Abstract
STUDY QUESTION
Which endometriosis classification, staging and reporting systems have been published and validated for use in clinical practice?
SUMMARY ANSWER
Of the 22 endometriosis classification, staging and reporting systems identified in this historical overview, only a few have been evaluated, in 46 studies, for the purpose for which they were developed.
WHAT IS KNOWN ALREADY
In the field of endometriosis, several classification, staging and reporting systems have been developed.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A systematic PUBMED literature search was performed. Data were extracted and summarized.
MAIN RESULTS AND THE ROLE OF CHANCE
Twenty-two endometriosis classification, staging and reporting systems have been published between 1973 and 2021, each developed for specific, and different, purposes. There still is no international agreement on how to describe the disease. Studies evaluating the different systems are summarized showing a discrepancy between the intended and the evaluated purpose, and a general lack of validation data confirming a correlation with pain symptoms or quality of life for any of the current systems. A few studies confirm the value of the ENZIAN system for surgical description of deep endometriosis. With regards to infertility, the endometriosis fertility index has been confirmed valid for its intended purpose.
LARGE SCALE DATA
NA.
LIMITATIONS, REASONS FOR CAUTION
The literature search was limited to PUBMED. Unpublished classification, staging or reporting systems, or those published in books were not considered.
WIDER IMPLICATIONS OF THE FINDINGS
It can be concluded that there is no international agreement on how to describe endometriosis or how to classify it, and that most classification/staging systems show no or very little correlation with patient outcomes. This overview of existing systems is a first step in working toward a universally accepted endometriosis classification.
STUDY FUNDING/COMPETING INTEREST(S)
The meetings and activities of the working group were funded by the American Association of Gynecologic Laparoscopists, European Society for Gynecological Endoscopy, European Society of Human Reproduction and Embryology and World Endometriosis Society. A.W.H. reports grant funding from the MRC, NIHR, CSO, Wellbeing of Women, Roche Diagnostics, Astra Zeneca, Ferring, Charles Wolfson Charitable Trust, Standard Life, Consultancy fees from Roche Diagnostics, AbbVie, Nordic Pharma and Ferring, outside the submitted work. In addition, A.W.H. has a patent Serum biomarker for endometriosis pending. N.P.J. reports personal fees from Abbott, Guerbet, Myovant Sciences, Vifor Pharma, Roche Diagnostics, outside the submitted work; he is also President of the World Endometriosis Society and chair of the trust board. S.M. reports grants and personal fees from AbbVie, and personal fees from Roche outside the submitted work. C.T. reports grants, non-financial support and other from Merck SA, non-financial support and other from Gedeon Richter, non-financial support from Ferring Pharmaceuticals, outside the submitted work and without private revenue. K.T.Z. reports grants from Bayer Healthcare, MDNA Life Sciences, Roche Diagnostics Inc, Volition Rx, outside the submitted work; she is also a Board member (Secretary) of the World Endometriosis Society and World Endometriosis Research Foundation, Research Advisory Board member of Wellbeing of Women, UK (research charity), and Chair, Research Directions Working Group, World Endometriosis Society. The other authors had nothing to disclose.
TRIAL REGISTRATION NUMBER
NA.
Keywords: endometriosis, infertility, classification, staging, reporting, surgery
What does this mean for patients?
Since 1973, clinicians have proposed classification systems for endometriosis, and so far 22 different systems have been developed. Some of these systems focus on symptoms, while others have been developed to document the surgical observations, or predict the outcomes after treatment. Ideally, classification systems are evaluated in a research project to confirm it is useful in clinical management. We found that of the 22 classification systems, few have been evaluated for the purpose for which they were developed. From this review, it can be concluded that there is no international agreement on how to describe endometriosis or how to classify it.
Introduction
Endometriosis is an inflammatory estrogen-dependent disease associated with chronic pelvic pain and/or infertility that is characterized by lesions of endometrial-like tissue outside of the uterus (Johnson et al., 2017). The disease is usually confined to the abdominal cavity but, rarely, extra-abdominal lesions have been detected in the lungs, brain and even in the eye. Within the pelvic cavity, the variety of presentations is extensive with lesions detected on the peritoneum, within the ovaries (endometrioma), around the uterus, but also affecting the urinary tract, bowel, and vagina. Most definitions, but not all, consider adenomyosis (similar lesions arising within the myometrium) as a separate disease (Zegers-Hochschild et al., 2017).
Traditionally, three phenotypes of endometriosis lesions are recognized; peritoneal, ovarian (endometrioma) and deep endometriosis (DE) (Working group of ESGE ESHRE and WES et al., 2020a,b; Working group of ESGE ESHRE and WES et al., 2017a,b). Symptoms include chronic pelvic pain (dysmenorrhea, acyclic pelvic pain, dyspareunia, dyschezia, dysuria) with severity ranging from mild to debilitating, infertility, and non-specific symptoms (fatigue), but endometriosis can also be asymptomatic (Zondervan et al., 2020). Treatment options for pain include different medical and hormonal treatments or surgery, while for infertility, surgery and/or ART have been used.
Since the first descriptions of endometriosis, this spectrum of lesions and symptoms has urged clinicians to attempt to classify the disease into informative subgroups or hierarchical stages. By definition, classification entails a systematic arrangement of similar entities on the basis of certain differing characteristics (Miller-Keane and O’Toole, 2005). When disease classification can be related to treatment outcomes or prognosis, the system is considered a staging system.
In the field of endometriosis, several classification, staging and reporting systems have been developed. The current paper provides, based on an assessment of published studies, a historical overview of these different systems. Validation studies and published reports on the implementation of the different classification, staging and reporting systems have been summarized to highlight the uptake, benefits and drawbacks of published systems for endometriosis.
Materials and methods
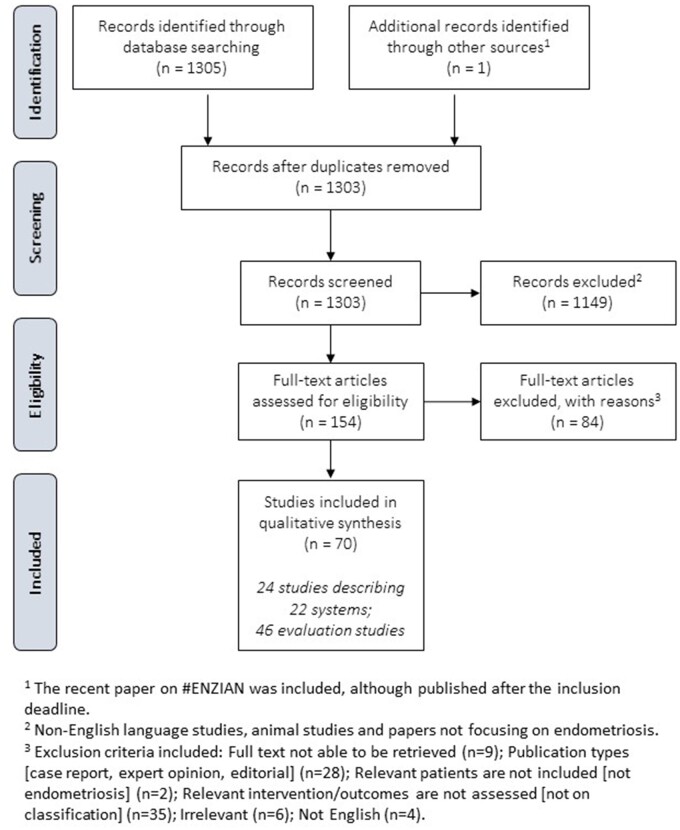
A literature review was performed collecting studies and reports focusing on ‘endometriosis’ and ‘classification, staging, or scoring’. PUBMED/MEDLINE was searched, and studies were included from inception (1966) up to 08 May 2020; all retrieved references were checked for relevance. Non-English language studies, animal studies and papers not focusing on endometriosis, including those focusing specifically on adenomyosis, were excluded from the retrieved references. Papers and classifications systems focusing on endometriosis but including adenomyosis were not excluded. For the remaining references, the full-text papers were collected and assessed. Inclusion criteria included original studies focusing on endometriosis and classification, staging or reporting systems. The results of the literature search are summarized in a PRISMA flowchart (Fig. 1). The details of the final set of papers are summarized in evidence tables. The draft paper was published for stakeholder review by all societies involved; 81 comments were tabulated in a review report and, where relevant, incorporated in the final version of the paper.
Figure 1.
PRISMA flow diagram for the selection of studies describing endometriosis classification, staging and reporting systems.
Results
The literature review retrieved 1305 references; one reference was added at a later stage. After applying the exclusion criteria, 154 full papers were assessed, of which 84 papers were excluded for the following reasons: full-text papers could not be retrieved (n = 9), not written in English (n = 4), inappropriate publication types (case report, expert opinion, editorial) (n = 28) and relevant patients and/or intervention/outcomes are not assessed (not endometriosis or not classification) (n = 43). Seventy papers were included for either describing a classification, staging or reporting system in endometriosis (n = 24) or evaluating one (n = 46) (Fig. 1). The systems in endometriosis described in this paper have been published as classification, staging or reporting systems, even though some were developed for stratification or subgrouping rather than classification.
Table I provides an overview of the 22 classification, staging or reporting systems identified in the literature and included in this report. The 46 studies reporting an evaluation of the different systems are listed in Table II.
Table I.
Historical overview of endometriosis classification/staging systems.
| Endometriosis classification/ staging system | Publication year | Classification/staging based on | Intended purpose |
Details | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis/pre-operativeassessment | Description | Staging | Treatment selection | Prediction of difficultyof surgery | Prediction of pain remediation/improved QoL | Prediction of conception | |||||
| #ENZIAN classification | 2021 | Surgical observation or imaging | √ | √ | Non-invasive and surgical description system for endometriosis | Keckstein et al. (2021) | |||||
| Adhesion scoring system | 2020 | US | √ | √ | Pre-operative prediction of the pelvic adhesion status | Ichikawa et al. (2020) | |||||
| ENDOGRAM | 2019 | Disease markers in biopsy sample | √ | Analysis of endometriotic tissues supporting therapeutic decisions | Bouquet de Joliniere et al. (2019) | ||||||
| ENDORECT | 2019 | Clinical examination, US, MRI | √ | Preoperative score to predict rectosigmoid involvement | Chattot et al. (2019) | ||||||
| Bowel Endometriosis Syndrome (BENS) score | 2017 | Symptoms | √ | Identify bowel endometriosis syndrome based on patient reported symptoms and QoL | Riiskjær et al. (2017) | ||||||
| Preoperative ultrasound-based endometriosis staging system (UBESS) | 2016 | US | √ | √ | Pre-operative staging and prediction of the level of complexity of laparoscopic surgery. | Menakaya et al. (2016) | |||||
| Classification of ureteral endometriosis | 2015 | Surgical observation | √ | √ | √ | Clinical classification of urinary tract endometriosis | Knabben et al. (2015) | ||||
| EPHect SSF—EPHect MSF (surgical form) | 2014 | Surgical observation | √ | Recording of surgical phenotypic information and related sample collections obtained at laparoscopy | Becker et al. (2014) | ||||||
| Clinical score | 2014 | Symptoms | √ | √ | Predict DE presence before endometrioma surgery | Lafay Pillet et al. (2014) | |||||
| LSD/MURO Classification | 2013 | Modified Virtual Colonoscopy | √ | Descriptive imaging classification, with implied severity for rectogenital and disseminated endometriosis | van der Wat et al. (2013) | ||||||
| ECO system | 2012 | Extent, symptoms and objectives | √ | Determine most appropriate management | Lasmar et al. (2012), Lasmar et al. (2015) | ||||||
| Deep endometriosis staging form | 2011 | US | √ | Staging system for DE based on ultrasonographic finding | Coccia and Rizzello (2011) | ||||||
| Endometriosis Fertility Index (EFI) | 2010 | Surgical observation + Patient parameters | √ | Prediction of (non-IVF) pregnancy after surgery | Adamson and Pasta (2010) | ||||||
| ENZIAN classification | 2005 | Surgical observation (or MRI) | √ 1 | √ | Surgical classification for DE | Keckstein et al. (2021), Tuttlies et al. (2005) | |||||
| Chapron classification | 2003 | Surgical observation | √ | √ | Surgical classification for DE with suggested operative procedure | Chapron et al. (2003a), Chapron et al. (2003b) | |||||
| Revised ASRM classification | 1997 | Surgical observation | √ | √ | Adapted from rAFS score | American Society for Reproductive Medicine (1997) | |||||
| TOP classification | 1993 | Surgical observation | √ | √ | Evaluate the severity of endometriosis by site, i.e., fallopian tubes (T), ovaries (O) and the peritoneum (P) and impact on PR | Kurata et al. (1993) | |||||
| Revised American Fertility Society (rAFS) classification | 1985 | Surgical observation | √ | Point system to determine stage/degree of endometriosis involvement | American Fertility Society (1985) | ||||||
| American Fertility Society (AFS) classification | 1979 | Surgical observation | √ | American Fertility Society (1979) | |||||||
| Buttram classification | 1978 | Surgical observation | √ | √ | Classification of endometriosis in the infertile female (expanded from Acosta et al. (1973)) | Buttram (1978) | |||||
| Kistner classification | 1977 | Surgical observation | √ | √ | Classification as a tool to link pregnancy rates with the presence/extent of disease | Kistner et al. (1977) | |||||
| Acosta classification | 1973 | Surgical observation | √ | √ | Classify the extent of disease and relationship with pregnancy rate | Acosta et al. (1973) | |||||
ENZIAN, the recently updated ENZIAN classification, which incorporates all types of endometriosis; DE, deep endometriosis; ECO, disease extent complaints, objectives system; PR, pregnancy rate; QoL, quality of life; US, ultrasound.
In case of ENZIAN score based on MRI.
Table II.
Overview of replication, validation and clinical value of published systems.
| Diagnosis/pre-operative assessment | Description | Staging | Treatment selection | Prediction of difficulty of surgery/complication | Prediction of pain remediation/QoL | Prediction of conception | Feasibility | Interobserver agreement | Aim of the study | Sample size | Age mean (range or SD) | Population source | Endometriosis case definition | Main results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENZIAN | |||||||||||||||
|
Correlation with Clavien-Dindo complication grading | 401 | 34.8 years (SD 8.73) | Single center | Histologic confirmation | ENZIAN A2, C1, C3 and FA were risk factors for the length of hospital stay. | Nicolaus et al. (2020) | ||||||||
|
| |||||||||||||||
| + |
|
63 | 33.5 years (22–49) | 2 centers | Surgical confirmation | Sensitivity and NPV of MRI confirmed by surgery were 95.2% and 91.7% (lesions in the vaginal/rectovaginal space), 78.4% and 56% (utero-sacral ligaments), 91.4% and 89.7% (rectum/sigmoid colon), 57.1% and 94.1% (myometrium), 85.7% and 98.3% (bladder), and 73.3% and 92.2% (intestine), respectively. | Burla et al. (2019) | ||||||||
|
| |||||||||||||||
| No analysis | Application of the rENZIAN system | 60 | 30.5 years (28.6–32.3) | Single center | Laparoscopic diagnosis | Medial compartment was found as the most affected one in 80% of the cases (mainly ovarian endometriomas), followed by posterior compartment in 65% and less frequent, anterior compartment. | Morgan-Ortiz et al. (2018) | ||||||||
|
| |||||||||||||||
| + |
|
115 | 36 years (20.3–48) | Single center | Histologic confirmation | The sensitivity, specificity, accuracy, PPV and NPV of MRI were 94%, 97%, 95%, 99%, 86%, respectively. The highest accuracy was for adenomyosis (100%) and endometriosis of utero-sacral ligaments (98%), slightly lower for vagina-rectovaginal septum and colorectal walls (96%), and the lowest for bladder endometriosis (92%). The concordance with histopathology was excellent. | Di Paola et al. (2015) | ||||||||
| ENZIAN | |||||||||||||||
|
Preoperative estimation of laparoscopic operating time | 151 |
|
Single center | Histologic confirmation | An ENZIAN-based model for estimating operating time for DE, assuming complication-free procedures (model’s predictive power: P < 0.001). The error of estimation for the operating time prediction is 0 ± 35.35 min (range −83 to +117 min). | Haas et al. (2013a) | ||||||||
|
| |||||||||||||||
| + | Identification of duplicate classifications of the same lesions | 219 | Not reported | Single center | Histologic confirmation | Comparison to rAFS: The severity of DE according to ENZIAN was as follows: grade 1: 45%; grade 2: 26%; grade 3: 19%; grade 4: 10%. Fifty-eight patients were classified according to ENZIAN although they did not fulfill the criteria of DE and had previously been classified according to the rAFS classification. Adaptation of the ENZIAN score would reduce the diagnoses of DE by 36% (95% CI: 29–44%). | Haas et al. (2011) | ||||||||
| UBESS | |||||||||||||||
| + | Correlation with RCOG laparoscopic level of complexity 1, 2, and 3 | 293 | Not reported | Multi-center | history of chronic pelvic pain, or endometriosis. |
|
Espada et al. (2020) | ||||||||
|
| |||||||||||||||
| − | Correlation with the difficulty of the surgery | 33 | 32.8 years (SD 7.7) | Single center | Histologic confirmation | Weak concordance between pre-operative UBESS score and the difficulty of the surgery (RCOG, concordance Kendall Tau 0.22) and between UBESS and CHI (concordance 0.30). | Chaabane et al. (2019) | ||||||||
| UBESS | |||||||||||||||
|
Validation for predicting the correct RANZCOG/AGES’ laparoscopic skill level. | 155 | 32.7 years (SD 8.6) | Multi-center | history of chronic pelvic pain and/or endometriosis | The accuracy, sensitivity, specificity, PPV and NPV, and positive and negative likelihood ratios of the UBESS I to predict the RANZCOG/AGES surgical skill levels 1/2 were 99.4%, 98.9%, 100%, 100%, 98.5%, not applicable, and 0.011; those of UBESS II to predict surgical skill levels 3/4 were: 98.1%, 96.8%, 98.4%, 93.8%, 99.2%, 60 and 0.033, and those for UBESS III to predict surgical skill level 6 were: 98.7%, 97.2%, 99.2%, 97.2%, 99.2%, 115.7, and 0.028. The rate of correctly predicting the exact level of skills needed was 98.1%, and Cohen’s kappa statistic for the agreement between UBESS prediction and levels of training required at surgery was 0.97, indicating almost perfect agreement. | Tompsett et al. (2019) | ||||||||
| EFI | |||||||||||||||
| + | Accuracy for the prediction of non-ART pregnancy | 4598 | NA | NA | Cumulative non-ART pregnancy rate at 36 months increased from 10% (95% CI: 3, 16%) in women with EFI score 0–2 to 69% (95% CI: 58, 79%) in women with EFI score 9–10, with a significant increase for each score category (0–2, 3–4, 5–6, 7–8, 9–10) |
Meta-analysis |
|||||||||
| EFI | |||||||||||||||
| Acceptable | Reproducibility among three experts | 82 | Reproductive age as inclusion criterium | Single-center | Surgical confirmation | A near ‘inter-expert’ clinical agreement rate (1.000, 95% CI 0.956–1.000; P = 0.0149) was observed. The numerical agreement between two experts was also high (0.988, 95% CI 0.934–1.000); similarly, high agreement rates were observed for both ‘junior-expert’ comparisons (clinical 0.963, 95% CI 0.897–0.992; numerical 0.988, 95% CI 0.934–1.000) and ‘intra-expert’ comparisons (clinical 0.988, 95% CI 0.934–1.000; numerical 1.000, 95% CI 0.956–1.000). | Tomassetti et al. (2020) | ||||||||
|
| |||||||||||||||
|
Accuracy for the prediction of pregnancy | 123 | 32.4 years (no range) | Single-center | Surgical confirmation | 8 (40%) patients with low, 20 (58.82%) with moderate, and 26 (96.29%) with high EFI conceived. EFI score showed statistically significant positive correlation with pregnancy outcome P = 0.001. Patients conceived spontaneously, after ovulation induction (±IUI) or after IVF. | Negi et al. (2019) | ||||||||
|
| |||||||||||||||
| + | Accuracy for the prediction of non-ART pregnancy in recurrent endometriosis | 107 | 31.1 years (SD 0.39) | Single-center | Surgical confirmation—recurrent endometriosis | Cumulative pregnancy rates (CPR) during the first 2 years were 51.86% in women with EFI ≥5, and 26.00% in women with EFI <5. At 3- and 5-year post-surgery, the CPR increased further in women with EFI ≥5, but not in women with EFI <5. The EFI score had good predictive power for postoperative pregnancy in women with recurrent endometriosis. | Zhou et al. (2019) | ||||||||
| EFI | |||||||||||||||
| + | Accuracy for the prediction of non-ART pregnancy | 68 | XX | Single-center | Surgical confirmation | The mean EFI scores of 68 women who were not pregnant and pregnant were 5.43 ± 0.36 and 6.88 ± 0.28, respectively. The relation between EFI and natural pregnancy was significant (cumulative overall PR, p = 0.006), whereas rAFS stage was not (univariate logistics, P = 0.853). The cut-point for maximum natural pregnancy outcomes was 6 (area under ROC curve = 0.710, 95% CI 0.586–0.835). | Kim et al. (2019) * | ||||||||
|
| |||||||||||||||
| + | Accuracy for the prediction of non-ART pregnancy | 1097 | 29.8 years (20–46) | Single-center | Surgical confirmation | The difference in cumulative pregnancy incidence among EFI scores 10, 7–9, 4–6, and 2–3 was statistically significant (Kaplan–Meier survival analysis). A significant relationship was found between EFI and time to achieving pregnancy. | Zhang et al. (2018) * | ||||||||
|
| |||||||||||||||
| + | Accuracy for the prediction of non-ART pregnancy | 235 | 34 years (20–47) | 2 centers | Histologic confirmation | The EFI was highly associated with live births (P < 0.001): for EFI of 0–2, the estimated cumulative non-ART LBR at 5 years was 0% and steadily increased up to 91% with an EFI of 9–10, while the proportion of women who attempted ART and had a live birth, steadily increased from 38% to 71% among the same EFI strata (P = 0.1). A low least function score was the most significant predictor of failure, followed by having had a previous resection or incomplete resection, being older than 40 compared to <35 years, and having leiomyomas. | Maheux-Lacroix et al. (2017) * | ||||||||
| EFI | |||||||||||||||
| + | Accuracy for the prediction of non-ART pregnancy and ART pregnancy | 196 | 32.3 years (SD 4.8) | Single center | Surgical confirmation | The cumulative PR was 76%. The PR, non-ART PR and ART PR for EFI ≤4 were 42.3%, 0% and 50%; for EFI 5–6, 67.9%, 30.5% and 60.6%; and for EFI ≥7, 87.7%48.2% and 80.3%, respectively. The benefit of ART was inversely correlated with the mean EFI score. On multivariate analysis, the EFI score was significantly associated with non-ART pregnancy (OR 1.629, 95% CI 1.235–2.150). | Boujenah et al. (2017) * | ||||||||
|
| |||||||||||||||
|
+ | Accuracy for the prediction of non-ART pregnancy + use for treatment selection (Surgery vs surgery + IVF-ET) | 345 | 32.2 years (22.0–45.0) | Single center | Histologic confirmation | Significant differences in spontaneous PRs among different EFI scores were identified (chi2 = 29.945, P < 0.05). The least function score was proved to be the most important factor for EFI. In patients with an EFI score ≥5 after 12 months from surgery, the cumulative PRs of those who received both surgery and IVF-ET were much higher than the spontaneous PRs of those who received surgery alone (chi2 = 4.16, ns). | Li et al. (2017) * | |||||||
|
| |||||||||||||||
|
+ | Accuracy for the prediction of non-ART pregnancy + use for treatment selection (Surgery vs surgery + IVF-ET) | 412 | 32.5 years (SD 4.6) | Single center | Histologic confirmation | A significant relationship between EFI and spontaneous PR was observed at 12 months (P = 0.001). The least function score and complete removal of endometriotic lesions and pelvic adhesions were significantly associated with spontaneous pregnancy (P = 0.006). Cumulative PR at 18 months was 78.8%. ART benefits were higher for patients with poor EFI. | Boujenah et al. (2015) * | |||||||
| EFI | |||||||||||||||
|
Accuracy for the prediction of non-ART pregnancy and ART pregnancy | 104 | 34.5 years (SD 4.5) | Single center | Surgical confirmation | Differences in time to non-ART pregnancy for the six EFI groups were statistically significant (log-rank, P = 1.4 × 10(−4)). The AUC for EFI as ART outcome predictor was 0.75 (95% CI 0.61–0.89, P = 6.2 × 10(−3)), while the best cut-point for pregnancy was 5.5. | Garavaglia et al. (2015) * | ||||||||
|
| |||||||||||||||
| + | Accuracy for the prediction of non-ART pregnancy | 161 | 32.08 years (22–40) | Single center | Surgical confirmation | Comparison to rAFS: Significant differences in cumulative PRs were observed among EFI scores (EFI score 0–3, 8.3%; EFI score 4–7 41.2%, and EFI score 8–10 60.9%; chi2 = 16.254, P < 0.001). EFI scores, but not rAFS stage, predict PRs in patients with endometriosis-associated infertility. | Zeng et al. (2014) * | ||||||||
|
| |||||||||||||||
|
Ability of the EFI score and rAFS classification for predicting IVF outcomes | 199 | 32.0 years (SD 4.2) | Single center | Histologic confirmation | Comparison to rAFS: The AUC of the EFI score (AUC = 0.641, standard error (SE) = 0.039, 95% CI = 0.564–0.717, cutoff score = 6) was significantly larger than that of the r-AFS classification (AUC = 0.445, SE = 0.041, and 95% CI = 0.364–0.526). The antral follicle count, estradiol level on day of hCG, number of oocytes retrieved, number of oocytes fertilised, number of cleaved embryos, implantation rate, CPR, and cumulative pregnancy rate were greater in the ≥6 EFI score group compared to the ≤5 EFI score group. EFI has more predictive power for IVF outcomes than r-AFS. | Wang et al. (2013) | ||||||||
| EFI | |||||||||||||||
| + | Accuracy for the prediction of non-ART pregnancy | 233 | 31.3 years (SD 3.9) | Single center | Surgical confirmation | Highly significant relationship between EFI and the time to non-ART pregnancy (P = 0.0004), with the K-M estimate of cumulative overall PR at 12 months after surgery equal to 45.5% (95% CI 39.47–49.87) ranging from 16.67% (95% CI 5.01–47.65) for EFI scores 0–3, to 62.55% (95% CI 55.18–69.94) for EFI scores 9–10. For each increase of 1 point in the EFI score, the relative risk of becoming pregnant increased by 31% (95% CI 16–47%; i.e. HR 1.31). The ‘least function score’ was found to be the most important contributor to the total EFI score. | Tomassetti et al. (2013) * | ||||||||
| ECO system | |||||||||||||||
| + | Validation | 166 | 34.0 years (SD 7.2) | 2 centers | Histologic confirmation | Among patients, 78 (47.0%) were medically treated and 88 (53.0%) underwent therapeutic laparoscopy. All three patients scoring two had undergone hormonal treatment. Among 51 patients scoring 3, 49 (96.1%) were clinically managed and 2 (3.9%) underwent surgery. Among 52 patients scoring 4, 26 (50.0%) had undergone medical treatment and 26 (50.0%) surgical treatment. All 56 patients who scored 5 and the 4 patients who scored 6 underwent surgery. | Lasmar et al. (2015) | ||||||||
| rASRM/rAFS/AFS | |||||||||||||||
| − | Accuracy for the prediction of non-ART pregnancy | 161 | 32.08 years (22–40) | Single center | Surgical confirmation | Comparison to EFI: The cumulative PR 36 months after surgery was 46.6% (stage I, 53.6%; stage II, 36.0%; stage III, 51.7%, and stage IV, 41.7%; chi2 = 4.143, P = 0.246). In the 1st year, PRs significantly differed between patients with rAFS stage IV and those with stages I–III (chi2 = 6.024, P = 0.014). rAFS stage did not predict PR in patients with endometriosis-associated infertility. | Zeng et al. (2014) | ||||||||
|
| |||||||||||||||
|
Ability of rAFS (vs EFI) to predict IVF outcomes | 199 | 32.0 years (SD 4.2) | Single center | Histologic confirmation | Comparison to EFI: The AUC of the EFI score was significantly larger than that of the r-AFS classification (AUC = 0.445, SE = 0.041, and 95% CI = 0.364–0.526). | Wang et al. (2013) | ||||||||
|
| |||||||||||||||
| + | Correlation with Clavien-Dindo complication grading | 401 | 34.8 years (SD 8.73) | Single center | Histologic confirmation | rASRM IV was a risk factors for the length of hospital stay. Clavien-Dindo Grade III complications were significantly associated with rASRM stage IV. | Nicolaus et al. (2020) | ||||||||
| rASRM/rAFS/AFS | |||||||||||||||
| Acceptable | Inter-observer agreement | 148 | 32.0 years (SD 6.7) | Single center | 105 women with and 43 women without a postoperative endometriosis | Surgeons and expert reviewers had substantial agreement on diagnosis and staging after viewing digital images (n = 148; mean j = 0.67, range 0.61–0.69; mean j = 0.64, range 0.53–0.78, respectively) and after additionally viewing operative reports (n = 148; mean j = 0.88, range 0.85–0.89; mean j = 0.85, range 0.84–0.86, respectively). Although additionally viewing MRI findings (n = 36) did not greatly impact agreement, agreement substantially decreased after viewing histological findings (n = 67), with expert reviewers changing their assessment from a positive to a negative diagnosis in up to 20% of cases. | Schliep et al. (2017) | ||||||||
|
| |||||||||||||||
| + | Prognostic value of individual adhesion scores for recurrence | 379 | 31.8 years (SD 6.7) | Single center | histologic confirmation | In endometriosis of advanced stage, younger age at the time of surgery, bilateral ovarian cysts at the time of diagnosis, a rAFS ovarian adhesion score >24, and complete cul-de-sac obliteration were independent risk factors of poor outcomes, and a rAFS ovarian adhesion score >24 had the highest risk of recurrence [hazard ratio = 2.948 (95% CI: 1.116–7.789), P = 0.029]. | Yun et al. (2015) | ||||||||
| rASRM/rAFS/AFS | |||||||||||||||
|
Correlation with the number of follicles, the level of FSH | 39 | 28.7 years (22–34) | Single center | Surgical confirmation | No statistically significant correlation between the AFC, the level of FSH and the stage of endometriosis was found. | Posadzka et al. (2014) | ||||||||
|
| |||||||||||||||
|
+ ART outcome |
Prediction of IVF outcome | 40 | 34.7 years (SD 4.3) | Single center | Surgical confirmation | Higher cancelation rates, higher total gonadotropin requirements, and lower oocyte yield were found in women with endometriosis Stage III and IV compared with both the Stage I/II and control groups. The fertilization rate was higher in Stage III/IV endometriosis compared to Stage I/II. CPR and LBR were comparable between patients with endometriosis Stage I/II and control group, whereas they were significantly lower in patients with endometriosis Stage III/IV compared to other two groups. | Pop-Trajkovic et al. (2014) | ||||||||
|
| |||||||||||||||
|
Correlation of rASRM stage with outcome ART treatment | 1764 (11) | Not applicable | Not applicable | Not reported | Comparison of women with Stage-III/IV vs Stage-I/II endometriosis: LBR, RR = 0.94 (95% CI, 0.80–1.11); CPR, RR = 0.90 (95% CI, 0.82–1.00); miscarriage, RR = 0.99 (95% CI, 0.73–1.36); number of oocytes retrieved, MD = −1.03 (95% CI, −1.67 to −0.39). No relevant difference between Stage-III/IV and Stage-I/II in LBR following ART. |
Meta-analysis |
||||||||
| rASRM/rAFS/AFS | |||||||||||||||
| Acceptable (surgeons) | Interrater and intrarater reliability (8 experts) | 148 | Not reported | Single center | Not reported | The interrater reliability for endometriosis diagnosis among the 8 surgeons was substantial: Fleiss kappa = 0.69 (95% CI 0.64–0.74). Surgeons agreed on revised ASRM endometriosis staging criteria after experienced assessment in a majority of cases (mean 61%, range 52–75%) with moderate interrater reliability: Fleiss kappa = 0.44 (95% CI 0.41–0.47). | Schliep et al. (2012) | ||||||||
|
| |||||||||||||||
| − | Correlation with symptoms | 319 | Age categories reported | Single center | Surgical confirmation, histologic confirmation in 72.9% | A correlation between endometriosis stage and severity of symptoms was observed only for dysmenorrhea (chi2 = 5.14, P = 0.02) and non-menstrual pain (chi2 = 5.63, P = 0.018). However, the point estimates of ORs were very close to unity (respectively, 1.33, 95% CI 1.04–1.71, and 1.01, 95% CI 1.00–1.03). The association between endometriosis stage and severity of pelvic symptoms was marginal and inconsistent. | Vercellini et al. (2007) | ||||||||
| rASRM/rAFS/AFS | |||||||||||||||
|
|
Predictive value for response to surgical treatment | 537 | Age categories reported | Single center | Histologic confirmation | The cumulative probability of pregnancy at 3 years from surgery was 47% (51% at stage I, 45% at stage II, 46% at stage III and 44% at stage IV; chi2 = 1.50, ns). The cumulative probability of moderate or severe dysmenorrhoea recurrence in 425 symptomatic subjects was 24% (32% at stage I, 24% at stage II, 21% at stage III and 19% at stage IV; chi2 = 6.39, ns). The cumulative probability of disease relapse was 12% (3% at stage I, 11% at stage II, 11% at stage III and 23% at stage IV; chi2 = 24.95, P = 0.0001). | Vercellini et al. (2006) | |||||||
|
| |||||||||||||||
| + | + |
|
Association with type and severity of pain, and with symptoms after laparoscopic surgery | 95 | Not reported | Single center | Surgical confirmation | In patients with AFS ≥16; preoperative pain scores were significantly higher for dysmenorrhea (P = 0.0022) and deep dyspareunia (P < 0.0001) but not for non-menstrual pain. After surgery, dysmenorrhea improved in 43% of cases in patients with AFS <16 vs 66% with AFS ≥16 (P = 0.0037). For deep dyspareunia, improvement was reported by 33% and 67%, respectively (ns). Improvement in non-menstrual pain was not significantly different (67% vs 56%). Cases with advanced disease benefit the most from laparoscopy. | Milingos et al. (2006) | ||||||
| rASRM/rAFS/AFS | |||||||||||||||
| − | − | Impact of treatments on pain + association pain scores | 181 | Not reported | Single center | Histologic confirmation | No correlation was found between the stage of endometriosis according to R-AFS score and the severity of CPP. | Szendei et al. (2005) | |||||||
|
| |||||||||||||||
| Variable | Comparison of laparoscopic and laparotomic scoring | 84 | Not reported | Single center | Surgical confirmation | There was considerable variability in laparoscopic vs laparotomic scoring by the same observer, with largest variability in ovarian endometriosis and cul-de-sac obliteration subscores, and least variability for peritoneum endometriosis. The inter-method variation was sufficient to alter the staging in 34.5% of patients, with a difference of two stages in 3.6% of patients. In general, there was fair-to-good agreement (kappa coefficient 0.49). | Lin et al. (1998) | ||||||||
|
| |||||||||||||||
|
Impact of severity of endometriosis on the outcome of IVF | 61 |
|
Single center | Surgical confirmation | Response to COH and the number, maturity, and quality of the oocytes was comparable between stages. Fertilization rates for oocytes of patients with stages III/IV were significantly impaired compared to those in stage I/II (P = 0.004). The implantation rate, CPR, and miscarriage rate were comparable between stages I/II and stages III/IV. | Pal et al. (1998) | ||||||||
| rASRM/rAFS/AFS | |||||||||||||||
| Variable | Intraobserver and interobserver variability—5 experts | 20 | Not reported | Single center | Not reported | The grand total score varied with an SD of 13.44 when the videotape of a single patient was rated twice by the same observer and varied with an SD of 17.12 when rated by two observers. The greatest variability occurred in endometriosis of the ovary and cul-de-sac obliteration, with less variability for peritoneum endometriosis and for ovarian and tubal adhesions. Comparison of intraobserver and interobserver scores resulted in a change in endometriosis stage in 38% and 52% of patients, respectively. | Hornstein et al. (1993) | ||||||||
|
| |||||||||||||||
| Acceptable | Reproducibility—2 experts | 315 | Not reported | Single center | Not reported | Good to fair agreement scoring endometriosis between the investigator and the blinded reviewer was noted. | Rock (1995) | ||||||||
|
| |||||||||||||||
| No analysis | Feasibility of AFS and adnexal score | 89 | Not reported | Single center | Surgical confirmation | Suggestion to split class IV in class IV and class V (with higher rate of bilateral adnexal disease/adhesions). | Canis et al. (1992) | ||||||||
|
| |||||||||||||||
|
Relation with endometriosis-associated symptoms and patients’ age. | 206 | 30 years (18–44) | Single center | Surgical confirmation |
No significant differences were found in total endometriosis scores, in active scores or in adhesion scores in different age groups. There was no significant difference in prevalence rate of symptoms for different aspects of endometriosis (implants, cysts or adhesions). AFS score does not reflect the intensity of symptoms. |
Marana et al. (1991) | ||||||||
| rASRM/rAFS/AFS | |||||||||||||||
| Variable | Feasibility of measuring endometrioma | 52 | 29.5 years (24–39) | Single center | NA | Cyst diameter was calculated using the geometric formula radius = 3 square root of 3 V/4 pi where V = volume of liquid aspirated. Eight patients with apparently normal pelvis had endometriosis, and 14 with apparent minimal or mild endometriotic lesions were restaged. Laparoscopic ovarian puncture of enlarged ovaries was important for correct diagnosis and staging of endometriosis. | Candiani et al. (1990) | ||||||||
|
| |||||||||||||||
|
Relation with pregnancy after therapy | 214 | Not reported | Single center | Surgical confirmation | The AFS scale poorly specifies the relation between severity of disease and pregnancy outcome after therapy. A nonparametric monotonic estimator, generating a relationship between AFS score and pregnancy following treatment is shown to improve the discriminatory power of the AFS scale. | Guzick et al. (1982) | ||||||||
|
| |||||||||||||||
|
(+ Kistner, Buttram) Prediction of pregnancy | 214 | 28.6 years (17–37) | Single center | Surgical confirmation | The AFS score revealed significant differences in pregnancy rate only if categories were combined (mild plus moderate versus severe plus extensive, P ≤ 0.05). The AFS system revealed that pregnancy success was significantly reduced if an ovarian endometrioma was greater than 3 cm or had ruptured (P ≤ 0.01). | Rock et al. (1981) | ||||||||
The symbols should be interpreted as follows; + indicates a significant positive result in a correlation (or similar) test, − indicates a significant negative result in a correlation (or similar) test, ns indicates a non-conclusive/non-significant result in a correlation (or similar) test. The highlighted columns represent the intended purpose of the classification/staging system (as in Table I).
AFC, antral follicle count; AFS, American Fertility Society; AGES, Australasian Gynaecological Endoscopy and Surgery; COH, controlled ovarian hyperstimulation; CPP, chronic pelvic pain; CPR, clinical pregnancy rate; DE, deep endometriosis; EFI: endometriosis fertility index; HR, hazard ratio; IVF-ET, IVF embryo transfer; K-M, Kaplan–Meier; LBR, live birth rate; NPV, negative predictive value; PPV, positive predictive value; RANZCOG, Royal Australian and New Zealand College of Obstetricians and Gynaecologists; RR, relative risk.
Study included in meta-analysis (Vesali et al., 2020).
Classification and staging systems
In the 1970s, the first ‘classification’ system for endometriosis originated from a study attempting to describe the results of conservative surgical treatment of endometriosis and hereby classify the extent of the disease and its relationship with the pregnancy rate (Acosta et al., 1973). Later, this classification system was further expanded and submitted for consideration to the American Fertility Society (AFS) (Buttram, 1978). Similarly, a system published by Kistner et al., (1977) was submitted for endorsement by AFS and the International Federation of Fertility Societies (IFFS). In 1979, AFS published a classification system on behalf of a group of experts including the leading authors of the previous systems (American Fertility Society, 1979). The AFS classification for endometriosis, and later published revised AFS (rAFS) and revised American Society for Reproductive Medicine (rASRM) classification, have been the main standard for classifying endometriosis ever since (American Fertility Society, 1979, 1985; American Society for Reproductive Medicine, 1997). The different versions of the AFS/ASRM classification system reflect the progress made in the knowledge on endometriosis.
Later attempts of surgical disease description or staging have focused on disease location—such as urinary tract endometriosis (Knabben et al., 2015)—or subtypes of the disease—such as DE (Chapron et al., 2003a; Tuttlies et al., 2005; Coccia and Rizzello, 2011): the latter group includes the ENZIAN-Score for classifying DE (Tuttlies et al., 2005). The recently updated #ENZIAN classification extends the previous ENZIAN score to incorporate all types of endometriosis (Keckstein et al., 2021). The EPHect standard recommended (SSF) and minimum required (MSF) were developed for recording of surgical phenotypic information on endometriosis (Becker et al., 2014).
While these classification systems mainly focused on describing the extent of disease during surgery, some attempted to link these observations to outcomes, such as pregnancy rates, after surgery (American Fertility Society, 1979, 1985; Kurata et al., 1993; American Society for Reproductive Medicine, 1997), or indicators for disease management (Chapron et al., 2003a). Another group of classification systems focused on pre-operative assessment of the extent of the disease (van der Wat et al., 2013; Lafay Pillet et al., 2014; Knabben et al., 2015; Menakaya et al., 2016; Riiskjær et al., 2017; Chattot et al., 2019; Ichikawa et al., 2020), based on either patient-reported symptoms or pre-operative imaging, or a combination of both. The ultrasound-based endometriosis staging system (UBESS) additionally aimed at predicting the complexity of endometriosis surgery (Menakaya et al., 2016), as does the adhesion scoring system in case of pelvic adhesions (Ichikawa et al., 2020).
Two systems aimed specifically at outcome prediction for endometriosis: the ‘disease extent, complaints, objectives (ECO)-system’, aiming to select the most appropriate management based on reported symptoms (Lasmar et al., 2012, 2015); and the endometriosis fertility index (EFI), aiming to predict the probability of natural conception after surgery (Adamson and Pasta, 2010). Finally, a recently published study ‘Endogram’ sets out to ‘profile’ endometriosis heterogeneity, based on the assessment of several disease markers in a biopsy sample, with the ultimate aim of guiding therapeutic options (Bouquet de Joliniere et al., 2019).
Replication, validation and clinical value of published systems
We retrieved 46 studies, mostly observational, reporting an evaluation of the different classification, staging or reporting systems (Table II). The aims and outcomes of the different studies varied significantly.
Of the included studies, eight reported on the practical aspects of the classification systems, being either the feasibility, or the inter-observer and intra-observer variability. Of these, seven studies focused on the rASRM classification system (Candiani et al., 1990; Canis et al., 1992; Hornstein et al., 1993; Rock, 1995; Lin et al., 1998; Schliep et al., 2012, 2017), while the most recent one evaluated the reproducibility of the EFI (Tomassetti et al., 2020). Early studies (1990s) reported significant variability in rAFS classification by five independent experts reviewing surgery recordings, specifically with regards to endometriosis of the ovary and cul-de-sac obliteration (Hornstein et al., 1993), although another study from the same period reported good to fair agreement in scoring endometriosis between two experts using photographs or recordings (Rock, 1995). In more recent studies, the rASRM classification system was found to have acceptable inter-observer agreement and inter-rater reliability among surgeons and experts reviewing surgical photographs and/or recordings (Schliep et al., 2012, 2017). Studies have also focused on the feasibility of specific aspects of the AFS/rAFS/rASRM classification, specifically classifying bilateral adnexal disease (Canis et al., 1992), measuring cyst diameter (Candiani et al., 1990), or the reliability of laparoscopic versus laparotomic scoring (Lin et al., 1998). For the EFI, a near perfect clinical agreement rate between two independent experts (1.000, 95% CI 0.956–1.000) and high agreement between two assessments by the same expert (0.988, 95% CI 0.934–1.000) has been reported (Tomassetti et al., 2020).
The remaining studies (n = 37) applied the classification or staging systems to a cohort of patients, evaluating whether the system was reliable with regards to its proposed aim, or evaluating whether the classification could be used for other purposes. The latter was mainly the case for the AFS/rAFS/rASRM classification system, which was developed for surgical staging, but has been evaluated for predicting symptom relief and recurrence after surgery (Milingos et al., 2006; Vercellini et al., 2006), complications after surgery (Nicolaus et al., 2020), ovarian reserve (Posadzka et al., 2014), time to non-ART pregnancy (Yun et al., 2015), pregnancy outcomes (Rock et al., 1981; Guzick et al., 1982) and the outcomes of ART treatment (Pal et al., 1998; Barbosa et al., 2014; Pop-Trajkovic et al., 2014). Furthermore, correlation of the AFS/rAFS/rASRM classification system with symptoms before surgery was evaluated (Marana et al., 1991; Szendei et al., 2005; Vercellini et al., 2007). To our knowledge, there are no studies specifically evaluating the feasibility or reliability of the AFS/rAFS/rASRM classification system for its proposed aim, being a descriptive system of surgical documentation of disease.
The EFI, a 10-point scoring system grouped into five categories of risk, has been assessed in 12 studies and one review. It has been mainly assessed for its intended purpose, being prediction of the probability of natural conception after surgery (Tomassetti et al., 2013; Wang et al., 2013; Zeng et al., 2014; Boujenah et al., 2015; Garavaglia et al., 2015; Boujenah et al., 2017; Li et al., 2017; Maheux-Lacroix et al., 2017; Zhang et al., 2018; Kim et al., 2019; Negi et al., 2019; Zhou et al., 2019). Interestingly, in some of these studies, an evaluation of the prognostic value of the different factors included in the EFI score was also performed. A meta-analysis summarized these validation studies and evaluated the performance of the EFI score for predicting non-ART pregnancy after endometriosis surgery, observing good predictive value with a pooled estimate for AUC of 0.71 (95%CI 0.65-0.80) (Vesali et al., 2020). Some authors have (additionally) evaluated whether its purpose can be extended to guide patient management, by using it to select patients that would benefit from ART treatments (Boujenah et al., 2015; Li et al., 2017), and/or predicting the chances of pregnancy from ART treatments (Wang et al., 2013; Garavaglia et al., 2015).
The ECO system has been validated for prediction of management (surgery or medical treatment) in a single study, by the same authors that developed the tool (Lasmar et al., 2015).
The UBESS system, developed for pre-operative staging and prediction of the complexity of surgery, was evaluated in three studies reporting on the latter purpose, i.e. difficulty of surgery (Chaabane et al., 2019; Espada et al., 2020) and prediction of surgical skill levels (Tompsett et al., 2019).
Finally, the ENZIAN classification system, developed as a descriptive system for surgical staging of DE, was evaluated for its purpose in two studies (Haas et al., 2011; Morgan-Ortiz et al., 2018). Another evaluation reported on the correlation between the ENZIAN classification and complications after surgery, classified according to the Clavien-Dindo complication grading (Nicolaus et al., 2020). The use of the ENZIAN classification system was further extrapolated for its use in pre-operative assessment with imaging. Two studies evaluated this MRI-based ENZIAN system (Di Paola et al., 2015; Burla et al., 2019), and a third study reported on a model to predict operation time based on the MRI-based ENZIAN classification (Haas et al., 2013a).
In general, published classification or staging systems have been developed with various intended purposes, ranging from diagnosis (including symptoms) and pre-operative assessment, surgical description or staging, to prediction of surgical difficulty and treatment outcomes (both for pain and infertility). The studies summarized above confirm the surgical value of the ENZIAN system for description and pre-operative assessment of DE, and of UBESS for predicting laparoscopic difficulty. However, most classification/staging systems show no or very little correlation with patient outcomes. The exception is the EFI, which has been consistently shown to provide good predictive value for natural conception after endometriosis surgery. It is notable that the development of the EFI was data driven, whereas the development of most other classification/staging systems was based on expert opinion.
Discussion
The current paper provides an overview of currently available and published classification, staging and/or reporting systems for endometriosis. We include 22 systems published between 1973 and 2021. Each of the systems was developed for a specific and different purpose. The first systems tried to classify the various forms of endometriosis that were encountered (at the time), and this remains the purpose of more recent systems as there still is no international agreement on how to describe the disease. Next, we summarize published studies evaluating the different classification, staging or reporting systems. From this, we show a discrepancy between the intended and the evaluated purpose, and a general lack of validation data confirming correlation with pain symptoms or quality of life for any of the current endometriosis classification systems. With regards to infertility, the EFI has been confirmed valid for its intended purpose of predicting the probability of natural conception after surgery.
Classification and staging systems are widely used in medicine and have been shown to be valuable in guiding clinical management. Examples include the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging systems for cancer, the Gleason score for prostate cancer, the Braak Staging for Parkinson’s disease, and the ACR/EULAR Classification Criteria for Rheumatoid Arthritis. The ACR/EULAR Classification Criteria for Rheumatoid Arthritis were developed based on data analysis of 3115 patients followed by a consensus process in which determinants for risk of rheumatoid arthritis were selected and grouped into a classification system, which was further refined, and the feasibility was optimized (Aletaha et al., 2010). A review published 2 years afterwards identified 17 articles (total 6816 patients) and 17 meeting abstracts (total 4004 patients) investigating the classification criteria. Only a minority of the articles aimed to validate the system in the intended population, while the other studies extended the target population, used different reference standards or adapted the criteria in the system (Radner et al., 2014). The review findings are similar to the findings of the current review, although in a different field of medicine. The TNM staging system for cancer was developed in the early 1950s, aiming to guide clinical classification of cancer cases by anatomical extent. The philosophy and technique of TNM staging were developed by Professor Denoix and later adopted by international societies (Denoix, 1952; Sellers, 1971). The system is currently at its eighth edition (Edge et al., 2010). The system is revised in a 6- or 8-year cycle and changes are implemented based on high-level evidence collected through large datasets. Specifications are available for different types of cancer, and the system has been complemented with a summary staging or classification linked to prognosis and used for treatment planning. In the TNM system for lung cancer, as an example, TNM staging adaptations included the removal of rare findings from the system, and corrections in stage grouping based on survival outcomes (Lim et al., 2018). In addition, the TNM system has been increasingly complemented by molecular marker data that more accurately stratify risk in patients and guide appropriate treatment options. The longevity and update systems applied for the TNM staging, and the value of additional molecular subtype identification, are likely to be important guides for the design of future endometriosis classification and staging systems that correlate with relevant patient outcomes.
Specifically, for endometriosis, previous reviews have summarized and commented on existing classification systems, mainly rASRM, ENZIAN and EFI. It has previously been concluded that the rASRM system has poor correlation with pain, fertility outcomes or prognosis, and that the ENZIAN system has poor correlation with symptoms and infertility (Haas et al., 2013b; Johnson et al., 2017; Andres et al., 2018). The EFI system needs further evaluation with regards to the importance of the different parameters and whether to include the completeness of surgical treatment (Maheux-Lacroix et al., 2017). The conclusion of previous reviews of classification systems and our overview is consistently phrased as a need for a generally accepted classification with a clear goal/purpose (Adamson, 2011; Haas et al., 2013b; Johnson et al., 2017; Andres et al., 2018; Rolla, 2019). Yet, as presented in this paper, the goal and purpose of published classification, staging or reporting systems for endometriosis is often ignored when evaluating classification or staging systems, limiting the value of the evaluation studies and of the systems in general.
To our knowledge, this is the first report comparing the outcomes assessed in the studies with the intended purposes of the classification systems. Indeed, we show that the rASRM system has been widely evaluated, often with negative conclusions, but we found no studies evaluating the system for its intended goal, which is descriptive surgical staging. ENZIAN and EFI have been evaluated for their intended purpose, but studies have also evaluated whether they can be applied more widely and for other outcomes. Apart from these three systems, only two other classification systems (UBESS and ECO) have been evaluated for their intended purpose, with no evaluations of the remaining 17 classification systems, preventing them from further dissemination and uptake.
The current review provides an overview of published classification systems and studies evaluating them, but no detailed assessment of all positive and negative aspects of the classification systems, so as not to repeat previous reviews (Johnson et al., 2017). In addition, we have restricted our overview to classification systems published in peer-reviewed papers and available through PUBMED/MEDLINE. Although locally used and/or unpublished systems are available and can be valuable, the relevance of including them in the current review was considered low, as they would not be widely applied, nor evaluated by (independent) researchers. For universal use of a classification system, it is pivotal that the system is accessible, validated, reliable and reproducible.
Our report includes a summary of evaluation studies assessing these aspects in the different classification systems. Even though we retrieved 46 studies, the value of these evaluations is limited. Apart from the EFI score, the current classification systems have not been thoroughly assessed for validity, feasibility and reproducibility. Moreover, a significant proportion of the evaluation studies have examined the classification systems for purposes other than the one for which they were designed and initially evaluated.
Endometriosis is a challenging disease to classify, as it is known to have different phenotypes and presentations (both with regards to the type of lesions and their location), and various symptoms without a clear link to phenotype or presentation. Moreover, the natural progression of the disease is unknown. There is a perceived need for a validated classification or descriptive system for endometriosis that could support further progress in defining subgroups and more importantly guiding the therapeutic options for women with pain and/or infertility. Such a system would certainly also progress endometriosis research by unifying patient subgroups and facilitating the development of prognostic and predictive tools.
From this overview, it can be concluded that several classification, staging and reporting systems have been developed for endometriosis. A universally accepted categorization of the disease using the experience from the already existing proposals seems to be needed for clinical and research purposes.
Data availability
All data are incorporated into the article.
Acknowledgements
The authors would like to acknowledge the experts on endometriosis that contributed to the stakeholder review and thank them for their useful comments.
Authors’ roles
N.V. performed the literature review and summarized the results. All other authors contributed to conception and design, drafting the content and critically revising it. All authors approved the final version.
Funding
The meetings and activities of the working group were funded by the American Association of Gynecologic Laparoscopists, European Society for Gynaecological Endoscopy, European Society of Human Reproduction and Embryology and World Endometriosis Society.
Conflict of interest
A.W.H. reports grant funding from the MRC, NIHR, CSO, Wellbeing of Women, Roche Diagnostics, Astra Zeneca, Ferring, Charles Wolfson Charitable Trust, Standard Life, Consultancy fees from Roche Diagnostics, AbbVie, Nordic Pharma and Ferring, outside the submitted work. In addition, A.W.H. has a patent Serum biomarker for endometriosis pending. N.P.J. reports personal fees from Abbott, Guerbet, Myovant Sciences, Vifor Pharma, Roche Diagnostics, outside the submitted work; he is also President of the World Endometriosis Society and chair of the trust board. S.M. reports grants and personal fees from AbbVie, and personal fees from Roche outside the submitted work. C.T. reports grants, non-financial support and other from Merck SA, non-financial support and other from Gedeon Richter, non-financial support from Ferring Pharmaceuticals, outside the submitted work and without private revenue. K.T.Z. reports grants from Bayer Healthcare, MDNA Life Sciences, Roche Diagnostics Inc, Volition Rx, outside the submitted work; she is also a Board member (Secretary) of the World Endometriosis Society and World Endometriosis Research Foundation, Research Advisory Board member of Wellbeing of Women, UK (research charity), and Chair, Research Directions Working Group, World Endometriosis Society. The other authors had nothing to disclose.
Footnotes
ESHRE pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
This article is published simultaneously, with permission, in HROpen, FACTS, VIEWS & VISION in Obgyn and Journal of Minimally Invasive Gynecology.
References
- Acosta AA, Buttram VC Jr, Besch PK, Malinak LR, Franklin RR, Vanderheyden JD.. A proposed classification of pelvic endometriosis. Obstet Gynecol 1973;42:19–25. [PubMed] [Google Scholar]
- Adamson GD, Pasta DJ.. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril 2010;94:1609–1615. [DOI] [PubMed] [Google Scholar]
- Adamson GD. Endometriosis classification: an update. Curr Opin Obstet Gynecol 2011;23:213–220. [DOI] [PubMed] [Google Scholar]
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–1588. [DOI] [PubMed] [Google Scholar]
- American Fertility Society. Classification of endometriosis. The American Fertility Society. Fertil Steril 1979;32:633–634. [PubMed] [Google Scholar]
- American Fertility Society. Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril 1985;43:351–352. [DOI] [PubMed] [Google Scholar]
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–821. [DOI] [PubMed] [Google Scholar]
- Andres MP, Borrelli GM, Abrao MS.. Endometriosis classification according to pain symptoms: can the ASRM classification be improved? Best Pract Res Clin Obstet Gynaecol 2018;51:111–118. [DOI] [PubMed] [Google Scholar]
- Barbosa MA, Teixeira DM, Navarro PA, Ferriani RA, Nastri CO, Martins WP.. Impact of endometriosis and its staging on assisted reproduction outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2014;44:261–278. [DOI] [PubMed] [Google Scholar]
- Becker CM, Laufer MR, Stratton P, Hummelshoj L, Missmer SA, Zondervan KT, Adamson GD; WERF EPHect Working Group. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil Steril 2014;102:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boujenah J, Bonneau C, Hugues JN, Sifer C, Poncelet C.. External validation of the Endometriosis Fertility Index in a French population. Fertil Steril 2015;104:119–123.e1. [DOI] [PubMed] [Google Scholar]
- Boujenah J, Cedrin-Durnerin I, Herbemont C, Bricou A, Sifer C, Poncelet C.. Use of the endometriosis fertility index in daily practice: a prospective evaluation. Eur J Obstet Gynecol Reprod Biol 2017;219:28–34. [DOI] [PubMed] [Google Scholar]
- Bouquet de Joliniere J, Major A, Ayoubi JM, Cabry R, Khomsi F, Lesec G, Frydman R, Feki A.. It is necessary to purpose an add-on to the American Classification of Endometriosis? This disease can be compared to a malignant proliferation while remaining benign in most cases. EndoGram® is a new profile witness of its evolutionary potential. Front Surg 2019;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burla L, Scheiner D, Samartzis EP, Seidel S, Eberhard M, Fink D, Boss A, Imesch P.. The ENZIAN score as a preoperative MRI-based classification instrument for deep infiltrating endometriosis. Arch Gynecol Obstet 2019;300:109–116. [DOI] [PubMed] [Google Scholar]
- Buttram VC Jr. An expanded classification of endometriosis. Fertil Steril 1978;30:240–242. [DOI] [PubMed] [Google Scholar]
- Candiani GB, Vercellini P, Fedele L.. Laparoscopic ovarian puncture for correct staging of endometriosis. Fertil Steril 1990;53:994–997. [DOI] [PubMed] [Google Scholar]
- Canis M, Pouly JL, Wattiez A, Manhes H, Mage G, Bruhat MA.. Incidence of bilateral adnexal disease in severe endometriosis (revised American Fertility Society [AFS], stage IV): should a stage V be included in the AFS classification? Fertil Steril 1992;57:691–692. [DOI] [PubMed] [Google Scholar]
- Chaabane S, Nguyen Xuan HT, Paternostre A, Du Cheyron J, Harizi R, Mimouni M, Fauconnier A.. [Endometriosis: assessment of the Ultrasound-Based Endometriosis Staging System score (UBESS) in predicting surgical difficulty]. Gynecol Obstet Fertil Senol 2019;47:265–272. [DOI] [PubMed] [Google Scholar]
- Chapron C, Dubuisson JB, Chopin N, Foulot H, Jacob S, Vieira M, Barakat H, Fauconnier A.. [Deep pelvic endometriosis: management and proposal for a “surgical classification”]. Gynecol Obstet Fertil 2003a;31:197–206. [DOI] [PubMed] [Google Scholar]
- Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B, Pansini V, Vacher-Lavenu MC, Dubuisson JB.. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod 2003b;18:157–161. [DOI] [PubMed] [Google Scholar]
- Chattot C, Huchon C, Paternostre A, Du Cheyron J, Chouillard E, Fauconnier A.. ENDORECT: a preoperative score to accurately predict rectosigmoid involvement in patients with endometriosis. Hum Reprod Open 2019;2019:hoz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia ME, Rizzello F.. Ultrasonographic staging: a new staging system for deep endometriosis. Ann N Y Acad Sci 2011;1221:61–69. [DOI] [PubMed] [Google Scholar]
- Denoix P. Nomenclature classification des cancers. Bull Inst Nat Hyg(Paris) 1952;7:743–748. [Google Scholar]
- Di Paola V, Manfredi R, Castelli F, Negrelli R, Mehrabi S, Pozzi Mucelli R.. Detection and localization of deep endometriosis by means of MRI and correlation with the ENZIAN score. Eur J Radiol 2015;84:568–574. [DOI] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F.. AJCC Cancer Staging Manual. New York: Springer, 2010. [Google Scholar]
- Espada M, Leonardi M, Aas-Eng K, Lu C, Reyftmann L, Tetstall E, Slusarczyk B, Ludlow J, Hudelist G, Reid S. et al. A multicenter international temporal and external validation study of the ultrasound-based endometriosis staging system. J Minim Invasive Gynecol 2020;28:57–62. [DOI] [PubMed] [Google Scholar]
- Garavaglia E, Pagliardini L, Tandoi I, Sigismondi C, Vigano P, Ferrari S, Candiani M.. External validation of the endometriosis fertility index (EFI) for predicting spontaneous pregnancy after surgery: further considerations on its validity. Gynecol Obstet Invest 2015;79:113–118. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Bross DS, Rock JA.. Assessing the efficacy of The American Fertility Society's classification of endometriosis: application of a dose-response methodology. Fertil Steril 1982;38:171–176. [DOI] [PubMed] [Google Scholar]
- Haas D, Chvatal R, Habelsberger A, Schimetta W, Wayand W, Shamiyeh A, Oppelt P.. Preoperative planning of surgery for deeply infiltrating endometriosis using the ENZIAN classification. Eur J Obstet Gynecol Reprod Biol 2013a;166:99–103. [DOI] [PubMed] [Google Scholar]
- Haas D, Chvatal R, Habelsberger A, Wurm P, Schimetta W, Oppelt P.. Comparison of revised American Fertility Society and ENZIAN staging: a critical evaluation of classifications of endometriosis on the basis of our patient population. Fertil Steril 2011;95:1574–1578. [DOI] [PubMed] [Google Scholar]
- Haas D, Shebl O, Shamiyeh A, Oppelt P.. The rASRM score and the Enzian classification for endometriosis: their strengths and weaknesses. Acta Obstet Gynecol Scand 2013b;92:3–7. [DOI] [PubMed] [Google Scholar]
- Hornstein MD, Gleason RE, Orav J, Haas ST, Friedman AJ, Rein MS, Hill JA, Barbieri RL.. The reproducibility of the revised American Fertility Society classification of endometriosis. Fertil Steril 1993;59:1015–1021. [PubMed] [Google Scholar]
- Ichikawa M, Akira S, Kaseki H, Watanabe K, Ono S, Takeshita T.. Accuracy and clinical value of an adhesion scoring system: A preoperative diagnostic method using transvaginal ultrasonography for endometriotic adhesion. J Obstet Gynaecol Res 2020;46:466–478. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R, Sharpe-Timms KL. et al. ; World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod 2017;32:315–324. [DOI] [PubMed] [Google Scholar]
- Keckstein J, Saridogan E, Ulrich UA, Sillem M, Oppelt P, Schweppe Kw, Krentel H, Janschek E, Exacoustos C, Malzoni M. et al. The #Enzian classification: a comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet Gynecol Scand 2021;100:1165–1175. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee CW, Yun J, Lee JH, Yun BH, Park JH, Seo SK, Cho S, Choi YS, Lee BS.. Use of the endometriosis fertility index to predict natural pregnancy after endometriosis surgery: a single-center study. Gynecol Obstet Invest 2019;84:86–93. [DOI] [PubMed] [Google Scholar]
- Kistner RW, Siegler AM, Behrman SJ.. Suggested classification for endometriosis: relationship to infertility. Fertil Steril 1977;28:1008–1010. [PubMed] [Google Scholar]
- Knabben L, Imboden S, Fellmann B, Nirgianakis K, Kuhn A, Mueller MD.. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril 2015;103:147–152. [DOI] [PubMed] [Google Scholar]
- Kurata S, Ishimaru T, Masuzaki H, Yamabe T.. Relationship between the prognosis of conception and the location of pelvic involvement in endometriosis: significance of the TOP (tube, ovary, peritoneum) classification. Asia Oceania J Obstet Gynaecol 1993;19:391–399. [DOI] [PubMed] [Google Scholar]
- Lafay Pillet MC, Huchon C, Santulli P, Borghese B, Chapron C, Fauconnier AA.. clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod 2014;29:1666–1676. [DOI] [PubMed] [Google Scholar]
- Lasmar BR, Simoes Abraao M, Lasmar RB, Leon Dewilde R.. Simplified approach to the treatment of endometriosis–ECO system. Minerva Ginecol 2012;64:331–335. [PubMed] [Google Scholar]
- Lasmar RB, Lasmar BP, Celeste RK, Larbig A, De Wilde RL.. Validation of a score to guide endometriosis therapy for the non-specialized gynecologist. Int J Gynaecol Obstet 2015;131:78–81. [DOI] [PubMed] [Google Scholar]
- Li X, Zeng C, Zhou YF, Yang HX, Shang J, Zhu SN, Xue Q.. Endometriosis fertility index for predicting pregnancy after endometriosis surgery. Chin Med J 2017;130:1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W, Ridge CA, Nicholson AG, Mirsadraee S.. The 8(th) lung cancer TNM classification and clinical staging system: review of the changes and clinical implications. Quant Imaging Med Surg 2018;8:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Lee RK, Hwu YM, Lin MH.. Reproducibility of the revised American Fertility Society classification of endometriosis using laparoscopy or laparotomy. Int J Gynaecol Obstet 1998;60:265–269. [DOI] [PubMed] [Google Scholar]
- Maheux-Lacroix S, Nesbitt-Hawes E, Deans R, Won H, Budden A, Adamson D, Abbott JA.. Endometriosis fertility index predicts live births following surgical resection of moderate and severe endometriosis. Hum Reprod 2017;32:2243–2249. [DOI] [PubMed] [Google Scholar]
- Marana R, Muzii L, Caruana P, Dell'Acqua S, Mancuso S.. Evaluation of the correlation between endometriosis extent, age of the patients and associated symptomatology. Acta Eur Fertil 1991;22:209–212. [PubMed] [Google Scholar]
- Menakaya U, Reid S, Lu C, Gerges B, Infante F, Condous G.. Performance of ultrasound-based endometriosis staging system (UBESS) for predicting level of complexity of laparoscopic surgery for endometriosis. Ultrasound Obstet Gynecol 2016;48:786–795. [DOI] [PubMed] [Google Scholar]
- Milingos S, Protopapas A, Kallipolitis G, Drakakis P, Loutradis D, Liapi A, Antsaklis A.. Endometriosis in patients with chronic pelvic pain: is staging predictive of the efficacy of laparoscopic surgery in pain relief? Gynecol Obstet Invest 2006;62:48–54. [DOI] [PubMed] [Google Scholar]
- Miller-Keane OTM, O’Toole MT.. Miller-Keane Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health. Philadelphia, PA, USA: Elsevier Health Sciences, 2005. [Google Scholar]
- Morgan-Ortiz F, Lopez-de la Torre MA, Lopez-Zepeda MA, Morgan-Ruiz FV, Ortiz-Bojorquez JC, Bolivar-Rodriguez MA.. Clinical characteristics and location of lesions in patients with deep infiltrating endometriosis: using the revised Enzian classification. J Turk Ger Gynecol Assoc 2018;20:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi N, Roy KK, Kumar S, Nair VG, Vanamail P.. Clinical outcome analysis and correlation of reproductive outcome with endometriosis fertility index in laparoscopically managed endometriosis patients: a retrospective cohort study. J Hum Reprod Sci 2019;12:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaus K, Zschauer S, Bräuer D, Jimenez-Cruz J, Lehmann T, Rengsberger M, Diebolder H, Runnebaum IB.. Extensive endometriosis surgery: rASRM and Enzian score independently relate to post-operative complication grade. Arch Gynecol Obstet 2020;301:699–706. [DOI] [PubMed] [Google Scholar]
- Pal L, Shifren JL, Isaacson KB, Chang Y, Leykin L, Toth TL.. Impact of varying stages of endometriosis on the outcome of in vitro fertilization-embryo transfer. J Assist Reprod Genet 1998;15:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop-Trajkovic S, Popović J, Antić V, Radović D, Stefanović M, Stavanovic M, Vukomanović P.. Stages of endometriosis: does it affect in vitro fertilization outcome. Taiwan J Obstet Gynecol 2014;53:224–226. [DOI] [PubMed] [Google Scholar]
- Posadzka E, Jach R, Pityński K, Nocuń A.. Ovarian reserve assessment in women with different stages of pelvic endometriosis. Ginekol Pol 2014;85:446–450. [DOI] [PubMed] [Google Scholar]
- Radner H, Neogi T, Smolen JS, Aletaha D.. Performance of the 2010 ACR/EULAR classification criteria for rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2014;73:114–123. [DOI] [PubMed] [Google Scholar]
- Riiskjær M, Egekvist AG, Hartwell D, Forman A, Seyer-Hansen M, Kesmodel US.. Bowel Endometriosis Syndrome: a new scoring system for pelvic organ dysfunction and quality of life. Hum Reprod 2017;32:1812–1818. [DOI] [PubMed] [Google Scholar]
- Rock JA, Guzick DS, Sengos C, Schweditsch M, Sapp KC, Jones HW Jr.. The conservative surgical treatment of endometriosis: evaluation of pregnancy success with respect to the extent of disease as categorized using contemporary classification systems. Fertil Steril 1981;35:131–137. [DOI] [PubMed] [Google Scholar]
- Rock JA. The revised American Fertility Society classification of endometriosis: reproducibility of scoring. ZOLADEX Endometriosis Study Group. Fertil Steril 1995;63:1108–1110. [DOI] [PubMed] [Google Scholar]
- Rolla E. Endometriosis: advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Res 2019;8:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliep KC, Chen Z, Stanford JB, Xie Y, Mumford SL, Hammoud AO, Boiman Johnstone E, Dorais JK, Varner MW, Buck Louis GM. et al. Endometriosis diagnosis and staging by operating surgeon and expert review using multiple diagnostic tools: an inter-rater agreement study. BJOG 2017;124:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliep KC, Stanford JB, Chen Z, Zhang B, Dorais JK, Boiman Johnstone E, Hammoud AO, Varner MW, Louis GM, Peterson CM.. Interrater and intrarater reliability in the diagnosis and staging of endometriosis. Obstet Gynecol 2012;120:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers AH. The clinical classification of malignant tumours: the TNM system. Can Med Assoc J 1971;105:836 passim. [PMC free article] [PubMed] [Google Scholar]
- Szendei G, Hernadi Z, Devenyi N, Csapo Z.. Is there any correlation between stages of endometriosis and severity of chronic pelvic pain? Possibilities of treatment. Gynecol Endocrinol 2005;21:93–100. [DOI] [PubMed] [Google Scholar]
- Tomassetti C, Bafort C, Meuleman C, Welkenhuysen M, Fieuws S, D'Hooghe T.. Reproducibility of the Endometriosis Fertility Index: a prospective inter-/intra-rater agreement study. BJOG 2020;127:107–114. [DOI] [PubMed] [Google Scholar]
- Tomassetti C, Geysenbergh B, Meuleman C, Timmerman D, Fieuws S, D'Hooghe T.. External validation of the endometriosis fertility index (EFI) staging system for predicting non-ART pregnancy after endometriosis surgery. Hum Reprod 2013;28:1280–1288. [DOI] [PubMed] [Google Scholar]
- Tompsett J, Leonardi M, Gerges B, Lu C, Reid S, Espada M, Condous G.. Ultrasound-based endometriosis staging system: validation study to predict complexity of laparoscopic surgery. J Minim Invasive Gynecol 2019;26:477–483. [DOI] [PubMed] [Google Scholar]
- Tuttlies F, Keckstein J, Ulrich U, Possover M, Schweppe KW, Wustlich M, Buchweitz O, Greb R, Kandolf O, Mangold R. et al. [ENZIAN-score, a classification of deep infiltrating endometriosis]. Zentralbl Gynakol 2005;127:275–281. [DOI] [PubMed] [Google Scholar]
- van der Wat J, Kaplan MD, Roman H, Da Costa C.. The use of modified virtual colonoscopy to structure a descriptive imaging classification with implied severity for rectogenital and disseminated endometriosis. J Minim Invasive Gynecol 2013;20:543–546. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Fedele L, Aimi G, De Giorgi O, Consonni D, Crosignani PG.. Reproductive performance, pain recurrence and disease relapse after conservative surgical treatment for endometriosis: the predictive value of the current classification system. Hum Reprod 2006;21:2679–2685. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG.. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod 2007;22:266–271. [DOI] [PubMed] [Google Scholar]
- Vesali S, Razavi M, Rezaeinejad M, Maleki-Hajiagha A, Maroufizadeh S, Sepidarkish M.. Endometriosis Fertility Index for predicting non-assisted reproductive technology pregnancy after endometriosis surgery: a systematic review and meta-analysis. BJOG 2020;127:800–809. [DOI] [PubMed] [Google Scholar]
- Wang W, Li R, Fang T, Huang L, Ouyang N, Wang L, Zhang Q, Yang D.. Endometriosis fertility index score maybe more accurate for predicting the outcomes of in vitro fertilisation than r-AFS classification in women with endometriosis. Reprod Biol Endocrinol 2013;11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Working group of ESGE ESHRE and WES Keckstein J, Becker CM, Canis M, Feki A, Grimbizis GF, Hummelshoj L, Nisolle M, Roman H, Saridogan E.. Recommendations for the surgical treatment of endometriosis Part 2: deep endometriosis. Facts Views Vis Obgyn 2020a;11:269–297. [PMC free article] [PubMed] [Google Scholar]
- Working group of ESGE ESHRE and WES Keckstein J, Becker CM, Canis M, Feki A, Grimbizis GF, Hummelshoj L, Nisolle M, Roman H, Saridogan E. et al. Recommendations for the surgical treatment of endometriosis. Part 2: deep endometriosis. Hum Reprod Open 2020b;2020:hoaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Working group of ESGE ESHRE and WES Saridogan E, Becker CM, Feki A, Grimbizis GF, Hummelshoj L, Keckstein J, Nisolle M, Tanos V, Ulrich UA, Vermeulen N. et al. Recommendations for the surgical treatment of endometriosis-part 1: ovarian endometrioma. Gynecol Surg 2017a;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Working group of ESGE ESHRE and WES Saridogan E, Becker CM, Feki A, Grimbizis GF, Hummelshoj L, Keckstein J, Nisolle M, Tanos V, Ulrich UA. et al. Recommendations for the Surgical Treatment of Endometriosis. Part 1: Ovarian Endometrioma. Hum Reprod Open 2017b;2017:hox016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun BH, Jeon YE, Chon SJ, Park JH, Seo SK, Cho S, Choi YS, Lee JS, Lee BS.. The prognostic value of individual adhesion scores from the Revised American Fertility Society Classification system for recurrent endometriosis. Yonsei Med J 2015;56:1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID. et al. The international glossary on infertility and fertility care, 2017. Hum Reprod 2017;32:1786–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Xu JN, Zhou Y, Zhou YF, Zhu SN, Xue Q.. Reproductive performance after surgery for endometriosis: predictive value of the revised American Fertility Society classification and the endometriosis fertility index. Gynecol Obstet Invest 2014;77:180–185. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu D, Huang W, Wang Q, Feng X, Tan J.. Prediction of Endometriosis Fertility Index in patients with endometriosis-associated infertility after laparoscopic treatment. Reprod Biomed Online 2018;37:53–59. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lin L, Chen Z, Wang Y, Chen C, Li E, Wu R.. Fertility performance and the predictive value of the endometriosis fertility index staging system in women with recurrent endometriosis: a retrospective study. Medicine (Baltimore) 2019;98:e16965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Missmer SA.. Endometriosis. N Engl J Med 2020;382:1244–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are incorporated into the article.