Abstract
Aims
Persistence with direct oral anticoagulants (DOACs) has become a concern in non-valvular atrial fibrillation (NVAF) patients, but whether this affects prognosis is rarely studied. We investigated the persistence with oral anticoagulants (OACs) and its association with prognosis among a nationwide cohort of NVAF patients.
Methods and results
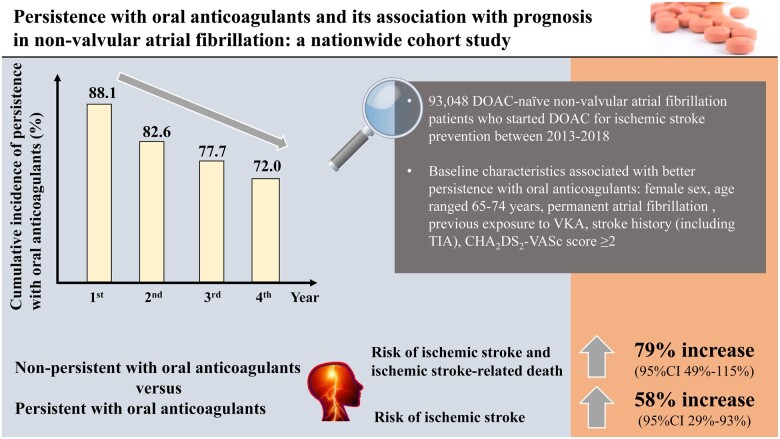
DOAC-naive NVAF patients who started to use DOACs for ischaemic stroke prevention between 2013 and 2018 were included using Dutch national statistics. Persistence with OACs was determined based on the presence of a 100-day gap between the last prescription and the end of study period. In 93 048 patients, 75.7% had a baseline CHA2DS2-VASc score of ≥2. The cumulative incidence of persistence with OACs was 88.1% [95% confidence interval (CI) 87.9–88.3%], 82.6% (95% CI 82.3–82.9%), 77.7% (95% CI 77.3–78.1%), and 72.0% (95% CI 71.5–72.5%) at 1, 2, 3, and 4 years after receiving DOACs, respectively. Baseline characteristics associated with better persistence with OACs included female sex, age range 65–74 years, permanent atrial fibrillation, previous exposure to vitamin K antagonists, stroke history (including transient ischaemic attack), and a CHA2DS2-VASc score ≥2. Non-persistence with OACs was associated with an increased risk of the composite outcome of ischaemic stroke and ischaemic stroke-related death [adjusted hazard ratio (aHR) 1.79, 95% CI 1.49–2.15] and ischaemic stroke (aHR 1.58, 95% CI 1.29–1.93) compared with being persistent with OACs.
Conclusion
At least a quarter of NVAF patients were non-persistent with OACs within 4 years, which was associated with poor efficacy of ischaemic stroke prevention. The identified baseline characteristics may help identify patients at risk of non-persistence.
Keywords: Atrial fibrillation, Direct oral anticoagulants, Vitamin K antagonists, Medication, Persistence, Stroke
Graphical Abstract
See page 4138 for the editorial comment for this article ‘Persistence with treatment in atrial fibrillation: still a pressing issue in the era of direct oral anticoagulants’, by P.V Rasmussen and E. Hylek, https://doi.org/10.1093/eurheartj/ehab524.
Introduction
Non-valvular atrial fibrillation (NVAF), the most common cardiac arrhythmia globally,1,2 is associated with a five-fold increased risk of ischaemic stroke.3,4 Long-term oral anticoagulation is therefore recommended for NVAF patients at moderate to high risk of thromboembolic events to prevent thromboembolism according to current guidelines.5–7 Vitamin K antagonists (VKAs) were the most frequently used oral anticoagulants (OACs) over the past 60 years, but direct oral anticoagulants (DOACs) are replacing VKAs to be the main therapeutic option in NVAF.8,9 Compared with VKAs, DOACs have attractive characteristics including predictable pharmacokinetics and pharmacodynamics, low drug–drug and food interactions, and no need for laboratory monitoring in general.10 Evidence from large randomized controlled trials indicates at least noninferiority for the combined endpoint of stroke or systemic embolism but a superior safety profile of DOACs compared with VKAs.11–14 However, concerns have been expressed about treatment persistence (i.e., the duration of time from initiation to discontinuation of therapy15) with DOACs. Since ‘drugs don’t work in patients who don’t take them’,16 the lack of regular control visits may be a double-edged sword. Suboptimal persistence with DOACs in atrial fibrillation (AF) patients was observed in numerous real-world studies, although a precise estimation is challenging (and maybe not necessary) to obtain given the variations of settings and definitions of non-persistence across studies.17–24 Unlike the persistence pattern, the impact of non-persistence with OACs on prognosis has not been well investigated. As far as we know, only one study looked at the association between DOAC persistence (instead of adherence) and clinical outcomes.21 For these reasons, we conducted a nationwide study to investigate OAC persistence pattern and its association with prognosis among a nationwide cohort of NVAF patients who initiated a DOAC between 2013 and 2018 in the Netherlands.
Methods
Data sources and study population
The study comprised a nationwide cohort of adult DOAC-naive NVAF patients who started to use DOAC for ischaemic stroke prevention between January 1, 2013, and September 30, 2018, in the Netherlands, using data accessed from Statistics Netherlands (‘Centraal Bureau voor de Statistiek’, CBS). A detailed introduction of the data sources and inclusion and exclusion criteria of the study population are presented in the Supplementary material online, methods. Several code systems were used for data extraction, as presented in Supplementary material online, Table S1. The study complied with the Declaration of Helsinki and received an ethical approval from the Department of Clinical Epidemiology of the Leiden University Medical Center with a waiver of participant consent due to the use of pre-existing, de-identified data only.
Baseline characteristics
The date of the first DOAC prescription was defined as the index date, which was the baseline of the patients. The following baseline characteristics were studied: sex, age, subtype of AF (i.e. permanent or not; only available for AF diagnosed after 2015 and the diagnosis that was closest to the index date was used if a patient had more than one AF diagnosis records), previous exposure to VKA (i.e. VKA prescribed before the index date but after the first AF diagnosis), stroke history [including transient ischaemic attack (TIA)] and some other comorbidities (identified by screening diagnosis data within 3 years before the index date), immigration status, marital status, standardized household income, the baseline CHA2DS2-VASc score,25 and the adapted (8-item, removing the item ‘Labile INR’) HAS-BLED score.26 Detailed information about how the two scores were calculated is presented in the Supplementary material online, methods.
In addition to the above characteristics, for patients who had participated in the ‘Dutch Health Monitor’ (DHM) surveys before the index date (i.e. DHM participants), the following characteristics were also studied: highest education level, body mass index (BMI), physical health, feeling of loneliness, feeling of depression, ability to meet financial needs, alcohol use, smoking history, living alone, and employment status. If a patient participated in the surveys more than once before the index date, data from the latest survey were used.
Persistence pattern
Non-persistence with OAC (i.e. stop receiving DOAC or VKA therapy) and non-persistence with the initial DOAC (i.e. stop receiving DOAC therapy, with/without switch from DOAC to VKA) were identified in the study (illustrated in Supplementary material online, Figures S1 and S2). Since data about the amount of medication for each prescription were unavailable, a conservative definition was used to determine non-persistence. In brief, to determine non-persistence with OAC, OAC prescription records between the index date and December 31, 2018, or date of death (whichever came first) were examined to identify the last OAC prescription during this period. If the last OAC was prescribed between October 1, 2018, and December 31, 2018 (i.e. an interval of about 100 days), the patient would be considered as persistent with OAC between the index date and September 30, 2018. If the last OAC was prescribed before September 30, 2018, the patient would be considered as non-persistent with OAC from the date when the last OAC was prescribed, unless the patient died within 100 days after the last OAC prescription. We chose 100 days as the length of gap based on the fact that a patient could only receive medication for a maximum of 90 days each time in the Netherlands27 and extra 10 days were added to allow some flexibility. Non-persistence with the initial DOAC was determined in a similar way, but instead of the last OAC prescription, the last DOAC prescription (before the first VKA prescription, if it existed) was used to determine the persistence pattern to the initial DOAC. Since information on specific types of DOAC was unavailable, the initial DOAC referred to all types of DOACs rather than VKA.
Clinical outcomes
The following clinical outcomes were studied: (i) a composite outcome of ischaemic stroke and ischaemic stroke-related death; (ii) ischaemic stroke; and (iii) all-cause mortality. To determine the studied clinical outcomes, all patients were followed from the index date until the first occurrence of the studied outcome, date of death, or the end of the study period (i.e. September 30, 2018), whichever came first. For the composite outcome, ischaemic stroke was examined in the diagnoses data, and at the same time, death caused by ischaemic stroke was also examined in the data about causes of death. For the outcome ischaemic stroke, only diagnoses data were examined for ischaemic stroke. TIA was not included when identifying ischaemic stroke.
Statistical analysis
Continuous variables were presented as means ± standard deviations and categorical variables were presented as numbers and percentages. Kaplan–Meier estimator was used to estimate the cumulative incidences of persistence with OAC (or persistence with the initial DOAC). To explore predictors of non-persistence with OAC (or non-persistence with the initial DOAC), a Cox proportional hazard model was employed. In addition to the crude association of each predictor with non-persistence, the association after adjusting for age and sex was evaluated, with or without restricting the follow-up time to up to 1 year and patients with a baseline CHA2DS2-VASc score ≥2. For variables extracted from the DHM surveys, only DHM participants were included in the analyses. To evaluate the associations between persistence pattern (i.e. persistence pattern with OAC) and clinical outcomes, incidence rates of the studied outcomes between persistent stage and non-persistent stage were calculated and the Mantel–Byar method was used to estimate the associations. In brief, persistence status was treated as a time-dependent exposure in multivariable Cox regression models, and for non-persistent patients, the follow-up time before becoming non-persistent was classified into the effect of being persistent instead of being non-persistent. The following adjustment models were used: (i) adjusting for age and sex and (ii) adjusting for age, sex, subtype of AF, previous exposure to VKA, stroke history (including TIA), and standardized household income. The associations were also evaluated after being stratified by CHA2DS2-VASc score at baseline. To examine the robustness of the associations, a sensitivity analysis was planned which first used different lengths of the gap to define non-persistence with OAC (i.e. 14, 30, 60, 90, 120, 180 days) and then evaluated again the associations between non-persistence with OAC and the studied outcomes. As an examination of data quality, we examined the associations between the baseline characteristics and the studied outcomes. We also investigated time distributions of the studied outcomes during the non-persistent stage with OAC using the cumulative incidence competing risk method (for the composite outcome and ischaemic stroke) and Kaplan–Meier estimator (for all-cause mortality). All statistical analyses were performed with SPSS® Statistics (IBM Corp. Released 2017; IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY) and R program (R Core Team 2018, R Foundation for Statistical Computing, Vienna, Austria; available online at https://www.R-project.org/).
Results
Baseline characteristics
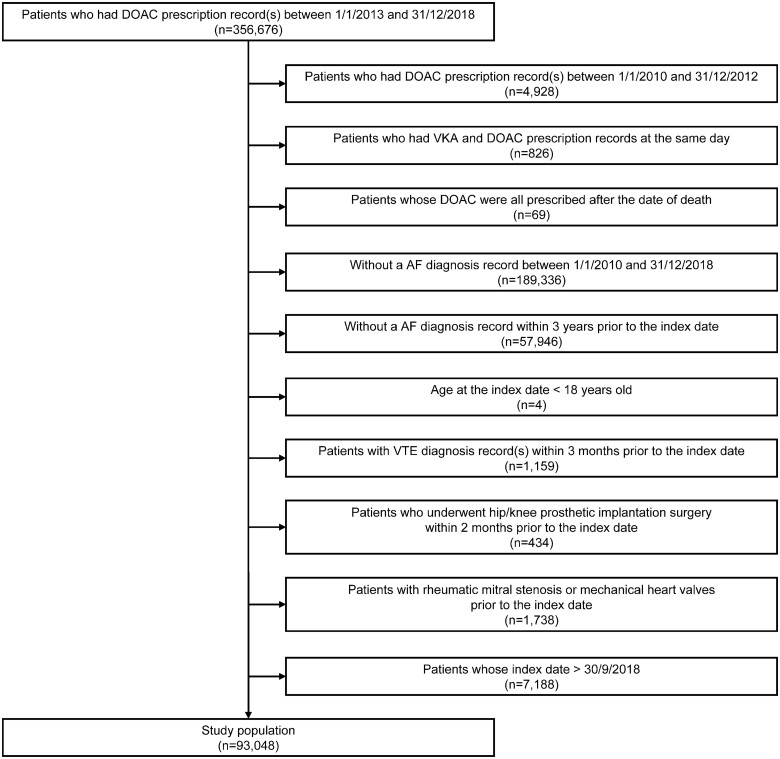
After applying the inclusion and exclusion criteria, 93 048 adult DOAC-naive NVAF patients who started to use DOAC for ischaemic stroke prevention between January 1, 2013, and September 30, 2018, in the Netherlands were included (Figure 1). The study population had an mean age of 72.2 ± 11.1 years, 56.2% were male, 87.5% were native Dutch, 10.7% had a stroke history (including TIA), and 28.4% were switched from VKA. Among patients whose information about subtype of AF was available, 25.6% had permanent AF. The mean baseline CHA2DS2-VASc score was 2.8 ± 1.7, and 75.7% patients had a baseline CHA2DS2-VASc score ≥2. Information about other baseline characteristics is presented in Table 1 and Supplementary material online, Table S2. A total of 10 188 patients had participated in at least one of the DHM surveys, which comprised the DHM participants. Similar baseline characteristics (for variables available for the entire cohort) were observed among the DHM participants compared with the entire cohort, except that the DHM participants were slightly older (mean age 74.8 ± 9.3 years). According to variables only available for the DHM participants, 43.6% had the highest education level of ‘High school underclassman’, 44.0% had a BMI that ranged between 25 and 30 kg/m2, and 92.7% had a fair or good physical health. Information about other baseline characteristics is presented in Supplementary material online, Table S3.
Figure 1.
Flow chart of the study population. Note: 1 month was counted as 30 days, and 1 year was counted as 360 days. AF, atrial fibrillation; DOAC, direct oral anticoagulant; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Table 1.
Baseline characteristics of the study population
| Variable | |
| Patients, n | 93 048 |
| Sex | |
| Male | 52 285 (56.2) |
| Female | 40 763 (43.8) |
| Age (years) | 72.2 ± 11.1 |
| Age group (years) | |
| 18–34 | 316 (0.3) |
| 35–44 | 994 (1.1) |
| 45–54 | 5133 (5.5) |
| 55–64 | 15 455 (16.6) |
| 65–74 | 32 439 (34.9) |
| 75–84 | 27 478 (29.5) |
| ≥85 | 11 233 (12.1) |
| Subtype of AFa | |
| Permanent | 4845 (25.6) |
| Paroxysmal | 14 073 (74.4) |
| Previous exposure to VKA | |
| No | 66 619 (71.6) |
| Yes | 26 429 (28.4) |
| Stroke history (including TIA) | |
| No | 83 101 (89.3) |
| Yes | 9947 (10.7) |
| Immigration status | |
| Native | 81 433 (87.5) |
| First generation | 6208 (6.7) |
| Second generation | 5407 (5.8) |
| Marital status | |
| Married or in partnership | 56 385 (60.6) |
| Unmarried or single | 6664 (7.2) |
| Divorced | 10 307 (11.1) |
| Widowed | 19 692 (21.2) |
| Standardized household incomeb | |
| First quintile (0–20%) | 13 274 (14.5) |
| Second quintile (20–40%) | 24 761 (27.1) |
| Third quintile (40–60%) | 22 908 (25.1) |
| Fourth quintile (60–80%) | 16 593 (18.1) |
| Fifth quintile (80–100%) | 13 906 (15.2) |
| CHA2DS2-VASc score | |
| Mean ± SD | 2.8 ± 1.7 |
| 0 (low risk) | 7470 (8.0) |
| 1 (moderate risk) | 15 160 (16.3) |
| ≥2 (high risk) | 70 418 (75.7) |
| HAS-BLED scorec | |
| Mean ± SD | 1.7 ± 1.0 |
| 0 | 10 095 (10.8) |
| 1 | 32 371 (34.8) |
| 2 | 32 363 (34.8) |
| ≥3 (high risk) | 18 219 (19.6) |
Notes: Missing data (if any) are not presented.
AF, atrial fibrillation; INR, international normalized ratio; TIA, transient ischaemic attack; SD, standard deviation; VKA, vitamin K antagonist.
Subtypes of AF were only available for AF diagnosed after 2015. The subtype of unspecified AF was not included into the analysis. The subtypes of persistent AF, chronic AF, type I atrial flutter, and type II atrial flutter were categorized as ‘Permanent’.
Private household with an unknown income and institutional household are not presented. Percentile groups were determined based on disposable income of private households of the whole target population in the database (instead of the study population only).
Labile INR was not included.
Predictors of persistence pattern
The cumulative incidences of persistence with OAC were 88.1% [95% confidence interval (CI) 87.9–88.3%], 82.6% (95% CI 82.3–82.9%), 77.7% (95% CI 77.3–78.1%), and 72.0% (95% CI 71.5–72.5%) at 1, 2, 3, and 4 years after the index DOAC prescription, respectively (Supplementary material online, Table S4). Variables associated with poor persistence with OAC included male sex [hazard ratio (HR) 1.25, 95% CI 1.21–1.29, compared to female sex), a younger age (HRs all >1 for the age groups below 65 years, compared to the age group 65–74 years), paroxysmal AF (HR 1.15, 95% CI 1.06–1.25, compared to permanent AF), no exposure to VKA (HR 1.50, 95% CI 1.45–1.56, compared to previous exposure to VKA), without stroke/TIA history (HR 1.40, 95% CI 1.32–1.49, compared to those with stroke/TIA history), other marital status except for marriage (HR 1.72, 95% CI 1.63–1.81 for those unmarried or single, HR 1.29, 95% CI 1.23–1.35 for those divorced, and HR 0.99, 95% CI 0.95–1.03 for those widowed, compared to marriage), and a lower baseline CHA2DS2-VASc score (HR 3.35, 95% CI 3.21–3.48 for a score of 0, and HR 1.43, 95% CI 1.37–1.48 for a score of 1, compared to a baseline CHA2DS2-VASc score ≥2) (Table 2). The results were broadly consistent after adjusting for age and sex, without/with restricting the follow-up time to a maximum of 1 year after the index date, and without/with excluding patients who had a baseline CHA2DS2-VASc score of <2. For other baseline characteristics, the associations with non-persistence with OAC were not consistent across all strata, but some strata still showed an increased risk of non-persistence with OAC, including first-generation immigrant (compared with native Dutch) and the first and fifth quintiles of standardized household income (compared with the third quintile). Results of factors associated with non-persistence with the initial DOAC (presented in Supplementary material online, Table S5) suggested similar predictors, but some crude associations became statistically non-significant in the adjustment and/or restriction analyses. For variables only available in the DHM participants, as presented in Supplementary material online, Table S6, some were associated with an increased risk of non-persistence with OAC, including living alone, a poor or very poor physical health (compared with a very good or good physical health), often feeling depression (compared with never feeling depression), and having some difficulties in meeting financial needs (compared with having no difficulties in meeting financial needs). A similar profile of predictors of non-persistence with the initial DOAC in the DHM participants was observed (Supplementary material online, Table S7).
Table 2.
Risk of being non-persistent with oral anticoagulant according to baseline characteristics of the study population
| Variables | Observation time (PY) | No. events | Hazard ratio | Hazard ratio | Hazard ratio | Hazard ratio |
|---|---|---|---|---|---|---|
| (95% CI) | (95% CI)c | (95% CI)c,d | (95% CI)c,d,e | |||
| Sex | ||||||
| Female | 67 804 | 5965 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Male | 88 266 | 9656 | 1.25 (1.21–1.29) | 1.10 (1.07–1.14) | 1.13 (1.08–1.17) | 1.06 (1.01–1.12) |
| Age (years) | 156 069 | 15 621 | 0.97 (0.97–0.97) | 0.97 (0.97–0.97) | 0.96 (0.96–0.97) | 1.02 (1.02–1.02) |
| Age group (years) | ||||||
| 18–34 | 266 | 234 | 11.14 (9.76–12.71) | 10.82 (9.48–12.35) | 12.96 (11.19–15) | 3.70 (1.39–9.86) |
| 35–44 | 1307 | 587 | 6.43 (5.89–7.01) | 6.29 (5.76–6.86) | 7.87 (7.11–8.71) | 2.96 (1.94–4.51) |
| 45–54 | 8650 | 2044 | 3.60 (3.41–3.80) | 3.54 (3.35–3.73) | 4.26 (3.98–4.55) | 1.55 (1.29–1.86) |
| 55–64 | 29 088 | 3535 | 1.89 (1.81–1.98) | 1.87 (1.79–1.96) | 2.23 (2.10–2.36) | 1.30 (1.17–1.44) |
| 65–74 | 61 673 | 3967 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 75–84 | 42 597 | 3464 | 1.20 (1.14–1.25) | 1.22 (1.16–1.27) | 1.12 (1.06–1.19) | 1.19 (1.11–1.27) |
| ≥85 | 12 488 | 1790 | 1.90 (1.79–2.01) | 1.97 (1.86–2.08) | 1.92 (1.79–2.05) | 2.01 (1.87–2.16) |
| Subtype of AFa | ||||||
| Permanent | 6080 | 692 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Paroxysmal | 18 411 | 2378 | 1.15 (1.06–1.25) | 1.17 (1.07–1.27) | 1.24 (1.12–1.37) | 1.19 (1.03–1.36) |
| Previous exposure to VKA | ||||||
| Yes | 54 273 | 3897 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| No | 101 796 | 11 724 | 1.50 (1.45–1.56) | 1.55 (1.50–1.61) | 1.85 (1.76–1.95) | 1.60 (1.50–1.71) |
| Stroke history (including TIA) | ||||||
| Yes | 15 445 | 1163 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| No | 140 624 | 14 458 | 1.40 (1.32–1.49) | 1.26 (1.18–1.33) | 1.44 (1.33–1.56) | 1.21 (1.12–1.32) |
| Immigration status | ||||||
| Native | 136 916 | 13 425 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| First generation | 9928 | 1223 | 1.24 (1.17–1.32) | 1.19 (1.12–1.26) | 1.09 (1.01–1.17) | 1.29 (1.17–1.42) |
| Second generation | 9225 | 973 | 1.08 (1.01–1.15) | 1.08 (1.02–1.16) | 1.04 (0.96–1.13) | 1.07 (0.95–1.19) |
| Marital status | ||||||
| Married or in partnership | 100 400 | 9185 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Unmarried or single | 10 269 | 1673 | 1.72 (1.63–1.81) | 1.38 (1.30–1.45) | 1.34 (1.26–1.43) | 1.51 (1.36–1.69) |
| Divorced | 16 870 | 2033 | 1.29 (1.23–1.35) | 1.24 (1.18–1.30) | 1.16 (1.09–1.23) | 1.29 (1.19–1.41) |
| Widowed | 28 530 | 2730 | 0.99 (0.95–1.03) | 1.41 (1.35–1.48) | 1.40 (1.32–1.48) | 1.14 (1.06–1.22) |
| Standardized household incomeb | ||||||
| First quintile (0–20%) | 18 440 | 2095 | 1.31 (1.24–1.38) | 1.44 (1.36–1.52) | 1.39 (1.30–1.49) | 1.27 (1.17–1.38) |
| Second quintile (20–40%) | 39 390 | 3408 | 1.04 (0.99–1.09) | 1.14 (1.09–1.20) | 1.07 (1.01–1.14) | 1.04 (0.96–1.12) |
| Third quintile (40–60%) | 39 866 | 3245 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Fourth quintile (60–80%) | 30 599 | 3011 | 1.23 (1.17–1.29) | 1.11 (1.05–1.17) | 1.09 (1.03–1.16) | 0.98 (0.90–1.08) |
| Fifth quintile (80–100%) | 26 078 | 3529 | 1.70 (1.62–1.78) | 1.37 (1.30–1.44) | 1.39 (1.30–1.47) | 1.15 (1.04–1.28) |
| CHA2DS2-VASc score | ||||||
| Clinical categories | ||||||
| ≥2 (high risk) | 113 078 | 9075 | 1 (reference) | – | – | – |
| 1 (moderate risk) | 30 232 | 3240 | 1.43 (1.37–1.48) | – | – | – |
| 0 (low risk) | 12 760 | 3306 | 3.35 (3.21–3.48) | – | – | – |
| Any category | ||||||
| 7–9 | 2013 | 232 | 1.34 (1.17–1.53) | – | – | – |
| 6 | 5086 | 491 | 1.14 (1.04–1.26) | – | – | – |
| 5 | 11 536 | 1057 | 1.11 (1.04–1.20) | – | – | – |
| 4 | 21 476 | 1758 | 1.02 (0.96–1.08) | – | – | – |
| 3 | 34 092 | 2638 | 1.00 (0.95–1.05) | – | – | – |
| 2 | 38 874 | 2899 | 1 (reference) | – | – | – |
| 1 | 30 232 | 3240 | 1.47 (1.40–1.54) | – | – | – |
| 0 | 12 760 | 3306 | 3.44 (3.27–3.62) | – | – | – |
AF, atrial fibrillation; CI, confidence interval; OAC, oral anticoagulant; PY, person-year; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
Subtypes of AF were only available for AF diagnosed after 2015. The subtype of unspecified AF was not included into the analysis. The subtypes of persistent AF, chronic AF, type I atrial flutter, and type II atrial flutter were categorized as “Permanent”.
Private household with an unknown income and institutional household were not included into the analysis. Percentile groups were determined based on disposable income of private households of the whole target population in the database (instead of the study population only).
Adjusted for age and sex.
Restrict the follow-up to up to 1 year after the first DOAC prescription.
Restrict to patients who had a baseline CHA2DS2-VASc score ≥2.
Associations between non-persistence with OAC and clinical outcomes
After adjusting for age, sex, subtype of AF, previous exposure to VKA, stroke history (including TIA), and standardized household income, non-persistence with OAC was associated with an increased risk of the composite outcome (HR 1.79, 95% CI 1.49–2.15), ischaemic stroke (HR 1.58, 95% CI 1.29–1.93), and all-cause mortality (HR 2.32, 95% CI 2.18–2.47) when compared with being persistent with OAC (Table 3). When stratified by levels of baseline CHA2DS2-VASc score (Table 4), the associations between non-persistence with OAC and the studied outcomes were consistent within levels of baseline CHA2DS2-VASc score ≥2, while for patients with a baseline CHA2DS2-VASc score <2, except for all-cause mortality, non-persistence with OAC was found to be associated with a reduced risk of the composite outcome (HR 0.37, 95% CI 0.20–0.69, adjusting for the adapted HAS-BLED score) and ischaemic stroke (HR 0.38, 95% CI 0.20–0.70, adjusting for the adapted HAS-BLED score).
Table 3.
Associations between non-persistence with OAC and clinical outcomes
| Clinical outcomes | Observation time (PY) | No. events | Incidence rateb | Hazard ratio | Hazard ratio | Hazard ratio |
|---|---|---|---|---|---|---|
| (95% CI) | (95% CI)c | (95% CI)d | ||||
| Composite outcomea | ||||||
| Persistent stage | 154 496 | 1596 | 1.03 | 1 (reference) | 1 (reference) | 1 (reference) |
| Non-persistent stage | 17 359 | 207 | 1.19 | 1.41 (1.22–1.63) | 1.69 (1.46–1.96) | 1.79 (1.49–2.15) |
| Ischaemic stroke | ||||||
| Persistent stage | 154 496 | 1446 | 0.94 | 1 (reference) | 1 (reference) | 1 (reference) |
| Non-persistent stage | 17 359 | 157 | 0.90 | 1.17 (0.99–1.38) | 1.39 (1.17–1.64) | 1.58 (1.29–1.93) |
| All-cause mortality | ||||||
| Persistent stage | 156 069 | 8187 | 5.25 | 1 (reference) | 1 (reference) | 1 (reference) |
| Non-persistent stage | 17 598 | 1877 | 10.67 | 2.38 (2.26–2.51) | 2.77 (2.63–2.91) | 2.32 (2.18–2.47) |
AF, atrial fibrillation; CI, confidence interval; OAC, oral anticoagulant; PY, person-year; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
Ischaemic stroke and ischaemic stroke-related death.
Per 100 PY.
Adjusted for age and sex.
Adjusted for age, sex, subtype of AF, previous exposure to VKA, stroke history (including TIA), and standardized household income.
Table 4.
Associations between non-persistence with oral anticoagulant and clinical outcomes stratified by baseline CHA2DS2-VASc score
| Clinical outcomes | Observation time (PY) | No. events | Incidence rateb | Hazard ratio | Hazard ratio |
|---|---|---|---|---|---|
| (95% CI) | (95% CI)c | ||||
| Composite outcomea | |||||
| 0–1 | |||||
| Persistent stage | 42 727 | 176 | 0.41 | 1 (reference) | 1 (reference) |
| Non-persistent stage | 9 371 | 11 | 0.12 | 0.31 (0.17–0.57) | 0.37 (0.20–0.69) |
| 2–4 | |||||
| Persistent stage | 93 538 | 872 | 0.93 | 1 (reference) | 1 (reference) |
| Non-persistent stage | 6625 | 118 | 1.78 | 2.20 (1.81–2.68) | 2.27 (1.87–2.75) |
| ≥5 | |||||
| Persistent stage | 18 231 | 548 | 3.01 | 1 (reference) | 1 (reference) |
| Non-persistent stage | 1363 | 78 | 5.72 | 2.49 (1.95–3.17) | 2.51 (1.97–3.20) |
| Ischaemic stroke | |||||
| 0–1 | |||||
| Persistent stage | 42 727 | 174 | 0.41 | 1 (reference) | 1 (reference) |
| Non-persistent stage | 9371 | 11 | 0.12 | 0.31 (0.17–0.58) | 0.38 (0.20–0.70) |
| 2–4 | |||||
| Persistent stage | 93 538 | 813 | 0.87 | 1 (reference) | 1 (reference) |
| Non-persistent stage | 6625 | 87 | 1.31 | 1.74 (1.40–2.18) | 1.79 (1.43–2.24) |
| ≥5 | |||||
| Persistent stage | 18 231 | 459 | 2.52 | 1 (reference) | 1 (reference) |
| Non-persistent stage | 1363 | 59 | 4.33 | 2.20 (1.67–2.89) | 2.22 (1.68–2.92) |
| All-cause mortality | |||||
| 0–1 | |||||
| Persistent stage | 42 991 | 641 | 1.49 | 1 (reference) | 1 (reference) |
| Non-persistent stage | 9394 | 217 | 2.31 | 1.71 (1.47–2.00) | 2.29 (1.95–2.69) |
| 2–4 | |||||
| Persistent stage | 94 443 | 5258 | 5.57 | 1 (reference) | 1 (reference) |
| Non-persistent stage | 6754 | 1195 | 17.69 | 3.62 (3.39–3.85) | 3.72 (3.49–3.97) |
| ≥5 | |||||
| Persistent stage | 18 635 | 2288 | 12.28 | 1 (reference) | 1 (reference) |
| Non-persistent stage | 1450 | 465 | 32.06 | 3.03 (2.74–3.36) | 3.05 (2.75–3.37) |
CI, confidence interval; OAC, oral anticoagulant; PY, person-year.
Ischaemic stroke and ischaemic stroke-related death.
Per 100 PY.
Adjusted for the adapted HAS-BLED score.
Sensitivity analysis
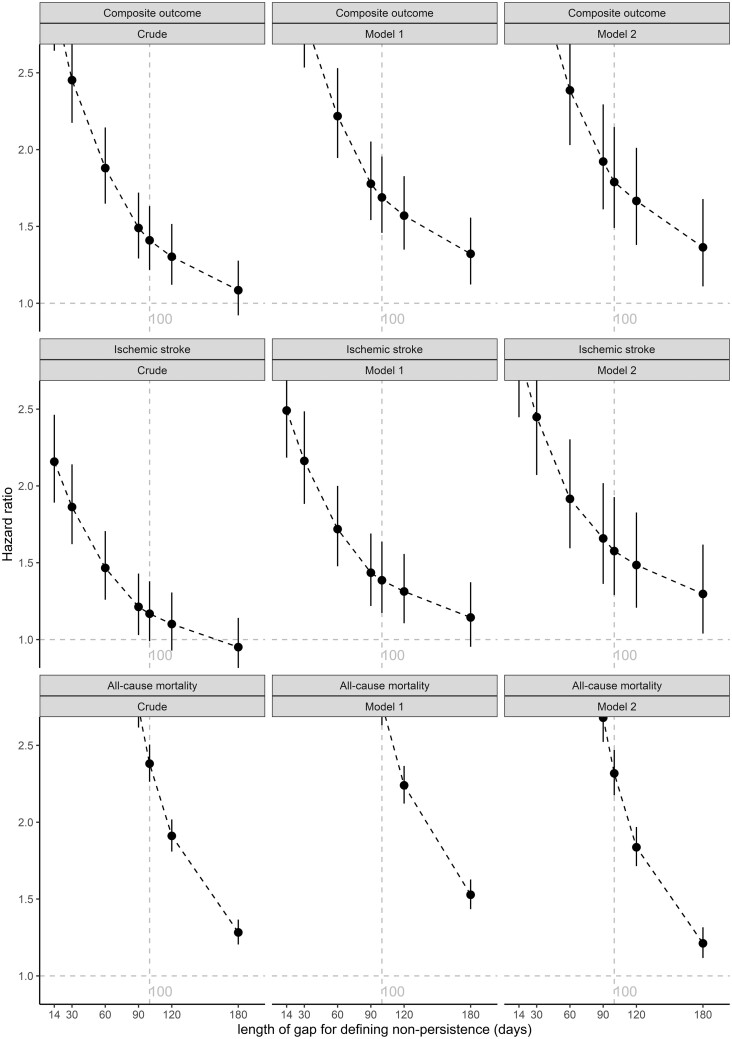
The associations of non-persistence with OAC with the studied clinical outcomes were robust after changing the length of the gap (i.e. from 100 to 14, 30, 60, 90, 120, 180 days) to define non-persistence with OAC (Figure 2). The shorter the length of the gap to define non-persistence with OAC, the higher HRs could be observed for the associations of non-persistence with OAC with the studied clinical outcomes. When using the longest length of the gap (i.e. 180 days) to define non-persistence with OAC, non-persistence with OAC was still associated with worse prognosis. As presented in Supplementary material online, Table S8, a younger age, male sex, paroxysmal AF, previous exposure to VKA, absence from stroke history (including TIA), the fifth quintile of standardized household income, a lower baseline CHA2DS2-VASc score, and a lower baseline adapted HAS-BLED score were associated with reduced risk of the studied clinical outcomes.
Figure 2.
Associations between non-persistence with oral anticoagulant and clinical outcomes using different lengths of gap to define non-persistence with oral anticoagulant. Notes: The composite outcome refers to ischaemic stroke and ischaemic stroke-related death. Model 1 was adjusted for age and sex, Model 2 was adjusted for age, sex, subtype of atrial fibrillation, previous exposure to vitamin K antagonist, stroke history (including transient ischaemic attack), and standardized household income. OAC, oral anticoagulant; AF, atrial fibrillation; VKA, vitamin K antagonist; TIA, transient ischaemic attack.
Occurrence of the studied outcomes during the non-persistent stage with OAC
As presented in Supplementary material online, Figures S3–S5, the curves are steeper at the early stage when compared to the later stage, showing that the studied clinical outcomes occurred more frequently at the early stage after a patient became non-persistent with OAC than the later stage. This could also be observed from the cumulative incidences of the studied outcomes at different stages after a patient became non-persistent with OAC (Supplementary material online, Table S9).
Discussion
This study investigated a nationwide cohort of adult DOAC-naive NVAF patients who started to use DOAC for ischaemic stroke prevention between 2013 and 2018 in the Netherlands. In the study population, persistence pattern of OAC was assessed, together with its potential associated risk factors and potential impact on clinical outcomes. The main findings were: (i) the persistence with OAC or with the initial DOAC was suboptimal, and a higher risk of non-persistence was observed in the early stage after the start of DOAC use compared to the later stage; (ii) several baseline characteristics including baseline CHA2DS2-VASc score were associated with persistence pattern; and (iii) being non-persistent with OAC was associated with poor efficacy of ischaemic stroke prevention (Graphical abstract).
There are several studies that investigated the persistence pattern among AF patients who were receiving DOAC in real-world settings. A recent meta-analysis24 reported an overall pooled proportion of persistence with DOAC of 71% (95% CI 69–74%) at 6 months and 62% (95% CI 56–68%) at 1 year. Our study found a higher proportion of persistence with DOAC (86.7% and 82.6% at 6 months and 1 year, respectively), which can be attributed to the conservative criterion we used to define non-persistence. In our study, we defined non-persistence based on a 100-day gap applied after the last OAC (or the initial DOAC) prescription. Although there are several methods for defining persistence in pharmacoepidemiology,28 a definition based on a gap is often used in anticoagulant studies. However, the lengths of gap applied vary between studies, ranging from 14 to 90 days,18,19,21,29 and usually longer gaps result in reporting higher persistence, which could also be observed in the sensitivity analysis of our study. Theoretically, a gap should be determined according to the pharmacologic properties of the medication and the treatment situation,15 but such data are usually unavailable, especially for population-based databases like ours. Therefore, we chose a conservative definition with the belief that an underestimated proportion of non-persistence might be more informative than an overestimated one (limiting the issue of a statistical type I finding), and as a result, better persistence was observed in our study than in others. In addition to non-persistence with DOAC, our study also investigated non-persistence with OAC (either DOAC or VKA) and a suboptimal persistence with OAC was observed. Although the result is not surprising, an investigation on this endpoint, which was usually not included in other studies, is at least important given that switching from DOAC to VKA is possible.
The several baseline characteristics we identified as predictors of non-persistence are worth being discussed. We found that male sex was associated with poor persistence with OAC (HR 1.06, 95% CI 1.01–1.12). This is consistent with a large study from Germany,30 which found that male sex was associated with higher risk of DOAC discontinuation (odds ratio 1.12, 95% CI 1.06–1.18) and a study from Australia.31 However, there are also studies that reported contrary results,22,32 which might be due to the fact that the reported relative increase in non-persistence in men vs. women is often <10%. Unlike sex, the associations between age and persistence pattern are consistent in most studies, indicating that an older age (usually above 65 years) is associated with better persistence.30,33,34 Our study had a similar finding that patients aged 65–74 years were the most persistent with OAC, but we found that the risk of non-persistence increased with age above 74 years. Paroxysmal AF was associated with increased risk of non-persistence with OAC in our study, which was also observed in a study that investigated the non-persistent use of warfarin.35 Patients who had used VKA before a DOAC had better persistence with OAC; however, an inverse association was observed that patients who had used VKA before a DOAC had worse persistence with the initial DOAC among patients who had a baseline CHA2DS2-VASc score ≥2. A potential explanation is that patients who had used VKA before were more able to understand the importance of anticoagulation therapy (through experience with VKA therapy) and therefore were less likely to become non-persistent with OAC when compared to those without experience of VKA use. For the same reason, they may also be more likely to switch back to VKA (knowing the medication and its use) after using DOAC, so they showed worse persistence with DOAC, but better persistence with OAC. As this explanation is speculative, it should be handled with caution. Stroke history has been reported to be associated with better persistence,33,35 and as expected, a similar association was found in our study. Apart from stroke history, we also found that marriage and not living alone were associated with better persistence with OAC, which is consistent with other studies36,37 and suggests the role that family support and involvement might play in facilitating anticoagulant compliance.38,39 Instead of exploring various individual comorbidities, we explored the association between the baseline CHA2DS2-VASc score and persistence pattern. The result we found is comparable to other studies,34,40 suggesting that a baseline CHA2DS2-VASc score >2 is associated with better persistence and adherence with DOAC. Our study also found some other variables to be associated with an increased risk of non-persistence with OAC, including immigration status, standardized household income, physical health, feeling of depression, unemployment, and the ability to meet financial needs. Interpretations of these results should be cautious, as some results were based on small sample sizes and some variables have not been investigated in prior studies. The association we found for household income, showed a U-shaped curve where those who had the lowest income and the highest income levels were those who were most likely to become non-persistent. Interestingly, we showed in a previous paper on adherence with DOAC that patients with a high education (who were most likely also to have a high income) were amongst the groups that were most likely not to adhere with DOAC.41 However, a low income as a risk factor for being non-persistent is unlikely to be related with pure financial distress as in the Netherlands DOACs are reimbursed by insurance companies for which every Dutch citizen must be a member. It can however not be completely ruled out that financial distress is a reason for not being persistent with DOAC in those with a low household income as medication is on a 385€ deductible. We cannot further comment on this issue due to the design, since our study can only look at predicting variables for persistence with OAC (or the initial DOAC). It is worth mentioning that our exploration of potential predictors of persistence pattern is only an initial investigation, which was mainly based on univariable regression analysis. Future studies may further develop a prediction model incorporating many baseline characteristics we identified to predict the persistence pattern.
The suboptimal persistence we observed would matter only when it impacts prognosis. It has been confirmed in other classes of long-term used medications42,43 that poor medication-taking behaviours in real-world settings cannot guarantee the same efficacy of that observed in randomized controlled trials. This could intuitively apply to anticoagulation therapy in NVAF patients, but evidence on this issue is very limited. The results of our study indicate that non-persistence with OAC was associated with a 79% higher risk of ischaemic stroke and ischaemic stroke-related death in NVAF patients initiated on DOAC. These results are in line with the only available study that investigated persistence pattern and clinical outcomes in NVAF patients,21 which found being non-persistent with DOAC was associated with an increased risk of stroke/TIA among dabigatran users (HR 3.75, 95% CI 2.59–5.43) as well as among rivaroxaban users (HR 6.25, 95% CI 3.37–11.58). However, the study had a relatively small sample size (as shown by the broad CIs), with outdated data (between 1998 and 2014) and a short follow-up time (i.e. 6 months),21 which made our study necessary for confirmation.
Our findings warrant an improvement of OAC persistence to achieve optimal outcomes in the anticoagulation management of NVAF patients. Additional consideration on potential risk of being non-persistent with OAC might be necessary when making decisions on types of anticoagulant prescribed to NVAF patients. After we stratified the analysis by baseline CHA2DS2-VASc score, we found that for patients with a baseline CHA2DS2-VASc score <2, non-persistence with OAC was associated with a reduced risk of the composite outcome (HR 0.37, 95% CI 0.20–0.69). This finding can possibly be explained by the fact that NVAF patients with a CHA2DS2-VASc score <2 are not recommended by guidelines to receive long-term OAC therapy.44 These patients, who were at low risk of ischaemic stroke at baseline, might receive short-term OAC therapy due to other indications such as preparation for cardioversion or left atrial appendage closure. After the short-term use of OAC, patients who are still at low risk of ischaemic stroke are likely to stop using OAC, while OAC therapy is still indicated for patients who developed into high risk of ischaemic stroke (i.e. a CHA2DS2-VASc score of ≥2). In our study, it could be observed that ischaemic stroke events appeared to occur more frequently in the early stage after patients became non-persistent with OAC when compared with that in a later stage. This suggests that more attention should be paid to patients who just stopped using OAC as could be done, for instance, by pharmacies, with some success.45 However, it should also be noted that association does not imply causation. Since the exact reasons for becoming non-persistent with OAC are unknown in our study, the observed increased risk of adverse outcomes associated with non-persistence with OAC should be interpreted cautiously. Unmeasured confounding and reverse causality cannot be ruled out in our study. For example, the observed increased risk of an outcome event after a patient became non-persistent with OAC could also be explained by the reason for stopping OAC treatment itself (such as frailty, need for a major surgery, or some other life-threatening conditions), which was not necessarily a consequence of the absence of anticoagulation therapy. It is worth mentioning that a recent randomized clinical trial46 reported that a multilevel motivational intervention increased persistence and adherence with OAC in NVAF patients, but no significant impact on clinical outcomes was observed, although this could also be due to a relatively short follow-up and few outcome events (chance of a type II error).
Our study has several strengths. First, as a nationwide study, it provided the largest sample size so far with updated data and was nationally representative. Second, under the conservative definition of non-persistence, the reported persistence pattern is not overestimated as might have been the case in other studies. Similarly, the study design could only underestimate the association between non-persistence with OAC and ischaemic stroke-related clinical outcomes, since persistent stage has longer follow-up time under the conservative definition of non-persistence, which led to that more ischaemic stroke events would be attributed to the persistent stage. This suggests the negative impact of non-persistence with OAC on prognosis can only be worse than what we observed. In addition, when evaluating the association between non-persistence with OAC and clinical outcomes, various confounding factors were considered, including socioeconomic status (i.e. standardized household income) and the two scoring systems for evaluating risks of stroke and major bleeding (i.e. CHA2DS2-VASc score and the adapted HAS-BLED score), which also increases the robustness of our findings.
Limitations
There are also some limitations of the study. First, due to a data limitation, information about OAC prescribed in hospitals was unavailable, which could lead to misclassification of persistence as non-persistence. This concern can be relieved for the following reasons: (i) the 100-day gap we used to determine non-persistence is likely to be longer than the average length of a hospitalization and (ii) such a misclassification would only underestimate the risk of ischaemic stroke associated with non-persistence with OAC. We aimed to include naive DOAC users in the study, but it cannot be ruled out that a few non-naive DOAC users were also included, since phase III trials about DOAC use in AF patients were conducted in the Netherlands before 2013, and these DOAC prescription data were not included in the medication data we obtained. Given the limited sample size of this patient group compared to the whole study population, it should not be rendered problematic. In addition, since DOAC prescription data about the amount of medication for each prescription were unavailable, and variables such as diagnoses and covariates were identified or calculated mainly based on International Classification of Diseases codes, the study could be prone to measurement error. For example, the covariates CHA2DS2-VASc score and the adapted HAS-BLED score were calculated from individual components such as hypertension, alcohol abuse, and the use of nonsteroidal anti-inflammatory drug, which may not be well captured in administrative data, and lead to an underestimated frequency of risk factors in the study population. However, in the analyses (Supplementary material online, Table S8), it could be observed that the covariates such as CHA2DS2-VASc score were well associated with clinical outcomes, suggesting that the concern is limited.
Second, in our study, only diagnoses made in hospitals were examined, while in the Netherlands it is possible that a patient got the first NVAF diagnosis and DOAC from a general practitioner. These patients were excluded from our study population due to the lack of an NVAF diagnosis in hospitals before the first DOAC prescription. Considering in real practice most of these patients will be further referred to cardiologists,47 we conducted a sensitivity analysis that included patients with NVAF diagnosed within 1 month after the first DOAC prescription, and consistent results were found (but not presented in the manuscript).
Third, due to the lack of information about specific types of DOAC, we were unable to study variations in the persistence patterns of different types of DOAC, which were reported in another study.48 Therefore, our results of cumulative incidence of non-persistence with DOAC could be seen as an average estimation over the different types of DOAC. However, for the results of the association between being non-persistent with OAC and the studied clinical outcomes, this limitation should not be problematic since the efficacy of ischaemic stroke prevention between different types of DOAC is considered to be equivalent.44
Fourth, the reasons for OAC discontinuation were unknown which made analysis of why patients became non-persistent unavailable. Without this information, some patients might be misclassified as being non-persistent with anticoagulation therapy. For example, a patient might suffer cancer during the follow-up and be switched to low-molecular-weight heparin, or a patient might receive left atrial appendage closure during the follow-up and stopped using OAC 6–12 weeks after the procedure. Our definition would consider these patients as non-persistent patients, but such misclassification would only lead to an overall underestimation of the risk of ischaemic stroke associated with being non-persistent with OAC. In addition, there were four other studies that examined the associations between adherence patterns and clinical outcomes among AF patients receiving DOAC, and they all showed that non-adherence was associated with increased risk of several thromboembolic clinical outcomes,33,49–51 but due to data limitation, our study cannot investigate adherence patterns and its potential impact on prognosis.
Conclusions
In conclusion, persistence with OAC decreased with time to ∼70% after 4 years for adult DOAC-naive NVAF patients who started to use DOAC for ischaemic stroke prevention between 2013 and 2018 in the Netherlands, which was associated with poor efficacy of ischaemic stroke prevention. Interventions might be needed to improve poor persistence, and the baseline characteristics we identified could be helpful to identify those who tend to be non-persistent.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
Results are based on calculations by the authors using non-public microdata made available by Statistics Netherlands. The authors thank Statistics Netherlands for making data (including data from the Dutch Hospital Data registry) available. The authors also thank the Community Health Services, Statistics Netherlands, and the National Institute for Public Health and the Environment, for making data from the Public Health Monitor Adults and Elderly (2012 and 2016) available. Q.C. is supported by the Chinese Government Scholarship (No. 201906380148) for his PhD study in the Leiden University Medical Center.
Conflict of interest: V.Y.I.G.T. works as the head of Thrombosis Service of Certe-MDA, Groningen, The Netherlands. He also works as post-doc at the Division of Hemostasis and Thrombosis, Department of Hematology, University of Groningen, Groningen, The Netherlands. The other authors declare that there is no conflict of interest.
Data availability
The data underlying this article cannot be shared publicly due to the policy of Statistics Netherlands.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr., Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ.. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conen D. Epidemiology of atrial fibrillation. Eur Heart J 2018;39:1323–1324. [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB.. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 4. Wolf PA, Dawber TR, Thomas HE Jr., Kannel WB.. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology 1978;28:973–977. [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW.. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–132. 2019; [DOI] [PubMed] [Google Scholar]
- 6. Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C, Healey JS, Bell A, Cairns J, Connolly S, Cox J, Dorian P, Gladstone D, McMurtry MS, Nair GM, Pilote L, Sarrazin JF, Sharma M, Skanes A, Talajic M, Tsang T, Verma S, Wyse DG, Nattel S, Macle L, Committee C; CCS Atrial Fibrillation Guidelines Committee. 2018 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol 2018;34:1371–1392. [DOI] [PubMed] [Google Scholar]
- 7. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbuchel H, Group E; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 8. Huiart L, Ferdynus C, Renoux C, Beaugrand A, Lafarge S, Bruneau L, Suissa S, Maillard O, Ranouil X.. Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population-based cross-sectional study in the French health insurance databases. BMJ Open 2018;8:e018180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Heuvel JM, Hovels AM, Buller HR, Mantel-Teeuwisse AK, de Boer A, Maitland-van der Zee AH.. NOACs replace VKA as preferred oral anticoagulant among new patients: a drug utilization study in 560 pharmacies in The Netherlands. Thromb J 2018;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mekaj YH, Mekaj AY, Duci SB, Miftari EI.. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag 2015;11:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 12. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 13. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 14. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener H-C, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 15. Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK.. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–47. [DOI] [PubMed] [Google Scholar]
- 16. Bonaccorso S, Sturchio JL.. What information do patients need about medicines? Perspectives from the pharmaceutical industry. BMJ 2003;327:863–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamberts M, Staerk L, Olesen JB, Fosbol EL, Hansen ML, Harboe L, Lefevre C, Evans D, Gislason GH.. Major bleeding complications and persistence with oral anticoagulation in non-valvular atrial fibrillation: contemporary findings in real-life Danish patients. J Am Heart Assoc 2017;6:e004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banerjee A, Benedetto V, Gichuru P, Burnell J, Antoniou S, Schilling RJ, Strain WD, Ryan R, Watkins C, Marshall T, Sutton CJ.. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: a population-based study. Heart 2020;106:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferroni E, Gennaro N, Costa G, Fedeli U, Denas G, Pengo V, Corti MC.. Real-world persistence with direct oral anticoagulants (DOACs) in naive patients with non-valvular atrial fibrillation. Int J Cardiol 2019;288:72–75. [DOI] [PubMed] [Google Scholar]
- 20. Harper P, Pollock D, Stephens M.. Dabigatran persistence and adherence in New Zealand: a nationwide retrospective observational study. BMJ Open 2018;8:e020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackevicius CA, Tsadok MA, Essebag V, Atzema C, Eisenberg MJ, Tu JV, Lu L, Rahme E, Ho PM, Turakhia M, Humphries KH, Behlouli H, Zhou L, Pilote L.. Early non-persistence with dabigatran and rivaroxaban in patients with atrial fibrillation. Heart 2017;103:1331–1338. [DOI] [PubMed] [Google Scholar]
- 22. Zielinski GD, Rein N, Teichert M, Klok FA, Rosendaal FR, Meer FJM, Huisman MV, Cannegieter SC, Lijfering WM.. Persistence of oral anticoagulant treatment for atrial fibrillation in the Netherlands: a surveillance study. Res Pract Thromb Haemost 2020;4:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paquette M, Franca LR, Teutsch C, Diener HC, Lu S, Dubner SJ, Ma CS, Rothman KJ, Zint K, Halperin JL, Olshansky B, Huisman MV, Lip GYH, Nieuwlaat R.. Dabigatran persistence and outcomes following discontinuation in atrial fibrillation patients from the GLORIA-AF Registry. Am J Cardiol 2020;125:383–391. [DOI] [PubMed] [Google Scholar]
- 24. Ozaki AF, Choi AS, Le QT, Ko DT, Han JK, Park SS, Jackevicius CA.. Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2020;13:e005969. [DOI] [PubMed] [Google Scholar]
- 25. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ.. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 26. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY.. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 27.Ministerie van Volksgezondheid WeS. Voor hoeveel dagen mag mijn arts medicijnen voorschrijven? https://www.rijksoverheid.nl/onderwerpen/geneesmiddelen/vraag-en-antwoord/voor-hoeveel-dagen-mag-mijn-arts-medicijnen-voorschrijven (accessed 1 July 2021).
- 28. Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M.. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007;10:3–12. [DOI] [PubMed] [Google Scholar]
- 29. Maura G, Billionnet C, Alla F, Gagne JJ, Pariente A.. Comparison of treatment persistence with dabigatran or rivaroxaban versus vitamin K antagonist oral anticoagulants in atrial fibrillation patients: a competing risk analysis in the French National Health Care Databases. Pharmacotherapy 2018;38:6–18. [DOI] [PubMed] [Google Scholar]
- 30. Hohnloser SH, Basic E, Nabauer M.. Changes in oral anticoagulation therapy over one year in 51,000 atrial fibrillation patients at risk for stroke: a practice-derived study. Thromb Haemost 2019;119:882–893. [DOI] [PubMed] [Google Scholar]
- 31. Obamiro KO, Chalmers L, Lee K, Bereznicki BJ, Bereznicki LR.. Adherence to oral anticoagulants in atrial fibrillation: an Australian survey. J Cardiovasc Pharmacol Ther 2018;23:337–343. [DOI] [PubMed] [Google Scholar]
- 32. Beyer-Westendorf J, Ehlken B, Evers T.. Real-world persistence and adherence to oral anticoagulation for stroke risk reduction in patients with atrial fibrillation. Europace 2016;18:1150–1157. [DOI] [PubMed] [Google Scholar]
- 33. Borne RT, O’Donnell C, Turakhia MP, Varosy PD, Jackevicius CA, Marzec LN, Masoudi FA, Hess PL, Maddox TM, Ho PM.. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the Veterans health administration. BMC Cardiovasc Disord 2017;17:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zalesak M, Siu K, Francis K, Yu C, Alvrtsyan H, Rao Y, Walker D, Sander S, Miyasato G, Matchar D, Sanchez H.. Higher persistence in newly diagnosed nonvalvular atrial fibrillation patients treated with dabigatran versus warfarin. Circ Cardiovasc Qual Outcomes 2013;6:567–574. [DOI] [PubMed] [Google Scholar]
- 35. Wang ZZ, Du X, Wang W, Tang RB, Luo JG, Li C, Chang SS, Liu XH, Sang CH, Yu RH, Long DY, Wu JH, Bai R, Liu N, Ruan YF, Dong JZ, Ma CS.. Long-term persistence of newly initiated warfarin therapy in Chinese patients with nonvalvular atrial fibrillation. Circ Cardiovasc Qual Outcomes 2016;9:380–387. [DOI] [PubMed] [Google Scholar]
- 36. Orensky IA, Holdford DA.. Predictors of noncompliance with warfarin therapy in an outpatient anticoagulation clinic. Pharmacotherapy 2005;25:1801–1808. [DOI] [PubMed] [Google Scholar]
- 37. Aggarwal B, Liao M, Mosca L.. Medication adherence is associated with having a caregiver among cardiac patients. Ann Behav Med 2013;46:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang M, Holbrook A, Lee M, Liu J, Leenus A, Chen N, Mbuagbaw L, Thabane L.. Barriers and facilitators to optimal oral anticoagulant management: a scoping review. J Thromb Thrombolysis 2020;50:697–714. [DOI] [PubMed] [Google Scholar]
- 39. Pandya EY, Bajorek B.. Factors affecting patients' perception on, and adherence to, anticoagulant therapy: anticipating the role of direct oral anticoagulants. Patient 2017;10:163–185. [DOI] [PubMed] [Google Scholar]
- 40. Gorst-Rasmussen A, Skjoth F, Larsen TB, Rasmussen LH, Lip GY, Lane DA.. Dabigatran adherence in atrial fibrillation patients during the first year after diagnosis: a nationwide cohort study. J Thromb Haemost 2015;13:495–504. [DOI] [PubMed] [Google Scholar]
- 41. Toorop MMA, Rein N, Nierman MC, Vermaas HW, Huisman MV, Meer FJM, Cannegieter SC, Lijfering WM.. Self-reported therapy adherence and predictors for nonadherence in patients who switched from vitamin K antagonists to direct oral anticoagulants. Res Pract Thromb Haemost 2020;4:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flory JH, Mushlin AI.. Effect of cost and formulation on persistence and adherence to initial metformin therapy for type 2 diabetes. Diabetes Care 2020;43:e66–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burnier M, Egan BM.. Adherence in hypertension. Circ Res 2019;124:1124–1140. [DOI] [PubMed] [Google Scholar]
- 44. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 45. Shore S, Ho PM, Lambert-Kerzner A, Glorioso TJ, Carey EP, Cunningham F, Longo L, Jackevicius C, Rose A, Turakhia MP.. Site-level variation in and practices associated with dabigatran adherence. JAMA 2015;313:1443–1450. [DOI] [PubMed] [Google Scholar]
- 46. Tzikas A, Samaras A, Kartas A, Vasdeki D, Fotos G, Dividis G, Paschou E, Forozidou E, Tsoukra P, Kotsi E, Goulas I, Karvounis H, Giannakoulas G.. Motivational Interviewing to Support Oral AntiCoagulation adherence in patients with non-valvular Atrial Fibrillation (MISOAC-AF): a randomised clinical trial. Eur Heart J Cardiovasc Pharmacother 2021;7:f63–f71. [DOI] [PubMed] [Google Scholar]
- 47. Verbiest-van Gurp N, van Mil D, van Kesteren HAM, Knottnerus JA, Stoffers H.. How do Dutch general practitioners detect and diagnose atrial fibrillation? Results of an online case vignette study. BMC Fam Pract 2019;20:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sorensen R, Jamie Nielsen B, Langtved Pallisgaard J, Ji-Young Lee C, Torp-Pedersen C.. Adherence with oral anticoagulation in non-valvular atrial fibrillation: a comparison of vitamin K antagonists and non-vitamin K antagonists. Eur Heart J Cardiovasc Pharmacother 2017;3:151–156. [DOI] [PubMed] [Google Scholar]
- 49. Alberts MJ, Peacock WF, Fields LE, Bunz TJ, Nguyen E, Milentijevic D, Schein JR, Coleman CI.. Association between once- and twice-daily direct oral anticoagulant adherence in nonvalvular atrial fibrillation patients and rates of ischemic stroke. Int J Cardiol 2016;215:11–13. [DOI] [PubMed] [Google Scholar]
- 50. Shore S, Carey EP, Turakhia MP, Jackevicius CA, Cunningham F, Pilote L, Bradley SM, Maddox TM, Grunwald GK, Baron AE, Rumsfeld JS, Varosy PD, Schneider PM, Marzec LN, Ho PM.. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans health administration. Am Heart J 2014;167:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deshpande CG, Kogut S, Laforge R, Willey C.. Impact of medication adherence on risk of ischemic stroke, major bleeding and deep vein thrombosis in atrial fibrillation patients using novel oral anticoagulants. Curr Med Res Opin 2018;34:1285–1292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the policy of Statistics Netherlands.