Abstract
Background
Pulse arrival time (PAT) is commonly used to estimate blood pressure response. We hypothesized that PAT response to obstructive respiratory events would be associated with increased cardiovascular risk in people with obstructive sleep apnea (OSA).
Methods
PAT, defined as the time interval between electrocardiography R wave and pulse arrival by photoplethysmography, was measured in the Multi-Ethnic Study of Atherosclerosis Sleep study participants. The PAT response to apneas/hypopneas was defined as the area under the PAT waveform following respiratory events. Cardiovascular outcomes included markers of subclinical cardiovascular disease (CVD): left ventricular mass, carotid plaque burden score, and coronary artery calcification (CAC) (cross-sectional) and incident composite CVD events (prospective). Multivariable logistic and Cox proportional hazard regressions were performed.
Results
A total of 1407 participants (mean age 68.4 years, female 47.5%) were included. Higher PAT response (per 1 SD increase) was associated with higher left ventricular mass (5.7 g/m2 higher in 4th vs. 1st quartile, p<0.007), higher carotid plaque burden score (0.37 higher in 4th vs. 1st quartile, p=0.02), and trended to greater odds of CAC (1.44 [0.98, 2.15], p=0.06). A total of 65 incident CVD events were observed over the mean of 4.1(2.6) years follow up period. Higher PAT response was associated with increased future CVD events (HR: 1.20 [1.02, 1.42], p=0.03).
Conclusion
PAT is independently associated with markers of subclinical CVD and incident CVD events. Respiratory-related PAT response is a novel and promising polysomnography metric with cardiovascular implications.
Keywords: cardiovascular disease, polysomnography, pulse arrival time, pulse transit time, sleep study, sleep apnea
Introduction
Obstructive sleep apnea (OSA) is associated with incident cardiovascular (CV) disease (CVD) and major adverse CV events among patients with CVD.1–4 Obstructive apneas and hypopneas elicit an immediate CV response, including acute sympathetic surge and blood pressure (BP) increase.5,6 Such CV responses are believed to be an important mediating mechanism of OSA’s long-term association with adverse CV outcomes.6 However, these physiological consequences may be overlooked by conventional metrics used in the diagnosis of OSA. Alternative or additional phenotypic markers are needed to better characterize the acute CV consequences of OSA and predict its long-term CV risk. Therefore, information about nocturnal BP may be useful in better understanding the risk associated with OSA. However, nocturnal BP monitoring, even during sleep studies, is rarely performed because of the burden and potential sleep-disrupting effects of such monitoring.5
Pulse transit time (PTT) is the time delay of pulse propagation between two points in the arterial tree, primarily determined by BP in a given arterial stiffness.7 Arterial stiffening resulting from increased BP leads to a rise in pulse wave velocity and a fall in PTT.8,9 Pulse arrival time (PAT), a widely used surrogate of PTT, which is known to correlate well with systolic BP, can be estimated using electrocardiography (ECG) and photoplethysmography (PPG) waveforms from peripheral pulse oximetry.10Polysomnography (PSG)-derived PAT has been evaluated for various purposes including characterization of respiratory pattern, detection of microarousals, and BP estimation in sleep.11–15 For example, acute BP surge following an obstructive apneic event is manifested as acutely shortened PAT. Nonetheless, these signals, are not routinely used for monitoring changes in BP during sleep.16 Since nocturnal sleep BP is an important prognostic marker of CV health,17 PAT assessment during sleep may provide useful information related to CV health in patients with OSA.5,18 In this study, we tested the hypothesis that obstructive respiratory event-related PAT response as measured by PAT change would be associated with established subclinical markers of CV risk.
Methods
Participants
We included participants of the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep study. MESA is a six-center cohort study of community-dwelling men and women aged 45–84 years recruited between 2000–2002 when they were free of known CVD.19 A subset of participants were recruited to undergo in-home PSG (full Type II study) as a part of the MESA Sleep ancillary study in conjunction with MESA exam 5 (year 2010–2012).20 The research protocols were approved by the Institutional Review Boards at each participating institution, and all participants gave written informed consent.
Polysomnography
An overnight in-home sleep study was performed using a 15-channel PSG (Somté PSG, Compumedics Ltd., Abbotsford, AU) as part of the MESA Sleep Study (year 2010–2013), applying standard channels recommended by American Academy of Sleep Medicine. PPG signal was derived from pulse oximetry. The sleep records were electronically transmitted to a centralized reading center (Brigham and Women’s Hospital, Boston, MA) for manual scoring by trained polysomnologists, who were blinded to all clinical data. Apnea was scored as >90% airflow reduction based on a thermocouple signal in reference to the pre event baseline for at least 10 s, and was further specified as obstructive or central on the basis of the presence of respiratory effort recorded using inductance plethysmography. Hypopneas were scored as >30% reduction in nasal pressure-measured airflow in reference to the pre event baseline for at least 10 s; hypopneas with either >3% desaturation or accompanying arousal were included in the apnea-hypopnea index (AHI) calculation. The AHI was defined as the number of total apneas and hypopneas per hour (hr) of sleep. Inter and intra-scorer intraclass correlation coefficients for the AHI ranged from 0.95 to 0.99.
Pulse Arrival Time (PAT) response as a surrogate measure of BP response to OSA events
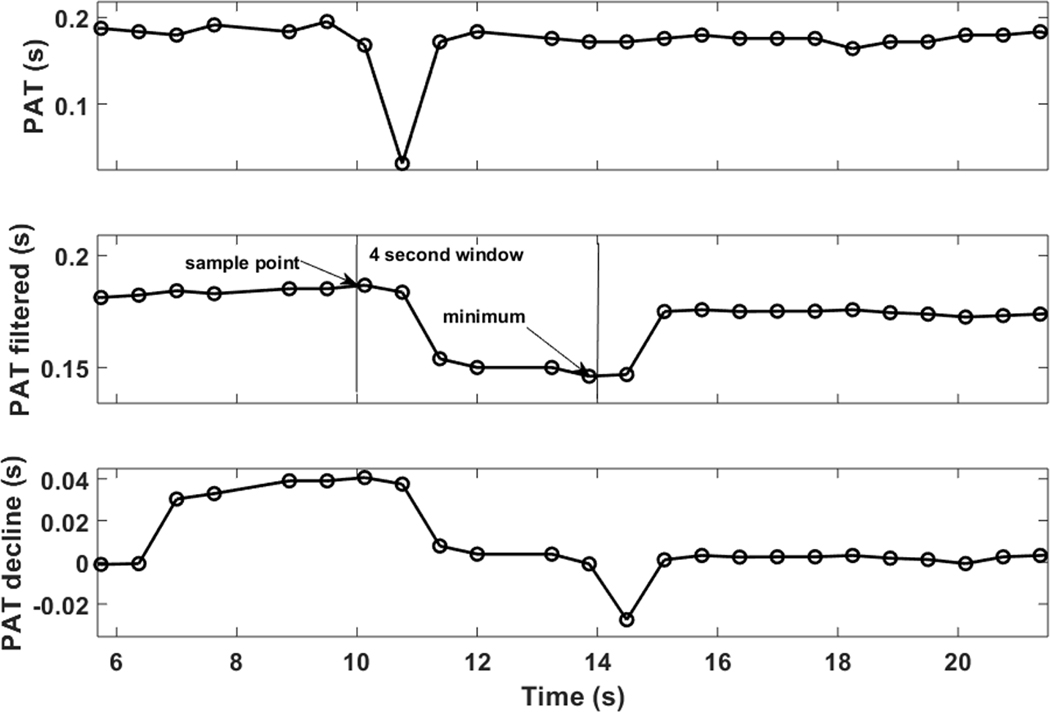
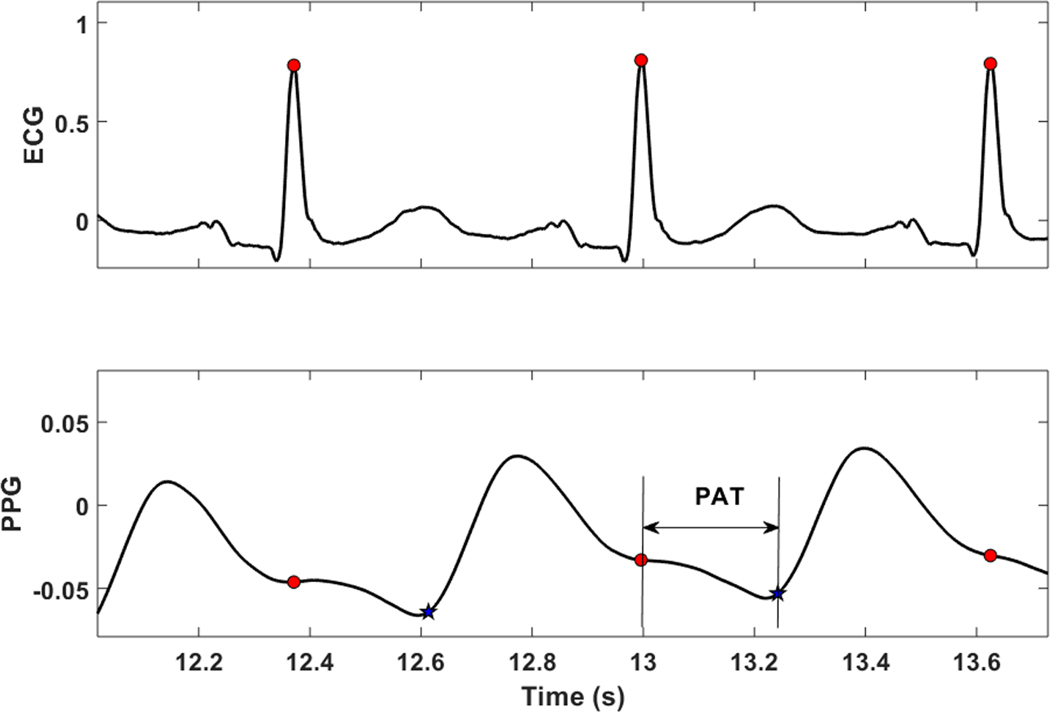
Data segments containing movement artifacts were automatically excluded from the analysis. ECG R-R interval outliers were removed by comparison with a running average threshold.21 Noisy PPG segments was removed by time-frequency analysis and application of criteria on the frequency of the maximum peak and power concentration for each time window. Thereafter, PAT was extracted from the raw synchronously-captured ECG and PPG signals (Sampling frequency: 1Hz). PAT was defined as the time delay between the ECG R-wave peak and the onset (foot) of the PPG waveform (Figure 1). The ECG R-wave was extracted using an adaptation of the single-channel QRS algorithm.22 The PPG foot point was determined as the maximum of the second derivative of the PPG waveform within a window of time after the ECG R-wave peak within which a physiologically possible PPG nadir could exist. The first differential of the PPG signal was filtered using a 0.13 second moving-average filter. The differential was repeated on the positive values of the filtered signal, producing spikes in the second derivative signal. Finally, the maximum value of the spikes was determined as the PPG foot, and the time between the Rwave peak and PPG foot was defined as PAT. Since our focus was on the PAT response to obstructive respiratory event, which is typically a decrease (i.e., shorter PAT), we focused on the degree of PAT decline. To measure the “PAT decline,” the PAT signal was smoothed using a 4-second moving average (Figure 2). At each PAT point, the PAT decline (in units of seconds) was defined as the maximum decrease in PAT over the subsequent 5 seconds (Figure 2). A more pronounced PAT decline would imply a higher BP increase.
Figure 1.
An example of an individual PAT measure. ECG, electrocardiography; PPG, photoplethysmography; PAT, pulse arrival time was defined as the time interval between ECG R-wave peak and the foot of PPG signal.
Figure 2.
An example of the PAT decline measure. After the PAT signal was obtained (top panel, see Figure 1 for more details), it was filtered using a 4-second moving average filter (middle panel). The PAT decline was defined as the maximum decrease in PAT within the subsequent 5 seconds from the current PAT value (bottom panel). PAT, pulse arrival time.
Obstructive sleep apnea-specific PAT response
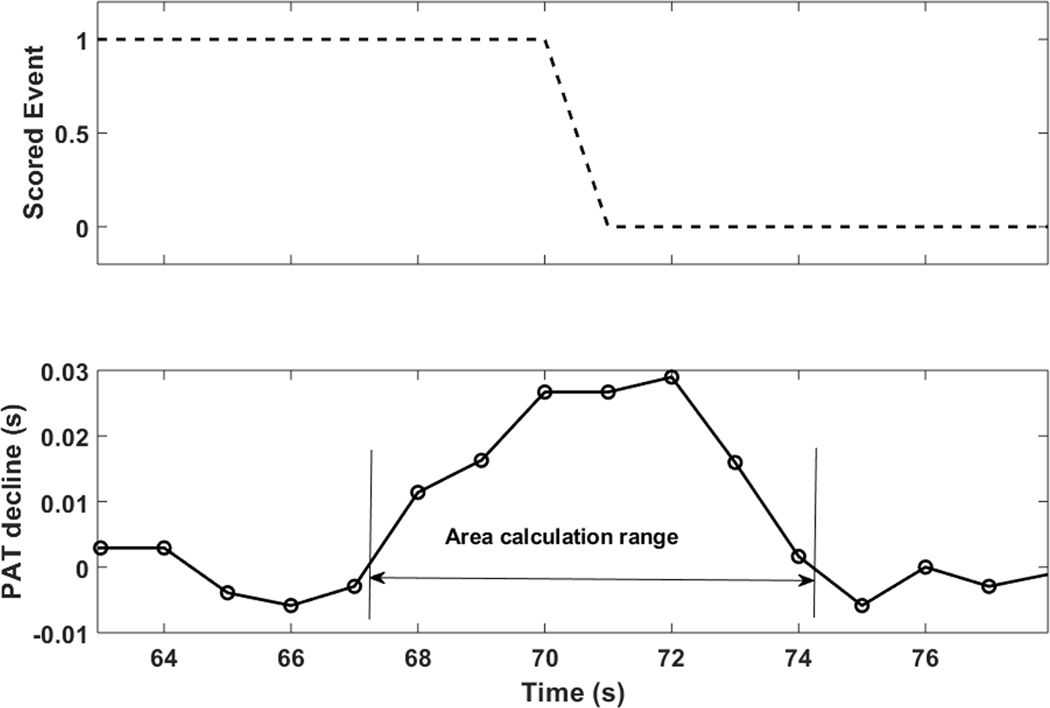
To obtain the OSA-specific PAT response (reflecting OSA-specific BP response), scored respiratory events were used for further analysis. First, all “significant” event-related PAT declines within a search window of +/− 3 seconds from the end of events were determined. To be considered significant, the maximum decline had to be larger than a subject-specific threshold equal to the mean plus 2 standard deviations (SD) of all obstructive respiratory event related PAT declines outside the search window. For each significant event-related PAT decline, the PAT response was defined as the area under the PAT decline curve between the later of the actual zero crossing or any local minimum immediately prior to the peak decline and the earlier of the actual signal zero crossing or first local minimum immediately after (Figure 3). The OSA-specific PAT response was defined as the average of all individual areas.23 Since the distribution of the PAT areas may be skewed, we also derived median value to use it as an alternative representative aggregate measure for each subject. PAT analysis was performed using MATLAB (MathWorks, Natick, Massachusetts, USA)
Figure 3.
An example of PAT response to a respiratory event (top panel). ‘1’ denotes ongoing an apneic or hypopneic event. “0” denotes termination of an apneic or hypopneic event. After determining “significant” PAT declines, an individual event-related PAT response was defined as the area under the PAT decline curve from the later of the immediate local minimum or zero crossing before the peak to the sooner of the immediate local minimum or zero crossing after the peak (bottom panel). The PAT response was defined as the mean value of all significant areas in each participant. PAT, pulse arrival time.
Outcomes
Well-established subclinical CVD markers were chosen a priori. The primary outcome was left ventricular hypertrophy (LVH), given its established relationship with BP. Secondary outcomes included coronary artery calcium (CAC) and carotid plaque burden (CPB). LVH was determined by cardiac magnetic resonance imaging if LV mass indexed to body surface area was greater than 122 g/m2 for women and 149 g/m2 for men.24 CPB was assessed by carotid ultrasound and was expressed as CPB score (0–12 scale); 1 point per plaque was allocated for the near and far walls of each segment (common carotid artery, bulb, and internal carotid artery) of each carotid artery (i.e., total of 12 segments) that was interrogated. 25 CAC was assessed by cardiac computerized tomography using standardized protocols. ‘Prevalent’ CAC was determined if the CAC (Agatston) score was greater than 0.26 These subclinical CVD markers were all obtained at MESA exam 5. All measures were calculated by high-quality core laboratories and have undergone rigorous quality control. Incident CVD events following MESA Sleep ancillary study were identified through regular telephone interview. Individuals who had an event prior to MESA sleep ancillary study were excluded. The following events were adjudicated by medical record review by blinded physicians from the events committee using pre-specified criteria: coronary heart disease, stroke, and heart failure or composite CVD.19 For this study, we included any of the following events: myocardial infarction, resuscitated cardiac arrest, angina, stroke, heart failure, and any death related to CVD. Prevalent CVD was determined by reported history of these events prior to MESA exam 5.
Statistical analysis
Average PAT response was modeled using both continuous and categorical (quartiles) variables. Baseline characteristics were described based on the high vs. low average PAT response by median value. All continuous variables were described by mean (standard deviation [SD]). Outcomes, including LV mass and CPB, were modeled as continuous variables, whereas prevalent CAC was modeled as a categorical variable. Given that the CPB score showed non-normal distribution, we repeated analysis using a log-transformed CPB score.27 Pearson product-moment correlation (r) was used to describe the correlation between the average PAT response and AHI. Multiple linear regression analyses were performed to determine the independent association of average PAT response with LV mass and CPB. Multivariable logistic regressions were performed for prevalent CAC. Because of the skewed distribution of PAT response, median PAT response was also tested as an alternative predictor. To account for potential confounding factors, all models were adjusted for site, age, sex, race/ethnicity, body mass index (BMI), systolic BP and smoking status. For incident CVD, after excluding any individuals with prevalent CVD at baseline, we performed Cox proportional hazard regression to derive the hazard ratio (HR) of incident CVD per 1 SD increase in average PAT response, adjusting for the same covariates. For this Cox analysis, we created two additional models; First, additionally adjusting for AHI to determine whether the association remained when accounting for the most commonly used OSA severity metric. Finally, we repeated the analyses by including AHI as an exposure, instead of PAT. Proportional hazards assumption was met based on scaled Schoenfeld residuals. Statistical analysis was performed using the R software environment (http://www.r-project.org).
Results
After excluding 628 individuals whose PSG either did not yield adequate-quality PAT measurement (N=310), or were missing covariates (N=105) or any outcome (N= 213), the cohort consisted of 1407 participants (mean age 68.4 years, female 47.5%). In participants included in the final cohort, 73.3% [66.7%−78.0%] (median [IQR]) of the segments per participant were analyzed after noise/artifact removal. Participants that were included had similar distributions of demographics and CV risk factors but were more obese compared to those excluded (BMI: 28.8 kg/m2 vs. 28.2, p=0.02) (data not shown).
OSA was common, as evidenced by the mean AHI of 19.5/hr. Nearly all respiratory events were obstructive (vs. central). The group with high PAT response was older, had higher BMI, and were likely to be male compared to the low PAT response group (Table 1a). The high PAT group had greater LV mass and CPB, and an increased prevalence of CAC (Table 1b). Overall, PAT response showed right-skewed distribution (Mean PAT response was 33.6 [31.2–35.9] % larger than the median PAT response). Mean PAT response was highly variable across the different severity groups of OSA by AHI (Figure 4). There was a weak relationship between PAT response and AHI (r=0.1, p<0.001).
Table 1.
Characteristics of the study participants
| Table 1a | |||||
|---|---|---|---|---|---|
| ALL | Mean PAT response | ||||
| [ALL] | <median | >median | P value | ||
| N | 1407 | 704 | 703 | ||
| Age (years) | 68.4 (9.09) | 67.8 (8.92) | 69.1 (9.22) | 0.007 | |
| Race/Ethnicity: | 0.001 | ||||
| 1: White | 506 (36.0%) | 267 (37.9%) | 239 (34.0%) | ||
| 2: Chinese American | 180 (12.8%) | 107 (15.2%) | 73 (10.4%) | ||
| 3: African American | 392 (27.9%) | 168 (23.9%) | 224 (31.9%) | ||
| 4: Hispanic | 329 (23.4%) | 162 (23.0%) | 167 (23.8%) | ||
| Sex (Male) | 668 (47.5%) | 294 (41.8%) | 374 (53.2%) | <0.001 | |
| BMI (kg/m2) | 28.8 (5.47) | 28.4 (5.38) | 29.2 (5.53) | 0.012 | |
| Height (m2) | 166 (10.1) | 164 (9.75) | 167 (10.4) | <0.001 | |
| Current smoking | 93 (6.64%) | 34 (4.86%) | 59 (8.43%) | 0.019 | |
| Diabetes | 162 (11.5%) | 74 (10.5%) | 88 (12.5%) | 0.182 | |
| Hypertension | 811 (57.6%) | 389 (55.3%) | 422 (60.0%) | 0.079 | |
| Hypertension Medication | 769 (54.7%) | 360 (51.1%) | 409 (58.2%) | 0.009 | |
| SBP (mmHg) | 123 (19.9) | 123 (20.0) | 123 (19.9) | 0.776 | |
| Lipid lowering medication | 531 (37.7%) | 255 (36.2%) | 276 (39.3%) | 0.262 | |
| Baseline CVD | 90 (6.42%) | 35 (4.99%) | 55 (7.86%) | 0.038 | |
| HDL (mg/dL) | 54.8 (15.8) | 55.3 (15.6) | 54.3 (16.0) | 0.264 | |
| LDL | 107 (31.9) | 109 (31.7) | 105 (32.0) | 0.028 | |
| AHI (/hr) | Median [IQR] | 19.5 [10.8;34.0] | 16.9 [8.76;31.2] | 22.0 [12.8;36.3] | <0.001 |
| Mean (SD) | 25.0 (19.4) | 22.8 (19.2) | 27.2 (19.4) | <0.001 | |
| OAI (/hr) | Median [IQR] | 1.1 [0.2;4.7] | 1.0 [0.2;4.0] | 1.3 [0.2;5.4] | 0.003 |
| Mean (SD) | 4.3 (8.5) | 3.8 (8.3) | 4.8 (8.7) | 0.026 | |
| Table 1b | |||||
| ALL | Mean PAT response | ||||
| [ALL] | <median | >median | P value | ||
| N=1407 | N=704 | N=703 | |||
| LV mass (g/m2) | 148 (35.4) | 143 (31.6) | 154 (38.4) | <0.001 | |
| CPB Score | 2.18 (2.35) | 1.98 (2.22) | 2.37 (2.46) | 0.004 | |
| Presence of CAC | 820 (67.2%) | 381 (62.4%) | 439 (72.1%) | <0.001 | |
BMI, body mass index; CVD, cardiovascular disease; HDL, high density lipoprotein; LDL, low density lipoprotein; AHI, apnea hypopnea index; OAI, obstructive apnea index; PAT, pulse arrival time. ‘Average PAT response’ denotes mean area under the PAT response curve following obstructive respiratory events that are associated with significant PAT decline.
LV, left ventricle; CPB, carotid plaque burden; CAC, coronary artery calcium
‘Average PAT response’ denotes mean area under the PAT response curve following obstructive respiratory events that are associated with significant PAT decline.
Values expressed as mean (SD) or N (%)
Figure 4.
Relationship between pulse arrival time (PAT, sec) change and apnea hypopnea index (AHI, /hr).
Average PAT response vs. Subclinical CVD
In the multivariable analysis (Table 2), a 1 SD increase in average PAT response was associated with 1.5 g/m2 higher LV mass (p=0.038). When participants were divided into quartile based on their average PAT response time, those in the 4th quartile had 5.7 g/m2 higher LV mass as compared with those in the 1st quartile. The results remained similar when height (vs. BMI) was included in the model (data not shown). Using the median PAT response as a predictor yielded similar results (6.7 g/m2 higher LV mass in 4th vs 1st quartile). The CPB score increased by 0.13 for every 1 SD increase in average PAT response (p=0.036). Individuals in the 4th quartile of average PAT response had 0.41 higher CPB score. The results remained similar when log-transformed CPB was used (data not shown). Similarly, every 1 SD increase in average PAT response was associated with prevalent CAC (OR: 1.19 [1.02, 1.41], p=0.03). In the categorical-based analysis, subjects in the 4th quartile of average PAT response had a trend of higher odds of having prevalent CAC as compared with those in the 1st quartile (OR: 1.44 [0.98, 2.15, p=0.06].
Table 2.
Left ventricular mass, carotid plaque burden score and coronary artery calcification by average PAT response (quartile-based)
| LV mass (g/m2) | CPB score | Presence of CAC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles | beta estimate | low CI | high CI | P value | beta estimate | low CI | high CI | P value | OR | low CI | high CI | P value |
| 1 | ref | ref | ref | |||||||||
| 2 | −1.2 | −5.1 | 2.8 | 0.56 | 0.27 | −0.07 | 0.62 | 0.12 | 1.10 | 0.76 | 1.59 | 0.60 |
| 3 | 0.08 | −3.9 | 4.1 | 0.97 | 0.32 | −0.03 | 0.67 | 0.07 | 1.33 | 0.92 | 1.95 | 0.13 |
| 4 | 5.7 | 1.5 | 9.8 | 0.007 | 0.41 | 0.05 | 0.77 | 0.02 | 1.44 | 0.98 | 2.15 | 0.06 |
Adjusted for site, age, sex, race/ethnicity, body mass index, systolic blood pressure and smoking status. LV, left ventricle; CPB, carotid plaque burden; CAC, coronary artery calcium
Average PAT response vs. Incident CVD
There was a total of 65 incident CVD events over the mean of 4.1(2.6) years follow up period. As shown in Table 3, a 1 SD increase in average PAT response was associated with 18% higher risk of incident CVD. A model that additionally controlled for AHI (Model 2) attenuated the significance.
Table 3.
Incident cardiovascular event by average PAT response (per 1 SD)
| HR | low CI | high CI | P value | ||
|---|---|---|---|---|---|
| Model 1 | per 1 SD average PAT response | 1.20 | 1.02 | 1.42 | 0.03 |
| Model 2 | per 1 SD average PAT response | 1.18 | 0.99 | 1.40 | 0.06 |
Model 1 adjusted for site, age, sex, race/ethnicity, body mass index, systolic blood pressure and smoking status. Model 2 additionally adjusted for apnea hypopnea index.
‘Average PAT response’ denotes mean area under the PAT response curve following obstructive respiratory events that are associated with significant PAT decline.
Discussion
We found that in ethnically diverse community dwelling adults with OSA, PSG-based respiratory event-related PAT response was associated with key subclinical CVD markers as well as with incident CVD. These findings were independent of potential confounding factors, and the association remained significant after controlling for AHI, the conventional metric most commonly used in the diagnosis and severity assessment of OSA. Specifically, higher average PAT response to respiratory events was associated with higher likelihood of having increased LV mass, CPB, and CAC, all of which are well-established and clinically important subclinical CVD markers predictive of CVD and mortality. Moreover, PAT response to obstructive respiratory events was predictive of future composite CVD events consisting of acute coronary events, angina, stroke and CVD related deaths.
OSA, and to a lesser degree central sleep apnea, produce sympathetic surges in association with arousal or transient hypoxemia. Such acute physiological responses lead to an abrupt response in heart rate, BP, and arterial stiffness. Our presumption was that PAT responses can be used to quantify such CV responses and that it would more strongly associate with CV outcomes than the standard and less physiologically informative PSG metrics currently used. Prior studies have shown the association of OSA with overall increased baseline arterial stiffness as measured by standard pulse wave velocity or estimated by ‘static’ single PAT measurement using the ECG R wave to pulse wave peripheral PPG. 20,28 In this study, we were interested in whether the ‘dynamic PAT change’, which may be reflective of dynamic sympathetic activity, would be predictive of adverse CV outcomes. Although PAT from PSG has been examined as a tool to detect autonomic arousal (so called “subcortical arousal”) and to estimate BP change across the sleep period, it has not been examined in the context of clinical outcomes.10,11,16,29–31 The higher LV mass occurring with shorter PAT responses may reflect greater event-related sympathetic surges, leading to unfavorable nocturnal BP resulting in higher afterload and subsequently to structural remodeling of the LV. LVH is a potent predictor of stroke, coronary heart disease, and heart failure.32,33. Association with higher CPB or CAC may be explained by the well-known impact of sympathetic activity on the progression of atherosclerosis.34 Both CPB and CAC are strong predictors of future CVD and mortality.35–38 Increased CVD events can be explained by the aforementioned mechanisms. Due to small number of composite CVD events, we did not have power to look into the individual components of CVD.
Given the consistent finding across several key measures of subclinical CVD as well as with incident CVD, it is plausible that PAT response may be a novel physiological measurement that may provide additional physiological information and better predict a subgroup of OSA patients who are at a higher risk of CVD. One of the most challenging aspects of contemporary sleep apnea management relates to risk stratification and therapeutic decision making. Additional information, such as OSA-specific hypoxic burden23 and pulse rate response,39 directly available from a sleep study, either alone or in combination, could provide clinically useful information beyond the ‘diagnosis’ of OSA. Better utilization of PSG or other aspects of the sleep study to characterize sleep apnea and personalization of care is an area of unmet need and deserves additional investigation.40 We propose that if confirmed by additional studies, the described respiratory event-related average PAT response has the potential to be a clinically useful physiological marker to guide clinicians to better characterize sleep apnea and help with therapeutic decision-making.
We focused on respiratory event-related PAT responses that were a priori defined as significant and computed the average integral of those responses to capture the exposure across the sleep period. However, there are other simpler measurements that may be worthy of evaluation, such as maximum PAT response or an index using a set threshold PAT response. Since there are multiple respiratory events and the related PAT responses in a given subject, an optimal method to best represent the PAT response needs to be explored. Moreover, although not assessed in our study, PAT response in association with periodic limb movements, spontaneous arousals, or during sleep-wake transition could also contribute to CVD and deserves investigation. Therefore, future investigation may consider entire PAT in sleep. We also recognize that to be more applicable in clinical practice, measurement methods need to be better refined and night-to-night stability of the measurement should be examined.
PAT is a proxy estimator of the PTT as it includes a short, but highly variable, pre-ejection period (PEP) that consists of electromechanical delay, isovolumetric contraction and the opening of the aortic valve.7 Therefore, theoretically, PTT is a superior metric than PAT. However, measuring PTT is technically challenging as it requires two pulse detection sites and PAT is a reasonable surrogate to PTT and is easier to extract from commonly acquired continuous physiological signals. Even so, it is important to note that manual measurement of PAT is not possible. In our study, we employed a fiducial point-based algorithm that automatically calculates the time interval between ECG R wave and the foot of the rise in PPG signal from marked annotated respiratory events. Thus, automated program is necessary to edit frequent artifacts. Moreover, PPG signal can be easily lost due to improper contact of pulse oximeter. In this study, about 18% of participants were not included because of overall inadequate quality of PPG signal, and thus PAT measurement. Lastly, there can be inherent measurement bias when devices with different sampling rate or filtering techniques are used.
Several strengths of our study are noteworthy. Our study cohort consisted of an ethnically-diverse unselected population and thus, selection bias is minimized. The design of the study allowed us to temporally link PSG data to subclinical CVD measures obtained at a similar timing by state-of-the-art and clinically relevant imaging techniques including, cardiac magnetic resonance imaging, cardiac computerized tomography, and carotid duplex ultrasound. Although PAT was associated with LV mass, the effect size was small and the clinical significance of this finding warrants further investigation. While, the cross-sectional associations with subclinical CVD measures limit causal inference, the prospective findings with CV outcomes supports PAT response as a predictor of later CVD. However, the number of CVD outcomes were small.
In conclusion, we found that the higher average PAT response to respiratory events manifested in PSG is predictive of markers of subclinical CVD and future CVD.
What is the key question?
Does sleep study-derived pulse arrival time indicate high cardiovascular risk in patients with sleep apnea?
What is the bottom line?
Sleep apnea-specific pulse arrival time change is associated with subclinical cardiovascular disease and an increased risk of cardiovascular events in patients with sleep apnea.
Why read on?
This study offers a new insight into how novel sleep study-derived physiological markers may improve risk stratification of patients with sleep apnea.
Acknowledgement:
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This publication was developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. YK, AA and SM were supported by NIH R21HL140432. AA was supported by the NIH R01HL153874 American Heart Association (19CDA34660137) and the American Academy of Sleep Medicine Foundation (188-SR-17). SR, AW, and AA were partially supported by NHLBI R35HL135818.
We thank all the MESA participants and MESA staffs
Footnotes
Conflicts of Interest: AW works as a consultant for Apnimed, Somnifix, and Nox and he has received grants from Somnifix and Sanofi. AW has a financial interest in Apnimed, a company developing pharmacologic therapies for sleep apnea. His interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies; SR received grant support and consulting fees and grant support from Jazz Pharmaceutical, and consulting fees from Eisai Pharma and Respicardia; AA serves as consultant for Somnifix and Apnimed and reports grant from Somnifix.
References
- 1.Kwon Y, Logan J, Pusalavidyasagar S, et al. Sleep Apnea and Heart. Sleep Med Res 2019;10(2):67–74. doi: 10.17241/smr.2019.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010;122(4):352–60. doi: 10.1161/CIRCULATIONAHA.109.901801 CIRCULATIONAHA.109.901801 [pii] [published Online First: 2010/07/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaggi HK, Concato J, Kernan WN, et al. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. New England Journal of Medicine 2005;353(19):2034–41. doi: 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- 4.Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation 2003;107(12):1671–8. doi: 10.1161/01.cir.0000061757.12581.15 [published Online First: 2003/04/02] [DOI] [PubMed] [Google Scholar]
- 5.Kwon Y, Stafford PL, Lim DC, et al. Blood pressure monitoring in sleep: time to wake up. Blood Press Monit 2019. doi: 10.1097/mbp.0000000000000426 [published Online First: 2019/12/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96(4):1897–904. doi: 10.1172/JCI118235 [published Online First: 1995/10/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukkamala R, Hahn JO, Inan OT, et al. Toward Ubiquitous Blood Pressure Monitoring via Pulse Transit Time: Theory and Practice. IEEE transactions on bio-medical engineering 2015;62(8):1879–901. doi: 10.1109/tbme.2015.2441951 [published Online First: 2015/06/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kounalakis SN, Geladas ND. The role of pulse transit time as an index of arterial stiffness during exercise. Cardiovasc Eng 2009;9(3):92–7. doi: 10.1007/s10558-009-9081-4 [published Online First: 2009/08/07] [DOI] [PubMed] [Google Scholar]
- 9.Gribbin B, Steptoe A, Sleight P. Pulse Wave Velocity as a Measure of Blood Pressure Change. Psychophysiology 1976;13(1):86–90. doi: 10.1111/j.1469-8986.1976.tb03344.x [DOI] [PubMed] [Google Scholar]
- 10.Patzak A, Mendoza Y, Gesche H, et al. Continuous blood pressure measurement using the pulse transit time: Comparison to intra-arterial measurement. Blood pressure 2015;24(4):217–21. doi: 10.3109/08037051.2015.1030901 [published Online First: 2015/04/11] [DOI] [PubMed] [Google Scholar]
- 11.Pepin JL, Delavie N, Pin I, et al. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest 2005;127(3):722–30. doi: 10.1378/chest.127.3.722 [published Online First: 2005/03/15] [DOI] [PubMed] [Google Scholar]
- 12.Pepin JL, Tamisier R, Borel JC, et al. A critical review of peripheral arterial tone and pulse transit time as indirect diagnostic methods for detecting sleep disordered breathing and characterizing sleep structure. Curr Opin Pulm Med 2009;15(6):550–8. doi: 10.1097/MCP.0b013e3283318585 [published Online First: 2009/09/03] [DOI] [PubMed] [Google Scholar]
- 13.Pitson DJ, Sandell A, van den Hout R, et al. Use of pulse transit time as a measure of inspiratory effort in patients with obstructive sleep apnoea. The European respiratory journal 1995;8(10):1669–74. [published Online First: 1995/10/01] [DOI] [PubMed] [Google Scholar]
- 14.Argod J, Pepin JL, Levy P. Differentiating obstructive and central sleep respiratory events through pulse transit time. American journal of respiratory and critical care medicine 1998;158(6):1778–83. doi: 10.1164/ajrccm.158.6.9804157 [published Online First: 1998/12/16] [DOI] [PubMed] [Google Scholar]
- 15.Gesche H, Grosskurth D, Kuchler G, et al. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol 2012;112(1):309–15. doi: 10.1007/s00421-011-1983-3 [published Online First: 2011/05/11] [DOI] [PubMed] [Google Scholar]
- 16.Smith RP, Argod J, Pepin JL, et al. Pulse transit time: an appraisal of potential clinical applications. Thorax 1999;54(5):452–7. doi: 10.1136/thx.54.5.452 [published Online First: 1999/04/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kario K, Kanegae H, Tomitani N, et al. Nighttime Blood Pressure Measured by Home Blood Pressure Monitoring as an Independent Predictor of Cardiovascular Events in General Practice. Hypertension 2019;73(6):1240–48. doi: 10.1161/hypertensionaha.118.12740 [published Online First: 2019/04/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsioufis C, Andrikou I, Thomopoulos C, et al. Increased nighttime blood pressure or nondipping profile for prediction of cardiovascular outcomes. Journal of human hypertension 2011;25(5):281–93. doi: 10.1038/jhh.2010.113 [published Online First: 2010/12/03] [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. American journal of epidemiology 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 20.Kwon Y, Jacobs DR Jr., Lutsey PL, et al. “Sleep disordered breathing and ECG R-wave to radial artery pulse delay, The Multi-Ethnic Study of Atherosclerosis”. Sleep medicine 2018;48:172–79. doi: 10.1016/j.sleep.2018.05.005 [published Online First: 2018/07/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Detection of obstructive sleep apnea from cardiac interbeat interval time series. IEEE transactions on bio-medical engineering; 2000. [Google Scholar]
- 22.Engelse WAH, Zeelenberg C. A single scan algorithm for QRS-detection and feature extraction. Computers in Cardiology 1979;6:37–42. [Google Scholar]
- 23.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. European heart journal 2019;40(14):1149–57. doi: 10.1093/eurheartj/ehy624 [published Online First: 2018/10/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. The American journal of cardiology 1986;57(6):450–8. [published Online First: 1986/02/15] [DOI] [PubMed] [Google Scholar]
- 25.Tattersall MC, Gassett A, Korcarz CE, et al. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke 2014;45(11):3257–62. doi: 10.1161/strokeaha.114.005669 [published Online First: 2014/09/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon Y, Duprez DA, Jacobs DR, et al. Obstructive sleep apnea and progression of coronary artery calcium: the multi-ethnic study of atherosclerosis study. Journal of the American Heart Association 2014;3(5):e001241. doi: 10.1161/jaha.114.001241 [published Online First: 2014/09/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gepner AD, Young R, Delaney JA, et al. Comparison of Carotid Plaque Score and Coronary Artery Calcium Score for Predicting Cardiovascular Disease Events: The Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association 2017;6(2):e005179. doi: doi: 10.1161/JAHA.116.005179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips CL, Butlin M, Wong KK, et al. Is obstructive sleep apnoea causally related to arterial stiffness? A critical review of the experimental evidence. Sleep medicine reviews 2013;17(1):7–18. doi: 10.1016/j.smrv.2012.03.002 [published Online First: 2012/06/05] [DOI] [PubMed] [Google Scholar]
- 29.Gehring J, Gesche H, Drewniok G, et al. Nocturnal blood pressure fluctuations measured by using pulse transit time in patients with severe obstructive sleep apnea syndrome. Sleep & breathing = Schlaf & Atmung 2018;22(2):337–43. doi: 10.1007/s11325-017-1555-9 [published Online First: 2017/08/23] [DOI] [PubMed] [Google Scholar]
- 30.Katz ES, Lutz J, Black C, et al. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res 2003;53(4):580–8. doi: 10.1203/01.pdr.0000057206.14698.47 [published Online First: 2003/03/04] [DOI] [PubMed] [Google Scholar]
- 31.Pitson DJ, Stradling JR. Value of beat-to-beat blood pressure changes, detected by pulse transit time, in the management of the obstructive sleep apnoea/hypopnoea syndrome. The European respiratory journal 1998;12(3):685–92. [published Online First: 1998/10/08] [DOI] [PubMed] [Google Scholar]
- 32.Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. The New England journal of medicine 1990;322(22):1561–6. doi: 10.1056/nejm199005313222203 [published Online First: 1990/05/31] [DOI] [PubMed] [Google Scholar]
- 33.Gosse P. Left ventricular hypertrophy as a predictor of cardiovascular risk. J Hypertens Suppl 2005;23(1):S27–33. doi: 10.1097/01.hjh.0000165625.79933.9a [published Online First: 2005/04/12] [DOI] [PubMed] [Google Scholar]
- 34.Karakas M, Koenig W. Sympathetic Nervous System. Circulation Research 2013;112(1):13–16. doi: doi: 10.1161/CIRCRESAHA.112.281097 [DOI] [PubMed] [Google Scholar]
- 35.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. Journal of the American College of Cardiology 2007;49(3):378–402. doi: 10.1016/j.jacc.2006.10.001 [published Online First: 2007/01/24] [DOI] [PubMed] [Google Scholar]
- 36.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. Jama 2012;308(8):788–95. doi: 10.1001/jama.2012.9624 [published Online First: 2012/08/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Störk S, Beld AWvd, Schacky Cv, et al. Carotid Artery Plaque Burden, Stiffness, and Mortality Risk in Elderly Men. Circulation 2004;110(3):344–48. doi: doi: 10.1161/01.CIR.0000134966.10793.C9 [DOI] [PubMed] [Google Scholar]
- 38.Sillesen H, Sartori S, Sandholt B, et al. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. European heart journal cardiovascular Imaging 2018;19(9):1042–50. doi: 10.1093/ehjci/jex239 [published Online First: 2017/10/24] [DOI] [PubMed] [Google Scholar]
- 39.Azarbarzin A, Sands SA, Younes M, et al. The Sleep Apnea-specific Pulse Rate Response Predicts Cardiovascular Morbidity and Mortality. American journal of respiratory and critical care medicine 2021. doi: 10.1164/rccm.202010-3900OC [published Online First: 2021/01/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzotti DR, Lim DC, Sutherland K, et al. Opportunities for utilizing polysomnography signals to characterize obstructive sleep apnea subtypes and severity. Physiological measurement 2018;39(9):09TR01–09TR01. doi: 10.1088/1361-6579/aad5fe [DOI] [PMC free article] [PubMed] [Google Scholar]