Abstract
Context
While adrenal adenomas have been linked with cardiovascular morbidity in convenience samples of patients from specialized referral centers, large-scale population-based data are lacking.
Objective
To determine the prevalence and incidence of cardiometabolic disease and assess mortality in a population-based cohort of patients with adrenal adenomas.
Design
Population-based cohort study.
Setting
Olmsted County, Minnesota, USA.
Patients
Patients diagnosed with adrenal adenomas without overt hormone excess and age- and sex-matched referent subjects without adrenal adenomas.
Main outcome measure
Prevalence, incidence of cardiometabolic outcomes, mortality.
Results
(Adrenal adenomas were diagnosed in 1004 patients (58% women, median age 63 years) from 1/01/1995 to 12/31/2017. At baseline, patients with adrenal adenomas were more likely to have hypertension [adjusted odds ratio (aOR) 1.96, 95% CI 1.58-2.44], dysglycemia (aOR 1.63, 95% CI 1.33-2.00), peripheral vascular disease (aOR 1.59, 95% CI 1.32-2.06), heart failure (aOR 1.64, 95% CI 1.15-2.33), and myocardial infarction (aOR 1.50, 95% CI 1.02-2.22) compared to referent subjects. During median follow-up of 6.8 years, patients with adrenal adenomas were more likely than referent subjects to develop de novo chronic kidney disease [adjusted hazard ratio (aHR) 1.46, 95% CI 1.14-1.86], cardiac arrhythmia (aHR 1.31, 95% CI 1.08-1.58), peripheral vascular disease (aHR 1.28, 95% CI 1.05-1.55), cardiovascular events (aHR 1.33, 95% CI 1.01-1.73), and venous thromboembolic events (aHR 2.15, 95% CI 1.48-3.13). Adjusted mortality was similar between the 2 groups.
Conclusion
Adrenal adenomas are associated with an increased prevalence and incidence of adverse cardiometabolic outcomes in a population-based cohort.
Keywords: adrenal incidentaloma, adrenal mass, epidemiology, incidence, prevalence, cardiovascular outcomes, cardiovascular events
Adrenal tumors are commonly encountered in clinical practice and are predominantly incidental findings, observed in 5% to 7% of adults undergoing cross-sectional abdominal imaging with increased prevalence and incidence with older age (1-4). The majority of incidentally discovered adrenal tumors—adrenal incidentalomas—are benign adrenocortical adenomas. Overt hormone excess is rare, but mild autonomous cortisol secretion (MACS) may be present in 30% to 50% of adrenal adenomas (2,5). Patients with MACS lack the classic signs and symptoms of glucocorticoid excess that are seen in Cushing’s syndrome (CS) but demonstrate abnormal glucocorticoid autonomy that may still lead to clinical consequences over time.
In a recent systematic review and meta-analysis on the natural history of adrenal incidentalomas, patients with adrenal adenomas and no overt hormone excess [nonfunctioning adrenal tumors (NFAT) or MACS] were found to have a high pooled prevalence and incidence of cardiometabolic disease at baseline and at follow-up, respectively, with increased morbidity in patients with MACS compared to NFAT (6). The conclusions from the systematic review and meta-analysis, however, were limited by significant heterogeneity in the definitions of MACS and of the clinical outcomes across the individual studies. Many previous studies were also smaller in size, lacked referent subjects without adrenal tumors for comparison, and had insufficient follow-up time for the assessment of de novo cardiovascular events and mortality. Furthermore, the existing data on adrenal adenomas and cardiometabolic risk have only been derived from specialized referral centers, which limit the generalizability of the findings to the general population. Referred patients may differ substantially from those who are not referred in terms of comorbidities and subtle hormone abnormalities.
Here, we conducted a population-based cohort study to (1) determine the prevalence and incidence of metabolic and cardiovascular disease and (2) assess mortality in a large, population-based sample of patients with adrenal adenomas.
Methods
Study Design and Setting
We conducted a population-based cohort study in Olmsted County, Minnesota, USA, using the Rochester Epidemiology Project (REP). REP is a medical records-linkage system that captures health information across different care providers and care settings (primary to tertiary) for residents of Olmsted County. Researchers are able to identify persons with specific medical diagnoses and surgical procedural codes of interest indexed in the REP and to retrieve medical records for persons who have provided Minnesota research authorization (overall participation 98% among Olmsted County residents) (7). Additional information on the Olmsted County population and on the REP are available in other publications (7-9). Study approval was obtained from the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Study Population and Assessment of Exposure Status
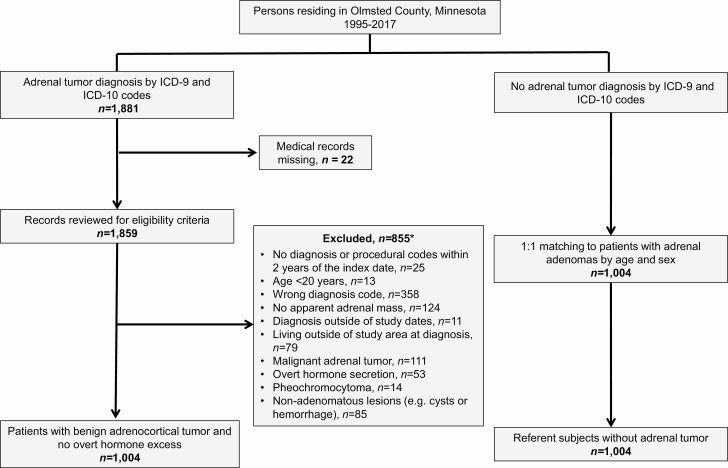
We previously assembled a population-based cohort of all Olmsted County residents diagnosed with an adrenal tumor from January 1, 1995 to December 31, 2017 (3). In brief, we identified every patient who received an International Classification of Diseases, 9th or 10th edition, diagnosis code for adrenal neoplasm [see Supplementary Table 1 in (10)] during the study timeframe using the REP infrastructure. Individual medical records were reviewed to confirm the presence of an adrenal tumor, assess eligibility criteria, and extract clinical information. For this study, we excluded patients younger than 20 years old, patients lacking diagnosis or procedural codes within 2 years prior to the adrenal tumor diagnosis, and patients with malignant adrenal disease, primary aldosteronism, CS, androgen-producing adrenal tumor, pheochromocytoma, or nonadenomatous lesions (eg, adrenal cysts or hemorrhage). Adrenal tumors were classified as benign adenomas based on imaging characteristics (unenhanced computed tomography tumor attenuation <10 Hounsfield units, rapid contrast washout, chemical shift on magnetic resonance imaging, lack of growth on imaging after 6 months), clinical follow-up of at least 24 months or histopathology if surgically removed. The final cohort consisted of 1004 patients with adrenal adenomas and no suspected overt hormone excess (Fig. 1).
Figure 1.
Flowchart showing the identification of study participants. *Multiple participants (n = 18) met more than 1 exclusion criteria.
Patients with adrenal adenomas were then 1:1 matched by age (±1 year) and sex to referent subjects randomly selected from Olmsted County residents without diagnosis codes for adrenal neoplasm. The index date was defined as the date of the earliest adrenal adenoma diagnosis based on imaging for patients and the date of matching for referent subjects.
To assess the negative predictive value of the screening process for the referent cohort, a random sample of 60 referent subjects was selected for validation. Manual medical record and imaging review was performed and confirmed the absence of an adrenal adenoma in 60 out of 60 (100%) of the referent subjects examined.
Biochemical classification of adrenal adenomas
Adrenal adenomas were classified based on the results of the overnight 1 mg dexamethasone suppression test (DST), consistent with current clinical guidelines (5,11). MACS was diagnosed if the postdexamethasone serum cortisol concentration was >1.8 mcg/dL, whereas NFAT was diagnosed if the postdexamethasone serum cortisol concentration was ≤1.8 mcg/dL. Given the historical timeline of the study and the population-based study design, we suspected that a significant proportion of patients diagnosed with an adrenal adenoma would not have been evaluated by an endocrinologist and would not have undergone a DST. Thus, patients who did not undergo the 1-mg DST and had no features of overt hormonal excess were classified as having adenomas with unknown cortisol secretion.
Assessment of Outcomes
Definitions of cardiometabolic disease
The outcomes of interest were cardiovascular risk factors and disease: hypertension, dyslipidemia, dysglycemia (prediabetes or type 2 diabetes mellitus), chronic kidney disease, cardiovascular events, cardiac arrhythmia, peripheral vascular disease, and heart failure. We also assessed the prevalence and incidence of venous thromboembolic events. Cardiovascular events were a composite of myocardial infarction, coronary intervention, or ischemic stroke, whereas venous thromboembolic events were a composite of deep venous thrombosis or pulmonary embolism. Hypertension was defined as an office systolic blood pressure of ≥130 mmHg and/or diastolic blood pressure of ≥80 mmHg on 2 or more occasions obtained 3 months apart, consistent with the American Heart Association guidelines (12); anti-hypertensive medication prescription; and/or documented International Classification of Diseases, 9th or 10th edition, diagnosis code. Dyslipidemia was defined by low-density lipoprotein cholesterol of ≥130 mg/dL (3.4 mmol/L), lipid-lowering medication prescription, and/or documented diagnosis code. Prediabetes was defined by hemoglobin A1c of 5.7% to 6.4% on 2 or more separate occasions in patients not on diabetes treatment and/or documented diagnosis code. Diabetes mellitus was defined as hemoglobin A1c ≥ 6.5% on 2 or more separate occasions and/or documented diagnosis code. Chronic kidney disease, myocardial infarction, coronary intervention, ischemic stroke, heart failure, cardiac arrhythmia, peripheral vascular disease, pulmonary embolism, and deep venous thrombosis were defined based on the relevant diagnosis or surgical procedural codes [see Supplementary Tables 2 and 3 in (10)].
Calculation of prevalence and incidence of cardiometabolic disease and mortality
The prevalence of cardiometabolic disease was assessed over a baseline period, consisting of the 6 months preceding the index date, to minimize the risk of surveillance bias related to increased medical attention at the time of abdominal imaging. The incidence of cardiometabolic disease was assessed starting at 1 year following the index date until death, migration out of the community, last contact with the REP, or adrenalectomy (n = 30). Study participants with an event or comorbidity at baseline or prior to the 1-year mark were excluded from the follow-up analysis for the particular event or condition. As surveillance bias is not a concern with mortality, overall mortality was assessed starting at the index date for all study participants.
Statistical Analysis
Continuous variables were presented using the median and range, and comparisons were made between the exposed and the unexposed groups using the Kruskal-Wallis rank sum test. Categorical variables were presented using counts and proportions, and comparisons were made using the chi-square test. We used logistic regression models to test for baseline group differences in the prevalence of cardiometabolic disease univariately, and after adjustment for age, sex, body mass index (BMI), and smoking status (current or former smoker vs nonsmoker).
The cumulative incidence of cardiometabolic disease was estimated using the Aalen-Johansen approach, with death considered as a competing risk event. We used Cox regression models to test for group differences univariately and after adjustment for potential confounders (age, sex, BMI, smoking status, and prevalent cardiometabolic risk factors) using all events starting at 1 year following the index date. Multiple imputation using the mice package in R was employed to address any missing data for BMI and smoking status (13). A 2-tailed probability value of P < 0.05 was considered statistically significant for all tests. Statistical analysis was conducted in SAS, version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population and Prevalence of Cardiometabolic Disease
From 1995 to 2017, 1004 patients were diagnosed with an adrenal adenoma, and 22% underwent biochemical screening with the 1-mg DST. A total of 141 patients (14%) were diagnosed as NFAT, 81 (8%) as MACS, and the remainder (n = 782, 78%) as adenomas with unknown cortisol secretion. The median age of diagnosis was 63 years (range, 21-96), and 582 (58%) were women. Patients with adrenal adenomas had higher BMI (31 kg/m2vs 29 kg/m2, P < 0.001) and were more likely to be current or former smokers than age- and sex-matched referent subjects (70% vs 54%, P < 0.001) (Table 1). In the 5 years preceding the index date, rates of abdominal cross-sectional imaging were similar between the 2 cohorts (22% in cases and 20% in referents).
Table 1.
Clinical characteristics of study participants with or without adrenal adenomas at baseline
| Characteristic | Adrenal adenoma (n = 1004) | No adrenal adenoma (n = 1004) | P-value |
|---|---|---|---|
| Age, median (range), years | 63 (21-96) | 63 (21-96) | 0.976 |
| Sex, female, n (%) | 582 (58.0) | 582 (58.0) | 1.000 |
| Race, white, n (%) | 939 (93.5) | 945(94.1) | 0.578 |
| Body mass index, mean (SD), kg/m2 | 31.2 (7.8) | 29.0 (6.2) | <0.001 |
| Abdominal imaging within 5 years prior to index date, n (%) | 223 (22.2) | 200 (19.9) | 0.208 |
| Follow-up time, median (range), years | 6.8 (0-21.9) | 7.2 (0-22.0) | — |
| Adrenocortical adenoma | |||
| Unilateral adenoma, n (%) | 876 (87.3) | — | — |
| Adenoma size, median (range), mm | 15 (5-85)a | — | — |
| Serum cortisol following 1 mg DST, n (%) | |||
| ≤1.8 mcg/dL | 141 (14) | — | — |
| >1.8 mcg/dL | 81 (8) | — | — |
| No DST performed | 782 (78) | — | — |
| Cardiovascular risk factors, n (%) | |||
| Current or former smoker | 652 (70.1) | 434 (53.6) | <0.001 |
| Hypertension | 770 (78.5) | 614 (64.9) | <0.001 |
| Dyslipidemia | 644 (65.8) | 575 (58.9) | 0.002 |
| Prediabetes | 146 (15.4) | 101 (10.5) | <0.001 |
| Diabetes mellitus | 261 (27.5) | 167 (17.4) | <0.001 |
| Dysglycemiab | 410 (43.1) | 268 (28.0) | <0.001 |
| Chronic kidney disease | 65 (6.5) | 52 (5.2) | 0.216 |
| Cardiovascular disease, n (%) | |||
| Myocardial infarction | 78 (7.8) | 48 (4.8) | 0.006 |
| Coronary intervention | 75 (7.5) | 56 (5.6) | 0.086 |
| Ischemic stroke | 66 (6.6) | 55 (5.5) | 0.302 |
| Cardiovascular eventsc | 157 (15.6) | 116 (11.6) | 0.008 |
| Atrial fibrillation | 91 (9.1) | 73 (7.3) | 0.142 |
| Cardiac arrhythmia | 323 (32.2) | 226 (22.5) | <0.001 |
| Heart failure | 105 (10.5) | 60 (6.0) | <0.001 |
| Peripheral vascular disease | 208 (20.7) | 133 (13.2) | <0.001 |
| Deep venous thrombosis | 29 (2.9) | 17 (1.7) | 0.073 |
| Pulmonary embolism | 30 (3.0) | 13 (1.3) | 0.009 |
| Deep venous thrombosis or pulmonary embolism | 49 (4.9) | 26 (2.6) | 0.007 |
Abbreviations: DST, dexamethasone suppression test.
a An 85-mm adrenal adenoma was associated with hemorrhage and subsequently decreased in size on follow-up imaging scans.
b Dysglycemia: composite of prediabetes or diabetes mellitus.
c Cardiovascular events: composite of myocardial infarction, coronary intervention, or ischemic stroke.
At baseline, patients with adrenal adenomas had a higher unadjusted prevalence of hypertension (79% vs 65%, P < 0.001), dyslipidemia (66% vs 59%, P = 0.002), dysglycemia (43% vs 28%, P < 0.001), myocardial infarction (8% vs 5%, P = 0.006), cardiovascular events (16% vs 12%, P = 0.008), cardiac arrhythmia (32% vs 23%, P < 0.001), peripheral vascular disease (21% vs 13%, P < 0.001), and heart failure (11% vs 6%, P < 0.001) compared to referent subjects without adrenal adenomas. After adjusting for age, sex, BMI, and smoking status, the prevalence of hypertension (aOR 1.96, 95% CI 1.58-2.44), dyslipidemia (aOR 1.23, 95% CI 1.01-1.51), dysglycemia (aOR 1.63, 95% CI 1.33-2.00), myocardial infarction (aOR 1.50, 95% CI 1.02-2.22), peripheral vascular disease (aOR 1.59, 95% CI 1.32-2.06), and heart failure (aOR 1.64, 95% CI 1.15-2.33) remained increased in patients with adrenal adenomas compared to referent subjects (Table 2).
Table 2.
Associations between adrenal adenomas and cardiometabolic diagnoses at baseline (case-control analyses)
| Cardiometabolic conditions | Unadjusted ORa (95% CI) | Age and Sex-adjusted OR (95% CI) | Model 1b OR (95% CI) |
|---|---|---|---|
| Hypertension | 2.11 (1.74-2.57) | 2.36 (1.91-2.91) | 1.96 (1.58-2.44) |
| Dyslipidemia | 1.34 (1.12-1.60) | 1.39 (1.15-1.69) | 1.23 (1.01-1.51) |
| Dysglycemiac | 1.89(1.56-2.29) | 1.92 (1.58-2.34) | 1.63 (1.33-2.00) |
| Chronic kidney disease | 1.27 (0.87-1.85) | 1.28 (0.87-1.89) | 1.06 (0.71-1.57) |
| Myocardial infarction | 1.68 (1.16-2.43) | 1.72 (1.18-2.51) | 1.50 (1.02-2.22) |
| Coronary intervention | 1.27 (0.96-1.95) | 1.39 (0.96-2.01) | 1.19 (0.82-1.74) |
| Ischemic stroke | 1.21 (0.84-1.76) | 1.22 (0.84-1.79) | 1.19 (0.81-1.76) |
| Cardiovascular eventsd | 1.42 (1.10-1.84) | 1.48 (1.12-1.94) | 1.33 (1.00-1.76) |
| Atrial fibrillation | 1.27 (0.92-1.75) | 1.30 (0.93-1.81) | 1.16 (0.82-1.64) |
| Cardiac arrhythmia | 1.63 (1.34-1.99) | 1.72 (1.40-2.13) | 1.55 (1.24-1.92) |
| Heart failure | 1.84 (1.32-2.56) | 1.92 (1.36-2.69) | 1.64 (1.15-2.33) |
| Peripheral vascular disease | 1.71 (1.35-2.17) | 1.81 (1.41-2.33) | 1.59 (1.32-2.06) |
| Deep venous thrombosis | 1.72 (0.94-3.16) | 1.73 (0.94-3.18) | 1.70 (0.91-3.19) |
| Pulmonary embolism | 2.35 (1.22-4.53) | 2.35 (1.22-4.53) | 1.97 (1.00-3.87) |
| Deep venous thrombosis or pulmonary embolism | 1.93 (1.19-3.13) | 1.94 (1.19-3.14) | 1.70 (1.03-2.80) |
Abbreviation: OR, odds ratio.
a Odds ratios were calculated for each cardiometabolic diagnosis in patients with adrenal adenoma vs referent subjects.
b Model 1: adjusted for age, sex, body mass index, and cigarette smoking (ever/never). Imputed data were used for participants missing body mass index or smoking data (n = 89 for patients with adrenal adenomas and n = 252 for referent subjects).
c Dysglycemia: composite of prediabetes or diabetes mellitus.
d Cardiovascular events: composite of myocardial infarction, coronary intervention, or ischemic stroke.
Incidence of Cardiometabolic Disease
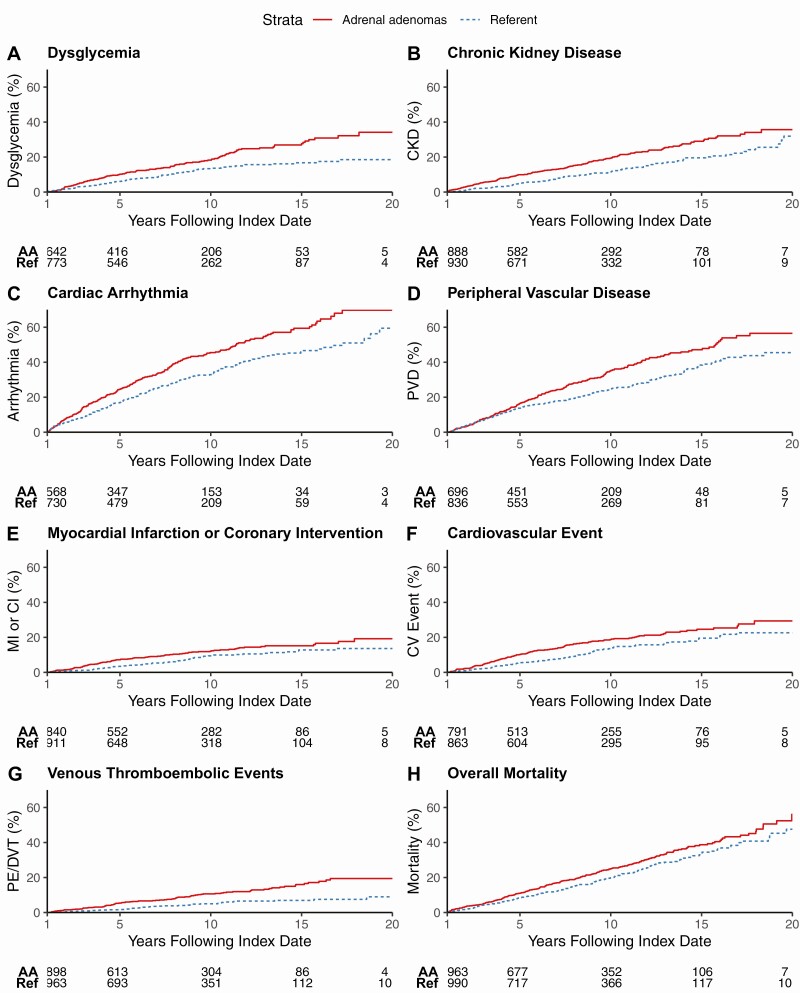
Patients with adrenal adenomas were followed for a median of 6.8 years (range, 0-22) and referent subjects for 7.2 years (range, 0-22) after the index date. The unadjusted 10-year cumulative incidence of new metabolic and cardiovascular disease was higher in patients with adrenal adenomas compared to referent subjects: dysglycemia [119/700 (18%) vs 88/818 (14%)], chronic kidney disease [167/891 (19%) vs 112/930 (12%)], cardiovascular events [131/793 (19%) vs 200/863 (14%)], atrial fibrillation [125/871 (16%) vs 93/911 (11%)], cardiac arrythmia [242/594 (46%) vs 224/730 (33%)], heart failure [148/849 (20%) vs 125/927 (13%)], and peripheral vascular disease [226/711 (35%) vs 200/836 (25%)] (Fig. 2).
Figure 2.
Cumulative incidence of cardiometabolic outcomes and mortality during follow-up, starting at 1 year after the index date. Study participants with the outcome of interest at baseline or prior to the 1-year mark were excluded. (A) Dysglycemia (composite of prediabetes or diabetes mellitus); (B) chronic kidney disease; (C) cardiac arrhythmia; (D) peripheral vascular disease; (E) myocardial infarction or coronary intervention; (F) cardiovascular events (composite of myocardial infarction, coronary intervention, or ischemic stroke); (G) venous thromboembolic events (composite of deep venous thrombosis or pulmonary embolism); and (H) overall mortality. Abbreviations: AA, adrenal adenoma group; CI, coronary intervention; CKD, chronic kidney disease; CV event, cardiovascular event; DVT, deep venous thrombosis; MI, myocardial infarction; PE, pulmonary embolism; PVD, peripheral vascular disease; Ref, referent group.
In a multivariable model adjusted for age, sex, BMI, smoking status, and prevalent cardiometabolic risk factors, adrenal adenomas were independently associated with an increased risk for chronic kidney disease [adjusted hazard ratio (aHR) 1.46, 95% CI 1.14-1.86], cardiovascular events (aHR 1.33, 95% CI 1.01-1.73), cardiac arrhythmia (aHR 1.31, 95% CI 1.08-1.58), and peripheral vascular disease (aHR 1.28, 95% CI 1.05-1.55) (Table 3). In addition, patients with adrenal adenomas were more than twice as likely to develop a new deep venous thrombosis or pulmonary embolism (aHR 2.15, 95% CI 1.48-3.13) compared to referent subjects.
Table 3.
Adrenal adenomas and the risk for incident cardiometabolic outcomes
| Outcome | Adrenal adenoma | No adrenal adenoma | Model 1a HRb (95% CI) | Model 2c HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| At risk, n | Events, n | 10-year cumulative incidence | At risk, n | Events | 10-year cumulative incidence | |||
| Hypertension | 109 | 50 | 0.86 | 271 | 137 | 0.76 | 0.81 (0.58-1.13) | 0.81 (0.58-1.13) |
| Dyslipidemia | 291 | 123 | 0.57 | 276 | 148 | 0.49 | 1.06 (0.82-1.37) | 1.04 (0.80-1.35) |
| Dysglycemiad | 700 | 119 | 0.18 | 818 | 88 | 0.14 | 1.41 (1.06-1.87) | 1.32 (0.99-1.76) |
| Chronic kidney disease | 891 | 167 | 0.19 | 930 | 112 | 0.12 | 1.57 (1.23-2.01) | 1.46 (1.14-1.86) |
| Myocardial infarction | 884 | 72 | 0.09 | 940 | 50 | 0.06 | 1.53 (1.06-2.22) | 1.45 (1.02-2.13) |
| Coronary intervention | 873 | 69 | 0.09 | 928 | 51 | 0.07 | 1.47 (1.02-2.13) | 1.37 (0.94-1.98) |
| Ischemic stroke | 890 | 83 | 0.10 | 930 | 63 | 0.08 | 1.28 (0.91-1.78) | 1.18 (0.84-1.64) |
| Cardiovascular eventse | 793 | 131 | 0.19 | 863 | 200 | 0.14 | 1.45 (1.11-1.89) | 1.33 (1.01-1.73) |
| Atrial fibrillation | 871 | 125 | 0.16 | 911 | 93 | 0.11 | 1.37 (1.04-1.79) | 1.29 (0.98-1.69) |
| Cardiac arrhythmia | 594 | 242 | 0.46 | 730 | 224 | 0.33 | 1.35 (1.12-1.63) | 1.31 (1.08-1.58) |
| Heart failure | 849 | 148 | 0.20 | 927 | 125 | 0.13 | 1.34 (1.05-1.70) | 1.23 (0.96-1.58) |
| Peripheral vascular disease | 711 | 226 | 0.35 | 836 | 200 | 0.25 | 1.35 (1.11-1.65) | 1.28 (1.05-1.55) |
| DVT | 921 | 74 | 0.09 | 970 | 35 | 0.04 | 2.10 (1.39-3.16) | 2.07 (1.37-3.14) |
| PE | 925 | 41 | 0.05 | 978 | 22 | 0.03 | 1.85 (1.09-3.13) | 1.89 (1.11-3.21) |
| DVT or PE | 898 | 90 | 0.16 | 963 | 43 | 0.05 | 2.16 (1.49-3.31) | 2.15 (1.48-3.13) |
| Overall mortality | 961 | 233 | 0.25 | 967 | 196 | 0.20 | 1.17 (0.96-1.42) | 1.11 (0.92-1.35) |
Abbreviations: DVT, deep venous thrombosis; HR, hazard ratio; PE, pulmonary embolism.
a Model 1: adjusted for age, sex, body mass index, and cigarette smoking (ever/never).
b Hazard ratios were calculated to assess the risk of incident outcomes in patients with adrenal adenomas vs referent subjects, starting at 1 year after the index date. Participants with the outcome of interest at baseline or prior to the one-year mark were excluded from the analysis for the particular condition.
c Model 2: adjusted for Model 1 variables plus number of prevalent cardiovascular risk factors (hypertension, dyslipidemia, dysglycemia, chronic kidney disease, peripheral vascular disease, heart failure, atrial fibrillation, and cardiovascular events).
d Dysgylcemia: composite of prediabetes or diabetes mellitus.
e Cardiovascular events: composite of myocardial infarction, coronary intervention, or ischemic stroke.
Mortality
During the study, 259 patients with adrenal adenomas and 196 referent subjects died. The unadjusted overall mortality was higher in patients with adrenal adenomas compared to referent subjects throughout the follow-up period (15% vs 10% at 5 years, 28% vs 21% at 10 years, and 41% vs 35% at 15 years; P < 0.001.) (Fig. 2), but there were no significant differences in mortality risk after adjusting for age, sex, BMI, and smoking status (aHR 1.17, 0.96-1.42) and number of prevalent cardiometabolic risk factors (aHR 1.11, 95% CI 0.92-1.35).
Comparison of PATIENTS with NFAT vs MACS
We examined cardiometabolic outcomes in patients with NFAT (n = 141) vs MACS (n = 81) for participants who underwent biochemical screening for cortisol excess [see Supplementary Tables 4 and 5 in (10)]. There were no notable differences in the prevalence and incidence of cardiometabolic disease between the two groups; however, the number of incident events in the analyses was small. The unadjusted overall mortality was higher in patients with MACS compared to NFAT throughout the follow-up period (3% vs 2% at 5 years, 20% vs 9% at 10 years, and 37% vs 19% at 15 years) with an age, sex, BMI, smoking, and prevalent cardiometabolic risk factors adjusted hazard ratio of 2.01 (95% CI 0.92-4.41).
Discussion
In a population-based setting, we found that patients with adrenal adenomas had both a higher prevalence at baseline and a higher risk of developing de novo cardiovascular events and comorbid conditions at follow-up than age- and sex-matched referent subjects. The majority of patients in our cohort did not undergo standard of care hormonal workup, reflecting the timeline of the study, the population-based study design, and the knowledge gaps regarding the diagnostic workup of adrenal adenomas in the general medical community. This prevented us from relating the findings to the presence and degree of MACS in the study participants.
This is the first study to assess the association of adrenal adenomas with cardiometabolic outcomes in a population-based cohort. We found that patients with adrenal adenomas without overt hormone excess had a high baseline prevalence of hypertension, dyslipidemia, dysglycemia, myocardial infarction, peripheral vascular disease, and heart failure, beyond that observed in age- and sex-matched referent subjects without adrenal tumors, even after adjustment for known confounders. These findings are consistent with the high cardiometabolic disease burden reported in convenience samples of patients with NFAT and MACS derived from university hospitals or specialized endocrinology clinics (2,6,14-18).
The prevalence of cardiometabolic conditions in our cohort was higher than the pooled prevalence reported in the recent systematic review and meta-analysis on the natural history of adrenal incidentalomas (6). This difference may be due to several reasons. We used comprehensive definitions for the classification of hypertension, diabetes mellitus, and dyslipidemia that incorporated laboratory results, medication prescription, and blood pressure measurements in addition to documented diagnosis codes. Furthermore, the REP infrastructure allowed us to follow study participants across multiple care providers and care settings, which likely resulted in more complete ascertainment of clinical outcomes. This capability may be particularly relevant for chronic diseases that are more likely to be diagnosed and followed in the primary care setting.
Patients with adrenal adenomas were more likely to be current or former smokers than age and sex-matched referent subjects (70% vs 54%). This finding is consistent with the results of 2 recent studies that examined the cross-sectional association of smoking with adrenal adenomas (19,20). In particular, Olsen et al found that patients with adrenal adenomas had a higher prevalence of current smoking compared to the general population and that smoking was associated with larger adrenal adenomas, more bilateral adrenal disease, and increased rates of autonomous cortisol secretion (19). Collectively, our studies suggest that smoking is a potential risk factor for the development of adrenal adenomas. Case selection bias could be an alternative explanation, because smokers may be more likely to undergo imaging evaluation for various reasons than non-smokers. However, the frequency of cross-sectional imaging was similar in our adrenal adenoma and referent cohorts in the 5 years preceding the index date. Smoking is also an established risk factor for the development of cardiovascular disease and was adjusted for in our analysis.
The existing data on the incidence of cardiometabolic outcomes in patients with adrenal adenomas are more limited than the data about prevalence. There are only a few longitudinal studies with extended follow-up time and most are smaller in size and lack referent subjects without adrenal tumors, making comparisons to our findings difficult (14-16,18,21). Among the larger studies, Lopez et al found an increased risk of incident composite diabetes (prediabetes or diabetes) among 166 patients with NFAT compared to patients without adrenal tumors (18). Di Dalmazi et al reported a higher risk of incident cardiovascular events (equivalent composite outcome as our study) in 198 patients with adrenal adenomas and MACS (14) and of incident atrial fibrillation in 102 patients with adrenal adenomas and MACS (15) compared to patients with NFAT.
In our cohort of 1004 patients, we found that patients with adrenal adenomas had a higher incidence of de novo chronic kidney disease, cardiovascular events, cardiac arrhythmia, and peripheral vascular disease than referent subjects without adrenal tumors, even after adjustment for known confounders. The risk for incident dysglycemia was lower than the 2-fold excess risk reported by Lopez et al in patients with NFAT compared to patients without adrenal tumors (18), possibly due to the already high baseline prevalence of diabetes in our cohort. These findings extend on the existing data from convenience samples of patients to characterize the cardiometabolic risk associated with adrenal adenomas on long-term follow-up in a population-based cohort.
We found that patients with adrenal adenomas also had a 2-fold increased risk of incident venous thromboembolic events compared to referent subjects without adrenal tumors. Because hypercoagulability is a well-known feature of CS (22), we hypothesize that venous thromboembolism risk occurs on a continuum, with higher rates of pulmonary embolism and/or deep venous thrombosis with greater degrees of glucocorticoid autonomy. However, we were unable to confirm this hypothesis in our study due to the low rates of hormonal screening.
In the 222 (22%) patients with adrenal adenomas and available DST results, we did not observe a meaningful difference in incident cardiometabolic disease or venous thromboembolism when patients with NFAT were compared to patients with MACS. However, the number of incident events in the analyses was smaller than that reported in a convenience sample of comparable size (14), which potentially limited our ability to detect an effect. In the 782 (78%) patients who did not undergo biochemical screening with the 1-mg DST, a significant proportion may have had MACS based on estimates of hormonal excess in previously published adrenal adenoma studies (2,5). In particular, a recent study found that 50% of patients with incidentally detected adrenal adenomas had postdexamethasone serum cortisol concentrations >1.8 mcg/dL in a prospective cohort (2). Thus, unrecognized cortisol excess may have contributed to the increased cardiometabolic burden observed in the adrenal adenoma group. However, we could not relate our findings to the presence and degree of mild cortisol secretion because of the low number of patients who underwent appropriate hormonal evaluation.
We found that the unadjusted overall mortality was higher in patients with adrenal adenomas compared to referent subjects without adrenal tumors. However, mortality did not differ after adjusting for smoking and cardiovascular morbidity, suggesting that these factors are potential contributors to the excess mortality risk in patients with adrenal adenomas. One previous study from an academic medical center found an increased all-cause mortality risk (aHR 1.14, 95% CI 1.003-1.29) in 969 patients with adrenal incidentalomas compared to controls without adrenal tumors (23). However, several important covariates, such as smoking status, were not considered in their analyses. A few studies have also reported increased mortality in patients with adrenal adenomas based on post-DST cortisol concentrations (14,17,24), but a systematic review and meta-analysis of 9e studies found that mortality was similar between patients with MACS and NFAT on a mean follow-up of 5.5 years (6).
The strengths of this study include the large sample size, extended longitudinal follow-up, and ability to capture clinical outcomes across health systems and care settings through the REP infrastructure. The population-based study design avoids the selection and referral biases of published studies from university hospital or specialized endocrinology clinics and increases the generalizability of our results.
The first limitation of this study was that a minority of patients (22%) underwent diagnostic workup with the 1-mg overnight DST. The low rate of hormone screening suggests a potential gap in the care of patients with adrenal adenomas. Although the onset of the study preceded the first published guidelines on the management of incidentally discovered adrenal tumors in 2002 (25), rates of hormonal workup continued to remain low (3) even after guidelines were readily available (5,11,25,26). This could be due to several reasons. First, most patients in our cohort were managed by nonspecialists, who may not be familiar with the standard adrenal workup. Second, adrenal adenomas may not be given appropriate attention, because providers and patients are more focused on the nonadrenal problem that prompted the imaging scan. However, timely evaluation for subtle cortisol excess is important because of the potential cardiometabolic consequences associated with adrenal adenomas.
Several other limitations apply to this study. First, there may be an inherent bias related to the need for cross-sectional imaging in patients with adrenal incidentalomas. Individuals who undergo imaging evaluation may be sicker or more likely to receive medical care than those who do not. Thus, the association observed between adrenal adenomas and cardiometabolic risk may in part be attributed to the underlying medical disorder that prompted the initial cross-sectional imaging, or to surveillance bias related to closer medical follow-up during that time. To minimize these biases, we assessed incidence starting at 1 year following the index date and excluded study participants who had the outcome of interest at baseline or prior to the 1-year mark. In addition, we examined the frequency of cross-sectional imaging in cases and referents in the 5 years preceding the index date and found no major differences. Second, because Olmsted County residents are predominately Caucasian (94%), our findings may not apply to populations with different ethnic and socioeconomic characteristics. Third, while the 1-mg DST is the guideline recommended test to screen for cortisol excess, additional data may be needed to verify the diagnosis of autonomous cortisol secretion. In particular, the DST may have false-positive results due to noncompliance, variability in dexamethasone absorption and metabolism, interference of certain medications, variability in cortisol assays and cutoffs used, and other factors. Last, there may be residual confounding in our analyses, including unrecognized primary aldosteronism, despite adjustment for important confounders in our models.
In summary, our findings suggest that adrenal adenomas are associated with a high prevalence of cardiometabolic disease at the time of diagnosis and with an increased incidence during follow-up of de novo cardiovascular events, peripheral vascular disease, cardiac arrhythmia, chronic kidney disease, and venous thromboembolic events compared to referent subjects without adrenal tumors. Overall mortality did not differ between the 2 groups after adjusting for the higher frequency of baseline smoking and cardiometabolic disease in patients with adrenal adenomas. Finally, screening rates for hormone excess were low in our population-based cohort, suggesting knowledge gaps in the workup of adrenal adenomas among general health care providers. Patients with adrenal incidentalomas should undergo timely screening for hormone excess at the time of diagnosis and assessment for cardiometabolic risk during follow-up.
Acknowledgments
Funding Support: This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) USA under award K23DK121888 (to I.B). The study was conducted using the Rochester Epidemiology Project medical record-linkage system, which is supported by the National Institute on Aging of the NIH under award numbers R01 AG034676 and AG052425. The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health USA.
Additional Information
Disclosures: I.B. reports advisory board participation and/or consulting with Corcept Therapeutics, Strongbridge, Sparrow Pharmaceutics, and HRA Pharma outside the submitted work. All other authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298-302. [DOI] [PubMed] [Google Scholar]
- 2. Reimondo G, Castellano E, Grosso M, et al. Adrenal incidentalomas are tied to increased risk of diabetes: findings from a prospective study. J Clin Endocrinol Metab. 2020;105(4):dgz284. [DOI] [PubMed] [Google Scholar]
- 3. Ebbehoj A, Li D, Kaur RJ, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(11):894-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol. 2008;190(5):1163-1168. [DOI] [PubMed] [Google Scholar]
- 5. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1-G34. [DOI] [PubMed] [Google Scholar]
- 6. Elhassan YS, Alahdab F, Prete A, et al. Natural history of adrenal incidentalomas with and without mild autonomous cortisol excess: a systematic review and meta-analysis. Ann Intern Med. 2019;171(2):107-116. [DOI] [PubMed] [Google Scholar]
- 7. St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sauver JLS, Grossardt BR, Leibson CL, Yawn BP, Melton LJ III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151-160. [DOI] [PMC free article] [PubMed]
- 9. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang CD, Li D, Kaur RJ. , et al. Supplemen tal data: cardiometabolic outcomes and mortality in patients with adrenal adenomas: a population-based cohort study. Uploaded April 13, 2021. Figshare. doi: 10.6084/m9.figshare.14408573.v3 [DOI] [Google Scholar]
- 11. Vaidya A, Hamrahian A, Bancos I, Fleseriu M, Ghayee HK. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25(2):178-192. [DOI] [PubMed] [Google Scholar]
- 12. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. [DOI] [PubMed] [Google Scholar]
- 13. van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw.2011;45(3):67. [Google Scholar]
- 14. Di Dalmazi G, Vicennati V, Garelli S, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396-405. [DOI] [PubMed] [Google Scholar]
- 15. Di Dalmazi G, Vicennati V, Pizzi C, et al. Prevalence and incidence of atrial fibrillation in a large cohort of adrenal incidentalomas: a long-term study. J Clin Endocrinol Metab. 2020;105(8):e2770-e2777. [DOI] [PubMed] [Google Scholar]
- 16. Petramala L, Olmati F, Concistrè A, et al. Cardiovascular and metabolic risk factors in patients with subclinical Cushing. Endocrine. 2020;70(1):150-163. [DOI] [PubMed] [Google Scholar]
- 17. Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” adrenal tumors and the risk for incident diabetes and cardiovascular outcomes: a cohort study. Ann Intern Med. 2016;165(8):533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olsen H, Kjellbom A, Löndahl M, Lindgren O. High prevalence of smoking in patients with adrenal incidentalomas: causality or case selection? Eur J Endocrinol. 2020;183(3):335-341. [DOI] [PubMed] [Google Scholar]
- 20. Yousaf A, Patterson J, Hobbs G, et al. Smoking is associated with adrenal adenomas and adrenocortical carcinomas: a nationwide multicenter analysis. Cancer Treat Res Commun. 2020;25:100206. [DOI] [PubMed] [Google Scholar]
- 21. Morelli V, Reimondo G, Giordano R, et al. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99(3):827-834. [DOI] [PubMed] [Google Scholar]
- 22. Wagner J, Langlois F, Lim DST, McCartney S, Fleseriu M. Hypercoagulability and risk of venous thromboembolic events in endogenous Cushing’s syndrome: a systematic meta-analysis. Front Endocrinol (Lausanne). 2018;9:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taya M, Paroder V, Bellin E, Haramati LB. The relationship between adrenal incidentalomas and mortality risk. Eur Radiol. 2019;29(11):6245-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patrova J, Kjellman M, Wahrenberg H, Falhammar H. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine. 2017;58(2):267-275. [DOI] [PubMed] [Google Scholar]
- 25. NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”). NIH Consensus State Sci Stat. 2002;19(2):1-25. [PubMed] [Google Scholar]
- 26. Zeiger M, Thompson G, Duh Q-Y, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(Suppl 1):1-20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.