Abstract
Context
Weight gain during adulthood increases cardiometabolic disease risk, possibly through adipocyte hypertrophy.
Objective
We aimed to study the specific metabolomic profile of adult weight gain, and to examine its association with adipocyte volume.
Methods
Nuclear magnetic resonance–based metabolomics were measured in the Netherlands Epidemiology of Obesity (NEO) study (n = 6347, discovery) and Oxford Biobank (n = 6317, replication). Adult weight gain was calculated as the absolute difference between body mass index (BMI) at middle age and recalled BMI at age 20 years. We performed linear regression analyses with both exposures BMI at age 20 years and weight gain, and separately with BMI at middle age in relation to 149 serum metabolomic measures, adjusted for age, sex, and multiple testing. Additionally, subcutaneous abdominal adipocyte biopsies were collected in a subset of the Oxford Biobank (n = 114) to estimate adipocyte volume.
Results
Mean (SD) weight gain was 4.5 (3.7) kg/m2 in the NEO study and 3.6 (3.7) kg/m2 in the Oxford Biobank. Weight gain, and not BMI at age 20 nor middle age, was associated with concentrations of 7 metabolomic measures after successful replication, which included polyunsaturated fatty acids, small to medium low-density lipoproteins, and total intermediate-density lipoprotein. One SD weight gain was associated with 386 μm3 (95% CI, 143-629) higher median adipocyte volume. Adipocyte volume was associated with lipoprotein particles specific for adult weight gain.
Conclusion
Adult weight gain is associated with specific metabolomic alterations of which the higher lipoprotein concentrations were likely contributed by larger adipocyte volumes, presumably linking weight gain to cardiometabolic disease.
Keywords: weight gain, body mass index, metabolomics, metabolic profile, cohort study, adipocyte volume
It is well established that weight gain during adulthood, as a result of an increase in body fat mass, is associated with a higher risk of type 2 diabetes, coronary artery disease, and (all-cause) mortality (1-3). Although there is a constant turnover of adipocytes throughout life, adipocyte number remains fixed during adulthood, and therefore the expansion of adipose tissue (AT) in response to weight gain in adults is due to an increase in adipocyte volume (4, 5). Previous studies have shown that adipocyte expansion, also known as adipocyte hypertrophy, is associated with increased systemic insulin resistance, and thereby a worsening metabolic profile (6-8).
According to the “lipid overflow” or “AT expandability” hypothesis, AT becomes dysfunctional when the capacity of hypertrophic adipocytes to expand is exceeded (9, 10). This in turn, leads to “lipid overflow” and the accumulation of triglycerides in visceral AT and ectopic fat deposition in normally lean organs such as the heart, skeletal muscles, pancreas, and liver (9-11). Compared to subcutaneous adipocytes, adipocytes in the visceral depot have a high secretion rate of nonesterified fatty acids, very low-density lipoproteins (VLDL), and cytokines, such as interleukin-6 and tumor necrosis factor-α, thereby inducing a systemic low-grade inflammatory state and oxidative stress (11-14). Finally, intracellular nonesterified fatty acid accumulation in non-ATs leads to impaired insulin signaling and insulin resistance (15).
We previously demonstrated in the Netherlands Epidemiology of Obesity (NEO) cohort that middle-aged individuals who gained body weight during adulthood had relatively more visceral fat and liver fat than weight-stable individuals (16). Additionally, participants with adult-onset weight gain were more insulin resistant, which was partly mediated by fat deposition in the visceral area and in the liver (17). Furthermore, body mass index (BMI) in adulthood has been associated with higher circulating very-low density lipoprotein, monounsaturated fatty acids, saturated fatty acids, and branched-chain amino acid levels, as well as lower plasma large high-density lipoprotein concentrations (18, 19). While studies have described the metabolic changes after short-term weight loss interventions (20, 21), the metabolomic profile associated with long-term adult weight gain has not been defined, but can produce detailed novel insights linking body weight at different stages over the life course, adult weight gain, and the development of cardiometabolic disease.
Here, we aimed to study the concentrations of metabolomic measures at middle age that were specifically associated with adult weight gain, as opposed to those associated with BMI at age 20 or BMI at middle age, in the NEO study (discovery) (22) and to replicate these findings in the Oxford Biobank (OBB) (23). Additionally, we aimed to examine the relation between adult weight gain and its specific metabolomic measures with abdominal adipocyte volume in a subpopulation of the OBB.
Materials and Methods
Study Design and Study Population
Netherlands Epidemiology of Obesity Study (discovery cohort)
The NEO study is a population-based cohort study of 6671 individuals aged 45 to 65 years, with an oversampling of individuals with BMI greater than or equal to 27, living in the greater area of Leiden (in the West of the Netherlands). All inhabitants aged between 45 and 65 years from one municipality (Leiderdorp) were invited to participate irrespective of their BMI, allowing for a reference distribution of BMI. The study design and population are described in detail elsewhere (22) and in the supplementary methods (24). The medical ethical committee of the Leiden University Medical Center approved the NEO study. All participants provided written informed consent.
Oxford Biobank (replication cohort) and abdominal adipose tissue biopsies
The OBB is a population-based cohort study of randomly selected healthy men and women living in Oxfordshire, UK. The study includes 7185 individuals aged 29 to 56 years. The exclusion criteria for the OBB were history of myocardial infarction, diabetes mellitus type 1 or 2, heart failure, untreated malignancy, other ongoing systemic diseases, or ongoing pregnancy. Study recruitment criteria and population characteristics are described in detail elsewhere (23) and in the supplementary methods (24). The OBB protocol is approved by the Oxfordshire Clinical Research Ethics Committee and all participants have provided informed consent.
In a subset of 114 participants in the OBB with data on recalled body weight at age 20 and metabolomic measures, subcutaneous abdominal AT biopsies were performed originally for purposes other than described in the present study, and were collected after the baseline assessment. All participants within the subset were recalled based on their genotype (25-27) and were matched for sex, age, and BMI. The subset and the subcutaneous abdominal AT biopsies, which were used to calculate adipocyte volume and adipocyte weight (28), are described in more detail in the supplementary methods (24).
Weight Change During Adulthood
In the NEO study, height without shoes was measured with a vertically fixed, calibrated tape measure. Body weight was measured and percentage of body fat was estimated by the Tanita bio impedance balance (TBF-310, Tanita International Division) without shoes, and 1 kg was subtracted to correct for weight of clothing. BMI at baseline was calculated by dividing the weight in kilograms by the height in meters squared.
Recalled body weight at age 20 years was based on self-report. The general questionnaire included the question “How much did you weigh (approximately) when you were 20 years old?” BMI at age 20 years was calculated by dividing body weight at age 20 in kilograms by the height in meters squared at middle age with the assumption that height did not majorly change during adulthood. Weight gain was calculated by subtracting recalled BMI at age 20 years from BMI at middle age. As a sensitivity analysis, we also calculated relative weight gain as (body weight at middle age [kg] – body weight at age 20 years [kg])/body weight at age 20 years [kg] * 100.
In the OBB, height and body weight were measured at study inclusion. Information on recalled body weight at age 20 was obtained using questionnaires, similar as in the NEO study, which was used to calculate BMI at age 20 years and adult weight gain.
Metabolomic Measures in the Netherlands Epidemiology of Obesity Study and the Oxford Biobank
Both in the NEO study and the OBB, a high-throughput proton nuclear magnetic resonance (NMR) metabolomics platform (29) (Nightingale Health Ltd) was used to quantify 149 lipid and metabolomic measures in blood plasma samples. Details of the experimentation and applications of the NMR metabolomics platform have been described previously (29), as well as coefficients of variation for the metabolomic measures (30), and can be found in the supplementary methods (24).
Statistical Analyses
In the NEO study, individuals with a BMI of 27 or higher are oversampled. To correctly represent associations for the general population, adjustments for the oversampling of participants with a BMI greater than or equal to 27 were made. This was achieved by weighting individuals toward the BMI distribution of participants from the Leiderdorp municipality (31), whose BMI distribution was similar to the BMI distribution of the general Dutch population (32). Consequently, the results from all analyses apply to a population-based study without oversampling of individuals with a high BMI. Baseline characteristics of the NEO study (discovery cohort) and the OBB (replication cohort) are presented as mean (SD), median (interquartile range), or proportion (%). We calculated Pearson correlations coefficients between adult weight gain, BMI at age 20, and BMI at middle age.
For the analyses in the discovery cohort using the metabolomic measures as outcome, we used a hypothesis-free approach. To correct for multiple testing, an α that has been corrected for the number of independent tests/metabolomic measures was used, obtained by considering the correlation matrix between the NMR metabolomic measures (33). In the present study, 37 out of the 149 metabolic measures were independent and therefore the α value was corrected by dividing 0.05 by 37 (α = 1.34 × 10–3). We used multivariable-adjusted linear regression models to examine the associations of adult weight gain with all NMR metabolomic measures adjusted for confounders. All concentrations were standardized to a mean of 0 and SD of 1. Linear regression modeling was performed with adult weight gain and BMI at age 20 years as exposure variables and the metabolomic measures as outcome variable, adjusted for sex and age. Adult weight gain and BMI at age 20 years were included in the same model, as adult weight gain since age 20 years is dependent on initial BMI at age 20 years. A second model was additionally adjusted for the considered confounders use of glucose-lowering medication, including both oral medication and insulin, and statins and fibrates. Other covariates (eg, food intake, physical activity) were considered as possible mediators and therefore not included. In addition, we performed linear regression analyses with the exposure BMI at middle age and the levels of metabolomic measures as the outcome, adjusted for sex and age. Finally, we repeated the linear regression analyses of adult weight gain and metabolomic measures separately for men and women, because we expected there could be differences in the concentrations of metabolomic measures between men and women based on the previous literature (34). In addition, we used relative adult weight gain (percentage) as exposure.
Based on these analyses, we selected the metabolomic measures specific for adult weight gain on the basis of statistical significance. First, we examined which metabolomic measures were only statistically significantly associated with adult weight gain, and not with BMI at age 20 or BMI at middle age. Similarly, we selected metabolomic measures specific for BMI at age 20 years or BMI at middle age. The remaining metabolomic measures showed overlap in their associations with adult weight gain, BMI at age 20 years, and BMI at middle age.
In the OBB, as our replication cohort, we performed the same linear regression analyses as in the NEO study, with adult weight gain and BMI at age 20 years as the exposures and NMR-based metabolomic measures as the outcome, adjusted for sex and age. In addition, we performed linear regression analyses with BMI at middle age as exposure and all NMR-based metabolomic measures as the outcome, adjusted for sex and age. For this analysis, we included all metabolomic measures that were associated with BMI at middle age, BMI at age 20 years or adult weight gain in the NEO cohort after considering multiple testing. We performed the replication analyses using a hypothesis-testing approach (P < .05). From the results in the OBB, we determined whether metabolomic measures were specifically associated with BMI at middle age, BMI at age 20 years, or adult weight gain. Metabolomic measures of particular interest to this study were those that were associated only with adult weight gain and not with BMI at age 20 years or BMI at middle age.
In the OBB subset with data on weight gain and abdominal adipocyte volume, linear regression was used to examine the association between BMI at age 20, adult weight gain, and BMI at middle age as exposures, and the abdominal adipocyte volume as outcome. Adult weight gain and BMI at age 20 years were included in the same model. The exposures were standardized to a mean of 0 and SD of 1 to allow comparisons. Similarly, we analyzed the relationship between adipocyte volume (exposure) and the metabolomic measures specific for adult weight gain.
Analyses were performed using Stata 14 (StataCorp LP).
Results
Characteristics of the Study Populations
We included 6347 individuals from the NEO study and 6317 individuals from the OBB in our analyses (Table 1). Both cohorts comprised a similar proportion of men (43%) and women. Mean recalled body weight at age 20 years was likewise similar (NEO: 65.7 kg [SD 11.3], OBB: 66.2 kg [SD 12.8]). Participants of the OBB were younger (mean age 42 years; range, 29-56 years) than those in NEO (mean 56 years; range, 45-65 years) at inclusion. Despite the difference in mean age between the cohorts, the annual absolute adult weight gain was similar in both cohorts (0.13 [SD 0.11] kg/year in the NEO study and 0.16 [SD 0.19] kg/year in the OBB).
Table 1.
Characteristics of the study populations: the Netherlands Epidemiology of Obesity Study (NEO, discovery cohort) and the Oxford Biobank (OBB, replication cohort)
| Characteristic | NEO (N = 6347) | OBB (N = 6317) |
|---|---|---|
| Sex, % men | 43 | 43 |
| At age 20 y | ||
| Body weight, kg | 65.7 (11.3) | 66.2 (12.8) |
| BMI | 21.9 (2.7) | 22.5 (3.4) |
| At middle age | ||
| Age, range, y | 56 (45-65) | 42 (29-56) |
| Time between age 20 and middle age, y | 36 (31-41) | 22 (17-26) |
| Body weight, kg | 79.1 (15.9) | 76.1 (15.9) |
| Height, m | 1.73 (0.1) | 1.71 (0.1) |
| BMI | 26.3 (4.5) | 25.8 (4.5) |
| Waist circumference, cm | 92.1 (13.4) | 86.7 (12.8) |
| Relative weight gain, % | 20.9 (17.5) | 15.7 (16.5) |
| Absolute weight gain, kg | 13.4 (11.1) | 9.9 (10.7) |
| Absolute weight gain, kg/m2 | 4.5 (3.7) | 3.6 (3.7) |
| Annual weight gain, kg/m2 | 0.13 (0.11) | 0.16 (0.19) |
| Fasting glucose, mmol/L | 5.3 (5.0-5.7) | 5.2 (4.9-5.5) |
| HOMA-IR | 1.9 (1.2-2.9) | 2.7 (2.0-3.6) |
| Triglycerides, mmol/L | 1.0 (0.7-1.5) | 0.9 (0.7-1.3) |
| HDL-C, mmol/L | 1.6 (0.5) | 1.4 (0.4) |
| LDL-C, mmol/L | 3.5 (1.0) | 3.3 (0.9) |
| Total cholesterol, mmol/L | 5.7 (1.1) | 5.2 (1.0) |
| Glucose-lowering medication, %a | 5.1 | 0 |
| Lipid-lowering medication, %b | 15.4 | 0 |
Data are presented as mean (SD or range), median (25th–75th percentile) or percentage. Results in the NEO study were based on analyses weighted toward the BMI distribution of the general population (N = 6347).
Abbreviations: BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
a Use of glucose-lowering medication included oral medication and insulin.
b Use of lipid-lowering medication included fibrates and statins.
Adult weight gain was positively correlated with BMI at middle age (0.78 in the NEO study, and 0.67 in the OBB), but not with BMI at age 20 (correlation coefficient –0.17 in NEO and –0.18 in the OBB). BMI at middle age was correlated with BMI at age 20 by 0.49 in the NEO study and 0.61 in the OBB.
Metabolomic Measures in the Netherlands Epidemiology of Obesity Study and Replication in the Oxford Biobank
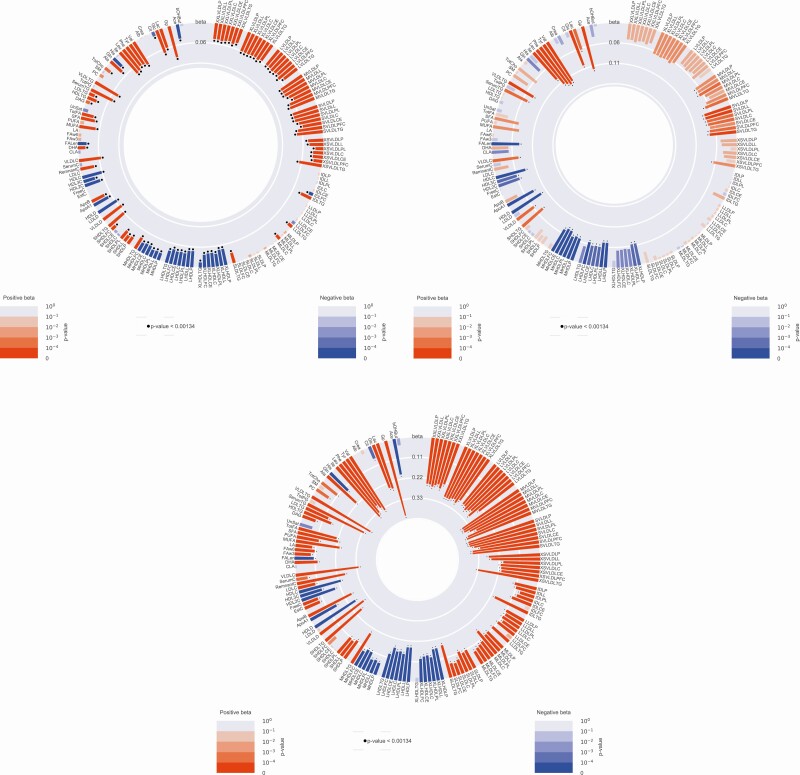
The circle plots presented in Fig. 1 show the associations between adult weight gain, BMI at age 20 years, and BMI at middle age with the metabolomic measures in the NEO study. In the model (age and sex adjusted) including BMI at age 20 years and adult weight gain simultaneously, absolute adult weight gain was associated with concentrations of 111 metabolomic measures at middle age. Furthermore, in the same model, BMI at age 20 years was associated with the concentrations of 48 metabolomic measures. BMI at middle age was associated with the levels of 105 metabolomic measures. Of the 111 metabolomic measures linked with adult weight gain during adulthood, 7 were not associated with BMI at either age 20 years or middle age and therefore considered as specific for adult weight gain, 47 were shared with metabolomic measures associated both with BMI at age 20 and BMI at middle age, and 53 were common with metabolomic measures associated with BMI at middle age (Supplementary Table 1) (24).
Figure 1.
The circle plots show the associations between body mass index (BMI) at age 20 years (upper left), BMI at middle age (down left), adult weight gain (right) and the individual metabolomic measures in the Netherlands Epidemiology of Obesity study. The metabolomic measures are indicated at the outer circle. A red bar indicates a positive association, whereas a blue bar indicates a negative association. The color intensity and height of the bar indicate the strength of the association; a black dot above a bar indicates statistical significance (P < .00134).
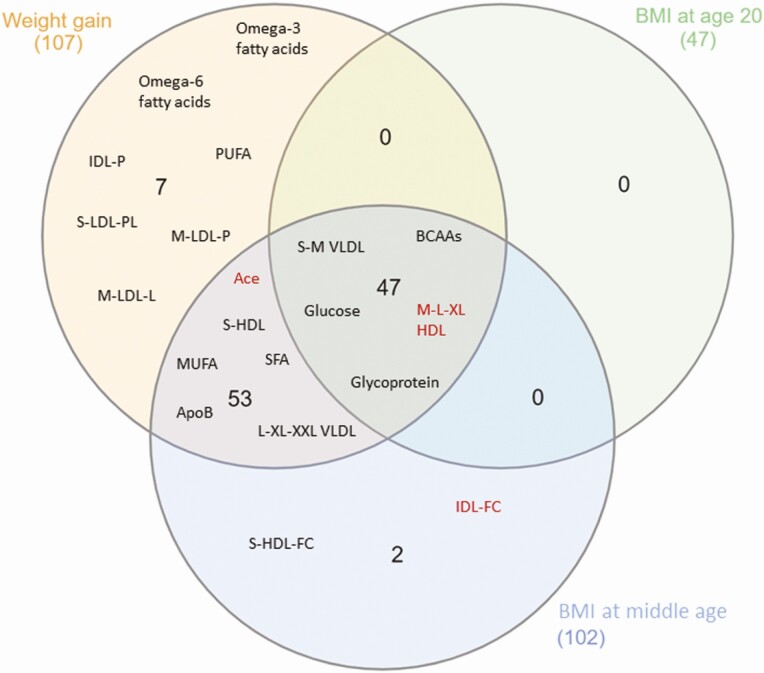
Of the 111 metabolomic measures that showed a significant association with adult weight gain in the NEO study, 107 (96%) were successfully replicated in the OBB. The Venn diagram (Fig. 2; detailed summary statistics data of OBB can be found in Supplementary Table 2 [24]) shows the overlap in the associations between adult weight gain, BMI at age 20 and BMI at middle age, and the 107 metabolomic measures replicated in the OBB (35).
Figure 2.
Venn diagram showing the overlap in the associations between adult weight gain, body mass index (BMI) at age 20 years and/or BMI at middle age, and the metabolomic measures after successful replication in the Oxford Biobank. Names in black indicate a positive association with this metabolomic measures, names in red indicate a negative association. Owing to the large number of included metabolomic measures, and for illustrative purposes, we listed the classes of the associated metabolomic measures instead of exact names. Adult weight gain and BMI at middle age both were associated with 52 metabolomic measures, whereas weight gain was specifically associated with 7 metabolomic measures. Forty-seven metabolomic measures showed overlapping associations with all 3 exposures.
Adult weight gain was specifically associated with the concentration of 7 metabolomic measures in the NEO study, which included omega-3 (0.02 SD per 1 kg/m2 weight gain [P = 5.6 × 10–5] and omega-6 fatty acids (0.02 SD [P = 6.5 × 10–4]), subparticles related to small to medium LDLs (eg, phospholipids in small LDL: 0.02 SD [P = 1.4 × 10–4]), and total intermediate-density lipoprotein (IDL; 0.01 [P = 6.5 × 10–4]), and were all successfully replicated in the OBB. In addition, none of the investigated metabolomic measures were associated only with BMI at age 20 years, and only 2 metabolomic measures were associated with the measured BMI at middle age (notably free cholesterol in small high-density lipoprotein (HDL) particles and free cholesterol in IDL particles).
To visualize consistency between the 2 data sets, we also plotted the regression coefficients (in SD per 1 kg/m2 gain in BMI) of the associations between adult weight gain and metabolomic measures in the NEO study and the OBB (Fig. 3). For both populations, we observed negative associations between weight gain and HDL (green dots), whereas positive associations were detected for LDL and VLDL particles (pink dots), as well as for most amino acids (red dots), triglycerides (light pink), and fatty acids (brown dots).
Figure 3.
Correlation plot between linear regression coefficients in the Netherlands Epidemiology of Obesity (NEO) study and the Oxford Biobank (OBB) of the association between weight gain and the metabolomic measures (in SD per 1 kg/m2 increase in body mass index [BMI]). The different colors of the dots indicate different subclasses of metabolomic measures.
Results were similar after additional adjustment for use of medication (Supplementary Table 3) (24). Absolute adult weight gain was associated with similar changes in concentrations of metabolomic measures both in men and women (Supplementary Table 4) (24). Results were also similar whether we used relative or absolute weight gain as exposures in the analyses (Supplementary Table 5) (24).
Cell Study in Subcutaneous Abdominal Adipocytes in a Subset of the Oxford Biobank
Associations between adult weight gain and abdominal adipocyte volume
Of the 114 participants with data on weight gain and abdominal AT histology, 50 (44%) were men and the mean age and BMI at tissue biopsy were 46 years (SD 6.6) and 26.2 (3.8). Mean adult weight gain was 3.8 kg/m2 (3.5), while median adipocyte volume was 3782 μm3 (2941-4450 μm3).
After adjustment for sex and age at biopsy, a 1-SD increase in adult weight gain was associated with 386 μm3 (95% CI, 143-629) larger median adipocyte volume, and a 1-SD increase in BMI at age 20 and BMI at middle age were associated with 240 μm3 (95% CI, 24-456) and 383 μm3 (95% CI, 160-605) increased adiposity volume, respectively (Table 2).
Table 2.
Associations between body mass index (BMI) at age 20 years, weight gain during adulthood and BMI at middle age, and abdominal adipose tissue cell volume in the Oxford Biobank (n = 114)
| Median AT cell volume, μm3 | |
|---|---|
| β (95% CI) | |
| Standardized BMI at age 20 y (SD)a | 240 (24-456) |
| Standardized adult weight gain (SD)b | 386 (143-629) |
| Standardized BMI at middle age (SD) | 383 (160-605) |
All analyses are adjusted for sex and age at adipose tissue biopsy. SD BMI at age 20: 2.7; SD adult weight gain: 3.5; SD BMI at middle age 3.8.
Abbreviation: AT, adipose tissue.
a Additionally adjusted for adult weight gain.
b Additionally adjusted for BMI at age 20 years.
Associations between abdominal adipocyte volume and adult weight gain-specific metabolomic measures
After adjustment for sex and age at biopsy, higher median cell volume was associated with higher circulating levels of phospholipids in small LDL (0.21 SD [0.05-0.37] per SD of adipocyte volume), total medium LDL particles (0.20 SD [0.02-0.37], and lipids in medium LDL (0.20 SD [0.02-0.37]) (see Table 3). In contrast, median cell volume was not associated with levels of either omega-3 or omega-6 fatty acids, or the sum thereof (ie, total polyunsaturated fatty acids).
Table 3.
Associations between adipocyte volume and 7 adult weight gain-specific metabolomic measures in the Oxford Biobank (n = 114)
| Omega-3 FA, SD | Omega-6 FA, SD | Polyunsaturated FA, SD | S-LDL-PL, SD | M-LDL-P, SD | IDL-P, SD | M-LDL-L, SD | |
|---|---|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Median cell volume, SD | 0.02 (–0.14 to 0.18) | 0.10 (–0.07 to 0.28) | 0.09 (–0.08 to 0.26) | 0.21 (0.05 to 0.38) | 0.20 (0.02 to 0.37) | 0.17 (–0.004 to 0.35) | 0.20 (0.02 to 0.37) |
The table shows the associations between standardized adipocyte volume as exposure, and 7 standardized adult weight gain–specific metabolomic measures as the outcomes, adjusted for sex and age.
Abbreviations: FA, fatty acids; IDL-P, total intermediate density lipoprotein; M-LDL-L, lipids in medium low-density lipoproteins; M-LDL-P, total medium low-density lipoproteins; S-LDL-PL, phospholipids in small low-density lipoproteins.
Discussion
The aim of this study was to investigate the metabolomic measures specifically associated with adult weight gain, because these metabolomic measures can provide insight into the mechanisms underlying the development of cardiometabolic disease as a consequence of adult weight gain.
Using data from 2 large cohorts used for discovery and replication, we showed that adult weight gain over a median period of 36 years was associated with 7 specific metabolomic measures, namely omega-3 fatty acids, omega-6 fatty acids, and their sum (polyunsaturated fatty acids), phospholipids within small LDL, total IDL particles, and total medium-sized LDL particles and lipids within these particles. The regression coefficients of the association between adult weight gain and the metabolomic measures in the NEO study and the OBB were similar in direction and size, which indicates robust replication of our findings in the OBB. While previous studies specifically focused on short-term changes in body weight, the analyses performed in the present study highlight specific biochemical disturbances associated with adult weight gain that could possibly link adult weight gain and the onset of cardiometabolic disease.
One mechanism thought to be responsible for the adverse cardiometabolic consequences of adult weight gain is adipocyte hypertrophy (4, 7, 8). We observed that adult weight gain was associated with larger adipocyte size at middle age irrespective of BMI at age 20 years. In addition, BMI at age 20 was associated with enlarged adipocytes at middle age as well, irrespective of adult weight gain, although this association was less strong than that of adult weight gain. BMI in adulthood is the aggregate of BMI at age 20, after growth and development during childhood and puberty have ceased, and subsequent weight gain during adulthood. Spalding et al observed that the number of adipocytes is set before adulthood; however, expansion of adipocyte number ends around age 16.5 years in individuals with obesity and 18.5 years in lean individuals (4). In line with our results, this suggests that weight gain during adolescence contributes to an increased adipocyte size as well, but to a lesser extent than weight gain during adulthood. As a result of hypertrophy, AT becomes dysfunctional, ultimately leading to insulin resistance and metabolic disturbances (6-8). Accordingly, we observed an association between adipocyte volume and 3 out of the 7 metabolomic measures that were specifically associated with adult weight gain, particularly phospholipids in small LDL, total medium LDL particles, and lipids in medium LDL. However, these metabolomic measures are highly correlated, as well as 3 other adult weight gain-specific metabolomic measures, omega-3 and omega-6 fatty acids and their sum (polyunsaturated fatty acids).
Overall, our findings are consistent with the results of previous longitudinal studies investigating the effects of body weight gain on the plasma metabolome (18, 36, 37). These studies identified increases in VLDL and LDL particles, monounsaturated and polyunsaturated fatty acids, saturated fatty acids, branched-chain amino acids, and products of glycolysis to be most strongly associated with weight gain during follow-up ranging from 6 to 9 years. In contrast, weight loss in older adults was associated with decreased circulating glycerol levels and increased HDL diameter (20). Additionally, a recent randomized clinical trial found a reduction in plasma branched-chain amino acids as a results of weight loss and showed that this was associated with decreased hepatic and intra-abdominal fat after the 2-year intervention (21).
Earlier studies have linked the metabolomic measures we found to be specifically associated with adult weight gain to cardiometabolic disease. LDL particles are a causal risk factor for cardiovascular disease identified by randomized controlled trials and mendelian randomization studies (38).
In observational studies, NMR-based measures of circulating LDL particles are strongly and consistently associated with the risk of cardiovascular disease in observational studies, also specifically small and medium LDL particles (39). On the other hand, in randomized controlled trials, the replacement of dietary saturated fatty acids or carbohydrates by polyunsaturated fatty acids resulted in lower levels of LDL cholesterol (40, 41). In the present study, we focused on circulating polyunsaturated fatty acids instead of dietary intake. The levels of polyunsaturated fatty acids in tissues and blood might not be an accurate biomarker of polyunsaturated fatty acid intake because they are affected by metabolic processes (41, 42).
In a large pooled analysis of individual data from prospective cohort studies (n = 45 637), higher concentrations of 3 circulating omega-3 fatty acids—α-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid—were associated with a lower risk of fatal coronary heart disease (43). In contrast, both intake of dietary omega-3 fatty acids and circulating levels of omega-3 fatty acids were not associated with risk of type 2 diabetes in a meta-analysis of longitudinal studies (44).
Omega-6 fatty acids mainly include linoleic acid. Higher circulating levels of linoleic acids were associated with a lower risk of cardiovascular disease across multiple prospective observational studies including a total of 68 659 individuals (45). In the same data set, higher levels of linoleic acid were associated with a lower risk of type 2 diabetes (46), which itself is an important risk factor for cardiovascular disease (47).
Strengths of our study included 2 large population-based studies, and robust replication of our study results in a younger cohort. A limitation that needs to be considered is the use of recalled body weight at age 20 years in both studies, which we used to calculate absolute adult weight gain. However, previous studies have shown that recalled body weight is strongly related with measured weight at the same age (48). By adjusting for age, we took into account the period between age 20 years and middle age, which differs between participants. Additionally, most of the participants of the NEO study and OBB were of White ethnicity. Therefore, the results of our study need to be confirmed in other ethnic groups. Another limitation is the lack of a measure of adipocyte hyperplasia, or the number of adipocytes, as both adipocyte hyperplasia and hypertrophy influence the amount of fat mass (4). However, previous research showed that after age 20 years adipocytes mainly respond by hypertrophy during weight gain (4). Our study has an observational cross-sectional design; however, our results are in line with longitudinal studies on adult weight gain and the metabolite profile (18, 36, 37). Another limitation is the use of the P value as an arbitrary cutoff to determine which metabolomic measures we considered being associated with our exposures in our discovery and replication cohort. Therefore, we might have missed metabolomic measures also associated with adult weight gain. Additionally, it is likely that metabolomic measures are shared between adult weight gain and BMI at middle age. And last, the present study used a rather limited metabolomics platform mostly containing lipoprotein (sub)particles. Investigating the metabolomic pathways altered by adult weight gain would require alternative platforms.
Our results indicate that adult weight gain was specifically and robustly associated with a higher concentration of 7 metabolomic measures, including some specific lipoproteins and polyunsaturated fatty acids. Although this should be further investigated in more detail in future studies, our observations may help explain how adult weight gain increases the risk of cardiometabolic disease.
Acknowledgments
We express our gratitude to all the individuals who participated in the Netherlands Epidemiology of Obesity study and Oxford Biobank. We are grateful for all participating general practitioners for inviting eligible participants. We furthermore thank P. R. van Beelen and all the research nurses for collecting the data, P. J. Noordijk and her team for sample handling and storage, and I. de Jonge, MSc, for data management of the NEO study.
Financial Support: The NEO study is supported by the participating departments, the division, and board of directors of the Leiden University Medical Center, and by the Leiden University Research Profile Area “Vascular and Regenerative Medicine.” We acknowledge the support from the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation (grant No. CVON2014-02 ENERGISE). The OBB is supported by the NIHR Oxford BRC Obesity and Lifestyle theme. F.K. is supported by the British Heart Foundation (grant No. RG/17/1/32663). C.C. is a BHF Intermediate clinical research fellow.
Glossary
Abbreviations
- AT
adipose tissue
- BMI
body mass index
- HDL
high-density lipoprotein
- IDL
intermediate-density lipoprotein
- LDL
low-density lipoprotein
- NEO
Netherlands Epidemiology of Obesity
- NMR
nuclear magnetic resonance
- OBB
Oxford Biobank
- PUFA
polyunsaturated fatty acid
- VLDL
very low-density lipoprotein
Additional Information
Disclosures: D.O.M.K. is a part-time research consultant at Metabolon, Inc. All other authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364(14):1315-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol. 2014;179(11):1353-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159(12):1150-1159. [DOI] [PubMed] [Google Scholar]
- 4. Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783-787. [DOI] [PubMed] [Google Scholar]
- 5. Salans LB, Horton ES, Sims EA. Experimental obesity in man: cellular character of the adipose tissue. J Clin Invest. 1971;50(5):1005-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLaughlin T, Lamendola C, Coghlan N, et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity. 2014;22(3):673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arner E, Westermark PO, Spalding KL, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59(1):105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50(3):625-633. [DOI] [PubMed] [Google Scholar]
- 9. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta. 2010;1801(3):338-349. [DOI] [PubMed] [Google Scholar]
- 10. Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359-404. [DOI] [PubMed] [Google Scholar]
- 11. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881-887. [DOI] [PubMed] [Google Scholar]
- 12. Bergman RN, Kim SP, Catalano KJ, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity. 2006;14(Suppl 1):16S-19S. [DOI] [PubMed] [Google Scholar]
- 13. Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity. 2006;14(Suppl 1):20S-24S. [DOI] [PubMed] [Google Scholar]
- 14. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840-846. [DOI] [PubMed] [Google Scholar]
- 15. Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45(9):1201-1210. [DOI] [PubMed] [Google Scholar]
- 16. Verkouter I, Noordam R, de Roos A, et al. Adult weight change in relation to visceral fat and liver fat at middle age: the Netherlands Epidemiology of Obesity Study. Int J Obes. 2019;43(4):790-799. [DOI] [PubMed] [Google Scholar]
- 17. Verkouter I, Noordam R, le Cessie S, et al. The association between adult weight gain and insulin resistance at middle age: mediation by visceral fat and liver fat. J Clin Med. 2019;8(10):1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Würtz P, Wang Q, Kangas AJ, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11(12):e1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rangel-Huerta OD, Pastor-Villaescusa B, Gil A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics. 2019;15(6):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beekman M, Schutte BAM, van den Akker EB, et al. Lifestyle-intervention-induced reduction of abdominal fat is reflected by a decreased circulating glycerol level and an increased HDL diameter. Mol Nutr Food Res. 2020;64(10):e1900818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Sun D, Zhou T, et al. Changes of branched-chain amino acids and ectopic fat in response to weight-loss diets: the POUNDS Lost trial. J Clin Endocrinol Metab. 2020;105(10):e3747-e3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Mutsert R, den Heijer M, Rabelink TJ, et al. The Netherlands Epidemiology of Obesity (NEO) study: study design and data collection. Eur J Epidemiol. 2013;28(6):513-523. [DOI] [PubMed] [Google Scholar]
- 23. Karpe F, Vasan SK, Humphreys SM, et al. Cohort profile: the Oxford Biobank. Int J Epidemiol. 2018;47(1):21-21g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verkouter I, Noordam R, Loh NY, et al. Supplementary material for The relation between adult weight gain, adipocyte volume and the metabolic profile at middle age. Uploaded May 3, 2021. https://figsharecom/account/articles/14528850 [DOI] [PMC free article] [PubMed]
- 25. Loh NY, Minchin JEN, Pinnick KE, et al. RSPO3 impacts body fat distribution and regulates adipose cell biology in vitro. Nat Commun. 2020;11(1):2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Small KS, Todorcevic M, Civelek M, et al. Regulatory variants at KLF14 influence type 2 diabetes risk via a female-specific effect on adipocyte size and body composition. Nat Genet. 2018;50(4):572-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verma M, Loh NY, Vasan SK, et al. TCF7L2 plays a complex role in human adipose progenitor biology which may contribute to genetic susceptibility to type 2 diabetes, bioRxiv. 2019:854661; posted November 26, 2019; preprint: not peer reviewed. [DOI] [PubMed] [Google Scholar]
- 28. Ashwell M, Priest P, Bondoux M, Sowter C, McPherson CK. Human fat cell sizing—a quick, simple method. J Lipid Res. 1976;17(2):190-192. [PubMed] [Google Scholar]
- 29. Soininen P, Kangas AJ, Würtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8(1):192-206. [DOI] [PubMed] [Google Scholar]
- 30. Kettunen J, Demirkan A, Würtz P, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016;7:11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81(9):1166-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ministerie van VWS. Hoeveel mensen hebben overgewicht?. 2013. www.rivm.nl/nldemaat. Accessed July 7, 2021. [Google Scholar]
- 33. Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95(3):221-227. [DOI] [PubMed] [Google Scholar]
- 34. Bell JA, Santos Ferreira DL, Fraser A, et al. Sex differences in systemic metabolites at four life stages: cohort study with repeated metabolomics. BMC Med. 2021;19(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics. 2015;16:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Menni C, Migaud M, Kastenmüller G, et al. Metabolomic profiling of long-term weight change: role of oxidative stress and urate levels in weight gain. Obesity. 2017;25(9):1618-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wahl S, Vogt S, Stuckler F, et al. Multi-omic signature of body weight change: results from a population-based cohort study. BMC Med. 2015;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holmes MV, Asselbergs FW, Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holmes MV, Millwood IY, Kartsonaki C, et al. ; China Kadoorie Biobank Collaborative Group . Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71(6):620-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katan MB, Zock PL, Mensink RP. Effects of fats and fatty acids on blood lipids in humans: an overview. Am J Clin Nutr. 1994;60(Suppl 6):1017S-1022S. [DOI] [PubMed] [Google Scholar]
- 41. Mensink RP. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. World Health Organization. 2016. Accessed May 3, 2021, https://wwwwhoint/nutrition/publications/nutrientrequirements/sfa_systematic_review/en/
- 42. Guan W, Steffen BT, Lemaitre RN, et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ Cardiovasc Genet. 2014;7(3):321-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Del Gobbo LC, Imamura F, Aslibekyan S, et al. ; for the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) . ω-3 Polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176(8):1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu JH, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107(Suppl 2):S214-S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marklund M, Wu JHY, Imamura F, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) . Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation. 2019;139(21):2422-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu JHY, Marklund M, Imamura F, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) . Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5(12):965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarwar N, Gao P, Seshasai SR, et al. ; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Casey VA, Dwyer JT, Berkey CS, Coleman KA, Gardner J, Valadian I. Long-term memory of body weight and past weight satisfaction: a longitudinal follow-up study. Am J Clin Nutr. 1991;53(6):1493-1498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.