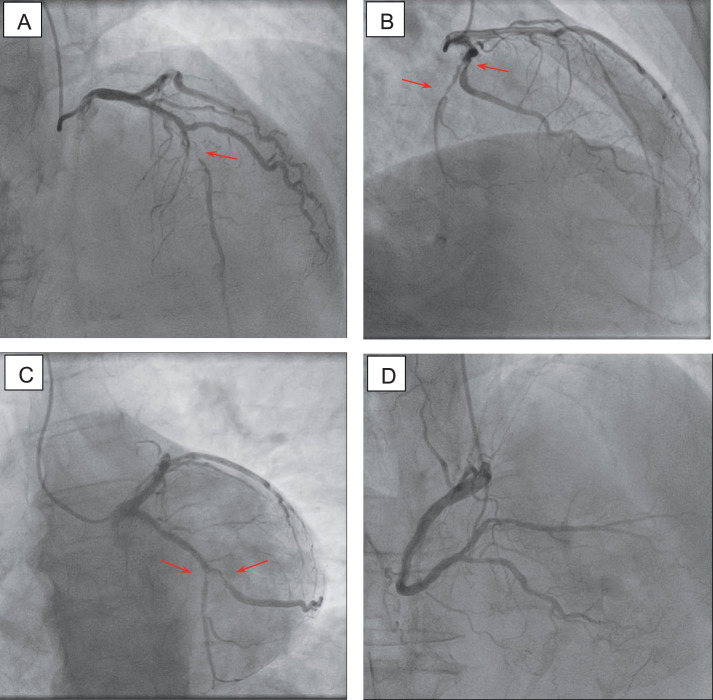
In light of the results, instead of performing stress echocardiography, we decided to immediately proceed to coronary angiography, which, to our surprise, revealed 2-vessel coronary disease in the presence of excessively calcified stenotic coronary lesions. In particular, a mid-left anterior descending artery subocclusion along with an 80% distal left circumflex artery stenosis and an 80% proximally occluded obtuse marginal branch were demonstrated; the right coronary artery was highly atherosclerotic without any critical stenosis (Figure 3).
Figure 3.
Coronary angiogram showing A, subocclusion of the mid-left anterior descending artery (arrow); B, C, 80% stenosis of the distal left circumflex artery and 80% stenosis of the proximal obtuse marginal branch (arrows); and D, highly atherosclerotic right coronary artery.
Considering the extent and complexity of coronary lesions as well as the existence of viable myocardium, as assessed echocardiographically, a surgical revascularization strategy was recommended to our patient. Two weeks later, the patient underwent successful coronary artery bypass graft surgery. The left internal mammary artery was anastomosed to the distal left anterior descending artery, and a saphenous venous graft was anastomosed to the obtuse marginal branch.
The athlete was mobilized shortly after the operation, performing short walks around the nurse's station. Four weeks after hospital discharge, he returned to outdoor physical activities, performing short-duration walks for up to 1 hour per day. He was allowed to start running at a comfortable pace and at a noncompetitive level 3 months after the operation and was advised to immediately report any chest pain or discomfort during exercise.
DISCUSSION
Despite the established and undeniable health benefits of regular physical training, sufficient evidence now exists to support the existence of a small but significant risk of atherosclerotic coronary artery disease among endurance athletes. Indeed, a paradoxical association of long-term participation in marathon running with increased calcified coronary plaque volume as assessed by computed tomography coronary angiography has been demonstrated.5,6 This association seems to be independent of the presence of cardiovascular risk factors and to exist even in athletes with low atherosclerotic risk profiles, as assessed by the commonly used risk score algorithms.
The precise pathogenetic substrate for the existence of an increased coronary calcification burden among endurance athletes remains largely unknown. A possible explanation is that hyperdynamic coronary blood flow during exercise is responsible for the development of increased shear stress forces in the coronary lumen, resulting in chronic endothelial injury. Repair of the injured coronary endothelium leads to increased calcium deposition and atherosclerotic plaque formation.7 The atherosclerotic process is likely to be further accelerated by increased vascular oxidative stress, after the excessive production of inflammatory cytokines accompanying endurance training.8
The clinical relevance of these findings is uncertain. Given the established role of coronary calcification as a strong marker for atherosclerotic coronary artery disease, a potentially higher cardiovascular risk than that predicted by conventional cardiovascular risk scores may be present in endurance athletes.6 In fact, ischemic heart disease is the most common cause of sudden death among athletes above the age of 35.9 Increased mechanical forces on calcified plaques during endurance training may induce plaque erosion or rupture, ultimately resulting in epicardial thrombus formation and microembolization.10 Nevertheless, the precise pathogenetic pathways for a possible association between an elevated epicardial plaque burden and ischemic myocardial damage remain to be established.
In this report, we emphasize that the detection of subclinical ischemic cardiomyopathy by routine screening tests may be quite challenging. A detailed assessment of the athlete's medical history may reveal crucial information, such as exercise-related palpitations, episodes of atypical angina, or even syncope. Apparently normal electrocardiograms should always be interpreted with caution, and echocardiography should be interpreted after considering the athlete's particular characteristics, including sport type, race, sex, age, and body size.11 Although exercise stress testing remains the established method for evaluating those wishing to participate in competitive sports, it is known to have low sensitivity for detecting coronary artery disease, especially in asymptomatic athletes older than 35 years of age with a low risk profile.12 Stress echocardiography may be more efficient in detecting ischemic heart disease by provoking an oxygen demand-supply mismatch in the myocardium, resulting in regional wall motion abnormalities.12 We believe that, in our patient's case, the demanding morning training preceding his echocardiographic evaluation may have contributed to wall motion changes due to an exercise-dependent deterioration of myocardial oxygenation. Our initial decision to further evaluate cardiac function with magnetic resonance and stress echocardiography was mainly prompted by the enlarged left ventricle with borderline contractility, which was observed on the initial echocardiography.
In a previous investigation of a deep vein thrombosis episode, heterozygosity for a 677T mutation in the MTHFR gene was revealed in our patient. The MTHFR gene encodes the MTHFR enzyme, which plays a central role in folate and homocysteine metabolism. Mutations of the MTHFR gene can lead to less active forms of the gene, resulting in mild-to-moderate elevations of plasma total homocysteine, an emerging factor for coagulation disorders, endothelial dysfunction, and atherosclerotic CVD. In particular, the 677T mutation is considered the most common MTHFR mutation, with a high prevalence in certain ethnic and geographic populations; heterozygosity for the mutation results in enzyme function that is approximately 65% of normal. A less active enzyme may not necessarily lead to elevated homocysteine levels. In addition, unlike with homozygosity, no robust data are available to confirm an association between heterozygosity for the 677T mutation and a higher CVD burden.13 Considering this information in total, it is unlikely that heterozygosity for the 677T mutation could have contributed to the extensive coronary artery disease observed in our patient.
Ventricular arrhythmias are rather common in athletes participating in competitive sports and are usually unrelated to underlying cardiovascular abnormalities.14 Nevertheless, an ischemic myocardium may constitute a substrate for the development of arrhythmias resulting from electrical instability and, ultimately, sudden cardiac death. A CMR scan often reveals areas with significant late gadolinium enhancement, which is a recognized marker of myocardial fibrosis. The subendocardial distribution of fibrosis usually indicates ischemic myocardial damage, and its extent is significantly associated with the risk for spontaneous ventricular arrhythmias.15 A strong association between late gadolinium enhancement and higher coronary artery calcium scores has been demonstrated in marathon runners, providing additional evidence for a pathogenetic link between subclinical coronary calcification and intramyocardial microvascular damage.6 However, beyond ischemic heart disease, structural cardiac abnormalities (such as hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and congenital coronary anomalies) as well as electrical abnormalities (including Wolff-Parkinson-White syndrome) and inherited cardiac channel-opathy are among the most common causes of fatal arrhythmias in younger athletes (<35 years old) leading to sudden cardiac death.10
A few weeks after the coronary artery bypass grafting operation, our athlete was encouraged to gradually return to outdoor physical activities. The beneficial effects of exercise on cardiovascular health overall survival are well recognized in patients undergoing percutaneous or surgical coronary artery revascularization. After a 2- to 6-week convalescence period, surgical patients are encouraged to participate in programs that include aerobic exercise using the large muscle groups, such as walking, jogging, and cycling, 3 to 5 times per week. The type and intensity of exercise are individualized according to the patient's heart rate, subjective feeling of exertion, and severity of the underlying heart disease and should be promptly modified in case of recurrent angina. Optimally, patients should be strongly encouraged to join exercise-based, medically supervised cardiac rehabilitation programs that provide a holistic approach to the CVD, including the management of risk factors and the stress and depression of the postoperative period.
Clinical Bottom Line
Atherosclerotic coronary artery disease is not uncommon in competitive athletes and may not be accurately predicted using conventional cardiovascular risk scores. Although the clinical significance of an increased subclinical plaque burden associated with endurance training is yet to be determined, we highlight the importance of detailed screening for ischemic heart disease independent of the presence of symptoms or relevant risk factors in this apparently low-risk population.
Special features include the following:
Left ventricular enlargement with borderline ejection fraction
Dynamic electrocardiographic changes postexercise
Excessive calcification of the coronary arteries
REFERENCES
- 1.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation . 2007;116(19):2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol . 2014;64(5):472–481. doi: 10.1016/j.jacc. 2014.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noakes TD, Opie LH, Rose AG, Kleynhans PH, Schepers NJ, Dowdeswell R. Autopsy-proved coronary atherosclerosis in marathon runners. N Engl J Med . 1979;301(2):86–89. doi: 10.1056/NEJM197907123010205. [DOI] [PubMed] [Google Scholar]
- 4.Kessler KM. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med . 2001;344(11):854–855. doi: 10.1056/NEJM200103153441114. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RS, Kraus SM, Schwartz JG, et al. Increased coronary artery plaque volume among male marathon runners. Mo Med . 2014;111(2):89–94. [PMC free article] [PubMed] [Google Scholar]
- 6.Möhlenkamp S, Lehmann N, Breuckmann F, et al. Running: the risk of coronary events : prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J . 2008;29(15):1903–1910. doi: 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest . 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Nakaji S, Yamada M, et al. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc . 2003;35(2):348–355. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- 9.Pigozzi F, Rizzo M. Sudden death in competitive athletes. Clin Sports Med . 2008;27(1):153–181. doi: 10.1016/j.csm. ix. doi: 2007.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Heusch G, Schulz R, Baumgart D, Haude M, Erbel R. Coronary microembolization. Prog Cardiovasc Dis . 2001;44(3):217–230. doi: 10.1053/pcad. 2001.26968. [DOI] [PubMed] [Google Scholar]
- 11.Lawless CE, Asplund C, Asif IM, et al. Protecting the heart of the American athlete: proceedings of the American College of Cardiology Sports and Exercise Cardiology Think Tank October 18, 2012, Washington, DC. J Am Coll Cardiol . 2014;64(2):2146–2171. doi: 10.1016/j.jacc.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Borjesson M, Dellborg M, Niebauer J, et al. Recommendations for participation in leisure time or competitive sports in athletes-patients with coronary artery disease: a position statement from the Sports Cardiology Section of the European Association of Preventive Cardiology (EAPC) Eur Heart J . 2019;40(1):13–18. doi: 10.1093/eurheartj/ehy408. [DOI] [PubMed] [Google Scholar]
- 13.Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J . 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biffi A, Pelliccia A, Verdile L, et al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol . 2002;40(3):446–452. doi: 10.1016/s0735-1097(02)01977-0. [DOI] [PubMed] [Google Scholar]
- 15.Scott PA, Morgan JM, Carroll N, et al. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol . 2011;4(3):324–330. doi: 10.1161/CIRCEP.110.959544. [DOI] [PubMed] [Google Scholar]