Abstract
Context
Continuous glucose monitoring (CGM) overcomes the limitations of glycated hemoglobin (HbA1c).
Objective
This study aimed to investigate the relationship between CGM metrics and laboratory HbA1c in pregnant women with type 1 diabetes.
Methods
An observational study enrolled pregnant women with type 1 diabetes who wore CGM devices during pregnancy and postpartum from 11 hospitals in China from January 2015 to June 2019. CGM data were collected to calculate time in range (TIR), time above range (TAR), time below range (TBR), and glycemic variability parameters. Relationships between the CGM metrics and HbA1c were explored. Linear and curvilinear regressions were conducted to investigate the best-fitting model to clarify the influence of HbA1c on the TIR-HbA1c relationship during pregnancy.
Results
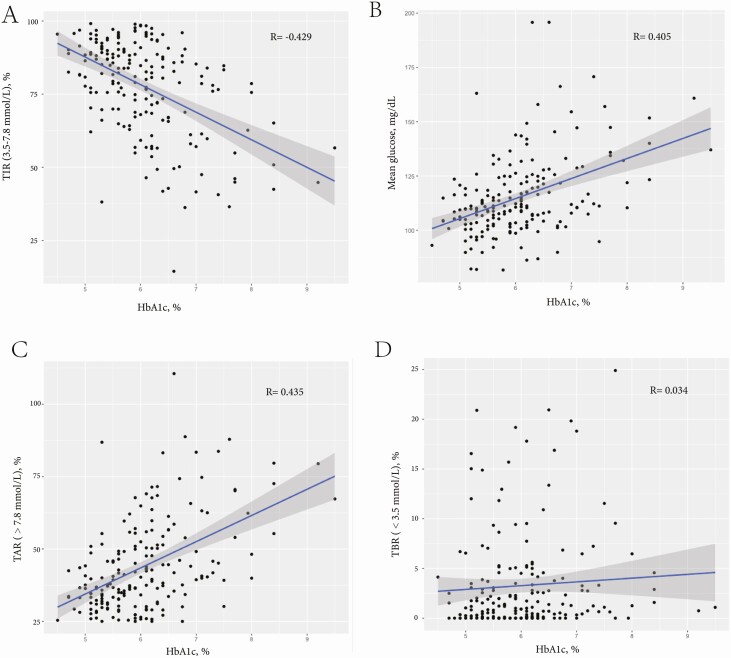
A total of 272 CGM data and corresponding HbA1c from 98 pregnant women with type 1 diabetes and their clinical characteristics were analyzed in this study. Mean HbA1c and TIR were 6.49 ± 1.29% and 76.16 ± 17.97% during pregnancy, respectively. HbA1c was moderately correlated with TIR3.5-7.8(R = –0.429, P = .001), mean glucose (R = 0.405, P = .001) and TAR7.8 (R = 0.435, P = .001), but was weakly correlated with TBR3.5 (R = 0.034, P = .001) during pregnancy. On average, a 1% (11 mmol/mol) decrease in HbA1c corresponded to an 8.5% increase in TIR3.5–7.8. During pregnancy, HbA1c of 6.0%, 6.5%, and 7.0% were equivalent to a TIR3.5–7.8 of 78%, 74%, and 69%, respectively.
Conclusion
We found there was a moderate correlation between HbA1c and TIR3.5–7.8 during pregnancy. To achieve the HbA1c target of less than 6.0%, pregnant women with type 1 diabetes should strive for a TIR3.5–7.8 of greater than 78% (18 hours 43 minutes) during pregnancy.
Keywords: glycated hemoglobin A1c, time in range, diabetes mellitus, type 1, pregnancy
Glycated hemoglobin (HbA1c), as a valuable metric for evaluating glycemic control, is widely used in clinical practice and research (1). However, the limitations of HbA1c in the evaluation of glycemic control have been recognized for a long time (2, 3). During pregnancy, HbA1c is affected by various common clinical conditions such as increased erythrocyte production, shortened erythrocyte metabolic cycle, and iron deficiency (2, 4, 5). Among pregnancies with type 1 diabetes, Murphy found an even larger decrease in HbA1c of 1% (11 mmol/mol) from the first trimester to the second trimester without a detectable improvement in self-monitored glucose levels (2), which indicated that HbA1c may inappropriately assess glycemic control during pregnancy (6).
A continuous glucose monitoring (CGM) system plays an increasingly important role in diabetes management, especially in antenatal care. CGM can provide abundant glycemic data to describe detailed glucose profiles. CGM-derived metrics, including time in range (TIR), time below range (TBR), time above range (TAR), mean amplitude of glycemic excursions, SD, and coefficient of variation, partially overcome the limitations of HbA1c. Among the aforementioned measurements, TIR is one of the critical metrics recommended by international guidelines and consensuses (7).
Several studies explored the relationship between TIR and HbA1c in patients with type 1 diabetes and revealed that an estimation of TIR (range, 3.9-10.0 mmol/L) was 70% for a given HbA1c of 6.5% (8-11). However, limited data were available in pregnancies with type 1 diabetes (12). Kristensen et al found there was a 0.3-mmol/L decrease in mean glucose and a 5% increase in TIR without a detectable change in HbA1c during pregnancy (13). In addition, only half the participants in the CONCEPTT study who met the target HbA1c of less than or equal to 6.5% maintained a TIR (range, 3.5-7.8 mmol/L) of greater than 70%, suggesting that the target of TIR (range, 3.5-7.8 mmol/L) of greater than 70% was more ambitious (14). The previous study proposed a pregnancy-specific calculation for translating CGM-derived mean glucose into an estimated HbA1c among pregnant women with diabetes (15). However, much is still unknown regarding the relationship between the CGM-derived metrics TIR and HbA1c in pregnant women with type 1 diabetes, which would be of clinical significance to provide them with comprehensive management.
Using the data from a prospective cohort study including pregnant women with type 1 diabetes with available CGM data in the first, second, third trimesters, and post partum (16), we aimed to evaluate the relationship between CGM metrics and laboratory HbA1c levels during pregnancy.
Materials and Methods
Study Design and Participants
This observational study enrolled pregnant women with type 1 diabetes who wore CGM devices (iPro2 Professional CGMS, Medtronic or 722 Medtronic-MiniMed) during pregnancy and post partum from 11 regional medical centers from 8 cities in China from January 2015 to June 2019. All participants received routine clinical care and comprehensive diabetes management twice in the first trimester (6-10 weeks, 12-13 weeks of gestation), once in the second trimester (22-24 weeks of gestation), twice in the third trimester (33-34 weeks, and 36 weeks of gestation), and post partum (17). We excluded those who had multiple intrauterine pregnancies and who had a termination of pregnancy for nonmedical reasons. The HbA1c target levels were less than 7.0% (53 mmol/mmol) for prepregnancy and less than 6.0% (42 mmol/mmol) without excessive hypoglycemia for pregnancy (1, 18). This study was approved by the ethics committee at each site in accordance with the Declaration of Helsinki ([2014]2-5 (1)). All participants provided written informed consent.
Data Collection
Maternal data, including age at conception, duration of diabetes, HbA1c, and hemoglobin (Hb) levels in the 3 trimesters, were collected from electronic medical records by trained physicians. CGM data were downloaded via Carelink iPro and were computed with GlyCulator 2.0 software (19). According to the guidance from Advanced Technologies and Treatments for Diabetes (ATTD), the main CGM metrics analyzed in this study were TIR of 3.5 to 7.8 mmol/L (TIR3.5–7.8), TIR of 3.9 to 10.0 mmol/L (TIR3.9–10.0), TBR of less than 3.0 mmol/L (TBR3.0), TBR of less than 3.5 mmol/L (TBR3.5), TAR of greater than 7.8 mmol/L (TAR7.8), TAR of greater than 10.0 mmol/L (TAR10.0), mean blood glucose, SD, coefficient of variation, and mean amplitude of glycemic excursions, high blood glucose indices, low blood glucose indices, and area under the curve (7). Records containing less than 80% of glucose data during the wearing time were excluded from the analysis. Laboratory HbA1c was measured centrally by an automated analyzer (Bio-Rad D10; Bio-Rad Laboratories) using the high-performance–liquid chromatography technique.
Statistical Analysis
R software (version 3.6.1) was used for all analyses. Data were presented as mean ± SD, median (interquartile range), or number (percentage) where appropriate. Spearman regression was performed to estimate the association between CGM-derived metrics and HbA1c measured the first day wearing the CGM devices. The mix-effect regression analysis was conducted to explore the best-fitting model to calculate the TIR from the HbA1c. Linear and curvilinear (squared) relations, as well as several covariates, including HbA1c levels, Hb, gestational weeks, and different trimesters, were included in the exploration for best-fitting model. A 2-sided P value of less than .05 was considered statistically significant.
Results
Population Characteristics
CGM data and corresponding HbA1c from 98 pregnant women with type 1 diabetes were collected. CGM and HbA1c data were collected on repeated occasions in the same woman throughout pregnancy; a total of 272 valid CGM-HbA1c profiles were obtained, including 89 CGM-HbA1c profiles in the first trimester, 61 in the second trimester, 83 in the third trimester, and 39 in post partum, respectively. Table 1 showed the characteristics and CGM parameters of the pregnant women with type 1 diabetes. The pregnant women had a mean age at conception of 28.80 ± 3.90 years, with diabetes duration of 8.72 ± 6.10 years. The mean HbA1c measurements were 6.68 ± 1.47% (range. 4.5%-13.5%) in the first trimester, 6.01 ± 0.57 (range, 5.1%-7.1%) in the second trimester, and 6.20 ± 0.82% (range, 5.1%-7.9%) in the third trimester (P = .001). The usage time of the CGM sensor was 98.44 ± 4.26%. The TIR3.5–7.8 measurements were 71.10 ± 18.93% in the first trimester, 78.34 ± 17.29% in the second trimester, and 79.91 ± 16.31% in the third trimester, respectively. Additionally, 39 CGM-HbA1c profiles in the postpartum were also collected and are shown in Table 1.
Table 1.
Continuous glucose monitoring system parameters of pregnant women with type 1 diabetes mellitus during pregnancy and postpartum
| During pregnancy | First trimester | Second trimester | Third trimester | Postpartum | P a | P b | |
|---|---|---|---|---|---|---|---|
| Pairs of CGMS-HbA1c | 233 | 89 | 61 | 83 | 39 | – | – |
| Age at pregnancy, y | 28.80 ± 3.90 | 29.23 ± 4.04 | 28.06 ± 3.52 | 28.50 ± 3.55 | 26.30 ± 4.23 | .146 | .846 |
| Diabetes duration at pregnancy, y | 8.72 ± 6.10 | 8.98 ± 5.95 | 8.78 ± 6.24 | 8.42 ± 6.22 | 8.65 ± 5.81 | .899 | .732 |
| HbA1c, % | 6.49 ± ± 1.29 | 6.68 ± 1.47 | 6.01 ± 0.57 | 6.20 ± 0.82 | 6.88 ± 1.31 | .189 | .131 |
| TIR (3.5-7.8mmol/L), %, median (IQR) | 76.16 ± 17.97 | 71.10 ± 18.93 | 78.34 ± 17.29 | 79.91 ± 16.31 | 63.94 ± 21.87 | .001 | .001 |
| TIR (3.9-10.0 mmol/L), %, median (IQR) | 86.65 ± 12.33 | 83.19 ± 13.77 | 87.53 ± 11.51 | 89.67 ± 10.37 | 79.73 ± 15.37 | .001 | .001 |
| TBR (< 3.5 mmol/L), %, median (IQR) | 1.23 (0.09-4.04) | 1.92 (0.19-4.46) | 1.38 (0-4.51) | 0.97 (0.11-2.79) | 1.39 (0.11-4.92) | .436 | .042 |
| TBR (< 3.0 mmol/L), %, median (IQR) | 0 (0-1.00) | 0(0-1.13) | 0(0-1.06) | 0 (0-0.42) | 0 (0-1.52) | .194 | .031 |
| TAR (> 7.8 mmol/L), %, median (IQR) | 15.92 (8.27-28.91) | 20.60 (11.54-24.97) | 13.73 (6.34-24.97) | 12.88 (5.90-24.68) | 29.75 (13.67-45.51) | .001 | .005 |
| Mean glucose, mmol/L | 6.49 ± 1.11 | 6.72 ± 1.27 | 6.34 ± 1.07 | 6.36 ± 0.93 | 7.11 ± 1.46 | .013 | .083 |
| SD, mmol/L | 0.10 ± 0.05 | 0.12 ± 0.05 | 0.09 ± 0.03 | 0.09 ± 0.04 | 0.13 ± 0.05 | .001 | .001 |
| CV, % | 27.60 ± 9.31 | 30.57 ± 10.78 | 26.74 ± 7.82 | 25.09 ± 7.71 | 31.53 ± 10.43 | .001 | .001 |
| MAGE, mmol/L | 4.56 ± 2.10 | 5.25 ± 2.64 | 4.34 ± 1.59 | 4.00 ± 1.56 | 5.83 ± 2.57 | .001 | .001 |
| HBGI, median (IQR) | 0.95 (0.45-2.14) | 1.37 (0.62-3.20) | 0.89 (0.41-1.67) | 0.72 (0.34-1.61) | 2.73 (0.86-4.05) | .001 | .005 |
| LBGI, median (IQR) | 1.43 (0.73-2.39) | 1.52 (0.66-2.47) | 1.58 (0.70-2.45) | 1.37 (0.80-2.15) | 2.21 (0.68-2.60) | .822 | .629 |
Data are presented in mean ± SD unless otherwise noted.
Abbreviations: CGMS, continuous glucose monitoring system; CV, coefficient of variation; HbA1c, glycated hemoglobin A1c; HBGI, high blood glucose indices; IQR, interquartile range; LBGI, low blood glucose indices; MAGE, mean amplitude of glycemic excursions; TAR, time above range; TBR, time below range; TIR, time in range.
a P value for comparison between 3 trimesters and postpartum.
b P value for comparison between 3 trimesters during pregnancy.
Relationship Between Time in Range and Glycated Hemoglobin A1c in Pregnancy
As shown in Table 2, HbA1c measurements were moderately correlated with the TIR3.5–7.8 (R = –0.429, P = .001, Fig. 1A), mean glucose (R = 0.405, P = .001, Fig. 1B), and the TAR7.8 (R = 0.435, P = .001, Fig. 1C), but were weakly correlated with the TBR3.5 (R = 0.034, P = .001, Fig. 1D) during pregnancy. Compared with the correlation between HbA1c and TIR3.5–7.8 during pregnancy, the correlation was stronger post partum (during pregnancy vs post partum: –0.429 vs –0.766, P = .001), where women were released from the pregnancy status. Supplementary Table 1 (20) shows the Spearman partial correlation among CGM metrics and HbA1c in type 1 diabetes pregnancies in the 3 trimesters.
Table 2.
Spearman partial correlation among glycated hemoglobin A1c and selected continuous glucose monitoring metrics during pregnancy and postpartum
| During pregnancy | HbA1c | TIR (3.5-7.8) | TIR (3.9-10.0) | TBR (3.5) | TBR (3.0) | TAR (7.8) | TAR (10.0) | Mean glucose | AUC > 7.8 | HBGI | LBGI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TIR (3.5-7.8 mmol/L) | –0.429 | ||||||||||
| TIR (3.9-10.0 mmol/L) | –0.316 | 0.727 | |||||||||
| TBR (< 3.5 mmol/L) | 0.034 | –0.191 | |||||||||
| TBR (< 3.0 mmol/L) | 0.019 | –0.255 | –0.581 | 0.829 | |||||||
| TAR (> 7.8 mmol/L) | 0.435 | –0.952 | –0.551 | –0.035 | 0.042 | ||||||
| TAR (> 10.0 mmol/L) | 0.365 | –0.848 | –0.686 | 0.050 | 0.113 | 0.869 | |||||
| Mean glucose, mg/dL | 0.405 | –0.763 | –0.281 | –0.322 | –0.205 | 0.883 | 0.763 | ||||
| AUC > 7.8 mmol/L | 0.391 | –0.925 | –0.631 | 0.024 | 0.101 | 0.959 | 0.951 | 0.851 | |||
| HBGI | 0.407 | –0.930 | –0.601 | –0.003 | 0.078 | 0.970 | 0.932 | 0.881 | 0.995 | ||
| LBGI | –0.114 | 0.095 | –0.414 | 0.860 | 0.709 | –0.304 | –0.154 | –0.613 | –0.217 | –0.257 | |
| HbA1c, % | 1.000 | –0.429 | –0.316 | 0.034 | 0.019 | 0.435 | 0.365 | 0.405 | 0.391 | 0.407 | –0.114 |
| Postpartum | HbA 1c | TIR (3.5-7.8) | TIR (3.9-10.0) | TBR (3.5) | TBR (3.0) | TAR (7.8) | TAR (10.0) | Mean glucose | AUC > 7.8 | HBGI | LBGI |
| TIR (3.5-7.8 mmol/L) | –0.766 | ||||||||||
| TIR (3.9-10.0 mmol/L) | –0.574 | 0.746 | |||||||||
| TBR (< 3.5 mmol/L) | –0.127 | 0.137 | –0.298 | ||||||||
| TBR (< 3.0 mmol/L) | –0.196 | 0.023 | –0.255 | 0.854 | |||||||
| TAR (> 7.8 mmol/L) | 0.739 | –0.964 | –0.617 | –0.336 | –0.208 | ||||||
| TAR (> 10.0 mmol/L) | 0.675 | –0.927 | –0.767 | –0.206 | –0.097 | 0.925 | |||||
| Mean glucose, mg/dL | 0.664 | –0.898 | –0.520 | –0.463 | –0.284 | 0.956 | 0.902 | ||||
| AUC > 7.8 mmol/L | 0.702 | –0.940 | –0.747 | –0.197 | –0.089 | 0.940 | 0.985 | 0.919 | |||
| HBGI | 0.707 | –0.944 | –0.736 | –0.199 | –0.080 | 0.944 | 0.980 | 0.922 | 0.998 | ||
| LBGI | –0.273 | 0.380 | –0.117 | 0.924 | 0.708 | –0.560 | -0.401 | –0.677 | –0.401 | –0.406 | |
| HbA1c, % | 1.000 | –0.766 | -0.574 | –0.127 | –0.196 | 0.739 | 0.675 | 0.664 | 0.702 | 0.707 | –0.273 |
Abbreviations: AUC, area under the curve; CGMS, continuous glucose monitoring system; CV, coefficient of variation; HbA1c, glycated hemoglobin A1c; HBGI, high blood glucose indices; , LBGI, low blood glucose indices; MAGE, mean amplitude of glycemic excursions; TAR, time above range; TBR, time below range; TIR, time in range.
Figure 1.
Relationships between glycated hemoglobin A1c (HbA1c) and A, time in range (TIR) 3.5 to 7.8 mmol/L; B, mean glucose; C, time above range (TAR) greater than 7.8 mmol/L; and D, time below range (TBR) less than 3.5 mmol/L.
The Best-Fitting Model to Account for the Time in Range–Glycated Hemoglobin A1c Relationship
For a given TIR3.5–7.8 level, a wide range of HbA1c levels corresponded to it when observing a graph of TIR3.5–7.8 vs HbA1c, and vice versa. The formulation between TIR and HbA1c may be influenced by other factors. To investigate the best-fitting model to account for the TIR–HbA1c relationship, different random variants were contained in the mixed-effects model (Table 3), including Hb, gestational weeks, and trimesters. No significant effect on the relationship of HbA1c and TIR was found in gestational weeks, gestational weeks squared, or trimesters, except Hb. There was no interaction between HbA1c and gestational weeks. The best-fitting model, with the highest criterion score, was shown as follows:
Table 3.
Comparison of an intercept-only mixed-effects model with models containing random effects to determine the best-fitting model to account for how gestational changes in glycated hemoglobin A1c (HbA1c) influence the time in range–HbA1c relationship
| Model | Fixed effects | β | 95% CI | P | F | P of the equation |
|---|---|---|---|---|---|---|
| 1 | Intercept | 131.00 | (118.56 to 143.44) | .001 | 77.15 | .001 |
| HbA1c | –8.93 | (–10.94 to –6.93) | .001 | |||
| 2 | Intercept | 125.74 | (110.71 to 140.77) | .001 | 39.41 | .001 |
| HbA1c, % | –8.57 | (–0.93 to 0.04) | .001 | |||
| Trimesters | 1.53 | (–0.93 to 3.99) | .222 | |||
| 3 | Intercept | 125.42 | (110.24 to 140.59) | .001 | 39.47 | .001 |
| HbA1c, % | –8.52 | –10.62 to –6.42) | .001 | |||
| Gestation, wk | 0.13 | (–0.07 to 0.34) | .208 | |||
| 4 | Intercept | 96.21 | (68.43 to 123.99) | .001 | 20.34 | .001 |
| HbA 1c , % | –8.46 | (–11.44 to –5.49) | .001 | |||
| Hb, g/L | 0.27 | (0.09 to 0.44) | .001 | |||
| 5 | Intercept | 132.75 | (111.76 to 153.74) | .001 | 26.65 | .001 |
| HbA1c, % | –8.82 | (–11.00 to –6.63) | .001 | |||
| Gestation, wk | 0.47 | (–1.68 to 0.74) | .443 | |||
| Gestation, wk2 | 0.01 | (–0.01 to 0.04) | .320 | |||
| 6 | Intercept | 90.51 | (50.90 to 130.13) | .001 | 10.62 | .001 |
| HbA1c, % | –8.02 | (–11.39 to –4.64) | .001 | |||
| Hb, g/L | 0.27 | (0.10 to 0.44) | .003 | |||
| Gestation, wk | –0.01 | (–1.68 to 1.66) | .991 | |||
| Gestation, wk2 | 0.01 | (–0.03 to 0.04) | .822 |
The values in bold shows the best fitting model of TIR-HbA1c relationship.
TIR (%) = 96.21 – 8.46 * HbA1c (%) + 0.27 * Hb (g/L)
The analysis showed a significant linear correlation between TIR3.5–7.8 and laboratory HbA1c (adjusted R2 = 0.217, P < .001) when considering the influence of Hb. This indicated that, for pregnancies with type 1 diabetes, a 1% (11 mmol/mol) decrease in HbA1c was equal to an 8.5% increase in percentage TIR during pregnancy. For instance, 6.0%, 6.5%, and 7.0% HbA1c were equivalent to a TIR of 78%, 74%, and 69%, respectively.
Discussion
To the best of our knowledge, this is the first study to explore the relationship between TIR and HbA1c in pregnant women with type 1 diabetes. Our results showed a significant linear relationship between TIR and HbA1c during pregnancy and provides a pregnancy-specific formulation for TIR according to HbA1c levels in pregnant women with type 1 diabetes while considering the influence of Hb.
The correlation coefficient of the relationship between TIR and HbA1c during pregnancy was weaker than that observed post partum. Similarly, previous studies showed that the correlations of TIR with HbA1c were 0.7 or greater among nonpregnant patients with type 1 diabetes (9). This may reflect a shift in the correlation of TIR-HbA1c from pregnancy to post partum that is due to the change of physiological status. Influenced by a shortened erythrocyte lifespan, altered red cell affinity for glucose, and iron deficiency, the HbA1c is slightly lower in pregnant than in nonpregnant patients, which explains the difference of the TIR-HbA1c relationship during pregnancy (5, 21). Providing a pregnancy-specific formulation for the relationship between HbA1c and TIR would benefit diabetes management clinically and precisely during pregnancy, especially for the estimates of hypoglycemia and hyperglycemia.
Guidance from the ATTD consensus recommends pregnancies with type 1 diabetes should strive to achieve a TIR3.5–7.8 greater than 70%, but the evidence is limited (7). For optimal glucose control and pregnancy outcomes (22), American Diabetes Association standards recommended the HbA1c target in pregnancy should be lower than 6.0% (42 mmol/mol) if it can be achieved without significant hypoglycemia (1, 18). Our study demonstrated that pregnant women with type 1 diabetes should strive for a TIR3.5–7.8 of greater than 78% (18 hours 43 minutes) to achieve an HbA1c of less than 6.0% during pregnancy.
Several studies have reported the conversion of HbA1c from TIR in patients with type 1 diabetes. However, considering the difference in physiological changes in HbA1c during pregnancy, all the studies excluded pregnant populations (2, 8, 9, 23). Previous studies indicated that an HbA1c of 6.0%, 6.5%, and 7.0% corresponded to a TIR3.9–10.0 of approximately 75%, 70%, and 64%, respectively, in nonpregnant patients with type 1 diabetes (9). Our study enrolled pregnant women with type 1 diabetes and included different confounders in the mixed-effects model to explore the conversion between TIR and HbA1c among this population. With the consideration of Hb, 6.0%, 6.5%, and 7.0% of HbA1c during pregnancy were equivalent to TIR measurements of 78%, 74%, and 69%, respectively. This suggested that to strive for the same HbA1c level, higher TIR levels were needed during pregnancy. The potential mechanism was that HbA1c lowers because of lower mean glucose, shortened erythrocyte life span, and increased erythropoiesis during pregnancy (2, 5, 24).
A recent study showed that the gestational week was essential for calculating the estimated average glucose from the mean glucose–HbA1c relationship during pregnancy. However, the influence of hematocrit levels or iron deficiency was not considered in the regression model (15). In our study, the Hb level rather than gestational week was included in the best-fitting model to determine the TIR-HbA1c relationship. This was possibly because the Hb levels strongly affected the physiological HbA1c changes during pregnancy, as the red blood cells were exposed to a lower time of blood glucose due to increased erythropoiesis, and the glycosylation of Hb was therefore attenuated during pregnancy (5).
There are several strengths in this study. First, this study corrected the influence of Hb in the conversion of HbA1c and TIR, which was critical for the HbA1c levels during pregnancy. Second, TIR and HbA1c data were collected on repeated occasions in the same women throughout pregnancy, which allowed the gestational week to be analyzed in this study. Third, all patients in this study wore the same brand of sensor, which reduced the bias from different monitoring technologies. Nonetheless, this study also has some limitations. First, this study included only Chinese participants. Considering the relationship of HbA1c and TIR may vary by race and ethnicity, and more clinical trials should be designed to explore the correlation among a larger population. Second, CGM profiles were obtained every 5 to 7 days, which might not be sufficient to reflect 3 months of glycemic profiles.
In conclusion, there is a significant correlation between HbA1c and TIR during pregnancy in patients with type 1 diabetes. This study suggests that women with type 1 diabetes should strive for a TIR3.5–7.8 greater than 78% (18 hours 43 minutes) during pregnancy to achieve the HbA1c target of 6.0% (1).
Acknowledgments
The authors are grateful to all participants of the study for the provision of the data. We thank the physicians and administrative staff for their assistance in data collection.
Financial Support: This work was supported by the National Key R&D Program of China (grant/award No. 2017YFC1309600 to J.W.), the National Natural Science Foundation of China (grant/award No. 81941022 to J.W.), the National Health and Family Planning Commission of the People’s Republic of China, and Foundation for Public Welfare Industry Research Project (grant/award No. 201502011 to J.W.), the Fundamental Research Funds for the Central Universities (grant/award No. wk9110000137 to X.Z.).
Clinical Trial Information: Clinical trial registry No. ChiCTR1900025955 (registered September 15, 2019).
Glossary
Abbreviations
- ATTD
Advanced Technologies and Treatments for Diabetes
- CGM
continuous glucose monitoring
- CV
coefficient of variation
- HBGI
high blood glucose indices
- Hb
hemoglobin
- HbA1c
glycated hemglobin A1c
- MAGE
mean amplitude of glycemic excursions
- TAR
time above range
- TBR
time below range
- TIR
time in range
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The data sets generated during and/or analyzed the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. American Diabetes Association. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44(Suppl 1):S200-S210. [DOI] [PubMed] [Google Scholar]
- 2. Murphy HR. Intensive glycemic treatment during type 1 diabetes pregnancy: a story of (mostly) sweet success! Diabetes Care. 2018;41(8):1563-1571. [DOI] [PubMed] [Google Scholar]
- 3. Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50(12):2553-2561. [DOI] [PubMed] [Google Scholar]
- 4. Worth R, Potter JM, Drury J, Fraser RB, Cullen DR. Glycosylated haemoglobin in normal pregnancy: a longitudinal study with two independent methods. Diabetologia. 1985;28(2):76-79. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27(5):1200-1201. [DOI] [PubMed] [Google Scholar]
- 6. Murphy HR. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia. 2019;62(7):1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vigersky RA, McMahon C. The relationship of hemoglobin A1c to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81-85. [DOI] [PubMed] [Google Scholar]
- 9. Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. JDiabetes Sci Technol. 2019;3(4):614-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirsch IB, Welsh JB, Calhoun P, Puhr S, Walker TC, Price DA. Associations between HbA1c and continuous glucose monitoring-derived glycaemic variables. Diabet Med. 2019;36(12):1637-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petersson J, Åkesson K, Sundberg F, Särnblad S. Translating glycated hemoglobin A1c into time spent in glucose target range: a multicenter study. Pediatr Diabetes. 2019;20(3):339-344. [DOI] [PubMed] [Google Scholar]
- 12. Advani A. Positioning time in range in diabetes management. Diabetologia. 2020;63(2):242-252. [DOI] [PubMed] [Google Scholar]
- 13. Kristensen K, Ögge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62(7):1143-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feig DS, Donovan LE, Corcoy R, et al. ; CONCEPTT Collaborative Group . Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390(10110):2347-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Law GR, Gilthorpe MS, Secher AL, et al. Translating HbA1c measurements into estimated average glucose values in pregnant women with diabetes. Diabetologia. 2017;60(4):618-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng X, Yang D, Luo S, et al. ; CARNATION Study Group . Association of implementation of a comprehensive preconception-to-pregnancy management plan with pregnancy outcomes among Chinese pregnant women with type 1 diabetes: the CARNATION Study. Diabetes Care. 2021;44(4):883-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cunningham FG, Leveno K, Bloom S, et al. Williams Obstetrics. 25th ed. McGraw-Hill; 2018. [Google Scholar]
- 18. American Diabetes Association. Standards of Medical Care in Diabetes–2015. Diabetes Care. 2015;38(Suppl 1):S1-S93. [PubMed] [Google Scholar]
- 19. Pagacz K, Stawiski K, Szadkowska A, Mlynarski W, Fendler W. GlyCulator2: an update on a web application for calculation of glycemic variability indices. Acta Diabetol. 2018;55(8):877-880. [DOI] [PubMed] [Google Scholar]
- 20. Ling P, Yang D. Supplementary data for: Spearman partial correlation among HbA1c and selected CGM metrics in pregnant women with T1DM in the three trimesters. Dryad data set, Deposited 27 March 2021. 10.5061/dryad.f7m0cfxvw [DOI]
- 21. Phelps RL, Honig GR, Green D, Metzger BE, Frederiksen MC, Freinkel N. Biphasic changes in hemoglobin A1c concentrations during normal human pregnancy. Am J Obstet Gynecol. 1983;147(6):651-653. [DOI] [PubMed] [Google Scholar]
- 22. Ahmed RG. Evolutionary interactions between diabetes and development. Diabetes Res Clin Pract. 2011;92(2):153-167. [DOI] [PubMed] [Google Scholar]
- 23. Kilpatrick ES. HbA1c measurement. J Clin Pathol. 2004;57(4):344-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El-Agouza I, Abu Shahla A, Sirdah M. The effect of iron deficiency anaemia on the levels of haemoglobin subtypes: possible consequences for clinical diagnosis. Clin Lab Haematol. 2002;24(5):285-289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed the present study are not publicly available but are available from the corresponding author on reasonable request.