Abstract
Context
Early initiation of continuous glucose monitoring (CGM) is advocated for youth with type 1 diabetes (T1D). Data to guide CGM use on time-in-range (TIR), hypoglycemia, and the role of partial clinical remission (PCR) are limited.
Objective
Our aims were to assess whether 1) an association between increased TIR and hypoglycemia exists, and 2) how time in hypoglycemia varies by PCR status.
Methods
We analyzed 80 youth who were started on CGM shortly after T1D diagnosis and were followed for up to 1-year post diagnosis. TIR and hypoglycemia rates were determined by CGM data and retrospectively analyzed. PCR was defined as (visit glycated hemoglobin A1c) + (4*units/kg/day) less than 9.
Results
Youth were started on CGM 8.0 (interquartile range, 6.0-13.0) days post diagnosis. Time spent at less than 70 mg/dL remained low despite changes in TIR (highest TIR 74.6 ± 16.7%, 2.4 ± 2.4% hypoglycemia at 1 month post diagnosis; lowest TIR 61.3 ± 20.3%, 2.1 ± 2.7% hypoglycemia at 12 months post diagnosis). No events of severe hypoglycemia occurred. Hypoglycemia was rare and there was minimal difference for PCR vs non-PCR youth (54-70 mg/dL: 1.8% vs 1.2%, P = .04; < 54mg/dL: 0.3% vs 0.3%, P = .55). Approximately 50% of the time spent in hypoglycemia was in the 65 to 70 mg/dL range.
Conclusion
As TIR gradually decreased over 12 months post diagnosis, hypoglycemia was limited with no episodes of severe hypoglycemia. Hypoglycemia rates did not vary in a clinically meaningful manner by PCR status. With CGM being started earlier, consideration needs to be given to modifying CGM hypoglycemia education, including alarm settings. These data support a trial in the year post diagnosis to determine alarm thresholds for youth who wear CGM.
Keywords: diabetes, hypoglycemia, management, type 1 diabetes, pediatric endocrinology
Type 1 diabetes (T1D) is a complex and difficult condition to manage, particularly for newly diagnosed children (1). Intensive glucose control and lower glycated hemoglobin A1c (HbA1c) targets recommended by the American Diabetes Association (ADA) and the International Society of Pediatric and Adolescent Diabetes (ISPAD) guidelines are aimed at reducing long-term microvascular and macrovascular complications (2, 3). However, as the Type 1 Diabetes Exchange has shown (4), only a small percentage of youth (and adults) with T1D meets HbA1c targets. Achieving optimal glycemic control during the early months of diagnosis is challenging but imperative because it sets the stage for future diabetes management habits as well as hypoglycemia-related beliefs and fears (5). Hypoglycemia is associated with subsequent increases in HbA1c (6), poorer quality of life outcomes (7), increased anxiety and fear (8), and barriers to exercise (9) in youth with long-standing T1D. With the increasing use of continuous glucose monitoring (CGM), hypoglycemia targets have been progressively adopted to optimize glycemic control. Current CGM targets include less than 4% of time spent at less than 70 mg/dL and less than 1% of time spent at less than 54 mg/dL (10).
Improvements in glucose values and HbA1c are transiently seen after the diagnosis of T1D and with the initiation of insulin treatment (11). This transient glycemic improvement is characterized by decreased insulin needs and is referred to as partial clinical remission (PCR) (11). Recent data have shown that youth without diabetes spend 1.1% of the day with blood glucose values at less than 70 mg/dL (12), suggesting that a small percentage of hypoglycemia may be physiologic. For these reasons, the ADA standards of care state that glycemic targets can be lower during PCR including a target HbA1c of 6.5%; however, little guidance is offered on the optimal CGM glucose targets (13, 14). We describe CGM data for 80 youth who were started on CGM shortly after diagnosis of T1D (15). The aims of this study were 2-fold:
1) To evaluate the relationship between glucose time-in-range (TIR), clinical hypoglycemia alert (< 70 mg/dL), and clinically serious hypoglycemia (csHypo, defined as < 54 mg/dL) in the first year after onset of diabetes. We hypothesized that an increase in TIR would not be associated with an increase in csHypo.
2) To describe CGM glucose values of less than 70 mg/dL by PCR status in order to assess the frequency and severity of hypoglycemia. We hypothesized that youth, irrespective of PCR status, would have similar time spent in csHypo.
Materials and Methods
Participants
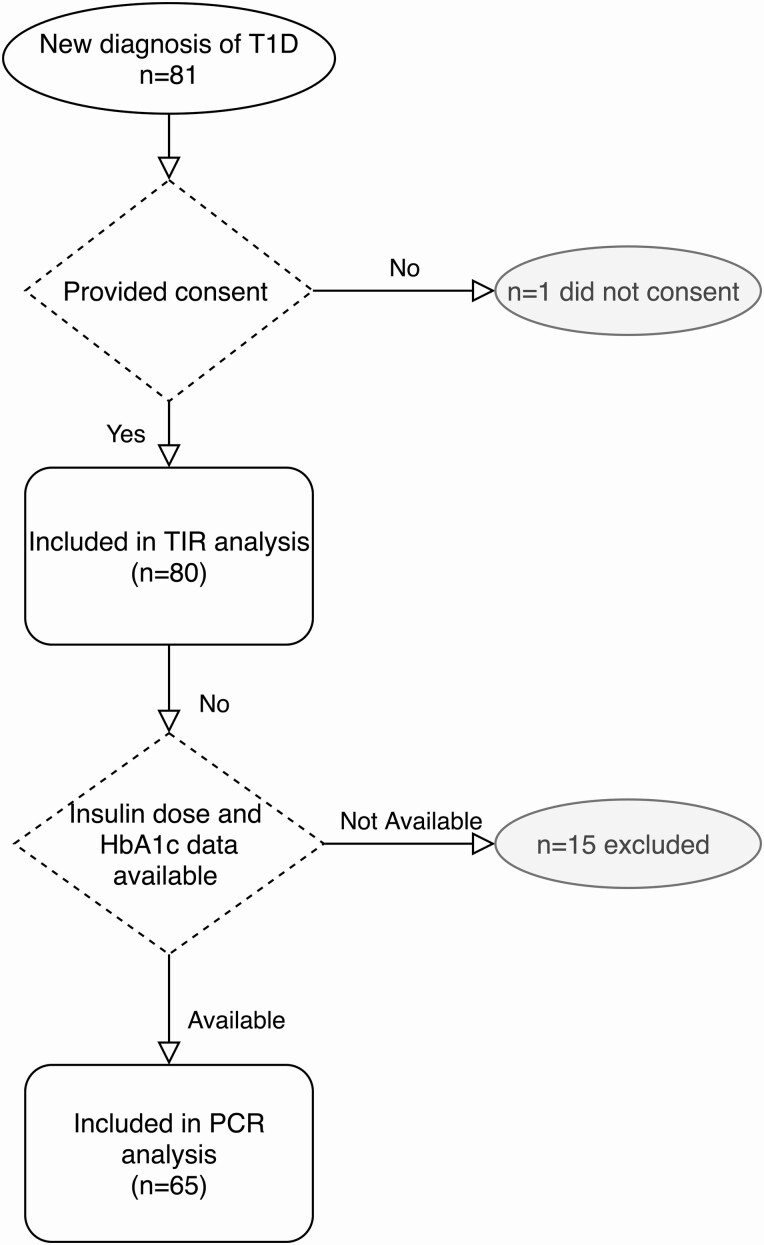
Newly diagnosed youth with T1D were started on CGM (Dexcom G6; Dexcom Inc) in the first month of T1D diagnosis. Between July 2018 and November 2019, 80 youth initiated CGM in the new-onset period as part of a pilot study (Fig. 1) (15). A small subset (n = 7) of participants included in this analysis was started on CGM more than 30 days post diagnosis (range, 57-129 days). This study was approved by the institutional review board at Stanford University.
Figure 1.
CONSORT diagram of study participants from eligibility through analyses. CONSORT indicates Consolidated Standards of Reporting Trials; HbA1c, glycated hemoglobin A1c; PCR, partial clinical remission; T1D, type 1 diabetes; TIR, time-in-range. Fifteen participants did not have complete data in the electronic medical records to determine PCR status.
Hypoglycemia Definitions
We defined hypoglycemia as recommended by the ISPAD consensus guidelines (8). Clinical hypoglycemia alert was defined as a glucose value of less than 70 mg/dL (< 3.9 mmol/L) and is the value that requires an intervention to prevent a further decrease in glucose values. csHypo was defined as a glucose value of less than 54 mg/dL (< 3.0 mmol/L), which is associated with hormonal dysregulation, neurological symptoms, and an increased risk of subsequent hypoglycemia. Severe hypoglycemia was defined as an event, such as coma and convulsions, requiring external assistance in the setting of a low blood sugar.
Statistical Methods
Two distinct, complementary analyses were executed to test our 2 hypotheses. The first analysis evaluated the relationship between TIR and hypoglycemia in all youth, irrespective of PCR status. Data were uploaded by participants to Dexcom Clarity (Dexcom Inc), downloaded by the researchers, and analyzed retrospectively. For percentage time spent at less than 54 mg/dL, less than 70 mg/dL, 70 to 180 mg/dL, 181 to 250 mg/dL, and at greater than 250 mg/dL, we collected CGM data 15 days before and after the following distinct periods: 1 month (n = 66), 3 months (n = 67), 6 months (n = 70), 9 months (n = 68), and 12 months (n = 67) post diagnosis. For baseline (n = 52), CGM data was collected only for the 15 days after the CGM start date. Sample size differences are apparent across time points because of periods of incomplete CGM data. The data were analyzed by a simple linear regression to evaluate the relationship between time from diagnosis and CGM glucose ranges. All statistical analyses for these data were conducted using GraphPad Prism version 9.1 (GraphPad Software).
In the second analysis, we aimed to evaluate hypoglycemia rates by PCR status. PCR was defined as an insulin dose-adjusted HbA1c value of less than 9 calculated from the Mortensen et al equation: [(Visit-HbA1c) + (4*units/kg/day)] (11). We conducted an analysis for those youth with data available on total daily insulin dose and HbA1c (n = 65, 15 participants did not have complete data in the electronic medical record to determine PCR status. We describe CGM glucose values at less than 70 mg/dL for youth wearing CGM by PCR status collected 7 days before and after each visit. Each participant’s raw CGM data were downloaded from Dexcom Clarity in 14-day increments (seven days before and after each available HbA1c measurement) to determine the percentage of time spent in 5 distinct hypoglycemia categories: 54 to 70 mg/dL, 65 to 70 mg/dL, 60 to 65 mg/dL, 54 to 60 mg/dL, and less than 54 mg/dL. This exploratory analysis aimed to determine if there were differences in the rates of hypoglycemia within these different ranges by PCR status. Glucose ranges are inclusive on the lower bound and exclusive on the upper bound (ie, 54-70 mg/dL = 54.00-69.99 mg/dL). These cut points were chosen to also inform a potential future clinical trial using these values for hypoglycemia alerts and action. We also evaluated CGM glucose values both for a 24-hour period and at nighttime (0000h to 0600h). In a multivariate model, we calculated the adjusted mean time spent in the 5 hypoglycemia categories by PCR status. This multivariate model included sex, age at visit, diabetes duration, CGM wear time, and PCR status as dependent variables and glucose values as the independent variable. All statistical analyses for these data were conducted using R 3.6.1 (The R Foundation for Statistical Computing).
Results
Baseline Characteristics
Newly diagnosed youth were all younger than 18 years, age at diabetes onset was 8.8 ± 4.6 years, 56% were male, 81% were on private insurance, 12.1 ± 1.9% HbA1c at diabetes diagnosis, and baseline standardized body mass index (BMIz) was –0.13 ± 1.22 (Table 1). In these 80 participants, CGM was started 8.0 days (range, 6.0-13.0 days) following diagnosis of T1D.
Table 1.
Participant demographic characteristics (n = 80)
| Demographics | |
|---|---|
| Age at diabetes onset, y | 8.8 ± 4.6 |
| Sex, M/F | M = 45/F = 35 |
| HbA1c at diagnosis, % | 12.1 ± 1.9 |
| Diabetic ketoacidosis at onset | 40/80 (50%) |
| Insurance type | 19% public/81% private |
| Height, cm | 136 ± 29 |
| Weight, kg | 35.7 ± 18.3 |
| BMIz | –0.13 ± 1.22 |
| Race and ethnicity | |
| Non-Hispanic White | 36/80 (45%) |
| Hispanic | 13/80 (16%) |
| Non-Hispanic Black | 0/80 (0%) |
| Asian/Pacific Islander | 11/80 (14%) |
| American/Indian/Alaska Native | 0/80 (0%) |
| Other | 8/80 (10%) |
| Unknown | 12/80 (15%) |
Data reported as mean ± SD.
Abbreviations: BMIz, standardized body mass index; F, female; HbA1c, glycated hemoglobin A1c; M, male.
Continuous Glucose Monitoring Analysis
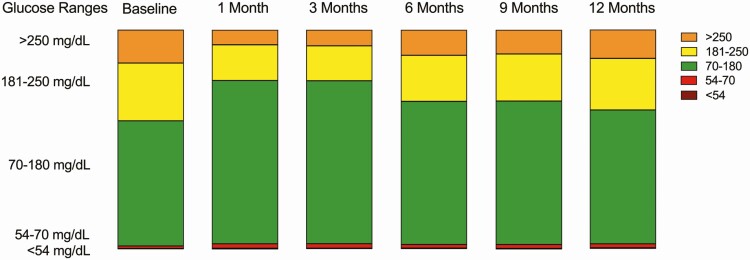
Mean glucose increased from 1 month post diagnosis (147 ± 28 mg/dL) through 12 months post diagnosis (168 ± 34 mg/dL; P < .0001). Similarly, glucose management indicator (GMI) increased from 1 month post diagnosis (6.8% ± 0.7%) through 12 months post diagnosis (7.3% ± 0.8%; P < .0001, Fig. 2). CGM wear time also increased from 85 ± 18% at baseline to 92% ± 20% at 12 months post diagnosis (P = .33). Time spent in hyperglycemia (ranges, 181-250 mg/dL and > 250 mg/dL) increased from 1 month to 12 months post diagnosis (P < .0001 and P = .0005, respectively). TIR declined as diabetes duration increased from a peak of 74.6% ± 16.7% 1 month post diagnosis to a nadir of 61.3 ± 20.3 12 months post diagnosis (P < .0001, Table 2). Percentage of time spent in hypoglycemia (< 70 mg/dL) was 1.2% ± 2.1% at baseline. At 1 month and 3 months post diagnosis, percentage of time at less than 70 mg/dL was 2.4% ± 2.4% and 2.3% ± 2.2%, respectively. Similarly, at 6 months and 9 months post diagnosis, percentage of time at less than 70 mg/dL was 1.9% ± 2.4% and 2.1% ± 2.1%, respectively. Percentage of time at less than 70 mg/dL remained low at 2.1% ± 2.7% at 12 months post diagnosis. In addition, percentage if time in csHypo (< 54 mg/dL) was 0.4% ± 0.5% at 1 month and remained minimal at 0.3% ± 0.6% at 12 months. Unlike the inverse relationship observed between TIR and months post diagnosis, percentage of time at less than 70 mg/dL and less than 54 mg/dL did not vary in the months post diagnosis (P = .47 and P = .83, respectively). No severe hypoglycemia events occurred in our cohort.
Figure 2.
Stacked bar graphs representing continuous glucose monitoring glucose ranges for all participants: percentage of time in hyperglycemia (181-250 mg/dL and > 250 mg/dL), percentage of time in range (70-180 mg/dL), and percentage of time in hypoglycemia (54-70 mg/dL and < 54 mg/dL). All data reported as mean ± SD.
Table 2.
Mean continuous glucose monitoring, glucose management indicator, and percentage of time spent in glucose ranges greater than 250 mg/dL, 181 to 250 mg/dL, 70 to 180 mg/dL, less than 70 mg/dL, and less than 54 mg/dL across all time points
| Baseline | 1 mo | 3 mo | 6 mo | 9 mo | 12 mo | P a | |
|---|---|---|---|---|---|---|---|
| n = 52 | n = 55 | n = 67 | n = 70 | n = 68 | n = 67 | ||
| Mean glucose, mg/dL | 176 ± 39 | 147 ± 28 | 147 ± 29 | 162 ± 35 | 161 ± 33 | 168 ± 34 | < .001 |
| GMI, % | 7.5 ± 0.9 | 6.8 ± 0.7 | 6.8 ± 0.7 | 7.2 ± 0.8 | 7.2 ± 0.8 | 7.3 ± 0.8 | < .001 |
| CGM glucose range, mg/dL, % | |||||||
| > 250 | 15.1 ± 17.1 | 6.7 ± 10.7 | 7.2 ± 9.8 | 11.5 ± 13.2 | 10.9 ± 12.3 | 13.0 ± 13.6 | < .001 |
| 181-250 | 26.4 ± 10.9 | 16.3 ± 8.6 | 16.0 ± 9.7 | 21.1 ± 10.8 | 21.5 ± 10.7 | 23.6 ± 9.7 | < .001 |
| 70-180 | 57.2 ± 22.5 | 74.6 ± 16.7 | 74.6 ± 17.7 | 65.5 ± 21.2 | 65.5 ± 20.1 | 61.3 ± 20.3 | < .001 |
| < 70 b | 1.2 ± 2.1 | 2.4 ± 2.4 | 2.3 ± 2.2 | 1.9 ± 2.4 | 2.1 ± 2.1 | 2.1 ± 2.7 | .47 |
| < 54 c | 0.2 ± 0.5 | 0.4 ± 0.5 | 0.3 ± 0.3 | 0.3 ± 0.6 | 0.3 ± 0.4 | 0.3 ± 0.6 | .83 |
All data reported as mean ± SD.
Abbreviations: CGM, continuous glucose monitoring; GMI, glucose management indicator; IQRs, interquartile ranges.
a P value represents slope from 1 month post diagnosis to 12 months post diagnosis for each category.
b Median (IQRs) for less than 70 mg/dL were 0.3 (0.0-1.6), 1.8 (0.7-3.2), 1.9 (0.8-3.0), 1.2 (0.4-2.5), 1.4 (0.7-3.2), and 1.4 (0.6-2.5) from baseline through 12 months post diagnosis, respectively.
c Median (IQRs) for less than 54 mg/dL were 0.0 (0.0-0.2), 0.2 (0.0-0.5), 0.2 (0.0-0.3), 0.1 (0.0-0.2), 0.2 (0.0-0.4), and 0.1 (0.1-0.3) from baseline through 12 months post diagnosis, respectively.
Partial Clinical Remission Analysis
We included 65 youth (38% female, age 9.7 ± 4.5 years, 39% non-Hispanic White, HbA1c at onset 12.2% ± 1.9%, diabetes duration 56.0 ± 79.8 days, CGM wear time 88.1% ± 19.7%) who had sufficient data to determine PCR status for this analysis. Participant characteristics (such as age, sex, HbA1c at onset) of the 65 youth included in the PCR analysis did not differ from the larger 80 youth sample. Of the 65 youth who were evaluated, 38 youth (29% female, age 9.4 ± 4.7 years, 34% non-Hispanic White, diabetes duration 39.5 ± 41.7 days, CGM wear time 88.2% ± 22.7%) had at least one visit that was classified as PCR whereas 27 youth never had a visit that qualified as PCR (52% female, age 10.2 ± 4.3 years, 44% non-Hispanic White, diabetes duration 79.2 ± 111.0 days, CGM wear time 87.9% ± 14.7%). Youth in PCR had lower HbA1c at T1D onset when compared to youth not in PCR (11.7% ± 2.2% vs 12.8% ± 1.2%, P = .01). Sex (P = .11), age (P = .51), race (P = .85), diabetes duration (P = .08), and CGM wear time (P = .96) did not differ significantly by PCR status.
Youth, both in and out of PCR, did not experience any episodes of severe hypoglycemia in the study period. In the multivariate regression (presented in Table 3) there was minimal clinical difference (0.6% or 8.6 minutes/day) in time spent between 54 to 70 mg/dL by PCR status in a 24-hour period (1.8% PCR vs 1.2% non-PCR, P = .04), although it was statistically significantly higher in the PCR group. The statistical difference in the 54 to 70 mg/dL range appears to be driven primarily by modest differences in time spent between 65 and 70 mg/dL (1.0% PCR vs 0.6% non-PCR, P = .02) as time spent in the remaining hypoglycemia ranges did not differ by PCR status. Time spent between 54 and 70 mg/dL did not statistically differ by PCR status at nighttime. csHypo did not vary by PCR status in a 24-hour period (0.3% PCR vs 0.3% non-PCR, P = .55) or at nighttime (0.7% PCR vs 0.7% non-PCR, P = .76). Notably, when evaluating the glucose values within the 54 to 70 mg/dL range, approximately half the time was spent between 65 and 70 mg/dL in a 24-hour period (52.3% for PCR and 52.0% for non-PCR) and at nighttime (50.8% for PCR and 52.4% for non-PCR).
Table 3.
Mean percentage time spent in hypoglycemic ranges with different thresholds in a 24-hour period and at nighttime
| Glucose range, mg/dL | Partial clinical remissiona n = 38 |
No partial clinical remissionb n = 27 |
P d | |||||
|---|---|---|---|---|---|---|---|---|
| % Time | (95% CI) | % Time | (95% CI) | |||||
| 24-h | 54-70 | 1.8 | 0.0 | 1.1 | 1.2 | –0.3 | 2.8 | .04 |
| 65-70 | 1.0 | 0.1 | 0.6 | 0.6 | –0.1 | 1.3 | .02 | |
| 60-65 | 0.5 | 0.0 | 0.4 | 0.3 | –0.2 | 0.8 | .06 | |
| 54-60 | 0.4 | 0.0 | 0.2 | 0.3 | –0.1 | 0.7 | .14 | |
| < 54 | 0.3 | –0.1 | 0.1 | 0.3 | 0.0 | 0.6 | .55 | |
| Nighttimec | 54-70 | 3.3 | –0.1 | 1.8 | 2.5 | –0.8 | 5.8 | .09 |
| 65-70 | 1.7 | 0.0 | 0.9 | 1.3 | –0.1 | 2.7 | .07 | |
| 60-65 | 0.8 | –0.1 | 0.6 | 0.6 | –0.5 | 1.7 | .12 | |
| 54-60 | 0.8 | –0.1 | 0.5 | 0.6 | –0.3 | 1.6 | .17 | |
| < 54 | 0.7 | –0.2 | 0.3 | 0.7 | –0.3 | 1.6 | .76 |
a Insulin dose-adjusted glycated hemoglobin A1c less than or equal to 9.
b Insulin dose-adjusted glycated hemoglobin A1c greater than 9.
c Nighttime is defined midnight to 6 am; percentags are presented for this 6-hour period.
d Regression model controls for sex, age at visit, diabetes duration, continuous glucose monitoring wear time, and partial clinical remission status.
Discussion
These data from 80 youth who were started on CGM shortly after T1D diagnosis demonstrate that csHypo was infrequent and not associated with TIR, consistent with literature indicating that improvements in HbA1c or TIR can be achieved without an increase in csHypo or severe hypoglycemia (16-20). Notably, there were no events of severe hypoglycemia in our cohort. In an analysis of hypoglycemia by PCR status, we found clinical hypoglycemia alert and csHypo to be rare, irrespective of PCR status. For youth in PCR, the return of endogenous pancreatic function may also play a role in the disassociation in the relationship between TIR and hypoglycemia. The transient return of pancreatic function observed during PCR likely results both in regulation of dysglycemia and a decrease in csHypo due to lower total daily insulin doses (21-23). Approximately 50% of the time spent between 54 and 70 mg/dL was spent between 65 and 70 mg/dL. Taken together, these 2 analyses offer insight into the relationship between TIR and hypoglycemia as well as the nature of hypoglycemia in youth by PCR status during the year after T1D diagnosis. Consistent with ADA guidelines (13, 14), these data suggest that lower CGM alarms may be safe during PCR, particularly as early CGM initiation becomes commonplace, and the use of automated insulin delivery becomes the standard for pump therapy. In addition, at nighttime, automated insulin delivery is also particularly effective at preventing severe hypoglycemia while optimizing TIR (24-27).
Currently, most low alarm alerts for youth wearing CGM are set at 70 mg/dL because this is the standard threshold for hypoglycemic treatment with fast-acting carbohydrates. Given that alarm fatigue with CGM is a commonly stated reason for CGM discontinuation (28-32), our findings suggest decreasing the threshold alarm to 65 mg/dL in youth who wear CGM might significantly decrease both alarm burden and overtreatment of hypoglycemia not reaching the clinically serious level of less than 54 mg/dL. However, this requires further investigation to determine safety and how often glucose values between 65 and 70 mg/dL self-resolve without rapid-acting carbohydrates to establish the benefit and safety of lower alarm targets. csHypo was rare both in youth with and without PCR, suggesting that a lowered CGM alert may be safe as well. The participants in our cohort who were recently diagnosed and used CGM had similar rates of hypoglycemia to youth without diabetes (12). Clinical trials comparing hypoglycemia, TIR, and HbA1c outcomes for hypoglycemia targets of 60 mg/dL, 65 mg/dL, and the standard 70 mg/dL in PCR and non-PCR youth may offer meaningful insight to improve glycemic outcomes, optimize hypoglycemia targets, and increase CGM uptake and retention while assessing safety.
The current standard of care to treat hypoglycemia includes administering fast-acting carbohydrates for any glucose value below 70 mg/dL. While some studies have offered suggestions on incorporating trend arrows in the treatment of hypoglycemia (33-35), national and international guidelines do not integrate the trend arrows provided by CGM into hypoglycemia treatment algorithms. In addition, new-onset diabetes education requires training families on the treatment of csHypo and severe hypoglycemia, which historically has included intramuscular glucagon—a common source of parental fear (36, 37). Education about the approach and treatment of hypoglycemia can affect the degree to which youth and their family experience fear of hypoglycemia (38). In one study evaluating CGM initiation in youth shortly after T1D diagnosis, parents did not report a decrease in fear of hypoglycemia. However, parents modified their behavior and did not preferentially maintain hyperglycemia to avoid hypoglycemia (39). The approach to new-onset diabetes education and changing CGM threshold alarms may reduce this worry and fear of hypoglycemia. In a qualitative study evaluating the parental experience of CGM initiation in youth shortly after T1D diagnosis, parents were overwhelmingly enthusiastic about early CGM initiation and found CGM to be essential in diabetes care (40). Accordingly, these newly diagnosed youth with T1D continued to wear CGM throughout the first year of diagnosis.
There is a strong relationship between TIR and HbA1c (41, 42) and HbA1c commonly increases as early as 6 months after the diagnosis of T1D (43). Similarly, in our cohort, sustained improvements in TIR beyond 3 months post diagnosis waned as TIR decreased and GMI increased by 12 months post diagnosis. The apparent increase in mean glucose and decrease in TIR as early as 6 months post diagnosis presents an opportunity for more frequent monitoring and unified education by health care providers to target tighter glycemic control for this population, which is currently under investigation (15).
These data are applicable only to youth who wear CGM as all study conclusions are derived from CGM data. This limitation in the use of these findings raises an important consideration in the equitable access of CGM technology. Emerging studies suggest that youth from low socioeconomic groups have less access to CGM and diabetes technology (44), with insurance as the primary driver for these inequities (45-49). Thus, this study and all emerging studies using diabetes technologies will have an inherent limitation to both generalizability and equitable provision of care if access to CGM is not addressed (50, 51).
There are limitations to this study that are worth noting, including its single-site design and differences within the CGM data sample size at each time point due to incomplete CGM data. To evaluate for potential biases, we compared baseline TIR for participants who completed the entire 12-month study duration with participants who completed less than 12 months’ duration and found no difference in TIR between the groups (P = .36). In addition, our findings are limited to youth currently using CGM technology and therefore cannot be generalized to individuals self-monitoring their blood glucose. Thus, larger studies with a more diverse and population representative sample, including heterogeneity in BMI, insurance status, and race/ethnicity, are warranted. Additionally, further research is also needed to determine whether these lower targets may need to be adjusted once individuals are no longer in PCR, although we found a minimal difference for csHypo, irrespective of PCR vs non-PCR youth or for different age groups.
In summary, CGM initiation in the first month of diabetes diagnosis in youth with T1D demonstrates a high persistence of use in addition to a low incidence of hypoglycemia (52). Time spent in clinical hypoglycemia alert (< 70 mg/dL) and csHypo (< 54 mg/dL) were rare, and no severe hypoglycemia episodes occurred. We found that approximately 50% of time spent in clinical hypoglycemia alert was in the 65 to 70 mg/dL range, and at the same frequency as described in people without diabetes. As such, a threshold alarm of 65 mg/dL may reduce hypoglycemia-related alarms without increasing the risk of csHypo or severe hypoglycemia and could be tested in a prospectively designed study to evaluate safety, quality of life, and CGM metrics.
Acknowledgments
The authors would like to acknowledge all the youth who participated in the 4T study.
Financial Support: This work was supported in part by the Stanford Diabetes Research Center and the National Institute of Diabetes and Digestive and Kidney Diseases (grant Nos. P30DK116074 and R18 DK122422) and the Maternal Child Health Research Institute and the National Institute of Diabetes and Digestive and Kidney Diseases (grant No. K12DK122550 to A.A.) at Stanford University. CGM supplies for the first month (transmitter, 3 sensors, and receiver per patient) were donated by Dexcom. Funding for iOS devices was provided by a grant through the Lucile Packard Children’s Hospital Auxiliaries Endowment.
Author Contributions: A.A., D.P.Z., and D.M.M. contributed to the concept and design of the study. A.A., D.P.Z., A.G., and D.S. performed the statistical analysis. A.A. and D.P.Z. wrote the manuscript with critical contributions from D.M.M., B.B., P.P., and D.S. All authors contributed to manuscript revision and approved the submitted manuscript.
Glossary
Abbreviations
- ADA
American Diabetes Association
- BMIz
standardized body mass index
- CGM
continuous glucose monitoring
- csHypo
clinically serious hypoglycemia
- HbA1c
glycated hemoglobin A1c
- ISPAD
International Society of Pediatric and Adolescent Diabetes
- PCR
partial clinical remission
- T1D
type 1 diabetes
- TIR
time in range
Additional Information
Disclosures: D.P.Z. has received speakers honoraria from Medtronic Diabetes, Ascensia Diabetes, and Insulet Canada; and research support from the Helmsley Charitable Trust and ISPAD-JDRF Research Fellowship. D.M.M. has received research support from the National Institutes of Health, JDRF, NSF, and the Helmsley Charitable Trust; and his institution has received research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. He has consulted for Abbott, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, and Insulet, and is supported by P30DK116074. B.B. has received research support from the National Institutes of Health, JDRF, Medtronic, Insulet, Tandem, Dexcom, Beta Bionics, Eli Lilly, and Convatec, and has consulted for Medtronic, Convatec, and Insulet. K.H. has received research support from Dexcom, Inc, for investigator-initiated research and consultant fees from the Lilly Innovation Center, LifeScan Diabetes Institute, and MedIQ. The remaining authors do not report any relevant disclosures.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Chiang JL, Maahs DM, Garvey KC, et al. Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care. 2018;41(9):2026-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44(Suppl 1):S73-S84. [DOI] [PubMed] [Google Scholar]
- 3. DiMeglio LA, Acerini CL, Codner E, et al. ISPAD clinical practice consensus guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):105-114. [DOI] [PubMed] [Google Scholar]
- 4. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdul-Rasoul M, Habib H, Al-Khouly M. ‘The honeymoon phase’ in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7(2):101-107. [DOI] [PubMed] [Google Scholar]
- 6. Pilgaard KA, Breinegaard N, Johannesen J, et al. Episodes of severe hypoglycemia is associated with a progressive increase in hemoglobin A1c in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2020;21(5):808-813. [DOI] [PubMed] [Google Scholar]
- 7. Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes. 2011;4:337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abraham MB, Jones TW, Naranjo D, et al. ISPAD clinical practice consensus guidelines 2018: assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 2018;19(Suppl 27):178-192. [DOI] [PubMed] [Google Scholar]
- 9. Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31(11):2108-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mortensen HB, Hougaard P, Swift P, et al. ; Hvidoere Study Group on Childhood Diabetes . New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32(8):1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Diabetes Association. 13. Children and adolescents: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44(Suppl 1):S180-S199. [DOI] [PubMed] [Google Scholar]
- 14. Redondo MJ, Libman I, Maahs DM, et al. The evolution of hemoglobin A1c targets for youth with type 1 diabetes: rationale and supporting evidence. Diabetes Care. 2021;44(2):301-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prahalad P, Zaharieva DP, Addala A, et al. Improving clinical outcomes in newly diagnosed pediatric type 1 diabetes: teamwork, targets, technology, and tight control—The 4T Study. Front Endocrinol (Lausanne). 2020;11:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haynes A, Hermann JM, Clapin H, et al. WACDD and DPV registries. Decreasing trends in mean HbA1c are not associated with increasing rates of severe hypoglycemia in children: a longitudinal analysis of two contemporary population-based pediatric type 1 diabetes registries from Australia and Germany/Austria between 1995 and 2016. Diabetes Care. 2019;42(9):1630-1636. [DOI] [PubMed] [Google Scholar]
- 18. Haynes A, Hermann JM, Miller KM, et al. T1D Exchange; WACDD and DPV registries. Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes. 2017;18(7):643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinstock RS, Xing D, Maahs DM, et al. T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab. 2013;98(8):3411-3419. [DOI] [PubMed] [Google Scholar]
- 20. Maahs DM, Hermann JM, DuBose SN, et al. Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D Exchange and German/Austrian DPV registries. Diabetologia. 2014;57(8):1578-1585. [DOI] [PubMed] [Google Scholar]
- 21. Akirav E, Kushner JA, Herold KC. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes. 2008;57(11):2883-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hramiak IM, Dupre J, Finegood DT. Determinants of clinical remission in recent-onset IDDM. Diabetes Care. 1993;16(1):125-132. [DOI] [PubMed] [Google Scholar]
- 23. Bowden SA. Partial remission (honeymoon phase) in type 1 diabetes mellitus. In: Atta-ur-Rahman, ed. Frontiers in Clinical Drug Research—Diabetes and Obesity. The Ohio State University; 2017;4:1-20. [Google Scholar]
- 24. Maahs DM, Calhoun P, Buckingham BA, et al. In Home Closed Loop Study Group . A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care. 2014;37(7):1885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forlenza GP, Li Z, Buckingham BA, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG Trial. Diabetes Care. 2018;41(10):2155-2161. [DOI] [PubMed] [Google Scholar]
- 26. Buckingham BA, Raghinaru D, Cameron F, et al. In Home Closed Loop Study Group . Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care. 2015;38(7):1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collyns OJ, Meier RA, Betts ZL, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44(4):969-975. [DOI] [PubMed] [Google Scholar]
- 28. Shivers JP, Mackowiak L, Anhalt H, Zisser H.. “Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Technol. 2013;7(3):789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lawton J, Blackburn M, Allen J, et al. Patients’ and caregivers’ experiences of using continuous glucose monitoring to support diabetes self-management: qualitative study. BMC Endocr Disord. 2018;18(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forlenza GP, Messer LH, Berget C, Wadwa RP, Driscoll KA. Biopsychosocial factors associated with satisfaction and sustained use of artificial pancreas technology and its components: a call to the technology field. Curr Diab Rep. 2018;18(11):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40(2):181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Messer LH, Johnson R, Driscoll KA, Jones J. Best friend or spy: a qualitative meta-synthesis on the impact of continuous glucose monitoring on life with type 1 diabetes. Diabet Med. 2018;35(4):409-418. [DOI] [PubMed] [Google Scholar]
- 33. Moser O, Riddell MC, Eckstein ML, et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia. 2020;63(12):2501-2520. [DOI] [PubMed] [Google Scholar]
- 34. Aleppo G, Laffel LM, Ahmann AJ, et al. A practical approach to using trend arrows on the Dexcom G5 CGM system for the management of adults with diabetes. J Endocr Soc. 2017;1(12):1445-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ziegler R, von Sengbusch S, Kröger J, et al. Therapy adjustments based on trend arrows using continuous glucose monitoring systems. J Diabetes Sci Technol. 2019;13(4):763-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sherr JL, Ruedy KJ, Foster NC, et al. ; T1D Exchange Intranasal Glucagon Investigators . Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. 2016;39(4):555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pontiroli AE, Ceriani V. Intranasal glucagon for hypoglycaemia in diabetic patients. An old dream is becoming reality? Diabetes Obes Metab. 2018;20(8):1812-1816. [DOI] [PubMed] [Google Scholar]
- 38. Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr Diab Rep. 2016;16(8):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Youngkin EM, Majidi S, Noser AE, Stanek KR, Clements MA, Patton SR.. Continuous glucose monitoring decreases hypoglycemia avoidance behaviors, but not worry in parents of youth with new onset type 1 diabetes. J Diabetes Sci Technol. Published online June 10, 2020. doi:10.1177/1932296820929420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tanenbaum ML, Zaharieva DP, Addala A, et al. ‘I was ready for it at the beginning’: parent experiences with early introduction of continuous glucose monitoring following their child’s type 1 diabetes diagnosis. Diabet Med. Published online March 27, 2021. doi: 10.1111/dme.14567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81-85. [DOI] [PubMed] [Google Scholar]
- 42. Valenzano M, Cibrario Bertolotti I, Valenzano A, Grassi G.. Time in range-A1c hemoglobin relationship in continuous glucose monitoring of type 1 diabetes: a real-world study. BMJ Open Diabetes Res Care. 2021;9(1):e001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prahalad P, Yang J, Scheinker D, Desai M, Hood K, Maahs DM. Hemoglobin A1c trajectory in pediatric patients with newly diagnosed type 1 diabetes. Diabetes Technol Ther. 2019;21(8):456-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Addala A, Maahs DM, Scheinker D, Chertow S, Leverenz B, Prahalad P. Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes. 2020;21(7):1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anderson JE, Gavin JR, Kruger DF. Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther. 2020;22(3):169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ravi SJ, Coakley A, Vigers T, Pyle L, Forlenza GP, Alonso T.. Pediatric Medicaid patients with type 1 diabetes benefit from continuous glucose monitor technology. J Diabetes Sci Technol. 2021;15(3):630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walker AF, Hood KK, Gurka MJ, et al. Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care. Published online May 17, 2021. doi: 10.2337/dc20-2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agarwal S, Kanapka LG, Raymond JK, et al. Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(8):e2960-e2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44(1):258-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lipman TH, Hawkes CP. Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care. 2021;44(1):14-16. [DOI] [PubMed] [Google Scholar]
- 52. Prahalad P, Addala A, Scheinker D, Hood KK, Maahs DM. CGM initiation soon after type 1 diabetes diagnosis results in sustained CGM use and wear time. Diabetes Care. 2020;43(1):e3-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.