Abstract
Introduction
Delays in cancer diagnosis arose from the commencement of non-pharmaceutical interventions (NPI) introduced in the UK in March 2020 in response to the COVID-19 pandemic. Our earlier work predicted this will lead to approximately 3620 avoidable deaths for four major tumour types (breast, bowel, lung, and oesophageal cancer) in the next 5 years. Here, using national population-based modelling, we estimate the health and economic losses resulting from these avoidable cancer deaths. We also compare these with the impact of an equivalent number of COVID-19 deaths to understand the welfare consequences of the different health conditions.
Methods
We estimate health losses using quality-adjusted life years (QALYs) and lost economic productivity using the human capital (HC) approach. The analysis uses linked English National Health Service (NHS) cancer registration and hospital administrative datasets for patients aged 15–84 years, diagnosed with breast, colorectal, and oesophageal cancer between 1st Jan to 31st Dec 2010, with follow-up data until 31st Dec 2014, and diagnosed with lung cancer between 1st Jan to 31st Dec 31 2012, with follow-up data until 31st Dec 2015. Productivity losses are based on the estimation of excess additional deaths due to cancer at 1, 3 and 5 years for the four cancer types, which were derived from a previous analysis using this dataset. A total of 500 random samples drawn from the total number of COVID-19 deaths reported by the Office for National Statistics, stratified by gender, were used to estimate productivity losses for an equivalent number of deaths (n = 3620) due to SARS-CoV-2 infection.
Results
We collected data for 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with oesophageal cancer, and 29,305 with lung cancer. We estimate that across the four site-specific cancers combined in England alone, additional excess cancer deaths would amount to a loss of 32,700 QALYs (95% CI 31,300-34,100) and productivity losses of £103.8million GBP (73.2–132.2) in the next five years. For breast cancer, we estimate a loss of 4100 QALYS (3900–4400) and productivity losses of £23.2 m (18.2–28.6); for colorectal cancer, 15,000 QALYS (14,100–16,000) lost and productivity losses of £35.7 m (22.4–48.7); for lung cancer 10,900 QALYS (9,900–11,700) lost and productivity losses of £38.3 m (14.0–59.9) for lung cancer; and for oesophageal cancer, 2700 QALYS (2300–3,100) lost and productivity losses of £6.6 m (–6 to –17.6). In comparison, the equivalent number of COVID-19 deaths caused approximately 21,450 QALYs lost, as well as productivity losses amounting to £76.4 m (73.5–79.2).
Conclusion
Premature cancer deaths resulting from diagnostic delays during the first wave of the COVID-19 pandemic in the UK will result in significant economic losses. On a per-capita basis, this impact is, in fact, greater than that of deaths directly attributable to COVID-19. These results emphasise the importance of robust evaluation of the trade-offs of the wider health, welfare and economic effects of NPI to support both resource allocation and the prioritisation of time-critical health services directly impacted in a pandemic, such as cancer care.
Keywords: COVID-19, Cancer, Avoidable deaths, Quality-adjusted life years, Economics, Productivity, Diagnostic delay
1. Introduction
The introduction of non-pharmaceutical interventions (NPIs) designed to curb the transmission of COVID-19, including national lockdowns, and other physical distancing measures, has had a significant impact on cancer pathways from presentation through to diagnosis and treatment.
In the UK, following the first wave of the pandemic, up to 3 million men and women did not receive screening investigations due to suspension of these services, fewer patients were referred with suspected cancers [1], and 3.2 million fewer investigations (e.g., colonoscopy, cystoscopy, gastroscopy, CT scans, MRI between March and July 2020) were performed due to cancellation or deferral [2,3]. This decrease in referrals and diagnostic investigation implies an eventual later diagnosis with more advanced-stage cancer, which will have a direct impact on long term prognosis [4,5]. As well as diagnostic pathways, it has also become rapidly apparent that treatment delays [6], especially for cancer surgery, were also occurring [7,8].
Presently, in the UK, we find ourselves amid a second pandemic wave, which started in November 2020. Once again, many diagnostic investigations have been deferred or delayed (particularly routine investigations, which account for 40% of all cancer diagnoses [9]), cancer surgery cancelled, and reductions have been observed in the number of patients presenting with suspected cancers [10]. These delays in the cancer pathway will undoubtedly have consequences on premature mortality [11].
A major question is whether any of these effects could have been mitigated, particularly with respect to resource allocation and prioritisation of non-COVID related health services for time-critical diseases, if the government had accurately weighed up the direct economic and health impacts of NPI across the spectrum of health care services.
In this respect, estimating the economic consequences of changes in disease burden is crucial for understanding these welfare trade-offs. Specifically, whether the costs of implementing different types of NPI are justified when considering both their expected benefits and their wider impacts on other health conditions.
Similar evaluations unrelated to the pandemic have been undertaken in the context of cancer [12] to quantify the economic impact of premature mortality from cancer in different international regions. The outputs have been used to directly influence policy, particularly prioritisation and financial investment into a different component of the cancer care pathway to reduce the number of avoidable deaths [13].
In this study, we model the economic impacts of diagnostic delay during the COVID-19 pandemic using excess mortality estimates derived from the study by Maringe et al., [14] published in July 2020, which predicted that for four major tumour types (breast, bowel, lung and oesophagus), delays in diagnosis due to NPI during the first pandemic wave would lead to approximately 3620 avoidable deaths and 60,000 life years lost within 5 years of diagnosis.
We use a human capital approach to estimate productivity losses from excess cancer deaths due to diagnostic delay from NPIs. We also compare estimates from excess cancer deaths with the same number of COVID-19 deaths to understand the health and economic consequences of mortality from different health conditions.
2. Methods
2.1. Populations
2.1.1. Cancer patients
Information on all adults with non-small cell lung cancer (NSCLC, ICD-10: C33, C34), cancers of the colon (ICD-10: C18) and rectum (ICD-10: C19), cancers of the oesophagus and gastro-oesophageal junction (ICD-10: C15, C16.0) and women with breast cancer (ICD-10: C50) were obtained from the National Cancer Registration Service (NCRS). The pre-pandemic cohort includes patients diagnosed in 2010 for cancers of the breast, colon, rectum and oesophagus and 2012 for patients diagnosed with NSCLC in England. We restricted the analyses to patients aged 15–84 years at diagnosis.
2.1.2. COVID-19 patients
COVID-19 deaths are deaths that occurred in England and Wales up to 6th October 2020, inclusive with any mention of COVID-19 on the death certificate, by gender and 5-year age groups, from the Office for National Statistics (ONS) [15]. National life tables are from the ONS, based on 2016–2018 data [16].
2.2. Approach
We value health and economic losses using two established Indicators: (i) quality-adjusted life years (QALYs) and (ii) the human capital (HC), which measures the lost productivity associated with death. QALYs are calculated by estimating the years of life remaining for each patient and weighting each year with a quality-of-life score. For example, a year lived in perfect health is worth 1 QALY [17].
The HC approach estimates the productivity losses incurred by sick or deceased workers assuming it is irreplaceable. In keeping with the literature, we assume average personal income as a proxy for the value of lost productivity; we calculated the total value of productivity loss as the cumulative sum of personal income lost over the duration of illness (morbidity) and the number of years lost due to premature death (mortality), assuming not all individuals are employed (age-sex-specific employment rates) and a retirement age of 66 [18].
Average age-sex-specific gross earnings are sourced from the 2019 (provisional) results of the Annual Survey of Hours and Earnings (ASHE) [19], and age-sex-specific employment rates are sourced from the 2019 results of the Labour Force Survey (LFS) [20], both published by the ONS. A proportional adjustment is made to isolate the earnings of 65–66-year olds in the 65–69 years age group based on the mid-2019 population estimates in the UK by discrete years [21].
Projected excess deaths due to delays in diagnosis for breast, lung, colorectal and oesophageal cancer, by 5-year age bands (e.g. 65–69), at 1, 3 and 5 years after diagnosis are based on scenario C of Maringe et al., which reallocated patients from non-urgent pathways to 2-week wait and emergency referral pathways in proportions designed to reflect the real-time and future anticipated changes in access to diagnostic services in the 12 months following commencement of the first wave lockdown 16th March 2020 [14]. The median age for each age band considered is, e.g. 22 years for the 20–24 years age group.
2.3. Statistical analyses
2.3.1. QALYS estimation
Based on age-sex average crude probabilities of death calculated at 1, 3 and 5 years post-diagnosis, we assume that cancer deaths at 1-year post-diagnosis occur in 2021, at 3-years in 2023 and at 5-years in 2025. The calendar year when deaths occur is important to know from an economic perspective because cause and health losses occurring further into the future are valued less (discounted) relative to those occurring in or closer to the present. Productivity losses and QALYs lost from 2021 onwards were discounted at 3.5% per annum back to the reference year (2019).
We estimate QALYs lost based on the age distribution at death due to cancer and apply age-specific UK quality of life norms, discounted to obtain their 2019 value [17]. QALYs lost due to the effect of the first lockdown are estimated by taking the difference between the QALYs estimated for the deaths due to cancer observed pre-pandemic and those estimated following the effects of the first lockdown on diagnostic patterns.
2.3.2. Productivity losses estimation
We multiply the individual crude probabilities of cancer death, estimated at 1, 3 and 5 years after diagnosis, with the economic costs of a death calculated using the HC approach, stratified by 5-year age group and gender. We sum these quantities to obtain the economic value of the cancer deaths across the entire sampled cohort, representing the calendar period 16th March 2020 to 15th March 2021. By taking the difference between this quantity and the total economic value of deaths in the pre-pandemic cohort, and averaging across 500 bootstrapped datasets, we obtain the average (and 95% CI, percentile method) economic costs of cancer deaths in addition to those to be expected pre-pandemic, i.e. the excess productivity losses due to additional excess cancer deaths.
We compared the economic burden of additional excess deaths in the cancer population with that of an equal estimated number of COVID-19 deaths (n = 3620 deaths), based on 500 random samples drawn from the total number of COVID-19 deaths up to October 2020 (stratified by age). COVID-19 deaths were converted in QALYs using the method and calculator developed by Briggs, which also adjusts for multiple comorbidities [22]. The productivity and QALYs losses associated with COVID-19 deaths were estimated by multiplying deaths with the age-sex adjusted HC multiplier described above.
3. Results
We analysed data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 29,305 with lung cancer, and 6744 with oesophageal cancer. Patients were all diagnosed in England. Patients were aged 15–84 years and the mean age at diagnosis was 60·5 years (Standard Deviation (SD) 12·6) for breast cancer, 68·5 years (10·7) for colorectal cancer, 68·5 years (10·3) for oesophageal cancer, and 69·8 years (9·3) for lung cancer. 10,441 (41·8%) of 24,975 patients diagnosed with colorectal cancer, 13,211 (45·1%) of 29,305 diagnosed with lung cancer, and 1894 (28·1%) of 6744 diagnosed with oesophageal cancer were women. Table 1 presents three measures of the estimated impact following COVID-19-related delay in diagnosis.
Table 1.
QALY and productivity losses associated with additional excess cancer deaths by cancer site.
| Cancer site | Approach | Pre-pandemic (observed) | First wave pandemic (estimated with 95% CI) | Differences in observed and estimated QALY and productivity losses due to additional excess cancer deaths |
|---|---|---|---|---|
| Breast | Deaths | 3564 | 3908 (3891–3926) | 344 (326–361) |
| QALYs (thousands) | 37.1 | 41.3 (40.1–41.6) | 4.1 (3.9–4.4) | |
| Productivity losses (GBP million) | ||||
| Human capital approach | 267.0 | 290.2 (285.2–295.6) | 23.2 (18.2–28.6) | |
| Colorectal | Deaths | 9416 | 11,062 (10,982–11,145) | 1646 (1566–1729) |
| QALYs (thousands) | 80.9 | 95.9 (95.0–96.8) | 15.0 (14.1–16.0) | |
| Productivity losses (GBP million) | ||||
| Human capital approach | 410.1 | 445.8 (432.6–458.8) | 35.7 (22.4–48.7) | |
| Lung | Deaths | 25,800 | 27,090 (27,081–27,099) | 1290 (1281–1299) |
| QALYs (thousands) | 220.1 | 231.0 (230.1–231.8) | 10.9 (9.9–11.7) | |
| Productivity losses (GBP million) | ||||
| Human capital approach | 805.3 | 843.6 (819.3–865.2) | 38.3 (14.0–59.9) | |
| Oesophagus | Deaths | 5713 | 6048 (6038–6047) | 335 (325–345) |
| QALYs (thousands) | 50.5 | 53.2 (52.8–53.7) | 2.7 (2.3–3.1) | |
| Productivity losses (GBP million) | ||||
| Human capital approach | 195.5 | 202.1 (191.4–213.1) | 6.6 (−4.0–17.6) | |
| Total cancer | Deaths | 44,493 | 48,109 (48,020–48,200) | 3615 (3526–3706) |
| QALYs (thousands) | 388.7 | 421.4 (419.9–422.8) | 32.7 (31.3–34.1) | |
| Productivity losses (GBP million) | ||||
| Human capital approach | 1677.9 | 1781.7 (1751.1–-1810.1) | 103.8 (73.2–132.2) |
Abbreviations: QALY – quality-adjusted life-years discounted to 2019 value.
Notes: 95% confidence intervals obtained from 500 bootstraps, percentile method. Totals may not add up due to rounding.
3.1. Cancer deaths
The number of additional cancer deaths resulting from the diagnostic delay due to the COVID 19 pandemic was 1646 (1566–1729 deaths) for colorectal cancer up to Year 5, 1290 (1281–1299 deaths) for lung cancer, 344 for breast cancer (326–361 deaths), and 335 (325–345 deaths) additional deaths for oesophageal cancer (Table 1).
3.2. COVID-19 deaths
Up to 06 October 2020, there had been 53,863 deaths in total. The age distribution of death occurrences where COVID-19 was mentioned on the death certificate (Appendix 1) is based on cumulative deaths up to and including week 41 (09 October 2021). 61% of deaths occurred among those aged over 80 years and a further 28% among those aged 65–79 years. 45% of COVID-19 deaths occurred in women. Estimates for QALYS lost and productivity losses were based on 3620 COVID-19 deaths.
3.3. QALYS
We estimate that the additional cancer deaths due to diagnostic delay during the COVID-19 pandemic would result in 2700 QALYs lost for oesophageal cancer, 4100 QALYs lost for breast cancer, 10,900 QALYs lost for lung cancer and 15,000 QALYs lost for colorectal cancer. Across the four cancer sites combined, additional excess deaths would amount to approximately 32,700 QALYs lost (Table 1).
3.4. Productivity losses
Under the human capital approach, across these four cancers, additional productivity losses would amount to 103.8 (95% confidence interval 73.2 to 132.2) million GBP. Lung and colorectal cancers account for the largest share of productivity losses (38.3 and 35.7 million respectively), followed by breast cancer (23.2 million) and oesophageal cancer (6.6 million) (Table 1).
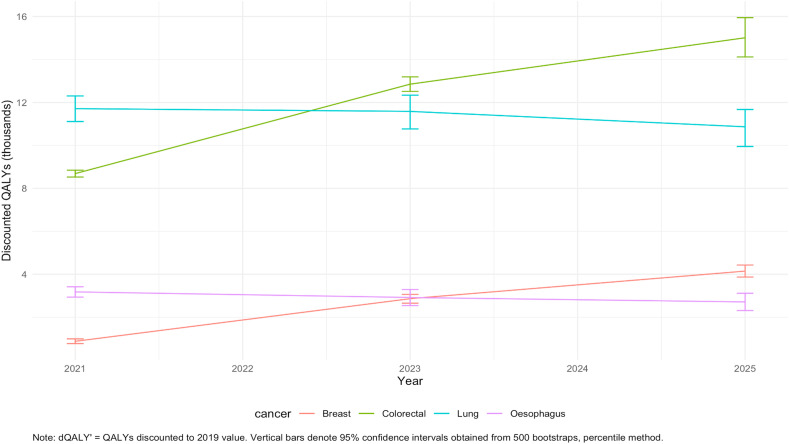
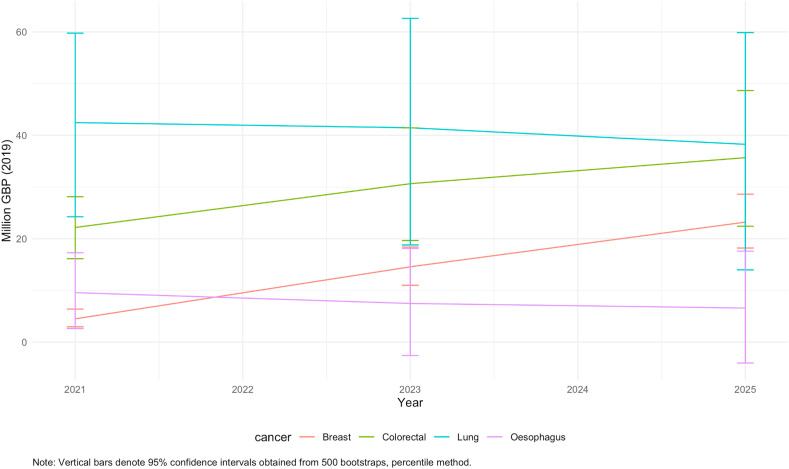
Fig. 1 and Fig. 2 demonstrate that for breast and colorectal cancers, excess losses in QALYs and productivity accumulate gradually from 2021 to 2025 at comparable and reasonably constant rates. For lung and oesophageal cancer, however, the burden is frontloaded within a year since diagnosis and losses are incurred by the end of 2021. This is due to differences across the four cancers in the cumulative probability of death over time since diagnosis translating in different temporal profiles of the excess burden (see Fig. 3).
Fig. 1.
Cumulative QALY losses associated with additional excess cancer deaths by cancer site and year.
Fig. 2.
Cumulative productivity losses associated with additional excess cancer deaths by cancer site and year.
Fig. 3.
Productivity and QALY losses associated with additional excess cancer deaths and an equal number of COVID-19 deaths stratified by age.
The uncertainty around the point estimates of productivity losses calculated under the human capital approach is high, particularly for lung and oesophageal cancers. Oesophageal cancer accounts for the least share of the excess burden among the four cancer types across all scenarios and metrics, and productivity losses are unlikely to be statistically different from pre-pandemic quantities.
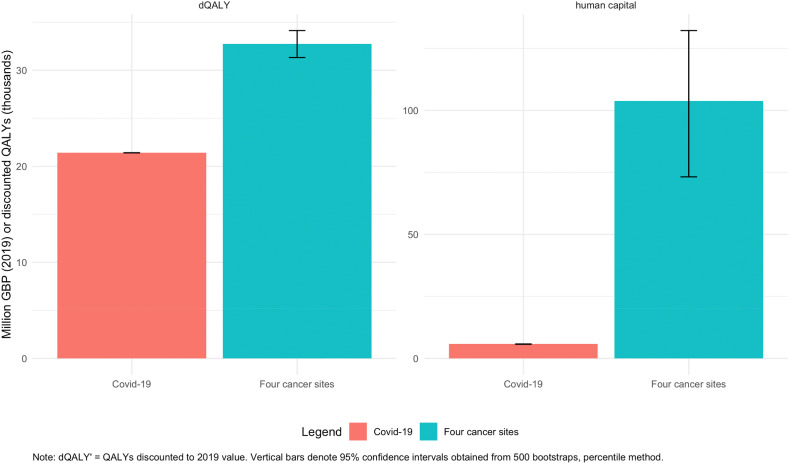
3.5. Indirect economic impact of COVID-19 versus cancer deaths
COVID-19 deaths were estimated to result in the loss of 21,450 QALYs, as well as 76.4 (73.5–79.2) million worth of lost economic output under the human capital approach. Additional excess deaths due to the four selected cancers are estimated to result in an additional 11,300 (9900 to 12,700) more QALYs lost compared to an equivalent number of COVID-19 deaths (Fig. 2). Differences in excess productivity losses amount to 27.6 (−0.8 to 53.3) million GBP.
4. Discussion
Our findings indicate that the projected impact of excess deaths due to delays in cancer diagnosis across England will lead to significant health and economic losses. The predicted excess deaths for four major tumour types combined (bowel, breast, lung and oesophagus) translate into an approximate loss of 32,700 quality-adjusted life years and productivity losses of between 73 and 132 million GBP over 5 years.
Colorectal cancer accounts for the largest productivity losses among the four cancers; from a third of excess productivity losses to just under half the total excess losses in QALYs. In addition, notable differences are evident in the magnitude and rate of accumulation of losses across the different cancers. Whilst delays due to diagnosis were expected to result in a similar number of additional cancer deaths for breast and oesophageal cancer, the difference in age profile (breast cancer with a mean age of 60.5 years versus oesophageal cancer with a mean age of 68.5 years) and prognosis of the two cancers result in higher estimated productivity losses and QALYs losses for breast cancer. In addition, we find that the burden of additional excess cancer deaths is likely to be higher than that of a comparable number of COVID-19 deaths due to the younger age profile of deaths from cancer [23].
The results complement those from an Australian Study that estimated the healthcare costs associated with stage migration (from Stage I to Stage II) of four cancers (breast, colorectal, lung and melanoma) due to 3 month and 6-month delays in diagnosis during the pandemic [24]. A 3-month delay is predicted to result in $12 million AUD excess health care costs over 5 years, and a 6-month delay $46 million AUD. Two other international studies have highlighted the increased risk of financial toxicity that cancer patients face during the COVID-19 pandemic due to rising unemployment levels and economic recession, meaning patients have to increasingly pay out of pocket due to loss of employment-based insurance or may forgo life-saving treatments to support their families financially [25,26].
Our results demonstrate that NPI such as national lockdowns will have substantial health and economic impacts and does call into question the “protect the NHS” messaging that was used to support NPI, as the NHS faces up to the prospect of managing the legacy (e.g. cancer diagnostic and treatment backlogs, significant waiting lists (>1–2 years) for benign health conditions) in the context of stagnating health budgets over the next few years. If these trade-offs had been considered more accurately and explicitly from the outset, it would have undoubtedly helped to support the mitigation strategies needed to avert this silent epidemic.
It could be argued that comprehensive decision frameworks and accompanying mathematical models that incorporate wider health, social and economic consequences were not available at the time when decisions were made. For example, it was only in mid-November 2020 that Imperial College released a UK-focused combined epidemiological and economic model that accounted for interdependencies between economic sectors [27].
However, the UK government’s ONS report published in July 2020, which was designed to estimate the indirect impacts of NPI on other health conditions, significantly under-estimated the true impact on cancer services of diagnostic delay (3500 QALYs lost across eighteen cancers) [28]. A more accurate and integrated assessment would have supported better trade-off decision-making around the extent of NPIs and the allocation of resources to mitigate the indirect impact of NPI on non-COVID healthcare pathways such as cancer.
It should be noted that our estimates are very conservative with regards to the health and economic impact. Recent data [5] suggest that the drop in expected cancer diagnoses based on yearly estimates is far greater than the estimates we have made in this paper [14]. For example, for bowel cancer between April to October 2020, over 3500 fewer people had been diagnosed and treated for colorectal cancer in England than would have been expected [7]. Likewise, urgent referrals for suspected lung cancer were 35% lower between March–November 2020 compared with the same time period in 2019, which equates to around 17,800 fewer referrals [29]. Many of these patients may never present or when they do, will present with advanced-stage disease.
The economic losses are predicted to be 103 million GBP in England alone. Therefore, extrapolating across the whole of the UK that has a population of 66 million, we estimate 121 million GBP in economic losses. When considering the impact of the second wave and the present national lockdown, which has persisted for over three months since December 2020 and is not expected to be fully scaled back till June 2021, it is not unreasonable to expect this figure to have doubled for just these four tumour types.
The estimates of productivity losses also do not consider the downstream impact of treatment delay, particularly of elective cancer surgery and changes to treatment doses and schedules. Even a 4-week delay in cancer treatment (surgery, chemotherapy and radiotherapy) increases the risk of mortality by approximately 10% [30], with a 3-month delay in cancer surgery alone across all incident solid tumours estimated to incur 4755 excess deaths [31].
Looking widely across Europe, substantial delays in diagnosis are a consistent finding. For example, in Paris alone, during the first wave (March to May 2020), new cancer presentations declined by 30% in comparison to the 2018/19 average [32]. Likewise, in the Netherlands, national-level data between Feb and April 2020 during their first wave demonstrated a persistent nearly 30% decline in the diagnosis of new cancers, which had not recovered by the end of the study [33].
Our results are significant for two major policy domains going forward. The first is that subsequent waves of COVID continue to add to overall system delays and excess death for cancer and other diseases [7]. The longer it takes to address these backlogs, the greater the clinical, economic and welfare costs will be, adding to an already indebted society. Greater investment to ensure resilience in the health system over the next few years will be a necessity, given clear evidence of the impact of previous economic downturns on rising mortality rates from diseases such as cancer [34]. The second policy issue relates to the importance of evidenced-based trade-off considerations around NPI. Our analysis demonstrates the importance of accurately quantifying the indirect effects of NPI on critical non-pandemic related health care services such as cancer care that require an integrated response from public health to hospital-based care and which are sensitive to changes in patient behaviour and their perception of risk.
A strength of this study is the use of linked national administrative health records of actual patients diagnosed and treated in the NHS for the four tumour types. While the data is retrospective, it still provides a robust template for modelling the economic productivity losses due to premature deaths from cancer, as it is representative of the age and sex distribution of patients diagnosed with these cancers in the NHS, which is essential when modelling economic losses.
In terms of limitations, our results are based on the predicted number of additional deaths within five years, for four cancer sites in England alone and not based on observed additional excess deaths, which will take a year(s) to show on official figures. However, it is imperative that further research establishes whether this has been the case. In addition, there is uncertainty in the productivity losses calculated, especially for oesophagus and lung cancer deaths. However, this uncertainty is unlikely to alter the essence of our findings.
Extrapolating our estimates across all cancers using this model would require a separate empirical analysis for each individual tumour. For example, the impact of diagnostic delay in prostate cancer on additional excess deaths is likely to be much smaller compared to the impact on head and neck and gastric cancers. Equally, extrapolating these economic losses across Europe is prone to significant bias as the predicted excess death is dependent on the burden, stage distribution and survival from these cancers, which we know is variable across different health systems in Europe [35]. In addition, the type and extent of NPIs introduced across Europe was variable and would again impact the number of additional excess deaths.
We do not directly consider the additional health care costs associated with an increasing proportion of patients presenting with late-stage disease [24]. As well as greater morbidity, it has been shown that Stage III/IV disease is more costly to manage than patients presenting with Stage I and II disease [36]. When clearer estimates for stage migration are available, it will be possible to model these impacts in the future, which will be important when considering how cancer budgets need to adapt to the increased proportion of patients presenting with advanced-stage disease. Finally, we capture only productivity losses due to premature mortality and not morbidity-related costs, and indirect effects on patient carers and disrupted family lives that will also increase the economic burden on countries [37].
From a wider health system perspective, cancers represent approximately 35% of all incident deaths in the UK and just over a third of all deaths from non-communicable diseases. As such, the four considered cancers amount to 10–15% of all NCD-related deaths [38]. While extrapolations are difficult at this point (within and beyond cancer) without additional data, even with simplifying assumptions, one can reasonably conclude that service disruptions in chronic disease pathways, e.g. cardiac and renal disease other than those considered here [39], are likely to have broader societal impacts many times over what we estimated in this study, spread over the following years.
5. Conclusions
In summary, we estimate that the additional excess cancer deaths for breast, colorectal, lung, and oesophageal cancer due to diagnostic delay in England resulting from NPIs, will translate into productivity losses of £104million over 5 years. The results are sobering when one considers we only focus on four tumour types and do not consider either the impact of treatment delays or NPIs that were initiated during the 2nd pandemic wave. There is an urgent need for investment to manage the rising cancer diagnostic and treatment backlog. In addition, the results emphasise the importance of accurate and transparent modelling of direct and indirect effects of NPIs on the wider health system prior to their introduction to support both resource allocation and prioritisation of time-critical health care services such as cancer care.
Author contributions
AA, AG, CM, KC, RS, MM and BR conceived and designed the study. AG and CM analysed the data. AG, CM, AA, RS, BR were involved in data interpretation. AG and AA wrote the first draft of the paper. AG produced the manuscript figures and tables. AG, CM, JS, AP, KC, BR, RS and AA were involved in reviewing and editing drafts of the paper and approving the manuscript.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was funded UK Research and Innovation Economic and Social Research Council GCRF. ES/P010962/1. This work uses data provided by patients and collected by the NHS as part of their care and support. AG acknowledges funding from the Research Councils United Kingdom through the Research for Health in Conflict in the Middle East and North Africa (ES/P010962/1) and is a member of AG is a member of the MRC Centre for Global Infectious Disease Analysis (MRC GIDA), jointly funded by the United Kingdom Medical Research Council (MRC) (MR/RO15600/1). CM and BR are funded through the Cancer Research UK Population Research Committee Funding Scheme: Cancer Research UK Population Research Committee - Programme Award (C7923/A20987 and C7923/A29018). RS is funded through the UK Research and Innovation GCRF RESEARCH FOR HEALTH IN CONFLICT (R4HC-MENA) ES/P010962/1. AA is supported by a National Institute for Health Research (NIHR) Advanced Fellowship (NIHR300599). JS is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King's College London (IS-BRC-1215-20006), and the Cancer Research UK King’s Health Partners Centre at King’s College London (C604/A25135). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.04.019.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Gathani T., Clayton G., E M, Horgan K. The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Canc. 2020;124:710–712. doi: 10.1038/s41416-020-01182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood E., Swanton C. Consequences of COVID-19 for cancer care — a CRUK perspective. Nat Rev Clin Oncol. 2021;18:3–4. doi: 10.1038/s41571-020-00446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutter M., Brookes M., Lee T., Rogers P., Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2020;70:537–543. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 4.McCormack V., Aggarwal A. Early cancer diagnosis: reaching targets across whole populations amidst setbacks. Br J Canc. 2021;124:1181–1182. doi: 10.1038/s41416-021-01276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purushotham A.R.G., Haire K., Dodkins J., Harvey-Jones E., Han L., Twinn C., et al. The impact of national non-pharmaceutical interventions (“lockdowns”) on the presentation of cancer patients. Ecancermedicalscience. 2021;15:1180–1187. doi: 10.3332/ecancer.2021.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer K., Jones C.M., Girdler R., et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol. 2021;22:309–320. doi: 10.1016/S1470-2045(20)30743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris E.J.A., Goldacre R., Spata E., et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6(3):199–208. doi: 10.1016/S2468-1253(21)00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle J., Kuryba A., Blake H., et al. The impact of the first peak of the COVID-19 pandemic on colorectal services in England and Wales; a national survey. Colorectal Dis. 2021:1–12. doi: 10.1111/codi.15622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliss-Brookes L., McPhail S., Ives A., et al. Routes to diagnosis for cancer - determining the patient journey using multiple routine data sets. Br J Canc. 2012;107(8):1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly L. Cancer surgery delays are biggest concern as services struglle with COVID, say NHS Chief. The Telegraph. 2021:4. [Google Scholar]
- 11.Hanna T.P., King W.D., Thibodeau S., et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilhorst S., Lockey A. Demos; 2019. Cancer Costs: a ‘ripple effect’ analysis of cancer's wider impact. [Google Scholar]
- 13.Rodin D., Burger E.A., Atun R., et al. Scale-up of radiotherapy for cervical cancer in the era of human papillomavirus vaccination in low-income and middle-income countries: a model-based analysis of need and economic impact. Lancet Oncol. 2019;20(7):915–923. doi: 10.1016/S1470-2045(19)30308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Office for National Statistics . June 2020. Deaths registered weekly in England and Wales, provisional.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/weeklyprovisionalfiguresondeathsregisteredinenglandandwales [Google Scholar]
- 16.Office for National Statistics . 2019. National life tables, UK.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables [Google Scholar]
- 17.Janssen B., Szende A. Springer; Netherlands: 2014. Population norms for the EQ-5D. Self-reported population health: an international perspective based on EQ-5D. [PubMed] [Google Scholar]
- 18.BBC News . BBC; 2020. State pension age hits 66 and set to rise further.https://www.bbc.co.uk/news/business-54421662 October 06, 2020. [Google Scholar]
- 19.Office for National Statistics . ASHE; 2019. Earnings and hours worked, age group.https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/agegroupashetable6 Table 6. [Google Scholar]
- 20.Office for National Statistics . 2020. Employment, unemployment and economic inactivity for people aged 16 years and over and aged from 16 to 64 years (not seasonally adjusted)https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/datasets/employmentunemploymentandeconomicinactivityforpeopleaged16yearsandoverandagedfrom16to64yearsnotseasonallyadjusted [Google Scholar]
- 21.Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland [Google Scholar]
- 22.Briggs A. Avalon Health Economics; 2020. Moving beyond ‘lives-saved’ from COVID-19.https://avalonecon.com/moving-beyond-lives-saved-from-covid-19/ [Google Scholar]
- 23.Cancer mortality by age. 2019. https://www.cancerresearchuk.org/health-professional/cancer-statistics/mortality/age#heading-Zero [Google Scholar]
- 24.Degeling K., Baxter N.N., Emery J., et al. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Asia Pac J Clin Oncol. 2021;1:1–9. doi: 10.1111/ajco.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baddour K., Kudrick L.D., Neopaney A., et al. Potential impact of the COVID-19 pandemic on financial toxicity in cancer survivors. Head Neck. 2020;42(6):1332–1338. doi: 10.1002/hed.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong Y.C., Sakti V.V., Sullivan R., Bhoo-Pathy N. Cancer and COVID-19: economic impact on households in Southeast Asia. Ecancermedicalscience. 2020;14:1134. doi: 10.3332/ecancer.2020.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haw D., Christen P., Forchini G., et al. Imperial College London; London: 2020. DAEDALUS: an economic-epidemiological model to optimize economic activity while containing the SARS-CoV-2 pandemic. [Google Scholar]
- 28.Office for National Statistics . 2020. Direct and indirect impacts of COVID-19 on excess deaths and morbidity: executive summary.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/907616/s0650-direct-indirect-impacts-covid-19-excess-deaths-morbidity-sage-48.pdf [Google Scholar]
- 29.Cancer Research UK . 2021. Recognition and referral of suspected lung cancer in the UK during the COVID-19 pandemic. [Google Scholar]
- 30.Hanna T., King W., Thibodeau S., et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. Br Med J. 2020:371. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sud A., Jones M., Broggio J., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempf E., Lame G., Layese R., et al. New cancer cases at the time of the sars-cov2 pandemic and related public health policies. Eur J Canc. 2021;150:260–267. doi: 10.1016/j.ejca.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Din Mohamed A.G., Visser O., Verhoeven R.H.A., et al. Fewer cancer diagnoses during the cancer epidemic in The Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maruthappu M., Watkins J., Noor A.M., et al. Economic downturns, universal health coverage, and cancer mortality in high-income and middle-income countries, 1990-2010: a longitudinal analysis. Lancet (London, England) 2016;388(10045):684–695. doi: 10.1016/S0140-6736(16)00577-8. [DOI] [PubMed] [Google Scholar]
- 35.De Angelis R, Sant M, Coleman M. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE-5 – a population-based study. Lancet Oncol;15(1):23-34. [DOI] [PubMed]
- 36.Sun L., Legood R., dos-Santos-Silva I., Gaiha S.M., Sadique Z. Global treatment costs of breast cancer by stage: a systematic review. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0207993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luengo-Fernandez R., Leal J., Gray A., Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–1174. doi: 10.1016/S1470-2045(13)70442-X. [DOI] [PubMed] [Google Scholar]
- 38.Institute for Health Metrics and Evaluation . 2021. GBD compare.http://www.healthdata.org/data-visualization/gbd-compare [Google Scholar]
- 39.Fersia O., Bryant S., Nicholson R., et al. The impact of the COVID-19 pandemic on cardiology services. Open Heart. 2020;7(2) doi: 10.1136/openhrt-2020-001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.