Abstract
Context
Gray matter morphology in the prefrontal cortex and subcortical regions, including the hippocampus and amygdala, are affected in youth with classical congenital adrenal hyperplasia (CAH). It remains unclear if white matter connecting these aforementioned brain regions is compromised in youth with CAH.
Objective
To examine brain white matter microstructure in youth with CAH compared to controls.
Design
A cross-sectional sample of 23 youths with CAH due to 21-hydroxylase deficiency (12.9 ± 3.5 year; 61% female) and 33 healthy controls (13.1 ± 2.8 year; 61% female) with 3T multishell diffusion-weighted magnetic resonance brain scans.
Main Outcome Measures
Complementary modeling approaches, including diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI), to examine in vivo white matter microstructure in six white matter tracts that innervate the prefrontal and subcortical regions.
Results
DTI showed CAH youth had lower fractional anisotropy in both the fornix and stria terminalis and higher mean diffusivity in the fornix compared to controls. NODDI modeling revealed that CAH youth have a significantly higher orientation dispersion index in the stria terminalis compared to controls. White matter microstructural integrity was associated with smaller hippocampal and amygdala volumes in CAH youth.
Conclusions
These patterns of microstructure reflect less restricted water diffusion likely due to less coherency in oriented microstructure. These results suggest that white matter microstructural integrity in the fornix and stria terminalis is compromised and may be an additional related brain phenotype alongside affected hippocampus and amygdala neurocircuitry in individuals with CAH.
Keywords: neurodevelopment, congenital adrenal hyperplasia, 21-hydroxylase deficiency, white matter, pediatrics, diffusion, tractography
Congenital adrenal hyperplasia (CAH) is the most common cause of primary adrenal insufficiency in children, with the majority of cases due to abnormal variations in CYP21A2 encoding the adrenal steroid 21-hydroxylase enzyme (P450c21) (1,2). This results in deficiencies in cortisol, aldosterone, and epinephrine, as well as excess production of androgens (1). It is known that fetal hormonal alterations can have a myriad of effects on developmental health, and the developing brain is not spared. In fact, about 46% of adults with CAH exhibit brain abnormalities, specifically within white matter, on brain imaging studies (3). However, the pathophysiology behind these abnormalities is unclear, and the studies addressing white matter changes in CAH are limited. Further, there is no consensus on the time course of white matter impairment in CAH. Diffusion studies in children with CAH begin to address some of these outstanding questions.
Four CAH case reports of suspected white matter abnormalities using magnetic resonance imaging (MRI) have illustrated diverse findings across age ranges, including diffuse low T2-weighted signal, periventricular T2-weighted hyperintensities, and signs of leukoencephalopathy, as well as a reported case of multiple sclerosis onset in a patient with CAH (4-7). Other studies have reported white matter abnormalities in approximately one third to one half of their CAH study populations (8-11). However, the most recent study to date examining white matter changes in CAH failed to find significant associations with white matter volumes after correcting for total brain volume (12). It is important to note that volumetric analysis itself is a limited characterization of white matter as it may not reflect the complex variety of cellular and architectural changes that can occur. Diffusion MRI provides a tool for probing these features, and one such observational study demonstrated compromised white matter microstructural integrity with extensive increases in mean diffusivity (MD), as well as decreases in fractional anisotropy (FA) in patients with CAH compared to controls (3). While there is evidence to suggest that some patients with CAH are vulnerable to white matter changes, a clear pattern has yet to emerge. Moreover, it remains unknown how white matter microstructure is impacted in patients with CAH during childhood and adolescence, a time period when the brain undergoes robust maturation of white matter pathways, particularly those connecting frontolimbic regions (eg, prefrontal, amygdala, and hippocampus), known to be important for both social and emotional function (13).
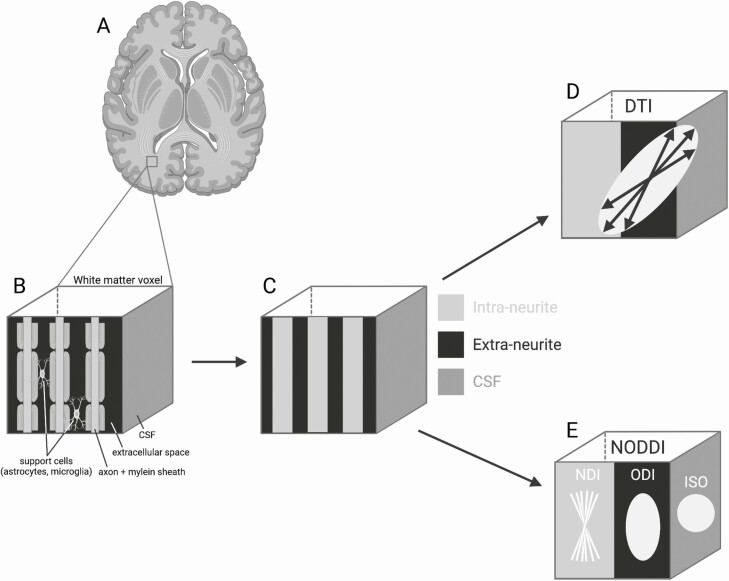
We therefore sought to identify potential microstructural abnormalities in frontolimbic white matter tracts in youth with CAH as compared to controls. These white matter tracts were expressly chosen because research indicates that the prefrontal cortex, amygdala, and hippocampus gray matter are altered in CAH (3,14,15). To this end, we implemented diffusion-weighted imaging and 2 biophysical modeling approaches, diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI) (Fig. 1) to examine in vivo information regarding characteristics of white matter microstructure in CAH (16-19). DTI parameters provide information on restriction of water diffusion and can provide insight on white matter microstructural properties, albeit in a nontissue specific fashion, whereas NODDI uses a multicompartment biophysical model that can provide tissue-specific microstructural information about intra- and extracellular processes (20). Specifically, NODDI allows for the estimation of a neurite density index (NDI), orientation dispersion index (ODI), and the free water fraction known as the isotropic component (ISO) (20). NDI describes the volume fraction of the highly restricted intraneurite compartment (within the axon), while ODI describes the volume fraction of the hindered diffusion in the extraneurite compartment (outside the axon) (Fig. 1E) (21). More specifically, ODI provides information about the dispersion of neurites around the principal diffusion direction and can be suggestive of the coherence of the axons in a single voxel (21). While DTI parameters are well-studied and provide a valuable connection to past findings, NODDI may be able to elucidate additional biological properties underlying microstructural changes (18), as NODDI parameters have been empirically found to be comparable to histological findings (22,23). This is the first study to utilize multiple diffusion models to examine impairments in microstructure in youth affected by CAH.
Figure 1.
White matter microstructural properties measured with diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). (A) White matter is the tissue through which neural messages pass between different areas of gray matter within the brain. (B) Using magnetic resonance imaging (MRI), a voxel (ie, the unit of 3D MRI) of white matter largely contains a larger number of myelinated axons and support cells (ie, astrocytes and glial cells). (C) With diffusion-weighted imaging we can measure water diffusion along multiple different directions in space and then utilize mathematical models to make inferences about the underlying biology in the brain. (D) DTI is the most commonly used model, which estimates an ellipsoid, with the size and shape of the ellipsoid capturing the amount and direction of diffusion, albeit this model cannot account for diffusion within different biological compartments. (E) NODDI uses a different type of tissue model that distinguished 3 microstructural environments including an intracellular neurite density index (NDI; bound by membranes of neurites and modeled as “sticks”), extracellular orientation dispersion index (ODI; space around the neurites occupied by various support cells and modeled as a simple anisotropic diffusion), and cerebral spinal fluid, also referred to as the isotropic fraction (ISO; modeled as isotropic diffusion) compartments. Abbreviation: CSF, cerebrospinal fluid. Created with BioRender.com.
Materials and Methods
Study Participants
The study was cross-sectional and approved by the Institutional Review Board of the University of Southern California (USC) and Children’s Hospital Los Angeles (CHLA). Written consent was obtained from all parents or legal guardians and/or participants, and all minors up to 14 years of age gave assent, in accordance with the code of ethics of the World Medical Association. Participants were recruited via flyers posted at CHLA and Keck School of Medicine of USC, with CAH participants recruited from the CHLA CAH Comprehensive Care Center. Health-related exclusionary criteria for all participants included prenatal drug or alcohol exposure, premature birth, serious medical illness (other than CAH), eating disorders, or psychotropic medication. Participants were screened for any significant neurological conditions (eg, epilepsy, traumatic head injury) and psychiatric/developmental disorders (eg, autism, attention-deficit/hyperactive disorder, schizophrenia, and self-harm tendencies), which, if present, barred participation. Participants were also screened for any factors that would prevent proper and safe usage of MRI, such as irremovable ferrous materials (eg, braces), uncorrectable vision impairments (eg, blind spots and colorblindness), need for hearing aids, or claustrophobia.
This study analyzed diffusion data from 25 youths with classical CAH and 34 controls between the ages of 8 and 18 years old at the time of their visit. However, 3 individuals (2 CAH, 1 control) did not pass quality control due to motion artifact, resulting in a final sample of 23 youths with CAH and 33 controls [Table 1; also see Supplemental Figure 1 (24)]. Youth with CAH had either the salt-wasting (n = 21) or simple-virilizing form (n = 2), as diagnosed by positive newborn screen and confirmatory serum analytes (n = 10), biochemically, +/− genotype (n = 13). At the time of the study visit, patients with CAH were on daily glucocorticoid dosing (16.6 ± 5.0 mg/m2/day) with glucocorticoid dose equivalencies calculated based on growth-suppressing effects of longer-acting glucocorticoids compared to hydrocortisone (dexamethasone dose was multiplied by 80; n = 7) (25). Almost all CAH youth (n = 22) were also treated with fludrocortisone (0.12 ± 0.05 mg/day). In terms of glucocorticoid type, 7 CAH patients were treated with a combination of fludrocortisone and dexamethasone, and 15 were treated with a combination of fludrocortisone and hydrocortisone. One patient was on hydrocortisone alone. Distribution of medication doses are presented for CAH youth by sex in Supplemental Figure 2 (24).
Table 1.
Participant characteristics for CAH and control youth
| CAH (n = 23) | Controls (n = 33) | Group difference (P-value) | |
|---|---|---|---|
| Age, yr | 12.95 (3.49) | 13.13 (2.77) | 0.83 |
| Female, n (%) | 14 (60.9%) | 20 (60.6%) | 0.98 |
| Right-handed, n (%) | 22 (95.7%) | 28 (84.8%) | 0.20 |
| IQa | 99.17 (17.35) | 104.27 (14.77) | 0.24 |
| Bone age, yearr | 13.67 (3.10) | 13.27 (2.76) | 0.61 |
| Bone age SD | 1.45 (3.35) | 0.13 (0.62) | 0.03 |
| Tanner stageb | 2.96 (1.69) | 3.39 (1.62) | 0.33 |
| TS 1-2: F = 5; M = 6; TS 3-5: F = 9; M = 3 | TS 1-2: F = 5; M = 7; TS 3-5: F = 15; M = 6 | ||
| BMIzc | 1.64 (0.83) | 0.82 (0.93) | 0.001 |
| Maternal education, year | n = 22; 13.36 (3.14) | N = 32; 15.06 (3.41) | 0.07 |
| Household income, n (%) | n = 21 | N = 32 | 0.54 |
| Less than $50k | 11 (52.4) | 14 (43.8) | |
| Greater than or equal to $50k | 10 (47.6) | 18 (56.2) | |
| Race, n (%)d | 0.71 | ||
| Asian | 1 (4.3) | 2 (6.1) | |
| Black or African American | 2 (8.7) | 4 (12.1) | |
| White | 10 (43.5) | 16 (48.5) | |
| More than 1 race | 2 (8.7) | 2 (6.1) | |
| Other | 0 (0) | 3 | |
| Ethnicity, n (%) | 0.20 | ||
| Hispanic or Latino | 9 (39.1) | 19 (57.6) | |
| Motione | 0.78 (0.37) | 0.77 (0.33) | 0.93 |
| Type, n (%) | |||
| Salt-wasting | 21 (91.3) | — | |
| Simple-virilizing | 2 (8.7) | — | |
| Newborn diagnosis, n (%) | 10 (43.5) | — | |
| Glucocorticoid total daily dose, mg/m2/day | |||
| Mean (SD) | 16.66 (5.01) | — | |
| Range | 7.94-29.60 | — | |
| Fludrocortisone total daily dose (mg) | n = 22 | ||
| Mean (SD) | 0.12 (0.04) | — | |
| Range | 0.05-0.20 | — | |
| Plasma renin activity, ng/mL/h or μg/L/h | |||
| Mean (SD) | 3.65 (2.58) | — | |
| Range | 0.12-9.19 | — | |
| 17-OHP, ng/dL [nmol/L] | n = 22 | ||
| Mean (SD) | 3761.27 (5084.67) [113.82 (153.87)] | — | |
| Range | 44.00-19966.00 [1.33-604.19] | — | |
| Testosterone, ng/dL [nmol/L] | |||
| Mean (SD) | 82.70 (166.97) [2.87 (5.79)] | — | |
| Range | 0.99-623.00 [0.03-21.6] | — | |
| Androstenedione, ng/dL [nmol/L] | |||
| Mean (SD) | 166.83 (243.49) [5.83 (8.50)] | — | |
| Range | 10.00-881.00 [0.35-30.76] | — |
Mean ± SD unless otherwise noted. Bold denotes P < 0.05 uncorrected.
Abbreviation: 17-OHP, 17-hydroxyprogesterone; CAH, congenital adrenal hyperplasia.
a General intelligence measured by the Wechsler Abbreviated Scale of Intelligence II.
b Pubertal development assessed by a physical using Tanner staging (TS) criteria.
c BMI z-score (BMIz) calculated using the SAS program based on the 2000 Centers for Disease Control and Prevention Growth Charts.
d Individuals were asked to self-identify by choosing as many of these options that may apply (white; black or African American; Asian; American Indian or Alaska Native; Native Hawaiian or Other Pacific Islanders; Other). More than 1 race indicates more than 1 option was selected.
e Motion calculated using an automated QC script measuring mean volume-to-volume translation (root mean square).
Anthropometric measures of height (cm) and weight (kg) were obtained in all participants. Pubertal (Tanner) staging was assessed by a pediatric endocrinologist. Body mass index (BMI) and BMI z-score (BMIz) were calculated by SAS based on 2000 Center for Disease Control and Prevention Growth Chart data (26,27). Bone age (BA) advancement can be a marker of prolonged and/or excess exposure to postnatal androgens, and the individual’s SD for BA can serve as an index of BA advancement as an average (mean) for their age and sex (28). To determine BA, a radiograph of the left hand was read by a single blinded pediatric endocrinologist (M.S.K.) using the Greulich-Pyle method (29). BA SD was determined utilizing digital software (30). BA was obtained at the time of the study visit or within 5 months of the visit if taken for clinical purposes. Individuals who had completed growth at the time of the study visit had their prior BA X-rays reviewed to confirm early full maturity.
There were no significant differences between the groups in terms of handedness, ethnicity/race composition, family income, or IQ as assessed by the Wechsler Adult Intelligence Scale IV 2-subtest test, which includes vocabulary and matrix reasoning (31). While there were trend-level differences in maternal education, they did not reach significance. Pubertal development was not significantly different between the 2 groups, although patients with CAH had higher BMIz (P = 0.001), as well as greater BA SD for their chronological age (P = 0.03) compared to controls. After an overnight fast (12 h) and prior to the administration of routine morning CAH medication, all participants had their blood drawn at the CHLA Clinical Trials Unit for measurement of analytes including 17-hydroxyprogesterone, androstenedione, total testosterone, and plasma renin activity by liquid chromatography-tandem mass spectrometry (Quest Diagnostics Nichols Institute, San Juan Capistrano, CA, USA). Distribution of 17-hydroxyprogesterone, androstenedione, and testosterone are presented for CAH youth by sex in Supplemental Figure 3 (24).
MRI Acquisition and Quality Control
All images were collected on a Siemens Magnetom Prisma 3 Tesla MRI scanner using a 32-channel head coil at the Center for Image Acquisition in the USC Stevens Neuroimaging and Informatics Institute at the Keck School of Medicine of USC. Three-dimensional T1- and T2-weighted structural images were acquired using sagittal whole-brain MPRAGE sequences MPRAGE and T2-SPACE [T1-weighted: repetition time (TR) = 2400 ms, echo time (TE) = 2.22 ms, flip angle = 8°, bandwidth (BW) = 220 Hz/pixel, field of view (FoV) = 256 mm, 208 slices, and 0.8-mm isotropic voxels with a GRAPPA phase-encoding acceleration factor of 2; T2-weighted: TR = 3200 ms, TE = 563 ms, BW = 744 Hz/pixel, FoV = 256 mm, 208 slices, 0.8-mm isotropic voxels, and 3.52 ms echo spacing with a GRAPPA phase-encoding acceleration factor of 2]. Anterior-posterior and posterior-anterior spin echo field maps were also obtained (TR = 8000 ms, TE = 66.0 ms, flip angle = 90°, BW = 2290 Hz/pixel, FoV = 208 mm, 72 slices, and 2.0-mm isotropic voxels).
Multishell diffusion-weighted MRI with b-values of 0, 1000, and 2500 s/mm2 were acquired to enable microstructural modeling (20,32). A 32-channel head array (Nova Medical Inc, Wilmington MA, USA) was used for radiofrequency signal reception with body-coil radiofrequency transmission. An optimally distributed multishell diffusion-encoding sampling scheme, consisting of 100 directions (33 and 67 directions for b-values of 1000 and 2500 s/mm2, respectively) was designed using the q-space sampling web application (33). Diffusion-encoding gradients were acquired in both anterior-posterior and posterior-anterior phase encoding directions to allow echo planar imaging distortion correction. Eleven interleaved unweighted diffusion (B0) gradients were acquired for each phase-encoding direction. Total diffusion MRI scan time was 15 min 22 sec (total of 222 images). Other diffusion MRI sequence parameters: 81 slices, 2.0 mm isotropic voxels, matrix size = 116 × 116, FoV = 232 × 232 mm, fixed TE of 72 ms and a TR of 4000 ms, and effective echo spacing of 0.55 ms, phase partial Fourier 6/8, iPAT acceleration factor 3 with a monopolar diffusion-encoding sequence.
Motion assessments and image exclusion
All scans were reviewed by a radiologist for incidental findings of abnormalities. All raw structural images were visually inspected to assess motion and passed quality control using the previously published Pass, Check, or Fail rating approach (34). Qualitative and quantitative methods were utilized to assess the effects of motion on the quality of the DWI images. First, all raw DWI images were visually examined for artifacts due to motion, including signal drop-out in the axial plane in each of the 222 gradient volumes. Next, an open-source automated quality control script was used to calculate motion using the mean relative volume-to-volume translation and rotation of the 11 interspersed b = 0 images (34). Roalf et al (35) sets an exclusion threshold on motion greater than 1.89 mm. Thus, 3 subjects initially enrolled in the study (1 control and 2 CAH participants) were excluded from further analyses as they exceeded this quality control criterion for motion.
MRI Preprocessing
DTI model
We implemented an analytic workflow for quantitative analysis of tissue microstructure and white matter connectivity using our collected structural and diffusion MRI data. Standard DWI preprocessing was performed using a combination of the FSL Diffusion Toolbox (36), DTI-TK (http://dti-tk.sourceforge.net/pmwiki/pmwiki.php) (37), and the Quantitative Imaging Toolkit (QIT) (http://cabeen.io/qitwiki) (38). First, anterior-posterior and posterior-anterior acquisitions were intensity-normalized, and images underwent echo planar imaging distortion correction using FSL TOPUP. Images were then corrected for eddy current effects, magnetic field inhomogeneities, and head motion using FSL EDDY (39). Brain masks were extracted from the average baseline diffusion scan using the Brain Extraction Tool (40). Importantly, each step of the diffusion MRI preprocessing stream underwent visual quality control by a master’s-level neuroimaging technician (A.A.). Next, we estimated diffusion tensor indices using FSL DTIFIT, including FA, MD, axial diffusivity (AD), and radial diffusivity (RD) (19). FA values describe the degree of anisotropy, with higher FA values indicating more myelin and/or axon caliber. MD is conceptualized as the average diffusivity of water in all directions and has an inverse relationship to FA—higher MD indicates damaged white matter microstructural integrity. AD describes the average diffusion of water parallel to the tract within a voxel, while RD refers to the average diffusion of water perpendicular to said tract (41).
NODDI model
To assess tissue microstructure, we performed NODDI (20) using the grid-search algorithm in the VolumeNoddiFit module of QIT. Compared to DTI, NODDI analyses offer even more specific insight into the anatomy of white matter abnormalities, while also overcoming known limitations of DTI including failures to accurately account for crossing fibers (20). For tractography analysis, we also estimated ball-and-stick models with FSL BEDPOSTX (42) using the multishell model and up to 3 fiber compartments per voxel. We then performed spatial normalization of each subject’s diffusion data to the IIT template (43) using the deformable tensor-based registration algorithm in DTI-TK; the resulting deformations were retained for subsequent analysis.
Tractography
Tractography methods can be employed to identify white matter tracts in the brain for each individual. Our multifiber tractography workflow performed automated modeling of fiber bundles and quantitative analysis of microstructure parameters along their length, the details of which can be found in supplemental text and Supplemental Figure 4 (24) and is summarized as follows: population-averaged fiber bundle models were delineated using a template-based approach, which were then used to guide the segmentation of analogous bundles in individual subject datasets (44). Our analysis used the IIT Human Brain Atlas (https://www.nitrc.org/projects/iit/), and we augmented the template using data from the Human Connectome Project to define more comprehensive bundle definitions. We created an IIT-space multifiber volume using a kernel regression framework for interpolating and averaging multifiber data from 88 Human Connectome Project participants, and we performed tractography in this population average to obtain a reference bundle and associated delineation masks. It can be assumed that the streamlines resulting from this process are analogous to the anatomical fibers, or bundles of axons, that compose white matter tracts between given gray matter regions within the brain. Tract regions of interest were chosen based on their connections with areas known to be affected in CAH [eg, amygdala, hippocampus, prefrontal regions (3,14,15)] and included the following: the anterior commissure, which connects the amygdala and temporal lobes of each hemisphere (45); the ventral amygdalofugal tract, which connects the amygdala to the nucleus accumbens and the thalamus (46,47); the fornix, the major output bundle of the hippocampus (48); the cingulum, which connects the cingulate gyrus to the entorhinal cortex; the stria terminalis, the major output bundle of the amygdala to the hypothalamus (48); and the uncinate fasciculus, which connects the orbitofrontal cortex with the anterior temporal lobe (48) (see Fig. 2). The resulting tract models were visualized at the single-subject level to ensure tract accuracy. During this quality assurance process, errors in the tractography process led to unusable data for 2 CAH participants for the anterior commissure and one CAH participant for the right stria terminalis. For each subject, accurately defined bundles were then quantitatively summarized with respect to volume, length, and average microstructure parameters for DTI (FA, MD, RD, and AD) and NODDI (NDI, ISO, and ODI). Despite unique differences in modeling of water diffusion in DTI vs NODDI, metrics from both techniques have similar patterns in white matter, with higher values of FA and NDI but lower values of MD and ODI in white matter regions as compared to gray matter tissue.
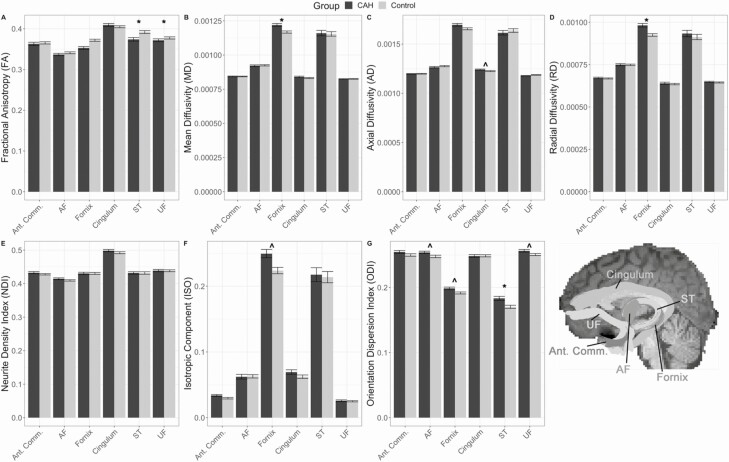
Figure 2.
White matter microstructural properties between congenital adrenal hyperplasia (CAH) and control youth: diffusion tensor imaging (DTI) outcomes (A-D) and neurite orientation density and dispersion imaging (NODDI) outcomes (E-G) for tracts of interest: anterior commissure (Ant. Comm.), cingulum, fornix, stria terminalis (ST), uncinate fasciculus (UF), and ventral amygdalofugal pathway (AF). Tracts are illustrated on a representative subject’s structural brain image. ^ denotes group difference is P < 0.05 uncorrected; and * denotes group differences are P < 0.05 corrected for multiple comparisons.
In addition to averaging diffusion parameters across the entire tract, we also performed along-tract analyses to determine if group differences were heterogeneous at various points along each white matter bundle. To perform along-tract analyses, each tract was subdivided into equidistant points using an automated approach based on the methodology of Colby et al (49) and implemented with the CurvesSegmentAlong module in QIT. A prototypical curve was first established in template space by identifying the “centroid” curve with the minimum Hausdorff distance to the other streamlines. The prototypical curve was then resampled with vertices every 5 mm to represent discretized regions along the length of the bundle and was registered to subject native space using DTI-TK. To create correspondence among cross-sectional points for a given tract, streamline vertices were interpolated to best match the prototypical curve for each subject’s tract. Diffusion parameters were then sampled and averaged over each group of shared vertex labels that match the reference prototypical curve and the subsequent along-tract parameter maps were used for statistical analysis.
Gray matter volumes
Amygdala and hippocampal segmentations used for exploratory volumetric analyses is described in full in Herting et al (50).
Statistical Analyses
All of the analyses were conducted in R (version 3.6.1) using the nlme package (version 3.1-148) (51). First, we compared demographics and motion by group (CAH vs control) using a 2-sample t test and/or chi-square test. Next, we utilized a model building approach to explore potential group by age interactions as well as potential main effects of group on microstructure properties derived from DTI (FA, MD, RD, and AD) and NODDI (ODI, ISO, and NDI). Specifically, using separate multilevel models, we first examined the effect of group, age, and a group-by-age interaction, while covarying for age, sex, and hemisphere (left or right), with the random effect of subject. In these multilevel models, hemisphere is used as a nested factor to account for greater similarity in white matter microstructure within an individual’s 2 hemispheres, rather than increasing the number of model comparisons if each hemisphere is treated separately. As the anterior commissure is not a bilateral tract, an identical linear regression was used without the repeated measure of hemisphere included in the model. Given that for all models examined we saw no significant associations between the group-by-age interaction term (uncorrected P-values > 0.05), we removed the interaction term to focus on reporting the main effects of group, still accounting for age, sex, and hemisphere in each model. Moreover, following the identification of significant group differences for a given tract, follow-up analyses were done using linear mixed-effect models to examine the microstructural properties along each tract between the groups while covarying for age, sex, and hemisphere (left or right), with the random effect of subject. Analysis of variance was then performed for each linear-mixed effect model to assess the significance of the interaction of group by diffusion parameter along the tract, and a simple slope (via R reghelper) was performed to determine the location of the significant effects along the tract. All P-values were grouped based on region of interest (ROI) outcome and corrected for multiple comparisons using false discovery rate correction across each set of microstructural outcomes of interest (5 bilateral hemisphere models for 4 DTI metrics = 20 tests; 5 bilateral hemisphere models for 3 NODDI metrics = 15 tests, as well as for the nonbilateral anterior commissure corrections = 4 and 3 tests, respectively).
Based on white matter microstructural differences found in the fornix and stria terminalis, we performed 2 exploratory follow-up analyses. The fornix is a major output bundle of the hippocampus, whereas the stria terminalis serves as a major output bundle of the amygdala to the hypothalamus (47). Thus, we examined if white matter microstructural differences related to our previous findings that CAH youth have smaller amygdala and hippocampus volumes as compared to controls (16). To accomplish this, we implemented a series of multiple regression analyses to examine: (1) how hippocampal volumes related to microstructural findings in the fornix and (2) how amygdala volumes related to microstructural findings in the stria terminalis across the entire sample. We included age, sex, and group (CAH, control) in our models. Given that plotted associations appeared slightly larger in CAH vs control youth, we also explored a potential volume-by-group interaction. Lastly, we also performed a within-group assessment of relationships between white matter microstructure and clinical features in CAH youth using multiple regression. These clinical features included BA SD, serum testosterone and androstenedione concentrations, glucocorticoid dose (mg/m2/day), and cognition as measured by IQ.
Results
Group Differences in White Matter Microstructure
The number of streamline fibers per tract was similar between the CAH and control groups, except for the left fornix where CAH youth had fewer streamlines on average compared to controls, albeit this difference did not pass multiple comparison correction (Table 2). Significant differences in white matter microstructural integrity were observed in both the fornix and stria terminalis tracts (Table 3; Fig. 2). In models using traditional DTI measures, CAH youth had lower fornix and stria terminalis FA (fornix: β = 0.019, P = 0.005; stria terminalis: β = 0.020, P = 0.007) as well as higher fornix MD (β = −5.21E-5, P = 0.026) and higher fornix RD (β = −5.75E-5, P = 0.009) compared to controls. In models utilizing NODDI measures, CAH youth had significantly higher stria terminalis ODI values as compared to controls (β = −0.013, P = 0.019).
Table 2.
Number of streamline fibers per white matter tract
| White matter tracts | CAH (n = 23) | Controls (n = 33) | P-value (uncorrected) | P-value (FDR correction) |
|---|---|---|---|---|
| Anterior commissure | 1236.43 (685.56)a | 1326.24 (577.40) | 0.61 | 0.84 |
| Left ventral amygdalofugal | 1202.04 (462.47) | 1378.36 (382.06) | 0.13 | 0.36 |
| Right ventral amygdalofugal | 1337.17 (329.10) | 1478.42 (259.36) | 0.08 | 0.36 |
| Left cingulum | 570.09 (362.16) | 599.67 (386.58) | 0.77 | 0.85 |
| Right cingulum | 3570.26 (809.52) | 3477.27 (1028.98) | 0.72 | 0.85 |
| Left fornix | 365.48 (242.01) | 520.09 (268.35) | 0.03 | 0.33 |
| Right fornix | 775.87 (341.39) | 764.42 (313.69) | 0.90 | 0.90 |
| Left stria terminalis | 536.39 (248.49) | 617.67 (324.57) | 0.32 | 0.50 |
| Right stria terminalis | 119.57 (83.93)b | 159.00 (101.66) | 0.17 | 0.37 |
| Left uncinate fasciculus | 3124.26 (334.54) | 3240.30 (406.38) | 0.26 | 0.48 |
| Right uncinate fasciculus | 6284.57 (1008.11) | 6704.15 (972.21) | 0.12 | 0.36 |
Data are given as mean (SD), along with P-values. Bold denotes P < 0.05 uncorrected.
Abbreviations: CAH, congenital adrenal hyperplasia; FDR, false discovery rate.
a n = 21;
b n = 22 due to errors in tractography (see Methods for details).
Table 3.
Group differences in white matter microstructure outcomes per white matter tract in congenital adrenal hyperplasia and control youth
| Diffusion tensor imaging outcomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tracts | Fractional anisotropy | Mean diffusivity | Radial diffusivity | Axial diffusivity | ||||||||
| Estimates | CI | P | Estimates | CI | P | Estimates | CI | P | Estimates | CI | P | |
| Anterior commissure | 0.003 | −0.01-0.02 | 0.71 | −0.000001 | −0.00001-0.00001 | 0.95 | −0.000001 | −0.00002-0.00001 | 0.74 | −0.000002 | −0.00001-0.00002 | 0.70 |
| Ventral amygdalofugal | 0.004 | −0.005-0.01 | 0.38 | 0.000003 | −0.000009-0.00002 | 0.59 | −0.0000008 | −0.00001-0.00001 | 0.91 | 0.00001 | −0.000002-0.00003 | 0.10 |
| Cingulum | −0.005 | −0.02-0.005 | 0.33 | −0.000008 | −0.00002-0.000003 | 0.14 | −0.000005 | −0.00002-0.000008 | 0.47 | −0.00001 | −0.00003-0.0000002 | 0.047 |
| Fornix | 0.02 | 0.009-0.03 | 0.0003* | −0.00005 | −0.00009-0.00002 | 0.01* | −0.00006 | −0.00009-0.00002 | 0.001* | −0.00004 | −0.00008-0.000002 | 0.06 |
| Stria terminalis | 0.02 | 0.007-0.03 | 0.002* | −0.00001 | −0.00004-0.00002 | 0.46 | −0.00003 | −0.00006-0.000006 | 0.11 | 0.00002 | −0.00002-0.00005 | 0.38 |
| Uncinate fasciculus | 0.005 | −0.002-0.01 | 0.12 | 0.0000007 | −0.000007-0.000008 | 0.85 | −0.000003 | −0.00001-0.000006 | 0.46 | 0.000009 | −0.000001-0.00002 | 0.09 |
| Neurite orientation dispersion density index outcomes | ||||||||||||
| Neurite density index | Isotropic component | Orientation dispersion index | ||||||||||
| Tracts | Estimates | CI | P-value | Estimates | CI | P-value | Estimates | CI | P-value | |||
| Anterior commissure | −0.006 | −0.02-0.004 | 0.21 | −0.005 | −0.01-0.0006 | 0.081 | −0.006 | −0.01-0.003 | 0.167 | |||
| Ventral amygdalofugal | −0.005 | −0.01-0.003 | 0.22 | 0.0004 | −0.005-0.006 | 0.887 | −0.006 | −0.01-0.0004 | 0.037 | |||
| Cingulum | −0.007 | −0.02-0.003 | 0.17 | −0.007 | −0.02-0.001 | 0.089 | 0.0003 | −0.006-0.006 | 0.918 | |||
| Fornix | −0.0006 | −0.01-0.01 | 0.92 | −0.03 | −0.05-0.005 | 0.015 | −0.007 | −0.01-0.0009 | 0.025 | |||
| Stria terminalis | 0.0004 | −0.01-0.01 | 0.95 | −0.006 | −0.03-0.01 | 0.512 | −0.012 | −0.02-0.005 | 0.002* | |||
| Uncinate fasciculus | −0.001 | −0.009-0.007 | 0.79 | −0.0009 | −0.004-0.003 | 0.593 | −0.006 | −0.01-0.001 | 0.015 | |||
Beta estimates, 95% CI, and P-values for group differences for DTI and NODDI outcomes. Bold values reflect P < 0.05 uncorrected, * denotes P-value remains significant after multiple comparison correction. For dichotomous grouping variable, congenital adrenal hyperplasia is the reference group (coded = 0 vs controls = 1).
To ensure group difference findings were not due to significant differences in BMIz or trend level differences in maternal education, we performed a sensitivity analysis including these as potential covariates. Importantly, all findings were similar and group differences in white matter microstructure remained significant (Ps < 0.05).
Along-Tract Microstructural Properties
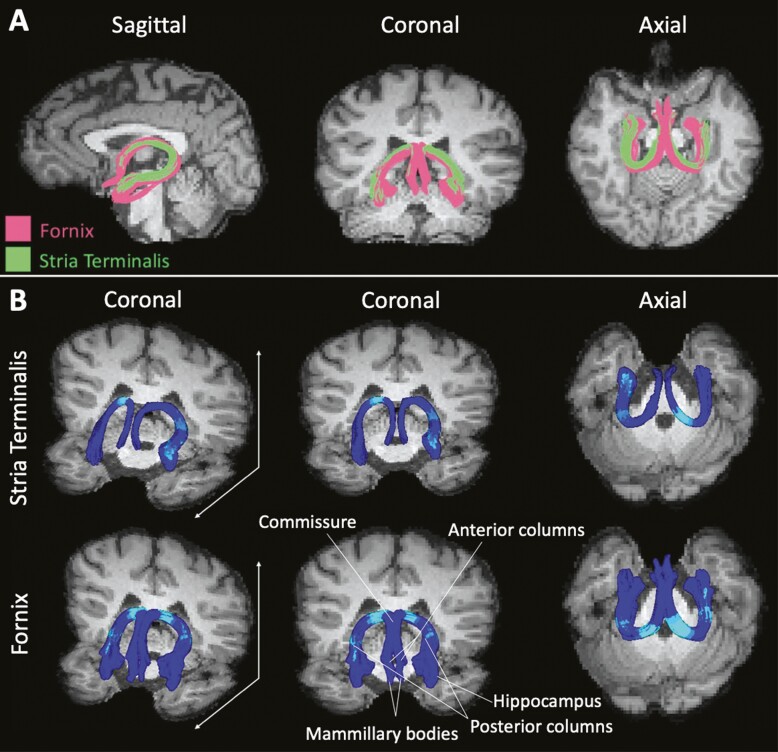
We found significant group effects along the left and right fornix tracts for FA [left: F(26, 1378) = 2.45, P < 0.0001; right: F(26, 1378) = 2.94, P < 0.0001], MD [left: F(26, 1378) = 4.83, P < 0.0001; right: F(26, 1378) = 3.63, P < 0.0001] and RD [left: F(26, 1378) = 4.77, P < 0.0001; right: F(26, 1378) = 3.85, P < 0.0001] (Fig. 3). Specifically, group differences were most striking along portions of the crus from each hemisphere that transition along the upper surface of the thalamus into the body of the fornix, in close proximity to the commissure of the fornix.
Figure 3.
White matter tract differences in congenital adrenal hyperplasia youth and controls. (A) Sagittal, coronal, and axial views of the fornix (pink) and stria terminalis (green). (B) Three-dimensional rendering of both the fornix and the stria terminalis. Cyan regions indicate areas within the fornix and stria terminalis where there are significant group effects between congenital adrenal hyperplasia and control youth, based on results from our multilevel models.
For the stria terminalis, significant group effects in CAH compared to control youth were only seen along the right hemisphere tract for FA [right: F(22, 1122) = 1.88, P < 0.008], with the effect located similarly along the upper surface of the thalamus near the significant region along the crus of the fornix. In contrast, FA difference in the left stria terminalis [F(22, 1165) = 0.9, P = 0.59], as well as the ODI in bilateral stria terminalis tracts, was not heterogeneous [left: F(22, 1165) = 1.51, P = 0.06; right: F(22, 1122) = 1.15, P = 0.29], meaning that the whole tract was affected similarly, suggesting lower FA in the left hemisphere and higher ODI in both hemispheres in CAH youth compared to controls were uniform across the stria terminalis.
White Matter Microstructure and Other Clinical Features in CAH Youth
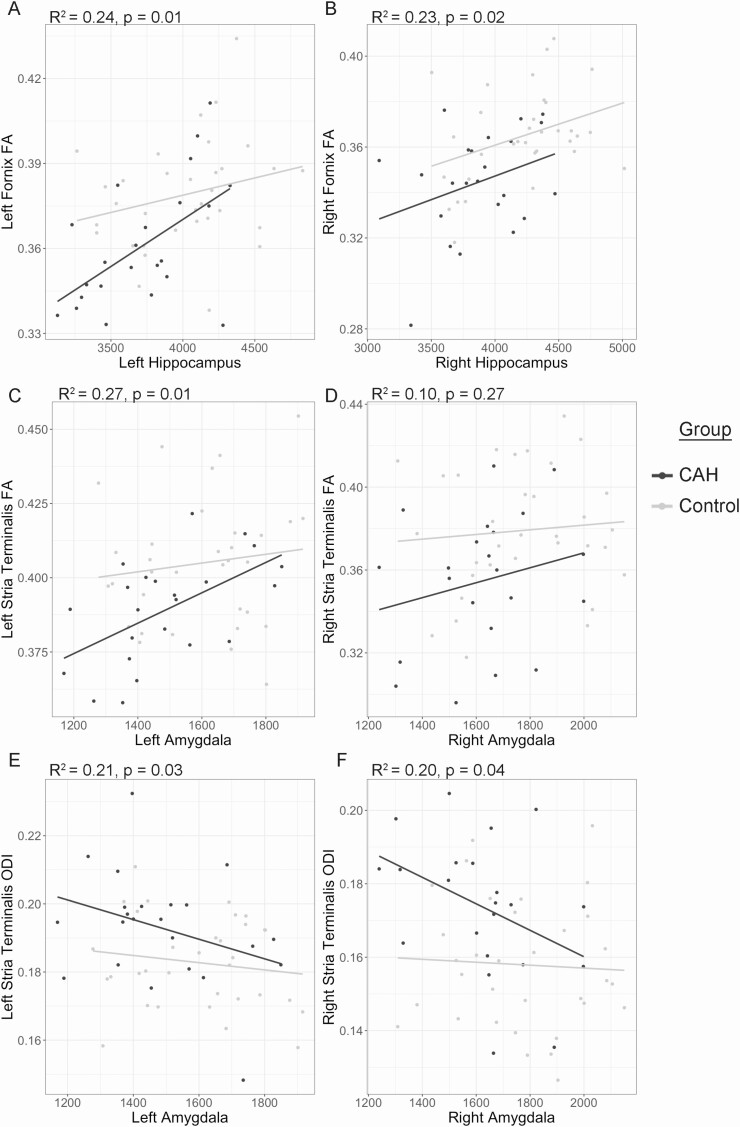
A significant association was seen between hippocampal volumes and FA in the fornix, with smaller hippocampal volumes related to lower FA (left: β = 1.919E-5, P = 0.00869; right: β = 1.877E-5, P = 0.0165) (Fig. 4A and 4B). Similarly, smaller amygdala volumes were also found to be associated with altered white matter integrity in the stria terminalis, as reflected by lower FA in the left, but not the right (left: β = 3.534E-5, P = 0.0115; right: β = 2.389E-5, P = 0.2650) and higher ODI bilaterally (left: β = −2.315E-5, P = 0.0269; right: β = −2.515E-5, P = 0.0382) (Fig. 4C-F). Although gray matter volume and white matter microstructure associations appeared slightly stronger in CAH as compared to control youth, these effects were not significant (ie, no significant group-by-volume interaction for these white matter differences; see Supplemental Table 1 (24)]. As for other clinical and biochemical CAH features, including markers of androgen excess (eg, BA SD); levels of testosterone, androstenedione, and 17-hydroxyprogesterone; glucocorticoid dose; or IQ; no associations were seen with white matter microstructure findings (Ps < 0.05).
Figure 4.
Exploratory associations between white matter microstructure and subcortical brain volumes for the left and right hemisphere in congenital adrenal hyperplasia (CAH) and control youth. (A and B) Relationships between bilateral hippocampus volumes and fractional anisotropy (FA) in the fornix in all youth. (C and D) Bilateral amygdala volumes and FA in the stria terminalis in all youth. (E and F) Bilateral amygdala volumes and orientation dispersion index (ODI) in the stria terminalis in all youth. Adjusted R2 and P-values for the overall model fit across the entire sample. Lines reflect associations in CAH (black) and controls (gray).
Discussion
This is the first study to utilize tractography and complementary diffusion models, DTI and NODDI, to identify white matter microstructure differences in youth with classical CAH due to 21-hydroxylase deficiency. We found that CAH youth have lower FA in both the fornix and stria terminalis and higher MD in the fornix (with DTI modeling), as well as higher ODI in the stria terminalis (with NODDI modeling), compared to controls. While both DTI and NODDI models provide information about water diffusion, NODDI can provide additional clarity to the identified white matter tissue abnormalities. In healthy white matter microstructure, FA values are high, while MD and ODI values are low (ie, both inversely related to FA values). Higher ODI values as assessed by NODDI, however, indicate increased heterogeneity among the fiber orientations with potential etiologies including fanning, disordered axon paths, or “undulating” axons, possibly due to an inflammatory-mediated increase in or swelling of support cells within the extraneurite space (52). This is important because many factors may reduce FA, such as reduced fiber density or partial volume mixing with cerebrospinal fluid, whereas NODDI provides a way to separate these factors from features of structural organization, depicted by ODI. Thus, taken together, the current patterns of microstructure in youth with CAH reflect less restricted water diffusion (as indicated by lower FA and higher MD values on DTI modeling) that are likely due to less coherency in oriented microstructure (as indicated by higher ODI on NODDI modeling). Compared to DTI, our NODDI analyses provide more specific information regarding neurite orientation and reveal higher levels of isotropy, or structural disorganization, in white matter tracts of CAH youth compared to controls.
We also examined white matter microstructure in tracts that connect key brain regions of interest (prefrontal cortex, amygdala, hippocampus) that are affected in CAH (15) to examine the location of microstructural alterations. CAH youth tended to have lower FA and higher ODI in the same 2 brain regions of interest: the fornix and stria terminalis. These findings suggest a regionality of white matter microstructure alterations specific to CAH. FA and ODI in these same tracts were also found to be associated with smaller hippocampal and amygdala volumes, structures that are anatomically associated with the fornix and stria terminalis respectively. The stria terminalis is characterized by a number of nerve fibers connecting the amygdala to the hypothalamus and is involved in the regulation of autonomic, neuroendocrine, and behavioral responses (53). The stria terminalis is situated just over the superior thalamostriate vein, and that same vein connects with numerous others just behind the crus of the fornix. The fornix is the primary outgoing pathway from the hippocampus, most known for its involvement in episodic memory (48). The commissure of the fornix is a triangular sheet of fibers that contains fibers from the fimbriae of the hippocampus and connects the crus of the fornix below the posterior corpus callosum (54).
The hippocampus, amygdala, and their accompanying white matter tracts are known to be important parts of the limbic system. Specifically, the hippocampus is crucial for learning and memory, and the fornix is especially important for episodic memory and cognition (48,54). The amygdala plays a role in processing fear-inducing or threatening stimuli and in regulating emotion, specifically anxiety (55). The stria terminalis, as the amygdala’s main outgoing pathway to the hypothalamus and thalamus, is also involved in both anxiety and threat detection. Given the increased rates of anxiety disorder noted in children with CAH (56), as well as the documented involvement of white matter microstructure changes in the etiology of psychiatric disorders (57), our findings regarding the white matter microstructure of these structures in CAH compared to controls could have significant clinical relevance. Damage to these structures, be it reductions in gray matter volume or changes in white matter microstructural integrity, could result in emotional dysregulation, dysexecutive function, and impairments in memory. While we did not examine neurocognition beyond IQ or emotional regulation in this study, it is important for future work to address differences in the aforementioned cognitive and emotional domains in patients with CAH compared to controls and to investigate the potential link between neurocognitive and emotional functions with aberrations in white matter microstructure associated with CAH.
While there is no consistent regionality of CAH-related white matter alterations in the current literature, we offer some potential explanations for why the stria terminalis and fornix might be specifically affected in our cohort of youth with CAH. Both the stria terminalis and the fornix carry fibers from regions that contain a multitude of hormone receptors. In the typically developing brain, the stria terminalis carries corticotropin releasing factor projections from the bed nucleus of the stria terminalis to the amygdala (58). The anterior bed nucleus of the stria terminalis is also inundated with androgen receptors (59). The fornix, on the other hand, carries fibers from the hippocampus, a brain region that has a high density of mineralocorticoid receptors (60-62). Further, mineralocorticoid receptors are glucocorticoid-activated receptors, making them particularly susceptible to fluctuations in glucocorticoid concentrations from glucocorticoid-replacement therapies in youth with CAH (12,63). In fact, previous research in human CAH cohorts has demonstrated that prolonged exposure to glucocorticoids has deleterious effects on hippocampal structure (64) as well as potential implications for amygdala structure (14). Likewise, we demonstrate a notable association between smaller amygdala and hippocampal volumes with impaired white matter microstructure in the stria terminalis and fornix in our sample. As per the standard of care for patients with CAH (1,65), all patients in our sample were treated with glucocorticoids over their lifetime. While glucocorticoids are known to be anti-inflammatory in high doses, previous literature on glucocorticoid treatment and associated changes in brain structure is mixed. Specifically, patients with higher glucocorticoid doses not only showed lower right hippocampal MD compared to controls in 1 study (66) but also showed increased mean FA and reduced mean RD in another study (12). In our study, patients with CAH did not exhibit associations between glucocorticoid dose (at the time of the brain scan) with white matter microstructural differences identified in these youth. Thus, while exogenous glucocorticoid replacement in patients with CAH cannot be ruled out as a potential mechanism through which limbic gray and white matter are affected, further study is needed to determine if an effect exists. Longitudinal studies could also help elucidate the order in which gray matter and white matter changes emerge in these patients.
Future studies might also consider the role of inflammation in possible brain alterations identified in CAH youth, as hormones play an important role in the regulation of systemic inflammatory activity (67), and inflammation has been heavily implicated in white matter microstructural damage overall (68,69). Of particular interest to the current study, mean ODI has been found to have a strong positive correlation with microglial density in an animal model utilizing both NODDI and histological confirmation of microglial density (52). Microglia are known to mediate neuroinflammation in both acute and chronic states. As stated above, higher ODI values represent compromised white matter microstructure, and it follows that higher levels of microglial density within the brain could negatively impact white matter microstructural integrity by way of increased neuroinflammation. Our models showed that CAH youth had higher ODI compared with controls, which may suggest poorly organized axons as well as possibly higher microglial density in the extraneurite spaces and thus increased neuroinflammation. Additional research is needed to replicate differences in ODI in patients with CAH, alongside additional advancements in mapping NODDI metrics to biological properties in various animal models and postmortem samples.
Strengths of our study include the focus on CAH youth and use of individual-level tractography to examine white matter bundles and 2 diffusion modeling approaches to probe white matter microstructural properties (cutting-edge multishell diffusion weighted MRI, coupled with tractography). Moreover, we used a multimodal approach (diffusion MRI and structural MRI) in exploratory analyses between white matter microstructure and brain volumes to further clarify how structural gray matter morphology is linked to white matter phenotypes in CAH. Among the limitations to consider when interpreting our results is our relatively small CAH sample, which may have limited our ability to detect small-to-medium effect sizes, similar to other studies reported for this condition. For example, a larger sample size could allow for results to remain significant following multiple comparison corrections. Additionally, our study population was largely comprised of patients with salt-wasting CAH, half of whom were diagnosed at birth, and almost all of which have been treated with a combination of both glucocorticoids and fludrocortisone. Moreover, although CAH and control youth were not significantly different in terms of pubertal maturation, the current study included a wide age range of youth. Thus, although of interest, our sample size was underpowered to examine important questions regarding the influence of CAH forms, age of diagnosis, and glucocorticoid treatment types or potential group differences in associations between pubertal maturation by sex on white matter microstructure outcomes in the current study. Thus, larger multicenter studies are likely needed to investigate different forms of CAH, including simple-virilizing and nonclassical CAH, age of diagnosis, and potential differences in the pubertal developmental processes on white matter development in youth affected by CAH. Lastly, the field of neuroimaging has recognized a number of important strengths and limitations of various postprocessing techniques. Although whole brain techniques, such as FSL’s TBSS, are widely used, probabilistic tractography, while more time-consuming due to manual predefinition of seed and target gray matter regions, can facilitate the ability to select white matter pathways based on a set of hypotheses. Moreover, tractography algorithms individually demonstrate high reproducibility across scanners and sessions and have been shown to provide high sensitivity to delineating tracts through crossing fiber regions (70). Nonetheless, it is important to note there have also been reported issues with variability and reproducibility between tractography algorithms (71-74). We tried to minimize these limitations and build upon the strengths of tractography in the current study by using a rigorous approach to defining key frontolimbic white matter tracts to allow for both participant- and tract-specific white matter microstructure to be examined along each tract. Given that each technique has its own limitations and recent studies show postprocessing techniques can lead to different findings within the same sample of patients and controls (72,75,76), future methodological research is needed to compare and contrast multiple diffusion postprocessing techniques (ie, TBSS, probabilistic tractography, etc.) in quantifying potential differences in white matter microstructure associated with CAH.
In conclusion, our findings provide evidence for the presence of white matter alterations in youth with classical CAH. In addition, we have examined white matter microstructure in tracts that connect the prefrontal cortex, amygdala, and hippocampus, providing a new level of spatial specificity as to the location of microstructural alterations. Moreover, we implemented novel NODDI modeling to gain additional information as to potential impairments in white matter tissue properties in youth with CAH. Overall, these results suggest that white matter microstructural integrity in the fornix and stria terminalis is compromised in youth with CAH and may be an additional, related brain phenotype to consider alongside affected hippocampus and amygdala neurocircuitry in these patients.
Acknowledgments
The authors gratefully thank all participating individuals and families. In addition, we would like to acknowledge Norma Martinez, Heather Ross, Christina Koppin, Michelle Canales, Kimberly Felix, and Eva Gabor for assisting with participant recruitment and data collection, as well as Dr. J. Michael Tyszka for his input on tractography methods, including strengths and limitations.
Financial Support: National Institutes of Health K01 MH1087610 (M.M.H.), K23HD084735 and R03 HD101718-01 (M.S.K.), CARES Foundation (M.E.G. and M.S.K.), and the Abell Foundation (M.E.G.). Portions of this study were also supported by the USC Diabetes & Obesity Research Institute (DORI) with funding from the Stewart Clifton Endowment (M.M.H.) and the the Southern California CTSI Clinical Trials Unit Grant from the National Center for Advancing Translational Sciences (NCATS) of the U.S. National Institutes of Health (UL1TR001855 and UL1TR000130).
Additional Information
Disclosures: M.E.G. receives research support from Novo Nordisk; consultant fees from Adrenas, Daiichi Sankyo, Eton Pharmaceuticals, Neurocrine Biosciences, Novo Nordisk, Pfizer, and QED; and royalties from McGraw-Hill and UpToDate; serves on a data safety monitoring board for Ascendis, Millendo, and Tolmar; and receives royalties from McGraw-Hill and UpToDate. M.S.K. receives research support from Neurocrine Biosciences.
Data Availability:
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Speiser PW, Azziz R, Baskin LS, et al. ; Endocrine Society . Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(9):4133-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim MS, Donohoue PA.. Adrenal disorders. In: Kappy MS, Allen DB, Geffner ME. eds. Pediatric Practice: Endocrinology. 2nd ed. McGraw Hill. https://accesspediatrics-mhmedical-com.libproxy1.usc.edu/content.aspx?bookid=1082§ionid=61462816. Accessed June 11, 2021. [Google Scholar]
- 3. Webb EA, Elliott L, Carlin D, et al. Quantitative brain MRI in congenital adrenal hyperplasia: in vivo assessment of the cognitive and structural impact of steroid hormones. J Clin Endocrinol Metab. 2018;103(4):1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergamaschi R, Livieri C, Candeloro E, Uggetti C, Franciotta D, Cosi V. Congenital adrenal hyperplasia and multiple sclerosis: is there an increased risk of multiple sclerosis in individuals with congenital adrenal hyperplasia? Arch Neurol. 2004;61(12):1953-1955. [DOI] [PubMed] [Google Scholar]
- 5. Gaudiano C, Malandrini A, Pollazzon M, et al. Leukoencephalopathy in 21-beta hydroxylase deficiency: report of a family. Brain Dev. 2010;32(5):421-424. [DOI] [PubMed] [Google Scholar]
- 6. Samia YM, Mahdi K, Baha Z, Saida JO, Tahar SM, Habib SM. Congenital adrenal hyperplasia and brain magnetic resonance imaging abnormalities. Clin Pediatr Endocrinol. 2010;19(4):109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaga A, Saito-Hakoda A, Uematsu M, et al. Brain white matter abnormality in a newborn infant with congenital adrenal hyperplasia. Clin Pediatr Endocrinol. 2013;22(4):77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mnif MF, Kamoun M, Mnif F, et al. Brain magnetic resonance imaging findings in adult patients with congenital adrenal hyperplasia: increased frequency of white matter impairment and temporal lobe structures dysgenesis. Indian J Endocrinol Metab. 2013;17(1):121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergamaschi R, Livieri C, Uggetti C, et al. Brain white matter impairment in congenital adrenal hyperplasia. Arch Neurol. 2006;63(3):413-416. [DOI] [PubMed] [Google Scholar]
- 10. Nass R, Heier L, Moshang T, et al. Magnetic resonance imaging in the congenital adrenal hyperplasia population: increased frequency of white-matter abnormalities and temporal lobe atrophy. J Child Neurol. 1997;12(3):181-186. [DOI] [PubMed] [Google Scholar]
- 11. Sinforiani E, Livieri C, Mauri M, et al. Cognitive and neuroradiological findings in congenital adrenal hyperplasia. Psychoneuroendocrinology. 1994;19(1):55-64. [DOI] [PubMed] [Google Scholar]
- 12. Van’t Westeinde A, Karlsson L, Thomsen Sandberg M, Nordenström A, Padilla N, Lajic S. Altered gray matter structure and white matter microstructure in patients with congenital adrenal hyperplasia: relevance for working memory performance. Cereb Cortex. 2020;30(5):2777-2788. [DOI] [PubMed] [Google Scholar]
- 13. Lebel C, Deoni S. The development of brain white matter microstructure. Neuroimage. 2018;182:207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88(4):1760-1765. [DOI] [PubMed] [Google Scholar]
- 15. Herting MM, Azad A, Kim R, Tyszka JM, Geffner ME, Kim MS. Brain differences in the prefrontal cortex, amygdala, and hippocampus in youth with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(4):1098-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mah A, Geeraert B, Lebel C. Detailing neuroanatomical development in late childhood and early adolescence using NODDI. PloS One. 2017;12(8):e0182340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geeraert BL, Lebel RM, Lebel C. A multiparametric analysis of white matter maturation during late childhood and adolescence. Hum Brain Mapp. 2019;40(15):4345-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards LJ, Pine KJ, Ellerbrock I, Weiskopf N, Mohammadi S. NODDI-DTI: estimating neurite orientation and dispersion parameters from a diffusion tensor in healthy white matter. Front Neurosci. 2017;11:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34(1):51-61. [DOI] [PubMed] [Google Scholar]
- 20. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000-1016. [DOI] [PubMed] [Google Scholar]
- 21. McCunn P, Gilbert KM, Zeman P, et al. Reproducibility of neurite orientation dispersion and density imaging (NODDI) in rats at 9.4 Tesla. PloS One. 2019;14(4):e0215974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sepehrband F, Clark KA, Ullmann JF, et al. Brain tissue compartment density estimated using diffusion-weighted MRI yields tissue parameters consistent with histology. Hum Brain Mapp. 2015;36(9):3687-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grussu F, Schneider T, Zhang H, Alexander DC, Wheeler-Kingshott CA. Neurite orientation dispersion and density imaging of the healthy cervical spinal cord in vivo. Neuroimage. 2015;111:590-601. [DOI] [PubMed] [Google Scholar]
- 24. Cotter D, Azad A, Cabeen RP, et al. Supplemental material for: White matter microstructural differences in youth with classical congenital adrenal hyperplasia. Figshare. Published November 2, 2020. 10.6084/m9.figshare.13934366.v3 [DOI] [PMC free article] [PubMed]
- 25. Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 2002;11(246):1-190. [PubMed] [Google Scholar]
- 27. Center for Disease Control and Prevent. A SAS program for the 2000 CDC Growth Charts (ages 0 to <20 years). Secondary A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years).https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm. Accessed June 11, 2021.
- 28. Creo AL, Schwenk WF II. Bone age: a handy tool for pediatric providers. Pediatrics. 2017;140(6):e20171486. [DOI] [PubMed] [Google Scholar]
- 29. Greulich WW, Pyle SI.. Radiologic Atlas of Skeletal Development of the Hand and Wrist. 2 ed. Stanford University Press; 1959. [Google Scholar]
- 30. Gilsanz V, Ratib O.. Hand Bone Age: A Digital Atlas of Skeletal Maturity. Springer; 2005. [Google Scholar]
- 31. Wechsler D. Wechsler Adult Intelligence Scale. 4th ed. Pearson; 2008. [Google Scholar]
- 32. Sepehrband F, O’Brien K, Barth M. A time-efficient acquisition protocol for multipurpose diffusion-weighted microstructural imaging at 7 Tesla. Magn Reson Med. 2017;78(6):2170-2184. [DOI] [PubMed] [Google Scholar]
- 33. Caruyer E, Lenglet C, Sapiro G, Deriche R. Design of multishell sampling schemes with uniform coverage in diffusion MRI. Magn Reson Med. 2013;69(6):1534-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Backhausen LL, Herting MM, Buse J, Roessner V, Smolka MN, Vetter NC. Quality control of structural MRI images applied using FreeSurfer: a hands-on workflow to rate motion artifacts. Front Neurosci. 2016;10:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roalf DR, Quarmley M, Elliott MA, et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790. [DOI] [PubMed] [Google Scholar]
- 37. Zhang H, Yushkevich PA, Alexander DC, Gee JC. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal. 2006;10(5):764-785. [DOI] [PubMed] [Google Scholar]
- 38. Cabeen R, Laidlaw D, Toga A. Quantitative imaging toolkit: software for interactive 3D visualization, data exploration, and computational analysis of neuroimaging datasets. Proc Int Soc Magn Reson Med. 2018;2018:2854. [Google Scholar]
- 39. Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193-197. [DOI] [PubMed] [Google Scholar]
- 40. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes-what do we know? Front Neurol. 2018;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang S, Peng H, Dawe RJ, Arfanakis K. Enhanced ICBM diffusion tensor template of the human brain. Neuroimage. 2011;54(2):974-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cabeen RP, Toga AW. Reinforcement tractography: a hybrid approach for robust segmentation of complex fiber bundles. 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI) 2020;999-103. doi:10.1109/ISBI45749.2020.9098371
- 45. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105-1132. [DOI] [PubMed] [Google Scholar]
- 46. Kamali A, Sair HI, Blitz AM, et al. Revealing the ventral amygdalofugal pathway of the human limbic system using high spatial resolution diffusion tensor tractography. Brain Struct Funct. 2016;221(7):3561-3569. [DOI] [PubMed] [Google Scholar]
- 47. Mori S, Kageyama Y, Hou Z, et al. Elucidation of white matter tracts of the human amygdala by detailed comparison between high-resolution postmortem magnetic resonance imaging and histology. Front Neuroanat. 2017;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pascalau R, Popa Stănilă R, Sfrângeu S, Szabo B. Anatomy of the limbic white matter tracts as revealed by fiber dissection and tractography. World Neurosurg. 2018;113:e672-e689. [DOI] [PubMed] [Google Scholar]
- 49. Colby JB, Soderberg L, Lebel C, Dinov ID, Thompson PM, Sowell ER. Along-tract statistics allow for enhanced tractography analysis. Neuroimage. 2012;59(4):3227-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herting MM, Azad A, Kim R, Tyszka JM, Geffner ME, Kim MS. Brain differences in the prefrontal cortex, amygdala, and hippocampus in youth with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(4):1098- 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. nlme: Linear and Nonlinear Mixed Effects Models (program). R package version 3.1-148 version. R Foundation; 2020.
- 52. Yi SY, Barnett BR, Torres-Velázquez M, et al. Detecting microglial density with quantitative multi-compartment diffusion MRI. Front Neurosci. 2019;13:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol. 2013;11(2):141-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Senova S, Fomenko A, Gondard E, Lozano AM. Anatomy and function of the fornix in the context of its potential as a therapeutic target. J Neurol Neurosurg Psychiatry. 2020;91(5):547-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. LeDoux J. The amygdala. Curr Biol. 2007;17(20):R868-R874. [DOI] [PubMed] [Google Scholar]
- 56. Mueller SC, Ng P, Sinaii N, et al. Psychiatric characterization of children with genetic causes of hyperandrogenism. Eur J Endocrinol. 2010;163(5):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Westlye LT, Bjørnebekk A, Grydeland H, Fjell AM, Walhovd KB. Linking an anxiety-related personality trait to brain white matter microstructure: diffusion tensor imaging and harm avoidance. Arch Gen Psychiatry. 2011;68(4):369-377. [DOI] [PubMed] [Google Scholar]
- 58. Uchida K, Otsuka H, Morishita M, et al. Female-biased sexual dimorphism of corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis. Biol Sex Differ. 2019;10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miles OW, Maren S. Role of the bed nucleus of the stria terminalis in PTSD: insights from preclinical models. Front Behav Neurosci. 2019;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Seckl JR, Dickson KL, Yates C, Fink G. Distribution of glucocorticoid and mineralocorticoid receptor messenger RNA expression in human postmortem hippocampus. Brain Res. 1991;561(2):332-337. [DOI] [PubMed] [Google Scholar]
- 61. López JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43(8):547-573. [DOI] [PubMed] [Google Scholar]
- 62. Watzka M, Beyenburg S, Blümcke I, Elger CE, Bidlingmaier F, Stoffel-Wagner B. Expression of mineralocorticoid and glucocorticoid receptor mRNA in the human hippocampus. Neurosci Lett. 2000;290(2):121-124. [DOI] [PubMed] [Google Scholar]
- 63. Le Menuet D, Lombès M. The neuronal mineralocorticoid receptor: from cell survival to neurogenesis. Steroids. 2014;91:11-19. [DOI] [PubMed] [Google Scholar]
- 64. Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry 1992;32:756-765. [DOI] [PubMed] [Google Scholar]
- 65. Kim MS, Ryabets-Lienhard A, Geffner ME. Management of congenital adrenal hyperplasia in childhood. Curr Opin Endocrinol Diabetes Obes. 2012;19(6):483-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Holm SK, Madsen KS, Vestergaard M, et al. Previous glucocorticoid treatment in childhood and adolescence is associated with long-term differences in subcortical grey matter volume and microstructure. Neuroimage Clin. 2019;23:101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Garcia-Leme J, Farsky SP. Hormonal control of inflammatory responses. Mediators Inflamm. 1993;2(3):181-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Altendahl M, Maillard P, Harvey D, et al. An IL-18-centered inflammatory network as a biomarker for cerebral white matter injury. Plos One. 2020;15(1):e0227835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Low A, Mak E, Rowe JB, Markus HS, O’Brien JT. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev. 2019;53:100916. [DOI] [PubMed] [Google Scholar]
- 70. Auriat AM, Borich MR, Snow NJ, Wadden KP, Boyd LA. Comparing a diffusion tensor and non-tensor approach to white matter fiber tractography in chronic stroke. Neuroimage Clin. 2015;7:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nath V, Schilling KG, Parvathaneni P, et al. Tractography reproducibility challenge with empirical data (TraCED): the 2017 ISMRM diffusion study group challenge. J Magn Reson Imaging. 2020;51(1):234-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schilling KG, Daducci A, Maier-Hein K, et al. Challenges in diffusion MRI tractography—lessons learned from international benchmark competitions. Magn Reson Imaging. 2019;57:194-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schilling KG, Nath V, Hansen C, et al. Limits to anatomical accuracy of diffusion tractography using modern approaches. Neuroimage. 2019;185:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thomas C, Ye FQ, Irfanoglu MO, et al. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci U S A. 2014;111(46):16574-16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bach M, Laun FB, Leemans A, et al. Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage. 2014;100:358-369. [DOI] [PubMed] [Google Scholar]
- 76. Kuchling J, Backner Y, Oertel FC, et al. Comparison of probabilistic tractography and tract-based spatial statistics for assessing optic radiation damage in patients with autoimmune inflammatory disorders of the central nervous system. Neuroimage Clin. 2018;19:538-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.