Pancreatic cancer has known precursor lesions with potential to develop into malignancy over time. At least 20% of pancreatic cancer evolves from mucinous cystic neoplasms and intraductal papillary mucinous neoplasms, which are often discovered incidentally.1,2 Current guidelines for the management of mucinous cystic neoplasms and intraductal papillary mucinous neoplasms include long-term surveillance, which is expensive and nontherapeutic, or surgical resection, which is associated with major risk and may not be an option for patients with significant concomitant illness.3
Endoscopic ultrasound (EUS)-guided chemoablation of pancreatic cysts has emerged as an attractive alternative. An innovative and minimally invasive technique, EUS-guided chemoablation has proven efficacy and a superior safety profile to surgery. Although several promising reports4–7 on the short-term outcomes of EUS-guided chemoablation have been published, its long-term durability is unclear. In a recent, large cohort study, Choi et al8 reported that 98.3% of patients with completely resolved pancreatic cysts following EUS-guided chemoablation with paclitaxel maintained their results at 6 years. Although these findings do indicate the excellent durability of chemoablation, this study included serous cystadenomas and pseudocysts, which are generally low-risk and do not require intervention. Our study, therefore, is the first to report exclusively on the long-term outcomes of EUS-guided chemoablation of mucinous or indeterminate type cysts. Here, we provide a long-term follow-up report of the Chemotherapy for Ablation and Resolution of Mucinous pancreatic cysts (CHARM) trial to assess the durability of treatment response following EUS-guided chemoablation.
Methods
This study evaluates patients who were treated in the CHARM trial (NCT01475331) at the Penn State Health Milton S. Hershey Medical Center. The complete study design and eligibility criteria of the CHARM trial have been reported elsewhere.4 Briefly, in a prospective, randomized, double-blind trial, patients with eligible 1.5–5 cm mucinous-type pancreatic cysts were randomized to either ethanol or saline lavage. Following lavage, all cysts were infused with a chemotherapeutic admixture of paclitaxel and gemcitabine. At 12 months postablation, patients underwent radiographic imaging, and CHARM treatment response was assessed.
Patients included in the present long-term analysis are those who had imaging performed at least 12 months following the CHARM treatment response assessment. The most recent images available for each patient were used for this report.
One study-involved radiologist read the baseline, 12-month, and long-term follow-up images for each of the included patients. Cysts were measured in at least 2 dimensions, and cyst volume was calculated using , where r is the mean cyst radius. Treatment response was defined according to percent reduction in cyst volume from baseline as follows: complete response, ≥95% reduction in cyst volume; partial response, 94%–75% reduction; and nonresponse, <75% reduction.
Results
Of the 39 patients treated in the CHARM trial, 36 were included in the long-term analysis. At baseline, mean cyst diameter was 27.6 mm, and most cysts were clinically diagnosed as intraductal papillary mucinous neoplasms (69.4%) or mucinous cystic neoplasms (22.2%). Demographics of the sample and additional baseline cyst characteristics are presented in Supplementary Table 1.
Follow-up imaging was performed at a mean of 36.5 months (range, 20–78) following CHARM treatment response assessment. Of the 23 patients who achieved complete resolution at the initial assessment, 20 (87.0%) sustained those results at their latest follow-up. The other 3 patients had largely maintained their response, demonstrating 91%–93% reductions in cyst volume at follow-up. All cyst volumes from baseline to follow-up are displayed in Figure 1.
Figure 1.
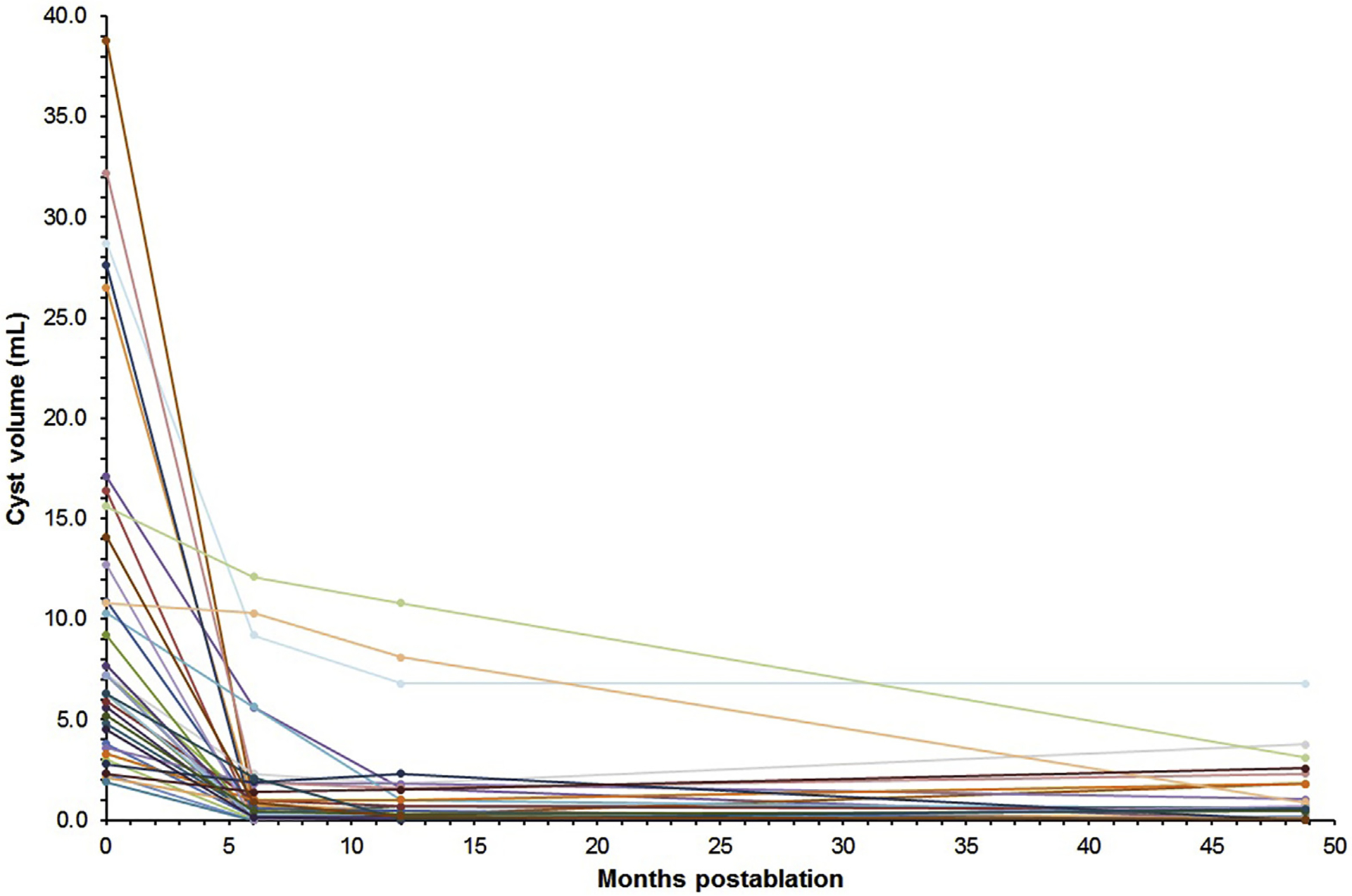
Volume of pancreatic cysts from baseline to long-term follow-up after a single EUS-guided chemoablation treatment in the CHARM trial.
Several initially persistent cysts proceeded toward resolution throughout the follow-up period: 4 cysts (30.8%) classified as partial responders (n = 3) or nonresponders (n = 1) at the CHARM assessment were found to have completely resolved at latest follow-up, and 2 cysts initially classified as nonresponders achieved partial resolution. One cyst regressed from a partial responder to nonresponder. The distribution of treatment responses at 12 months postablation and at long-term follow-up is shown in Supplementary Table 2.
Discussion
In this report, we show that complete cyst resolution after EUS-guided chemoablation with paclitaxel and gemcitabine is durable, with 87% of complete responders at 12 months having maintained resolution at their latest follow-up. Although our results highlight the long-term durability of a complete treatment response, the data also suggest the possibility of protracted treatment effects, because 31% of patients who do not achieve complete response initially may continue toward resolution.
The present study does have notable limitations. First, 2 of the patients lost to follow-up had initially achieved complete resolution, so it is possible that the absence of their long-term data affected the results. In addition, the CHARM trial used a small sample size and strict eligibility criteria because of its investigational nature. Therefore, the results presented here may not be representative of all mucinous-type pancreatic cysts.
Overall, these results are encouraging and clarify a key area of uncertainty surrounding EUS-guided ablation, increasing its appeal as a safe, low-cost, minimally invasive procedure that provides long-term control for premalignant pancreatic cysts. Although there is currently no long-term, prospective data to make the claim that EUS-guided chemoablation prevents pancreatic adenocarcinoma, our results demonstrate that chemoablation inhibits further progression of treated cysts over time and, thus, prevents invasive surgery. In addition, because surveillance following chemoablation is typically recommended at 12 months and then as per the new cyst dimensions thereafter, patients with complete resolution, and even many with partial resolution, experience a substantial reduction in post-treatment surveillance. EUS-guided chemoablation is, therefore, an attractive and effective treatment modality for appropriately selected patients, including those who are unable or unwilling to undergo major surgical resection.
Supplementary Material
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2021.03.041.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Distler M, Aust D, Weitz J, et al. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. Biomed Res Int 2014;2014:474905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crippa S, Castillo CF, Salvia R, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol 2010;8:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amini N, Spolverato G, Kim Y, et al. Trends in hospital volume and failure to rescue for pancreatic surgery. J Gastrointest Surg 2015;19:1581–1592. [DOI] [PubMed] [Google Scholar]
- 4.Moyer MT, Sharzehi S, Mathew A, et al. The safety and efficacy of an alcohol-free pancreatic cyst ablation protocol. Gastroenterology 2017;153:1295–1303. [DOI] [PubMed] [Google Scholar]
- 5.Moyer MT, Maranki JL, DeWitt JM. EUS-guided pancreatic cyst ablation: a clinical and technical review. Curr Gastroenterol Rep 2019;21:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canakis A, Law R, Baron T. An updated review on ablative treatment of pancreatic cystic lesions. Gastrointest Endosc 2020;91:520–526. [DOI] [PubMed] [Google Scholar]
- 7.Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology 2011;140:172–179. [DOI] [PubMed] [Google Scholar]
- 8.Choi JH, Seo DW, Song TJ, et al. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy 2017;49:866–873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


