Abstract
Context
For children with growth hormone deficiency (GHD), treatment burden with daily somatropin injections [human growth hormone (hGH)] is high, which may lead to poor adherence and suboptimal overall treatment outcomes. Lonapegsomatropin (TransCon hGH) is an investigational long-acting, once-weekly prodrug for the treatment of GHD.
Objective
The objective of this study was to evaluate the efficacy and safety of once-weekly lonapegsomatropin vs daily somatropin.
Design
The heiGHt trial was a randomized, open-label, active-controlled, 52-week Phase 3 trial (NCT02781727).
Setting
This trial took place at 73 sites across 15 countries.
Patients
This trial enrolled and dosed 161 treatment-naïve, prepubertal patients with GHD.
Interventions
Patients were randomized 2:1 to receive lonapegsomatropin 0.24 mg hGH/kg/week or an equivalent weekly dose of somatropin delivered daily.
Main Outcome Measure
The primary end point was annualized height velocity (AHV) at week 52. Secondary efficacy end points included change from baseline in height SD scores (SDS).
Results
Least squares (LS) mean (SE) AHV at 52 weeks was 11.2 (0.2) cm/year for lonapegsomatropin vs 10.3 (0.3) cm/year for daily somatropin (P = 0.009), with lonapegsomatropin demonstrating both noninferiority and superiority over daily somatropin. LS mean (SE) height SDS increased from baseline to week 52 by 1.10 (0.04) vs 0.96 (0.05) in the weekly lonapegsomatropin vs daily somatropin groups (P = 0.01). Bone age/chronological age ratio, adverse events, tolerability, and immunogenicity were similar between groups.
Conclusions
The trial met its primary objective of noninferiority in AHV and further showed superiority of lonapegsomatropin compared to daily somatropin, with similar safety, in treatment-naïve children with GHD.
Growth hormone (GH) promotes growth, maintenance of normal body composition, and overall endocrine health. GH exerts its effects both by directly binding to specific cell surface receptors and indirectly via insulin-like growth factor 1 (IGF-1). In addition to stimulating bone growth, GH is important for organ development, cardiovascular function, cognition, and metabolism (1-4).
Since 1987, children with GH deficiency (GHD) have been treated with daily injections of somatropin (recombinant human GH). Although treatment with daily somatropin is safe and provides children with the potential to achieve normal adult height, real-world outcomes have not matched expectations (5). Both children and their caregivers find the daily injection frequency to be burdensome, leading to nonadherence rates of 5% to 82% (5,6). In 2015, the Growth Hormone Research Society recognized the need for a long-acting growth hormone (LAGH) and agreed that by decreasing injection frequency and offering different pharmacokinetic properties, a LAGH would potentially improve adherence and outcomes (7).
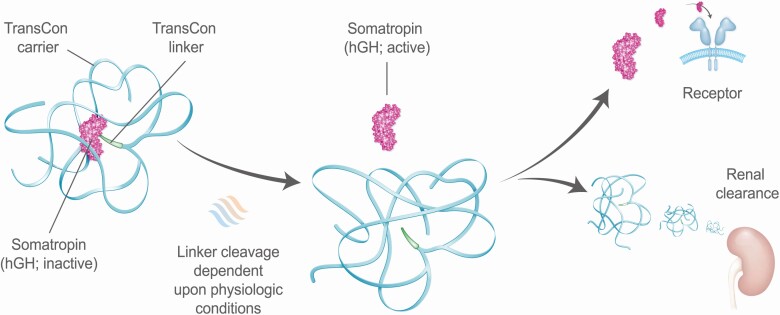
Lonapegsomatropin (TransCon hGH; Ascendis Pharma A/S) is a once-weekly long-acting prodrug in development for children and adults with GHD consisting of the parent drug, somatropin, an inert methyoxy polyethelene glycol carrier, and a TransCon® linker (Fig. 1) (8,9). Lonapegsomatropin is the only LAGH in Phase 3 development that releases somatropin with the identical 191 amino acid sequence and size (22 kDa) as both endogenous GH and daily somatropin therapy and is thus designed to maintain the same mode of action, distribution, and intracellular signaling (9). The apparent half-life of somatropin released from lonapegsomatropin is approximately 25 h, as established in clinical Phase 1 trials (data not shown), permitting the once-a-week dosing interval.
Figure 1.
Transient conjugation. Lonapegsomatropin is a long-acting prodrug consisting of the parent drug, unmodified somatropin; an inert carrier; and a proprietary linker that temporarily binds the somatropin and carrier. The carrier has a shielding effect that minimizes renal excretion and receptor-mediated clearance. Following autocleavage of the linker under physiologic conditions, lonapegsomatropin predictably releases somatropin within therapeutic levels over one week.
The purpose of the pivotal Phase 3 heiGHt trial was to evaluate the efficacy, safety, and tolerability of weekly lonapegsomatropin vs daily somatropin (Genotropin®, Pfizer) over 52 weeks in prepubertal children with GHD.
Materials and Methods
Study Oversight
This was a Phase 3 randomized, open-label, active-controlled trial comparing weekly lonapegsomatropin to daily somatropin (ClinicalTrials.gov: NCT02781727). The trial was approved by ethics and institutional review boards and conducted from December 2016 to January 2019 at 73 sites in 15 countries. All guardians of patients provided written informed consent.
Patients and Study Design
Treatment-naïve males and females (3-12 or 3-11 years old, respectively) at Tanner stage 1 with GHD (either isolated or as part of multiple pituitary hormone deficiency) were eligible (10). Diagnosis of GHD was defined as peak GH ≤10 ng/mL confirmed via 2 different GH stimulation tests ([insulin tolerance test (with cortisol response to hypoglycemia), arginine test, clonidine test, glucagon test (with or without propranolol, with cortisol response unless cortisol measured during an insulin tolerance test), or L-dopa test (with or without propranolol)], and subjects were primed with sex hormones at the discretion of the investigator. Subjects also had to have a height SD score (SDS) (11) ≤ −2.0, IGF-1 SDS ≤ −1.0, and body mass index (BMI) within ±2.0 SD of the mean, and bone age ≥6 months behind chronological age determined by a blinded radiologist. Exclusion criteria included prior exposure to GH or IGF-1 therapies; a history of malignancy (clinically cured tumors permitted); evidence of contemporaneous malnourishment, idiopathic short stature, small for gestational age, or other non-GHD related causes of short stature; or a prior diagnosis of or receiving treatment for other major medical conditions in which the disease and/or treatment might affect longitudinal growth.
In addition to screening, patients attended 6 visits at weeks 1 (baseline), 5, 13, 26, 39, and 52 for height and weight measurements, blood sampling for laboratory parameters, pharmacokinetic, IGF-1 and IGF binding protein 3 (IGFBP-3) measurements, immunogenicity [antihuman growth hormone (anti-hGH) binding and neutralizing antibodies] testing, and adverse event (AE) monitoring. Bone age was read by a blinded central bone age reader. At each site, auxology was to be performed by the same blinded auxologist at each visit when possible.
Eligible patients were randomized 2:1 to receive once-weekly lonapegsomatropin (0.24 mg hGH/kg/wk) or an equivalent dose of daily somatropin (0.034 mg hGH/kg/day) subcutaneously for 52 weeks. Randomization strata included age (≤6 and ˃6 years), peak stimulated GH levels (≤5 ng/mL or >5 ng/mL), and sex. Dosing was based on weight at the first visit and adjusted as patients grew. This trial was designed to be fixed-dose throughout the 52-week period, although doses could be adjusted at the discretion of the investigator after discussion with the medical monitor due to symptoms or lab results. Both drugs were recommended to be administered in a rotating fashion to the buttocks, thighs, and abdomen.
Study End Points
The primary efficacy end point was annualized height velocity (AHV; cm/year) after 52 weeks based on a wall-mounted, calibrated stadiometer and defined as
Secondary end points included parameters assessed at predefined timepoints over 52 weeks: AHV, height SDS (12), IGF-1, IGF-1 SDS, IGFBP-3, IGFBP-3 SDS, and bone age. Height velocity SDS was evaluated as an ad hoc analysis (13,14). IGF-I SDS and IGFBP-3 SDS were calculated according to Bidlingmaier et al (15) and Friedrich et al (16), respectively.
For the lonapegsomatropin group, IGF-1 was measured at baseline, at trough during week 5 (predose) and week 52 (6-7 days post dose), and at anticipated peak (2-3 days post dose) at weeks 13, 26, and 39. Furthermore, a pharmacokinetic/pharmacodynamic (PK/PD) subset of 11 patients had intensive pharmacokinetic and IGF-1 sampling during the dosing interval at week 13 (presumed steady state). All randomized subjects were medically eligible for the PK/PD subset. The heiGHt trial sought to enroll 8 subjects into the PK/PD subset and ultimately enrolled 11 subjects across 6 sites and 2 countries. The 6 sites where subjects were enrolled into the PK/PD subset had sufficient equipment, staff, and operating hours to conduct the rich sampling over the course of 1 week. Quantification of serum somatropin was conducted by Celerion (Lincoln, NE, USA) using an enzyme-linked immunosorbent assay. Quantification of serum IGF-1 and IGFBP-3 was performed by Laboratorium für Klinische Forschung GmbH (Schwentinental, Germany) using validated chemiluminescence immunoassays (Immunodiagnostics Systems iSYS, Boldon, UK).
Safety end points included incidence of AEs, local tolerability, laboratory parameters, and immunogenicity. Local injection site reactions were reported as AEs if considered abnormal with respect to duration and/or intensity. Anti-hGH-binding antibodies were detected by a validated assay (BioAgilytix, Durham, NC, USA); antibody positive samples were assessed for neutralizing activity using a cell-based assay (Eurofins, Abingdon, UK).
Average IGF-1 and Population Pharmacodynamic Model
Unlike daily somatropin with relatively constant IGF-1 values at steady state, a weekly LAGH like lonapegsomatropin has a characteristic weekly IGF-1 profile with levels that vary depending relative to time since the last dose. At steady state, the weekly average IGF-1 level best characterizes the overall IGF-1 exposure. The weekly average IGF-1 was defined as the area under the IGF-1 concentration curve over 0 to 168 h of the dose interval divided by 168 h, estimated as next described.
To calculate the average IGF-1 level at steady state for lonapegsomatropin, a nonlinear mixed effect population pharmacodynamic modeling was conducted based on all IGF-1 samples from this study and the Phase 2 study (8). The overall goal of the modeling was to predict full IGF-1 profiles at each visit for subjects in this study based on their sparse samples, so that the average IGF-1 values at any study visit can be calculated. An endogenous 1-compartment model with simultaneous zero- and first-order stimulation of IGF-1 production with first-order clearance was successfully selected as the final model, and a proportional error model fitted best. The structural kinetics for IGF-1 concentrations were established with no covariates identified as being statistically significant. The overall performance of the model met the required goal of predicting weekly IGF-1 profile and average IGF-1 concentration from the sparse sample subjects in the current Phase 3 heiGHt trial. The model was fit using NONMEM 7.4, and all modeling results were processed using R 3.6.0.
Average IGF-1 SDS for the daily somatropin group was represented by observed values given the known low fluctuation over the 24-h dose interval for daily somatropin. For lonapegsomatropin, where IGF-1 levels follow a predictable weekly curve, the weekly average IGF-1 was presented to reflect overall systemic exposure to IGF-1 and to allow direct comparison to the daily average for daily somatropin. Weekly model-derived profiles of IGF-1 for each individual and the thereof derived average IGF-1 for each of the post-baseline visits was generated based on model simulation of IGF-1 profiles for all study visits with an IGF-1 sample.
Statistical Analyses
Assuming a SD of 3.5 cm/year with a noninferiority margin of 2.0 cm, a sample size of 147 patients in the intent-to-treat population, randomized 2:1 to lonapegsomatropin:daily somatropin, were calculated to provide 90% power to show noninferiority of lonapegsomatropin compared to daily somatropin. An analysis of covariance (ANCOVA) with multiple imputations for missing data was used for the primary endpoint of AHV; a 2-sided 95% CI was used to determine noninferiority, followed by a test of superiority if noninferiority was established. The ANCOVA model included treatment and sex as factors and baseline age, peak stimulated GH levels (log transformed), and height corrected for genetic potential (height SDS − average parental height SDS) as covariates. The same ANCOVA model is also applied for height velocity SDS. Analyses of prespecified subgroups were selected based on factors known to contribute to growth potential and included age, peak stimulated GH levels, sex, and GHD etiology. Bone age/chronological age and IGF-1/IGFBP-3 ratios are reported as arithmetic means. For all other reported secondary end points, ANCOVA models were applied, including treatment and sex as factors, baseline age, and baseline values of the corresponding variables as covariates. Efficacy analyses were based on the intent-to-treat population, which included all randomized patients who received at least 1 dose of study medication. The safety analysis population included all randomized patients who received at least 1 dose of study medication. Overall treatment adherence for each group was defined as the ratio of total number of administered to planned doses as assessed by drug accountability records and patient diaries.
Results
Participants and Baseline Characteristics
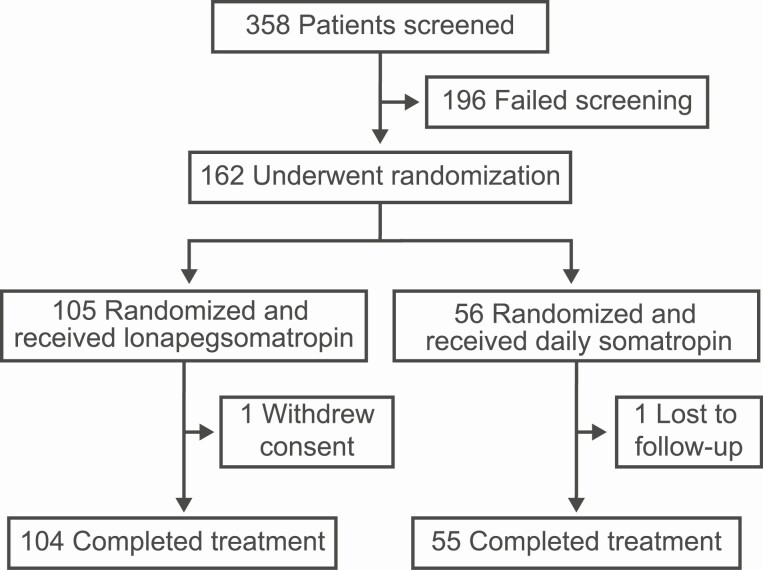
Of the 161 patients who enrolled and received at least 1 dose of study medication, 159 completed the trial (Fig. 2); 1 lonapegsomatropin patient withdrew consent and 1 daily somatropin patient was lost to follow-up. The groups were generally well-balanced with respect to baseline demographics and clinical characteristics, including approximately 17% in each arm with multiple pituitary hormone deficiency (Table 1). By week 52, 13.8% of all subjects entered the pubertal transition (lonapegsomatropin: 8/85 males entered gonadal stage 2, and 4/15 females entered breast stage 2; daily somatropin: 8/45 and 2/45 males entered gonadal stage 2 and 3, respectively, and 0/10 females entered breast stage 2). Mean adherence rates were 99.6% and 98.6% for the lonapegsomatropin and daily somatropin groups, respectively.
Figure 2.
Screening, randomization, and follow-up.
Table 1.
Baseline demographics and clinical characteristics
| Weekly lonapegsomatropin | Daily somatropin | Total | |
|---|---|---|---|
| 0.24 mg hGH/kg/wk | 0.24 mg hGH/kg/wk |
||
| n = 105 | n = 56 | n = 161 | |
| Demographics | |||
| Male, n (%) | 86 (82) | 46 (82) | 132 (82) |
| White, n (%) | 100 (95.2) | 52 (92.9) | 152 (94.4) |
| GHD etiology, n (%) | |||
| Isolated idiopathic | 68 (65) | 37 (66) | 105 (65) |
| Isolated organic | 19 (18) | 9 (16) | 28 (17) |
| MPHD | 18 (17) | 10 (18) | 28 (17) |
| Chronological age, yeara | 8.5 ± 2.7 | 8.5 ± 2.8 | 8.5 ± 2.7 |
| Auxological dataa | |||
| Bone age, year | 5.8 ± 2.6 | 6.0 ± 2.7 | 5.9 ± 2.6 |
| Bone age/chronological age ratio | 0.69 ± 0.16 | 0.70 ± 0.14 | 0.69 ± 0.15 |
| Weight, kg | 21.0 ± 6.5 | 21.2 ± 6.7 | 21.1 ± 6.6 |
| Height, cm | 112.9 ± 14.1 | 112.2 ± 15.3 | 112.7 ± 14.5 |
| Height SDS | −2.89 ± 0.85 | −3.00 ± 0.90 | −2.93 ± 0.87 |
| ∆ average parental height SDSb | −2.32 ± 1.14 | −2.55 ± 1.27 | −2.40 ± 1.19 |
| Historical growth rate, cm/yearc | 3.9 ± 2.0 | 3.9 ± 1.7 | 3.9 ± 1.9 |
| Height velocity SDS | −2.20 ± 2.22 | −2.14 ± 2.02 | −2.18 ± 2.14 |
| BMI, kg/m2 | 16.1 ± 1.8 | 16.5 ± 2.2 | 16.2 ± 1.9 |
| BMI SDS | −0.32 ± 0.95 | −0.14 ± 1.07 | −0.25 ± 0.99 |
| Laboratory assessmentsa | |||
| Peak GH stimulation test, ng/mL | 5.9 ± 2.8 | 5.5 ± 3.0 | 5.8 ± 2.8 |
| IGF-1, ng/mL | 78.4 ± 43.9 | 88.1 ± 56.8 | 81.7 ± 48.8 |
| IGF-1 SDS | −2.08 ± 0.88 | −1.96 ± 0.98 | −2.04 ± 0.92 |
| Hemoglobin A1c, % | 5.05 ± 0.32 | 5.00 ± 0.33 | 5.04 ± 0.33 |
| Fasting glucose, mg/dL | 87.1 ± 9.72 | 88.9 ± 9.15 | 87.7 ± 9.54 |
Abbreviations: BMI, body mass index; GH, growth hormone; GHD, growth hormone deficiency; hGH, human growth hormone (somatropin); IGF-1, insulin-like growth factor 1; MPHD, multiple pituitary hormone deficiencies; SDS, SD score.
a Plus-minus values are means ± SD.
b ∆ average parental height SDS is the difference between the patient’s height SDS and the average parental height SDS where average parental height SDS = [height SDSmother + height SDSfather]/2.
c Historical growth rates based on best available medical records (weekly lonapegsomatropin n = 94 and daily somatropin n = 54).
Efficacy
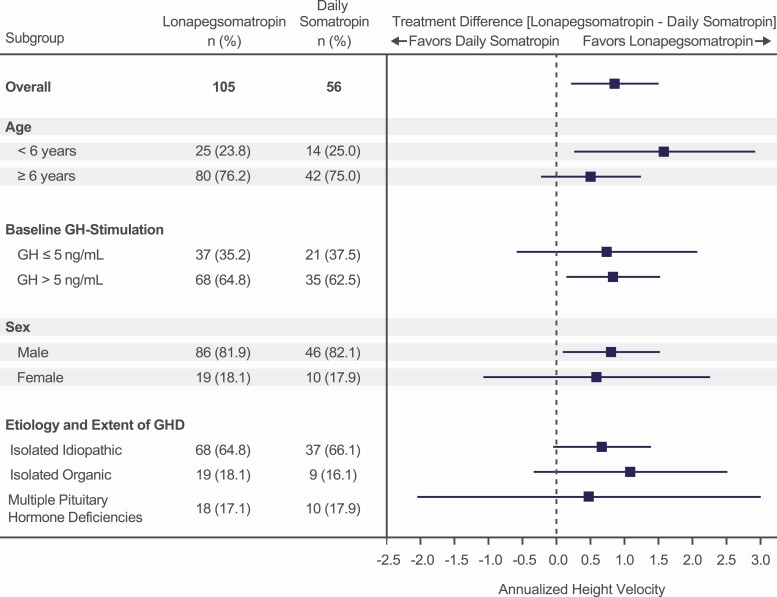
At week 52, patients receiving weekly lonapegsomatropin had an ANCOVA-adjusted least squares (LS) mean (SE) AHV of 11.2 (0.2) cm/year compared to 10.3 (0.3) cm/year for those receiving daily somatropin (P = 0.009) (Table 2), with lonapegsomatropin demonstrating noninferiority and superiority over daily somatropin. The observed AHV range was 5.9 to 18.0 cm/year and 4.7 to 16.3 cm/year for lonapegsomatropin and daily somatropin, respectively. The treatment difference in AHV favoring lonapegsomatropin started at week 5 and continued to the end of the trial, becoming statistically significant from week 26 onward (Table 3). In all prespecified subgroup analyses of AHV at week 52, the treatment difference favored lonapegsomatropin (Fig. 3).
Table 2.
End points at week 52 for growth, IGF-1, bone maturation, and metabolism
| Weekly lonapegsomatropin 0.24 mg hGH/kg/wk n = 105 LS meana (SE) |
Daily somatropin 0.24 mg hGH/kg/wk n = 56 LS meana (SE) |
Estimated treatment difference (95% CI) |
P-value | |
|---|---|---|---|---|
| Annualized height velocity, cm/year | 11.2 (0.2) | 10.3 (0.3) | 0.9 (0.2, 1.5) | 0.0088 |
| Change in height SDS from baseline | 1.10 (0.04) | 0.96 (0.05) | 0.14 (0.03, 0.26) | 0.0149 |
| Height velocity SDS | 5.88 (0.31) | 5.06 (0.39) | 0.82 (−0.04, 1.67) | 0.0616 |
| BMI SDSb | −0.03 (0.84) | −0.40 (1.00) | N/A | N/A |
| Average IGF-1 SDS | 0.72 (0.09) | −0.02 (0.12) | 0.74 (0.49, 1.0) | <0.0001 |
| Hemoglobin A1c,b % | 5.19 (0.34) | 5.11 (0.28) | N/A | N/A |
| Fasting glucose,b mg/dL | 87.9 (8.20) | 91.0 (9.14) | N/A | N/A |
| Bone age/chronological ageb | 0.75 (0.15) | 0.76 (0.14) | N/A | N/A |
Abbreviations: BMI, body mass index; CI, confidence interval; hGH, human growth hormone (somatropin); IGF-1 insulin-like growth factor 1; LS, least squares; SDS, SD score; SE, standard error.
a LS mean by analysis of covariance.
b Presented as mean (SD).
Table 3.
Annualized height velocity (cm/year) by visit
| Visit | Weekly lonapegsomatropin 0.24 mg hGH/kg/wk n = 105 LS meana (SE) |
Daily somatropin 0.24 mg hGH/kg/wk (n = 56) LS meana (SE) |
Mean treatment difference (95% CI) |
P-value |
|---|---|---|---|---|
| Week 5 | 13.5 | 12.8 | 0.7 (-2.3-3.7) | 0.6402 |
| Week 13 | 13.3 | 12.2 | 1.1 (-0.3-2.4) | 0.1286 |
| Week 26 | 12.7 | 11.2 | 1.4 (0.5-2.3) | 0.0017 |
| Week 39 | 11.9 | 10.9 | 1.0 (0.3-1.7) | 0.0061 |
| Week 52 | 11.2 | 10.3 | 0.9 (0.2-1.5) | 0.0088 |
Abbreviations: hGH, human growth hormone (somatropin).
aLS mean by analysis of covariance.
Figure 3.
Subgroup analysis of annualized height velocity. Lines represent 95% CI.
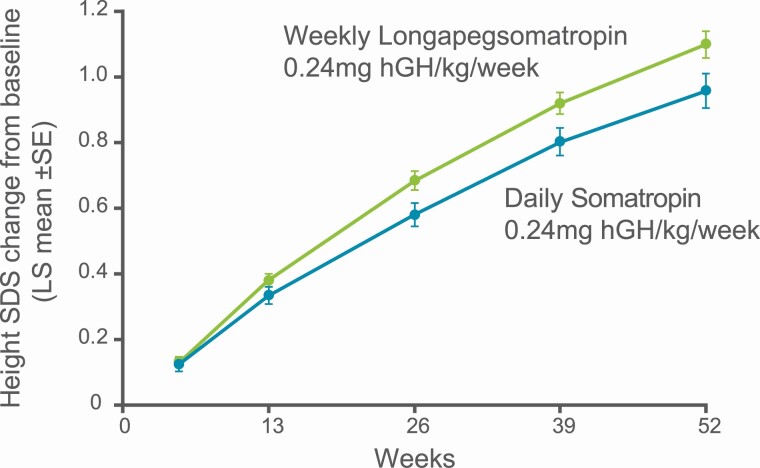
The change from baseline in height SDS LS mean increased in both treatment groups at every visit (Fig. 4), with an increase of 1.10 SDS for lonapegsomatropin and 0.96 SDS for daily somatropin at week 52 (estimated difference = 0.14 SDS; P = 0.01) (Table 2). Robust first year “catch-up” growth was observed with a LS mean height velocity SDS of 5.88 and 5.06 for lonapegsomatropin and daily somatropin, respectively. The bone age/chronological age ratio advanced similarly in both groups (Table 2).
Figure 4.
Change from baseline in height SD score.
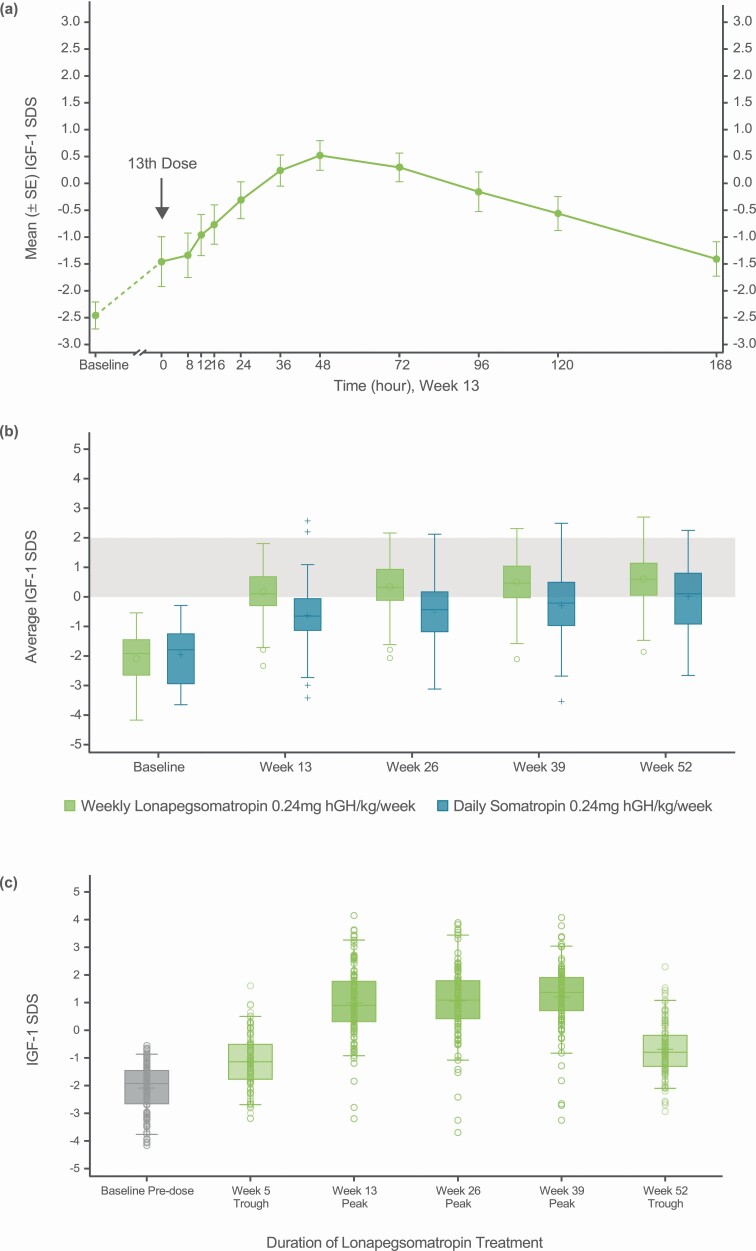
In the subset of 11 patients who underwent intensive blood sampling at week 13, serum hGH concentrations increased slowly following administration of lonapegsomatropin, followed by a prolonged decline, with a maximum concentration (geometric mean) of 15.2 ng/mL occurring at a median of 12 h. IGF-1 levels increased gradually following administration of lonapegsomatropin with maximum response at approximately 48 h post dose (Fig. 5A). IGF-1 levels returned to predose levels by the end of the dose interval, indicating that steady state was reached by week 13. As can be observed from the figure, the fluctuation over the week from peak to trough was approximately 2 SDS. The average IGF-1/average IGFBP-3 ratio for lonapegsomatropin-treated patients was similar to the IGF-1/IGFBP-3 ratio for daily somatropin-treated patients at week 13.
Figure 5.
(A) Insulin-like growth factor 1 (IGF-1) weekly profile following lonapegsomatropin administration. (B) Average IGF-1 SD score (SDS) over 52 weeks of treatment. (C) Observed IGF-1 SDS at baseline, peaks, and troughs for the lonapegsomatropin group. (A) shows the IGF-1 profile from a lonapegsomatropin population subset (n = 11) over 7 days following the 13th dose, with a steady state SDS difference between peak and trough of approximately 2. The box-and-whisker plots in (B) show the derived average (lonapegsomatropin) and observed (daily somatropin) IGF-1 SDS over 52 weeks in the intention-to-treat population. For the daily somatropin group, average IGF-1 SDS were represented by observed values given the relative stability of IGF-1 levels with daily somatropin. At all visits, the lonapegsomatropin group had a higher average IGF-1 SDS, thus reaching a range of IGF-1 SDS 0-2 (shaded grey area) sooner than did the daily somatropin group. For each box-and-whisker plot, the upper and lower box edges represent the 75th and 25th percentiles. Within the box, the horizontal bar indicates the median, while the circle (for lonapegsomatropin) or plus sign (for daily somatropin) corresponds to the mean. The upper and lower ends of the whiskers represent the highest and lowest observed values within 1.5 times the interquartile range above and below the 75th and 25th percentiles, respectively. Outside the box, open circles (lonapegsomatropin) and plus signs (daily somatropin) correspond to observed values beyond 1.5 times the interquartile range above and below the 75th and 25th percentiles. Similarly, the box-and-whisker plots for observed IGF-1 levels for lonapegsomatropin-treated subjects at baseline, troughs (weeks 5 and 52), and peaks (weeks 13, 26, and 39) are presented in (C). The circles represent individual subject levels.
Estimated average IGF-1 SDS increased in both groups relative to baseline and over time (Fig. 5B). Compared to the daily somatropin group, the lonapegsomatropin group reached an IGF-1 SDS range of 0 to 2 earlier and remained within this range, with a higher average IGF-1 SDS throughout the trial (LS mean for lonapegsomatropin +0.72 vs daily somatropin −0.02 at week 52). The augmented IGF-1 values in the lonapegsomatropin group compared to the daily somatropin group were consistent with greater longitudinal growth (AHV and change in height SDS from baseline) (Table 2). Estimated average IGF-1 SDS levels rarely exceeded 2.0 at any time [in subjects treated with lonapegsomatropin, 8 (7.6%) had ≥1 average IGF-1 >2.0 at any time during the trial; in subjects treated with daily somatropin, 2 (3.6%) had ≥1 average IGF-1 >2.0 at any time during the trial] and never exceeded 3.0 in either group. In the lonapegsomatropin group, the observed mean (SD) IGF-1 SDS level was +1.20 (1.22) at anticipated peak (2-3 days post dose) at week 39 and was −0.69 (0.98) at anticipated trough (6-7 days post dose) at week 52 (Fig. 5C).
Safety
There were no serious AEs (SAEs) related to the study drug and no AE led to treatment discontinuation or death. Rates of SAEs and AEs were similar between groups.
Two SAEs occurred. One patient administered lonapegsomatropin required an appendectomy for appendicitis, and 1 patient administered daily somatropin suffered a concussion requiring 1 night of hospitalization. Both SAEs were deemed unrelated to study drug and resolved without sequalae. AEs were reported in 81/105 (77.1%) and 39/56 (69.6%) patients administered lonapegsomatropin and daily somatropin, respectively, with 11.4% and 17.9% considered related to study treatment.
The most common AEs in patients administered lonapegsomatropin or daily somatropin were upper respiratory tract infection (37.1% and 41.4%, respectively), pyrexia (15.2% and 8.9%, respectively), and headache (12.4% and 12.5%, respectively). New onset or worsening deficiencies of other pituitary axes (ie, secondary hypothyroidism, secondary adrenal insufficiency, diabetes insipidus) were similar in both groups (lonapegsomatropin: secondary hypothyroidism 6.7%, secondary adrenal insufficiency 1.9%, diabetes insipidus 1.0%; daily somatropin: secondary hypothyroidism 7.1%, secondary adrenal insufficiency 1.8%, diabetes insipidus 1.8%). Dose reductions occurred in 2/105 (1.9%) lonapegsomatropin-treated patients due to elevated IGF-1 levels (asymptomatic), and in 1/56 (1.8%) daily somatropin-treated patient, associated with facial edema.
Two patients administered lonapegsomatropin reported injection site-related AEs. One experienced lipoatrophy after repeated injections to the same site, contrary to protocol recommendations. The other reported an episode of urticaria. One patient administered daily somatropin reported an injection site-related AE of swelling. All 3 AEs were isolated, resolved without sequelae, and the patients continued in the trial.
A low titer of anti-hGH binding antibodies were detected in 7/105 (6.7%) and 2/56 (3.6%) patients administered lonapegsomatropin and daily somatropin, respectively. No neutralizing antibodies were detected; detected antibodies did not appear to affect safety or efficacy.
Observed mean fasting glucose and hemoglobin A1c levels were generally stable over time and were maintained within the normal range for both groups throughout the trial (Tables 1 and 2). Observed mean BMI SDS remained within the normal range, with an increase from −0.32 to −0.03 in the lonapegsomatropin group and a decrease from −0.14 to −0.40 in the daily somatropin group.
Discussion
Development of a successful LAGH has been a long-standing and scientifically challenging endeavor, and it has been shown that altering the molecular structure of somatropin to prolong circulation time may result in suboptimal efficacy, unacceptable safety, and/or poor tolerability (17-20). The Phase 3 heiGHt trial of weekly lonapegsomatropin in treatment-naïve pediatric patients with GHD demonstrated superior AHV and statistically greater change in height SDS from baseline compared to a commercially available daily somatropin of equivalent weekly dose, with a similar safety and tolerability profile. Importantly, the bone age to chronological age ratio advanced similarly in both groups, suggesting that the increased rate of longitudinal growth did not occur at the expense of accelerated skeletal maturation.
The ultimate goal of developing a LAGH is to create a more convenient dosing regimen to potentially improve adherence and clinical outcomes (7). To allow for less frequent dosing, 2 basic approaches have been followed: (1) combining unmodified GH with a prolongation technology as with lonapegsomatropin or (2) extending the half-life through protein modification (eg, through albumin binding or protein enlargement). Two LAGHs in Phase 3 development for pediatric GHD that employ the latter approach include somapacitan (Novo Nordisk A/S), a LAGH containing recombinant hGH with a single point mutation to which a terminal fatty acid is attached with noncovalent albumin-binding properties, and somatrogon (OPKO Health and Pfizer Inc.), a LAGH containing hGH and 3 carboxyterminal peptide copies of the beta chain of human chorionic gonadotropin. Although somapacitan has recently gained approval for adult GHD, there are currently no LAGH products approved for pediatric GHD in the United States and Europe. Jintrolong® (Gene Science), a pegylated recombinant human GH delivered weekly, is available for use in China. These differing approaches to extend the half-life of GH also lead to different pharmacokinetic and pharmacodynamic profiles among LAGH products. The most easily measured indicator of the effectiveness of a GH therapy in children is linear growth; the evaluation of response during the first year (ie, catch-up growth) appears to be most important for prediction models calculating final height (21-23). In a Phase 2 study, mean AHV for the somapacitan 0.16 mg/kg/week group was 12.9 cm/year compared to 11.4 cm/year with daily somatropin 0.034 mg/kg/day at week 26 (not significantly different), with comparable safety (24). Somatrogon demonstrated noninferior AHV compared to daily somatropin (10.10 cm/year vs 9.78 cm/year) with a similar safety profile in a Phase 3 study (25). In comparison, AHV for lonapegsomatropin diverged early and was numerically greater at week 5, achieving significance and superiority at and beyond 26 weeks, confirming observations of the Phase 2 trial (8).
The lonapegsomatropin pharmacokinetic profile in this trial was similar to that observed in the Phase 2 trial, where ACP-001 (a bioequivalent predecessor to lonapegsomatropin) at 0.21 mg hGH/kg/wk demonstrated comparable somatropin exposure to daily somatropin at an equivalent weekly dose (8). Despite similar levels of exposure, it is hypothesized that the pharmacokinetic profile with sustained release of somatropin from lonapegsomatropin may lead to improved engagement of GH receptors in target tissues, including the growth plate, with resultant higher AHV compared to daily somatropin. Increased AHV as a result of improved receptor engagement is supported by higher IGF-1 levels in lonapegsomatropin-treated patients, suggesting preservation of the biological balance between direct GH and indirect IGF-1 effects. In a study by Surya et al, continuous infusion of somatropin was more effective at increasing both the plasma IGF-1 concentration and IGF-1 messenger RNA levels in human muscle compared to pulsatile exposure (26). These data are further supported by studies in adults with GHD, where continuous infusion of somatropin was associated with significantly higher IGF-1 levels compared to bolus injections at an equivalent daily dose (27,28).
The PK and PD profiles of a LAGH are expected to differ from daily somatropin, given the different characteristics of the molecules. The weekly average IGF-1 is reported for lonapegsomatropin to best represent the overall exposure and allow for comparison to daily somatropin. Although some subjects experienced IGF-1 SDS excursions above 2, usually as peak levels, IGF-1 levels would be expected to return to predose levels at the end of the dose interval, as seen in the seen in Figure 5A.
An optimal replacement therapy should achieve the same tissue distribution, receptor activation and effects as endogenous GH. The impact of molecular size on tissue penetration has been investigated in the tibial growth plate in mice, showing that dextrans weighing ≥40 kDa did not enter the growth plate (29). Therefore, modification of the GH protein through enlargement may restrict access to some target tissues while still stimulating hepatic IGF-1 production, resulting in altered tissue ratios of somatropin and IGF-1 that may adversely affect safety and efficacy (9). In contrast, somatropin released from lonapegsomatropin is expected to distribute into peripheral tissues and activate GH receptors in an identical manner as endogenous GH. The similar IGF-1/IGFBP-3 ratios between the lonapegsomatropin- and daily somatropin-treated patients observed in this study further implicate a preservation of the physiological relationship between IGF-1 and its binding protein (30). Consistent with historic data for somatropin therapy, both the lonapegsomatropin and daily somatropin arms maintained a normal BMI SDS, further suggesting the conservation of the balance of the metabolic actions of GH and IGF-1 (31).
Lonapegsomatropin was not associated with increased AEs, immunogenicity, metabolic complications, or injection site reactions compared to daily somatropin, alleviating concerns relative to LAGH safety considerations outlined by the Growth Hormone Research Society (7). The majority of AEs reported were mild or moderate and represented usual ailments in a pediatric population. While intracranial hypertension, slipped capital femoral epiphysis, and development or worsening of diabetes have been reported with somatropin therapy, none were observed during this trial (32-34). Furthermore, similar to daily somatropin and as expected given the identical nature of the released somatropin, only a low incidence of binding antibodies and no neutralizing antibodies were observed following lonapegsomatropin treatment. In contrast to previous LAGH attempts that were associated with high rates of pain or lipoatrophy (17,19,20), only a low rate of injection-site reactions were reported with lonapegsomatropin, likely because the somatropin in the prodrug state is essentially inactive (35) and administered with an injection of low volume through a small needle.
This trial had some limitations. Given the administration of a weekly vs daily injection, neither patients nor investigators were blinded. Doses of both drugs were fixed to 0.24 mg hGH/kg/week to allow for straightforward comparison, even though in the clinical setting doses may be titrated based on an individual patient’s sensitivity to GH and targeted treatment effect. Additional studies are needed to better understand the effect of weekly lonapegsomatropin on dosing adherence in a real-world setting. Although adherence rates were similarly high in both arms of this trial, high levels of adherence are anticipated in a controlled clinical trial and may not be reflective of a real-world setting. The decrease in the number of injections is expected to reduce the overall treatment burden and may improve adherence in a real-world setting, potentially translating to improved overall outcomes (36-38).
The fundamental challenge of developing a LAGH is to create a more convenient dosing regimen while retaining the excellent safety, efficacy, and tolerability of daily somatropin. Building on the concept of releasing unmodified somatropin to maintain physiologic distribution, weekly lonapegsomatropin is the first LAGH with data demonstrating superior efficacy compared to a daily somatropin, while maintaining similar bone age advancement, AE profile, and immunogenicity. Lonapegsomatropin may represent an important therapeutic option for children with GHD.
Acknowledgments
We thank the study participants and their families, investigators, and study site staff. We also wish to acknowledge Drs. Pierre Chatelain, Mitchell Geffner, and Ron Newfield; members of the trial’s independent safety committee; and the following employees of Ascendis Pharma: Karin Heidmann and Kenny Kamineni for clinical trial management; Jessica M. Peng and Allison S. Komirenko for scientific review; Per Holse Mygind for immunogenicity assessments; and Andrew Occiano and Eva Mortensen for writing and editorial assistance.
Funding: This study was sponsored by Ascendis Pharma Endocrinology Division A/S.
Clinical Trial Information:
Additional Information
Disclosures: P.S.T. has received research funding from Ascendis Pharma, Novo Nordisk, Pfizer, and OPKO. A.K.M. has received research funding and is an advisory board consultant for Ascendis Pharma, Novo Nordisk, OPKO, and Pfizer. E.A., E.C., T.K., E.G., M.K.S., and P.L.H. are research investigators for Ascendis Pharma. E.V. is a research investigator for Ascendis Pharma, OPKO, and Janssen and has received honoraria for lectures from Sandoz, Eli Lilly, and Novo Nordisk. Z.L., W.S., and A.D.S. are employees of Ascendis Pharma, Inc. D.B.K. was an employee of Ascendis Pharma, Inc. at time of work. E.D.C., V.M.B., and M.B. are employees of Ascendis Pharma A/S.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Deijen JB, van Driel MI, Drent ML. The involvement of the GH/IGF-I axis in cognitive functions of adult patients and healthy subjects. Open Endocrinol J. 2012;6:68-79. [Google Scholar]
- 2. Hazem A, Elamin MB, Bancos I. Body composition and quality of life in adults treated with GH therapy: a systematic review and meta-analysis. Eur J Endocrinol. 2012;166(1):13-20. [DOI] [PubMed] [Google Scholar]
- 3. Isgaard J, Arcopinto M, Karason K, Cittadini A. GH and the cardiovascular system: an update on a topic at heart. Endocrine. 2015;48(1):25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152-177. [DOI] [PubMed] [Google Scholar]
- 5. Guyda HJ. Four decades of growth hormone therapy for short children: what have we achieved? J Clin Endocrinol Metab. 1999;84(12):4307-4316. [DOI] [PubMed] [Google Scholar]
- 6. Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Horm Res Paediatr. 2013;79(4):189-196. [DOI] [PubMed] [Google Scholar]
- 7. Christiansen JS, Backeljauw PF, Bidlingmaier M, et al. Growth hormone research society perspective on the development of long-acting growth hormone preparations. Eur J Endocrinol. 2016;174(6):C1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chatelain P, Malievskiy O, Radziuk K, et al. ; TransCon GH Working Group . A randomized Phase 2 study of long-acting TransCon GH vs daily GH in childhood GH deficiency. J Clin Endocrinol Metab. 2017;102(5):1673-1682. [DOI] [PubMed] [Google Scholar]
- 9. Sprogøe K, Mortensen E, Karpf DB, Leff JA. The rationale and design of TransCon Growth Hormone for the treatment of growth hormone deficiency. Endocr Connect. 2017;6(8):R171-R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51(3):170-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;( 246):1-190. [PubMed] [Google Scholar]
- 12. Cole TJ. Galton’s midparent height revisited. Ann Hum Biol. 2000;27(4):401-405. [DOI] [PubMed] [Google Scholar]
- 13. Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child. 1966;41(220):613-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. I. Arch Dis Child. 1966;41(219):454-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bidlingmaier M, Friedrich N, Emeny RT, et al. Reference intervals for insulin-like growth factor-1 (IGF-I) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712-1721. [DOI] [PubMed] [Google Scholar]
- 16. Friedrich N, Wolthers OD, Arafat AM, et al. Age- and sex-specific reference intervals across life span for insulin-like growth factor binding protein 3 (IGFBP-3) and the IGF-I to IGFBP-3 ratio measured by new automated chemiluminescence assays. J Clin Endocrinol Metab. 2014;99(5):1675-1686. [DOI] [PubMed] [Google Scholar]
- 17. Reiter EO, Attie KM, Moshang T Jr, et al. ; Genentech, Inc.-Alkermes, Inc. Collaborative Study Group . A multicenter study of the efficacy and safety of sustained release GH in the treatment of naive pediatric patients with GH deficiency. J Clin Endocrinol Metab. 2001;86(10):4700-4706. [DOI] [PubMed] [Google Scholar]
- 18. de Schepper J, Rasmussen MH, Gucev Z, Eliakim A, Battelino T. Long-acting pegylated human GH in children with GH deficiency: a single-dose, dose-escalation trial investigating safety, tolerability, pharmacokinetics and pharmacodynamics. Eur J Endocrinol. 2011;165(3):401-409. [DOI] [PubMed] [Google Scholar]
- 19. Touraine P, D’Souza GA, Kourides I, et al. ; GH Lipoatrophy Study Group . Lipoatrophy in GH deficient patients treated with a long-acting pegylated GH. Eur J Endocrinol. 2009;161(4):533-540. [DOI] [PubMed] [Google Scholar]
- 20. Khadilkar V, Radjuk KA, Bolshova E, et al. 24-month use of once-weekly GH, LB03002, in prepubertal children with GH deficiency. J Clin Endocrinol Metab. 2014;99(1):126-132. [DOI] [PubMed] [Google Scholar]
- 21. Wit JM, Ranke MB, Albertsson-Wikland K, et al. Personalized approach to growth hormone treatment: clinical use of growth prediction models. Horm Res Paediatr. 2013;79(5):257-270. [DOI] [PubMed] [Google Scholar]
- 22. Straetemans S, De Schepper J, Thomas M, Verlinde F, Rooman R; BESPEED . Validation of prediction models for near adult height in children with idiopathic growth hormone deficiency treated with growth hormone: a belgian registry study. Horm Res Paediatr. 2016;86(3):161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ranke MB, Lindberg A; KIGS International Board . Observed and predicted growth responses in prepubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. 2010;95(3):1229-1237. [DOI] [PubMed] [Google Scholar]
- 24. Savendahl L, Battelino T, Brod M, et al. ; Real 3 Study Group . Once-weekly somapacitan vs daily GH in children with GH deficiency: results from a randomized phase 2 trial. J Clin Endocrinol Metab. 2020;105(4):e1847-e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deal C, Pastrak A, Silverman LA, Valluri SR, Wajnrajch M, Cara JF. OR10-06 somatrogon growth hormone in the treatment of pediatric growth hormone deficiency: results of the pivotal pediatric phase 3 clinical trial. J Endocr Soc. 2020;4(Suppl 1):OR10-06. [Google Scholar]
- 26. Surya S, Horowitz JF, Goldenberg N, et al. The pattern of growth hormone delivery to peripheral tissues determines insulin-like growth factor-1 and lipolytic responses in obese subjects. J Clin Endocrinol Metab. 2009;94(8):2828-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jørgensen JO, Møller N, Lauritzen T, Alberti KG, Orskov H, Christiansen JS. Evening versus morning injections of growth hormone (GH) in GH-deficient patients: effects on 24-hour patterns of circulating hormones and metabolites. J Clin Endocrinol Metab. 1990;70(1):207-214. [DOI] [PubMed] [Google Scholar]
- 28. Laursen T, Møller J, Jørgensen JO, Orskov H, Christiansen JS. Bioavailability and bioactivity of intravenous vs subcutaneous infusion of growth hormone in GH-deficient patients. Clin Endocrinol. 1996;45(3):333-339. [DOI] [PubMed] [Google Scholar]
- 29. Farnum CE, Lenox M, Zipfel W, Horton W, Williams R. In vivo delivery of fluoresceinated dextrans to the murine growth plate: imaging of three vascular routes by multiphoton microscopy. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(1):91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Löfqvist C, Andersson E, Gelander L, et al. Reference values for insulin-like growth factor-binding protein-3 (IGFBP-3) and the ratio of insulin-like growth factor-I to IGFBP-3 throughout childhood and adolescence. J Clin Endocrinol Metab. 2005;90(3):1420-1427. [DOI] [PubMed] [Google Scholar]
- 31. Reinehr T, Lindberg A, Koltowska-Häggström M, Ranke M. Is growth hormone treatment in children associated with weight gain?–longitudinal analysis of KIGS data. Clin Endocrinol. 2014;81(5):721-726. [DOI] [PubMed] [Google Scholar]
- 32. Darendeliler F, Karagiannis G, Wilton P. Headache, idiopathic intracranial hypertension and slipped capital femoral epiphysis during growth hormone treatment: a safety update from the KIGS database. Horm Res. 2007;68(Suppl 5):41-47. [DOI] [PubMed] [Google Scholar]
- 33. Harris M, Hofman PL, Cutfield WS. Growth hormone treatment in children: review of safety and efficacy. Paediatr Drugs. 2004;6(2):93-106. [DOI] [PubMed] [Google Scholar]
- 34. Stochholm K, Kiess W. Long-term safety of growth hormone-A combined registry analysis. Clin Endocrinol. 2018;88(4):515-528. [DOI] [PubMed] [Google Scholar]
- 35. Rau H, Hersel U, Wegge T, et al. Preclinical data for a novel once-weekly human growth hormone prodrug. Paper presented at: International Congress of Endocrinology; March 2010, Kyoto, Japan. [Google Scholar]
- 36. Amato G, Mazziotti G, Di Somma C, et al. Recombinant growth hormone (GH) therapy in GH-deficient adults: a long-term controlled study on daily versus thrice weekly injections. J Clin Endocrinol Metab. 2000;85(10):3720-3725. [DOI] [PubMed] [Google Scholar]
- 37. Iglay K, Cao X, Mavros P, Joshi K, Yu S, Tunceli K. Systematic literature review and meta-analysis of medication adherence with once-weekly versus once-daily therapy. Clin Ther. 2015;37(8):1813-21.e1. [DOI] [PubMed] [Google Scholar]
- 38. Shi L, Hodges M, Yurgin N, Boye KS. Impact of dose frequency on compliance and health outcomes: a literature review (1966-2006). Expert Rev Pharmacoecon Outcomes Res. 2007;7(2):187-202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.