Abstract
Context
The interaction of glycated hemoglobin A1c (HbA1c) and glycemic variability in relation to diabetes-related outcomes remains unknown.
Objective
To evaluate the relationship between HbA1c and all-cause mortality across varying degrees of glycemic variability in patients with type 2 diabetes.
Design, Setting, and Patients
This was a prospective study conducted in a single referral center. Data of 6090 hospitalized patients with type 2 diabetes was analyzed. Glucose coefficient of variation [coefficient of variation (CV)] was obtained as the measure of glycemic variability by using continuous glucose monitoring for 3 days. Cox proportional hazards regression models were used to estimate hazard ratios and 95% CIs for all-cause mortality.
Results
During a median follow-up of 6.8 years, 815 patients died. In patients with the lowest and middle tertiles of glucose CV, HbA1c ≥ 8.0% was associated with 136% (95% CI 1.46-3.81) and 92% (95% CI 1.22-3.03) higher risks of all-cause mortality, respectively, as compared with HbA1c 6.0%-6.9%, after adjusting for confounders. However, a null association of HbA1c with mortality was found in patients with the highest tertile of glucose CV.
Conclusions
HbA1c may not be a robust marker of all-cause mortality in patients with high degree of glycemic variability. New metrics of glycemic control may be needed in these individuals to achieve better diabetes management.
Keywords: glucose variability, HbA1c, mortality, continuous glucose monitoring, interaction
Diabetes is one of the fastest growing public health problems in both developing and developed countries (1). Much of the burden of diabetes is attributable to microvascular and macrovascular complications. Concerning diabetes management, glycated hemoglobin A1c (HbA1c) has been accepted as the most important surrogate marker for diabetic complications and the gold standard for long-term glycemic control. A target value of HbA1c below 7% is recommended by most guidelines published to date to minimize the risk of adverse outcomes (2-4). However, it is increasingly recognized that HbA1c may not be an optimal marker for the quality of glycemic control. From the perspective of clinical practice, it is noted that HbA1c does not provide enough information (eg, hyperglycemia and hypoglycemia) to inform the adjustment of treatment plans. Moreover, the use of HbA1c may sometimes be misleading (5), which is evident in patients with a high degree of glycemic variability (GV). This observation raises the question that whether HbA1c is a valid marker for all groups of patients with type 2 diabetes. To date, no outcome-based study has tried to address this issue.
As an important component of dysglycemia, GV has attracted a lot of attention recently. Mechanistic investigations indicated that glucose swings may induce oxidative stress and subsequent endothelial dysfunction (6-9), which are key players in the development of vascular complications of diabetes. Numerous observational studies also provided evidence on the relationship between GV and diabetes-related outcomes (10-12). However, it is not known if HbA1c can predict the risk of diabetes-related outcomes across a wide range of GV. Therefore, the aim of the present study was to investigate the interaction between HbA1c and GV with the risk of all-cause mortality among patients with type 2 diabetes.
Methods
Study Population
We used data from the INDices of contInuous Glucose monitoring and adverse Outcomes of diabetes (INDIGO) study (13). The ongoing INDIGO study aims to prospectively recruit inpatients admitted to the Department of Endocrinology and Metabolism of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital from January 2005. Patients who enrolled from January 2005 to December 2015 and fulfilled the following criteria were included in the analysis: (1) age ≥ 18 years with the diagnosis of type 2 diabetes; (2) a stable glucose-lowering regimen for the previous 3 months; (3) complete data on HbA1c and continuous glucose monitoring (CGM) parameters; and (4) a citizen of Shanghai, China. We excluded those with other types of diabetes (eg, gestational diabetes or type 1 diabetes), and those who had experienced severe and recurrent hypoglycemic events within the previous 3 months. Subjects included in the final analysis were admitted to the hospital for the evaluation of glucose control and diabetes-related complications and comorbidities but not for other specific reasons such as diabetic ketoacidosis. All patients provided written informed consent. The study protocol was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and complied with the principles of the Helsinki Declaration.
Measurements
Patients’ information on date of birth, sex, age of diabetes diagnosis, smoking status (current smoking or not), history of cancer and cardiovascular disease (CVD; angina, coronary heart disease, or stroke), and medication prescriptions such as antihypertensive drugs, glucose-lowering drugs, and lipid-lowering drugs was collected through a standardized electronic inpatient medical record data collection form. At admission, trained doctors measured height, weight, and blood pressure using a standard protocol. Height and weight were measured to the nearest 0.1 cm using a stadiometer with light clothing and without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured 3 times using a standard mercury sphygmomanometer after 5 min of sitting, and the measurements were averaged. Blood samples were drawn in the next morning after hospital admission with at least 10-h fasting. HbA1c, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein cholesterol, and triglycerides were assayed as previously described (13).
Assessment of CGM Parameters
A blinded CGM system (CGMS GOLD, Medtronic Inc, Northridge, CA, USA) was used for subcutaneous interstitial glucose monitoring. The sensor of the CGM system was inserted on the first day during hospital admission and removed after 72 h, generating a daily record of 288 continuous sensor values. At least 4 capillary blood glucose readings per day were measured by a SureStep blood glucose meter (LifeScan, Milpitas, CA, USA) to calibrate the CGM system. Glucose coefficient of variation (CV) was calculated as the measure of GV. Time in range (TIR) was defined as the percentage of time in the target glucose range of 3.9 to 10.0 mmol/L during a 24-h period. Time above ranges [TARs; TAR > 140 mg/dL (TAR>140), TAR > 180 mg/dL (TAR>180), and TAR > 250 mg/dL (TAR>250)], and time below ranges [TBRs; TBR < 54 mg/dL (TBR<54), and TBR < 70 mg/dL (TBR<70)] were also ascertained. During the CGM period, all participants adhered to a standard diet, as previously reported (14).
Prospective Follow-up
Causes and time of death were obtained from the database of the Shanghai Municipal Center for Disease Control and Prevention and were linked with study data through the personal identification number. The death causes were identified with the use of the codes in the International Classification of Diseases, 10th Revision. The rate of missing death events in Shanghai was 0.7‰ (T. Xia and J. Zhou, oral communication, September 2012). We used chart review to evaluate the confirmation of death (COD) via the Shanghai adaption of the Medical Data Audit Form. Trained physicians have reviewed the medical records of a death event and have reassigned the COD, which provided a gold standard to measure the quality of routine COD data. The death events identified by Shanghai Civil Registration Vital Statistics routine monitoring were thus reported with high sensitivity and specificity of 85.7% and 90.0%, respectively. All patients were followed up until a death event occurred or until December 31, 2018, whichever occurred first.
Statistical Analysis
The trends of continuous variables across the tertiles of glucose CV were assessed using linear polynomial contrasts in an analysis of variance for normally distributed variables and the Jonckheere-Terpstra test for nonnormally distributed data. The Cochran-Armitage trend test was used to examine the trends of rates across groups. The correlations between glucose metrics were evaluated by Spearman’s correlation coefficients. The Cox proportional hazard model was used to estimate the hazard ratio (HR) and 95% CI of HbA1c or other glycemic metrics on all-cause mortality across CV tertiles. HbA1c was categorized into 4 groups (<6.0%, 6.0%-6.9%, 7.0%-7.9%, and ≥8.0%). HbA1c 6.0%-6.9% was selected as the reference group, as our previous study revealed a J-shaped association of HbA1c with all-cause mortality in the same population, with HbA1c 6.0%-6.9% conferring minimal risk of death (13). Three different models were used in the analyses. Covariates in Model 1 included age and sex. In Model 2, we additionally adjusted for diabetes duration, BMI, systolic blood pressure, triglyceride, HDL cholesterol, low-density lipoprotein cholesterol, current smoking status, history of cancer, and history of cardiovascular diseases. Model 3 represented the fully adjusted model, where we additionally adjusted for the use of concomitant medications including aspirin, statins, insulin, oral hypoglycemic agents, and antihypertensive drugs. The potential interactions between glucose CV and glycemic metrics including HbA1c were evaluated by comparing the log-likelihood statistics of models that included interaction terms and models without interaction terms. A P-value of < 0.05 (2-tailed) was considered statistically significant. Statistical analyses were performed using the SPSS software version 17.0 (SPSS Inc, Chicago, IL, USA).
Results
A total of 6090 participants were finally included into the analysis. Table 1 shows baseline characteristics of participants stratified by the tertiles of glucose CV (≤21.5%, 21.6%-28.9%, and >28.9%). Age, diabetes duration, HDL cholesterol, HbA1c, and the use of insulin and aspirin were positively associated with glucose CV, and BMI, triglycerides, and the use of oral hypoglycemic agents and antihypertensive drugs were inversely associated with glucose CV.
Table 1.
Characteristics of participants by tertiles of glucose CV among patients with type 2 diabetes
| Characteristics | Total | Glucose CV | P for trend | ||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||
| Participants, n | 6090 | 2017 | 2060 | 2013 | / |
| Person-years | 43108.9 | 14391.6 | 14438.8 | 14278.5 | / |
| Age, years | 61.7 ± 11.9 | 60.8 ± 12.5 | 61.3 ± 11.6 | 62.9 ± 11.4 | <0.001 |
| Men, n (%) | 3326 (54.6) | 1044 (51.8) | 1170 (56.8) | 1112 (55.2) | 0.026 |
| Diabetes duration, years | 10 (4-15) | 8 (3-13) | 10 (4-14) | 10 (4-15) | <0.001 |
| SBP, mmHg | 132.9 ± 16.9 | 133.1 ± 17.1 | 132.5 ± 16.6 | 133.0 ± 17.0 | 0.856 |
| DBP, mmHg | 79.7 ± 9.5 | 80.1 ± 9.8 | 79.6 ± 9.2 | 79.5 ± 9.3 | 0.057 |
| Body mass index, kg/m2 | 24.9 ± 3.5 | 25.5 ± 3.6 | 24.9 ± 3.4 | 24.3 ± 3.4 | <0.001 |
| Total cholesterol, mmol/L | 4.7 ± 1.2 | 4.8 ± 1.3 | 4.7 ± 1.2 | 4.7 ± 1.1 | 0.225 |
| Triglycerides, mmol/L | 1.4 (0.9-2.0) | 1.5 (1.1-2.2) | 1.4 (1.0-2.1) | 1.2 (0.8-1.7) | <0.001 |
| HDL cholesterol, mmol/L | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.3 | <0.001 |
| LDL choleterol, mmol/L | 2.9 ± 0.9 | 2.9 ± 0.9 | 2.9 ± 1.0 | 3.0 ± 1.0 | 0.613 |
| HbA1c, % | 8.9 ± 2.2 | 8.5 ± 2.1 | 8.8 ± 2.1 | 9.3 ± 2.3 | <0.001 |
| History of CVD, n (%) | 1299 (21.3) | 430 (21.3) | 418 (20.3) | 451 (22.4) | 0.401 |
| History of cancer, n (%) | 279 (4.6) | 80 (4.0) | 106 (5.1) | 93 (4.6) | 0.321 |
| Current smoker, n (%) | 1441 (23.7) | 446 (22.1) | 529 (25.7) | 466 (23.1) | 0.438 |
| CGM parameter | |||||
| Glucose CV, % | 25.8 ± 8.4 | 17.0 ± 3.2 | 25.1 ± 2.1 | 35.2 ± 5.4 | <0.001 |
| TBR<54, % | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.9) | <0.001 |
| TBR<70, % | 0.0 (0.0-1.2) | 0.0 (0.0-0.0) | 0.0 (0.0-0.5) | 1.2 (0.0-5.2) | <0.001 |
| TAR>140, % | 62.9 (41.8-81.9) | 72.9 (37.7-93.4) | 64.4 (43.6-81.6) | 57.3 (42.7-70.9) | <0.001 |
| TAR>180 % | 29.5 (13.4-50.7) | 22.6 (4.9-57.3) | 28.8 (14.1-49.0) | 33.0 (20.3-47.9) | <0.001 |
| TAR>250, % | 4.0 (0.0-13.0) | 0.0 (0.0-5.8) | 3.3 (0.0-10.8) | 9.2 (3.6-18.5) | <0.001 |
| TIR, % | 64.5 ± 24.6 | 66.6 ± 31.2 | 65.5 ± 23.1 | 61.3 ± 17.1 | <0.001 |
| Medication, n (%) | |||||
| Oral hypoglycemic drugs | 4303 (70.7) | 1534 (76.1) | 1472 (71.5) | 1297 (64.4) | <0.001 |
| Insulin | 4072 (66.9) | 1080 (53.5) | 1378 (66.9) | 1614 (80.2) | <0.001 |
| Anti-hypertensive drugs | 3308 (54.3) | 1145 (56.8) | 1102 (53.5) | 1061 (52.7) | 0.010 |
| Aspirin | 2870 (47.1) | 909 (45.1) | 982 (47.7) | 979 (48.6) | 0.023 |
| Statins | 2349 (38.6) | 773 (38.3) | 795 (38.6) | 781 (38.8) | 0.757 |
Data shown are mean ± SD, median (interquartile range) or number (percentage) unless otherwise indicated.
Abbreviations: CGM, continuous glucose monitoring; CV, coefficient of variation; CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL cholesterol, high-density lipoprotein cholesterol; LDL cholesterol, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TBR<54, time below range (<54mg/dL); TBR<70, time below range (<70mg/dL); TAR>140, time above range (>140 mg/dL); TAR>180, time above range (>180 mg/dL); TAR>250, time above range (>250 mg/dL); TIR, time in range; HbA1c, glycated hemoglobin A1c.
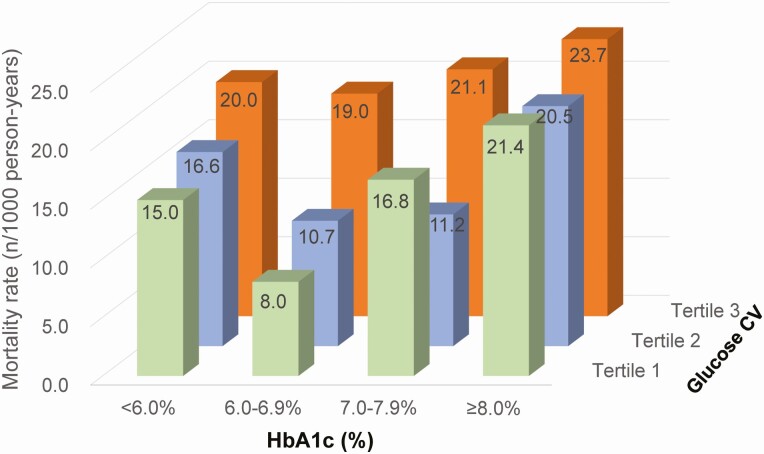
During a median follow-up of 6.8 years (43 108.9 total person-years), 815 people died. As illustrated in Figure 1, the crude mortality rates displayed a similar and obvious pattern in patients with the lowest and middle glucose CV tertiles, where the lowest mortality rate across HbA1c categories (<6.0%, 6.0%-6.9%, 7.0%-7.9%, and ≥8.0%) was observed in HbA1c 6.0%-6.9%. However, the pattern was less pronounced in those with the highest glucose CV tertile.
Figure 1.
Crude all-cause mortality rates stratified by glucose CV tertiles and HbA1c categories. Abbreviations: CV, coefficient of variation; HbA1c, glycated hemoglobin A1c.
In the total population, the multivariable-adjusted (model 3) HRs of all-cause mortality associated with HbA1c levels (<6.0%, 6.0%-6.9%, 7.0%-7.9%, and ≥8.0%) were 1.64 (95% CI 1.07-2.49), 1.00, 1.37 (95% CI 1.03-1.82), and 1.80 (95% CI 1.40-2.31), respectively (Table 2). In patients with the lowest and middle tertiles of glucose CV, HbA1c ≥ 8.0% was related to 136% (HR 2.36, 95% CI 1.46-3.81) and 92% (HR 1.92, 95% CI 1.22-3.03) higher risk of all-cause mortality as compared with HbA1c 6.0%-6.9% in the fully adjusted model, respectively. However, the 4 categories of HbA1c did not differ in the risk of all-cause mortality in subjects with the highest tertile of glucose CV. A significant interaction between HbA1c and CV (P < 0.05) was observed in Model 2 but not in Models 1 and 3 (both P > 0.10).
Table 2.
Hazard ratios of all-cause mortality according to categories of HbA1c among patients with type 2 diabetes by total samples and across glucose CV tertiles
| HbA1c | ||||
|---|---|---|---|---|
| <6.0% | 6.0%-6.9% | 7.0%-7.9% | ≥8.0% | |
| Total samples | ||||
| Participants/deaths, n | 291/33 | 988/83 | 1152/128 | 3659/571 |
| Person-years | 1996.6 | 7114.0 | 7976.1 | 26022.3 |
| Adjusted HRs (95% CIs) | ||||
| Model 1 | 1.51 (1.00-2.29) | 1 (reference) | 1.42 (1.07-1.88) | 2.06 (1.62-2.61) |
| Model 2 | 1.62 (1.06-2.46) | 1 (reference) | 1.40 (1.06-1.86) | 2.02 (1.59-2.56) |
| Model 3 | 1.64 (1.07-2.49) | 1 (reference) | 1.37 (1.03-1.82) | 1.80 (1.40-2.31) |
| Patients with tertile 1 of glucose CV | ||||
| Participants/Deaths, n | 132/14 | 407/24 | 408/47 | 1070/164 |
| Person-years | 932.6 | 2988.1 | 2805.7 | 7665.3 |
| Adjusted HRs (95% CIs) | ||||
| Model 1 | 1.98 (0.99-3.95) | 1 (reference) | 2.20 (1.31-3.68) | 3.11 (1.97-4.90) |
| Model 2 | 2.11 (1.05-4.25) | 1 (reference) | 2.09 (1.24-3.53) | 2.82 (1.78-4.48) |
| Model 3 | 1.98 (0.98-4.00) | 1 (reference) | 1.99 (1.18-3.36) | 2.36 (1.46-3.81) |
| Patients with tertile 2 of glucose CV | ||||
| Participants/deaths, n | 100/11 | 330/25 | 409/32 | 1221/176 |
| Person-years | 663.9 | 2337.4 | 2847.0 | 8590.5 |
| Adjusted HRs (95% CIs) | ||||
| Model 1 | 1.68 (0.82-3.45) | 1 (reference) | 1.09 (0.64-1.87) | 2.11 (1.36-3.26) |
| Model 2 | 1.94 (0.94-4.02) | 1 (reference) | 1.03 (0.60-1.76) | 2.05 (1.32-3.18) |
| Model 3 | 1.99 (0.96-4.12) | 1 (reference) | 1.04 (0.61-1.79) | 1.92 (1.22-3.03) |
| Patients with tertile 3 of glucose CV | ||||
| Participants/deaths, n | 59/8 | 251/34 | 335/49 | 1368/231 |
| Person-years | 400.1 | 1788.6 | 2323.4 | 9766.4 |
| Adjusted HRs (95% CIs) | ||||
| Model 1 | 1.21 (0.53-2.72) | 1 (reference) | 1.20 (0.78-1.87) | 1.40 (0.97-2.00) |
| Model 2 | 1.28 (0.56-2.92) | 1 (reference) | 1.20 (0.77-1.86) | 1.40 (0.98-2.02) |
| Model 3 | 1.29 (0.57-2.96) | 1 (reference) | 1.22 (0.78-1.91) | 1.38 (0.94-2.02) |
Model 1 adjusted for age and sex; Model 2 further adjusted for diabetes duration, smoking, body mass index, systolic blood pressure, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, history of cancer, and history of cardiovascular diseases; Model 3 adjusted for the covariates in Model 2 plus the use of aspirin, statins, oral hypoglycemic agents, insulin, and antihypertensive drugs.
Abbreviations: CV, coefficient of variation; HbA1c, glycated hemoglobin A1c; HR, hazard ratio.
In contrast to HbA1c, after multivariable adjustment (Model 3), each 10% decrease in TIR was related to 12% (HR 1.12, 95% CI 1.04-1.20) higher risk of all-cause mortality, while each 10% increment in TAR>140, TAR>180, and TAR>250 was associated with 11% (HR 1.11, 95% CI 1.04-1.19), 11% (HR 1.11, 95% CI 1.04-1.18), and 14% (HR 1.14, 95% CI 1.04-1.25) heightened risks of all-cause mortality among patients with the highest tertile of glucose CV, respectively (Table 3).
Table 3.
Associations of CGM metrics with all-cause mortality across tertiles of glucose CV
| CGM metrics/glucose CV | Adjusted HRs (95% CIs)a | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Time in range | |||
| Tertile 1 | 1.08 (1.04-1.12) | 1.06 (1.02-1.11) | 1.03 (0.98-1.08) |
| Tertile 2 | 1.09 (1.03-1.15) | 1.07 (1.02-1.13) | 1.05 (0.99-1.12) |
| Tertile 3 | 1.13 (1.06-1.21) | 1.12 (1.05-1.20) | 1.12 (1.04-1.20) |
| P for interaction | <0.025 | <0.025 | <0.025 |
| Time above range (>140 mg/dL) | |||
| Tertile 1 | 1.10 (1.05-1.15) | 1.08 (1.03-1.14) | 1.05 (1.00-1.11) |
| Tertile 2 | 1.09 (1.03-1.15) | 1.07 (1.01-1.13) | 1.05 (0.99-1.11) |
| Tertile 3 | 1.12 (1.06-1.19) | 1.11 (1.04-1.18) | 1.11 (1.04-1.19) |
| P for interaction | >0.10 | >0.25 | >0.25 |
| Time above range (>180 mg/dL) | |||
| Tertile 1 | 1.08 (1.04-1.12) | 1.06 (1.01-1.10) | 1.02 (0.98-1.07) |
| Tertile 2 | 1.09 (1.03-1.14) | 1.07 (1.02-1.13) | 1.05 (0.99-1.11) |
| Tertile 3 | 1.12 (1.06-1.19) | 1.11 (1.05-1.19) | 1.11 (1.04-1.18) |
| P for interaction | <0.05 | <0.05 | <0.05 |
| Time above range (>250 mg/dL) | |||
| Tertile 1 | 1.10 (1.02-1.19) | 1.06 (0.98-1.16) | 1.01 (0.92-1.10) |
| Tertile 2 | 1.14 (1.04-1.24) | 1.10 (1.01-1.21) | 1.07 (0.98-1.18) |
| Tertile 3 | 1.16 (1.07-1.26) | 1.15 (1.06-1.26) | 1.14 (1.04-1.25) |
| P for interaction | >0.10 | >0.10 | >0.10 |
| Time below range (<54 mg/dL) | |||
| Tertile 1 | 0.17 (0.01-2.72) | 0.23 (0.02-3.15) | 0.30 (0.03-3.39) |
| Tertile 2 | 0.92 (0.72-1.17) | 0.93 (0.73-1.18) | 0.95 (0.77-1.18) |
| Tertile 3 | 0.97 (0.93-1.01) | 0.97 (0.93-1.02) | 0.97 (0.92-1.01) |
| P for interaction | <0.05 | <0.05 | <0.05 |
| Time below range (<70 mg/dL) | |||
| Tertile 1 | 0.98 (0.90-1.07) | 0.99 (0.92-1.06) | 0.99 (0.94-1.05) |
| Tertile 2 | 0.98 (0.92-1.04) | 0.99 (0.93-1.05) | 0.99 (0.94-1.05) |
| Tertile 3 | 0.98 (0.96-1.01) | 0.98 (0.96-1.01) | 0.98 (0.96-1.01) |
| P for interaction | >0.50 | >0.50 | >0.25 |
Model 1 adjusted for age and sex; Model 2 further adjusted for diabetes duration, smoking, body mass index, systolic blood pressure, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, history of cancer, and history of cardiovascular diseases; Model 3 adjusted for the covariates in Model 2 plus the use of aspirin, statins, oral hypoglycemic agents, insulin, and antihypertensive drugs.
Abbreviations: CGM, continuous glucose monitoring; CV, coefficient of variation; HR, hazard ratio.
aCalculated for each 10% decrease in TIR, 10% increase in time above range and 1% increase in time below range.
Concerning the effect of glucose CV on morality risk, a significant association of glucose CV with all-cause mortality was only observed in individuals with HbA1c 6.0%-6.9%. Specially, relative to patients with the lowest tertile of glucose CV, the risk of mortality was increased by 15% (HR 1.15, 95% CI 0.63-2.11) and 89% (HR 1.89, 95% CI 1.06-3.35) in the middle and highest tertiles of glucose CV (P for trend = 0.027, Model 3) (Table 4).
Table 4.
Hazard ratios of all-cause mortality according to tertiles of glucose CV among patients with type 2 diabetes by total samples and across HbA1c categories
| HbA1c | Glucose CV | P for trend | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| Total | ||||
| Model 1 | 1 (reference) | 1.00 (0.83-1.19) | 1.20 (1.01-1.41) | 0.141 |
| Model 2 | 1 (reference) | 0.99 (0.83-1.19) | 1.17 (0.99-1.39) | 0.229 |
| Model 3 | 1 (reference) | 0.94 (0.78-1.13) | 1.05 (0.88-1.25) | 0.761 |
| <6.0% | ||||
| Model 1 | 1 (reference) | 1.29 (0.57-2.93) | 1.34 (0.53-3.37) | 0.489 |
| Model 2 | 1 (reference) | 1.11 (0.47-2.59) | 1.52 (0.58-3.99) | 0.425 |
| Model 3 | 1 (reference) | 1.40 (0.54-3.66) | 1.87 (0.68-5.18) | 0.222 |
| 6.0%-6.9% | ||||
| Model 1 | 1 (reference) | 1.40 (0.77-2.52) | 2.27 (1.31-3.93) | 0.003 |
| Model 2 | 1 (reference) | 1.35 (0.75-2.45) | 2.25 (1.29-3.93) | 0.004 |
| Model 3 | 1 (reference) | 1.15 (0.63-2.11) | 1.89 (1.06-3.35) | 0.027 |
| 7.0%-7.9% | ||||
| Model 1 | 1 (reference) | 0.71 (0.45-1.11) | 1.22 (0.82-1.84) | 0.343 |
| Model 2 | 1 (reference) | 0.68 (0.43-1.08) | 1.20 (0.78-1.83) | 0.419 |
| Model 3 | 1 (reference) | 0.67 (0.42-1.06) | 1.13 (0.73-1.74) | 0.572 |
| ≥8.0% | ||||
| Model 1 | 1 (reference) | 0.95 (0.77-1.18) | 0.98 (0.80-1.20) | 0.907 |
| Model 2 | 1 (reference) | 0.95 (0.77-1.18) | 0.96 (0.78-1.19) | 0.755 |
| Model 3 | 1 (reference) | 0.94 (0.76-1.17) | 0.93 (0.75-1.15) | 0.501 |
Model 1 adjusted for age and sex; Model 2 further adjusted for diabetes duration, smoking, body mass index, systolic blood pressure, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, history of cancer, and history of cardiovascular diseases; Model 3 adjusted for covariates in Model 2 plus the use of aspirin, statins, insulin, oral hypoglycemic agents, and antihypertensive drugs.
Abbreviations: CV, coefficient of variation; HbA1c, glycated hemoglobin A1c.
Discussion
In a large cohort of inpatients with type 2 diabetes, we explored the impact of HbA1c on all-cause mortality across varying degrees of GV, as measured by glucose CV through CGM. We found that the association of HbA1c with all-cause mortality seemed to be weakened in individuals with the highest tertile of glucose CV, suggesting that patients with a high degree of GV may not benefit from the measurement of HbA1c as much as others.
There is compelling evidence supporting the link between HbA1c and diabetes-related outcomes (15-18). Nevertheless, given that HbA1c reflects the mean glucose over the previous 2 to 3 months, a patient with an HbA1c “under control” could have concomitant uncontrolled hyperglycemia and hypoglycemia. The former is clearly detrimental to micro- and macrovasculatures (19,20), while the latter is related to the quality of life (21), dementia (22), CVD events (23), and mortality (24). Therefore, although HbA1c is undoubtedly a valid marker for diabetic complications in the general population with diabetes, it may not adequately address the burden of adverse outcomes in certain patient groups. In support of this notion, some clinical trials targeting HbA1c to specific goals, including ACCORD (25), ADVANCE (18), and VADT (26), did not show improved CVD end points in patients with advanced type 2 diabetes. Of note, no previous studies have investigated whether GV modifies the relationship between HbA1c and clinical outcomes among patients with type 2 diabetes. Our study demonstrated a weaker association of HbA1c with all-cause mortality in patients with relatively high levels of glucose fluctuations than in those with stable glucose values, suggesting that other glucose metrics in addition to HbA1c are needed for better diabetes care.
To date, CGM has been demonstrated to be effective in glucose management in patients with both type 1 and type 2 diabetes (27-29). Unfortunately, due to the high cost of CGM, it is used only in a small portion of patients with diabetes, especially in those with type 1 diabetes who have been struggling to achieve a specific HbA1c. Findings of our study implied that, apart from a useful tool for health education and guiding therapy changes, CGM could be implemented to assess GV and therefore to evaluate the suitability of HbA1c in diabetes management in certain subjects. Furthermore, we found consistent associations of TIR and TARs with all-cause mortality in patients with a high degree of GV, suggesting that CGM may provide additional information over HbA1c in predicting health outcomes in this subset of diabetic patients.
The results of the present study need to be interpreted within the context of several limitations. First, 3 days of CGM were used in the study, while 2 to 4 weeks of monitoring may be needed to robustly assess the actual GV (30,31). In addition, all patients received standard diets during CGM. Therefore, the glucose profiles obtained in this study may not represent the patients’ quality of glucose control in the real life, and the CGM parameters, including CV, should not be directly compared with other studies. However, this proof-of-concept study may provide insights into the potential caveats of HbA1c in predicting diabetes-related outcomes. Second, the data on socioeconomic and lifestyle were not available in the present study, and residual confounding was almost inevitable. Third, data on HbA1c was based on 1 measurement (ie, baseline), which was less precise than taking average HbA1c values during the study follow-up, and the investigation on the effect of visit-to-visit variability of HbA1c on mortality risk was precluded. Fourth, the data on the smoking status and history of CVD and cancer were collected by self-report, which can lead to potential misclassification bias. Since misclassification tends to bias the results to the null, our study may have underestimated the association between glucose CV and mortality. Finally, the subjects who enrolled in the current study were inpatients with type 2 diabetes admitted to a single center. To what degree these results could be generalized to other populations is unclear.
In conclusion, the relationship between HbA1c and all-cause mortality seemed to be blunted in diabetic patients with a relatively high degree of GV, suggesting that new markers of glycemia may be needed in this subset of patients with diabetes for better diabetes management.
Acknowledgments
We would like to thank all the involved clinicians, nurses, and technicians in the Shanghai Clinical Center for Diabetes for dedicating their time and skill to the completion of this study.
Author Contributions: J.Z., G.H., and T.X. conceived and designed the study. J.L., C.W., J.C., and Y.S. contributed to data collection, data analysis, and writing the manuscript. C.W., L.C., L.Z., J.C., W.L., and W.Z. contributed to data collection and analysis. W.L. and W.Z. contributed to conduction of study and data collection. T.X. and J.Z. contributed to interpretation of data and revision of the manuscript. G.H. contributed to revision of the manuscript. J.Z. and T.X. are the guarantors of this work and, as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Support: This work was funded by the National Key R&D Program of China (2018YFC2001004), the National Natural Science Foundation of China (31971485), the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20161430), the Shanghai Municipal Project for Academic Leaders Public Health (GWV-10.2-XD20), and Shanghai Municipal Key Clinical Specialty.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding authors will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S66-S76. [DOI] [PubMed] [Google Scholar]
- 3. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jia W, Weng J, Zhu D, et al. ; Chinese Diabetes Society . Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. [DOI] [PubMed] [Google Scholar]
- 5. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349-1354. [DOI] [PubMed] [Google Scholar]
- 7. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681-1687. [DOI] [PubMed] [Google Scholar]
- 8. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795-2804. [DOI] [PubMed] [Google Scholar]
- 9. Horváth EM, Benko R, Kiss L, et al. Rapid “glycaemic swings” induce nitrosative stress, activate poly(ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia. 2009;52(5):952-961. [DOI] [PubMed] [Google Scholar]
- 10. Picconi F, Parravano M, Ylli D, et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: the role of glycemic variability. Acta Diabetol. 2017;54(5):489-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Šoupal J, Škrha J Jr, Fajmon M, et al. Glycemic variability is higher in type 1 diabetes patients with microvascular complications irrespective of glycemic control. Diabetes Technol Ther. 2014;16(4):198-203. [DOI] [PubMed] [Google Scholar]
- 12. Wang X, Zhao X, Dorje T, Yan H, Qian J, Ge J. Glycemic variability predicts cardiovascular complications in acute myocardial infarction patients with type 2 diabetes mellitus. Int J Cardiol. 2014;172(2):498-500. [DOI] [PubMed] [Google Scholar]
- 13. Lu J, Wang C, Shen Y, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2021;44(2):549-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370-2376. [DOI] [PubMed] [Google Scholar]
- 15. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968-983. [PubMed] [Google Scholar]
- 16. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. [PubMed] [Google Scholar]
- 17. Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7(7):e015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572. [DOI] [PubMed] [Google Scholar]
- 19. Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108(12):1527-1532. [DOI] [PubMed] [Google Scholar]
- 20. Stehouwer CDA. Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes. 2018;67(9):1729-1741. [DOI] [PubMed] [Google Scholar]
- 21. McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Self-report of hypoglycemia and health-related quality of life in patients with type 1 and type 2 diabetes. Endocr Pract. 2013;19(5):792-799. [DOI] [PubMed] [Google Scholar]
- 22. Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301(15):1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. [DOI] [PubMed] [Google Scholar]
- 24. Zoungas S, Patel A, Chalmers J, et al. ; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410-1418. [DOI] [PubMed] [Google Scholar]
- 25. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duckworth W, Abraira C, Moritz T, et al. ; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. [DOI] [PubMed] [Google Scholar]
- 27. Battelino T, Conget I, Olsen B, et al. ; SWITCH Study Group . The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254-2263. [DOI] [PubMed] [Google Scholar]
- 29. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of flash glucose-sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther. 2017;8(3):573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herrero P, Alalitei A, Reddy M, Georgiou P, Oliver N. Robust determination of the optimal continuous glucose monitoring length of intervention to evaluate long-term glycemic control. Diabetes Technol Ther. 2021;23(4):314-319. [DOI] [PubMed] [Google Scholar]
- 31. Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20(4):314-316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding authors will on request detail the restrictions and any conditions under which access to some data may be provided.