Abstract
Computer simulations, using the “Pi double-threshold” mechanism of muscle fatigue postulated previously (the first threshold initiating progressive reduction in work efficiency and the second threshold resulting in exercise intolerance), demonstrated that several parameters of the skeletal muscle bioenergetic system can affect maximum oxygen consumption (V̇O2max), critical power (CP), and oxygen consumption (V̇O2) on-kinetics in skeletal muscle. Simulations and experimental observations together demonstrate that endurance exercise training increases oxidative phosphorylation (OXPHOS) activity and/or each-step activation (ESA) intensity, the latter, especially in the early stages of training. Here, new computer simulations demonstrate that an endurance training-induced increase in OXPHOS activity and decrease in peak Pi (Pipeak), at which exercise is terminated because of exercise intolerance, result in increased V̇O2max and CP, speeding of the primary phase II of V̇O2 on-kinetics, and decreases V̇O2 slow component magnitude, consistent with their observed behavior in vivo. It is possible, but remains unknown, whether there is a contribution to this behavior of an increase in the critical Pi (Picrit), above which the additional ATP usage underlying the slow component begins, and a decrease in the activity of the additional ATP usage (kadd). Thus, we offer a mechanism, involving Pi accumulation, Picrit and Pipeak, of the training-induced adaptations in V̇O2max, CP, and the primary and slow component phases of V̇O2 on-kinetics that was absent in the literature.
NEW & NOTEWORTHY A mechanism of the training-induced changes in V̇O2max, critical power, and V̇O2 on-kinetics in skeletal muscle reported in the literature is postulated. It involves the self-driving “Pi double-threshold” mechanism of muscle fatigue underlying exercise inefficiency, the slow component of the V̇O2 on-kinetics, and termination of exercise. It is proposed that an increase in OXPHOS activity and decrease in peak Pi at which exercise terminates are responsible for the training-induced changes in the muscle bioenergetic system.
Keywords: critical power, inorganic phosphate, muscle training, V̇O2max, V̇O2 on-kinetics
INTRODUCTION
Endurance training changes several kinetic properties of the skeletal muscle bioenergetic system. It elevates maximum oxygen consumption (V̇O2max) (1–14), increases critical power (CP) (5, 8, 10), speeds the primary phase II of the oxygen consumption (V̇O2) on-kinetics (1, 9, 13–15), and lowers the slow component of the V̇O2 on-kinetics (1–3, 16). It was shown that training-induced increase in oxidative phosphorylation (OXPHOS) activity and each-step activation (ESA) intensity (the activation of all OXPHOS complexes and the NADH supply block, in parallel with the activation of ATP usage) leads to speeded V̇O2 on-kinetics (17). However, the mechanisms underlying the rest of the observed intramuscular kinetic changes following endurance training are still not well understood.
Muscle performance during exercise is reduced by both peripheral and central fatigue mechanisms (18–22). Inorganic phosphate (Pi) is proposed as the main metabolite involved with peripheral fatigue, whereas other metabolites, for instance, H+ ions and ADP, as well as depletion of glycogen stores, play a less important role in peripheral fatigue (18).
Muscle fatigue is associated with changes in V̇O2 on-kinetics, and the emergence of the V̇O2 slow component (rev. ref. 22), but no quantitative cellular mechanism for this association was proposed until the recent development of “Pi double-threshold” mechanism of muscle fatigue and inefficiency (23). This hypothesis postulated that 1) additional ATP usage, underlying the slow component of the V̇O2 and intramuscular metabolic on-kinetics, appears when Pi exceeds a certain critical value, termed Picrit; 2) muscle work is terminated because of exercise intolerance when Pi reaches another, greater, peak value, termed Pipeak; and 3) Pi increase and additional ATP usage form a self-driving, reciprocal mechanism of positive feedback (23, 25), which, if sustained, leads to attainment of Pipeak, peripheral fatigue, and exercise intolerance. It was demonstrated that these assumptions could generate various, apparently unrelated, system properties, comprising (23): time courses of muscle V̇O2, cytosolic ADP, pH, PCr, and Pi during rest-to-work transition; end-exercise constancy of these variables at various power outputs; hyperbolic shape of the power-duration curve with critical power (CP) as an asymptote; and hyperoxia-induced increase in CP and acceleration of the principal phase II V̇O2 on-kinetics (decrease in the characteristic transition time t0.63, related to τp) observed in knee extension or supine cycling exercise (26, 27).
These previous simulations provide the first model of cellular events that determine whether a power output exceeds CP (the power output where a metabolic steady-state is lost). To test this model, here we use a computer simulation of the entire muscle bioenergetic system to test the hypothesis that changes in several parameters related to OXPHOS activity (rate constants of OXPHOS complexes and ESA intensity) and muscle fatigue (Picrit, Pipeak, and kadd), of the order observed or estimated following endurance exercise training, result in anticipated adaptations of the kinetic system properties of skeletal muscle. We aim to identify whether a simulated increase in OXPHOS activity (rate constants of OXPHOS complexes) and/or ESA intensity (in the early stages of training), as well as decrease in the Pipeak, are the main factors responsible for (the size of) the training-induced increase in V̇O2max, increase in CP, acceleration of the primary phase II of the V̇O2 on-kinetics, and reduction of the V̇O2 slow component. We also aim to identify whether simulated increases in Picrit and additional ATP usage activity (kadd) contribute to training-induced adaptations of the muscle kinetic system behavior. Overall, this study postulates a plausible mechanism of the training-induced increase in V̇O2max and CP, as well as acceleration of the phase II of the V̇O2 on-kinetics and reduction of its slow component that was absent in the literature.
THEORETICAL METHODS
Ethical Approval
This is a purely theoretical study that did not involve any experiments on humans or animals.
Computer Model
The computer model of OXPHOS and the entire bioenergetic system in intact skeletal muscle was used (23, 28–33). The model involves the ESA (or parallel activation) concept, according to which not only ATP usage but also the NADH supply block and all OXPHOS complexes are directly activated by some cytosolic factor/mechanism (probably involving cytosolic Ca2+ ions) during rest-to-work or low- to high-work transition in skeletal muscle and heart (28, 34). The complete model description is given in Korzeniewski’s study (25) and is located at http://awe.mol.uj.edu.pl/∼benio/.
This model was able to reproduce a wide range of, apparently unrelated, kinetic properties of the skeletal muscle bioenergetic system during exercise and was used for numerous theoretical studies (see ref. 34 for review; 23, 25, 29, 30).
Rate constants appear in kinetic equations for all OXPHOS complexes (complex I, complex III, complex IV, ATP synthase, ATP/ADP carrier, Pi carrier) and NADH supply block within the computer model. All these rate constants (kC1, kC3, kC4, kSN, kEX, kPI, kDH) are individually represented in the model and can be grouped into a single rate constant (activity) of OXPHOS: kOX, notionally proportional to the volume-density of mitochondria and concentration and activity of OXPHOS enzymes. In the standard model version, corresponding to moderately trained (physically active) individuals, the relative kOX is scaled to 1.
The intensity of ESA AOX determines how much OXPHOS is directly activated, or how many times kOX is elevated, during the rest-to-work transition. AOX is within the model, a function of the relative ATP usage activity AUT (29):
| (1) |
where AOXmax (unitless) is the maximum ESA activity minus one, AUT (unitless) is the relative (fold) increase in the activity (rate constant) of ATP usage during rest-to-work transition, and KAut (unitless) is the saturation constant for AUT. In the standard model version (23, 25, 29) AOXmax = 5 and KAut = 5.
During high-intensity exercise, an additional ATP usage is observed, which is in excess of regular ATP usage, proportional to power output (PO) for any given type of exercise. This additional ATP usage is the basis for the V̇O2 slow component. The kinetics of additional ATP usage is described by the following formula (23):
| (2) |
where vadd is the rate of additional ATP usage (mM·min−1), kadd is the “rate constant” of the additional ATP usage (mM−1), vUT is the rate of the regular ATP usage (mM·min−1), Pi is the current inorganic phosphate concentration (mM), Picrit is the critical Pi for the initiation of the additional ATP usage (mM), ta is the characteristic time of the activation of the additional ATP usage (min), and tex is the time after the onset of exercise (min). In the standard model version, additional ATP usage starts when Pi exceeds Picrit = 18 mM, kadd = 0.2 mM−1, ta = 2 min (23), and muscle work is terminated because of exercise intolerance when Pi reaches Pipeak = 25 mM (25).
The total absolute ATP usage flux vUTtot (in mM·min−1) is equal to the sum of the regular and additional absolute ATP usage rate:
| (3) |
Simulation Procedures
Power-duration relationships for different selected parameter values were obtained by subsequent running of simulations for increasing values of the ATP usage activity (AUT, corresponding to power output) at a given set of parameter values. The effect of the following parameters was studied: OXPHOS activity (kOX), ESA intensity (AOXmax), peak Pi (Pipeak), critical Pi (Picrit), and activity (rate constant) of the additional ATP usage (kadd). Power-duration curves for “standard” values of these parameters (see previous section) and for these values decreased or increased by 10% were simulated.
The standard version of the model (23), where the characteristic transition time of V̇O2 on-kinetics (t0.63, corresponding to τp) is equal to ∼25 s, corresponds to physically active, “moderately trained” individuals. To represent untrained (sedentary) individuals, the relative OXPHOS activity kOX was decreased to 0.8 of the standard value. On the other hand, in two sets of simulations representing well-trained individuals, kOX was increased to 1.1 of the standard value. Thus, the simulation of training of untrained individuals involved an increase in kOX from 0.8 to 1.1 (by 37.5%). In the first simulation (simulation set A), Pipeak = 25 mM was unchanged, whereas in the second simulation (simulation set B), Pipeak was decreased from 25 mM to 22 mM to reproduce more closely observations from experimental data (for justification see discussion).
The training-induced increase in OXPHOS activity by 37.5% (from 0.8 to 1.1) conforms well with the size of the training-induced increases in skeletal muscle mitochondrial volume-density or OXPHOS activity encountered in experimental studies as a result of long-term endurance training (4, 6, 12, 35).
Rest-to-work transitions at different ATP usage activities for voluntary constant-power exercise were simulated as described by Korzeniewski (29). The relative activity of ATP usage AUT (relative increase in its rate constant kUT in relation to rest) between 110 (maximum AUT) and AUT below the critical AUT (AUTcrit) was used in computer simulations. One AUT unit corresponds to ∼3 W during cycle ergometer exercise (29). This value may vary between ∼2 and 4 W, depending on, for example, working muscle mass and type of exercise.
THEORETICAL RESULTS
Our initial findings from simulating the effect of changes in selected parameter values on V̇O2max, AUTcrit (CP) and t0.63 are shown in Figs. 1 and 2 and Table 1. Parameters were manipulated in isolation to determine their effects on a range of system variables and kinetics.
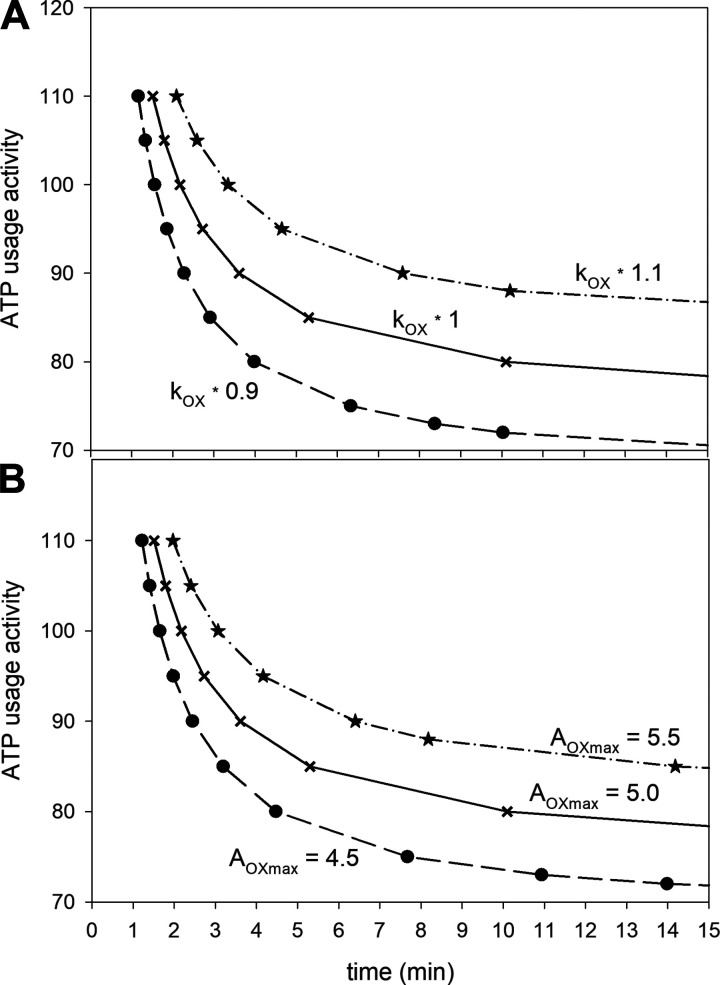
Figure 1.
A: simulated ATP usage activity-exercise duration curves (corresponding to power-duration curves) for “standard” oxidative phosphorylation (OXPHOS) activity kOX (kOX *1), kOX diminished by 10% (kOX * 0.9), and kOX enlarged by 10% (kOX * 1.1); B: simulated ATP usage activity-exercise duration curves for “standard” each-step activation (ESA) intensity AOXmax (AOXmax = 5), AOXmax diminished by 10% (AOXmax = 4.5), and AOXmax enlarged by 10% (AOXmax = 5.5).
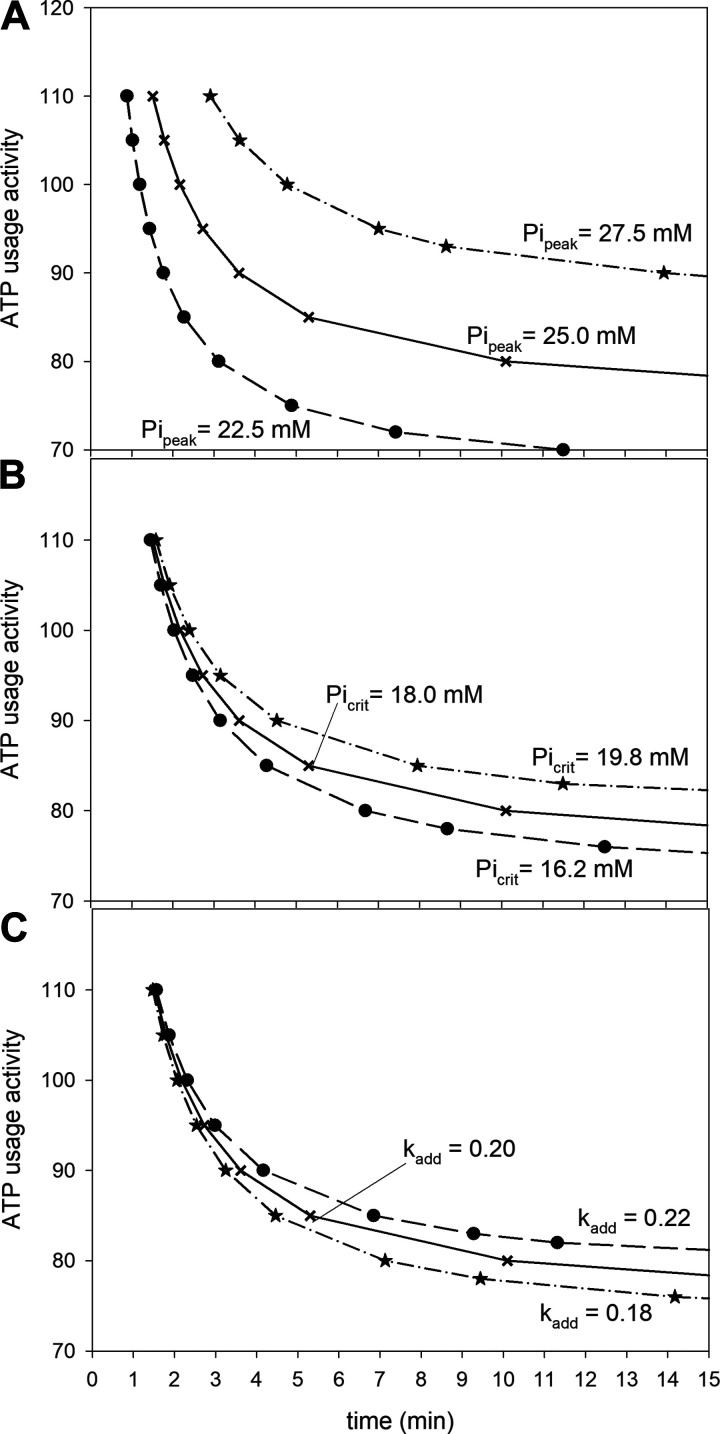
Figure 2.
A: simulated ATP usage activity-exercise duration curves (corresponding to power-duration curves) for “standard” peak inorganic phosphate (Pi) at which exercise is terminated Pipeak (Pipeak = 25.0 mM), Pipeak diminished by 10% (Pipeak = 22.5 mM), and Pipeak enlarged by 10% (Pipeak = 27.5 mM); B: simulated ATP usage activity-exercise duration curves for “standard” critical Pi (Picrit) at which the additional ATP usage [and thus slow components of the oxygen uptake (V̇O2) and metabolites on-kinetics] is initiated Picrit (Picrit = 18 mM), Picrit diminished by 10% (Picrit = 16.2 mM), and Picrit enlarged by 10% (Picrit = 19.8 mM); C: simulated ATP usage activity-exercise duration curves for “standard” activity (rate constant) of the additional ATP usage kadd (kadd = 0.20), kadd diminished by 10% (kadd = 0.18), and kadd enlarged by 10% (kadd = 0.22).
Table 1.
Simulated effect of a decrease or increase in chosen parameter values by 10% in relation to “standard” (100%) values on selected variables
| Parameter | Relative Parameter Value | V̇O2max, mM·min−1 | AUTcrit, unitless | t0.63, s |
|---|---|---|---|---|
| kOX | −10% | 11.9 (–10.2%) | 67.1 (–10.2%) | 26.5 (+8.2%) |
| 100% | 13.3 | 74.7 | 24.5 | |
| +10% | 14.7(+10.5%) | 83.3 (+10.5%) | 22.8 (–6.9%) | |
| AOXmax | −10% | 12.2 (–8.4%) | 69.4 (–7.1%) | 26.8(+9.4%) |
| 100% | 13.3 | 74.7 | 24.5 | |
| 10% | 14.4 (+8.6%) | 81.0 (+8.4%) | 22.5 (–8.2%) | |
| Pipeak | −10% | 11.1 (–16.2%) | 66.6 (–10.8%) | 24.5 (--) |
| 100% | 13.3 | 74.7 | 24.5 | |
| +10% | 15.7 (+18.1%) | 84.5 (+13.1%) | 24.5 (--) | |
| Picrit | −10% | 13.3 (--) | 71.5 (–4.3%) | 24.5 (--) |
| 100% | 13.3 | 74.7 | 24.5 | |
| +10% | 13.3 (--) | 78.7 (+5.4%) | 24.5 (--) | |
| kadd | −10% | 13.3 (--) | 72.1 (–3.5%) | 24.5 (--) |
| 100% | 13.3 | 74.7 | 24.5 | |
| +10% | 13.3 (--) | 77.5(+3.7%) | 24.5 (--) |
t0.63 was simulated for ATP usage activity AUT = 40 (moderate exercise). AOXmax, each-step activation (ESA) intensity; AUTcrit, critical ATP usage activity (corresponding to critical power); kadd, activity (rate constant) of the additional ATP usage; kOX, oxidative phosphorylation (OXPHOS) activity; Picrit, critical Pi at which the additional ATP usage (and thus the slow component of the V̇O2 and metabolites) appears; Pipeak, peak Pi at which exercise is terminated because of exercise intolerance; V̇O2max, maximal V̇O2; t0.63, characteristic transition time of the V̇O2 on-kinetics (analogous to τp).
In the simulations shown in Fig. 1, the values of parameters related to OXPHOS activity (OXPHOS activity, kOX, and ESA intensity, AOXmax) were either decreased or increased by 10% in relation to “standard” values, and the effect of this manipulation on the power-duration relationship was studied. Solid lines in Fig. 1 correspond to “standard” parameter values. Reducing kOX by 10% decreased V̇O2max by 10.2%, decreased AUTcrit by 10.2%, and lengthened t0.63 by 8.2% (Fig. 1A and Table 1). An increase in kOX by 10%, increased V̇O2max by 10.5%, increased AUTcrit by 10.5%, and shortened t0.63 by 6.9% (Fig. 1A and Table 1). On the other hand, a decrease in AOXmax by 10% decreased V̇O2max by 8.4% and AUTcrit by 7.1% and lengthened t0.63 by 9.4%, whereas an increase in AOXmax by 10% increased V̇O2max by 8.4% and AUTcrit by 8.4% and shortened t0.63 by 8.2% (Fig. 1B and Table 1). Therefore, while a change in the value of kOX has a slightly greater effect on V̇O2max and AUTcrit, a change in AOXmax has a slightly greater effect on t0.63.
All of the three tested parameters related to the additional ATP usage and muscle fatigue influenced AUTcrit (CP), but only one influenced V̇O2max and none influenced t0.63. This is demonstrated in Fig. 2 and Table 1. In Fig. 2 the values of Pipeak, Picrit, and kadd, were independently decreased or increased by 10%, and the effect of this manipulation on the power-duration relationship was studied. Solid lines in Fig. 2 correspond to “standard” parameter values. A decrease in Pipeak by 10% decreased V̇O2max by 16.2%, decreased AUTcrit by 10.8%, and did not affect t0.63, whereas an increase in Pipeak by 10% increased V̇O2max by 18.1%, increased AUTcrit by 13.1%, and did not affect t0.63 (Fig. 2A and Table 1). A decrease in Picrit by 10% did not affect V̇O2max, decreased AUTcrit by 4.3%, and did not affect t0.63, whereas an increase in Picrit by 10% did not affect V̇O2max, increased AUTcrit by 5.4%, and did not affect t0.63 (Fig. 2B and Table 1). Finally, a decrease in kadd by 10% did not affect V̇O2max, decreased AUTcrit by 3.5%, and did not affect t0.63, whereas an increase in kadd by 10% did not affect V̇O2max, increased AUTcrit by 3.7%, and did not affect t0.63 (Fig. 2C and Table 1). Therefore, although the relative impact of the value of Pipeak on AUTcrit (CP) and V̇O2max is the strongest of all the parameters tested, the impact of the value of Picrit and kadd is rather small or none at all.
Overall Figs. 1 and 2 and Table 1 demonstrate that ±10% alterations in key system parameters (e.g., as in response to training or detraining) have very diverse magnitudes of effect on V̇O2max, AUTcrit, and t0.63.
In the first set of simulations of the effect of endurance training (set A), where only the activity of OXPHOS (kOX) was increased from 0.8 (untrained state) to 1.1 (trained state) of the “standard” value (37.5% increase), V̇O2max increased by ∼40%, AUTcrit (corresponding to CP) increased by ∼35%, and the characteristic transition time of the phase II of the V̇O2 on-kinetics (t0.63) decreased by ∼22%. This is demonstrated in Table 2 Although qualitatively appropriate, set A simulations of the training-induced increase in kOX alone produced changes in V̇O2max and AUTcrit (CP) that were quantitatively too large compared with in vivo observations (see discussion), and therefore, a second set of simulations was carried out.
Table 2.
Simulated effect of training when only OXPHOS activity is elevated (set A of simulations)
| Parameter | Untrained | Trained |
|---|---|---|
| OXPHOS activity, kOX | 0.8 | 1.1 (↑37.5%) |
| (in relation to standard) | ||
| Variable | ||
| V̇O2max, mM·min−1 | 10.43 | 14.61 (↑40.1%) |
| AUTcrit, unitless | 60.6 | 82.0 (↑35.3%) |
| t0.63, s | 29.2 | 22.8 (↓21.9%) |
The change in the parameter value causes changes in variable values. AUTcrit, critical ATP usage activity (corresponding to critical power); kOX, oxidative phosphorylation (OXPHOS) activity; V̇O2max, maximal V̇O2; t0.63, characteristic transition time of the V̇O2 on-kinetics (analogous to τp).
In the second set of simulations of the effect of endurance training (set B), the activity of OXPHOS (kOX) was increased from 0.8 (untrained state) to 1.1 (trained state) of the “standard” value (37.5% increase) and peak Pi (Pipeak) was simultaneously decreased from 25 mM (untrained state) to 22 mM (trained state) (12% decrease). This resulted in increased V̇O2max by ∼13% and AUTcrit by ∼22% and the characteristic transition time of the phase II of the V̇O2 on-kinetics (t0.63) decreased by ∼22%. The decrease in Pipeak was chosen to lower the training-induced rise in V̇O2max and AUTcrit (CP), as the effect of an increase in Picrit and increase in kadd on these variables was too small or none at all (compare Fig. 2 and Table 1).
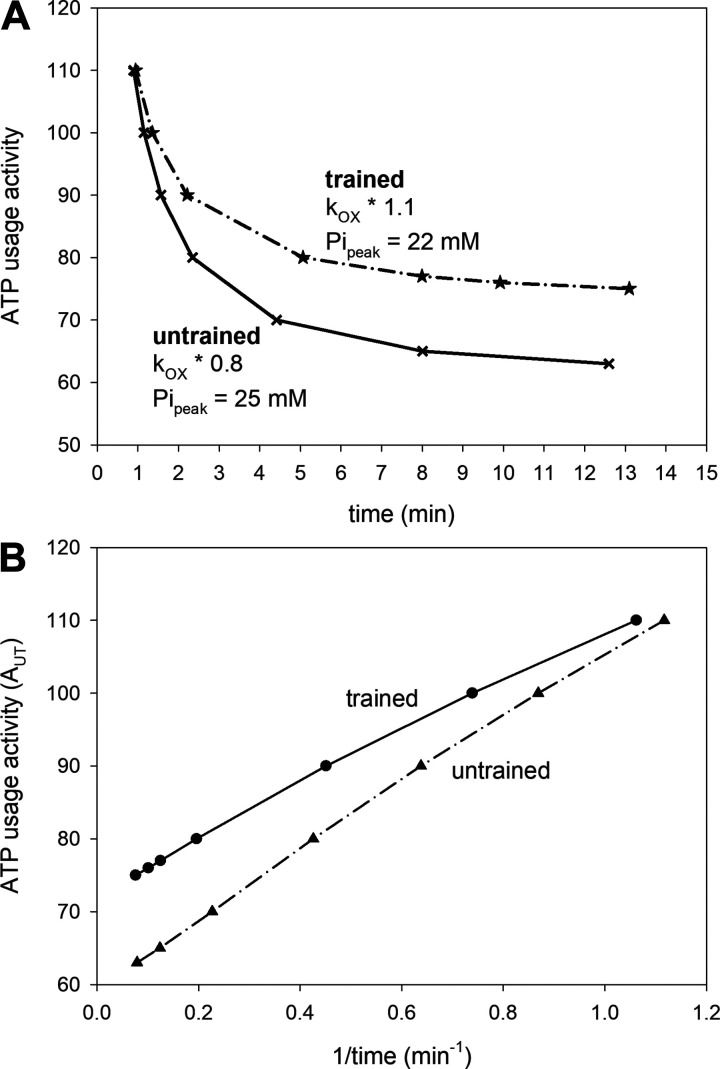
Using set B of simulation parameters, the ATP usage activity-duration of exercise (time) relationship was very close to hyperbolic, both before and after training. This is shown in Fig. 3A; at the same time, the critical ATP usage activity (corresponding to CP) increased significantly as a result of training (by ∼22%; compare Table 3). Consequently, the dependence of the critical ATP usage on 1/time (exercise duration) was near-linear, and the line representing this dependence for trained muscle was situated above the line representing the dependence for untrained muscle (Fig. 3B). Also of note, the slope of this relationship in the trained state is somewhat shallower (Fig. 3B). This represents a smaller W′ (the curvature constant of the power-duration relationship) by ∼22% in the trained state.
Figure 3.
A: simulated ATP usage activity-exercise duration curves (corresponding to power-duration curves) for untrained muscle and trained muscle under the assumption that training causes an increase in oxidative phosphorylation (OXPHOS) activity (kOX) from 0.8 to 1.1 of the standard value and a decrease of the peak Pi at which exercise is terminated (Pipeak) from 25 mM to 22 mM (setB of simulations); B: simulated ATP usage activity minus 1/duration curves (corresponding to power minus 1/duration curves) for untrained muscle and trained muscle constituting transformed data from A.
Table 3.
Simulated effect of training when OXPHOS activity is increased and peak Pi is decreased (set B of simulations)
| Parameter | Untrained | Trained |
|---|---|---|
| OXPHOS activity, kOX | 0.8 | 1.1 (↑37.5%) |
| (in relation to standard) | ||
| Peak Pi (Pipeak), mM | 25 | 22 (↓12%) |
| Variable | ||
| V̇O2max, mM·min−1 | 10.50 | 11.83 (↑12.7%) |
| AUTcrit, unitless | 59 | 72 (↑22%) |
| t0.63, s | 29.2 | 22.8 (↓21.9%) |
The changes in the parameter values cause changes in variable values. AUTcrit, critical ATP usage activity (corresponding to critical power); V̇O2max, maximal V̇O2; t0.63, characteristic transition time of the V̇O2 on-kinetics (analogous to τp).
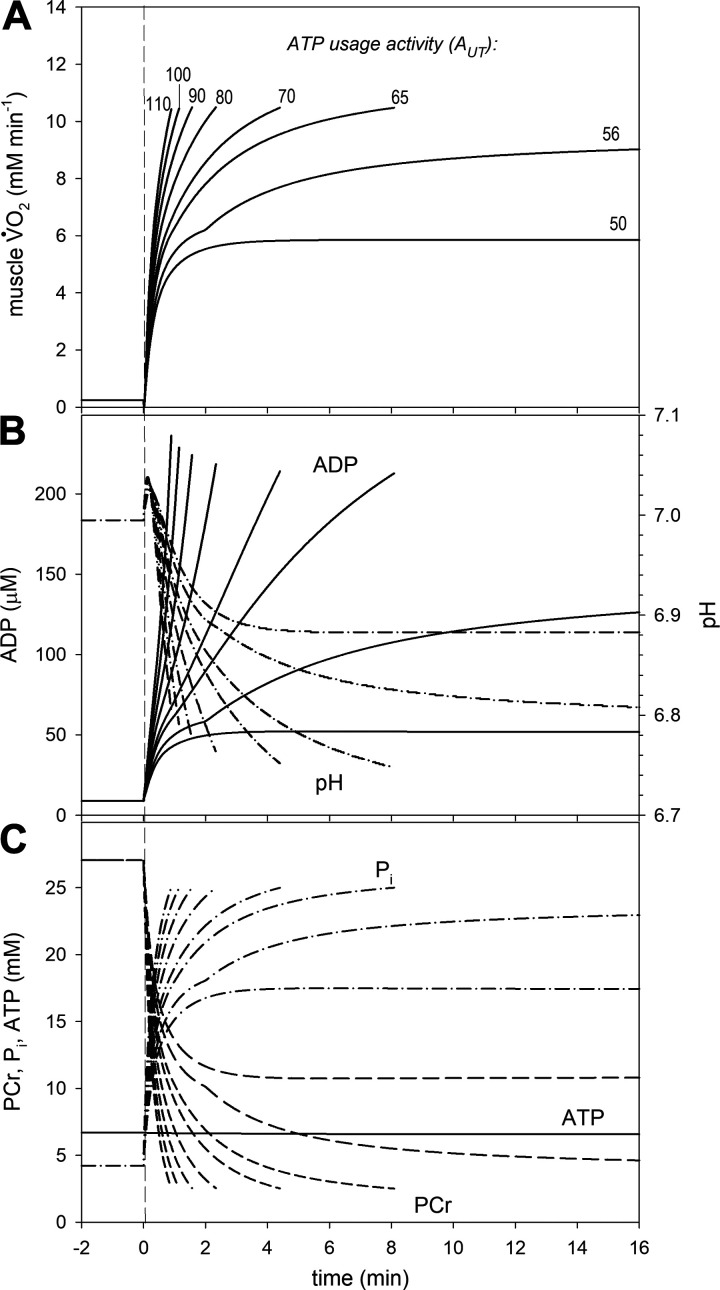
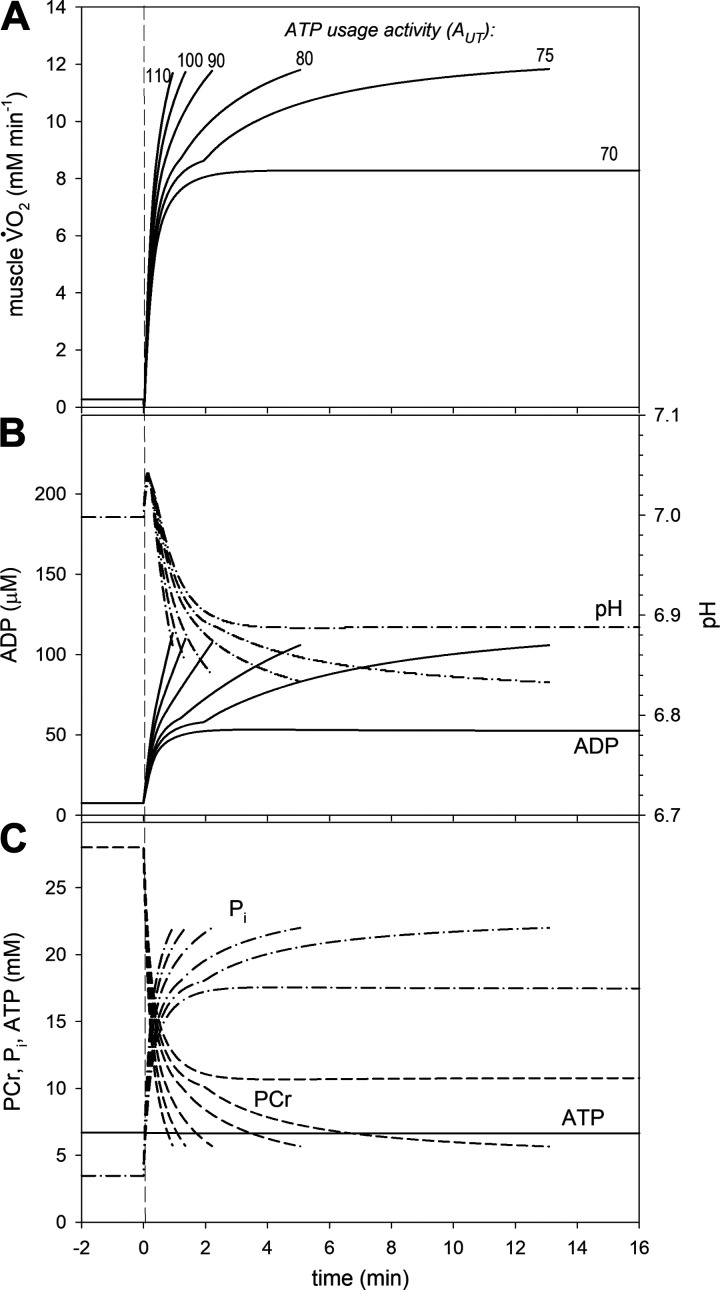
The simulated time courses of V̇O2 and metabolites during the on-transient over a range of ATP usage activities for the untrained state and trained state generally agree well with experimental data (see refs. 23, 25 for a detailed discussion of the “standard” state). They are presented in Figs. 4 and 5. Generally speaking, identical V̇O2max, PCr, and Pi (by definition) were present at the end of exercise in both cases (but significantly different between cases), whereas the levels of ADP and pH were similar, but not identical in each case. A characteristic “notch” in time-course of simulated variables was observed for some values of the ATP usage, encountered in several experimental studies (see ref. 23 for discussion). The “notch” appears at the point in time when Pi exceeds Picrit. The characteristic transition time t0.63 for the phase II of the V̇O2 on-kinetics for AUT = 40 (moderate exercise) equaled ∼29 s for untrained state and ∼23 s for trained state (compare Table 3). A steady-state, without a V̇O2 or metabolite slow component, was quickly reached for ATP usage activities corresponding to moderate exercise, whereas a slow component appeared for higher ATP usage activities that corresponded to heavy and very heavy (and severe) exercise. In untrained muscle, for AUT = 56, which is below the critical ATP usage activity AUTcrit = 59, the slow component appeared, but a delayed steady-state was observed without reaching V̇O2max. Therefore, this simulation corresponded to heavy exercise. None of the AUT values above AUTcrit resulted in a steady-state, and variable values continued to change up to the termination of exercise. Thus, these simulations represented very heavy and severe exercise. On the other hand, in simulations of well-trained muscle, the range of AUT values that represented heavy intensity was greatly diminished. At AUT = 70, which was just below AUTcrit = 72, there was no V̇O2 or metabolite slow component observed (moderate exercise), whereas above AUTcrit, V̇O2 increased progressively toward V̇O2max (very heavy and severe exercise).
Figure 4.
Simulated muscle oxygen uptake (V̇O2) and metabolites on-kinetics for various ATP usage activities (AUT) in untrained muscle (kOX * 0.8, Pipeak = 25 mM). A: time courses of muscle V̇O2; B: time courses of cytosolic free ADP and pH; C: time courses of cytosolic phosphocreatine (PCr), inorganic phosphate (Pi), and ATP.
Figure 5.
Simulated muscle oxygen uptake (V̇O2) and metabolites on-kinetics for various ATP usage activities (AUT) in trained muscle (kOX * 1.1, Pipeak = 22 mM). A: time courses of muscle V̇O2; B: time courses of cytosolic free ADP and pH; C: time courses of cytosolic phosphocreatine (PCr), inorganic phosphate (Pi), and ATP.
The metabolic perturbations caused by simulated exercise were smaller in the trained than in the untrained state. The magnitude of changes in metabolites relative to ATP usage activity (increase in ADP and Pi, decrease in PCr, and decrease in pH after an initial increase), and the values for these metabolites at end-exercise when exercise was terminated were much smaller in trained muscle than in untrained muscle (Fig. 5 versus Fig. 4).
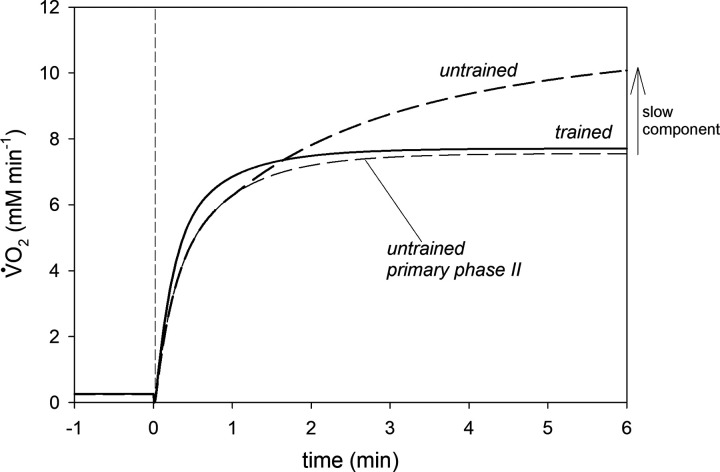
Training simulations demonstrated that the same ATP usage activity (and therefore, PO) can result in very different states of metabolic strain. This is demonstrated in Fig. 6, which represents the simulated V̇O2 on-kinetics for ATP usage activity AUT = 65 before and after training. The principal phase II of the V̇O2 on-kinetics in untrained muscle is also presented. Training principally changes the behavior of the system. Although before training a clear slow component can be observed and no steady-state can be achieved before reaching V̇O2max, there is no slow component and a steady-state is quickly reached after training at the same AUT activity. Therefore, whereas in untrained muscle this power output clearly corresponds to very heavy-intensity exercise, in trained muscle, the same power output results in moderate-intensity metabolic behavior. In addition, training causes a clear acceleration of the primary phase II of the V̇O2 on-kinetics. Therefore, at this particular AUT (PO), training brings the system from nonsteady (very heavy-exercise) to steady-state (moderate exercise)-like behavior.
Figure 6.
Simulated oxygen uptake (V̇O2) on-kinetics for ATP usage activity AUT = 65 in untrained and trained muscle. In untrained muscle, the V̇O2 primary phase II component and the slow component are distinguished.
DISCUSSION
This theoretical study postulates that the changes in V̇O2max, CP, and V̇O2 on-kinetics in skeletal muscle induced by long-term endurance training can be caused by an increase in OXPHOS activity and a decrease in the peak Pi, at which exercise is terminated because of exercise intolerance. No such mechanism that unifies the training-induced increase in these muscle bioenergetic behaviors had been proposed in the literature.
Comparison with Experimental Data
Endurance training programs typical of research studies lead to an average increase in V̇O2max (and maximal work) in the region of 10%–15%, increase in CP by 15%–20%, decrease in t0.63 by ∼25%, and significant decrease in the slow component of the V̇O2 on-kinetics (1–16). Therefore, the increase in V̇O2max by 40% and increase of AUTcrit (corresponding to CP) by 35% obtained in set A of simulations (solely resulting from a 37.5% increase in OXPHOS activity) differ significantly from experimental data. On the other hand, the increase in V̇O2max by 13%, increase of AUTcrit by 22%, and decrease in t0.63 by 22% obtained in set B of simulations (37.5% increase in OXPHOS activity and decrease in Pipeak from 25 mM to 22 mM) agree well with experimental data. This leads to the prediction that long-term endurance training leads to not only an increase in mitochondrial volume-density and oxidative activity but also a decrease of the peak Pi at which exercise is terminated.
Within the computer model used, the training-induced increase in V̇O2max is caused by the attenuation of Pi accumulation during exercise owing to an increased OXPHOS activity (and/or ESA intensity). This, is turn, delays Pi reaching Pipeak. The increase in CP is also related to a slower Pi accumulation, as this delays attainment of Picrit and also reduces the magnitude of the V̇O2 slow component for any given ATP usage activity above CP. The increase in AUTcrit (CP) causes some POs that were unsustainable before training to reach steady-state after training, where Pipeak is not reached at all. On the other hand, the decrease in Pipeak in the trained state exerts an opposite effect to the increase of OXPHOS activity: this has the action of suppressing an increase in both V̇O2max and CP, as it somewhat accelerates the reaching by Pi of Pipeak. The lowering Pipeak has the effect of reducing AUTcrit (CP), as Pi reaches Pipeak sooner at POs that cause [Pi] to exceed Picrit, and also reduces the range of supra-CP POs over which [Pi] can stabilize at a steady-state (heavy-intensity exercise).
To the best of our knowledge, no direct experimental data exist concerning the end-exercise changes in metabolites in the same individuals before and after training. An indirect cue can be found in the study by McCully at al. (36), who showed that in ramp exercise of the wrist flexors that was terminated because of exercise intolerance, the end-exercise, H2PO4− is over two times higher (13.0 mM vs. 5.7 mM) in controls than in trained rowers. Also, the finding by Sundberg et al. (37) that in maximum-intensity kicking exercise associated with fatigue (decline of generated force during exercise), Pi increases significantly more in old versus young individuals. In our simulations, the exact molar values of Picrit and Pipeak were chosen to obtain system kinetics that best represented the experimental observations. However, their values are likely to vary among individuals or fiber types, and their range remains to be determined. Nevertheless, the chosen exact molar values representing these thresholds do not qualitatively influence simulations results (23, 25).
Significantly different end-exercise [PCr] (and thus, as it can be inferred, end-exercise [Pi]), during exercise terminated because of exercise intolerance were encountered in different experimental conditions using (an identical model of) single-leg knee extension exercise in the prone position. Although in Vanhatalo et al.’s study (27), end-exercise [PCr] fell to 5%–12% of the rest value in subjects with a single leg knee-extensor CP of 16 W, it was only diminished to 26% of the rest value in Jones et al.’s study (38), in subjects who had 25% greater CP. This suggests a much lower end-exercise [Pi] in the latter case, where subjects had a greater CP (and presumably V̇O2max).
It has been proposed that in the early stage of training the acceleration of the V̇O2 on-kinetics is caused by an increase in ESA intensity, rather than in OXPHOS activity (13). However, it is likely that after longer-term training, the former is replaced by and/or supplemented with the latter, as long-term training causes an increase in OXPHOS activity, as discussed above.
Mechanisms Underlying Training-Induced Changes in the Bioenergetic System
Several factors, including O2 supply limitations (and thus lowered myocyte O2 concentration) or OXPHOS capacity for ATP synthesis, and central fatigue contribute to task-specific V̇O2max in vivo. The greater the muscle mass involved in the exercise, the greater is the likelihood that an individual will be limited less by peripheral fatigue (intramuscular limitations) and more by convective or diffusive O2 delivery or motor-neural mechanisms (11, 24). In isolated muscle exercise, for example, forearm flexion, plantar flexion, or perhaps single-leg knee extension, according to the concept presented, the “Pi double-threshold” mechanism defines the response, kinetics, and limitations of the muscle system. A “direct peripheral” mechanism of action of Pi is not known, although some possibilities have been proposed (see ref. 25 for discussion). In larger muscle mass exercise, or in environmental or pathologic conditions that alter O2 delivery, it is proposed that system behavior also acts through the “Pi double-threshold” mechanism (and perhaps other associated factors, for instance H+ ions). In these situations, earlier exercise limitation would occur because of more rapid Pi accumulation, earlier attainment of Picrit, and greater V̇O2 slow component, resulting in earlier attainment of Pipeak (determined, among others, by factors related to muscle training). An “indirect peripheral” (or central) control loop, involving Pi (or some related factor, e.g., H+), which can participate in both control operations—peripheral (direct) and central (indirect) control—is unknown. For this, neural feedback loops would need to sense (e.g., through metaboreceptors) wide-ranging effects of enhanced intramuscular Pi accumulation for any given AUT and exacerbate central fatigue contributions to exercise limitation. Although other models of central control could explain the observed system behavior, independent of the muscle metabolic state, the “Pi double-threshold” mechanism reproduces to a startling degree a broad range of diverse system properties (including time courses of V̇O2 and metabolites, constancy of end-exercise V̇O2max and metabolites, the origin and form of the slow component, the hyperbolic shape of the power-duration relationship, the dependence of CP and t0.63 on oxygen concentration and the effect of endurance training on the system properties), at least for exercise durations up to 20 min (23, 25; current data).
According to the proposed “Pi double-threshold” mechanism, moderate exercise can be described as exercise in which Picrit is not exceeded, heavy exercise as exercise in which Picrit is exceeded, but Pipeak is not reached, whereas very heavy (and severe) exercise as exercise in which Picrit is exceeded and Pipeak is reached (in severe exercise there is too little time for the slow component to fully develop, which is demonstrated by our simulations).
Computer simulations show that while the space (the range of AUT values) for heavy exercise between moderate exercise and very heavy exercise is wide in untrained muscle (Fig. 4), this becomes smaller in the “standard” state of moderately trained muscle (23), and there is little or no space for heavy exercise in well-trained muscle (Fig. 5). This is because, for power outputs just below critical power, moderate exercise-like behavior is observed. This is caused by an increased metabolite, especially Pi, stability for a given power output as a result of elevated OXPHOS activity (17). The analogue of this in vivo is a greater influence of exercise training on the lactate threshold than on CP, i.e., with endurance training, the lactate threshold (LT) becomes a greater fraction of CP. This concept is supported in a recent meta-analysis, which found that LT varies between 57% and 83% of CP among subjects (39).
Endurance training can shift the relative intensity of a given power output from very heavy-intensity exercise to moderate-intensity exercise. This is shown in Fig. 6, where the V̇O2 on-kinetics for AUT = 65 for untrained and trained muscle is presented (also the primary component of the V̇O2 on-kinetics for untrained muscle is presented). The slow component of the V̇O2 on-kinetics, which is present and large in untrained muscle, vanishes completely in trained muscle. One can also see that training leads to acceleration of the primary phase of the V̇O2 on-kinetics. A very similar effect of training for the same absolute work intensity was shown by Casaburi et al. (3) (see Fig. 1D therein).
Simulations for very heavy/severe exercise also showed that W′ was reduced following endurance training. Lower W′ after endurance-training is not a consistent finding in vivo. Nevertheless, W′ is typically unchanged or reduced, rather than increased by training (5, 8, 10). In our simulations, W′ (like CP) constitutes an emergent property of the system, and therefore, its decrease in response to training does not have a simple physiological interpretation. The combination of increase in in OXPHOS activity (kOX) and lower Pipeak in the trained state would predispose toward a reduced W′ following endurance training. W′ can also depend on Picrit and kadd that are kept constant in computer simulations of the effect of training, but they can be affected by training in real systems.
It should be stressed that all these simulated adaptations of the system behavior following training are consequences only of increased OXPHOS activity. Decreased Pipeak only diminishes effects on V̇O2max and CP. In other words, changes in other parameters are in principle not necessary to induce the considered effects observed by endurance training, although some contribution of an increase in Picrit and decrease in kadd cannot be excluded. Training-induced decrease in the additional ATP usage activity (kadd) and/or increase in the critical Pi (Picrit), would also have the effect of decreasing the magnitude of the slow component (not shown). However, at this time, it is not clear whether endurance muscle training actually causes these effects.
V̇O2 at task failure (V̇O2max) is influenced by any factor delaying or accelerating the reaching of Pipeak by Pi. V̇O2max is increased by the increase in OXPHOS activity, as this delays the reaching of Pipeak by Pi, and decreased by the decrease in Pipeak, as this accelerates the reaching Pipeak by Pi.
It has been proposed that the greater vulnerability of skeletal muscle than heart to mitochondrial diseases caused by OXPHOS complex deficiencies elicited by mutations in mitochondrial or nuclear DNA is caused by a greater maximum increase in Pi in the former than in the latter (40). This conforms well with the “Pi double-threshold” mechanism of muscle fatigue postulated in this article and previously (23, 25).
These theoretical predictions concerning training-induced decrease in Pipeak could be tested experimentally, for example, by measurement of end-exercise Pi in constant-power exercise or ramp-incremental exercise before and after training. Thus, this theoretical study can stimulate and direct future experimental studies. The effect of the training-induced increase in OXPHOS activity (and/or ESA intensity) is sufficiently supported by the experimental studies discussed in this article. Further research is needed to identify whether training alters Picrit (or kadd), and therefore, whether these parameters form part of the adaptations that improve muscle metabolic stability and enhanced exercise tolerance in the trained state.
Study Limitations
Of course, this model, as any model of this kind, has limitations.
First, the model involves explicitly only Pi as a fatigue-generating factor and fixed Pi concentrations representing the “Pi double-threshold.” These are recognized simplifications of the fatigue process, which most probably includes action of other variables, especially H+ and alteration in Ca2+ release and sensitivity, and which also differ among fiber types. However, for instance, pH changes in parallel with Pi (Figs. 4 and 5). Central fatigue also contributes to reducing maximal muscle force and/or velocity, and may contribute to, or cause, intolerance at the limit of whole-body exercise. Central fatigue is not a component of this single-cell model. On the other hand, Pi as a trigger of fatigue may act either directly within the cell or indirectly through central nervous system, for example, through metaboreceptors. The model involves constant cellular O2 concentration and, therefore, does not take into account potential O2 transport limitations. This undoubtedly constitutes a significant simplification, as O2 concentration changes during rest-to-work transition are well described. Nevertheless, this is a limitation of the model. It was shown in our previous article (23) that lowered/elevated O2 can affect CP (and V̇O2max) through an elevation or lowering of Pi accumulation, but it is by no means the only factor determining (“limiting”) these variables.
Despite these simplifications, the model was able to explain diverse behaviors of the whole muscle bioenergetic system during exercise in the trained and deconditioned state, providing a cellular mechanism by which fatigue acts as a mediator of CP, inefficiency, and intolerance. The precise concentration where the Pi “thresholds” occur is not the primary focus, rather the novelty of our findings is contained in the observed behavior of the system when the proposed mechanism is simulated.
Second, the model uses typical values of training-induced changes in the OXPHOS activity, V̇O2max, and PO. Although the experimental studies report a wide range of adaptive response to endurance training, we used an average of these training-induced changes to inform model parameter values. For example, in one extreme, Wibom et al. (35) found that cytochrome-c oxidase activity (a major component of OXPHOS activity) increased by 78% (although complex III activity increased by only 18%), whereas whole-body V̇O2max increased by only 9% in response to training; a disconnect that could reflect regional adaptations in biopsied muscle and/or the active muscle mass, but it can also be explained in our simulations through reduced Pipeak. Thus, the variability among experimental studies can be emulated by appropriate manipulation of parameter values used in the model.
Third, the model used is a one-compartment model that does not consider different muscle fiber types. However, the model is compared with “one-compartment” experimental data of whole-muscle V̇O2, cytosolic PCr, Pi, pH, and ATP using MRS and biopsy studies that themselves represent responses of multiple cells of a range of fiber types. Therefore, we selected parameter values representing a “chimeric” muscle cell with lumped parameter values representative of a large human working muscle complex, such as the quadriceps.
Fourth, the model assumes that the training-induced increase in the activity of all OXPHOS complexes is identical. However, this can be only an approximation: the activity of some complexes increases more, whereas the activity of other complexes increases less. Therefore, the assumed increase in the OXPHOS activity by 37.5% represents only an average increase in complex activity. This does not affect significantly the predictions made in this study.
Conclusions
This theoretical study postulates a simple mechanism underlying the training-induced increase in V̇O2max, increase in critical power, and changes in the V̇O2 on-kinetics (acceleration of its principal phase II and reduction of the slow component) in skeletal muscle. According to this mechanism, these changes are mostly caused by an increase in OXPHOS activity and decrease in the peak Pi, at which exercise is terminated because of exercise intolerance (in fact, the latter simply lessens the changes). An increase in ESA activity can play an important role in early stages of training. There may also be some minor role played by an increase in the critical Pi, above which the additional ATP usage, underlying the slow component, appears, and decrease of the additional ATP usage activity; however, further research is needed to identify contribution of these mechanisms. Therefore, the “Pi double-threshold” mechanism can explain a wide range of training-induced changes in skeletal muscle behavior during exercise. No such mechanism that quantitatively unifies the training-induced increase in muscle bioenergetic behaviors had been postulated in the literature.
GRANTS
No specific funding was received.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTION
B.K. conceived and designed research; B.K. performed computer simulations; B.K. and H.B.R. analyzed data; B.K. and H.B.R. interpreted results of experiments; B.K. prepared figures; B.K. and H.B.R. drafted manuscript; B.K. and H.B.R. edited and revised manuscript; B.K. and H.B.R. approved final version of manuscript.
REFERENCES
- 1.Berger NJ, Tolfrey K, Williams AG, Jones AM. Influence of continuous and interval training on oxygen uptake on-kinetics. Med Sci Sports Exerc 38: 504–512, 2006. doi: 10.1249/01.mss.0000191418.37709.81. [DOI] [PubMed] [Google Scholar]
- 2.Carter H, Jones AM, Barstow TJ, Burnley M, Williams C, Doust JH. Effect of endurance training on oxygen uptake kinetics during treadmill running. J Appl Physiol 89: 1744–1752, 2000. doi: 10.1152/jappl.2000.89.5.1744. [DOI] [PubMed] [Google Scholar]
- 3.Casaburi R, Storer TW, Ben-Dov I, Wasserman K. Effect of endurance training on possible determinants of V̇O2 during heavy exercise. J Appl Physiol 62: 199–207, 1987. doi: 10.1152/jappl.1987.62.1.199. [DOI] [PubMed] [Google Scholar]
- 4.Fernström M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol 554: 755–763, 2004. doi: 10.1113/jphysiol.2003.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaesser GA, Wilson LA. Effects of continuous and interval training on the parameters of the power-endurance time relationship for high-intensity exercise. Int J Sports Med 9: 417–421, 1988. doi: 10.1055/s-2007-1025043. [DOI] [PubMed] [Google Scholar]
- 6.Hoppeler H, Howald H, Conley K, Lindsted SL, Claassen H, Vock P, Weibel ER. Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol (1985) 59: 320–327, 1985. doi: 10.1152/jappl.1985.59.2.320. [DOI] [PubMed] [Google Scholar]
- 7.Hottenrott K, Ludyga S, Schulze S. Effects of high intensity training and continuous endurance training on aerobic capacity and body composition in recreationally active runners. J Sports Sci Med 11: 483–488, 2012. [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins DG, Quigley BM. Endurance training enhances critical power. Med Sci Sports Exerc 24: 1283–1289, 1992. [PubMed] [Google Scholar]
- 9.Phillips SM, Green HJ, MacDonald MJ, Hughson RL. Progressive effect of endurance training on V̇O2 kinetics at the onset of submaximal exercise. J Appl Physiol (1985) 79: 1914–1920, 1995. doi: 10.1152/jappl.1995.79.6.1914. [DOI] [PubMed] [Google Scholar]
- 10.Poole DC, Ward SA, Whipp BJ. The effects of training on the metabolic and respiratory profile of high-intensity cycle ergometer exercise. Eur J Appl Occup Physiol 59: 421–429, 1990. doi: 10.1007/BF02388623. [DOI] [PubMed] [Google Scholar]
- 11.Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodrigez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at VO2max. J Appl Physiol (1985) 73: 1067–1076, 1992. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- 12.Suter E, Hoppeler H, Claassen H, Billeter R, Aebi U, Horber F, Jaeger P, Marti B. Ultrastructural modification of human skeletal-muscle tissue with 6-month moderate-intensity exercise training. Int J Sports Med 16: 160–166, 1995. doi: 10.1055/s-2007-972985. [DOI] [PubMed] [Google Scholar]
- 13.Zoladz JA, Grassi B, Majerczak J, Szkutnik Z, Korostyński M, Karasiński J, Kilarski W, Korzeniewski B. Training-induced acceleration of O2 uptake on-kinetics precedes mitochondrial biogenesis in humans. Exp Physiol 98: 883–898, 2013. doi: 10.1113/expphysiol.2012.069443. [DOI] [PubMed] [Google Scholar]
- 14.Zoladz JA, Grassi B, Majerczak J, Szkutnik Z, Korostyński M, Grandys M, Jarmuszkiewicz W, Korzeniewski B. Mechanisms responsible for the acceleration of pulmonary VO2 on-kinetics in humans after prolonged endurance training. Am J Physiol Regul Integr Comp Physiol 307: R1101–R1114, 2014. doi: 10.1152/ajpregu.00046.2014. [DOI] [PubMed] [Google Scholar]
- 15.Burnley M, Jones AM. Oxygen uptake kinetics as a determinant of sports performance. Eur J Sport Sci 7: 63–79, 2007. doi: 10.1080/17461390701456148. [DOI] [Google Scholar]
- 16.Womack CJ, Davis SE, Blumer JL, Barrett E, Weltman AL, Gaesser GA. Slow component of O2 uptake during heavy exercise: adaptation to endurance training. J Appl Physiol (1985) 79: 838–845, 1995. doi: 10.1152/jappl.1995.79.3.838. [DOI] [PubMed] [Google Scholar]
- 17.Korzeniewski B, Zoladz JA. Factors determining the oxygen consumption rate (VO2) on-kinetics in skeletal muscle. Biochem J 379: 703–710, 2004. doi: 10.1042/bj20031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 19.Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol 536: 657–665, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 22.Grassi B, Rossiter HB, Zoladz JA. Skeletal muscle fatigue and decreased efficiency: two sides of the same coin? Exerc Sport Sci Rev 43: 75–83, 2015. doi: 10.1249/JES.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 23.Korzeniewski B, Rossiter HB. Exceeding a “critical” muscle Pi: implications for V̇O2 and metabolite slow components, muscle fatigue and the power-duration relationship. Eur J Appl Physiol 120: 1609–1619, 2020. doi: 10.1007/s00421-020-04388-4. [DOI] [PubMed] [Google Scholar]
- 24.Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 59: 1647–1653, 1985. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- 25.Korzeniewski B. Pi-induced muscle fatigue leads to near-hyperbolic power–duration dependence. Eur J Appl Physiol 119: 2201–2213, 2019. doi: 10.1007/s00421-019-04204-8. [DOI] [PubMed] [Google Scholar]
- 26.Goulding RP, Roche DM, Marwood S. Hyperoxia speeds pulmonary uptake kinetics and increases critical power during supine cycling. Exp Physiol 104: 1061–1073, 2019. doi: 10.1113/EP087599. [DOI] [PubMed] [Google Scholar]
- 27.Vanhatalo A, Fulford J, DiMenna FJ, Jones AM. Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol 95: 528–540, 2010. doi: 10.1113/expphysiol.2009.050500. [DOI] [PubMed] [Google Scholar]
- 28.Korzeniewski B. Regulation of ATP supply during muscle contraction: theoretical studies. Biochem J 330: 1189–1195, 1998. doi: 10.1042/bj3301189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korzeniewski B. Regulation of oxidative phosphorylation is different in electrically- and cortically-stimulated skeletal muscle. PLoS One 13: e0195620, 2018. doi: 10.1371/journal.pone.0195620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korzeniewski B. Muscle V̇O2-power output nonlinearity in constant-power, step-incremental, and ramp-incremental exercise: magnitude and underlying mechanisms. Physiol Rep 6: e13915, 2018. doi: 10.14814/phy2.13915. [DOI] [Google Scholar]
- 31.Korzeniewski B, Liguzinski P. Theoretical studies on the regulation of anaerobic glycolysis and its influence on oxidative phosphorylation in skeletal muscle. Biophys Chem 110: 147–169, 2004. doi: 10.1016/j.bpc.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Korzeniewski B, Rossiter HB. Each-step activation of oxidative phosphorylation is necessary to explain muscle metabolite kinetic responses to exercise and recovery in humans. J Physiol 593: 5255–5268, 2015. doi: 10.1113/JP271299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korzeniewski B, Zoladz JA. A model of oxidative phosphorylation in mammalian skeletal muscle. Biophys Chem 92: 17–34, 2001. doi: 10.1016/S0301-4622(01)00184-3. [DOI] [PubMed] [Google Scholar]
- 34.Korzeniewski B. Regulation of oxidative phosphorylation through each-step activation (ESA): evidences from computer modeling. Prog Biophys Mol Biol 125: 1–23, 2017. doi: 10.1016/j.pbiomolbio.2016.12.001. [27939921] [DOI] [PubMed] [Google Scholar]
- 35.Wibom R, Hultman E, Johansson M, Matherei K, Constantin-Teodosiu D, Schantz PG. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J Appl Physiol 73: 2004–2010, 1992. doi: 10.1152/jappl.1992.73.5.2004. [DOI] [PubMed] [Google Scholar]
- 36.McCully KK, Boden BP, Tuchler M, Fountain MR, Chance B. Wrist flexor muscles of elite rowers measured with magnetic resonance spectroscopy. J Appl Physiol (1985) 67: 926–932, 1989. doi: 10.1152/jappl.1989.67.3.926. [DOI] [PubMed] [Google Scholar]
- 37.Sundberg CW, Prost RW, Fitts RH, Hunter SK. Bioenergetic basis for the increased fatigability with ageing. J Physiol 597: 4943–4957, 2019. doi: 10.1113/JP277803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol 294: R585–R593, 2008. doi: 10.1152/ajpregu.00731.2007. [DOI] [PubMed] [Google Scholar]
- 39.Galán-Rioja MÁ, González-Mohíno F, Poole DC, González-Ravé JM. Relative proximity of critical power and metabolic/ventilatory thresholds: systematic review and meta-analysis. Sports Med 50: 1771–1783, 2020. doi: 10.1007/s40279-020-01314-8. [DOI] [PubMed] [Google Scholar]
- 40.Korzeniewski B. Faster and stronger manifestation of mitochondrial diseases in skeletal muscle than in heart related to cytosolic inorganic phosphate (Pi) accumulation. J Appl Physiol 121: 424–437, 2016. doi: 10.1152/japplphysiol.00358.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]