Abstract
Context
We previously reported that anti-Müllerian hormone (AMH), a marker of ovarian reserve, is positively associated with breast cancer risk, consistent with other studies.
Objective
This study assessed whether risk factors for breast cancer are correlates of AMH concentration.
Methods
This cross-sectional study included 3831 healthy premenopausal women (aged 21-57, 87% aged 35-49) from 10 cohort studies among the general population.
Results
Adjusting for age and cohort, AMH positively associated with age at menarche (P < 0.0001) and parity (P = 0.0008) and inversely associated with hysterectomy/partial oophorectomy (P = 0.0008). Compared with women of normal weight, AMH was lower (relative geometric mean difference 27%, P < 0.0001) among women who were obese. Current oral contraceptive (OC) use and current/former smoking were associated with lower AMH concentration than never use (40% and 12% lower, respectively, P < 0.0001). We observed higher AMH concentrations among women who had had a benign breast biopsy (15% higher, P = 0.03), a surrogate for benign breast disease, an association that has not been reported. In analyses stratified by age (<40 vs ≥40), associations of AMH with body mass index and OCs were similar in younger and older women, while associations with the other factors (menarche, parity, hysterectomy/partial oophorectomy, smoking, and benign breast biopsy) were limited to women ≥40 (P-interaction < 0.05).
Conclusion
This is the largest study of AMH and breast cancer risk factors among women from the general population (not presenting with infertility), and it suggests that most associations are limited to women over 40, who are approaching menopause and whose AMH concentration is declining.
Keywords: anti-Müllerian hormone, breast cancer risk factors, AMH correlates
Epidemiological studies, including our pooled analysis of data from 10 prospective cohorts, have consistently reported a positive association between the concentration of anti-Müllerian hormone (AMH), produced in ovarian follicles and a marker of ovarian reserve, and risk of breast cancer (1-4). It is therefore of interest to examine the association of AMH concentration with known breast cancer risk factors. Reports of associations of AMH with parity (4-8), age at menarche (4-6), body mass index (BMI) (4-7, 9-12), and oral contraceptive (OC) use (5, 13-24) have provided inconsistent results. Limited data are available about the association of AMH with family history of breast cancer (4) and gynecological surgeries such as partial oophorectomy and hysterectomy (25-27). To our knowledge, no study has examined whether a history of breast biopsy that did not result in a diagnosis of breast cancer (referred to as benign breast biopsy hereafter) is associated with AMH concentration. While a number of studies have examined the association of AMH concentration with reproductive and lifestyle factors, these have primarily focused on women experiencing infertility (14, 28). Few large studies have assessed AMH correlates in women from the general population (5, 6, 9). We assessed the relationship between breast cancer risk factors and AMH concentrations in women who were presumed healthy in a large international collaborative study including 10 prospective cohorts.
Methods
The majority (81.5%) of women included in this cross-sectional analysis were controls from nested case-control studies of AMH and risk of breast cancer (1, 2, 4, 29) in 10 cohorts (the Generations Study, CLUE II, Columbia MO Serum Bank, Guernsey Study, Nurses’ Health Study [NHS], Nurses’ Health Study II [NHSII], Northern Sweden Mammography Screening Cohort, New York University Women’s Health Study, Study of Hormones and Diet in the Etiology of Breast Tumors, and the Sister Study). The NHSII cohort also provided samples from 2 other studies of AMH, one in relation to early menopause and the other to race/ethnicity. For all studies except the race/ethnicity study, controls were selected using individual matching (with cases defined as women diagnosed with breast cancer or women with early menopause), with matching factors including cohort, age, and date of blood draw. The race/ethnicity study selected women from 3 race/ethnicity categories: African American, Asian, and Caucasian, as described in (30). Only women who provided blood samples before menopause were eligible. We excluded women who reported use of menopausal hormone replacement therapy at blood draw.
We assessed the association of AMH concentrations with BMI, smoking status, OC use, hysterectomy, partial oophorectomy, age at menarche, parity, age at first full-term pregnancy, first-degree family history of breast cancer, and history of benign breast biopsy. Variables were self-reported by participants at the time of, or on the questionnaire that was closest in time to, the blood draw. Weight and height were self-reported in most studies but were measured by study personnel in 3 cohorts (Guernsey, Study of Hormones and Diet in the Etiology of Breast Tumors [ORDET], and Sister Study). All variables were harmonized across contributing studies (1).
Laboratory Measurement
AMH was measured using the picoAMH assay (Ansh Labs, Webster, TX) for all cohorts except the NHSII race/ethnicity study, which used the AMH Gen II ELISA kit (Beckman Coulter) and a subset of the Sister Study, which used a combination of the Ultrasensitive AMH and picoAMH ELISA kits (Ansh Labs) (2). Details about the assays and their detection limits for each study have been described previously (1, 2, 29).
Statistical Analysis
AMH concentrations below the lowest detectable value of the assay were assigned the midpoint between zero and the lowest detectable value of the assay used for that cohort. AMH concentration was log2-transformed to normalize its distribution. The strong association between AMH and age is well documented (31) and we planned, a priori, to adjust all analyses for age. Specifically, we adjusted for both age and age-squared (age2), which provided a good fit to the data (see Supplemental Figure 1 (32)) and has been used by others to capture the age-AMH association (29, 33, 34). We observed a strong R2 (0.45) and an almost complete overlap of the locally-estimated-scatterplot-smoothing (LOESS) (35) and quadratic curves, further confirming that a quadratic function appropriately captures the age-AMH association.
AMH geometric means were calculated for each of the categories of the potential correlates, adjusting for age, age2, and cohort. F-tests from the analysis of variance were used to assess heterogeneity across the categories for each variable. We assessed the trend in AMH concentration across categories of age at menarche, parity, and age at first full-term pregnancy by modeling these as ordered categorical variables. Tests for heterogeneity between cohorts were conducted by comparing models including/excluding a cohort-by-variable interaction term. All variables that were individually associated with AMH were included simultaneously in an analysis of variance model to calculate multivariable-adjusted AMH geometric means. AMH-covariate associations were also assessed in age subgroups by stratifying by age <40 vs ≥40 years at the time of blood draw. All analyses were performed using SAS v9.4 and R software. A 2-sided P value < 0.05 was considered statistically significant.
Results
A total of 3831 premenopausal women were included in this cross-sectional analysis. Most (75.9%) were over age 40 (Table 1). About 90% of women were White, 6% Black/African American, and 3% Asian. Most had a BMI < 25 kg/m2 (59%), though 25% were overweight (BMI 25-29.9 kg/m2), and about 16% were obese (BMI ≥ 30 kg/m2). Few women reported current use of OCs at blood draw (5%), which was expected since this was an exclusion criterion for most cohorts. About 26% had a first-degree family history of breast cancer, although after excluding the Sister Study, in which all participants have a family history by design, this proportion was 11.5%. Approximately 17% of women had a history of benign breast biopsy and about 11% were current smokers at the time of blood donation. Distributions of breast cancer risk factors by cohort are shown in Supplemental Table 1 (32).
Table 1.
Descriptive characteristics
| N | % | |
|---|---|---|
| 3831 | ||
| Age at blood draw, years | ||
| 21-35 | 134 | 3.5% |
| 35-39 | 789 | 20.6% |
| 40-44 | 1365 | 35.6% |
| 45-49 | 1183 | 30.9% |
| 50-57 | 360 | 9.4% |
| Race/ethnicity | ||
| White | 3313 | 89.8% |
| Black/African American | 218 | 5.9% |
| Asian | 120 | 3.3% |
| Other | 39 | 1.1% |
| Missing | 141 | |
| BMI, kg/m 2 | ||
| <18.5 | 69 | 1.8% |
| 18.5-24.9 | 2197 | 57.7% |
| 25-29.9 | 944 | 24.8% |
| 30-34.9 | 345 | 9.1% |
| 35+ | 255 | 6.7% |
| Missing | 21 | |
| Age at menarche, years | ||
| 9-10 | 305 | 8.0% |
| 11 | 581 | 15.2% |
| 12 | 1016 | 26.7% |
| 13 | 1099 | 28.8% |
| 14 | 459 | 12.0% |
| 15+ | 351 | 9.2% |
| Missing | 20 | |
| Parity | ||
| Nulliparous | 865 | 23.0% |
| 1 | 514 | 13.7% |
| 2 | 1404 | 37.4% |
| 3 | 709 | 18.9% |
| 4+ | 266 | 7.1% |
| Missing | 73 | |
| Age at first full-term pregnancy, years | ||
| Nulliparous/missing | 938 | |
| <20 | 254 | 6.6% |
| 21-24 | 939 | 24.5% |
| 25-29 | 1066 | 27.8% |
| 30-34 | 484 | 12.6% |
| 35+ | 150 | 3.9% |
| Oral contraceptive use | ||
| Never user | 911 | 24.4% |
| Former user | 2639 | 70.7% |
| Current user | 184 | 4.9% |
| Missing | 97 | |
| Hysterectomy and/or partial oophorectomy | ||
| No | 3576 | 93.7% |
| Hysterectomy without oophorectomy | 107 | 2.8% |
| Partial oophorectomy with or without hysterectomy | 133 | 3.5% |
| Missing | 15 | |
| Family history of breast cancer | ||
| No | 2830 | 73.9% |
| Yes | 1001 | 26.1% |
| Benign breast biopsy | ||
| No | 3128 | 83.2% |
| Yes | 633 | 16.8% |
| Missing | 70 | |
| Smoking status a | ||
| Never | 2340 | 63.9% |
| Former | 912 | 24.9% |
| Current | 412 | 11.2% |
| Missing | 167 |
Abbreviation: BMI, body mass index.
a Variable was reported as ever/never in 2 cohorts (Northern Sweden Mammography Screening Cohort and Northern Sweden Mammography Screening Cohort). “Ever” was set to missing as these individuals could not be distinguished as former vs current (n = 54)
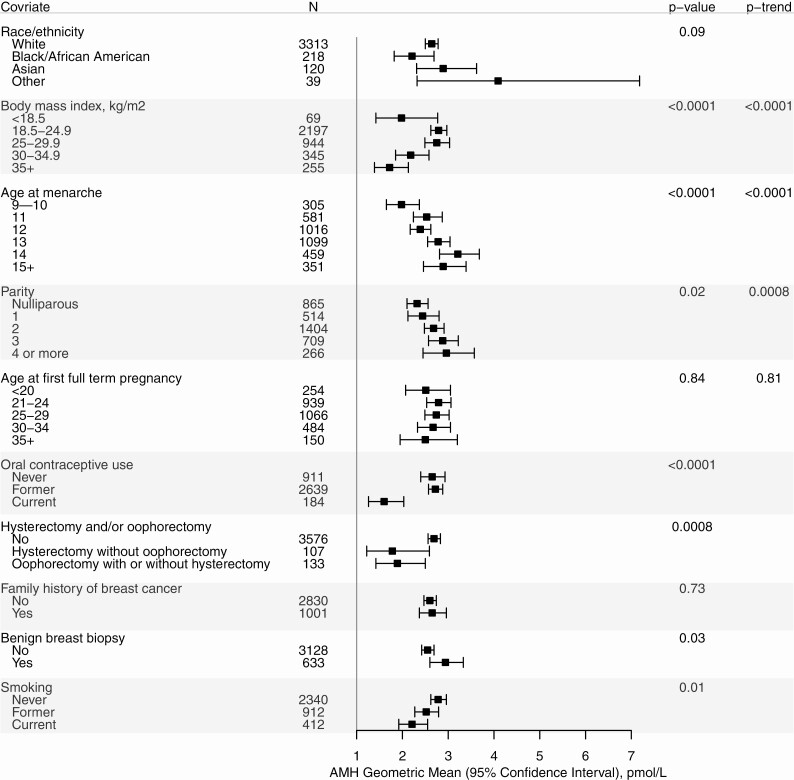
AMH geometric means are shown in Fig. 1 for each variable, in models adjusted for cohort, age, and age-squared. In women with BMI ≥ 18.5 kg/m2, AMH concentration decreased with increasing BMI (Ptrend < 0.0001). The AMH geometric mean was lower among women who were underweight than for women with BMI of 18.5-25 kg/m2. AMH concentrations increased with increasing age at menarche (P < 0.0001) and with increasing parity (Ptrend = 0.02 and for parous vs nulliparous P = 0.03, data not shown). We did not observe an association of AMH with age at first full-term pregnancy (Ptrend = 0.84). Current OC users had lower AMH geometric mean than former and never users (P < 0.0001). Women who had had a simple hysterectomy or a partial oophorectomy had lower geometric mean AMH than women without these surgeries (P = 0.0008). Family history of breast cancer was not associated with AMH concentration (P = 0.73), including in analyses that excluded the Sister Study (data not shown). AMH concentrations varied by smoking status: current smokers had the lowest AMH geometric mean, and former smokers had a geometric mean intermediate between current smokers and nonsmokers (P = 0.01). History of benign breast biopsy was associated with a higher AMH geometric mean (P = 0.03). There was no evidence of heterogeneity by cohort for any of the AMH associations with these variables.
Figure 1.
Age- and cohort-adjusted geometric mean AMH concentration (pmol/L) by individual characteristics. Note: P values are from the ANOVA F-tests for differences across categories. Ptrend for ordered categories are shown for BMI, age at menarche, parity, and age at first full-term pregnancy. All models are adjusted for age, age-squared, and cohort.
Table 2 shows age- and cohort-adjusted, as well as multivariate-adjusted, AMH geometric means. The first 2 columns show that age- and cohort-adjusted results were similar in analyses including all participants and in analyses limited to participants without missing data for any of the variables included in the multivariate model. The last 2 columns show that all variables that were associated with AMH in the model adjusting only for age and cohort remained associated with AMH in the multivariate model.
Table 2.
Geometric mean AMH concentration (pmol/L) adjusted for risk factors by which AMH concentrations vary across levels
| Age- and cohort-adjusteda | Age- and cohort-adjusted in dataset with no missing covariatesb | Fully adjusted for each of the other covariates, age, and cohortc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Geo mean | 95% CI | P value | N | Geo mean | 95% CI | P value | N | Geo mean | 95% CI | P value | |
| Body mass index, kg/m2 | <0.0001 | <0.0001 | <0.0001 | |||||||||
| <18.5 | 69 | 1.98 | (1.42-2.77) | 62 | 2.35 | (1.66-3.31) | 62 | 2.20 | (1.58-3.08) | |||
| 18.5-24.9 | 2197 | 2.79 | (2.62-2.97) | 2057 | 2.88 | (2.71-3.08) | 2057 | 2.83 | (2.65-3.01) | |||
| 25-29.9 | 944 | 2.75 | (2.49-3.03) | 881 | 2.80 | (2.53-3.09) | 881 | 2.85 | (2.57-3.15) | |||
| 30-34.9 | 345 | 2.18 | (1.85-2.58) | 325 | 2.20 | (1.85-2.62) | 325 | 2.31 | (1.95-2.75) | |||
| 35+ | 255 | 1.72 | (1.39-2.13) | 243 | 1.87 | (1.51-2.31) | 243 | 1.97 | (1.59-2.43) | |||
| Age at menarched | <0.0001 | <0.0001 | 0.0002 | |||||||||
| 9-10 | 305 | 1.98 | (1.65-2.37) | 280 | 2.02 | (1.67-2.44) | 280 | 2.09 | (1.73-2.52) | |||
| 11 | 581 | 2.53 | (2.24-2.87) | 559 | 2.63 | (2.32-2.99) | 559 | 2.71 | (2.39-3.07) | |||
| 12 | 1016 | 2.39 | (2.17-2.62) | 949 | 2.43 | (2.21-2.68) | 949 | 2.44 | (2.22-2.70) | |||
| 13 | 1099 | 2.78 | (2.55-3.04) | 1025 | 2.86 | (2.61-3.14) | 1025 | 2.86 | (2.61-3.13) | |||
| 14 | 459 | 3.21 | (2.81-3.68) | 429 | 3.35 | (2.91-3.85) | 429 | 3.25 | (2.83-3.73) | |||
| 15+ | 351 | 2.89 | (2.46-3.39) | 326 | 3.09 | (2.63-3.63) | 326 | 2.98 | (2.53-3.51) | |||
| Parityd | 0.0008 | 0.0037 | 0.0284 | |||||||||
| Nulliparous | 865 | 2.32 | (2.10-2.56) | 809 | 2.45 | (2.22-2.72) | 809 | 2.52 | (2.27-2.79) | |||
| 1 | 514 | 2.44 | (2.12-2.80) | 491 | 2.49 | (2.16-2.86) | 491 | 2.52 | (2.19-2.90) | |||
| 2 | 1404 | 2.68 | (2.48-2.91) | 1340 | 2.75 | (2.54-2.99) | 1340 | 2.74 | (2.53-2.97) | |||
| 3 | 709 | 2.88 | (2.57-3.22) | 678 | 2.95 | (2.63-3.31) | 678 | 2.90 | (2.59-3.25) | |||
| 4 or more | 266 | 2.96 | (2.45-3.57) | 250 | 3.06 | (2.52-3.71) | 250 | 2.94 | (2.43-3.55) | |||
| Hysterectomy and/or oophorectomy | 0.0008 | 0.0066 | 0.0084 | |||||||||
| No | 3576 | 2.69 | (2.56-2.83) | 3349 | 2.76 | (2.62-2.90) | 3349 | 2.76 | (2.62-2.90) | |||
| Hysterectomy without oophorectomy | 107 | 1.78 | (1.22-2.59) | 96 | 1.83 | (1.24-2.70) | 96 | 1.86 | (1.26-2.74) | |||
| Partial oophorectomy | 133 | 1.89 | (1.42-2.50) | 123 | 2.12 | (1.58-2.84) | 123 | 2.13 | (1.60-2.85) | |||
| Oral contraceptive use | <0.0001 | <0.0001 | <0.0001 | |||||||||
| Never | 911 | 2.65 | (2.40-2.93) | 854 | 2.77 | (2.50-3.07) | 854 | 2.75 | (2.49-3.04) | |||
| Former | 2639 | 2.72 | (2.57-2.88) | 2521 | 2.78 | (2.62-2.95) | 2521 | 2.78 | (2.63-2.95) | |||
| Current | 184 | 1.60 | (1.26-2.03) | 172 | 1.61 | (1.26-2.06) | 172 | 1.60 | (1.24-2.06) | |||
| Benign breast disease | 0.0314 | 0.0201 | 0.0193 | |||||||||
| No | 3128 | 2.55 | (2.42-2.69) | 2934 | 2.63 | (2.49-2.78) | 2934 | 2.63 | (2.49-2.77) | |||
| Yes | 633 | 2.94 | (2.60-3.33) | 603 | 3.08 | (2.71-3.49) | 603 | 3.08 | (2.71-3.49) | |||
| Smoking | 0.0103 | 0.0087 | 0.0058 | |||||||||
| Never | 2340 | 2.78 | (2.62-2.96) | 2275 | 2.84 | (2.67-3.02) | 2275 | 2.85 | (2.68-3.04) | |||
| Former | 912 | 2.52 | (2.27-2.79) | 894 | 2.59 | (2.34-2.87) | 894 | 2.55 | (2.31-2.82) | |||
| Current | 412 | 2.21 | (1.92-2.55) | 399 | 2.23 | (1.93-2.58) | 399 | 2.24 | (1.94-2.59) |
a Model adjusted for age, age-squared, and cohort only
b Subjects with missing data on any of the covariates were excluded from this analysis.
c Model adjusted for age, age-squared, and cohort in addition to all other variables in the table.
d Ordered categories of age at menarche and parity were used to estimate P values
The associations of AMH with age at menarche, parity, hysterectomy and/or partial oophorectomy, smoking, and benign breast disease were primarily limited to women 40 years of age and over, while the associations of AMH with BMI and OCs were similar for women aged <40 and ≥40 (Supplemental Table 2 (32)).
Discussion
We observed the well-known relationship of decreasing AMH with increasing age, with the greatest AMH decrease after age 40, as ovarian function declines. Overall, we observed positive associations of AMH with age at menarche, parity, and history of benign breast biopsy, and inverse associations with obesity, history of hysterectomy and/or partial oophorectomy, current OC use, and current or former smoking. These associations were independent of each other, as they were observed in a multivariate-adjusted model. BMI and OCs were the only 2 variables whose association with AMH was similar in women <40 and ≥ 40 years of age. For all other variables, the association was observed only in older women. We did not observe an association of AMH with family history of breast cancer or age at first full-term pregnancy.
Our observation that AMH concentration was lower in women who had had a partial oophorectomy than in women with both ovaries intact was consistent with expectation, given the reduction in number of follicles following partial oophorectomy. The only other study to examine the effect of partial oophorectomy on AMH concentrations found an increase in AMH concentrations, but it included only 19 women with partial oophorectomy (36). We also observed that women who had undergone hysterectomy without oophorectomy had lower AMH concentration than women who had not had any of these surgeries. This observation is consistent with several previous studies which reported that hysterectomy in premenopausal women results in reductions in AMH compared with the concentration observed prior to surgery (25-27, 37-39), though not all studies agreed (40, 41). Such an effect could be due to reduced blood flow to the ovaries or altered signaling from the uterus following surgery (26, 42), a hypothesis supported by some (26, 43, 44), though not all (40), studies. Another possible explanation is that the pathology leading to hysterectomy was a cause of early ovarian failure and thus, lower AMH concentration. However, in the studies that observed a decline in AMH concentrations after hysterectomy, AMH concentrations prior to hysterectomy were similar to those of women who did not have the surgery (26, 27).
We observed a higher mean AMH concentration in parous than in nulliparous women, and a trend of increasing AMH with increasing parity. The largest study to date (n = 2320), like ours, observed higher concentrations of AMH in parous women than in nulliparous women (5). One smaller study also found a positive association with parity (8), while others found no association (7, 18, 19), and one study of young women (n = 294 women, ages 20-22) reported an inverse association (21). These discrepant results could be due to the differences in age distribution among studies. Because AMH increases until ages 20 to 25 and decreases thereafter (33, 45), the association of parity with AMH may differ in younger women compared with women close to menopause. We were not able to directly examine this hypothesis, as there were only very few women below the age of 25 in our study. However, we note that we observed a positive association between AMH and parity among women ≥40 years of age, but not among women younger than 40 years. AMH concentration has been shown to decrease during pregnancy; it is about 50% lower by the third trimester than in the nonpregnant state (46, 47). This suggests that primary recruitment of follicles is reduced during pregnancy and, as a result, there is slower depletion of the pool of primordial follicles. A larger pool of remaining primordial follicles in parous women compared with nulliparous women of the same age may be associated with greater number and quality of pre-antral and small antral follicles, which are the source of AMH. However, another possible explanation for the association we observed between parity and AMH is that nulliparous women include women with infertility problems, some of which are associated with low AMH concentrations. We were not able to disentangle the roles of parity and infertility because data on infertility are not available from most of the cohorts included in this study. The trend we observed of increasing AMH geometric means with parity in analyses limited to parous women, though, suggests that the association we observed cannot be fully explained by the inclusion of infertile women in the group of nulliparous women.
Use of OCs was also associated with AMH concentration; while there was no difference between never and past users, the geometric mean AMH concentration among current users of OCs was substantially lower than in the 2 other groups. Our finding of reduced AMH concentration in current, but not past, users of OCs is consistent with most (5, 13-21, 48, 49), though not all (22-24), studies. It is also consistent with the observation that the number of follicles measured by ultrasound, which includes the small growing follicles that secrete AMH, is reduced in women using OCs (14-16). It is of interest that, though the lower AMH concentrations observed during pregnancy and OC use appears to have a similar cause, that is, a smaller number of growing follicles; pregnancy has a long-term effect (since parity is positively associated with AMH), while the effect of OC use appears transient (since AMH is not related to past use).
We found a trend of increasing AMH with increasing age at menarche. Two studies (n = 671, P = 0.04 and n = 2320, P = 0.09) reported a similar trend (5, 6), while a third study (n = 204) did not observe any association (4). Furthermore, 2 studies (n < 300 women per study) found an inverse association (20, 21). Participants in these 2 latter studies were women in their early 20s (20, 21), which is younger than the participants in our study. Our results showed that age at menarche was positively associated with AMH in women over 40 but was not associated with AMH concentration in women who were younger than age 40. Differences in age distributions across studies may, therefore, explain the inconsistent results. There is a negative correlation between AMH and follicular recruitment between the ages of 15 and 25 because AMH increases as a greater proportion of follicles reach later stages of development. After age 25, both AMH and follicular recruitment begin to decline as a reflection of declining ovarian reserve (50). AMH concentrations are thus thought to reflect different reproductive processes before and after age 25 (51, 52).
In the studies that examined the relationship between BMI and AMH among healthy women (ie, not among women with infertility), observations are mixed, with studies reporting both inverse (9-11, 48, 53-55) and null associations (4-7, 18, 19, 56). The largest studies that did not report any association included few obese women (5, 6). Most studies did not assess AMH concentrations among underweight women separately (4-7, 9-11, 18, 19, 48, 53-56), or like our study, had a small number of underweight women (5, 6), and associations were mixed across studies. Obesity can result in follicular atresia through the damaging effects of insulin resistance on granulosa cells, as a result of obesity-associated polycystic ovarian syndrome, or through altered hormone signaling due to leptin and adiponectin dysregulation (57, 58). Underweight (59, 60), and obesity, in some (59), but not all (60) studies, have been associated with risk of early menopause, consistent with our observations of lower AMH among women with low or high BMI.
Similar to several prior studies, we observed that current, and to a lesser extent, former smoking was associated with lower AMH concentrations than never smoking (5, 48, 61, 62). Studies that did not find an association of smoking with AMH included mostly younger women (ages 20-40) (7, 10, 63), in agreement with our observation that the smoking-AMH association was limited to women over age 40 (see Supplemental Table 2 (32)). The association we observed with smoking is supported by literature that has demonstrated earlier age at menopause among women who smoke cigarettes (64), as smoking is thought to reduce ovarian follicle density (65).
Higher AMH is a predictor of later age at menopause (29, 66), which could explain the AMH-breast cancer risk association, since age at menopause is a risk factor for breast cancer (67). Late age at menopause is thought to be associated with an increased risk of breast cancer because it reflects longer exposure to sex hormones. Longer exposure to sex hormones is also thought to explain, in part, the association of younger age at menarche with breast cancer risk (67). Similarly, premenopausal obesity, which is associated with reduced breast cancer risk (68, 69), is associated with anovulation and younger age at menopause, both of which are thought to be associated with reduced exposure to sex hormones. The directions of the associations between premenopausal obesity, AMH, and risk of breast cancer are consistent (premenopausal obesity is associated with lower AMH concentration, and both premenopausal obesity and lower AMH are inversely associated with risk of breast cancer). On the other hand, the association between age at menarche and AMH is not consistent with the direction of their associations with breast cancer risk (higher age at menarche is associated with higher AMH concentration, which is associated with increased risk of breast cancer, while higher age at menarche is associated with decreased risk). Thus, while the association between AMH and premenopausal obesity might help explain the association of premenopausal obesity with breast cancer risk, the association of AMH with age at menarche does not help explain the association of age at menarche with risk of breast cancer.
The association between parity and breast cancer risk is complex, as each full-term pregnancy results first in an increase, followed by a long-term decrease, in risk (70). A large consortium study recently reported that the initial increase in breast cancer risk is observed up to 24 years after the most recent full-term pregnancy (71). The positive association we observed between AMH and parity is consistent with this initial positive association of parity with breast cancer risk.
A novel finding in our study is that women with a history of benign breast biopsy, a surrogate for benign breast disease, had higher AMH concentrations than women without previous biopsy. The AMH-breast biopsy association was restricted to women who were over age 40, suggesting that a slower decline in ovarian function among women approaching menopause may co-occur with benign breast disease (72-74). Among women <40, we found some suggestion that benign breast biopsy was associated with lower AMH concentration. We note that the number of women <40 was small and the majority likely underwent mammography for clinical reasons (ie, strong family history of breast cancer, identification of a palpable mass or other symptoms), while women over 40 could be referred to breast biopsy for any of the same reasons but are also part of the broader screening population. The reason for the positive association observed in women ≥ 40 is unclear. Since this is the first study reporting an association of AMH with history of breast biopsy and we were not able to examine the association by type of benign breast disease, additional studies are needed.
This study had several strengths. Women included in this study were not selected for history of infertility or other pathology. The large sample size of this study allowed us to detect clear trends of AMH with several variables (BMI, age at menarche, and parity) because we were able to use finer categorization than previous studies. Further, we had enough data to assess whether AMH-risk factor associations varied according to age at blood donation (<40 vs ≥40). Finally, AMH was measured for a majority of the participants (75%) using the same high sensitivity picoAMH assay.
Our study also had several limitations. Because of its cross-sectional design, the temporal relationship between breast cancer risk factors and AMH concentration could not be assessed. The cross-sectional design also prevented us from assessing the AMH-age at menopause association. Over 80% of the women included in the study were controls who were individually selected to match to cases of breast cancer using incidence density sampling and thus are not a random sample of the cohorts and the cohorts themselves are not a random sample of the general population. We confirmed, though, several associations of AMH with age, smoking, oral contraceptive use, and hysterectomy/oophorectomy that other large studies have reported, giving credibility to our other results. In addition, though the characteristics of the populations of the 10 cohorts were quite different, there was no evidence of heterogeneity between cohorts, which also supports the generalizability of our results. We therefore believe our results to be generalizable to healthy premenopausal women. Only 24% of the women were less than 40 years of age, which could explain why we observed fewer associations in this group than in the group of older women. We note that sample handling and processing procedures varied across cohorts and there remained some variability in AMH concentration by cohort after controlling for technical sample handling variables (1). To eliminate this nuisance effect, we controlled all analyses for cohort. Although we only had a single AMH measurement from each woman, we and others have observed that temporal reproducibility is high over a period of a few months to a few years (3, 75, 76). Finally, we did not have breast biopsy results and thus could not examine whether the association we observed between breast biopsy and AMH concentration was specific to some benign breast disease diagnoses. We used benign breast biopsy because it was available from the majority of participating cohorts and because it is a clearly defined event, which women are likely to remember and report accurately, while they may not remember the results of the biopsy beyond its benign characteristic.
In a large study of premenopausal women aged 30 to 57, we observed that lower AMH concentrations were associated with obesity, younger age at menarche, parity, hysterectomy and partial oophorectomy, current OC use, and current and former smoking. This is the largest study of the association of AMH with breast cancer risk factors in women from the general population, who were not selected based on fertility status. Our results help to clarify associations with several hormone- and reproduction-related risk factors and AMH that were previously inconsistent across studies with smaller sample size. It also supports that the associations of AMH with reproductive and lifestyle variables are different in late premenopausal women than in women in the early and middle stages of reproductive life. History of benign biopsy, a surrogate for benign breast disease, was associated with higher AMH in women over age 40. Since this is the first study to report the association with benign breast biopsy, it requires confirmation.
Acknowledgments
We thank the NCI Cohort Consortium. CLUE authors would like to thank the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries of the Centers for Disease Control and Prevention for the funds that helped support the collection and availability of the cancer registry data. The CLUE authors would also like to thank the CLUE participants and staff at the George W. Comstock Center for Public Health Research and Prevention. NHS authors thank the participants and staff of the NHS and NHSII for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Financial Support: This work was supported by grant National Institutes of Health (NIH) R01 CA178949. Support for the individual cohorts included: The Generations Study: This work was supported by Breast Cancer Now and The Institute of Cancer Research, United Kingdom. We acknowledge National Health System funding to the Royal Marsden and The Institute of Cancer Research NIHR Biomedical Research Centre. Columbia, MO Serum Bank: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute (NCI), and the Department of Defense Breast Cancer Research Program (BC062367). Guernsey cohort: Cancer Research United Kingdom C570/A16491. Availability of data and materials: Data access policies for the Guernsey study are available on the Cancer Epidemiology Unit website at https://www.ceu.ox.ac.uk/policies2. Nurses Health Study: NCI UM1 CA186107; R01 CA49449. Nurses’ Health Study II (NHSII): NCI UM1 CA176726; R01 CA67262. New York University Women’s Health Study (NYUWHS): NIH R01 CA098661, UM1 CA182934 and center grants P30 CA016087 and P30 ES000260. Sister Study: This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES044005) to D.P. Sandler and the Avon Foundation (02-2012-085) to H.B. Nichols and D.P. Sandler.
Glossary
Abbreviations
- AMH
anti-Müllerian hormone
- BMI
body mass index
- NHS
Nurses’ Health Study
- NHSII
Nurses’ Health Study II
- OC
oral contraceptive
Additional Information
Disclosures: The authors have nothing to disclose. The authors do not declare any conflicts of interest.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Supplementary information and tables are available online at (32).
References
- 1. Ge W, Clendenen TV, Afanasyeva Y, et al. Circulating anti-Müllerian hormone and breast cancer risk: a study in ten prospective cohorts. Int J Cancer. 2018;142(11):2215-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nichols HB, Baird DD, Stanczyk FZ, et al. Anti-Mullerian hormone concentrations in premenopausal women and breast cancer risk. Cancer Prev Res. 2015;8(6):528-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eliassen AH, Zeleniuch-Jacquotte A, Rosner B, Hankinson SE. Plasma anti-mullerian hormone concentrations and risk of breast cancer among premenopausal women in the nurses’ health studies. Cancer Epidemiol Biomark Prev. 2016;25(5):854-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorgan JF, Stanczyk FZ, Egleston BL, et al. Prospective case-control study of serum mullerian inhibiting substance and breast cancer risk. J Natl Cancer Inst. 2009;101(21):1501-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dólleman M, Verschuren WM, Eijkemans MJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115. [DOI] [PubMed] [Google Scholar]
- 6. Jung S, Allen N, Arslan AA, et al. Demographic, lifestyle, and other factors in relation to antimüllerian hormone levels in mostly late premenopausal women. Fertil Steril. 2017;107(4):1012-1022.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. La Marca A, Spada E, Grisendi V, et al. Normal serum anti-Müllerian hormone levels in the general female population and the relationship with reproductive history. Eur J Obstet Gynecol Reprod Biol. 2012;163(2):180-184. [DOI] [PubMed] [Google Scholar]
- 8. Moini A, Hedayatshodeh M, Hosseini R, Rastad H. Association between parity and ovarian reserve in reproductive age women. Eur J Obstet Gynecol Reprod Biol. 2016;207:184-187. [DOI] [PubMed] [Google Scholar]
- 9. Du X, Ding T, Zhang H, et al. Age-specific normal reference range for serum anti-müllerian hormone in healthy chinese han women: a nationwide population-based study. Reprod Sci. 2016;23(8):1019-1027. [DOI] [PubMed] [Google Scholar]
- 10. Dafopoulos A, Dafopoulos K, Georgoulias P, et al. Smoking and AMH levels in women with normal reproductive history. Arch Gynecol Obstet. 2010;282(2):215-219. [DOI] [PubMed] [Google Scholar]
- 11. Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF 3rd. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87(1):101-106. [DOI] [PubMed] [Google Scholar]
- 12. Buyuk E, Seifer DB, Illions E, Grazi RV, Lieman H. Elevated body mass index is associated with lower serum anti-mullerian hormone levels in infertile women with diminished ovarian reserve but not with normal ovarian reserve. Fertil Steril. 2011;95(7):2364-2368. [DOI] [PubMed] [Google Scholar]
- 13. Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310. [DOI] [PubMed] [Google Scholar]
- 14. Bentzen JG, Forman JL, Pinborg A, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619. [DOI] [PubMed] [Google Scholar]
- 15. Birch Petersen K, Hvidman HW, Forman JL, et al. Ovarian reserve assessment in users of oral contraception seeking fertility advice on their reproductive lifespan. Hum Reprod. 2015;30(10):2364-2375. [DOI] [PubMed] [Google Scholar]
- 16. van den Berg MH, van Dulmen-den Broeder E, Overbeek A, et al. Comparison of ovarian function markers in users of hormonal contraceptives during the hormone-free interval and subsequent natural early follicular phases. Hum Reprod. 2010;25(6):1520-1527. [DOI] [PubMed] [Google Scholar]
- 17. Kristensen SL, Ramlau-Hansen CH, Andersen CY, et al. The association between circulating levels of antimüllerian hormone and follicle number, androgens, and menstrual cycle characteristics in young women. Fertil Steril. 2012;97(3):779-785. [DOI] [PubMed] [Google Scholar]
- 18. Shaw CM, Stanczyk FZ, Egleston BL, et al. Serum antimüllerian hormone in healthy premenopausal women. Fertil Steril. 2011;95(8):2718-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. La Marca A, Sighinolfi G, Giulini S, et al. Normal serum concentrations of anti-Müllerian hormone in women with regular menstrual cycles. Reprod Biomed Online. 2010;21(4):463-469. [DOI] [PubMed] [Google Scholar]
- 20. Kerkhof GF, Leunissen RW, Willemsen RH, et al. Influence of preterm birth and small birth size on serum anti-Müllerian hormone levels in young adult women. Eur J Endocrinol. 2010;163(6):937-944. [DOI] [PubMed] [Google Scholar]
- 21. Bragg JM, Kuzawa CW, Agustin SS, Banerjee MN, McDade TW. Age at menarche and parity are independently associated with Anti-Müllerian hormone, a marker of ovarian reserve, in Filipino young adult women. Am J Hum Biol. 2012;24(6):739-745. [DOI] [PubMed] [Google Scholar]
- 22. Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-Müllerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2012;39(5):574-580. [DOI] [PubMed] [Google Scholar]
- 23. Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90(2):395-400. [DOI] [PubMed] [Google Scholar]
- 24. Li HW, Wong CY, Yeung WS, Ho PC, Ng EH. Serum anti-müllerian hormone level is not altered in women using hormonal contraceptives. Contraception. 2011;83(6):582-585. [DOI] [PubMed] [Google Scholar]
- 25. Cho HY, Park ST, Kyung MS, Park SH. Assessment of ovarian reserve after hysterectomy: laparoscopic vs. non-laparoscopic surgery. Eur J Obstet Gynecol Reprod Biol. 2017;210:54-57. [DOI] [PubMed] [Google Scholar]
- 26. Trabuco EC, Moorman PG, Algeciras-Schimnich A, Weaver AL, Cliby WA. Association of ovary-sparing hysterectomy with ovarian reserve. Obstet Gynecol. 2016;127(5):819-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atabekoğlu C, Taşkin S, Kahraman K, et al. The effect of total abdominal hysterectomy on serum anti-Müllerian hormone levels: a pilot study. Climacteric. 2012;15(4):393-397. [DOI] [PubMed] [Google Scholar]
- 28. Dólleman M, Faddy MJ, van Disseldorp J, et al. The relationship between anti-Müllerian hormone in women receiving fertility assessments and age at menopause in subfertile women: evidence from large population studies. J Clin Endocrinol Metab. 2013;98(5):1946-1953. [DOI] [PubMed] [Google Scholar]
- 29. Bertone-Johnson ER, Manson JE, Purdue-Smithe AC, et al. Anti-Müllerian hormone levels and incidence of early natural menopause in a prospective study. Hum Reprod. 2018;33(6):1175-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, Hankinson SE. Racial differences in premenopausal endogenous hormones. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2147-2153. [DOI] [PubMed] [Google Scholar]
- 31. de Kat AC, van der Schouw YT, Eijkemans MJ, et al. Back to the basics of ovarian aging: a population-based study on longitudinal anti-Müllerian hormone decline. BMC Med. 2016;14(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clendenen TV, Ge W, Koenig KL, et al. Data from: Breast cancer risk factors and circulating anti-Müllerian hormone concentration in healthy premenopausal women: Supplementary Information and Tables [Data set]. Zenodo. Posted May 26, 2021. 10.5281/zenodo.4813348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JY, Jee BC, Lee JR, et al. Age-related distributions of anti-Müllerian hormone level and anti-Müllerian hormone models. Acta Obstet Gynecol Scand. 2012;91(8):970-975. [DOI] [PubMed] [Google Scholar]
- 34. Lee JY, Ahn S, Lee JR, et al. Reference values for the revised anti-müllerian hormone generation II assay: infertile population-based study. J Korean Med Sci. 2017;32(5):825-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829-836. [Google Scholar]
- 36. Wilkosz P, Greggains GD, Tanbo TG, Fedorcsak P. Female reproductive decline is determined by remaining ovarian reserve and age. Plos One. 2014;9(10):e108343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tehranian A, Zangbar RH, Aghajani F, Sepidarkish M, Rafiei S, Esfidani T. Effects of salpingectomy during abdominal hysterectomy on ovarian reserve: a randomized controlled trial. Gynecol Surg. 2017;14(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song T, Kim MK, Kim ML, et al. Impact of opportunistic salpingectomy on anti-Müllerian hormone in patients undergoing laparoscopic hysterectomy: a multicentre randomised controlled trial. BJOG. 2017;124(2):314-320. [DOI] [PubMed] [Google Scholar]
- 39. van Lieshout LAM, Steenbeek MP, De Hullu JA, et al. Hysterectomy with opportunistic salpingectomy versus hysterectomy alone. Cochrane Database Syst Rev. 2019;8:CD012858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee DY, Park HJ, Kim BG, Bae DS, Yoon BK, Choi D. Change in the ovarian environment after hysterectomy as assessed by ovarian arterial blood flow indices and serum anti-Müllerian hormone levels. Eur J Obstet Gynecol Reprod Biol. 2010;151(1):82-85. [DOI] [PubMed] [Google Scholar]
- 41. Ankum WM, Hehenkamp WJK, Reekers JA, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007;22(7):1996-2005. [DOI] [PubMed] [Google Scholar]
- 42. Siddle N, Sarrel P, Whitehead M. The effect of hysterectomy on the age at ovarian failure: identification of a subgroup of women with premature loss of ovarian function and literature review. Fertil Steril. 1987;47(1):94-100. [DOI] [PubMed] [Google Scholar]
- 43. Petri Nahás EA, Pontes A, Nahas-Neto J, Borges VT, Dias R, Traiman P. Effect of total abdominal hysterectomy on ovarian blood supply in women of reproductive age. J Ultrasound Med. 2005;24(2):169-174. [DOI] [PubMed] [Google Scholar]
- 44. Xiangying H, Lili H, Yifu S. The effect of hysterectomy on ovarian blood supply and endocrine function. Climacteric. 2006;9(4):283-289. [DOI] [PubMed] [Google Scholar]
- 45. Shebl O, Ebner T, Sir A, et al. Age-related distribution of basal serum AMH level in women of reproductive age and a presumably healthy cohort. Fertil Steril. 2011;95(2):832-834. [DOI] [PubMed] [Google Scholar]
- 46. Nelson SM, Stewart F, Fleming R, Freeman DJ. Longitudinal assessment of antimüllerian hormone during pregnancy-relationship with maternal adiposity, insulin, and adiponectin. Fertil Steril. 2010;93(4):1356-1358. [DOI] [PubMed] [Google Scholar]
- 47. Moini A, Hedayatshodeh M, Hosseini R, Rastad H. Association between parity and ovarian reserve in reproductive age women. Eur J Obstet Gynecol Reprod Biol. 2016;207:184-187. [DOI] [PubMed] [Google Scholar]
- 48. Anderson C, Mark Park YM, Stanczyk FZ, Sandler DP, Nichols HB. Dietary factors and serum antimüllerian hormone concentrations in late premenopausal women. Fertil Steril. 2018;110(6):1145-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Landersoe SK, Birch Petersen K, Sørensen AL, et al. Ovarian reserve markers after discontinuing long-term use of combined oral contraceptives. Reprod Biomed Online. 2020;40(1):176-186. [DOI] [PubMed] [Google Scholar]
- 50. Lie Fong S, Visser JA, Welt CK, et al. Serum anti-Müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weghofer A, Kim A, Barad DH, Gleicher N. Age at menarche: a predictor of diminished ovarian function? Fertil Steril. 2013;100(4):1039-1043. [DOI] [PubMed] [Google Scholar]
- 52. Fleming R, Kelsey TW, Anderson RA, Wallace WH, Nelson SM. Interpreting human follicular recruitment and antimüllerian hormone concentrations throughout life. Fertil Steril. 2012;98(5):1097-1102. [DOI] [PubMed] [Google Scholar]
- 53. Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis T, Gracia CR. Body size affects measures of ovarian reserve in late reproductive age women. Menopause. 2008;15(5):857-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Steiner AZ, Stanczyk FZ, Patel S, Edelman A. Antimullerian hormone and obesity: insights in oral contraceptive users. Contraception. 2010;81(3):245-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bernardi LA, Carnethon MR, de Chavez PJ, et al. Relationship between obesity and anti-Müllerian hormone in reproductive-aged African American women. Obesity (Silver Spring). 2017;25(1):229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bertrand KA, Baer HJ, Orav EJ, et al. Early life body fatness, serum anti-müllerian hormone, and breast density in young adult women. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1151-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Robker RL, Akison LK, Bennett BD, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. 2009;94(5):1533-1540. [DOI] [PubMed] [Google Scholar]
- 58. Lie Fong S, Schipper I, Valkenburg O, de Jong FH, Visser JA, Laven JS. The role of anti-Müllerian hormone in the classification of anovulatory infertility. Eur J Obstet Gynecol Reprod Biol. 2015;186:75-79. [DOI] [PubMed] [Google Scholar]
- 59. Szegda KL, Whitcomb BW, Purdue-Smithe AC, et al. Adult adiposity and risk of early menopause. Hum Reprod. 2017;32(12):2522-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hardy R, Mishra GD, Kuh D. Body mass index trajectories and age at menopause in a British birth cohort. Maturitas. 2008;59(4):304-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Plante BJ, Cooper GS, Baird DD, Steiner AZ. The impact of smoking on antimüllerian hormone levels in women aged 38 to 50 years. Menopause. 2010;17(3):571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93(9):3478-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Marsh EE, Steinberg ML, Parker JB, Wu J, Chakravarti D, Bulun SE. Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil Steril. 2016;106(3):766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Westhoff C, Murphy P, Heller D. Predictors of ovarian follicle number. Fertil Steril. 2000;74(4):624-628. [DOI] [PubMed] [Google Scholar]
- 66. Dólleman M, Verschuren WM, Eijkemans MJ, Broekmans FJ, van der Schouw YT. Added value of anti-Müllerian hormone in prediction of menopause: results from a large prospective cohort study. Hum Reprod. 2015;30(8):1974-1981. [DOI] [PubMed] [Google Scholar]
- 67. Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ma H, Ursin G, Xu X, et al. Body mass index at age 18 years and recent body mass index in relation to risk of breast cancer overall and ER/PR/HER2-defined subtypes in white women and African-American women: a pooled analysis. Breast Cancer Res. 2018;20(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Premenopausal Breast Cancer Collaborative Group; Schoemaker MJ, Nichols HB, Wright LB, et al. Association of body mass index and age with subsequent Breast Cancer risk in premenopausal women. JAMA Oncol. 2018;4(11):e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Breast cancer risk after recent childbirth. Ann Intern Med. 2019;170(1):22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nichols HB, Schoemaker MJ, Cai J, et al. Breast cancer risk after recent childbirth: a pooled analysis of 15 prospective studies. Ann Intern Med. 2019;170(1):22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Santen RJ. Benign breast disease in women. [Updated 2018 May 25]. In: Feingold KR, Anawalt B, Boyce A, et al. , ed. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. [Google Scholar]
- 73. Johansson H, Gandini S, Bonanni B, et al. Relationships between circulating hormone levels, mammographic percent density and breast cancer risk factors in postmenopausal women. Breast Cancer Res Treat. 2008;108(1):57-67. [DOI] [PubMed] [Google Scholar]
- 74. Samoli E, Trichopoulos D, Lagiou A, et al. The hormonal profile of benign breast disease. Br J Cancer. 2013;108(1):199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dorgan JF, Spittle CS, Egleston BL, Shaw CM, Kahle LL, Brinton LA. Assay reproducibility and within-person variation of Müllerian inhibiting substance. Fertil Steril. 2010;94(1):301-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20(4):923-927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Supplementary information and tables are available online at (32).