Abstract
Context
Neurodevelopmental disorders are more prevalent in childhood-onset type 1 diabetes than in the general population, and the symptoms may limit the individual’s ability for diabetes management.
Objective
This study investigated whether comorbid neurodevelopmental disorders are associated with long-term glycemic control and risk of diabetic complications.
Methods
This population-based cohort study used longitudinally collected data from Swedish registers. We identified 11 326 individuals born during 1973-2013, diagnosed with type 1 diabetes during 1990-2013 (median onset age: 9.6 years). Among them, 764 had a comorbid neurodevelopmental disorder, including attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder, and intellectual disability. We used multinomial logistic regression to calculate odds ratios (ORs) of having poor glycemic control (assessed by glycated hemoglobin [HbA1c]) and Cox regression to estimate hazard ratios (HRs) of nephropathy and retinopathy.
Results
The median follow-up was 7.5 years (interquartile range [IQR] 3.9, 11.2). Having any neurodevelopmental disorder (ORadjusted 1.51 [95% CI 1.13, 2.03]), or ADHD (ORadjusted 2.31 [95% CI 1.54, 3.45]) was associated with poor glycemic control (mean HbA1c > 8.5%). Increased risk of diabetic complications was observed in patients with comorbid neurodevelopmental disorders (HRadjusted 1.72 [95% CI 1.21, 2.44] for nephropathy, HRadjusted 1.18 [95% CI 1.00, 1.40] for retinopathy) and patients with ADHD (HRadjusted 1.90 [95% CI 1.20, 3.00] for nephropathy, HRadjusted 1.33 [95% CI 1.07, 1.66] for retinopathy). Patients with intellectual disability have a particularly higher risk of nephropathy (HRadjusted 2.64 [95% CI 1.30, 5.37]).
Conclusion
Comorbid neurodevelopmental disorders, primarily ADHD and intellectual disability, were associated with poor glycemic control and a higher risk of diabetic complications in childhood-onset type 1 diabetes.
Keywords: neurodevelopmental disorders, glycemic control, nephropathy, retinopathy, type 1 diabetes
Type 1 diabetes is one of the most common endocrine diseases in childhood (1). The management of diabetes is a challenging task for pediatric patients, even with assistance from their parents, as effective neurocognitive skills are required to execute and comply with the management.
Neurodevelopmental disorders, such as attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and intellectual disability, are a group of conditions categorized together by the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) (DSM-5). Neurodevelopmental disorders are more prevalent in childhood-onset type 1 diabetes than in the general population (2, 3). The main features of neurodevelopmental disorders include learning difficulties, attention and memory problems, and limitations in social communication (4). These impairments can undermine patients’ abilities to execute complex tasks and may compromise glycemic control and increase the risk of diabetic complications (5). Yet, there is no prospective study investigating the association of comorbid neurodevelopmental disorders with diabetes management in childhood-onset type 1 diabetes.
A few cross-sectional studies have examined glycemic control in type 1 diabetes patients with comorbid neurodevelopmental disorders (6-11) (summarized in Supplemental Table 1 (12)). Generally, poorer glycemic control was observed in patients with comorbid ADHD (6-9), but not in those with ASD (10, 11, 13), compared with patients with type 1 diabetes alone. However, earlier research suffers from different definitions of ADHD and ASD, mostly based on self- or parent-reported questionnaire data (7, 8) or medical charts where diagnostic criteria have not been specified (6, 9, 13). Additionally, glycemic control in these studies was generally assessed by a point value of glycated hemoglobin (HbA1c). To our knowledge, there is also no study on glycemic control in type 1 diabetes with comorbid intellectual disability that is not due to a chromosomal abnormality.
Other psychiatric disorders, such as depression and eating disorders, have been associated with worse long-term diabetes outcomes (14, 15). Current diabetes guidelines also have emphasized that pediatric patients with psychological difficulties are more likely to develop diabetic complications (16, 17). Yet, there is a distinct lack of research on the comorbid neurodevelopmental disorders with the risk of long-term diabetic complications in childhood-onset type 1 diabetes.
In this study, we used Swedish registers, which have high coverage and prospectively collected information on medical diagnoses, HbA1c measurements, and diabetes follow-ups, to investigate the association of comorbid neurodevelopmental disorders with glycemic control and risk of diabetic complications in childhood-onset type 1 diabetes.
Methods
Study Design
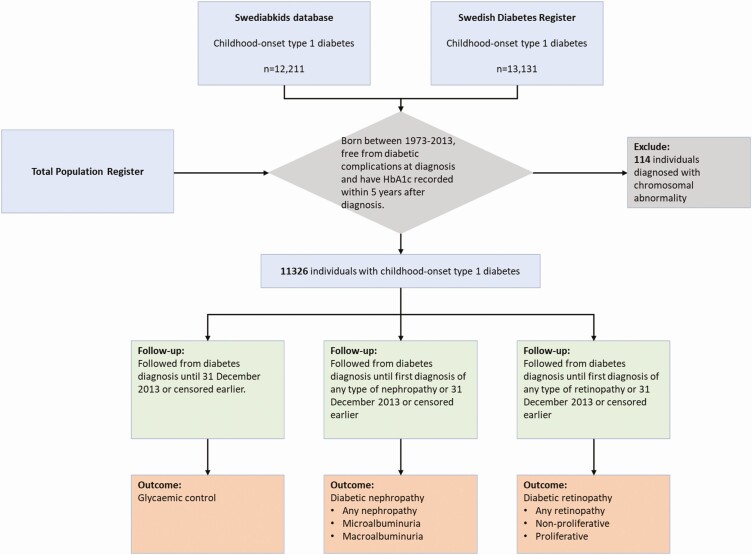
For this population-based cohort study, being consistent with previous studies (18, 19), we used data from Swedish registers to compose the type 1 diabetes patient cohort and retrieve relevant information. Details on registers involved in this study are described in Supplemental Table 2 (12). Individuals born in Sweden from 1973 and onwards with childhood-onset type 1 diabetes diagnosed before 18 years of age were identified from Swediabkids database and the Swedish Diabetes Register. The cohort was restricted to those who were free from diabetic complications at diabetes diagnosis and have documented HbA1c value within 5 years after a diabetes diagnosis. We have excluded patients with chromosomal abnormalities (International Classification of Disease [ICD] codes in Supplemental Table 3 (12)).
The start of follow-up was the date of diabetes diagnosis. For the analysis of glycemic control, the end of follow-up was the end of the study (December 31, 2013) or censoring (death or emigration), whichever occurred first. For the analyses of diabetic complications, the end of follow-up was the first diagnosis of nephropathy or retinopathy, the end of the study or censored earlier (Fig. 1).
Figure 1.
Study sample and follow-up of the cohort.
Neurodevelopmental Disorders
Lifetime diagnoses of neurodevelopmental disorders were identified from 5 registries: the National Patient Register (NPR) (20), the Clinical databases for Child and Adolescent Mental Health Services (21), the Habilitation Register (21) and the Halmstad University Register on Pupils with Intellectual Disability (22), and the Swedish Prescribed Drug Register (23). Diagnoses were defined in accordance with ICD codes for ASD (ICD-9: 299A, 299.8; ICD-10: F84.0, F84.1, F84.5, F84.8, F84.9) and intellectual disability (ICD-8: 310-315; ICD-9: 317-319; ICD-10: F70-F79). ADHD was defined as being diagnosed with ICD code (ICD-9: 314; ICD-10: F90) or prescribed with ADHD medication (Anatomical Therapeutic Chemical classification system codes: N06BA01-02, N06BA04, N06BA09). We decided to study neurodevelopmental disorders as a group of conditions based on the high comorbidity and phenotypic overlap across the diagnoses (4, 24). We grouped the patients into any neurodevelopmental disorders (at least 1 of the above diagnoses) and specific types of neurodevelopmental disorders, including (1) ADHD; (2) ASD; and (3) intellectual disability.
Glycemic Control
Glycemic control was evaluated using the mean of HbA1c measured between diabetes diagnosis and end of follow-up. HbA1c was reported according to the International Federation of Clinical Chemistry standard in millimoles per mole (mmol/mol) and converted into a percentage (%) according to the Diabetes Control and Complication Trial (25). Accuracy of HbA1c measurement in involved care units has been ensured by an external quality assessment scheme (26). The mean of HbA1c was calculated as the area under the curve divided by the time interval between the first and last recorded HbA1c. The area under the curve was estimated using the trapezoidal method, which accounted for time intervals between HbA1c measurements. Mean HbA1c levels were categorized as ≤6.5% (≤48 mmol/mol), 6.6% to 7.5% (49-58 mmol/mol), 7.6% to 8.5% (59-69 mmol/mol) and >8.5% (>69 mmol/mol).
Nephropathy and Retinopathy
Diabetic nephropathy and retinopathy were defined based on relevant ICD codes in the NPR and stated diagnosis criteria in the Swediabkids database and the Swedish Diabetes Register (corresponded ICD codes and diagnosis criteria in Supplemental Table 3 (12)). Endpoints of nephropathy were classified as any nephropathy, microalbuminuria, and macroalbuminuria. Endpoints of retinopathy were classified as any retinopathy, nonproliferative diabetic retinopathy, and proliferative diabetic retinopathy. Any retinopathy included any relevant diagnosis of retinopathy (simplex, preproliferative, or proliferative retinopathy or macular pathology). Proliferative diabetic retinopathy was defined as proliferation or earlier laser photocoagulation (18).
Other Measurements
Other variables obtained from the Swediabkids database and/or the Swedish Diabetes Register were systolic and diastolic blood pressure (mmHg), body mass index (BMI, kg/m2), high-density lipoprotein cholesterol (HDL, mmol/l), low-density lipoprotein cholesterol (LDL, mmol/l), triglycerides (mmol/l) and smoking status (18). Smoking status was defined as current or former (has ever reported ≥1 cigarette/day) or never. From the NPR, lifetime diagnoses of the following psychiatric diagnoses were retrieved: anxiety, mood disorder, psychotic disorders, eating disorder, psychoactive substance misuse, and other behavioral disorder (ICD codes in Supplemental Table 3 (12)) and summarized in a combined other psychiatric morbidity variable. Biological parents of the patients were identified through the Multi-Generation Register. Parental highest education level was acquired from the Longitudinal Integration Database for Health Insurance and Labour Studies and the population censuses from the years of 1970, 1975, and 1985. Parental psychiatric morbidity was defined as receiving any psychiatric diagnosis prior to the diabetes diagnosis date of the child (ICD codes in Supplemental Table 3 (12)).
Statistical Analysis
Baseline characteristics were compared between childhood-onset type 1 diabetes patients with and without comorbid neurodevelopmental disorders and are presented as means (SD) or median (interquartile range [IQR]) for continuous variables or percentage for categorical variables.
Comorbid Neurodevelopmental Disorders and Glycemic Control
We used multinomial logistic regression models to estimate the association between comorbid neurodevelopmental disorders and HbA1c levels using odds ratios (ORs) and 95% CIs. The multinomial regression model allows us to simultaneously estimate the odds of each of several possible outcomes (ie, having a higher HbA1c level) vs a single reference outcome (ie, having a reference HbA1c level) while taking into account that these outcomes are mutually exclusive. Hence, we estimated separate ORs for HbA1c 6.6% to 7.5% (49-58 mmol/mol) vs HbA1c ≤ 6.5% (≤48 mmol/mol); HbA1c 7.6% to 8.5% (59-69 mmol/mol) vs HbA1c ≤ 6.5% (≤48 mmol/mol); and HbA1c > 8.5% (>69 mmol/mol) vs HbA1c ≤ 6.5% (≤48 mmol/mol). We also fitted a model adjusted for sex, age at diabetes diagnosis, year of birth and year of diabetes diagnosis, other psychiatric morbidity, parental highest education level, parental psychiatric morbidity, smoking status, mean BMI, and mean systolic and diastolic blood pressure.
Comorbid Neurodevelopmental Disorders and Diabetic Nephropathy and Retinopathy
We used Cox regression models to estimate hazard ratios (HRs) of neurodevelopmental disorders for nephropathy and retinopathy in type 1 diabetes patients, using time since diabetes diagnosis as the underlying time metric. In addition to a crude model, Model 1 was adjusted for sex, age at diabetes diagnosis, year of birth and year of diabetes diagnosis, other psychiatric morbidity, parental highest education level, and parental psychiatric morbidity. To better understand the potential pathway that might mediate the association between comorbid neurodevelopmental disorders and diabetic complications, we further adjusted for mean HbA1c levels (Model 2) and additionally adjusted for mean BMI, mean systolic and diastolic blood pressure, and smoking status (Model 3). In patients who had records in the Swedish Diabetes Register, that is, had follow-up data through adulthood, we repeated these analyses with another model (Model 4), adjusting for means of HDL, LDL, and triglycerides.
We also fitted a model with additional adjustment for the baseline insulin administration method (multiple daily injection or insulin pump therapy) and repeated the analyses in individuals who used the same insulin administration method throughout the follow-up, attempting to capture possible influences from insulin regimen in the associations between comorbid neurodevelopmental disorders and diabetes outcomes.
Tests were 2-tailed and conducted at a 0.05 significance level. All data management was conducted in SAS software version 9.4 (SAS Institute, Cary, NC) and statistical analyses were performed using R version 3.6.1.
Results
Characteristics of the Study Sample
A total of 11 326 individuals with childhood-onset type 1 diabetes (diagnosed < 18 years of age) fulfilled the inclusion criteria and were included in this study (Fig. 1). All patients were diagnosed with type 1 diabetes during 1990-2013. Among them, 764 (6.8%) had at least one neurodevelopmental disorder, including 415 (3.7%) who were diagnosed with ADHD, 89 (0.8%) with ASD, 71 (0.6%) with intellectual disability, and 189 (1.6%) who were diagnosed with more than one disorder.
Table 1 summarizes the characteristics of type 1 diabetes patients with and without neurodevelopmental disorders. There were more males among patients with neurodevelopmental disorders (69.8%), especially in those with ADHD (68.4%) and ASD (74.2%), compared with those without neurodevelopmental disorders (54.3%). At baseline, the insulin administration method was not statistically significant between patients with and without neurodevelopmental disorders. However, at the end of the follow-up, there was a statistically significantly higher proportion of patients using insulin pump therapy among those without neurodevelopmental disorders. Notably, type 1 diabetes patients with comorbid intellectual disability had the lowest proportion of using insulin pump, with only 18.3% of patients using this therapy at the end of the observation. Patients with neurodevelopmental disorders were also more likely to be diagnosed with other psychiatric disorders and to smoke, especially those with comorbid ADHD. The highest parental education level was lower (40.8% postsecondary education) in the group of patients with neurodevelopmental disorders compared with those without (53.3% postsecondary education). Parents of patients with neurodevelopmental disorders were more likely to have psychiatric morbidity themselves (maternal: 13.6%, paternal: 12.3%) compared with parents of those without (maternal: 7.3%, paternal: 6.1%).
Table 1.
Characteristics of childhood-onset type 1 diabetes (diagnosed < 18 years of age) with and without comorbid neurodevelopmental disorders
| No neurodevelopmental disorder | Any neurodevelopmental disordera | Attention-deficit/ hyperactivity disorder | Autism spectrum disorder | Intellectual disability | |
|---|---|---|---|---|---|
| N (%) | 10,562 (93.3) | 764 (6.8) | 415 (3.7) | 89 (0.8) | 71 (0.6) |
| Sex, n (%) | |||||
| Male | 5737 (54.3) | 533 (69.8) | 284 (68.4) | 66 (74.2) | 40 (56.3) |
| Female | 4825 (45.7) | 231 (30.2) | 131 (31.6) | 23 (25.8) | 31 (43.7) |
| Calendar year of diabetes diagnosis | |||||
| 1990-1999 | 1273 (12.0) | 72 (9.4) | 40 (9.6) | 7 (7.9) | 7 (9.9) |
| 1999-2013 | 9289 (88.0) | 692 (90.6) | 375 (90.4) | 82 (92.1) | 64 (90.1) |
| Age at diabetes diagnosis, years | |||||
| Mean (SD) | 9.5 (4.6) | 9.9 (4.3) | 9.8 (4.1) | 9.9 (4.5) | 10.4 (4.2) |
| Median (IQR) | 9.5 (5.7,13.4) | 10.3 (6.6,13.2) | 9.9 (6.5,12.9) | 10.1 (6.6,13.6) | 11.0 (7.1,13.2) |
| Age at the end of follow-up, years b | |||||
| Mean (SD) | 17.4 (6.6) | 18.1 (5.0) | 17.8 (4.6) | 18.5 (5.8) | 19.1 (5.6) |
| Median (IQR) | 17.7 (12.7,21.7) | 18.3 (14.6,21.5) | 18.1 (14.6,21.1) | 18.0 (15.2,22.2) | 19.6 (15.3,23.1) |
| Length of follow-up, years b | |||||
| Mean (SD) | 7.9 (4.9) | 8.2 (4.4) | 8.1 (4.4) | 8.5 (4.6) | 8.7 (4.3) |
| Median (IQR) | 7.4 (3.8,11.2) | 8.4 (4.8,11.3) | 8.2 (4.5,11.1) | 8.2 (5.0,11.0) | 9.3 (5.1,11.8) |
| Baseline insulin administration method, n (%) | |||||
| Multiple daily injections | 7821 (74.0) | 578 (75.7) | 301 (72.5) | 66 (74.2) | 63 (88.7) |
| Insulin pump | 2545 (24.1) | 176 (23.0) | 111 (26.7) | 22 (24.7) | 6 (8.45) |
| Missing | 196 (1.9) | 10(1.3) | 3 (0.7) | 1 (1.12) | 2 (2.82) |
| Last recorded insulin administration method, n (%) | |||||
| Multiple daily injections | 6267 (59.3) | 498 (65.2) | 265 (63.9) | 48 (53.9) | 56 (78.9) |
| Insulin pump | 4105 (38.9) | 256 (33.5) | 147 (35.4) | 40 (44.9) | 13 (18.3) |
| Missing | 189 (1.8) | 10 (1.3) | 3 (0.7) | 1 (1.1) | 2 (2.8) |
| Other psychiatric morbidity prior to the end of follow-up, n (%) | |||||
| Yes | 1229 (11.6) | 330 (43.2) | 197 (47.5) | 39 (43.8) | 15 (21.1) |
| Parental highest education level, n (%) | |||||
| Primary and lower secondary education | 270 (2.6) | 38 (5.0) | 21 (5.0) | 1 (1.2) | 6 (8.5) |
| Upper secondary education | 4663 (44.1) | 414 (54.2) | 234 (56.4) | 40 (44.9) | 58 (67.6) |
| Postsecondary education | 5628 (53.3) | 312 (40.8) | 160 (38.6) | 48 (53.9) | 17 (23.9) |
| Missing | 1 | - | - | - | - |
| Maternal psychiatric morbidity, n (%) | |||||
| Yes | 768 (7.3) | 104 (13.6) | 59 (14.2) | 8 (9.0) | 6 (8.5) |
| Missing | 23 (0.2) | 10 (1.3) | 8 (1.9) | 1 (1.1) | - |
| Paternal psychiatric morbidity, n (%) | |||||
| Yes | 641 (6.1) | 94 (12.3) | 50 (12.0) | 10 (11.2) | 7 (9.9) |
| Missing | 26 (0.2) | 4 (0.5) | 2 (0.5) | - | - |
| Smoking status, n (%) | |||||
| Current or previous smoker | 1193 (11.3) | 182 (23.8) | 126 (30.4) | 11 (12.4) | 10 (14.1) |
| Missing | 1515 (14.3) | 79 (10.3) | 45 (10.8) | 7 (7.9) | 9 (12.7) |
| Mean BMI, kg/m 2 | |||||
| Mean (SD) | 20.5 (3.8) | 21.0 (4.0) | 20.7 (3.6) | 21.3 (4.6) | 21.8 (4.7) |
| Median (IQR) | 20.0 (17.7,22.7) | 20.2 (18.3,23.0) | 20.0 (18.4,22.6) | 20.1 (18.6,22.6) | 21.7 (18.0,24.7) |
| Missing, n (%) | 47 (0.4) | 9 (1.2) | 3 (0.7) | 1 (1.1) | 4 (5.6) |
| Mean systolic blood pressure, mmHg | |||||
| Mean (SD) | 112.3 (10.2) | 112.6 (9.6) | 112.5 (9.6) | 112.9 (9.7) | 112.6 (8.8) |
| Median (IQR) | 112.4 (105.6,118.9) | 112.6 (106.3,118.7) | 112.6 (106.2,118.4) | 112.5 (107.0,119.4) | 114.2 (106.7,118.3) |
| Missing, n (%) | 1436 (13.6) | 63 (8.3) | 31 (7.5) | 8 (9.0) | 9 (12.7) |
| Mean diastolic blood pressure, mmHg | |||||
| Mean (SD) | 66.9 (6.3) | 68.0 (5.9) | 67.6 (6.0) | 68.1 (5.3) | 68.7 (6.1) |
| Median (IQR) | 66.9 (62.9,71.0) | 67.9 (64.1,72.1) | 67.2 (63.4,72.0) | 68.2 (65.3,71.2) | 68.3 (64.4,72.9) |
| Missing, n (%) | 1436 (13.6) | 63 (8.3) | 31 (7.5) | 8 (9.0) | 9 (12.7) |
All characteristics were statistically significantly (P value < 0.05) different between patients without and with any or specific diagnosis of neurodevelopmental disorders, except for age of diabetes diagnosis (P = 0.21) and baseline insulin administration method (P = 0.14).
aAny neurodevelopmental disorder group comprised patients diagnosed with at least one of attention-deficit/hyperactivity disorder, autism spectrum disorder, and intellectual disability. For the 189 patients who received more than 1 diagnosis, characteristics are presented in Supplemental Table 4.
bFollow-up period for glycemic control as the outcome, calculated from the date of type 1 diabetes diagnosis to the end of follow-up, defined as the end of study (December 31, 2013) or censored earlier due to migration or death.
Glycemic Control
For the study sample, HbA1c was measured every 3.7 months (median; IQR, 3.0, 5.2). A higher mean HbA1c value was observed in patients with neurodevelopmental disorders, especially those with ADHD and intellectual disability, but not in patients with ASD, compared with patients without neurodevelopmental disorders (Table 2). Poor glycemic control (mean HbA1c > 8.5% [>69 mmol/mol]) presented in 18.9% of patients without neurodevelopmental disorders and was more commonly seen in patients with comorbid neurodevelopmental disorders: 31.8% of patients with any neurodevelopmental disorders, 38.1% of those with ADHD, 19.0% of those with ASD, and 31% of those with intellectual disability. Unadjusted and adjusted multinomial logistic regression models estimated the odds of having different levels of mean HbA1c. Compared with patients without neurodevelopmental disorders, those with any neurodevelopmental disorders had an odds 1.51 times higher of having mean HbA1c > 8.5% (>69 mmol/mol) than having mean HbA1c ≤ 6.5% (≤48 mmol/mol). A similar pattern was also observed in patients with ADHD but with a higher odds (ORadjusted 2.31 [95% CI 1.54, 3.45]), but not in those with ASD or intellectual disability.
Table 2.
Comparison of glycemic control between childhood-onset type 1 diabetes patients with and without comorbid neurodevelopmental disorders
| Comorbid diagnosis of neurodevelopmental disorder | Mean HbA1c (SD) | Crude modela | Adjusted modelb | |||||
|---|---|---|---|---|---|---|---|---|
| % | mmol/mol | 6.6%-7.5% (49-59 mmol/mol) vs ≤6.5% (≤48 mmol/mol) | 7.6%-8.5% (60-69 mmol/mol) vs ≤6.5% (≤48 mmol/mol) | >8.5% (>69 mmol/mol) vs ≤6.5% (≤48 mmol/mol) | 6.6%-7.5% (49-59 mmol/mol) vs ≤6.5% (≤48 mmol/mol) | 7.6%-8.5% (60-69 mmol/mol) vs ≤6.5% (≤48 mmol/mol) | >8.5% (>69 mmol/mol) vs ≤6.5% (≤48 mmol/mol) | |
| No neurodevelopmental disorder | 7.7 (1.1) | 60.6 (11.6) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Any neurodevelopmental disorder | 8.1 (1.3) | 64.8 (13.7) | 0.81 (0.62, 1.06) | 1.36 (1.06, 1.76) | 2.12 (1.64,2.75) | 0.84 (0.63, 1.12) | 1.30 (0.98, 1.72) | 1.51 (1.13, 2.03) |
| Attention-deficit/hyperactivity disorder | 8.3 (1.3) | 66.7 (14.1) | 0.79 (0.54, 1.16) | 1.49 (1.04, 2.14) | 2.90 (2.03, 4.15) | 0.87 (0.58, 1.30) | 1.56 (1.05, 2.30) | 2.31 (1.54, 3.45) |
| Autism spectrum disorder | 7.7 (1.1) | 60.8 (11.7) | 0.89 (0.45, 1.75) | 1.13 (0.58, 2.20) | 1.02 (0.48, 2.13) | 0.78 (0.38, 1.57) | 0.88 (0.43, 1.78) | 0.58 (0.26, 1.30) |
| Intellectual disability | 8.0 (1.2) | 63.5 (13.0) | 0.73 (0.44, 1.20) | 1.29 (0.81, 2.05) | 1.37 (0.83, 2.26) | 1.00 (0.42, 2.41) | 1.08 (0.45, 2.60) | 1.41 (0.57, 3.49) |
aMultinomial logistic regression model.
bAdditionally adjusted for sex, age at diabetes diagnosis, year of birth and year of diabetes diagnosis, other psychiatric morbidity, smoking status, mean BMI, mean systolic and diastolic blood pressure, parental highest education level, and parental psychiatric morbidity.
Risk of Diabetic Nephropathy and Retinopathy
Table 3 shows the risk of diabetic nephropathy and retinopathy in relation to comorbid neurodevelopmental disorders in type 1 diabetes patients using unadjusted and adjusted Cox models.
Table 3.
Incidence rates (IRs) and hazard ratios (HRs) with 95% CIs for diabetic complications between childhood-onset type 1 diabetes with and without comorbid neurodevelopmental disorders.
| Comorbid diagnosis of neurodevelopmental disorder | Diabetic complications | |||||
|---|---|---|---|---|---|---|
| Any nephropathy | ||||||
| Number of outcomes/ person-years | IR (95% CI) | Crude model, HR (95% CI) | Model 1a, HR (95% CI) | Model 2b, HR (95% CI) | Model 3c, HR (95% CI) | |
| No neurodevelopmental disorder | 416/81,141 | 5.13 (4.65, 5.64) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Any neurodevelopmental disorder | 43/6154 | 6.99 (5.06, 9.41) | 1.41 (1.03, 1.93) | 1.69 (1.21, 2.35) | 1.66 (1.20, 2.31) | 1.72 (1.21, 2.44) |
| Attention-deficit/hyperactivity disorder | 24/3266 | 7.35 (4.71, 10.93) | 1.50 (0.99, 2.26) | 1.94 (1.26, 2.99) | 1.81 (1.17, 2.79) | 1.90 (1.20, 3.00) |
| Autism spectrum disorder | 4/726 | 5.51 (1.50, 14.10) | 1.11 (0.42, 2.98) | 1.27 (0.47, 3.43) | 1.39 (0.51, 3.74) | 0.79 (0.19, 3.18) |
| Intellectual disability | 8/604 | 13.24 (5.72, 26.09) | 2.57 (1.28, 5.17) | 2.85 (1.13, 4.62) | 2.27 (1.12, 4.59) | 2.64 (1.30, 5.37) |
| Any retinopathy | ||||||
| Number of outcomes /person-years | IR (95% CI) | Crude model, HR (95% CI) | Model 1a, HR (95% CI) | Model 2b, HR (95% CI) | Model 3c, HR (95% CI) | |
| No neurodevelopmental disorder | 2429/74,082 | 32.79 (31.50, 34.12) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Any neurodevelopmental disorder | 199/5632 | 35.33 (30.60, 40.60) | 1.11 (0.96, 1.29) | 1.23 (1.06, 1.43) | 1.18 (1.01, 1.37) | 1.18 (1.00, 1.40) |
| Attention-deficit/hyperactivity disorder | 110/3001 | 36.66 (30.13, 44.18) | 1.18 (0.97, 1.43) | 1.39 (1.13, 1.69) | 1.30 (1.06, 1.58) | 1.33 (1.07, 1.66) |
| Autism spectrum disorder | 27/668 | 40.42 (26.63, 58.81) | 1.28 (0.88, 1.87) | 1.50 (1.02, 2.20) | 1.43 (0.96, 2.13) | 1.34 (0.85, 2.12) |
| Intellectual disability | 16/633 | 28.93 (16.53, 46.98) | 0.90 (0.55, 1.46) | 0.79 (0.48, 1.29) | 0.75 (0.45, 1.26) | 0.73 (0.41, 1.28) |
aModel 1: Cox model adjusted for sex, age at diabetes diagnosis, year of birth and year of diabetes diagnosis, other psychiatric morbidity, parental highest education level and parental psychiatric morbidity.
bModel 2: Additionally adjusted for mean HbA1c levels.
cModel 3: Additionally adjusted for mean BMI, systolic and diastolic blood pressure, and smoking status.
Over a median follow-up period of 7.30 years, 459 (4.1%) patients with childhood-onset type 1 diabetes were diagnosed with any diabetic nephropathy. The median time from the onset of diabetes to nephropathy was 8.10 years (IQR, 5.75, 11.33). Incidence rates of any diabetic nephropathy were higher in those with comorbid neurodevelopmental disorders than that those without. After adjustment for covariates, patients with any neurodevelopmental disorders were at a statistically significantly higher risk of diabetic nephropathy (HRadjusted 1.72 [95% CI 1.21, 2.44]). HRs differed across specific types of neurodevelopmental disorders: patients with ADHD and patients with intellectual disability have statistically significantly higher risks, with HRadjusted of 1.90 (95% CI 1.20, 3.00) and 2.64 (95% CI 1.30, 5.37), respectively.
When looking at specific diagnoses of microalbuminuria and macroalbuminuria, 373 patients were diagnosed with microalbuminuria, and 72 with macroalbuminuria. We found that patients with any neurodevelopmental disorders had statistically significantly higher risks of both microalbuminuria (HRadjusted 1.61 [95% CI 1.07, 2.43]) and macroalbuminuria (HRadjusted 2.45 [95% CI 1.07, 5.59]). Notably, patients with intellectual disability also showed statistically significantly increased risks of both microalbuminuria (HRadjusted 2.89 [95% CI 1.35, 6.20]) and macroalbuminuria (HRadjusted 8.18 [95% CI 1.90, 35.28]) (Supplemental Table 5 (12)).
Over a median follow-up of 6.80 years, 2628 (23.2%) patients were diagnosed with any diabetic retinopathy. The median interval between diabetes and retinopathy was 8.59 years (IQR, 6.09, 11.25). The incidence rate of any retinopathy was higher in those with any neurodevelopmental disorders, ADHD, and ASD but lower in those with intellectual disability compared with those without neurodevelopmental disorders. We found statistically significant increased risks of retinopathy in those with any neurodevelopmental disorders (HRadjusted 1.18 [95% CI 1.00, 1.40]) and those with ADHD (HRadjusted 1.33 [95% CI 1.07, 1.66]). There were 1818 patients diagnosed with nonproliferative retinopathy. Only 36 patients were diagnosed with proliferative nephropathy. Six of these had a neurodevelopmental disorder, and no case was observed in patients with ASD or with intellectual disability (Supplemental Table 6 (12)).
Analyses in patients who have records in Swedish Diabetes Register showed that additional adjustment for mean HDL, LDL, and triglycerides, the associations for any and specific diabetic nephropathy and retinopathy were attenuated to the null, but the statistically significantly increased risk of any nephropathy remained in patients with intellectual disability (HRadjusted 3.24 [95% CI 1.41, 7.47]) (Supplemental Table 7 (12)).
Discussion
This is the first prospective nationwide register-based cohort study with longitudinally collected data investigating the association of comorbid neurodevelopmental disorders with glycemic control and diabetic complications in patients with childhood-onset type 1 diabetes. We found that type 1 diabetes patients with neurodevelopmental disorders were more likely to have poor glycemic control compared with those without neurodevelopmental disorders. The highest risk was associated with comorbid ADHD. We also observed increased risks of diabetic nephropathy and retinopathy in patients with neurodevelopmental disorders, where the risk depended on the specific types of neurodevelopmental disorders.
In general, patients with any neurodevelopmental disorders have higher mean HbA1c values, which were evaluated using longitudinally collected repeated measurement, compared with those without neurodevelopmental disorders. Children with neurodevelopmental disorders were more likely to have poor glycemic control (HbA1c > 8.5% [>69 mmol/mol]) rather than optimal glycemic level (HbA1c ≤ 6.5% [≤48 mmol/mol]). A few previous studies have cross-sectionally examined glycemic control in type 1 diabetes with comorbid ADHD (6-8) and ASD (10, 11, 13). In line with these studies, using patients without neurodevelopmental disorders as the reference group, we observed that patients with ADHD have a higher HbA1c level and a statistically significantly higher odds of experiencing poor glycemic control. In contrast, patients with ASD displayed a similar glycemic control as those without neurodevelopmental disorders. Our study is also the first to provide an insight into glycemic control in type 1 diabetes patients with intellectual disability without chromosomal abnormalities. Although these patients demonstrated higher mean HbA1c, their risk of having suboptimal glycemic control was not statistically significantly different from those without neurodevelopmental disorders.
These observed different associations with glycemic control across specific types of neurodevelopmental disorders may be explained by the distinct features of each neurodevelopmental diagnosis. For instance, ADHD is characterized by varying degrees of inattention, inability to plan or memorize tasks, impulsivity, and hyperactivity. Impairment of executive dysfunction has been previously recognized as a risk factor of poor glycemic control in type 1 diabetes patients (27). Although similar symptoms may also occur in ASD, it is more distinguishable that many ASD patients have restricted and repetitive behaviors, prefer routines, and tend to reduce unpredictability (28). These features may facilitate glycemic control. Moreover, children and adolescents with ASD or intellectual disability are more likely to depend on caregivers to manage their diabetes (13, 29). Presumably, caregivers may estimate the patients’ insulin needs more accurately and carry out a more structured management routine. Therefore, patients could maintain a similar glycemic control level as those without neurodevelopmental disorders.
To date, no study has investigated the association of comorbid neurodevelopmental disorders with the risk of chronic diabetic complications in patients with childhood-onset type 1 diabetes. Our findings suggest that patients with childhood-onset type 1 diabetes and neurodevelopmental disorders were at increased risk of microvascular complications. The observed increased risk of nephropathy and retinopathy in patients with neurodevelopmental disorders, especially in those with any type and ADHD, are to some extent in line with our observed glycemic control in these patients. Given that glycemic control is one of the most important risk factors for diabetic complications (18), we hypothesized that comorbid neurodevelopmental disorders might influence the risk of diabetic complications via interference with glycemic control. As expected, we observed decreased magnitudes of risk estimates for nephropathy and retinopathy after control for glycemic control levels in patients with any or specific types of neurodevelopmental disorders. Nevertheless, the increased risk of both complications in patients with any neurodevelopmental disorders and ADHD remained statistically significant even after adjusting for HbA1c levels. Similarly, the observed higher risk of nephropathy in patients with intellectual disability remained statistically significant.
To better understand the potential role of nonglycemic factors in these associations, we further adjusted for BMI, blood pressure, and smoking status, but risk estimates remained statistically significant. We have also considered possible influences from abnormal cholesterol and/or triglycerides, which are linked to neurodevelopmental disorders (30-33). Elevated LDL/HDL ratio and higher triglycerides level have been implicated in an increased risk of nephropathy and retinopathy (34, 35). However, due to the availability of information on LDL, HDL, and triglycerides, we can merely adjust for lipids profile in adulthood ( >18 years of age). This led to a substantial loss of statistical power, but the increased risk of nephropathy in those with intellectual disability remained statistically significant. These findings somewhat support earlier evidence on the existence of risk factors other than glycemic control in the pathogenesis of diabetic complications in children and adolescents with type 1 diabetes (36, 37).
In our study sample, very few cases of advanced-stage complications occurred. This is in line with existing evidence that these complications are uncommonly found in pediatric patients (38). However, we did observe a statistically significantly increased risk of macroalbuminuria in patients with any neurodevelopmental disorders and intellectual disability. However, as mentioned above, given the small number of patients who developed the outcome, further studies are still warranted.
Strengths of this study include the population-based design with prospectively collected information on diabetes-related measurements and medical diagnoses. Given that neurodevelopmental disorders sometimes co-occur and have few comparable symptoms, diagnoses from psychiatric experts are vital for disease classification. Our diagnoses of neurodevelopmental disorders using ICD codes from high-quality registers minimized potential classification bias (20, 22).
This study also has some limitations. Due to the observational nature of this register-based study, we could not eliminate potential residual confounding. We had no data on parental involvement or detailed diabetes regimens, such as insulin dosage and use of continuous glucose monitoring devices or therapeutic interventions for neurodevelopmental disorders—this lack of data restricted our ability to comprehensively examine their potential mediation role in the observed associations. However, our fitted model with adjustment of baseline insulin administration method did not materially change the observed association (Supplemental Table 8 (12)), and neither did the additional analyses in patients who use the same insulin administration method throughout the follow-up (Supplemental Table 9 (12)), regardless of a substantial loss of statistical power. Despite our large sample size of childhood-onset type 1 diabetes, statistical power for comorbid ASD or intellectual disability was still not sufficient. For a similar reason, we were not able to comprehensively examine the outcomes in patients with more than one neurodevelopmental disorder. Nevertheless, for those with both ADHD and ASD (n = 111), we performed the analyses (Supplemental Table 10 (12)) and found the magnitudes of estimates being similar to those of ADHD alone but with widened confidence intervals. Furthermore, considering that symptoms of neurodevelopmental disorders change over time and that diagnoses often are stated years after the first symptoms are presented, the exact time of onset is not as precise as diabetes diagnosis. Thus, we cannot make any conclusion on the causality.
Given the adverse long-term outcome from neurodevelopmental disorders, special attention from the diabetes care team is needed for pediatric patients with type 1 diabetes with comorbid neurodevelopmental disorders. Disparities across types of neurodevelopmental disorders should be noticed and would be important in diabetes complication prevention. Further, diabetes education adjusted to the needs of patients with neurodevelopmental disorders should be provided to the patients and their parents. Family and social support would be beneficial for improving the adherence to diabetes regimen in children with diabetes and neurodevelopmental disorders, as their ability to manage the disease may be hampered by the comorbidity.
In conclusion, the current study suggests that childhood-onset type 1 diabetes patients with neurodevelopmental disorders, especially those with ADHD or intellectual disability, are more prone to poor glycemic control and a higher risk of chronic diabetic complications compared with those without neurodevelopmental disorders. Comorbid neurodevelopmental disorders in type 1 diabetes should be considered while planning intervention and follow-ups in patients. Further longitudinal studies with a more comprehensive evaluation of diabetes management and molecular data are needed to provide insight into potential mediators in the association between comorbid neurodevelopmental disorder and diabetes complications in type 1 diabetes.
Acknowledgments
Financial Support: This study was funded by the Swedish Research Council (No 2017-00788), Stockholm Region Council (No 20180718) and Karolinska Institutet, Strategic Research Program in Neuroscience (StratNeuro).
Glossary
Abbreviations
- ADHD
attention-deficit/hyperactivity disorder
- ASD
autism spectrum disorder
- BMI
body mass index
- HbA1c
glycated hemoglobin A1c
- HDL
high-density lipoprotein
- HR
hazard ratio
- ICD
International Classification of Disease
- IQR
interquartile range
- LDL
low-density lipoprotein
- NPR
National Patient Register
- OR
odds ratio
Additional Information
Disclosures: H. Larsson has served as a speaker for Evolan Pharma and Shire and has received research grants from Shire; all outside the submitted work. J. Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG) and has received funding from Janssen Corporation; all outside the submitted work. Other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Data Availability
The data that support the findings of this study are available from the National Board of Health Welfare in Sweden, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of the National Board of Health Welfare in Sweden (www.socialstyrelsen.se).
Ethics Approval: This study was approved by the Regional Ethical Review Board in Stockholm (Dnr: 2013/862-31/5).
References
- 1. International Diabetes Federation. IDF Diabetes Atlas, 9th ed. Brussels, Belgium: International Diabetes Federation;2019. [Google Scholar]
- 2. Butwicka A, Frisén L, Almqvist C, Zethelius B, Lichtenstein P. Risks of psychiatric disorders and suicide attempts in children and adolescents with type 1 diabetes: a population-based cohort study. Diabetes Care. 2015;38(3):453-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dybdal D, Tolstrup JS, Sildorf SM, et al. Increasing risk of psychiatric morbidity after childhood onset type 1 diabetes: a population-based cohort study. Diabetologia. 2018;61(4):831-838. [DOI] [PubMed] [Google Scholar]
- 4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth ed. American Psychiatric Association; 2013. [Google Scholar]
- 5. Duke DC, Harris MA. Executive function, adherence, and glycemic control in adolescents with type 1 diabetes: a literature review. Curr Diab Rep. 2014;14(10):532. [DOI] [PubMed] [Google Scholar]
- 6. Hilgard D, Konrad K, Meusers M, et al. ; German/Austrian DPV Study Group, the Working Group on Psychiatric, Psychotherapeutic Psychological Aspects of Paediatric Diabetology (PPAG e.V.) and the BMBF Competence Network Diabetes, Germany . Comorbidity of attention deficit hyperactivity disorder and type 1 diabetes in children and adolescents: Analysis based on the multicentre DPV registry. Pediatr Diabetes. 2017;18(8):706-713. [DOI] [PubMed] [Google Scholar]
- 7. Macek J, Battelino T, Bizjak M, et al. Impact of attention deficit hyperactivity disorder on metabolic control in adolescents with type1 diabetes. J Psychosom Res. 2019;126:109816. [DOI] [PubMed] [Google Scholar]
- 8. Yazar A, Akın F, Akça ÖF, et al. The effect of attention deficit/hyperactivity disorder and other psychiatric disorders on the treatment of pediatric diabetes mellitus. Pediatr Diabetes. 2019;20(3):345-352. [DOI] [PubMed] [Google Scholar]
- 9. Vinker-Shuster M, Golan-Cohen A, Merhasin I, Merzon E. Attention-Deficit Hyperactivity Disorder in Pediatric Patients With Type 1 Diabetes Mellitus: Clinical Outcomes and Diabetes Control. J Dev Behav Pediatr. 2019;40(5):330-334. [DOI] [PubMed] [Google Scholar]
- 10. Lemay JF, Lanzinger S, Pacaud D, et al. ; German/Austrian DPV Initiative . Metabolic control of type 1 diabetes in youth with autism spectrum disorder: A multicenter Diabetes-Patienten-Verlaufsdokumentation analysis based on 61 749 patients up to 20 years of age. Pediatr Diabetes. 2018;19(5):930-936. [DOI] [PubMed] [Google Scholar]
- 11. Stanek KR, Youngkin EM, Pyle LL, Raymond JK, Driscoll KA, Majidi S. Prevalence, characteristics, and diabetes management in children with comorbid autism spectrum disorder and type 1 diabetes. Pediatr Diabetes. 2019;20(5):645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu S, Kuja-Halkola R, Larsson H, et al. Neurodevelopmental disorders, glycaemic control and diabetic complications in type 1 diabetes a nationwide cohort study_Supplemental Tables.docx. figshare. Posted May 19, 2021. doi: 10.6084/m9.figshare.14616945.v1 [DOI] [PMC free article] [PubMed]
- 13. Bethin KE, Kanapka LG, Laffel LM, et al. ; T1D Exchange Clinic Network . Autism spectrum disorder in children with Type 1 diabetes. Diabet Med. 2019;36(10):1282-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nielsen S. Eating disorders in females with type 1 diabetes: an update of a meta-analysis. Eur Eat Disord Rev. 2002;10(4):241-254. [Google Scholar]
- 15. de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619-630. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association (2020) . 13. Children and adolescents: Standards of Medical Care in Diabetes. Diabetes Care 2020;43(Suppl. 1):S163-S182. [DOI] [PubMed] [Google Scholar]
- 17. Delamater AM, de Wit M, McDarby V, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Psychological care of children and adolescents with type 1 diabetes. Pediatr Diabetes. 2018;19(Suppl 27):237-249. [DOI] [PubMed] [Google Scholar]
- 18. Lind M, Pivodic A, Svensson AM, Ólafsdóttir AF, Wedel H, Ludvigsson J. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ. 2019;366:l4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu S, Kuja-Halkola R, Larsson H, et al. Poor glycaemic control is associated with increased risk of neurodevelopmental disorders in childhood-onset type 1 diabetes: a population-based cohort study. Diabetologia. 2021;64(4):767-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Idring S, Rai D, Dal H, et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. Plos One. 2012;7(7):e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lundh A, Forsman M, Serlachius E, Lichtenstein P, Landén M. Outcomes of child psychiatric treatment. Acta Psychiatr Scand. 2013;128(1):34-44. [DOI] [PubMed] [Google Scholar]
- 23. Sun X, Wu Z, Cao Q, et al. Genetic variant for behavioral regulation factor of executive function and its possible brain mechanism in attention deficit hyperactivity disorder. Sci Rep. 2018;8(1):7620. doi:10.1038/s41598-018-26042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris-Rosendahl DJ, Crocq MA. Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin Neurosci. 2020;22(1):65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoelzel W, Weykamp C, Jeppsson JO, et al. ; IFCC Working Group on HbA1c Standardization . IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50(1):166-174. [DOI] [PubMed] [Google Scholar]
- 26. Nordin G. Accuracy of HbA1c as Monitored by External Quality Assessment and Compared With Patient Mean Values. J Diabetes Sci Technol. 2018;12(4):771-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duke DC, Harris MA. Executive function, adherence, and glycemic control in adolescents with type 1 diabetes: a literature review. Curr Diab Rep. 2014;14(10):532. [DOI] [PubMed] [Google Scholar]
- 28. Fakhoury M. Autistic spectrum disorders: A review of clinical features, theories and diagnosis. Int J Dev Neurosci. 2015;43:70-77. [DOI] [PubMed] [Google Scholar]
- 29. McVilly K, McGillivray J, Curtis A, Lehmann J, Morrish L, Speight J. Diabetes in people with an intellectual disability: a systematic review of prevalence, incidence and impact. Diabet Med. 2014;31(8):897-904. [DOI] [PubMed] [Google Scholar]
- 30. Kim EK, Neggers YH, Shin CS, Kim E, Kim EM. Alterations in lipid profile of autistic boys: a case control study. Nutr Res. 2010;30(4):255-260. [DOI] [PubMed] [Google Scholar]
- 31. Trollor J, Salomon C, Curtis J, et al. Positive cardiometabolic health for adults with intellectual disability: an early intervention framework. Aust J Prim Health. 2016;22(4):288-293. [DOI] [PubMed] [Google Scholar]
- 32. Tyler CV, Schramm SC, Karafa M, Tang AS, Jain AK. Chronic disease risks in young adults with autism spectrum disorder: forewarned is forearmed. Am J Intellect Dev Disabil. 2011;116(5):371-380. [DOI] [PubMed] [Google Scholar]
- 33. Ugur C, Uneri OS, Goker Z, Sekmen E, Aydemir H, Solmaz E. The assessment of serum lipid profiles of children with attention deficit hyperactivity disorder. Psychiatry Res. 2018;264:231-235. [DOI] [PubMed] [Google Scholar]
- 34. Mäkinen VP, Soininen P, Kangas AJ, et al. ; Finnish Diabetic Nephropathy Study Group . Triglyceride-cholesterol imbalance across lipoprotein subclasses predicts diabetic kidney disease and mortality in type 1 diabetes: the FinnDiane Study. J Intern Med. 2013;273(4):383-395. [DOI] [PubMed] [Google Scholar]
- 35. Chaturvedi N, Fuller JH, Taskinen MR; EURODIAB PCS Group . Differing associations of lipid and lipoprotein disturbances with the macrovascular and microvascular complications of type 1 diabetes. Diabetes Care. 2001;24(12):2071-2077. [DOI] [PubMed] [Google Scholar]
- 36. Amin R, Williams RM, Frystyk J, et al. Increasing urine albumin excretion is associated with growth hormone hypersecretion and reduced clearance of insulin in adolescents and young adults with type 1 diabetes: the Oxford Regional Prospective Study. Clin Endocrinol (Oxf). 2005;62(2):137-144. [DOI] [PubMed] [Google Scholar]
- 37. Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K. Microvascular and macrovascular complications associated with diabetes in children and adolescents. Pediatr Diabetes. 2009;10(Suppl 12):195-203. [DOI] [PubMed] [Google Scholar]
- 38. Sochett E, Daneman D. Early diabetes-related complications in children and adolescents with type 1 diabetes. Implications for screening and intervention. Endocrinol Metab Clin North Am. 1999;28(4):865-882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the National Board of Health Welfare in Sweden, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of the National Board of Health Welfare in Sweden (www.socialstyrelsen.se).
Ethics Approval: This study was approved by the Regional Ethical Review Board in Stockholm (Dnr: 2013/862-31/5).